Abstract
In the United States, ~1.4 million individuals identify as transgender. Many transgender adolescents experience gender dysphoria related to incongruence between their gender identity and sex assigned at birth. This dysphoria may worsen as puberty progresses. Puberty suppression by gonadotropin-releasing hormone agonists (GnRHa), such as leuprolide, can help alleviate gender dysphoria and provide additional time before irreversible changes in secondary sex characteristics may be initiated through feminizing or masculinizing hormone therapy congruent with the adolescent’s gender experience. However, the effects of GnRH agonists on brain function and mental health are not well understood. Here, we investigated the effects of leuprolide on reproductive function, social and affective behavior, cognition, and brain activity in a rodent model. Six-week-old male and female C57BL/6J mice were injected daily with saline or leuprolide (20 μg) for 6 weeks and tested in several behavioral assays. We found that leuprolide increases hyperlocomotion, changes social preference, and increases neuroendocrine stress responses in male mice, while the same treatment increases hyponeophagia and despair-like behavior in females. Neuronal hyperactivity was found in the dentate gyrus (DG) of leuprolide-treated females, but not males, consistent with the elevation in hyponeophagia and despair-like behavior in females. These data show for the first time that GnRH agonist treatment after puberty onset exerts sex-specific effects on social- and affective behavior, stress regulation, and neural activity. Investigating the behavioral and neurobiological effects of GnRH agonists in mice will be important to better guide the investigation of potential consequences of this treatment for youth experiencing gender dysphoria.
Subject terms: Limbic system, Developmental disorders
Introduction
An estimated 0.5 percent of U.S. adults identify as transgender [1]. Many transgender individuals experience gender dysphoria, i.e., psychological distress associated with incongruence between their gender identity and their sex assigned at birth [2, 3]. Gender dysphoria often leads to feelings of anxiety, depression, and social isolation at a young age, impacting school performance, cognition, and emotional maturation [3, 4]. In early adolescence, gender dysphoria may worsen when unwanted secondary sex characteristics develop during puberty [5]. In order to alleviate dysphoria, an increasing number of adolescents and their families seek medical and social interventions to affirm a gender expression more congruent with their experienced gender [6–9].
Early gender-affirming medical intervention most commonly is a two-step process. The first step is aimed at halting puberty development to suppress further emergence of secondary sex characteristics consistent with sex assigned at birth. The second step is therapy with feminizing or masculinizing hormones to initiate puberty progression consistent with the adolescent’s experienced gender [6, 10]. Pharmacological suppression of puberty is generally initiated in or after Tanner stage II, that is, shortly after the onset of puberty and the first emergence of secondary sex characteristics [11, 12]. Treatment with gonadotropin-releasing hormone agonists (GnRHa), such as leuprolide, are commonly used to halt normal progression of sexual development [13, 14]. The clinical purpose of GnRHa administration to transgender youth in early adolescence is: (1) to prevent further development of secondary sex characteristics consistent with sex assigned at birth, which risks increasing or perpetuating anatomic dysphoria and related social stigma; and (2) to gain time for (therapist-assisted) consideration of the transgender identity before starting hormone treatments that lead to irreversible development of secondary sex characteristics consistent with the individual’s gender identity.
While puberty suppression is therefore a crucial step to alleviate gender dysphoria and associated psychological distress [15, 16], the broader biological impact of interfering with GnRH signaling and with the crucial developmental process of puberty are largely unknown, particularly with respect to their impact on the brain. Clinical and preclinical studies have shown that GnRH agonists can have long-term consequences on bone density and visuospatial-, executive-, and memory function [17–23]. However, neurobiological effects of GnRHa treatment with regards to mental health have not yet been extensively explored.
The GnRH receptor (GnRHR) is found in many limbic brain regions involved in emotional and cognitive function, such as the hippocampus [24, 25]. This expression profile raises concerns that GnRHa treatment for puberty suppression may have neuropsychiatric consequences beyond those related to alleviating gender dysphoria and related anxiety. Specifically, GnRHa treatment has been shown to alter gene expression in the amygdala and in the hippocampus in sheep in a sex-dependent manner, and structural MRI studies have shown that GnRHa administration during puberty onset increases amygdala size in adulthood with greater effect sizes in females than in males [26, 27]. These previous studies indicate potential effects of GnRHa on the development of brain regions important for affective behavior, and highlight the need for more in-depth research in this vastly understudied area of mental health.
While modeling gender dysphoria and puberty suppression in rodents is challenging due to the widely unknown biological factors that influence gender identity, rodent models have the potential to isolate the biological effects of GnRHa treatment on brain function and behavior from the dysphoria and psychological distress associated with an incongruence between gender identity and sex assigned at birth. We, therefore, developed a mouse model to halt puberty progression by chronically treating adolescent mice with leuprolide after puberty onset. We then tested the effects of leuprolide on rodent behavioral phenotypes that are commonly associated with anxiety-like or depressive-like behavior, including social behavior, despair-like behavior, hyponeophagia, avoidance, cognitive function, and stress reactivity. In addition, we investigated neural activity in the hippocampus as a crucial brain structure for cognition, social behavior, stress processing and affective behavior [28, 29]. Our results show for the first time that chronic leuprolide treatment after puberty onset has sex-dependent effects on behavior, regional brain activity, and reproductive organ weights. In particular, leuprolide changes social preference and increases hyperlocomotion in male mice, while leuprolide-treated female mice show increased despair-like behavior, hyponeophagia, and hyperactivity of the dentate gyrus (DG) region of the hippocampus, which is a crucial regulator of stress responses and negative affect [29–31]. If we can improve our understanding of how GnRHa treatment affects puberty progression, brain function and behavior in an animal model, we may be able to better predict and understand both hormonal influences during adolescent critical periods where sex-specific emotional behaviors emerge, and the impact of pubertal suppression in human adolescents.
Materials and methods
Mice
Male and female C57BL/6J mice were purchased from Jackson Laboratories (Hudson, NY) at 4 weeks of age. Mice were housed 5 per cage in a 12-h (06:00–18:00) light-dark colony room at 22 °C. Food and water were provided ad libitum. Behavioral testing was performed during the light phase. Body weight measurements were taken throughout the study to assess the impact of leuprolide on weight gain or loss. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the New York State Psychiatric Institute (NYSPI).
Drugs
Leuprolide (GnRH agonist) treatment
Mice were injected subcutaneously (s.c.) with leuprolide antigens (20 μg, Sigma-Aldrich, MO) daily for 6 weeks. This dose was based on previous rodent studies investigating the effects of the GnRH agonist, triptorelin, on uterine development and hormone receptor expression [32]. Clinical doses in humans vary from 7.5 to 45 mg injections every 4 weeks (i.e., ~250–1450 μg/day). Considering that the basal metabolic rate of mice is about seven times that of a human, our dose of 20 μg/day would correspond to 140 μg/day in humans, which is slightly lower than the clinical dose. Control mice were injected with saline (0.9% NaCl) daily for 6 weeks.
Vaginal cytology
Vaginal cytology was evaluated for the first 2 weeks of leuprolide treatment using vaginal lavage and cytological identification (Fig. S1). Briefly, 50 μl of sterile saline was washed along the vaginal canal and then pipetted onto a glass microscope slide. Smears were imaged using the ×10 objective of a standard brightfield microscope (Zeiss, Oberkochen, Germany). Vaginal smears were classified into one of four estrous stages (e.g., proestrus, estrus, diestrus, or metestrus) depending on vaginal cell morphology [33].
Behavioral assays
Behavioral testing commenced after 2 weeks of leuprolide treatment. At this time point, all leuprolide-treated female mice had stopped cycling and were in metestrus or proestrus, indicating that leuprolide was effective in suppressing gonadal hormone release (Fig. S1). Please see Supplemental Methods for a full description of behavioral assays.
Tissue collection and processing
Three days following the last behavioral test, mice were exposed to a novel cage for 10 min. Blood samples were obtained by submandibular blood draw 30 min following novelty exposures, and mice were deeply anesthetized with (R,S)-ketamine (100 mg/kg) and xylazine (15 mg/kg) 30 min following blood collection. Half of all mice that underwent behavioral testing were perfused with 4% paraformaldehyde (PFA) for brain collection and immunohistochemistry. Brains were processed as previously described [34] and sliced into 50-μm sections using a vibratome. The remaining mice were euthanized by decapitation and reproductive organs were carefully dissected and weighed.
Immunohistochemistry
For c-fos immunohistochemistry, sections were washed in 1X phosphate-buffered saline (PBS) in three increments of 10 min each. Sections were then blocked in 10% normal donkey serum (NDS) in 1X PBS with 0.3% TritonX-100 (1X PBST) solution for 45 min. After blocking, sections were incubated in rabbit polyclonal IgG anti-c-fos (1:5000/3% NDS/97% PBST, SySy, Goettingen, Germany) for 3 days at 4 °C. Sections were then washed in three increments of 10 min each in 1X PBS and incubated in secondary antibody solution consisting of Alexa 488 conjugated Donkey Anti-Rabbit IgG (1:500, Life Technologies, Carlsbad, CA) for 2 h. Sections were washed again in 3 increments of 10 min each in 1X PBS. Finally, sections were mounted on slides and dried for ~30 min before Fluoromount G (Electron Microscopy Sciences, Hatfield, PA) and a cover slip were added.
Statistical analysis
All data were analyzed using Prism 7.0. Normality was tested using a Shapiro–Wilk test. Generally, the effect of drug or sex in parametric data was analyzed using two-way analysis of variance (ANOVA) where appropriate. Post hoc Sidak’s or Tukey’s multiple comparison’s test was used to correct for multiple comparisons where appropriate. For the novelty-suppressed feeding (NSF) test, Kaplan–Meier survival analysis and Mantel-Cox log-rank test was used to evaluate differences between the experimental groups with non-parametric data. To correct for multiple comparisons of Kaplan–Meier survival curves, the alpha error probability of = 0.05 was divided by the number of comparisons (k = 4) to generate a significance threshold of = 0.0125 for each individual comparison. For comparisons of two groups (e.g., to determine the impact of leuprolide on uterine horn weight), student’s t-tests were performed. Please see Table S1 for a summary of all statistical results.
Results
Leuprolide alters locomotion and social preference in male, but not in female mice
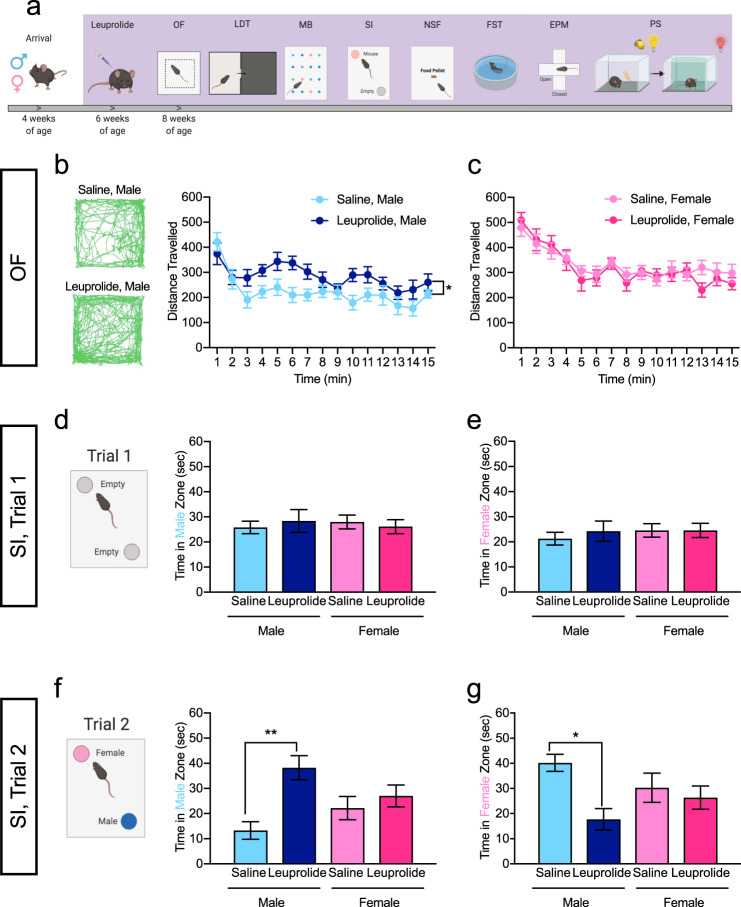
Male and female C57BL/6J mice were administered daily injections of saline or leuprolide starting at 6 weeks of age (Fig. 1a). At 8 weeks of age, leuprolide treatment halted estrous cycle changes in female mice (Fig. S1) without affecting body weight in either sex (Fig. S2). We first sought to determine if leuprolide administration changes locomotion in a sex-specific manner. During a 15-min assessment in the open-field (OF) arena, leuprolide-treated male mice exhibited increased locomotion when compared with saline-treated male mice and this effect was not observed in leuprolide-treated female mice (Fig. 1b, c; S3a). The percent of time spent in the center or periphery did not significantly differ between saline- and leuprolide-treated groups (Fig. S3b, c).
Fig. 1. Leuprolide increases locomotion and changes social preference in male mice, but not female mice.
a Timeline of leuprolide administration and behavioral testing. b Leuprolide increased total distance traveled in the OF in male mice. c Leuprolide did not increase the distance traveled in the OF in female mice. d During the 1st trial of the SI assay, all mice investigated the empty male zone equally. e During the 1st trial of the SI assay, all mice investigated the empty female zone equally. f During the 2nd trial of the SI assay, leuprolide-treated male mice spent more time in the male zone when compared with saline-treated male mice. Leuprolide treatment did not impact social preference of female mice. g During the 2nd trial of the SI assay, leuprolide-treated male mice spent less time in the female zone when compared with saline-treated male mice. Leuprolide treatment did not impact social preference of female mice. n = 9–10 mice per group. Error bars represent ± SEM. *p < 0.05; **p < 0.01. min minutes, sec seconds, OF open field test, SI social interaction test.
We next investigated whether leuprolide treatment affects social investigation of a same-sex and other-sex conspecific. Social preference for an other-sex conspecific has been shown to develop in adolescent mice at the time of puberty onset [35]. In our social investigation assay, during the habituation trial, two empty wire-mesh cups were placed in the arena (Fig. 1d) [36]. All mice investigated the empty male zone (Fig. 1d) and the empty female zone (Fig. 1e) equally during the first minute of trial 1, and the total time spent in the empty male- and female zones did not differ between groups (data not shown). These data indicate that mice were not biased towards one or the other zone of the arena. During the testing session (trial 2), saline-treated male mice investigated the female zone more than the male zone, consistent with previous studies (Fig. 1f, g, light blue bar) [35]. However, leuprolide-treated male mice spent more time in the male zone (Fig. 1f, dark blue bars) and less time in the female zone (Fig. 1g, dark blue bars) when compared with saline-treated male mice, indicating that leuprolide may reverse social preference in males. Moreover, we found a significant Treatment × Sex interaction on social interaction behavior (Fig. S1), suggesting that leuprolide-treated male mice may behave more similar to female mice in our social interaction task. No effect of leuprolide was observed in female mice (Fig. 1f, g). These data indicate that leuprolide impacts locomotion and social preference of male, but not of female mice.
Leuprolide increases hyponeophagia and despair-like behavior in female, but not in male mice
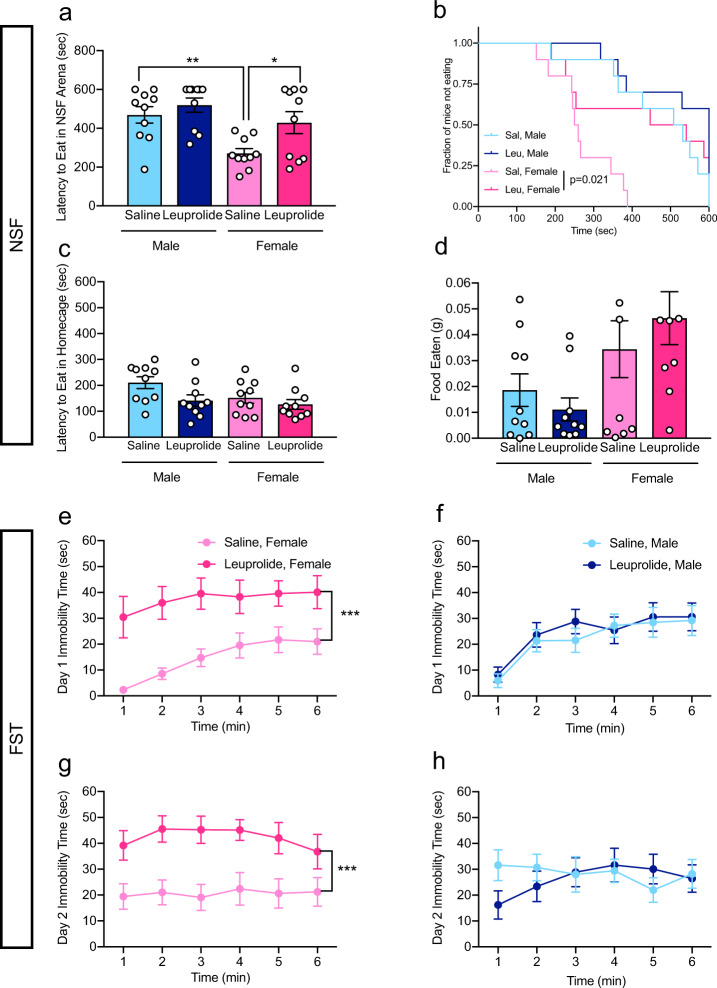
Considering that the GnRH receptor is expressed in limbic brain regions involved in affective behavior, such as the hippocampus [29–31], we next investigated whether leuprolide may affect behaviors that are commonly used to assess despair-like behavior and antidepressant responses. We therefore tested leuprolide-treated mice in the NSF paradigm and in the forced swim test (FST). These two antidepressant-responsive tests are commonly used to assess hyponeophagia and despair-like behavior, respectively [37, 38]. In the NSF paradigm, saline-treated female mice showed significantly lower latencies to feed in the novel arena compared with saline-treated male mice (Fig. 2a, b). Leuprolide treatment increased the latency to eat in the novel arena in female mice (similar to saline-treated male latencies), indicating elevated levels of hyponeophagia upon leuprolide treatment (Fig. 2a, b). This effect was absent in male mice. Leuprolide did not increase the latency to eat in the home cage (Fig. 2c) or the amount of food eaten in the home cage (Fig. 2d) in either sex, indicating that the differences in the latency to feed in the novel arena are indicative of hyponeophagia and not driven by differences in hunger between saline- and leuprolide-treated female mice.
Fig. 2. Leuprolide increases hyponeophagia and despair-like behavior in females, but not in males.
a, b Leuprolide increased the latency to eat in the NSF arena in female, but not in male mice. c Leuprolide did not impact the latency to eat in the home cage in either sex. d Leuprolide did not impact the amount of food eaten in the home cage in either sex. e Leuprolide increased immobility time of female mice in the FST on day 1. f Leuprolide did not impact immobility time of male mice in the FST on day 1. g Leuprolide increased immobility time of female mice in the FST on day 2. h Leuprolide did not impact immobility time of male mice in FST on day 2. n = 9–10 mice per group. Error bars represent ± SEM. *p < 0.05; **p < 0.01; ***p < 0.0001. sec seconds, g grams, NSF novelty-suppressed feeding, FST forced swim test, min minutes.
We next tested mice in the FST, a commonly used test to measure despair-like behavior, active coping, and antidepressant effectiveness in rodents [38]. In female mice, leuprolide treatment increased immobility time on day 1 (Fig. 2e) and on day 2 (Fig. 2g), indicating that leuprolide consistently increases despair-like behavior in female mice. This effect was absent in male mice (Fig. 2f, h). Taken together, these data suggest that leuprolide treatment increases hyponeophagia and despair-like behavior in female mice, without an effect in male mice.
Leuprolide does not impact avoidance behavior in female or male mice
To assess avoidance behavior, we next tested mice in the light-dark test (LDT), the marble burying assay (MB), and in the elevated plus maze (EPM). In the LDT, neither male nor female mice exhibited differences between the time spent in the light and dark side of the LDT (Fig. S3d, e), indicating that leuprolide did not impact avoidance behavior in the LDT. In the MB assay, all groups buried a comparable number of marbles (Fig. S3f). In the EPM, female mice spent more time in the open arms and closed arms and traveled longer distances than male mice. Leuprolide treatment had no effect on these measures in either sex (Fig. S3g–i). In summary, these data indicate that leuprolide administration does not significantly impact three complementary measures of avoidance behavior in either sex.
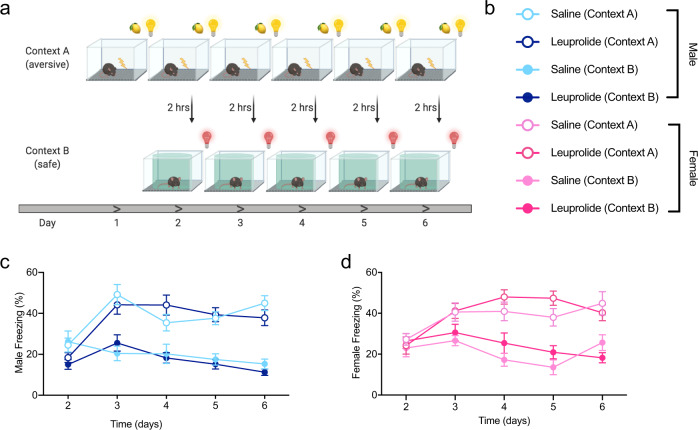
Leuprolide does not impact contextual fear discrimination learning in male or female mice
In light of previously reported effects of puberty suppression with GnRH agonists on cognitive function [21–23], we wanted to test whether cognitive functions that are related to mental health might be altered in leuprolide-treated mice. We, therefore, used a contextual fear discrimination (CFD) paradigm to test behavioral pattern separation (PS) ability in saline- and leuprolide-treated mice [39]. Throughout a 6-day CFD learning paradigm (Fig. 3a, b), all groups froze at comparable levels in the fear-associated context A, in which a shock was delivered every day. While overall freezing levels were lower in the non-fear associated context B (no-shock context), there was no difference in freezing between saline- and leuprolide-treated groups (Fig. 3c, d). These data indicate that leuprolide does not significantly impact CFD learning in male and female mice.
Fig. 3. Leuprolide does not impact contextual fear discrimination learning in male or female mice.
a Experimental design. b Experimental groups. c, d All groups of mice distinguish similarly between the aversive and safe contexts. n = 9–10 mice per group. Error bars represent ± SEM. *p < 0.05; hrs hours.
Leuprolide increases the corticosterone response to novelty exposure in male, but not in female mice
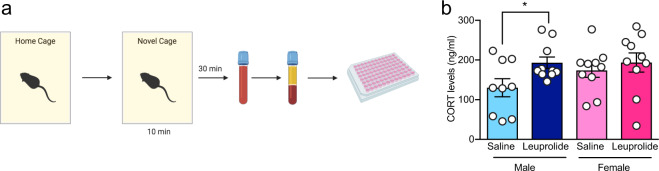
To test whether leuprolide has an effect on the neuroendocrine response to stress, we collected peripheral blood samples 30 min after mice were exposed to the mild stress of a novel cage environment (Fig. 4a). Leuprolide treatment increased the corticosterone response to novelty exposure (main effect of drug). Fisher’s LSD post hoc test revealed that this effect is significant in male mice, but not in female mice (Fig. 4b). These data suggest an elevated neuroendocrine response to a mild stressor in male mice, but not in female mice.
Fig. 4. Leuprolide increases the corticosterone response to novelty exposure in male, but not in female mice.
a Experimental design. b Leuprolide treatment increased the corticosterone response to novelty exposure in male, but not in female mice. n = 9–10 mice per group. Error bars represent ± SEM. *p < 0.05; ng nanogram, ml milliliters.
Leuprolide increases neural activity in the dentate gyrus of females, but not of male mice
We then wanted to investigate whether the increased despair-like behavior and hyponeophagia that we observed in leuprolide-treated females (shown in Fig. 3) is associated with changes in the activity of the hippocampus, a brain region that we previously found to be hyperactive in mice that show high levels of stress-induced behavioral abnormalities [31]. We quantified the number of DG neurons that were immunoreactive for the immediate early gene (IEG), c-fos, as a proxy marker for neural activity in response to the mild stress of a novel cage environment (Fig. 5a, b). Indeed, the number of c-fos+ cells was increased in the DG of leuprolide-treated female mice when compared with saline-treated mice (Fig. 5c), which is consistent with our previous studies that have shown an increase in the number of c-fos+ cells in the DG of the hippocampus in mice with increased stress vulnerability [31]. Moreover, we found a significant Treatment × Sex interaction on c-fos+ cells (Fig. S1), suggesting that leuprolide treatment may make female DG responses to mild stress more similar to DG activity of male mice. Leuprolide administration did not impact the number of c-fos+ cells in the DG of male mice, consistent with the lack of despair-like behavior and hyponeophagia in males (Fig. 5c).
Fig. 5. Leuprolide increases neural activity in the DG of female, but not male mice.
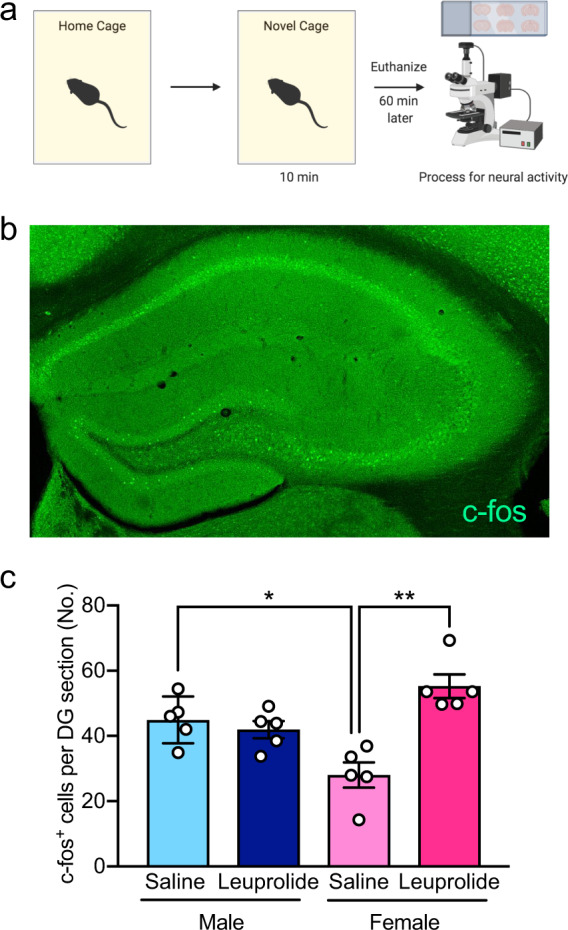
a Experimental design. b Representative image of c-fos+ cells in the hippocampus. c Leuprolide administration did not impact the number of c-fos+ cells in the DG of male mice when compared with saline administration. However, leuprolide administration increased the number of c-fos+ cells in the DG of female mice when compared with saline administration. Saline-treated female mice had significantly less c-fos+ cells in the DG when compared with saline-treated male mice. n = 5 mice per group. Error bars represent ± SEM. *p < 0.05; ***p < 0.001. DG dentate gyrus, min minutes, No number.
Leuprolide treatment reduces reproductive organ weight in female mice
Following behavioral assays after 6 weeks of treatment, we also assessed leuprolide effects on reproductive organ weights. Leuprolide decreased the weight of uterine horn tissue and ovaries in female mice (Fig. S4a–c) and decreased the gonadal somatic index (testes weight/body weight) of male mice (Fig. S4e) without affecting seminal vesicles (Fig. S4d) or fat pad weights (Fig. Sf). These data indicate that GnRHa treatment affects female and male reproductive systems.
Discussion
Treatment with the GnRH agonist, leuprolide, is commonly used in gender dysphoric youth after the initial onset of puberty to delay endogenous puberty progression and to provide more time for individuals to decide whether to initiate feminizing or masculinizing hormone therapy to affirm their gender identity [11, 12]. Leuprolide treatment in gender dysphoric adolescents to stop progressive development of secondary sex characteristics incongruent with their experienced gender identity has been shown to alleviate anxiety and depression [15]. However, the biological consequences associated with pubertal GnRH agonist treatment have been poorly characterized, and have focused primarily on physiological effects of leuprolide on bone density [17, 18]. In this rodent study, we thus wanted to determine the effects of leuprolide on negative affect, cognitive function, and activity changes in brain regions known to be important for mood and cognition. Our behavioral and neurobiological characterization reveals for the first time that chronic leuprolide treatment, starting after the onset of puberty, exerts sex-specific effects on social preference, despair-like behavior and hyponeophagia, neuroendocrine responses to mild stress, and hyperactivity of the DG, a crucial neurobiological regulator of stress responses in mice.
An important hallmark of puberty in mice is the development of male social preference for female mice [35]. We, thus, first used a social interaction assay to assess social preference. We found that saline-treated male mice prefer interacting with a female mouse, indicated by more time spent in the female zone than in the male zone. However, in leuprolide-treated male mice, social preference was reversed, as leuprolide-treated male mice spent more time interacting with the male mouse than the female mouse. This reversal of social preference could potentially be due to differences in aggressive behavior between saline- and leuprolide-treated mice, as previous studies have shown that male mice with disturbances in GnRH release show a lack of interest in females and displayed no aggressive behavior toward other male mice [40]. Interestingly, the effects of leuprolide on social preference seem to be specific to males, as female mice did not show any preference for the male- or the female target mouse regardless of treatment.
Our data show that saline-treated female mice exhibited decreased latencies to feed in the NSF arena and spent less time immobile in the FST than male mice. While it is important to emphasize that it is challenging to translate rodent behavior into mental health-related human phenotypes, these two behavioral tests are responsive to antidepressant treatment and have commonly be interpreted as indicators of hyponeophagia and despair-like behavior, respectively. However, in females but not in males, leuprolide increased the latency to feed in the NSF test and the time spent immobile in the FST, suggesting that leuprolide treatment increases hyponeophagia and despair-like behavior, respectively. Our findings are consistent with previous data in sheep, which have shown that leuprolide treatment impacts mood regulation in females, but not in males [41]. In humans, in addition to being a treatment for gender dysphoria, leuprolide is prescribed for several conditions, such as endometriosis pain, uterine fibrosis, precocious puberty, or prostate cancer [42–45]. Depression is a commonly reported side effect of leuprolide therapy when the treatment effects of leuprolide are not targeting a disorder that in itself can lead to psychological distress (such as gender dysphoria) [46–48]. It is important to emphasize that our rodent model of chronic leuprolide administration is characterized by an absence of gender dysphoria. While this model therefore has the disadvantage that it cannot accurately predict leuprolide effects on mental health of gender dysphoric adolescents, it has the advantage that it can disentangle the resolution of the psychological distress associated with the development of secondary sex characteristics incongruent with gender identity from the neurobiological effects of chronic GnRH agonist treatment. While our data indicate that leuprolide increases despair-like behavior and hyponeophagia in female mice, leuprolide treatment in adolescents suffering from gender dysphoria may indeed decrease depressive symptoms associated with the development of unwanted secondary sex characteristics. It is thus important to emphasize that for humans undergoing gender affirmation, the benefits of leuprolide treatment on mental health, particularly alleviation of gender dysphoria, may outweigh any detriments indicated by our rodent experiments. This ought to be carefully tested in future human studies. As we continue to improve our knowledge of the biological determinants of gender dysphoria in transgender youth [49], future studies should be aimed at administering GnRH agonists to rodent models that mimic biological aspects of gender incongruence.
While we found no effects of chronic leuprolide treatment on avoidance behaviors and contextual fear discrimination learning in either sex, leuprolide treatment increased neuroendocrine responses to mild stress, particularly in male mice. This modulatory effect of leuprolide on hypothalamus-pituitary adrenal (HPA) axis responses is likely due to the expression of GnRHR in major brain areas involved in HPA axis regulation, including the hippocampus, hypothalamus, or anterior pituitary. GnRH has been shown to activate unliganded glucocorticoid receptors (GR) via a GnRHR-dependent mechanism [50], suggesting that chronic GnRH treatment may alter HPA axis negative feedback regulation indirectly by affecting GR function, which could explain the increased corticosterone levels in novelty-exposed male mice.
In order to gain first insight into the neurobiological changes associated with chronic leuprolide treatment, we also investigated neural activity in the hippocampus, a brain region that is crucial for mood and anxiety regulation, stress resilience, and cognition [29–31, 51, 52]. In particular, we have previously shown that hyperactivity of the DG can promote stress-induced behavioral abnormalities in mice [31]. We, therefore, hypothesized that DG activity would be increased in leuprolide-treated female mice that showed higher levels of hyponeophagia and despair-like behavior. Indeed, we found an increased number of c-fos+ cells in the DG of leuprolide-treated female mice when compared with saline-treated female mice, but no effect in males, suggesting that the leuprolide-induced increase in hyponeophagia and despair-like behavior in females may be associated with DG hyperactivity.
It is important to note that some of our behavioral, neuroendocrine, and neurobiological findings could also be interpreted as changes in sex-specific behavior. In the NSF paradigm and in the FST, saline-treated females show lower latency to feed and less immobility than male mice, respectively. While an increase in these metrics is generally interpreted as an increase in hyponeophagia and despair-like behavior, an alternative interpretation could be that female mice actually behave more “male-like” in these tasks after GnRHa treatment. A similar interpretation could be applied to our findings on social preference, corticosterone levels, and DG activity: the male-specific change in social preference and the increase in corticosterone release following leuprolide treatment more closely resembles social preference and corticosterone levels in control female mice; and the female-specific increase in DG activity following leuprolide treatment more closely resembles the activity seen in saline-treated male mice. It is thus possible that our findings could indicate that chronic leuprolide treatment may reduce sex differences in behavior, neuroendocrine responsiveness, and hippocampus neural activity. It will be important for future studies to test this possible reduction in sex differences following treatment with leuprolide and other GnRH agonists in larger cohorts of mice.
In conclusion, we report that chronic leuprolide treatment in mice has profound effects on female behaviors that are commonly interpreted as depression-like, as well as on neural activity in the hippocampus—a brain region crucially involved in stress processing, depression, and cognition. While these mood-related effects are specific to females, leuprolide causes pronounced differences in locomotion and social preference in males and increases neuroendocrine responses to mild stress. Our results in an animal model shed new light on the effects of chronic GnRHa treatment on behavioral, neuroendocrine, and neurobiological effects of a drug that is commonly used for many clinical conditions, and particularly to alleviate gender dysphoria in transgender youth. Further research is needed to better understand the molecular and neurobiological mechanisms of leuprolide administration in a transgender context, in order to generate evidence to inform guidelines and clinical decision-making in transgender care. The present study demonstrates the potential of animal work to both guide and inform the interpretation of studies of pubertal suspension in humans.
Funding and disclosure
This project was supported by a generous Director’s Pilot Award from the Department of Psychiatry at Columbia University Irving Medical Center (CUIMC) to all authors. CA received funding from the National Institutes of Health (NIH) (R00 MH108719-04; 2P50MH090964-08) and from the Department of Psychiatry’s Depression Center at Columbia University. RD and RS received funding from NIH Research Supplements to Promote Diversity in Health-Related Research (2P50 MH090964-08S3 to RD; R00MH108719-04S1 to RS). ES was supported by an Amgen Scholars Summer Research Program scholarship. CAD receives funding from a Whitehall Foundation Grant, an NIH Transformative Award 1R01HD101402-01, an NIA 1R56AG058661-01A1, an NIA 1R21AG064774-01, and an NINDS 1R21NS114870-01. CA receives funding from Sunovion Pharmaceuticals to investigate compounds unrelated to this study. BKC and CAD are named on provisional patent applications for the prophylactic use of (R,S)-ketamine and other compounds against stress-related psychiatric disorders. ES, CCL, JCM, AM, HCH, RS, RD, BSM, WB, HFLMB, WB, and AAE have nothing to disclose.
Supplementary information
Acknowledgements
We thank members of the laboratories for insightful comments on this project and on the manuscript.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Deceased: Bruce S. McEwen.
Contributor Information
Christoph Anacker, Email: ca2635@cumc.columbia.edu.
Christine A. Denny, Email: cad2125@cumc.columbia.edu
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-020-00826-1).
References
- 1.Meyer IH, Brown TN, Herman JL, Reisner SL, Bockting WO. Demographic characteristics and health status of transgender adults in select US regions: behavioral risk factor surveillance system, 2014. Am J Public Health. 2017;107:582–9. doi: 10.2105/AJPH.2016.303648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byne W, Bradley SJ, Coleman E, Eyler AE, Green R, Menvielle E, et al. Treatment of gender identity disorder. Am J Psychiatry. 2012;169:875–6. doi: 10.1176/appi.ajp.2012.169.8.875. [DOI] [PubMed] [Google Scholar]
- 3.Leibowitz S, de Vries AL. Gender dysphoria in adolescence. Int Rev Psychiatry. 2016;28:21–35. doi: 10.3109/09540261.2015.1124844. [DOI] [PubMed] [Google Scholar]
- 4.de Vries AL, Steensma TD, Doreleijers TA, Cohen-Kettenis PT. Puberty suppression in adolescents with gender identity disorder: a prospective follow-up study. J Sex Med. 2011;8:2276–83.. doi: 10.1111/j.1743-6109.2010.01943.x. [DOI] [PubMed] [Google Scholar]
- 5.Steensma TD, Kreukels BP, de Vries AL, Cohen-Kettenis PT. Gender identity development in adolescence. Horm Behav. 2013;64:288–97.. doi: 10.1016/j.yhbeh.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 6.de Vries AL, Cohen-Kettenis PT. Clinical management of gender dysphoria in children and adolescents: the Dutch approach. J Homosex. 2012;59:301–20.. doi: 10.1080/00918369.2012.653300. [DOI] [PubMed] [Google Scholar]
- 7.Olson-Kennedy J, Cohen-Kettenis PT, Kreukels BP, Meyer-Bahlburg HF, Garofalo R, Meyer W, et al. Research priorities for gender nonconforming/transgender youth: gender identity development and biopsychosocial outcomes. Curr Opin Endocrinol Diabetes Obes. 2016;23:172–9. doi: 10.1097/MED.0000000000000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson-Kennedy J. Mental health disparities among transgender youth: rethinking the role of professionals. JAMA Pediatr. 2016;170:423–4. doi: 10.1001/jamapediatrics.2016.0155. [DOI] [PubMed] [Google Scholar]
- 9.Zucker KJ. Epidemiology of gender dysphoria and transgender identity. Sex Health. 2017;14:404–11. doi: 10.1071/SH17067. [DOI] [PubMed] [Google Scholar]
- 10.Byne W, Bradley SJ, Coleman E, Eyler AE, Green R, Menvielle EJ, et al. Disorder APATFoToGI. report of the american psychiatric association task force on treatment of gender identity disorder. Arch Sex Behav. 2012;41:759–96.. doi: 10.1007/s10508-012-9975-x. [DOI] [PubMed] [Google Scholar]
- 11.Shumer DE, Nokoff NJ, Spack NP. Advances in the care of transgender children and adolescents. Adv Pediatr. 2016;63:79–102. doi: 10.1016/j.yapd.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman E, Bockting W, Botzer M, Cohen-Kettenis P, DeCuypere G, Feldman J, et al. Standards of care for the health of transsexual, transgender, and gender-nonconforming people, version 7. Int J Transgenderism. 2012;13:165–232. [Google Scholar]
- 13.Costa R, Dunsford M, Skagerberg E, Holt V, Carmichael P, Colizzi M. Psychological support, puberty suppression, and psychosocial functioning in adolescents with gender dysphoria. J Sex Med. 2015;12:2206–14.. doi: 10.1111/jsm.13034. [DOI] [PubMed] [Google Scholar]
- 14.Chew D, Anderson J, Williams K, May T, Pang K. Hormonal treatment in young people with gender dysphoria: a systematic review. Pediatrics. 2018;141:e20173742. doi: 10.1542/peds.2017-3742. [DOI] [PubMed] [Google Scholar]
- 15.de Vries AL, McGuire JK, Steensma TD, Wagenaar EC, Doreleijers TA, Cohen-Kettenis PT. Young adult psychological outcome after puberty suppression and gender reassignment. Pediatrics. 2014;134:696–704. doi: 10.1542/peds.2013-2958. [DOI] [PubMed] [Google Scholar]
- 16.Abramowitz J. Hormone therapy in children and adolescents. Endocrinol Metab Clin North Am. 2019;48:331–9. doi: 10.1016/j.ecl.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Mohamad NV, Che Zulkepli MAA, May Theseira K, Zulkifli N, Shahrom NQ, Ridzuan NAM, et al. Establishing an animal model of secondary osteoporosis by using a gonadotropin-releasing hormone agonist. Int J Med Sci. 2018;15:300–8. doi: 10.7150/ijms.22732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klink D, Caris M, Heijboer A, van Trotsenburg M, Rotteveel J. Bone mass in young adulthood following gonadotropin-releasing hormone analog treatment and cross-sex hormone treatment in adolescents with gender dysphoria. J Clin Endocrinol Metab. 2015;100:E270–5. doi: 10.1210/jc.2014-2439. [DOI] [PubMed] [Google Scholar]
- 19.Schagen SE, Cohen-Kettenis PT, Delemarre-van de Waal HA, Hannema SE. Efficacy and safety of gonadotropin-releasing hormone agonist treatment to suppress puberty in gender dysphoric adolescents. J Sex Med. 2016;13:1125–32.. doi: 10.1016/j.jsxm.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Joseph T, Ting J, Butler G. The effect of GnRH analogue treatment on bone mineral density in young adolescents with gender dysphoria: findings from a large national cohort. J Pediatr Endocrinol Metab. 2019;32:1077–81. doi: 10.1515/jpem-2019-0046. [DOI] [PubMed] [Google Scholar]
- 21.Bryan KJ, Mudd JC, Richardson SL, Chang J, Lee HG, Zhu X, et al. Down-regulation of serum gonadotropins is as effective as estrogen replacement at improving menopause-associated cognitive deficits. J Neurochem. 2010;112:870–81.. doi: 10.1111/j.1471-4159.2009.06502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102:3869–903. doi: 10.1210/jc.2017-01658. [DOI] [PubMed] [Google Scholar]
- 23.Schneider MA, Spritzer PM, Soll BMB, Fontanari AMV, Carneiro M, Tovar-Moll F, et al. and others. Brain maturation, cognition and voice pattern in a gender dysphoria case under pubertal suppression. Front Hum Neurosci. 2017;11:528. doi: 10.3389/fnhum.2017.00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosati F, Sturli N, Cungi MC, Morello M, Villanelli F, Bartolucci G, et al. Gonadotropin-releasing hormone modulates cholesterol synthesis and steroidogenesis in SH-SY5Y cells. J Steroid Biochem Mol Biol. 2011;124:77–83. doi: 10.1016/j.jsbmb.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Schang AL, Ngô-Muller V, Bleux C, Granger A, Chenut MC, Loudes C, et al. GnRH receptor gene expression in the developing rat hippocampus: transcriptional regulation and potential roles in neuronal plasticity. Endocrinology. 2011;152:568–80.. doi: 10.1210/en.2010-0840. [DOI] [PubMed] [Google Scholar]
- 26.Skinner DC, Albertson AJ, Navratil A, Smith A, Mignot M, Talbott H, et al. Effects of gonadotrophin-releasing hormone outside the hypothalamic-pituitary-reproductive axis. J Neuroendocrinol. 2009;21:282–92.. doi: 10.1111/j.1365-2826.2009.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nuruddin S, Wojniusz S, Ropstad E, Krogenæs A, Evans NP, Robinson JE, et al. SOBER SOBERG. Peri-pubertal gonadotropin-releasing hormone analog treatment affects hippocampus gene expression without changing spatial orientation in young sheep. Behav Brain Res. 2013;242:9–16. doi: 10.1016/j.bbr.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 28.Nuruddin S, Bruchhage M, Ropstad E, Krogenæs A, Evans NP, Robinson JE, et al. and others. Effects of peripubertal gonadotropin-releasing hormone agonist on brain development in sheep-a magnetic resonance imaging study. Psychoneuroendocrinology. 2013;38:1994–2002. doi: 10.1016/j.psyneuen.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Anacker C, Hen R. Adult hippocampal neurogenesis and cognitive flexibility - linking memory and mood. Nat Rev Neurosci. 2017;18:335–46. doi: 10.1038/nrn.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anacker C, Scholz J, O’Donnell KJ, Allemang-Grand R, Diorio J, Bagot RC, et al. Neuroanatomic differences associated with stress susceptibility and resilience. Biol Psychiatry. 2016;79:840–9. doi: 10.1016/j.biopsych.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anacker C, Luna VM, Stevens GS, Millette A, Shores R, Jimenez JC, et al. Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature. 2018;559:98–102. doi: 10.1038/s41586-018-0262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei S, Guo H, Gong Z, Zhang F, Ma Z. Triptorelin and cetrorelix induce immune responses and affect uterine development and expressions of genes and proteins of ESR1, LHR, and FSHR of mice. Immunopharmacol Immunotoxicol. 2016;38:197–204. doi: 10.3109/08923973.2016.1168432. [DOI] [PubMed] [Google Scholar]
- 33.McLean AC, Valenzuela N, Fai S, Bennett SA. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp. 2012;67:e4389. doi: 10.3791/4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pavlova IP, Shipley SC, Lanio M, Hen R, Denny CA. Optimization of immunolabeling and clearing techniques for indelibly labeled memory traces. Hippocampus. 2018;28:523–35. doi: 10.1002/hipo.22951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panksepp JB, Jochman KA, Kim JU, Koy JJ, Wilson ED, Chen Q, et al. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS ONE. 2007;2:e351. doi: 10.1371/journal.pone.0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaidanovich-Beilin O, Lipina T, Vukobradovic I, Roder J, Woodgett JR. Assessment of social interaction behaviors. J Vis Exp. 2011;48;e2473. [DOI] [PMC free article] [PubMed]
- 37.Blasco-Serra A, González-Soler EM, Cervera-Ferri A, Teruel-Martí V, Valverde-Navarro AA. A standardization of the novelty-suppressed feeding test protocol in rats. Neurosci Lett. 2017;658:73–78. doi: 10.1016/j.neulet.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 38.Slattery DA, Cryan JF. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protoc. 2012;7:1009–14. doi: 10.1038/nprot.2012.044. [DOI] [PubMed] [Google Scholar]
- 39.Kheirbek MA, Klemenhagen KC, Sahay A, Hen R. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat Neurosci. 2012;15:1613–20.. doi: 10.1038/nn.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimshek DR, Bus T, Grinevich V, Single FN, Mack V, Sprengel R, et al. Impaired reproductive behavior by lack of GluR-B containing AMPA receptors but not of NMDA receptors in hypothalamic and septal neurons. Mol Endocrinol. 2006;20:219–31.. doi: 10.1210/me.2005-0262. [DOI] [PubMed] [Google Scholar]
- 41.Wojniusz S, Vögele C, Ropstad E, Evans N, Robinson J, Sütterlin S, et al. and others. Prepubertal gonadotropin-releasing hormone analog leads to exaggerated behavioral and emotional sex differences in sheep. Horm Behav. 2011;59:22–7. doi: 10.1016/j.yhbeh.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 42.Wex J, Sidhu M, Odeyemi I, Abou-Setta AM, Retsa P, Tombal B. Leuprolide acetate 1-, 3- and 6-monthly depot formulations in androgen deprivation therapy for prostate cancer in nine European countries: evidence review and economic evaluation. Clinicoecon Outcomes Res. 2013;5:257–69. doi: 10.2147/CEOR.S44855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown J, Farquhar C. An overview of treatments for endometriosis. JAMA. 2015;313:296–7. doi: 10.1001/jama.2014.17119. [DOI] [PubMed] [Google Scholar]
- 44.Neely EK, Lee PA, Bloch CA, Larsen L, Yang D, Mattia-Goldberg C, et al. Leuprolide acetate 1-month depot for central precocious puberty: hormonal suppression and recovery. Int J Pediatr Endocrinol. 2010;2010:398639. doi: 10.1155/2010/398639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jasonni VM, D’Anna R, Mancuso A, Caruso C, Corrado F, Leonardi I. Randomized double-blind study evaluating the efficacy on uterine fibroids shrinkage and on intra-operative blood loss of different length of leuprolide acetate depot treatment before myomectomy. Acta Obstet Gynecol Scand. 2001;80:956–8. doi: 10.1034/j.1600-0412.2001.801014.x. [DOI] [PubMed] [Google Scholar]
- 46.Freeman MP, Freeman SA. Treatment of leuprolide-induced depression with intramuscular testosterone: a case report. J Clin Psychiatry. 2003;64:341–3. doi: 10.4088/jcp.v64n0317h. [DOI] [PubMed] [Google Scholar]
- 47.Warnock JK, Bundren JC, Morris DW. Depressive symptoms associated with gonadotropin-releasing hormone agonists. Depress Anxiety. 1998;7:171–7. doi: 10.1002/(sici)1520-6394(1998)7:4<171::aid-da5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 48.Warnock JK, Bundren JC. Anxiety and mood disorders associated with gonadotropin-releasing hormone agonist therapy. Psychopharmacol Bull. 1997;33:311–6. [PubMed] [Google Scholar]
- 49.Mohammadi MR, Khaleghi A. Transsexualism: a different viewpoint to brain changes. Clin Psychopharmacol Neurosci. 2018;16:136–43. doi: 10.9758/cpn.2018.16.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kotitschke A, Sadie-Van Gijsen H, Avenant C, Fernandes S, Hapgood JP. Genomic and nongenomic cross talk between the gonadotropin-releasing hormone receptor and glucocorticoid receptor signaling pathways. Mol Endocrinol. 2009;23:1726–45.. doi: 10.1210/me.2008-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mastrodonato A, Martinez R, Pavlova IP, LaGamma CT, Brachman RA, Robison AJ, et al. Ventral CA3 activation mediates prophylactic ketamine efficacy against stress-induced depressive-like behavior. Biol Psychiatry. 2018;84:846–56. doi: 10.1016/j.biopsych.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Denny CA, Kheirbek MA, Alba EL, Tanaka KF, Brachman RA, Laughman KB, et al. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron. 2014;83:189–201. doi: 10.1016/j.neuron.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.