ABSTRACT
Background: SARS-CoV-2 is the new virus, and Streptococcus pneumoniae is one of the most important pathogens affecting humans. However, we do not yet know whether these microorganisms interact. Thus, we aimed to evaluate the relationship between Streptococcus pneumoniae and SARS-CoV-2 in pediatric patients.
Methods: This study was conducted retrospectively by means of medical records of pediatric patients who were tested for SARS-CoV-2 between March 11 and June 04, 2020, in the University of Health Sciences, Ankara Educating and Training Hospital and Hacettepe University Faculty of Medicine.
Results: We evaluated 829 pediatric patients for S. pneumoniae and SARS-CoV-2 from their nasopharyngeal specimen. Of 115 children positive for SARS-CoV-2, 32.2% had a positive S. pneumoniae test, whereas of 714 children negative for SARS-CoV-2, 14.1% had a positive S. pneumoniae test (p < .01). We compared patients with positive vs. negative SARS-CoV-2 tests according to S. pneumoniae positivity There were no statistically significant differences in terms of gender, underlying disease, fever, cough, leukocytosis, lymphopenia, increased CRP, increased procalcitonin, findings of chest x-ray, severity of disease, and treatment.
Conclusion: The nasopharyngeal S. pneumoniae carriage rate in patients with COVID-19 was higher than in non-infected children, while S. pneumoniae carriage did not affect the course of COVID-19 disease. Pneumococcal vaccination is significant, such that we do not know the outcomes of increased pneumococcal carriage for the upcoming months of pandemic.
KEYWORDS: COVID-19, children, S. pneumoniae
Introduction
With the devastating outbreak of a new infectious disease called novel coronavirus disease 2019 (COVID-19), people began to investigate treatment, for which there remains a huge knowledge gap.1 Most studies only focus on the causative virus SAR-coronavirus-2 (SARS-CoV-2), while it is known that many pathogens interact and that the coinfecting pathogens should also be considered for appropriate treatment of patients.2 Infection with one pathogen can affect the severity, infectivity or susceptibility to subsequent infection with other pathogens and these effects can have profound clinical and epidemiological results.3 We observed the results of one of the devastating interactions over the last century, influenza virus with bacterial pneumonia.4 Thus, previous viral pandemics provide lessons to be learned for the SARS-CoV-2 pandemic.
The clinical significance of viral/bacterial co-infections has been a complex issue, particularly among pediatric population. It is known that the upper respiratory tract is colonized by both commensals and potential pathogens. However, respiratory viruses are generally thought to promote bacterial superinfection by viral disruption of the respiratory epithelial barrier, viral-induced dysfunction of bacterial phagocytosis by innate immune cells, virus-mediated bacterial adherence to the upper respiratory epithelium.5,6 On the other hand, the presence of bacteria may promote viral infection in the respiratory tract system by contributing to increased virus production or releasing from infected epithelial cells.7,8 Understanding the mechanisms and clinical significance of co-infection is important to ensure the best possible patient care, proper usage of antibiotics.
Many articles have been published related to the epidemiological and clinical data of patients with COVID-19, unfortunately, limited data are available for co-infections for SARS-CoV-2 in the literature. Wang et al. reported that 5.8% of patients with COVID-19 infection had other respiratory viral pathogens however they did not evaluate patients for bacterial pathogens.9 In a recent study, Zhu et al. stated that Streptococcus pneumoniae was the most common bacteria with the evaluation of patients using real-time PCR and tested for 39 respiratory pathogens. Streptococcus pneumoniae is a significant pathogen causing invasive diseases such as sepsis, meningitis, and pneumonia. However, asymptomatic carriage of pneumococcus in the upper respiratory tract is common and thought to be the source of subsequent invasive pneumonia.10 The first question is whether Streptococcus pneumoniae carriage increases in patients with COVID-19. Secondly, understanding if the impact of the increased carriage because of the risk of pneumococcal invasion via the interaction of SARS-CoV-2. Under the guidance of these experiences, we attempted to share the rate and clinical features of pediatric patients with COVID-19 carrying Streptococcus pneumoniae in this study.
Material and methods
This study was conducted with medical records of pediatric patients aged under 18 y, who were tested for SARS-CoV-2 between March 11, and June 04, 2020, in the University of Health Sciences, Ankara, and Training Hospital and Hacettepe University Faculty of Medicine. This study was approved both by the University of Health Science and the Ankara Educating and Training Hospital Review Board, Ankara, Turkey.
We diagnosed suspected and confirmed COVID-19 cases, according to national COVID-19 guidelines which are prepared by Coronavirus Scientific Advisory Board in Turkey. Suspected cases with positive reverse transcriptase-polymerase chain reaction (RT-PCR) against 2019-nCoV were accepted as confirmed cases.11 We analyzed only the clinical data of children who had positive test for SARS-CoV-2 in this study. Nasopharyngeal (NP) specimens were collected by pediatricians for both S. pneumoniae and SARS-CoV-2. The children with antibiotic therapy within the last four weeks were decided to exclude from the study. None of the patients who were tested for S. pneumoniae received antibiotic treatment within the last four weeks. S. pneumoniae specific DNA was determined with a qualitatively multiplex Realtime PCR (RT-PCR) method that targets LytA gene (Montanide to 4896, Bosphore respiratory pathogens v4 kit Anatole Geneworks, Turkey) with 50 thermal cycles. Samples were considered positive for S. pneumoniae when CT value was <30.
Turkey implemented a 7-valent pneumococcal conjugate vaccine (PCV7) vaccination in the National Immunization Program (NIP) in 2009. It was changed to 13-valent pneumococcal conjugate vaccine (PCV13) with the same 3 + 1 vaccination schedule (2, 4, 6 months, and a booster at 12 months) in 2011.12 It was modified to a 2 + 1 schedule (3rd dose was removed from the schedule) in 2019.13 All children born in an after 2009 were properly vaccinated according to their age, with the PCV in use for the NIP and we checked the vaccination status of children.
Data regarding the demographic and clinical characteristics of patients were obtained from the hospital medical records of both hospitals and the records from the Pediatric Infectious Diseases Committee of the hospitals. In the first months of the pandemic, all children who had a positive test for SARS-CoV-2 were hospitalized for isolation due to the health care policy of our hospital. Now, we could evaluate patients who have an asymptomatic or mild disease in outpatient clinics. Due to the different health care politics, we thought that separating patients into two groups as hospitalized and nonhospitalized is not appropriate. We evaluated the clinical severity of patients.
We categorized the severity of pediatric COVID-19 cases, based on the clinical characteristics and the results of laboratory examinations and radiologic imaging, as defined by Dong et al.,14 as follows: (a) asymptomatic infection included cases with positive diagnoses but without any clinical or radiological findings; (b) mild disease included cases with acute upper respiratory tract infections but without clinical and radiological pneumonia; (c) moderate disease included cases with pneumonia and symptoms of respiratory tract infection; (d) severe disease included cases with progressive respiratory disease, dyspnea, and central cyanosis; and (e) critically ill included cases presented with acute respiratory distress syndrome or respiratory failure, shock, and organ dysfunction, including encephalopathy, myocardial injury, coagulation abnormalities, and acute kidney injury. We used the experts` consensus statement defined by Dong et al.14 to evaluate chest imaging examination. We evaluated the study population as native or refugee due to the high refugee population from Iraq and Syria in Turkey. The term refugee is defined by the 1951 Refugee Convention in Geneva.15
Statistical analysis
Data were evaluated using SPSS version 23.0 (SPSS, Inc., Chicago, IL, USA). Descriptive statistics were used to summarize the primary characteristics of patients, including the median and minimum-maximum for continuous variables. Frequency distribution was used for categorical variables. Chi-squared or Fisher’s exact tests were performed to compare the categorical variables. A p-value ≤0.05 was considered significant.
Results
During the study period, total of 1915 NP swaps were obtained from children for SARS-CoV-2 and 829 of them tested for S. pneumoniae at the same time. Of 115 children who had positive SARS-CoV-2, 37 (32.2%) had positive S. pneumoniae test whereas, of 714 children who had negative SARS-CoV-2, 101 (14.1%) had positive S. pneumoniae test. The difference between groups was statistically significant (p < .01) (Figure 1). Patients who had positive SARS-CoV-2 test were classified according to severity of disease, with the percentages of asymptomatic, mild, moderate, and critical/severe cases determined to be 23.5% (n = 27), 44.3% (n = 51), 29.6% (n = 34), and 2.6% (n = 3), respectively. The proportions of pneumococcal carriage in asymptomatic, mild, moderate, and severe/critical disease courses were 33%, 31,4%, 32,4%, and 33%, respectively. The relationship between NP pneumococcal carriage and the severity of children is shown in Figure 2.
Figure 1.
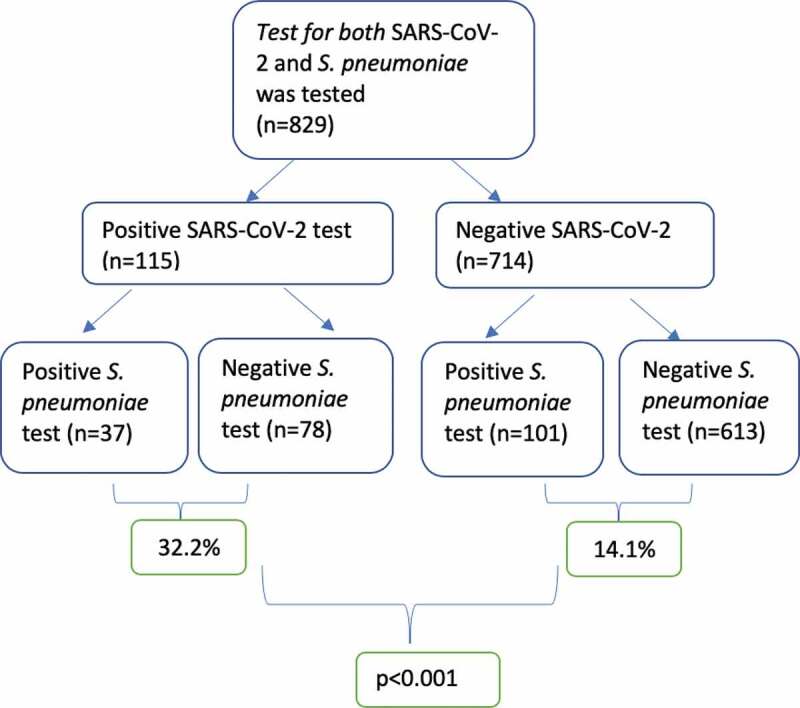
The number of NP swaps during the study period
Figure 2.
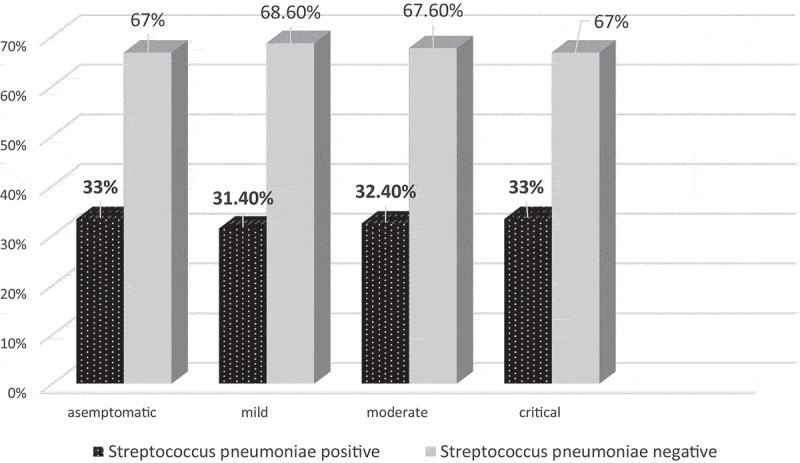
The relationship between NP Streptococcus pneumoniae carriage and the severity of children
We compared 115 children with COVID-19 in two groups as S. pneumoniae positive and negative and the characteristics of the groups are shown in Table 1. Of 115 children, 61 (50.8%) children were male, the median age was 9 y (IQR; 3–14). The median age was 4 y (IQR; 1–11) in children with S. pneumoniae positive group and 10 (IQR; 4–14) in children S. pneumoniae negative group. The difference between groups according to median age was statistically significant (p = .009). Only three (2.6%) patients had a concurrent viral infection; one had bocavirus, one had bocavirus and rhinovirus and the other had parechovirus. All three patients were S. pneumoniae positive.
Table 1.
Demographic and clinical data of patients with COVID-19
| Total (n = 115) |
S. pneumonia positive (n = 37) | S. pneumonia negative (n = 78) | p value | |
|---|---|---|---|---|
| Age, years (median, min-max) | 9 (3–14) | 4 (1–11) | 10 (4–14) | 0.009 |
| Male (%) | 61 (50.8) | 20 (52.6) | 41 (50) | 0.59 |
| Rate of Refugees, n (%) | 31 (26.9) | 12 (32.4) | 19 (24.4) | 0.36 |
| Underlying disease, n (%) | 9 (7.5) | 2 (5.3) | 7 (8.5) | 0.71 |
| Symptoms, n (%) | ||||
| Fever | 43 (35.8) | 15 (39.5) | 28 (34.1) | 0.63 |
| Cough | 43 (35.8) | 15 (39.1) | 28 (34.1) | 0.63 |
| Laboratory findings, n (%) | ||||
| Leukocytosis | 0 | 0 | 0 | NA |
| Lymphopenia | 24 (20) | 5 (13.2) | 19 (23.2) | 0.18 |
| Increased CRP | 28/88 (31.8) | 5/32 (15.6) | 12/70 (17.1) | 0.84 |
| Increased Procalcitonin | 0/77 (0) | 0/24 (0) | 0/53 (0) | NA |
| Increased LDH | 28/88 (31.8) | 13/26 (50) | 15/62 (24.2) | 0.03 |
| D dimer | 7/25 (28) | 4/10 (40) | 3/15 (20) | 0.37 |
| Chest x-ray, n (%) | 120 (100) | |||
| Abnormal | 38 (31.7) | 11 (28.9) | 27 (32.9) | 0.60 |
| Thorax CT, n (%) | 30 (40) | |||
| Normal | 19/30 (15.8) | 8/11 (72.7) | 11/19 (57.8) | 0.46 |
| Abnormal | 11/30 (9.2) | 3/11 (27.2) | 8/19 (42.1) | |
| Severity | ||||
| Asymptomatic | 32 (26.7) | 10 (26.3) | 22 (26.3) | NA |
| Mild | 51 (42.5) | 16 (42.1) | 31 (42.7) | |
| Moderate | 34 (28.3) | 11 (28.9) | 18 (28) | |
| Severe/Critical | 3 (2.5) | 1 (2.6) | 2 (2.4) | |
| Antibacterial treatment, n (%) | 23 (20) | 7 (18.9) | 16 (20.5) | 0.76 |
| Antiviral treatment, n (%) | 2 (1.7) | 1 (2.7) | 2 (1.3) | NA |
CRP; C-reactive protein, LDH; Lactate dehydrogenase, CT; Computer tomography,
There was no statistically significant difference in terms of gender and underlying disease and symptoms including fever and cough. None of the patients had leukocytosis. Lymphopenia was present in 5 (13.2%) of patients with S. pneumoniae positive group and 19 (23.2%) patients in S. pneumoniae negative group (p = .18). Procalcitonin did not increase in any patients. C-reactive protein was increased in 15.6% of patients with S. pneumoniae positive group and 17.1% patients in S. pneumoniae negative group. Increased LDH was detected in 50% of children with S. pneumoniae, 24.2% of children without S. pneumoniae and the difference between groups was statistically significant (p = .03). All of the children were evaluated with the chest x-ray and an abnormal chest x-ray rate was similar between groups. In the thorax CT evaluation, the abnormality rate was higher in patients without S.pneumoniae. However, all patients who had S.pneumoniae positivity with abnormal chest x-ray had thorax CT evaluation whereas, 70.3% of patients in S.pneumoniae negative had. Streptococcus pneumoniae positivity rate was similar between groups in each severity type. Antibacterial and antiviral treatment rates were similar in groups. One patient in each group received hydroxychloroquine and favipiravir as an antiviral treatment. Sixteen patients (20.5%) in Streptococcus pneumoniae negative group and seven patients (18.9%) in Streptococcus pneumoniae positive group received sulbactam ampicillin or ceftriaxone with or without combination clarithromycin or azithromycin.
According to the age distribution, more than half (60%) of the children under 1 year old were positive for Streptococcus pneumoniae in NP. The positivity rates were 38.9%, 27.8%, 21.4%, and 26.1% in 1–5 years of age, 6–10 years of age, 11–14 years of age, and 15–18 years of age, respectively. The NP pneumococcal carriage rate in each age groups was shown in Figure 3. Pneumococcal positivity rate was 38.7% in refugees and 29.7% in native population with no statistically difference between the two populations (p = .36).
Figure 3.
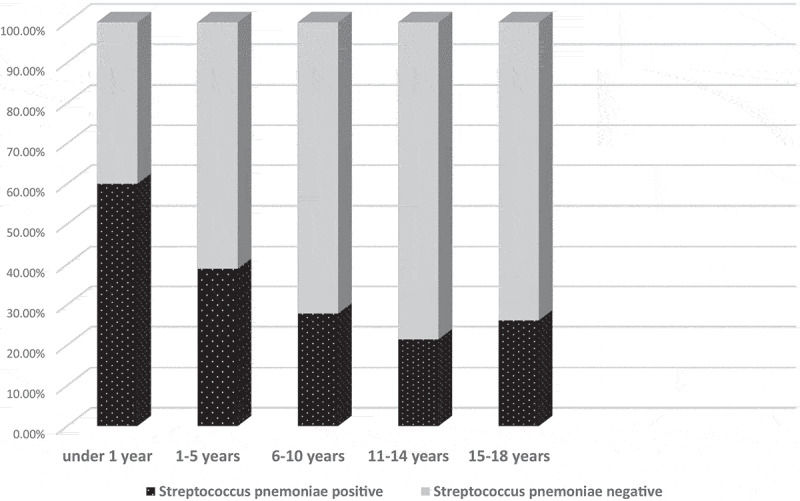
The NP pneumococcal carriage rate in each age groups
Discussion
We report a significant increase in S. pneumoniae carriage in pediatric patients with COVID-19. The proportion of pneumococcal carriage had no difference between the children according to the disease course (Figure 2); furthermore, there was no specific relationship between carriage and outcome. These findings are consistent with a prior report in patients with COVID-192 in which S. pneumoniae has been reported as the most coinfected pathogen in all COVID-19 patients among all age groups. However, S. pneumoniae has commonly colonized the upper respiratory tract of both 20–50% of healthy children and 8–30% of healthy adults.16 Although they have the potential to become pathogenic when the immune function of pediatric patients with COVID-19 was disrupted, many of them were considered as co-colonization because of the absence of any clinical presentation of children with COVID-19, including otitis media, community-acquired pneumonia, bacterial meningitis, and sepsis.12 Moreover, generally asymptomatic when colonizing the nasopharynx (lethal synergism-1), pneumococcus is also not correlated with an increase in intensive care unit hospitalizations and death in the present study contrarily with the previous reports.2,17
We believe that understanding of co-infection of S. pneumoniae during influenza pandemics will lead us to figure out contributors to the pathogenesis of the synergistic co-infection of S. pneumoniae with COVID-19. During the 20th century, S. pneumoniae was the predominant strain in all influenza pandemics dated in 1918, 1957, and in the late 1960s.4,18 Several more common pathogens in addition to S. pneumoniae such as Hemophilus influenzae, Staphylococcus spp. (in particular S. aureus), and other Streptococcus spp. were also identified during those pandemics [14] as in COVID-19 pandemic.2 Bacterial coinfections particularly caused by S. pneumoniae might play a considerable role in the outcome of seasonal influenza, although the coinfection rate was found to be significantly higher during a pandemic.17,19 Throughout all these pandemics including COVID-19 as well, co-infections have the potential to play a crucial role in the disease process, making it important to consider the effect of the bacterial co-pathogens when planning for a pandemic.20,21 Although the actual mechanism of the synergism seen with coinfection still is not clear, it has been shown in a mice model that animals treated with influenza have a decreased pneumococcal pulmonary clearance.22 In addition, the damage of respiratory epithelium results in an inability to repair and reproliferate itself. Therefore, pandemic viral infections might possibly lead to a high cytotoxic effect on epithelium and contribute to the increase in the rates of coinfections seen during pandemics.16,17,23,24 As a result, some aspects of this new emerging agent are similar to those of influenza: Both SARS-CoV and influenza preferentially infect type II cells compared to type I cells.25,26 A large number of viral particles are released after the propagation of the virus within type II cells, and the cells undergo apoptosis and die.27 This synergism seen with coinfection caused S. pneumoniae in children with COVID-19 might partly be explained by the mechanisms seen in influenza pandemics. The overlapping coinfection features between the condition observed in patients with COVID-19 and influenza may be due to partly similar pathophysiology, both of which the lungs are the primary targets. Influenza induces the expression of toll-like receptors (TLRs), which are members of the innate immune system, and this is resulted in both decreased bacterial clearance and increased the type I interferon (IFN) response. The induction of type I IFN might play a crucial role in the pathogenesis of coinfection as well as the disease process of influenza when coinfected with S.pneumoniae.28–30 Unfortunately, we had no chance to examine such kind of molecules that have a possibility to be a mediator of damage associated with pneumococci. Actually, further studies will have the potential to shed light on our understanding.
Nasopharyngeal carriage of S. pneumoniae prerequisite for disease and is the source of pneumococcal spread in the community. Since children are the main reservoir for pneumococci, it is reasonable that the peak age for colonization, transmission, and disease coincides with early childhood31,32 as in our study. Pneumococcal NP carriage rates may differ in various parts of the world and be caused by ethnic, demographic, and cultural differences. Poor and crowded living conditions, the hygienic status of living areas, breastfeeding history, and attendance of daycare center might also affect the carriage ratio.33–37 When considered the situation of Turkey in a short period, it is really difficult to discuss that the increased pneumococcal NP carriage in children with COVID-19 might be caused by the change of those mentioned population dynamics. However, relatively higher NP carriage of pneumococci in refugee children as compared to Turkish children in the present study may possibly be attributed to this poor societal level.
Pneumococcal conjugate vaccines (PCVs) have a direct effect on the incidence of pneumococcal disease in vaccinated individuals and therefore prevent transmission to unvaccinated children and susceptible adults, which is the indirect (herd) protection of PCVs.38,39 Since the lack of available evidence of a direct vaccine impact on the time, it takes to clear a colonization episode of pneumococci, the main strategy of reducing pneumococcal NP colonization should be to prevent the acquisition of new strains.40,41 Before the global vaccination with PCVs, the number of deaths among under five years old caused by pneumococcal pneumonia was estimated to be 642,000 in 2005 worldwide, which consists of a considerable proportion of the total number of 1,692,300 fatal cases.42 It was reported in a study from Turkey that more IPD cases were occurred in children under 5 y of age (64.2% of all cases) between 2008 and 2014, as well. In 2008, a vaccine protecting against seven serotypes (PCV7) was introduced into the Turkish Childhood Immunization Programme. The vaccine was replaced with the 13-valent vaccine (PCV13) in 2011, which protects against six additional serotypes. The vaccine is administered at two, four and twelve months of age and the national uptake of PCV13 was 97% in 2018.43 Despite being vaccinated with PCVs, pneumococcal positivity in NP suggests the possibility of colonization with non-vaccine serotypes. However, we could not determine the serotypes because of the PCR technique used in the present study.
One of the limitations of the present study was the lack of serotype distribution data of S. pneumoniae because of the method used. Future studies may focus on the pneumococcus serotypes in patients with COVID-19. Additionally, this will let us understand the real vaccine coverage of cases in the community. Respiratory viruses including influenza, respiratory syncytial virus, and metapneumo virus that affect the pneumococcal carriage are living with us in the community.44 Despite the mentioned limitations, we believe that our data is significant to understand the increased rate of S. pneumoniae carriage in viral-mentioned diseases in addition to infections caused by SARS-CoV-2.
As a conclusion, although S. pneumoniae carriage did not affect the course of COVID-19, S. pneumoniae carriage rate in patients infected with SARS-CoV-2 was higher than non-infected children. Therefore, pneumococcal vaccination has become more important than ever during a pandemic caused by a new pathogen.
References
- 1.World Health Organization . Pneumococcal diseases. 2015. November 16. [accessed 2015 Nov 26]. https://www.who.int/biologicals/vaccines/pneumococcal/en/
- 2.Zhu X, Ge Y, Tao Wua T, Zhao K, Chen Y, Wu B, Zhu F, Zhu B, Cui L.. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020;285:198005. doi: 10.1016/j.virusres.2020.198005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shrestha S, Foxman B, Daniel M, Weinberger DM, Viboud C, Rohani P. Identifying the interaction between influenza and pneumococcal pneumonia using incidence data. Sci Transl Med. 2013;5:191ra84. doi: 10.1126/scitranslmed.3005982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudd JM, Ashar HK, Chow VTK, Teluguakula N. Lethal synergism between influenza and Streptococcus pneumoniae. J Infect Pulm Dis. 2016;2. doi: 10.16966/2470-3176.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brealey JC, Sly PD, Young PR, Chappell KJ. Viral bacterial co-infection of the respiratory tract during early childhood. FEMS Microbiol Lett. 2015;362:fnv062. [DOI] [PubMed] [Google Scholar]
- 6.Ghoneim HE, Thomas PG, McCullers JA. Depletion of alveolar macrophages during influenza infection facilitates bacterial superinfections. J Immunol. 2013;191:1250–59. doi: 10.4049/jimmunol.1300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madhi SA, Klugman KP, Group TVT. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med. 2004;10:811–13. doi: 10.1038/nm1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith AM, Adler FR, Ribeiro RM, Gutenkunst RN, McAuley JL, McCullers JA, Perelson AS. Kinetics of coinfection with influenza A virus and streptococcus pneumoniae. PLoS Pathog. 2013;9:e1003238. doi: 10.1371/journal.ppat.1003238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Fu J, Liang Z, Chen J. Prevalence and serotype distribution of nasopharyngeal carriage of Streptococcus pneumoniae in China: a meta-analysis. BMC Infect Dis. 2017;17:765. doi: 10.1186/s12879-017-2816-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simell B, Auranen K, Käyhty H, Goldblatt D, Dagan R, O'Brien KL. Pneumococcal carriage group. The fundamental link between pneumococcal carriage and disease. Expert Rev Vaccines. 2012;11:841–55. doi: 10.1586/erv.12.53. [DOI] [PubMed] [Google Scholar]
- 11.The Coronavirus Scientific Advisory Board (Turkey) . 2020. April 14. [accessed 2020 April 14]. https://covid19bilgi.saglik.gov.tr/depo/rehberler/COVID-19_Rehberi.pdf.
- 12.Ceyhan M, Ozsurekci Y, Gurler N, Ozkan S, Sensoy G, Belet N, Hacimustafaoglu M, Celebi S, Keser M, Dinleyici EC, et al. Serotype distribution of streptococcus pneumoniae causing parapneumonic empyema in Turkey. Clin Vaccine Immunol. 2013;20:972–76. doi: 10.1128/CVI.00765-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turkey Ministery of Health . [accessed 2019. December 20]. https://www.saglik.gov.tr/tr,11588/istatistik-yilliklari.html
- 14.Dong Y, Mo X, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020;145:e20200702. doi: 10.1542/peds.2020-0702. [DOI] [Google Scholar]
- 15.[accessed 1951]. https://www.unhcr.org/1951-refugee-convention.html
- 16.McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19:571–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chertow DS, Memoli MJ. Bacterial coinfection in influenza: a grand rounds review. JAMA. 2013;309:275–82. doi: 10.1001/jama.2012.194139. [DOI] [PubMed] [Google Scholar]
- 18.Herzog H, Staub H, Richterich R. Gas-analytical studies in severe pneumonia; observation during the 1957 influenza epidemic. Lancet. 1959;1:593–97. doi: 10.1016/S0140-6736(59)92351-7. [DOI] [PubMed] [Google Scholar]
- 19.Wang XY, Kilgore PE, Lim KA, et al. Influenza and bacterial pathogen coinfections in the 20th century. Interdiscip Perspect Infect Dis. 2011;2011:146376. doi: 10.1155/2011/146376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCullers JA. Planning for an influenza pandemic: thinking beyond the virus. J Infect Dis. 2008;198:945–47. doi: 10.1086/592165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta RK, George R, Nguyen-Van-Tam JS. Bacterial pneumonia and pandemic influenza planning. Emerg Infect Dis. 2008;14:1187–92. doi: 10.3201/eid1408.070751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LeVine AM, Koeningsknecht V, Stark JM. Decreased pulmonary clearance of S. pneumoniae following influenza A infection in mice. J Virol Methods. 2001;94:173–86. doi: 10.1016/S0166-0934(01)00287-7. [DOI] [PubMed] [Google Scholar]
- 23.Loosli CG, Stinson SF, Ryan DP, Hertweck MS, Hardy JD, Serebrin R. The destruction of type 2 pneumocytes by airborne influenza PR8-A virus; its effect on surfactant and lecithin content of the pneumonic lesions of mice. Chest. 1975;67:7–14. doi: 10.1378/chest.67.2_Supplement.7S. [DOI] [PubMed] [Google Scholar]
- 24.Kash JC, Walters KA, Davis AS, Sandouk A, Schwartzman LM, Jagger BW, Chertow DS, Qi L, Kuestner RE, Ozinsky A, et al. Lethal synergism of 2009 pandemic H1N1 influenza virus and Streptococcus pneumoniae coinfection is associated with loss of murine lung repair responses. MBio. 2011;2:e00172–11. doi: 10.1128/mBio.00172-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mossel EC, Wang J, Jeffers S, Edeen KE, Wang S, Cosgrove GP, Funk CJ, Manzer R, Miura TA, Pearson LD, et al. SARS-CoV replicates in primary human alveolar type II cell cultures but not in type I-like cells. Virology. 2008;372:127–35. doi: 10.1016/j.virol.2007.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinheimer VK, Becher A, Tonnies M, Holland G, Knepper J, Bauer TT, Schneider P, Neudecker J, Ruckert JC, Szymanski K, et al. Influenza A viruses target type II pneumocytes in the human lung. J Infect Dis. 2012(206):1685–94. doi: 10.1093/infdis/jis455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian Z, Travanty EA, Oko L, Edeen K, Berglund A, Wang J, Ito Y, Holmes KV, Mason RJ. Innate immune response of human alveolar type II cells infected with severe acute respiratory syndrome-coronavirus. Am J Respir Cell Mol Biol. 2013;48:742–48. doi: 10.1165/rcmb.2012-0339OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spelmink L, Sender V, Hentrich K, Kuri T, Plant L, Henriques-Normark B. Toll-like receptors 3/TRIF-dependent IL-12p70 secretion mediated by streptococcus pneumoniae RNA and its priming by influenza A virus coinfection in human dendritic cells. MBio. 2016;7:e00168–16. doi: 10.1128/mBio.00168-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian X, Xu F, Lung WY, Meyerson C, Ghaffari AA, Cheng G, Deng JC. Poly I: C enhances susceptibility to secondary pulmonary infections by gram positive bacteria. PLoS One. 2012;7:e41879. doi: 10.1371/journal.pone.0041879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li W, Moltedo B, Moran TM. Type I interferon induction during influenza virus infection increases susceptibility to secondary streptococcus pneumoniae infection by negative regulation of gamma delta T cells. J Virol. 2012;86:12304–12. doi: 10.1128/JVI.01269-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Løvlie A, Vestrheim DF, Aaberge IS, Steens A. Changes in pneumococcal carriage prevalence and factors associated with carriage in Norwegian children, four years after introduction of PCV13. BMC Infect Dis. 2020;20:29. doi: 10.1186/s12879-019-4754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ben-Shimol S, Givon-Lavi N, Greenberg D, Dagan R. Pneumococcal nasopharyngeal carriage in children <5 years of age visiting the pediatric emergency room in relation to PCV7 and PCV13 introduction in southern Israel. Human Vaccines &Immunotherapeutics. 2016;12(2):268–76. doi: 10.1080/21645515.2015.1095414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore MR, Hyde TB, Hennessy TW, Parks DJ, Reasonover AL, Harker-Jones M, Gove J, Bruden DL, Rudolph K, Parkinson A, et al. Impact of a conjugate vaccine on community-wide carriage of nonsusceptible streptococcus pneumoniae in Alaska. J Infect Dis. 2004;190:2031–38. [DOI] [PubMed] [Google Scholar]
- 34.Loo JD, Conklin L, Fleming-Dutra KE, Knoll MD, Park DE, Kirk J, Goldblatt D, O’Brien KL, Whitney CG. Systematic review of the indirect effect of pneumococcal conjugate vaccine dosing schedules on pneumococcal disease and colonization. Pediatr Infect Dis J. 2014;33(Suppl 2):S161–171. doi: 10.1097/INF.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Usuf E, Badji H, Bojang A, Jarju S, Ikumapayi UN, Antonio M, Mackenzie G, Bottomley C. Pneumococcal carriage in rural Gambia prior to the introduction of pneumococcal conjugate vaccine: a population-based survey. Trop Med Int Health. 2015;20:871–79. doi: 10.1111/tmi.12505. [DOI] [PubMed] [Google Scholar]
- 36.Mackenzie GA, Leach AJ, Carapetis JR, Fisher J, Morris PS. Epidemiology of nasopharyngeal carriage of respiratory bacterial pathogens in children and adults: cross-sectional surveys in a population with high rates of pneumococcal disease. BMC Infect Dis. 2010;10:304. doi: 10.1186/1471-2334-10-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenberg D, Givon-Lavi N, Broides A, Blancovich I, Peled N, Dagan R. The contribution of smoking and exposure to tobacco smoke to streptococcus pneumoniae and haemophilus influenzae carriage in children and their mothers. Clin Infect Dis. 2006;42:897–903. doi: 10.1086/500935. [DOI] [PubMed] [Google Scholar]
- 38.van Hoek AJ, Sheppard CL, Andrews NJ, Waight PA, Slack MP, Harrison TG, Ladhani SN, Miller E. Pneumococcal carriage in children and adults two years after introduction of the thirteen valent pneumococcal conjugate vaccine in England. Vaccine. 2014;32:4349–55. doi: 10.1016/j.vaccine.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 39.Davis SM, Deloria-Knoll M, Kassa HT, O’Brien KL. Impact of pneumococcal conjugate vaccines on nasopharyngeal carriage and invasive disease among unvaccinated people: review of evidence on indirect effects. Vaccine. 2013;32:133–45. doi: 10.1016/j.vaccine.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Dagan R, Givon-Lavi N, Fraser D, Lipsitch M, Siber GR, Kohberger R. Serum serotype-specific pneumococcal anticapsular immunoglobulin G concentrations after immunization with a 9-valent conjugate pneumococcal vaccine correlate with nasopharyngeal acquisition of pneumococcus. J Infect Dis. 2005;192:367–76. doi: 10.1086/431679. [DOI] [PubMed] [Google Scholar]
- 41.Auranen K, Rinta-Kokko H, Goldblatt D, Nohynek H, O’Brien KL, Satzke C, Simell B, Tanskanen A, K€ayhty H. Colonisation endpoints in Streptococcus pneumoniae vaccine trials. Vaccine. 2013;32:153–58. doi: 10.1016/j.vaccine.2013.08.061. [DOI] [PubMed] [Google Scholar]
- 42.Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, Casey DC, Charlson FJ, Chen AZ, Coates MM, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388(10053):1459–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.[accessed 2020 July 15]. https://apps.who.int/immunization_monitoring/globalsummary/countries?countrycriteria%5Bcountry%5D%5B%5D=TUR&commit=OK
- 44.Morpeth SC, Munywoki P, Hammitt LL, Bett A, Bottomley C, Onyango CO, Murdoch DR, Nokes DJ, Scott JAG. Impact of viral upper respiratory tract infection on the concentration of nasopharyngeal pneumococcal carriage among Kenyan children. Sci Rep. 2018;8:11030. doi: 10.1038/s41598-018-29119-w. [DOI] [PMC free article] [PubMed] [Google Scholar]


