Myeloid-derived suppressor cells (MDSCs) are a group of heterogeneous cells1 that play crucial negative regulatory roles in immune-associated diseases, including infections,2 cancer,3 transplantation4, and autoimmunity.5 We and others have shown that mTOR signaling and its downstream metabolic pathways regulate MDSC recruitment and function in inflammation and autoimmunity.5–9 While AKT kinase family members, including ATK1, AKT2, and AKT3, are known downstream targets of mTOR, it is unclear whether any of them plays a role in regulating MDSC functions and metabolism. Herein, we report that AKT1 (also known as protein kinase B α, PKBα) regulates MDSC immunosuppressive activities by suppressing hypoxia-inducible factor 1α (HIF1α)-dependent glycolysis. Genetic ablation of AKT1 significantly impaired MDSC immunosuppressive functions, and concurrent deletion of HIF1α or pharmacological inhibition of glycolytic metabolic activities could partially restore MDSC function in vitro and in various pathological contexts. Our results implicate AKT1 as a critical signaling node that regulates both the metabolic activities and functions of MDSCs in the context of inflammation and cancer.
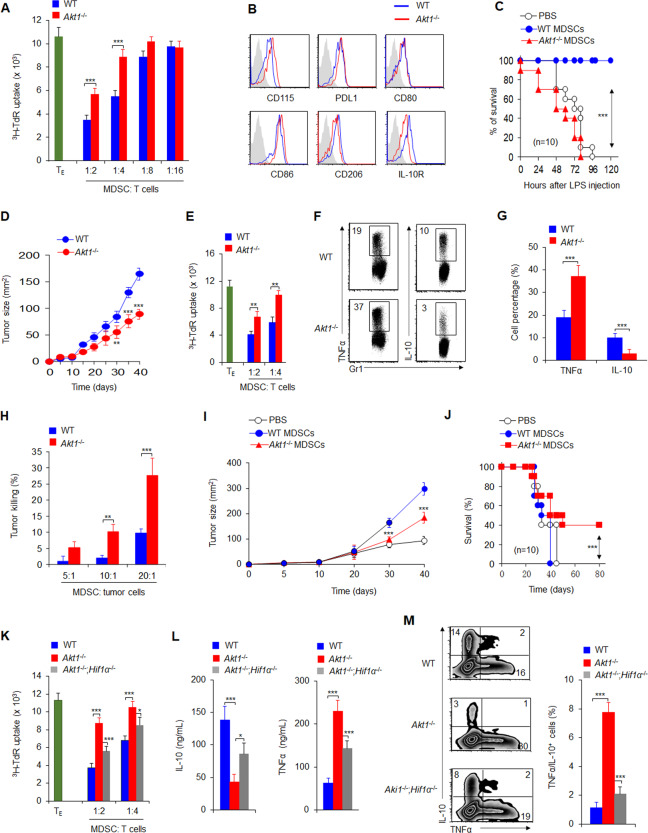
AKT1 but not AKT2 was preferentially induced in MDSCs in vitro, indicating a potential role of AKT1 in regulating MDSC activities (Fig. S1A–C). Supporting this idea, genetic ablation of AKT1 (Akt1−/−) in CD11b+Gr1+ MDSCs resulted in diminished immunosuppressive activities (Fig. 1A), increased expression of CD115, PDL1, and CD86, decreased expression of CD206 and IL-10R, and comparable expression of CD80 (Fig. 1B) compared to those of wild-type (WT) cells. Consistently, Akt1−/− MDSCs showed increased production of the proinflammatory cytokines TNFα and IL-12 and reduced production of the anti-inflammatory cytokine IL-10 (Fig. S2). These data suggest that AKT1 is critically involved in regulating the suppressive activities of MDSCs.
Fig. 1.
Akt1−/− inhibits the immunosuppressive effects of MDSCs through HIF1α-mediated glycolysis signaling in inflammation and cancer. A Analysis of the suppressive activities of splenic MDSCs. MDSCs were isolated from the indicated mice, T cells were stimulated with anti-CD3 mAbs and anti-CD28 mAbs in the presence of MDSCs, and T cell proliferation was assessed by the uptake of 3H-TdR (n = 6). B Expression of the indicated molecules in splenic MDSCs. C Donor groups were administered LPS (5 mg/kg) by i.p. injection for 48 h, and a total of 1 × 106 liver CD11b+Gr1+ cells were isolated and transferred into recipient wild-type (WT) C57BL/6 mice via i.v. injection. After 12 h, the mice were injected with LPS (10 mg/kg), and a survival curve was plotted (n = 10). (D) B16 tumor cells were implanted subcutaneously in WT and Akt1−/− mice (n = 10), and tumor size was measured every 5 days for 40 days. E Suppressive activity assays of tumor-infiltrating MDSCs from tumor-bearing mice (n = 5). F–G Intracellular staining of TNFα and IL-10 in infiltrating CD11b+Gr1+ cells in tumors. A representative figure is shown (F), and the data are summarized (G). H The tumor-killing activities at different ratios of MDSCs and tumor cells were determined as a percentage of tumor cell death. I–J Adoptive transfer of CD11b+Gr1+ cells from Akt1−/− tumor-bearing mice significantly delayed tumor growth. B16 tumor cells were first implanted subcutaneously in WT and Akt1−/− mice, and on day 40, a total of 2 × 106 infiltrating CD11b+Gr1+ cells were sorted from the tumors of the indicated mice and transferred into WT C57BL/6 recipient mice via i.v. injection. After 10–12 h, B16 tumor cells were implanted subcutaneously into recipient mice, and tumor growth (I) and survival (J) curves were plotted (n = 10). K The suppressive activities of MDSCs. L Splenic MDSCs were isolated from the indicated mice and stimulated with LPS (1 ng/mL) for 10–12 h, after which the serum levels of the indicated cytokines were measured. M Intracellular staining of TNFα and IL-10 in splenic MDSCs from the indicated mice. The data are representative of three to five individual experiments (n = 3–10). *P < 0.05; **P < 0.01 and ***P < 0.001, compared with the indicated groups
Akt1−/− mice showed a lower survival curve than WT mice in the murine endotoxic shock model (Fig. S3A). Importantly, hepatic Akt1−/− MDSCs showed reduce suppressive activities and increased levels of hepatic injury markers (Fig. S3B, C). Hepatic Akt1−/− CD11b+Gr1+ MDSCs showed increase TNFα and IL-12 production and reduced IL-10 production (Fig. S3D). Interestingly, adoptive transfer of sorted hepatic CD11b+Gr1+ MDSCs from Akt1−/− but not WT endotoxic shock model mice into WT C57BL/6 recipients significantly reduced endotoxic mouse survival (Fig. 1C) and exacerbated ALT levels (Fig. S4). Collectively, these data suggest that AKT1 is critical for MDSC-mediated protection against inflammatory hepatic injury in murine endotoxic shock.
We found that transplanted tumors grew slower in Akt1−/− mice than in WT mice (Fig. 1D). Akt1−/− MDSCs showed impaired suppressive activities and altered cytokine production (Fig. 1E–G). Importantly, tumor-infiltrating MDSCs from Akt1−/− mice showed enhanced tumor-killing activities (Fig. 1H). Finally, adoptive transfer of MDSCs isolated from tumor-bearing Akt1−/− mice into recipient mice significantly delayed tumor growth and improved host survival compared to the effects of MDSCs isolated from WT tumor-bearing mice (Fig. 1I, J). Thus, these data suggest that AKT1 ablation in MDSCs may confer improved antitumor immunity to the host.
We also found that enhanced glycolytic activities were associated with altered Akt1−/− MDSC functions (Fig. S5). Interestingly, treating Akt1−/− MDSCs with the glycolysis inhibitor 2-DG (Fig. S6A) partly reversed the functional changes caused by AKT1 deletion in MDSCs in vitro (Fig. S6A–C). Importantly, blocking glycolysis with 2-DG significantly reversed the changes in liver damage and inflammation in Akt1−/− mice following endotoxic shock (Fig. S7A–D). Taken together, these data indicate that glycolysis is responsible for the alterations in MDSCs induced by AKT1 deletion in vitro and in vivo.
We and others have previously shown that HIF1α is an important regulator of glycolysis and MDSC functions.10–12 HIF1α expression was higher in Akt1−/− MDSCs than in WT MDSCs (Fig. S8A). We then crossed Akt1−/− mice with Hif1αfl/fl and Lyz-Cre mice to generate an AKT1 and HIF1α double-knockout mouse strain (Akt1−/−Hif1α−/−). Similar to pharmacological inhibition of glycolysis (Fig. S6A–C), genetic ablation of HIF1α on an AKT1-null background (Akt1−/−Hif1α−/−) partly reversed many functional changes caused by AKT1 deletion in MDSCs in vitro (Fig. 1K–M and Fig. S8B). Collectively, these data suggest that HIF1α-dependent glycolysis is responsible for the functional changes in MDSCs induced by AKT1 deletion. We therefore conclude that AKT1 is a critical signaling node that regulates glycolysis and the function of MDSCs through HIF1α in the context of inflammation and cancer (Fig. S9).
In summary, we found that AKT1 governs the functional activities of MDSCs and regulates the alterations in glycolytic activities under physiological conditions and infection and in tumor pathological microenvironments. Glycolytic activities mediated by HIF1α were required for the AKT1-mediated regulation of MDSC activities, which protected against tumors and inflammatory injury (Fig. S9). Our results defined a new role of AKT1 in regulating MDSC functions, with implications for metabolic reprogramming as an immunotherapeutic approach in inflammation and cancer.
Supplementary information
Acknowledgements
This research is supported by grants from the National Natural Science Foundation for Key Programs of China (31730024, G.L.), the National Natural Science Foundation for General Programs of China (31671524, G.L.), and the Beijing Municipal Natural Science Foundation of China (5202013, GL).
Author contributions
A.J., Y.B., and Y.X.W. designed and conducted the mouse and cell experiment and analyzed the data; Y.F.W., Y.L., Q.Y. Y.C., Y.H., L.D., Y.D., Y.H., and R.W. participated in discussions; R.W. and Y.B. contributed to writing the manuscript; and G.L. developed the concept, designed and conducted the experiments, analyzed the data, wrote the manuscript, and provided overall direction.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of the College of Life Science of Beijing Normal University, Beijing China.
Footnotes
These authors contributed equally: Anna Jia, Yuexin Wang, Yufei Wang, Yan Li
Contributor Information
Ruoning Wang, Email: Ruoning.Wang@nationwidechildrens.org.
Yujing Bi, Email: byj7801@sina.com.
Guangwei Liu, Email: liugw@bnu.edu.cn.
Supplementary information
The online version of this article (10.1038/s41423-020-00610-7) contains supplementary material.
References
- 1.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weber C. Hepatitis: myeloid-derived suppressor cells in HBV infection. Nat. Rev. Gastroenterol. Hepatol. 2015;12:370–373. doi: 10.1038/nrgastro.2015.89. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, et al. Targeting myeloid-derived suppressor cells in cancer immunotherapy. Cancers. 2020;12:2626. doi: 10.3390/cancers12092626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, et al. The calcineurin-NFAT axis controls allograft immunity in myeloid-derived suppressor cells through reprogramming T cell differentiation. Mol. Cell Biol. 2015;35:598–609. doi: 10.1128/MCB.01251-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, et al. mTOR limits the recruitment of CD11b+Gr1+Ly6Chigh myeloid-derived suppressor cells in protecting against murine immunological hepatic injury. J. Leukoc. Biol. 2014;95:961–970. doi: 10.1189/jlb.0913473. [DOI] [PubMed] [Google Scholar]
- 6.Liu G, et al. SIRT1 limits the function and fate of myeloid-derived suppressor cells in tumors by orchestrating HIF-1alpha-dependent glycolysis. Cancer Res. 2014;74:727–737. doi: 10.1158/0008-5472.CAN-13-2584. [DOI] [PubMed] [Google Scholar]
- 7.Liu G, et al. Targeting S1P1 receptor protects against murine immunological hepatic injury through myeloid-derived suppressor cells. J. Immunol. 2014;192:3068–3079. doi: 10.4049/jimmunol.1301193. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, et al. mTOR signaling disruption from myeloid-derived suppressive cells protects against immune-mediated hepatic injury through the HIF1alpha-dependent glycolytic pathway. J. Leukoc. Biol. 2016;100:1349–1362. doi: 10.1189/jlb.2A1115-492R. [DOI] [PubMed] [Google Scholar]
- 9.Liao J, et al. Dexamethasone potentiates myeloid-derived suppressor cell function in prolonging allograft survival through nitric oxide. J. Leukoc. Biol. 2014;96:675–684. doi: 10.1189/jlb.2HI1113-611RR. [DOI] [PubMed] [Google Scholar]
- 10.Corzo CA, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, et al. Histone deacetylase SIRT1 negatively regulates the differentiation of interleukin-9-producing CD4(+) T cells. Immunity. 2016;44:1337–1349. doi: 10.1016/j.immuni.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Liu G, et al. Dendritic cell SIRT1-HIF1alpha axis programs the differentiation of CD4+ T cells through IL-12 and TGF-beta1. Proc. Natl Acad. Sci. USA. 2015;112:E957–E965. doi: 10.1073/pnas.1420419112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.