Abstract
Specific proteins present at telomeres ensure chromosome end stability, in large part through unknown mechanisms. In this work, we address how the Saccharomyces cerevisiae ORC-related Rif2 protein protects telomere. We show that the small N-terminal Rif2 BAT motif (Blocks Addition of Telomeres) previously known to limit telomere elongation and Tel1 activity is also sufficient to block NHEJ and 5’ end resection. The BAT motif inhibits the ability of the Mre11-Rad50-Xrs2 complex (MRX) to capture DNA ends. It acts through a direct contact with Rad50 ATP-binding Head domains. Through genetic approaches guided by structural predictions, we identify residues at the surface of Rad50 that are essential for the interaction with Rif2 and its inhibition. Finally, a docking model predicts how BAT binding could specifically destabilise the DNA-bound state of the MRX complex. From these results, we propose that when an MRX complex approaches a telomere, the Rif2 BAT motif binds MRX Head in its ATP-bound resting state. This antagonises MRX transition to its DNA-bound state, and favours a rapid return to the ATP-bound state. Unable to stably capture the telomere end, the MRX complex cannot proceed with the subsequent steps of NHEJ, Tel1-activation and 5’ resection.
Subject terms: Telomeres, Non-homologous-end joining, DNA metabolism, DNA synthesis
Different proteins localised at telomeres ensure chromosome end stability to prevent double strand-end break recognition. Here the authors provide new insight into how in S. cerevisiae the interaction between Rif2 and Rad50 inhibits MRX functions at telomeres.
Introduction
Telomeres are protein–DNA complexes ensuring that native chromosome ends escape the pathways acting on broken DNA ends1–5. The repressed pathways are non-homologous end joining (NHEJ), 5′ end resection, homologous recombination and the DNA damage checkpoint. In addition, telomeres in association with telomerase solve the problem of replicating chromosome ends by semiconservative DNA replication. They also control telomere length homoeostasis to avoid the occurrence of excessively short or long telomeres. These core telomere functions are established by a relatively small number of proteins specifically present or enriched at telomeres. How each factor acts at a molecular level is only partially deciphered6–19.
Telomere proteins are usually not restricted to a unique telomeric function and can control several pathways. In this regard, the budding yeast telomere factor Rif2 is paradigmatic. Rif2 is a globular 46 kDa protein with a single folded AAA+ domain originating from a duplication of the ORC4 gene20–22 (Fig. 1A, Supplementary Fig. 1). It is recruited to telomeres by Rap1, the protein covering telomere sequences in budding yeast species22,23. Rif2 interacts with Rap1 C-terminal domain through two distinct interfaces: the Rif2 AAA+ domain and a small Rap1 Binding Motif (RBM) in N-terminal position (residues 37–48)22. Both epitopes interact with Rap1 with similar affinities (id ~30–50 µM) and synergise to ensure an efficient Rif2 recruitment to telomeres. They may also allow Rif2 molecules to interconnect adjacent telomere-bound Rap1 molecules22. Rif2 contributes to several telomere functions. Rif2 limits telomere elongation by telomerase23–30. It also represses NHEJ, preventing telomere-telomere fusions21,31 and inhibits 5′ end resection, homologous recombination and checkpoint activation27,32–37.
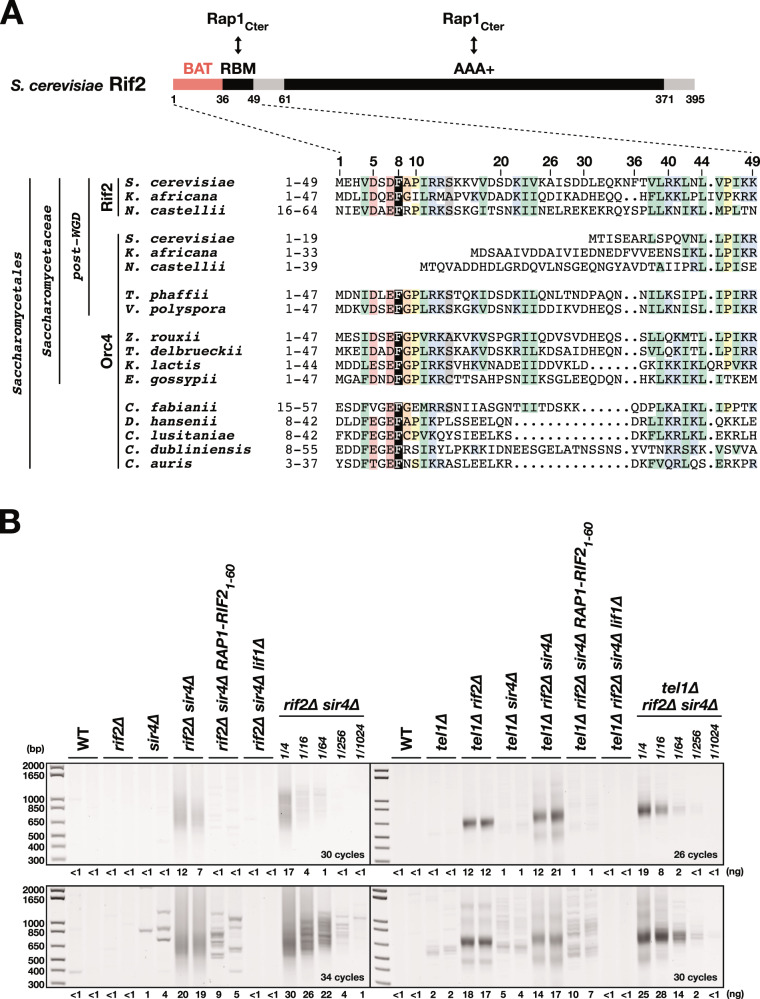
Fig. 1. Rif2 N-terminal region inhibits NHEJ at telomere.
A Schematic representation of S. cerevisiae Rif2 and sequence conservation of Rif2 and Orc4 N-terminal region in Saccharomycetales species21,22,28,30. In some post-WGD (whole genome duplication) Saccharomycetacae species, Rif2 and Orc4 are syntenic and only Rif2 possesses a BAT motif. In other Saccharomycetacae species, the motif is found in Orc4. Core residues of the BAT motif are also present in Orc4 N-terminal region in non-Saccharomycetacae species of the Saccharomycetales order30. The alignment was extended downstream towards a conserved motif, which might correspond to a Rap1-binding module (RBM)22. Alignment of the full-length proteins is shown in Supplementary Fig. 1. (Sequence Accession numbers: Rif2: Saccharomyces cerevisiae: Q06208, Kazachstania africana: XP_003954885.1, Naumovozyma_castellii: XP_003677686.1, Orc4: Saccharomyces cerevisiae: P54791, Kazachstania africana: XP_003955485.1, Naumovozyma_castellii: XP_003674441.1, Tetrapisispora_phaffii: XP_003686307.1, Vanderwaltozyma_polyspora: XP_001643535.1, Zygosaccharomyces_rouxii:XP_002496500.1, Torulaspora_delbrueckii: XP_003683125.1, Kluvyeromyces_lactis: XP_452959.1, Eremothecium_gossypii: NP_983126.1, Cyberlindnera fabianii: ONH65289.1, Debaryomyces hansenii: XP_459748.2, Clavispora XP_002615148.1, Candida dubliniensis XP_002420502.1, Candida auris: PIS52278.1). B Fusing Rif2 N-terminal region to Rap1 C-terminal end (RAP1-RIF21-60) protects telomeres against NHEJ-dependent fusions in cells lacking Rif2 and Sir4. Fusions between X and Y′ telomeres were detected by semi-quantitative PCR (upper panels: 30 and 26 cycles, lower panels: 34 and 30 cycles). Quantification of the amplified products indicated for each lane (ng). Serial 4-fold dilution of the template DNA from rif2∆ sir4∆ and tel1∆ rif2∆ sir4∆ cells provides an estimation of the method sensitivity. Rarer fusions are amplified as discrete bands. Experiment reproduced three times.
A shared feature of these pathways targeted by Rif2 is the involvement of the Mre11–Rad50–Xrs2NBS1 complex (MRXMRN), an ATPase related to the SMC family (Structural Maintenance of Chromosome). At double-strand breaks, the MRXMRN complex is recruited early to the broken ends where it has multiple roles. First, it promotes NHEJ repair through tethering of the broken ends and through interactions with the NHEJ factors KU, Lif1XRCC4 and Nej1XLF38–44. In contexts where NHEJ does not occur, MRXMRN promotes 5′ end resection, Mec1ATR/Rad3 checkpoint kinase activation and repair by homologous recombination through its nuclease activities45,46 and through interactions with chromatin remodellers and regulation of long-range resection actors47–49. At telomeres, MRXMRN is also essential to Tel1ATM kinase recruitment and activation, a key factor for chromosome ends maintenance by telomerase50–54.
The intersection between Rif2 and MRX functions suggests that the MRX complex is a Rif2 target at telomeres. Supporting this model, Rif2 inhibits the recruitment of the MRX complex to DNA ends either directly or by inhibiting Tel1, which stabilises the complex interaction with DNA29,33,55.
Previous studies addressing Rif2 molecular mechanisms focused on the inhibition of Tel1 and telomere elongation. This function of Rif2 involves a small BAT motif (Blocks Addition of Telomeres) present in the N-terminal position (residues 1–36) just upstream of the RBM motif28 (Fig. 1A). Targeting the BAT to telomeres through a covalent fusion with Rap1 shortens telomeres, even in the absence of full-length Rif2 or Rap1 C-terminal domain. This shows that the Rif2 BAT motif is sufficient to inhibit telomere elongation28. In vitro Rif2 represses DNA/MRX-dependent activation of Tel1, indicating that it can directly act on the MRX–Tel1 complex to inhibit telomere elongation30. Rif2 N-terminal region was initially proposed to interact with the Xrs2 C-terminal motif that binds to Tel1, therefore antagonising Tel1 recruitment and function at telomeres33. However, telomere shortening by the BAT still occurs in the absence of this Xrs2 C-terminal motif28. Furthermore, Rif2 N-terminal region interacts in vitro with Rad50 and stimulates its ATPase activity independently of Xrs229,30, suggesting that Rif2 acts on Rad50, and not on Xrs2, to repress telomere elongation.
Here we asked whether the function of the Rif2 BAT motif is restricted to telomere elongation inhibition. We found that the BAT is sufficient to inhibit NHEJ, 5′ resection and the stable interaction of the MRX complex with DNA ends. We showed that the BAT interacts with Rad50 ATP-binding domains. We defined a minimal active motif of 26 residues sufficient for both interaction and function, and showed that Rif2 action through the BAT is restricted to short distances in cis. Through genetic approaches guided by structural predictions, we identified residues at the surface of Rad50 that are essential for the interaction with Rif2 and for its inhibition. The position of these residues on Rad50 leads us to propose that Rif2 opposes MRX complex functions by precluding the formation of its DNA-bound active state.
Results
Rif2 N-terminal region inhibits chromosome end fusions
Targeting the Rif2 BAT motif to telomeres represses telomere elongation28. Can it also protect telomeres against the NHEJ pathway? In budding yeast, Rap1, Rif2 and another Rap1-interacting factor, Sir4, act in synergy to prevent NHEJ-dependent chromosome ends fusions21,31. The loss of both Rif2 and Sir4 is needed to result in telomere fusions frequent enough to be efficiently detected by PCR (Fig. 1B left panel, telomeres being heterogeneous in length, amplified telomere fusions appear as a smeared signal). Canonical NHEJ is the sole pathway producing these fusions since their occurrence requires Lif1XRCC4, an essential co-factor of Lig4 (Fig. 1B, compare rif2Δ sir4∆ with rif2Δ sir4∆ lif1∆)21. To test BAT ability to block NHEJ, we targeted Rif2 N-terminal region (1–60) to telomeres by fusing it to endogenous Rap1 C-terminal end. A 10-Glycine linker connects the two sequences (chimera from28, referred here as RAP1-RIF21-60). As expected28, fusing the BAT to Rap1 shortens telomeres (Supplementary Fig. 2A). More strikingly, it also inhibits the occurrence of chromosome ends fusions in cells lacking Rif2 and Sir4, bringing it back to levels observed in RIF2+ cells devoid of Sir4 (Fig. 1B left panel). Thus, targeting the Rif2 N-terminal region to telomeres is sufficient to inhibit NHEJ in the absence of the full-length protein. We also observed this inhibition in cells lacking Tel1, where telomeres are about half shorter and fusions more frequent (Fig. 1B right panel)21. This indicates that the Rif2 N-terminal region, in the same way as the full-length protein, can bypass Tel1 to repress NHEJ at telomeres.
Rif2 BAT motif inhibits NHEJ at broken ends
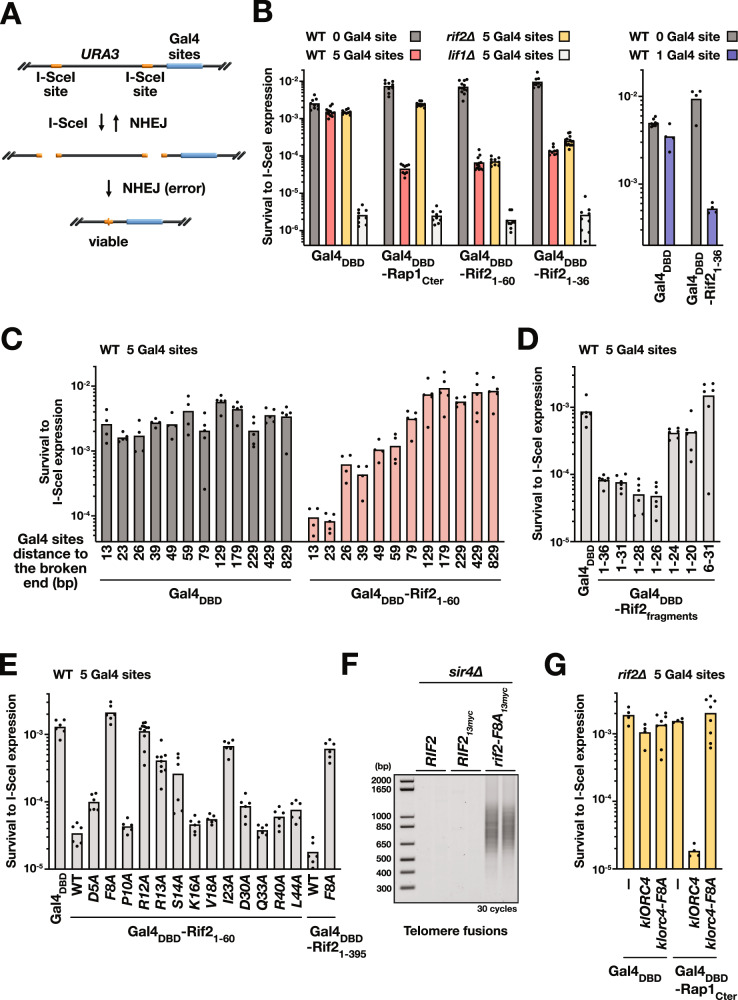
Next, we asked whether the Rif2 N-terminal region and more specifically the BAT motif can act at another location than telomeres. To assay NHEJ activity we used as a proxy survival to double-strand breaks (DSBs) induced by the continuously expressed I-SceI endonuclease (Fig. 2A, Supplementary Fig. 2B) (see figure legend for details)21,56,57. In these assays, Gal4 binding sites are present next to a I-SceI cutting site. Fusing peptides to Gal4 DNA binding domain (Gal4DBD) allows us to target them at a double-strand end to test their impact on NHEJ efficiency. Targeting full-length Rif2 through Rap1 C-terminal domain (Rap1Cter) inhibits NHEJ in these assays21. Targeting Rif2 N-terminal region (1-60) or a smaller Rif2 peptide only including the BAT motif, not the RBM (1-36) also lowers NHEJ-dependent survival relative to control situations where unfused Gal4DBD is bound to the Gal4 sites and where Gal4DBD-Rif21-36/60 proteins remain untargeted to broken end (0 Gal4 site) (Fig. 2B, Supplementary Fig. 2B). NHEJ inhibition by the BAT still takes place in cells lacking endogenous Rif2 (Fig. 2B) or Rap1 C-terminal domain (Supplementary Fig. 2C). This indicates that in these assays the BAT ability to repress NHEJ does not rely on the endogenous Rif2 protein nor on the Rap1 domain that recruits Rif2 to telomeres. The targeting of a single Gal4DBD-Rif21-36 dimer inhibits NHEJ (1 Gal4 site in Fig. 2B and Supplementary Fig. 2B), showing that one or two BAT molecules are sufficient to act.
Fig. 2. Rif2 BAT motif inhibits NHEJ at broken ends.
A I-SceI assay used to estimate NHEJ efficiency. Two inverted I-SceI sites are inserted at the endogenous URA3 gene. Most survivors to continuous I-SceI expression have eliminated the I-SceI sites by fusing the distal broken ends21. B NHEJ inhibition by Rap1 C-terminal domain and Rif2 N-terminal region targeted at broken ends (lif1∆: NHEJ-deficient cells). Means from independent cell cultures. C Increasing distances between the broken end and the Gal4 binding sites decrease NHEJ inhibition by Rif2. D Rif2 N-terminal truncations impacting the ability to inhibit NHEJ at broken ends. Gal4DBD and Gal4DBD-Rif2fragments expressed from a centromeric plasmid. E Rif2 mutations impacting its ability to inhibit NHEJ at broken ends. Gal4DBD and Gal4DBD-Rif21-60 expressed from an integrated plasmid. Gal4DBD-Rif21-395 expressed from a centromeric plasmid. F The rif2-F8A mutation exposes telomeres to NHEJ in cells lacking Sir4 (fusions between X and Y′ telomeres). Experiment reproduced three times. G K. lactis Orc4 expression complements Rif2 loss for NHEJ inhibition by Rap1 C-terminal domain in S. cerevisiae.
In the previous experiment, the edge of the Gal4 sites is 23 bp away from the broken end. To assess how far Rif2 BAT can act, we inserted sequences between the Gal4 sites and the I-SceI site. Strikingly, BAT ability to inhibit NHEJ rapidly weakens with increasing distances from the broken end (Fig. 2C, Supplementary Fig. 2D). Distances of 79 bp or more prevent BAT action. A similar threshold is found when NHEJ is inhibited by full-length Rif2 targeted through Rap1 C-terminal domain. In addition, Rif2 overexpression does not inhibit NHEJ when untargeted to DNA ends (Supplementary Fig. 3A&B). These results show that Rif2 and Rif2 BAT need to be actively concentrated in the immediate vicinity of the broken end to block NHEJ.
Next, we determined if smaller N-terminal Rif2 fragments retain the ability to inhibit NHEJ. C-terminal truncations down to position 26 have no significant effect (Fig. 2D, 23 bp between the edge of the Gal4 sites and the broken end). More severe C-terminal truncations (1–24, 1–20) and N-terminal truncations (6–31) reduce or abolish BAT function. Thus, the first 26 residues of Rif2 are sufficient to inhibit NHEJ when targeted to a broken end.
To better establish the specificity of BAT impact on NHEJ, we mutated conserved residues within the S. cerevisiae Rif2 N-terminal region (1–60) (Fig. 1A) and targeted these mutated fragments to a DSB (Fig. 2E). This mutagenesis identifies F8, R12, R13, S14 and I23 as important residues for NHEJ inhibition. Mutations altering the Rap1 Binding Motif (R40A and L44A) have no significant effect (Fig. 2E). Then we tested the role of F8, the most highly conserved residue, in the context of the full-length Rif2 protein. F8 is essential for NHEJ inhibition, both at a broken end when it is ectopically targeted there (1–395 vs 1–395 F8A) (Fig. 2E) and at telomeres through its native recruitment by Rap1 (Fig. 2F). As previously observed28, the F8A mutation elongates telomeres and does no impact protein stability (Supplementary Fig. 3C).
In addition to this mutagenesis, we asked if a Rif2 homologue from a distinct yeast species inhibits NHEJ. In S. cerevisiae and some other post-WGD (whole genome duplication) Saccharomycetaceae species, Rif2 and Orc4 are orthologs and only Rif2 possesses the BAT motif20,21,30 (Fig. 1A, Supplementary Fig. 1). In other Saccharomycetaceae species, including Kluyveromyces lactis, there is only one protein, Orc4, and it possesses both the BAT motif and the Rap1-binding motif. We overexpressed K. lactis Orc4 in S. cerevisiae cells lacking Rif2. This restores Rap1 ability to inhibit NHEJ at a broken end (Fig. 2G) and at telomeres (Supplementary Fig. 3D). As in S. cerevisiae Rif2, K. lactis Orc4 F8 is essential to this inhibition (Fig. 2G). Together, these results show that the conserved BAT motif blocks NHEJ.
Rif2 BAT blocks 5′ resection and inhibits MRX binding to broken ends
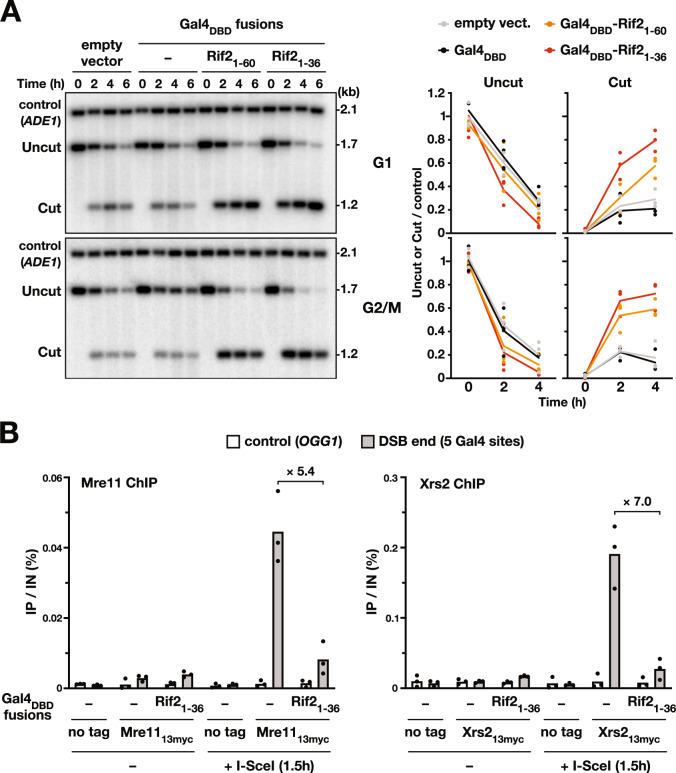
Since Rif2 antagonizes 5′ DNA end resection29,34–36, we asked if the BAT motif would be sufficient for this function too. To monitor resection, we used the I-SceI/Gal4 assay described above and a Southern blot approach (Supplementary Fig. 4A). Cells were arrested in G1 or in G2/M prior to induction of the I-SceI endonuclease. Targeting the Rif2 N-terminal region (1–60 and 1–36) (Fig. 3A, Supplementary Fig. 4B, C) or the Rap1 C-terminal domain (Supplementary Fig. 4C) stabilises the adjacent broken end relative to the control conditions (empty vector or Gal4DBD alone). These results show that Rif2 BAT limits 5′ end resection both in G1 and G2/M phases.
Fig. 3. Rif2 BAT blocks 5′ resection and MRX complex presence at broken ends.
A Rif2 N-terminal region stabilises broken ends. Left panel: the stability of I-SceI-induced broken ends with 5 Gal4 sites determined by Southern blot in G1 and G2/M arrested cells. Right panel: quantification of the uncut and cut signals normalised to the control ADE1 signal. Means from independent samples. Experiment reproduced three times. B Mre11 and Xrs2 presence at I-SceI-induced broken ends with 5 Gal4 sites determined by ChIP in G1 arrested cells. Quantification of immunoprecipitated DNA (IP) relative to the input DNA (IN). Means from independent samples. Quantification of I-SceI cleavage efficiency in Supplementary Fig. 4D.
Since Rif2 inhibits the interaction of MRX with DNA ends29,33,55, we next tested BAT ability to oppose this interaction using a ChIP approach. As shown in Fig. 3B, targeting Rif2 N-terminal region (1–36) limits Mre11 and Xrs2 presence at the adjacent broken end relative to the control condition (Gal4DBD alone). Since MRX functionality requires its three subunits, this result shows that the BAT motif antagonises MRX stable interaction with DNA ends. This property can explain its ability to inhibit telomere elongation28, NHEJ (Figs. 1, 2) and 5′ end resection (Fig. 3A).
Rif2 BAT interacts with Rad50 ATP-binding domains
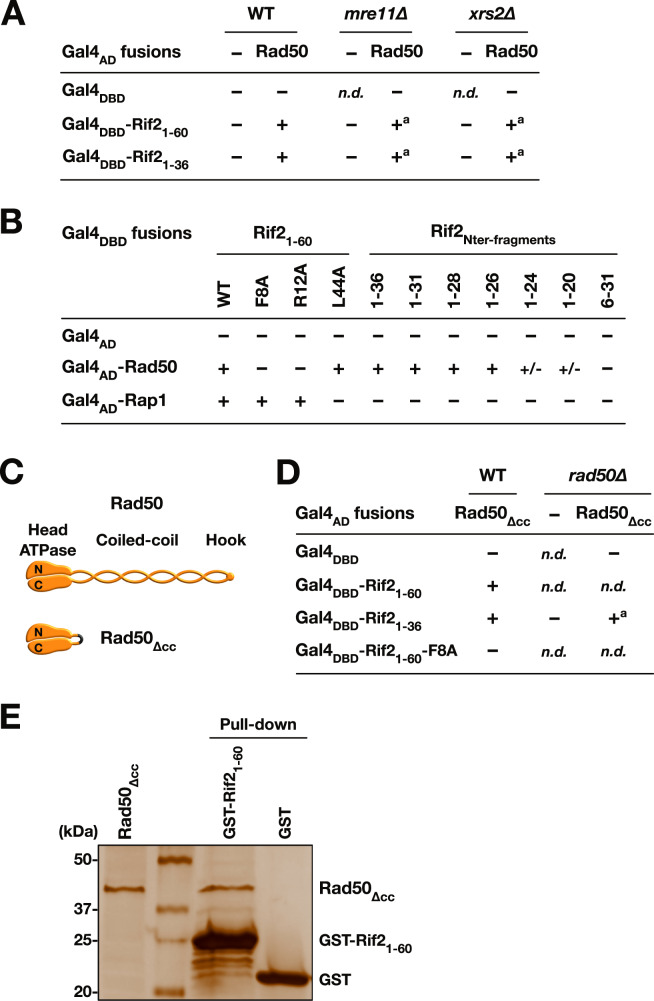
Next, we addressed the question of how the BAT motif opposes the MRX complex at DNA ends by searching for proteins interacting with the BAT. In vitro, Rif2 interacts with Rad5029,30. We used a Two-Hybrid approach to test for interactions with each full-length subunit of the MRX complex (Fig. 4, Supplementary Fig. 5). We found that Rif2 N-terminal region (1–60 and 1–36) interacts with Rad50. This interaction does not require Mre11 and Xrs2 (Fig. 4A).
Fig. 4. Rif2 BAT interacts with Rad50 ATPase Heads in vivo.
A 2-Hybrid interactions between Rif2 N-terminal region and full-length Rad50 in WT cells and in cells lacking Mre11 and Xrs2 (− no growth on plates supplemented with 3-AT, + growth on plates supplemented with 3-AT). A: slow growth in MRX-defective cells. B 2-Hybrid interactions between Rif2 N-terminal region and full-length Rad50 or Rap1 (fragment 366–827) in WT cells (+/- slow growth on plates supplemented with 3-AT). C Representation of full-length Rad50 and of the Rad50∆CC fragment lacking the coiled-coil arm. D 2-Hybrid interactions between Rif2 N-terminal region and Rad50 ATPase Head in WT cells and in cells lacking the endogenous Rad50. E GST pull-down interaction between Rif2 N-terminal region and Rad50 ATPase Head. Experiment reproduced three times.
To assess the significance of this interaction between the BAT motif and Rad50, we tested its sensitivity to mutations that impact BAT ability to inhibit NHEJ. Mutations in the F8 and R12 residues, C-terminal truncation past residue 26 and a N-terminal truncation (6-31) weaken or abolish the interaction with Rad50 (Fig. 4B). These data show a good correlation between the strength of NHEJ inhibition by Rif2 N-terminal region (Fig. 2D) and its ability to interact with Rad50 in vivo. Interestingly, Rif21-60 F8A and R12A fragments still interact with Rap1. By contrast, mutation L44A in the Rap1-binding motif prevents the interaction with Rap1, as expected22 but does not impact the interaction with Rad50. This further shows that Rif2 Rad50-binding motif (BAT) and Rap1-binding motif (RBM) are distinct and functionally separable.
Next, we asked which domain of Rad50 interacts with the BAT motif. Rad50 is an SMC protein with a fold-back structure58–62. Its N-terminal and C-terminal domains interact to form a globular ATPase Head. The rest of the protein assembles into a long coiled-coil with a median Zn-hook. Our attempt to arbitrarily truncate the protein into distinct domains failed in the Two-Hybrid assay. For this reason, we screened a library of random truncations within the Rad50 coiled-coil region and selected for clones still interacting with the BAT motif in Two-Hybrid. The shortest clone we obtained is a truncation of the coiled-coil region that connects the N-terminal and C-terminal Head domains (∆190–1124, hereafter Rad50∆cc) (Fig. 4C). Its interaction with Rif2 BAT does not require the endogenous full-length Rad50 and remains sensitive to the F8A mutation (Fig. 4D). The interaction between Rad50 Head domains and Rif21-60 can also be observed in vitro using a GST pull-down assay (Fig. 4E).
Identification of Rif2-resistant Rad50 mutants through a genetic screen
Rif2 BAT mutants defective for NHEJ inhibition are also defective for interaction with Rad50. Therefore, we expect Rad50 mutants specifically defective for interaction with Rif2 to be insensitive to Rif2 BAT inhibition. Finding such mutants will inform us on where the BAT motif interacts on Rad50, information that may give insights into the mechanism of inhibition. To this end, we first performed a genetic screen to identify Rad50 mutants that would be prone to telomere fusions, that is to say defective for telomere protection by Rif2.
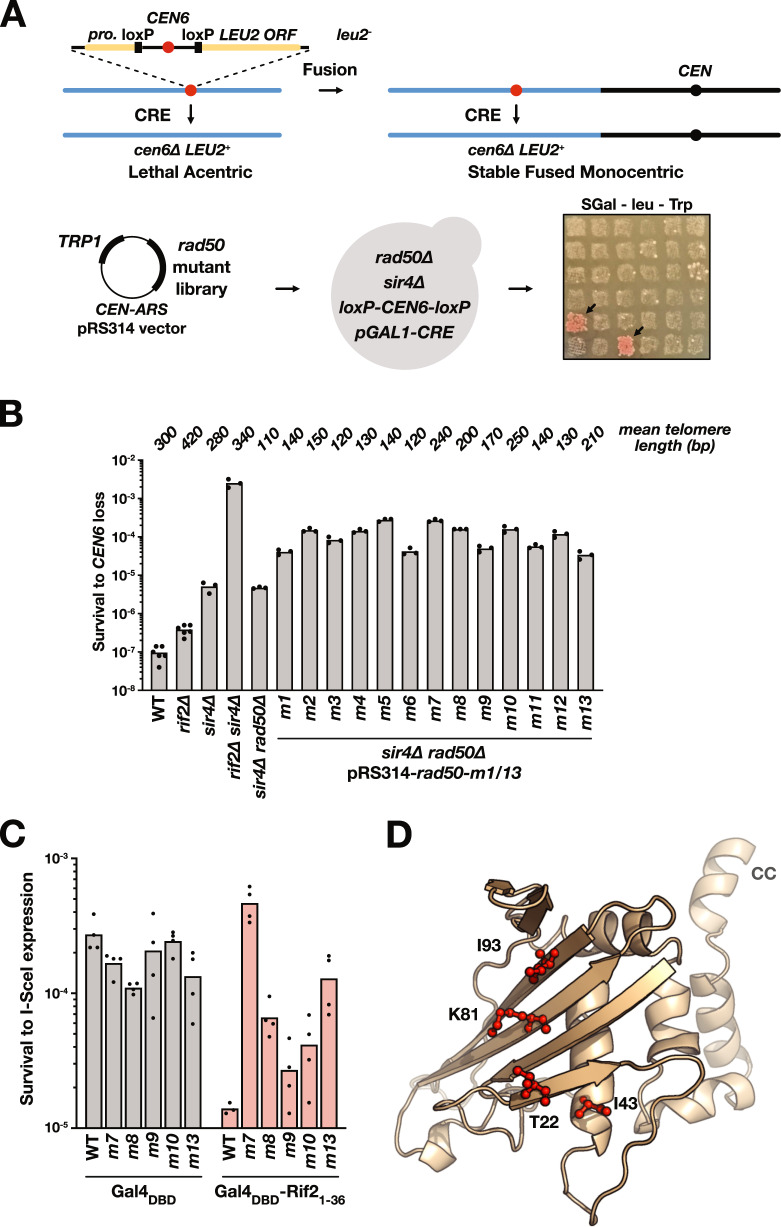
We used a genetic assay capable of capturing and quantifying chromosome fusions in budding yeast. This assay relies on the controlled inactivation of one centromere (CEN6) to select and rescue unstable dicentric chromosome fusions (Fig. 5A)63. Survival to centromere loss correlates with the frequency of chromosome fusions. Survival is low in wild-type cells (≈10−7 events/cell) and increases in cells lacking Rif2 and/or Sir4. To sensitise the assay, we choose to screen Rad50 mutants in cells lacking Sir4, where telomere protection relies more on Rif2 (Fig. 5A). Rad50 random PCR mutagenesis was performed on the sequence encoding the N-terminal part of the protein. We tested 300 mutants for survival to centromere loss. Among them, 13 increase the frequency of survival (referred as m1 to m13) (Fig. 5B).
Fig. 5. Identification of Rif2-resistant Rad50 mutants through a genetic screen.
A Schematic representation of the chromosome fusion capture assay used to identity Rif2-resistant Rad50 mutants. The loss of chromosome 6 centromere (CEN6) generates a lethal acentric chromosome unless chromosome 6 fused to another chromosome. The Cre recombinase is expressed from a galactose-inducible promoter. Cre-induced CEN6 loss generates a functional LEU2 gene at the CEN6 locus. The 5′ end of RAD50 ORF (−105 to +996) was mutagenized by PCR using the Taq polymerase. The mutant library was transformed in cells lacking Sir4 and Rad50. 300 individual transformants were patched to saturation on rich medium prior to being replicated on synthetic medium with galactose (2%) and lacking leucine to identify clones with increased survival rate to CEN6 loss. B Quantification of the survival to CEN6 loss in the 13 rad50 mutants identified in the screen (m1–m13). Cells were grown to saturation prior to plating on synthetic medium with galactose (2%) and lacking leucine. Colonies were counted after 5d at 30 °C. Means from independent cell cultures. Mean telomere length from Supplementary Fig. 5. C NHEJ inhibition by Rif2 BAT motif at I-SceI-induced broken ends in selected rad50 mutants (single I-SceI site assay (Supplementary Fig. 2B)). Means from independent cell cultures. D Position of rad50-m7 (K81) and rad50-m13 (T22, I43, I93) mutated residues on a model structure of S. cerevisiae Rad50 N-terminal Head domain (1–189, obtained from Chaetomium thermophilum Rad50 structure as template64 (PDB:5DAC)).
We expected two classes of mutants: (i) mutants whose increased fusion frequency is simply a consequence of a telomere shortening caused by a partial loss of function of MRX and (ii) mutants more specifically impacting Rif2 inhibition and better retaining MRX functions and the ability to elongate telomeres. Therefore, as a secondary screen, we determined the telomere length in the selected mutants. They display either very short telomeres (m1 to m6, m11, m12) or mildly short telomeres (m7 to m10, m13) (Fig. 5B, Supplementary Fig. 6A). We discarded the first group as unlikely to specifically resist Rif2. The candidates of the second group were assessed for their loss of sensitivity to Rif2 BAT using the I-SceI/Gal4 NHEJ assay. As shown in Fig. 5C, the 5 mutants maintain a frequency of survival to I-SceI expression close to the WT situation (Gal4DBD alone), indicating that NHEJ is still partially functional in these mutants. Targeting Rif2 BAT (1-36) to the break still represses NHEJ in the m8, m9 and m10 rad50 clones, indicating that these mutants are still sensitive to BAT inhibition. In the m7 and m13 clones, survival to I-SceI expression is not reduced by BAT targeting. This result shows that these two mutants lead to NHEJ resistance to the BAT in this assay.
The rad50-m7 allele carries three mutations, K81E, E242V and Q284L. The last two are within the N-terminal coiled-coil region. K81 is on a ß-sheet at the surface of Rad50 Head domain (Fig. 5D, model of S. cerevisiae Rad50∆cc Head generated using Chaetomium thermophilum Rad50 structure as template64 (PDB:5DAC 78% similarity/62% identity). The m13 allele carries five mutations, three in the Head (T22I, I43T, I93T) and two in the coiled-coil region (I292M and I382V). T22 and I93 are near K81 and part of the same ß-sheet at the surface of the Head. I43 is within the domain below T22. The clustering of the selected mutations in the Head suggests that this Rad50 ß sheet surface is important for Rif2 BAT function.
Identification of Rad50 residues essential for BAT function and interaction
In parallel with the genetic screen described above, we used a structural modelling approach to search for Rad50 residues interacting with the BAT motif. Since BAT conformation is unknown, we used the flexible docking approach CABSdock65 to perform the simulation search for the binding site, allowing full flexibility of the peptide and small fluctuations of the Rad50 backbone. To limit the combinatorial explosion due to the mainchain peptide flexibility, we used a short BAT sequence containing the most conserved amino acids (Rif2 residues 4 to 14, VDSDFAPIRRS; Fig. 1A). 10 000 structures were generated by CABSdock and the 1000 best scoring ones were clustered in 10 Rad50 NTD-BAT (4–14) peptide complexes. The clusters correspond to three interaction sites: one in the DNA-interaction site, one in the dimerisation site and one in the solvent-exposed ß-sheet where the mutations selected in the genetic screen are present. We therefore focused on this last cluster.
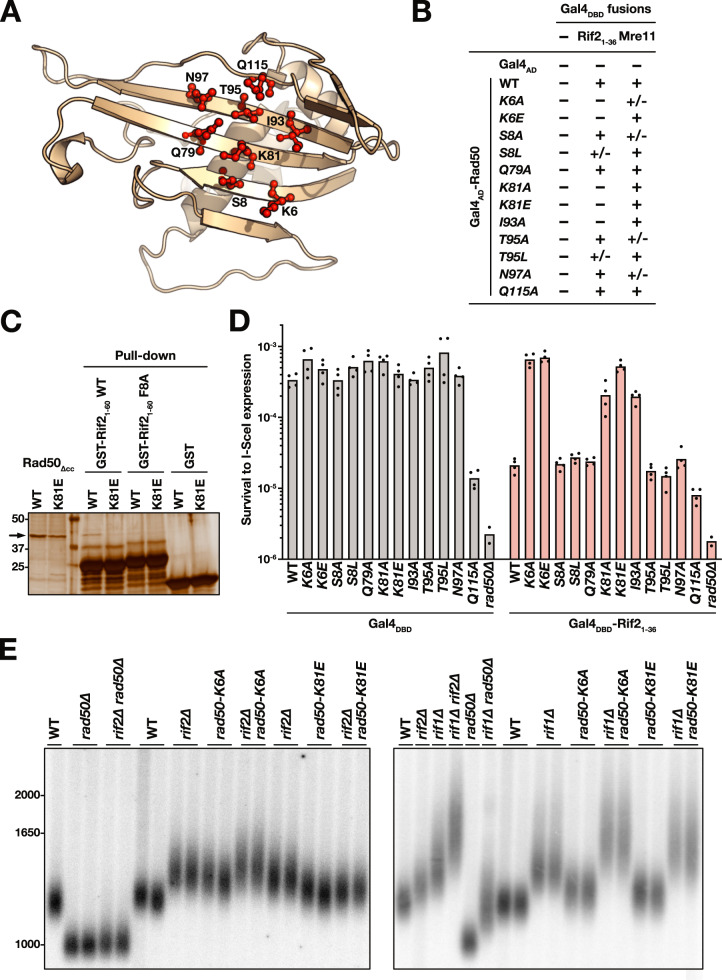
To validate this docking result, as well as to define more precisely the location of the BAT peptide, we used the Rad50∆cc–BAT4-14 complex models obtained with CABSdock to design mutations at several positions of the Rad50 ß-sheet (Fig. 6A). Mutations at positions K6, K81 and I93 abolish the Two-Hybrid interaction of Rad50 with Rif2 BAT (Fig. 6B, Supplementary Fig. 5). The same mutations preserve Rad50 interaction with Mre11. Mutations at surrounding positions on the ß sheet surface (S8, Q79, T95, N97 and Q115) maintain the Two-Hybrid interactions with Rif2 and Mre11 (S8L and T95L weakening the interaction with the BAT motif). In addition, purified Rad50∆cc harbouring the K81E mutation fails to interact with Rif2 BAT in vitro (Fig. 6C; the F8A mutation within Rif21-60 also prevents this interaction). These results show a specific requirement of K6, K81 and I93 for the Rad50–BAT interaction.
Fig. 6. Identification of Rad50 residues essential for BAT function and interaction.
A Position of the eight residues (red) selected with CABSdock as potentially interacting with Rif2 BAT. B 2-Hybrids interactions between mutant full-length Rad50 and Rif2 N-terminal region or full-length Mre11. C GST pull-down interaction between Rif2 N-terminal region and Rad50 ATPase Head. Experiment reproduced three times. D NHEJ inhibition by Rif2 BAT motif at I-SceI-induced broken ends in rad50 mutants (single I-SceI site assay (Supplementary Fig. 2B)). Means from independent cell cultures. E Impact on telomere length of mutants rad50-K6A and rad50-K81E in WT cells and in cells lacking Rif1 or Rif2 (Southern blot, Y′ probe, XhoI digest). Experiment reproduced three times.
Next, we tested the impact of the mutations on NHEJ inhibition by Rif2 BAT using the I-SceI/Gal4 assay. In the absence of the BAT motif at the broken end, the rad50 mutants remain NHEJ proficient, with one exception Q115A (Fig. 6D). Targeting Rif2 BAT to the break represses NHEJ in cells with mutations at positions S8, Q79, T95 and N97 but not in cells with mutations at positions K6, K81 and I93. Mutations that prevent Rad50–BAT interaction also prevent NHEJ inhibition by the BAT motif. Altogether, this result, the independent result of the genetic screen and the docking model show that Rif2 BAT likely contacts the solvent-exposed ß-sheet of Rad50 N-terminal Head domain, residues K6, K81 and I93 playing a central role in this interaction. Furthermore, our results show that this interaction is essential to inhibit Rad50.
Since Rif2 BAT shortens telomeres28 (Supplementary Figs. 2A, 3B, C), resistance to Rif2 BAT should cause telomere elongation. Among the numerous rad50 mutants previously generated and studied, the rad50S-K81I allele results in longer telomeres53,66,67. This indicates that at least one residue essential for BAT function is also essential for proper telomere length homoeostasis. To further address this point, we tested the impact of mutations K6A and K81E on telomere length. As expected, both cause telomere elongation (Fig. 6E left panel, Supplementary Fig. 6B). Telomeres in rad50-K6A and rad50-K81E cells are not as long as in cells lacking Rif2 or bearing the rif2-F8A mutation, perhaps in part because the rad50 mutations do not fully prevent Rif2 inhibition. In the absence of Rif2, the mutations K6A and K81E also lead to slightly different phenotypes. Telomeres are a little longer in rif2∆ rad50-K6A cells and a little shorter in rif2∆ rad50-K81E cells relative to rif2∆ cells. This suggests that the mutations moderately impact intrinsic Rad50 functions, in addition, to make it less sensitive to Rif2 (e.g. Tel1 activation53,68). In other words, they may not be perfect separation-of-function alleles. Rif1 is another repressor of telomere elongation that acts independently of Rif223,69–71. As expected for mutations impacting Rif2 function, the K6A and K81E mutations still result in longer telomeres in cells lacking Rif1 (Fig. 6E, right panel, compare rif1∆ with rif1∆ rad50-K6A and rif1∆ rad50-K81E). Together with the previous finding that rif2-F8A and rif2-R12A BAT mutants elongate telomeres28, these results show that telomere length homoeostasis relies in part on the Rad50–BAT interaction and its inhibitory function.
Predicted impact of BAT binding on the MRX complex
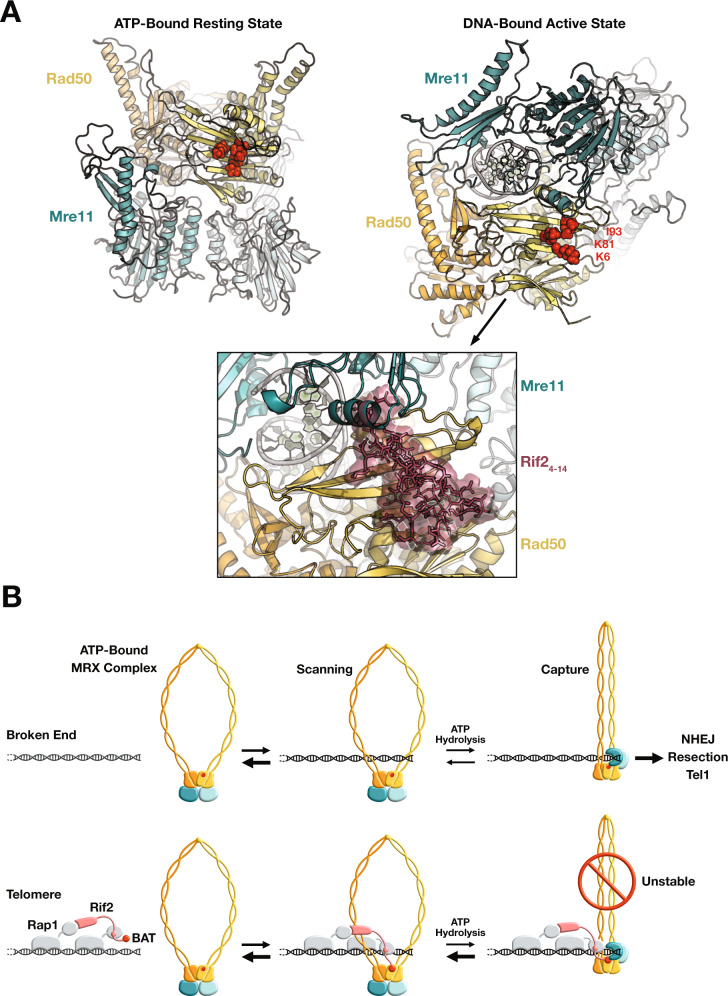
Next, we addressed the mechanism by which BAT binding on Rad50 Head inhibits MRX complex functions. First, we took advantage of the experimental results to build more accurate BAT-Rad50 models using the information-driven flexible docking approach HADDOCK72. In this method, ambiguous distance restraints that represent the spatial proximity between specific residues of the two partners are used to guide the docking. Residues K6, K81 and I93 of Rad50 and F8, R12, R13 and S14 of the BAT4-14 peptide were selected as the interacting residues. The HADDOCK calculation led to four clusters. The ambiguous character of the restraints and the fully flexible treatment of the BAT4-14 peptide explain the residual conformational spreading of the BAT peptide in these clusters. They all share the occupancy of a common region of the ß-sheet binding surface close to residue K6, K81 and I93. We then examined the consequences of this binding on the MRX complex structure.
How the MRX complex operates at a molecular level is not fully resolved yet but a recent cryo-EM study of Escherichia coli Rad50–Mre11SbcC-SbcD complex shows that it can adopt at least two states61. In an ATP-bound resting state, the Rad50 coiled-coils appear flexible and open. Upon DNA binding and consecutive ATP hydrolysis, the two coiled coils zip up into a rod and clamp DNA on the Rad50 Head. In this DNA-bound active state, Mre11 moves to the side of Rad50 to bind the DNA end. We used these structures to establish models of S. cerevisiae Rad50–Mre11 complex in the ATP-bound and DNA-bound states (Fig. 7A). To this end, we first built a model of S. cerevisiae Mre11 using Modeller73 and Chaetomium thermophilum Mre11 structure as template74 (PDB: 4YKE) (69% similarity/52% identity). To build the ATP-bound (resting state) and DNA-bound ScRad50-Mre11 models, we superimposed the ScRad50 and ScMre11 models on their E. coli homologues in the cryo-EM structures61 (PDB:6SV6 and 6S85).
Fig. 7. Predicted impact of BAT binding on the Rad50–Mre11 complex and model for MRX inhibition by Rif2 at telomeres.
A Models of S. cerevisiae Rad50–Mre11 complex in the ATP-bound resting state (left) and in the DNA-bound active state (right). Residues K6, K81 and I93 highlighted in red. Bottom panel: envelope of the BAT core peptide (Rif2 residues 4-14, in brown) belonging to the HADDOCK cluster 1 shown after superimposition of the Rad50 structures of the clusters on that of the DNA-bound Rad50–Mre11 complex model. B Model for telomere protection by Rif2 at telomeres (Rad50 orange/yellow, Mre11 teal/light blue, Rap1 grey, Rif2 pink, BAT and BAT interacting region on Rad50 red). For simplicity, Xrs2 and other factors present at broken ends and at telomeres are not represented.
In the predicted ATP-bound state, K6, K81 and I93, the three Rad50 residues essential to BAT interaction, are exposed and located away from Mre11. However, the DNA-bound state brings helix 181–192 of Mre11 near the ß-sheet surface including residues K6, K81 and I93. Furthermore, in the docking models of the BAT4-14 peptide on Rad50 obtained with HADDOCK, some BAT residues occupy the same region where helix 181–192 of Mre11 lies, as illustrated in Fig. 7A (bottom panel) (Supplementary Fig. 7). This suggests that the predicted BAT-bound state and DNA-bound state are not sterically compatible. In other words, the binding of the BAT peptide on Rad50 could prohibit the transition from the ATP-bound state to the DNA-bound state of the complex. This mechanism would explain how Rif2 BAT opposes MRX complex functions.
Discussion
Our results lead us to propose a mechanism for MRX inhibition by Rif2 at telomeres (Fig. 7B). Like other SMC proteins, MRX likely uses ATP-driven conformational changes of its coiled-coil arms to scan and handle DNA43,61,75,76. MRX does not extrude loops but captures broken ends60[,61,77. This end-capture is the first step that allows MRX to perform its functions in NHEJ repair, checkpoint activation, telomere elongation and 5′ end resection. Rad50 being a slow ATPase, both the ATP-bound resting state scanning for DNA ends and the DNA-bound active state are likely metastable61. At telomeres, Rap1 maintains Rif2 in close proximity to the DNA end22. We propose that, when an MRX complex approaches a telomere, the Rif2 BAT motif binds Rad50 ATPase Head in its ATP-bound resting state. This binding is favoured by Rif2 high local concentration at telomeres and Rad50 interaction surface accessibility in the resting state (Fig. 7 A). DNA binding stimulates ATP hydrolysis and the concomitant Mre11 move toward the DNA end. We propose that the BAT bound to Rad50 antagonises this last transition, plausibly by steric hindrance, and favours a rapid return to the more stable ATP-bound state. Unable to stably capture the telomere end, the MRX complex cannot proceed with the subsequent steps of NHEJ, Tel1-activation or 5′ resection. Once returned to the resting state, the complex will diffuse away from Rif2 and the telomere end. In agreement with this last step and a dynamic low-affinity BAT–Rad50 interaction, Rif2 BAT does not stably retain MRX where it binds on DNA (Fig. 3B). Furthermore, Rif2 BAT only acts at short distances (Fig. 2C) and Rif2 overexpression is insufficient to inhibit NHEJ in the absence of recruitment to DNA ends (Supplementary Fig. 3A, B). The need for close spatial proximity between the inhibitor and its target is a key feature of the proposed mechanism. It ensures that MRX inhibition is restricted to telomeres where Rif2 binds and does not oppose MRX functions at other positions on chromosomes
In this model, Rif2 selectively destabilises one metastable state of the MRX complex, therefore accelerating the ATP-driven cycle between the two states. This futile cycle can explain the paradoxical ~2-fold stimulation of S. cerevisiae Rad50 ATPase activity by Rif2 in vitro29,30. Further supporting this interpretation, mutations in E. coli Rad50–Mre11 complex that challenge Rad50-Mre11 contacts specifically in the active DNA-bound state also stimulate Rad50 ATPase activity ~2-fold in vitro, likely again by causing faster ADP-to-ATP exchange within Rad50 ATP cycle61. Interestingly, S. cerevisiae Rad50 Q115 residue, whose mutation impedes NHEJ (Fig. 6D), virtually contacts Mre11 in the docking model of the DNA-bound complex (Supplementary Fig. 7B). Rad50 Q115 mutation may specifically challenge the DNA-bound active state, as we predict BAT presence on the adjacent residues would do. Non-exclusively, Rif2 binding may also directly impact the Rad50 ATP-bound state by stimulating ATP hydrolysis by an unknown mechanism30.
Of the three residues identified as essential to interact with Rif2 BAT (Fig. 6), two (K6 and K81) also belong to a cluster of residues found mutated in the meiosis-defective rad50S alleles61,78,79 (the rad50-I93A allele remains sporulation proficient, Supplementary Fig. 8A). rad50S mutants specifically impair Mre11-dependent 5′ resection due to a loss of interaction with the Sae2CtIP protein80. Thus, Sae2 and Rif2 BAT interaction interfaces are likely to partially overlap on Rad50 Head, suggesting that Rif2 could also antagonize Sae2 binding. Note that this last hypothesis cannot alone account for BAT functions since NHEJ repair, Tel1 activation and NHEJ inhibition by Rif2 BAT remain proficient in the absence of Sae230,81 (Supplementary Fig. 8B).
In addition to MRX inhibition by the BAT, the full-length Rif2 protein has other regulatory properties. For instance, it downregulates DNA–RNA hybrids at telomeres, in part through the recruitment of RNaseH282. Rif2 was also proposed to protect telomeres with the help of the chromatin remodeler Rpd3L83,84. Since RNaseH2 loss has no impact on telomere fusions (Supplementary Fig. 8C) and Rif2 BAT still inhibits NHEJ in cells lacking Rpd3L subunits Sin3 and Rxt2 (Supplementary Fig. 8B), these additional Rif2 functions are separable from the BAT functions.
MRXMRN and Tel1ATM inhibition is a conserved feature of telomeres in eukaryotes3,7,15,17,42. Is the mechanism of MRXMRN inhibition established by the BAT motif maintained in evolution? Rad50 ß-sheet and K6/K81 residues are evolutionarily stable in eukaryotes (e.g. R6/K81 in fission yeast and K6/R83 in human)85–87, suggesting that this mechanism can be conserved. Since K. lactis Orc4 complements Rif2 loss in S. cerevisiae (Fig. 2G, Supplementary Fig. 3D), Orc4 could inhibit the MRX complex at telomeres in Saccharomycetaceae species lacking Rif2. In addition, MRX inhibition by Orc4 might have a function at replication origins, for instance protecting nascent strands from 5′ resection. Outside of the Saccharomycetaceae family, a conserved BAT motif is still present at Orc4 N-terminal end in some yeast species (e.g. Candida auris)30 (Fig. 1A, Supplementary Fig. 1). It is followed by another conserved motif, which may correspond to a Rap1-binding motif, even though it does not perfectly match the sequence pattern defined from the S. cerevisiae Rif2 sequence.
In other eukaryotes, we could not find the BAT motif, at least corresponding to the strict pattern (D/E–F–X–X–Φ–R/K) (Supplementary Fig. 1). Searching for a small evolving linear motif, which can be embedded in highly degenerated sequences within intrinsically disordered segments, is difficult88. A direct screen for protein fragments interacting with Rad50 will likely be required to further address the conservation of this pathway, a candidate in mammals being the MRN-interacting iDDR motif within TRF27. The yeast BAT core motif will also be a useful start to design artificial Rad50 inhibitors.
Methods
Strains, plasmids and molecular genetics
Strains, plasmids, and primers used in this study are listed in Supplementary Data 1. Telomeres and telomere fusions were amplified using primers X2, Y′2 and polyG1421,31. The I-SceI assay used here was first described in21. Sites are inserted at the endogenous URA3 locus. The I-SceI site inserted upstream of URA3 is (I-SceI site bold, native sequence underlined): GTCCATAAGATCCTAGGGATAACAGGGTAATAGATCTAAGCTTTT.
The I-SceI and Gal4 sites inserted downstream of URA3 are (I-SceI site bold, native sequence and Gal4 binding sites underlined): TATTACCCTCGACGGATCTATTACCCTGTTATCCCTAGGATCGATCCTCTAGAGTCGGAGTACTGTCCTCCGAGCGGAGTACTGTCCTCCGAGCGGAGTACTGTCCTCCGAGCGGAGTACTGTCCTCCGAGCGGAGTACTGTCCTCCGAGGACCTGC AGGCATGCAAGCTGATCCAATCTCGG
The peptide linker between Gal4DBD(1-147) and Rif2 fragments is -PELIPGDPGGGGGGGGGG.
To determine survival to I-SceI cleavage, cells were grown to saturation in synthetic medium lacking uracil with glucose (2%) (1×108 cells/ml), diluted in water and spread on synthetic medium plates with galactose (2%). Colonies were counted after 3d at 30 °C.
To determine end resection, cells grown to OD 0.4 in synthetic glycerol lactate medium lacking uracil were arrested in G1 with 10−7 M α-factor (from a 10−3 M stock solution in ethanol) or in G2/M with 5 µg/mL nocodazole (from 1.5 mg/mL stock solution in DMSO) for 4 h. I-SceI expression was induced by galactose addition (2%). Genomic DNA was cut with StuI prior to gel electrophoresis. Southern blots were performed with a mix of two 32P-labelled probes hybridising TIM9 and ADE1. Signal quantification was performed using a Typhoon 5 imager and the ImageQuant software.
Two-hybrid assays were performed using strain Y190 on synthetic medium plates with glucose (2%), adenine and His3 inhibitor 3-Amino-1,2,4-triazole (3AT) (25 and 50 mM). To screen for shorter Rad50 fragments interacting with the Rif2 BAT motif, plasmid pACT2-RAD50 was linearised by PvuII (situated in RAD50 ORF midzone), partially digested by BAL-31 exonuclease and re-circularised with T4 DNA ligase. The library of random RAD50 truncations was amplified in E. coli and transformed in yeast strain Y190 containing plasmid pRS414-Gal4DBD-Rif21-36. Positive clones retaining the interaction were selected on plates with 50 mM 3AT. Plasmids recovered from yeast were sequenced. The shortest one encodes the Rad50∆CC allele (Rad501–189-KILCY-1125-1312).
The 5′ end of RAD50 ORF (−105 to +996; codons 1–332) was mutagenized by PCR using the Taq polymerase, a pRS314-RAD50 plasmid as a template and standard buffer condition (~104-5-fold amplification). The amplified fragment was reintegrated into PstI/StuI-digested pRS314-RAD50 by gap repair in yeast cells lacking Sir4 and Rad50.
Protein purification and GST pull-down assay
Rad50∆CC and Rad50∆CC-K81E fused to His6-SUMO (N-terminal tag) were induced with 1 mM isopropyl-ß-D-thiogalactoside (IPTG) at 20 °C overnight into E. coli strain BL21 (DE3). All of the subsequent protein purification steps were carried out at 4 °C. Cells were harvested, suspended in lysis buffer (20 mM KPO4 pH7.8, 500 mM KCl, 1 mM DTT, 10% glycerol, 0.2% NP40, 1 mg/mL lysozyme, 1 mM 4-(2-aminoethyl) benzenesulphonyl fluoride (AEBSF), 10 mM benzaminide, 2 µM pepstatin) and disrupted by sonication. Extract was cleared by centrifugation at 186,000g for 1 hour at 4 °C and then incubated at 4 °C with NiNTA resin (QIAGEN) for 3 h. Mixture was poured into an Econo-Column® Chromatography column (BIO-RAD). After extensive washing of the resin with buffer A (20 mM KPO4 pH7.8, 150 mM KCl, 1 mM DTT, 10% glycerol, 0.2% NP40) complemented with 40 mM imidazole, protein was eluted with buffer A complemented with 400 mM imidazole. Fractions containing purified His-SUMO-Rad50∆CC were pooled and applied to a PD10 column (GE Healthcare) equilibrated with buffer A to remove imidazole. Purified His-SUMO-Rad50∆CC concentration was adjusted to 40 µM before storage at −80 °C.
GST-Rif21-60 and GST-Rif21-60-F8A were induced with 0.5 mM IPTG at 30 °C for 4 h into E. coli strain BL21 (DE3) and cells were disrupted by sonication into lysis buffer (50 mM Tris HCl [pH8@4 °C], 150 mM NaCl, 1 mM DTT, 1 mM EDTA, 0.2% NP40, 1 mg/mL lysozyme, 1 mM AEBSF, 10 mM benzaminide, 2 µM pepstatin). After centrifugation, the extract was incubated with GSH Sepharose resin (GE Healthcare) overnight at 4 °C and then poured into an Econo-Column® Chromatography column (BIO-RAD). After extensive washing of the resin with buffer B (50 mM Tris HCl [pH8@4 °C], 150 mM NaCl, 1 mM DTT), proteins bound to the resin were eluted with buffer B complemented with 30 mM Glutathion. Fractions containing GST-protein were pooled and applied to a 2×1 ml Hitrap Heparine column (GE Healthcare) equilibrated with buffer C (50 mM Tris HCl [pH8@4 °C], 50 mM NaCl, 1 mM DTT, 1 mM EDTA). Protein was eluted with a 20 mL linear gradient of 0.05–0.4 M NaCl. Purified GST-ScRif21-60 was stored at −80 °C.
His-SUMO-Rad50∆CC WT or His-SUMO-Rad50∆CC-K81E were cleaved with His-SUMO-Protease at a ratio of 1/20 (W/W) at 4 °C overnight. The mixtures were then incubated with NiNTA bead (BioRad) and Rad50∆CC lacking the His-SUMO tag was recovered directly in the flow through. GST-Rif21-60 (10 µg), GST-Rif21-60-F8A (10 µg) or GST (10 µg) was immobilised on 10 µL Glutathione Sepharose 4B in 300 µl of buffer A (50 mM Tris HCl [pH8@4 °C], 150 mM NaCl, 1 mM DTT, 0.5 mM EDTA, 10% Glycerol) complemented with 2 mM MgCl2 and 25 units of benzonase for 60 minutes at 4 °C. The beads were collected by centrifugation, washed three times with 300 µl of buffer A. Rad50∆CC (30 µg) was then added to the beads in 100 µL buffer A complemented with 2 mM MgCl2 and 25 units of benzonase) and incubation was pursued for 120 minutes at 4 °C with gentle agitation. The supernatant was removed and the beads were washed two times with 300 µL of buffer A. Proteins bound to the beads were then eluted by addition of 20 µL of 50 mM Tris-HCl [pH8@4 °C], 150 mM NaCl, 1 mM DTT, 30 mM glutathione. Proteins bound to the beads were resolved by 12% SDS-PAGE and detected by silver staining.
Chromatin immunoprecipitation
Cells grown to OD 0.4 in synthetic glycerol lactate medium lacking uracil were arrested in G1 with 10−7 M α-factor for 4 h. I-SceI expression was induced for 1.5 h by galactose addition (2%). Cells were crosslinked for 10 min with formaldehyde (1%) at 30 °C. Cell lysis and chromatin sonication were performed using a Bioruptor. Immunoprecipitation was performed using the 4A6 anti-myc antibody. Input and immunoprecipitated DNA concentrations were determined by qPCR.
Homology modelling
The N-terminal and C-terminal domains of S. cerevisiae Rad50 (Sc-Rad50) were modelled separately. Both domains were built by homology using Modeller 9.1773. We identified Rad50 from Chaetomium thermophilum (Ct-Rad50) as highly homologous to Sc-Rad50. The similarity between N-terminal domains of Sc-Rad50 (residue 2–189) and Ct-Rad50 is very high (62.8% identity/78% similarity). The sequence of the C-terminal domain of Sc-Rad50 (residue 1105–1312) is also highly similar to the one of Ct-Rad50 (residues 1105–1311; 61.2 % identity/73.7 % similarity). This made it possible to use the structure 5DAC64 as a structural template for modelling both N- and C-terminal domains of S. cerevisiae Rad50. The best scoring models returned by Modeller were used for further investigations. Then, we build a model of Sc-Rad50 using the models obtained for its N- and C-terminal domains. The sequence used corresponds to the Rad50∆CC fragment lacking the coiled-coil region.
A similar approach was used to model the catalytic domain of S. cerevisiae Mre11 (residue 1–415) involved in the interaction with Rad50. We used HMMER89 in the bioinformatics toolkit90 to identify a structural template in the protein database91. The X-ray structure 4YKE corresponding to the structure of Mre11 from Chaetomium thermophilum (Ct-Mre11) possesses a high sequence similarity with Mre11 from S. cerevisiae (Sc-Mre11) (52.2 % identity/69.7% similarity). Thus, 4YKE was selected as template to build the Sc-Mre11 model. 100 models were generated with Modeller 9.17 and the best scoring models were retained.
To build S. cerevisiae Rad50-Mre11 dimer models, we used the recently solved E. coli Rad50–Mre11 dimer complexes61 in the resting state (structure 6S6V) and in the cutting state (structure 6S85). These EM structures allowed us to orient the two partners and to build the Rad50-Mre11 dimer in the resting state and the cutting one. As the sequence homology between Sc-Rad50 and Ec-Rad50 and between Ec-Mre11 and Sc-Mre11 is low (below 15%), the RMSD on the coordinates after superimposition of the whole structures is high (>2.5 Å). However, this permitted to obtain initial Sc-Rad50-Mre11 dimer models in the ATP-bound state and DNA-bound state. These initial complexes were refined by molecular dynamics in explicit water solvent. The starting structure was immersed in a cubic water box. The box was set using the Tcl plugin Solvate of VMD92 so that the water layer around the solute (protein or DNA) was at least 12 Å. Then the system was neutralised with Na+ or Cl- ions using the autoionize Tcl plugin of VMD. All MD calculations were carried out with the NAMD software93 in the charmm36 forcefield94. The first step of the MD calculation consisted in a 1 ns restrained molecular dynamics simulation in the NPT ensemble. To this end, in addition to the standard energy terms (bonds, angles, dihedral angles, improper, van der Waals and electrostatic terms), we applied a harmonic potential (with constant k = 1 kcal mol−1 Å−2) restraining the CA atoms of the protein to their initial position while optimising sidechain atoms and water position. Then, a production step of 15 ns MD in the NPT ensemble was run without positional restraints. Frames were extracted from the last 5 ns MD trajectory and analysed. During MD, pressure control was achieved by a Nose-Hoover Langevin piston. MD trajectories were analysed with VMD v 1.9.4. Modelling figures were produced with PyMol95.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
The authors thank Carol Greider for Rap1-Rif21-60 plasmid, Hélène Bordelet for pGBT9-RAD50 plasmid, Julie Soutourina for the 2H library, Elea Dizet (CIGEx platform) for plasmid construction and Stefano Mattarocci, Karine Dubrana, Laure Crabbé, Jean-Baptiste Boulé, Miguel Godinho Ferreira, Eric Coïc, Laurent Maloisel, Anna Campalans, Pablo Radicella, Teresa Teixeira, Hélène Bordelet, Alice Deshayes, Dan Throsby and Thomas M. Guérin, for fruitful discussions and suggestions. This work was supported by grants from Agence Nationale de la Recherche (ANR-14-CE10-021 DICENS, ANR-15-CE12-0007 DNA-Life, FRISBI ANR-10-INSB-0005), Fondation ARC pour la Recherche sur le Cancer, CEA Radiation biology programme and GGP CEA EDF programme. F.R.H. was supported by a PhD fellowship from ANR, a Fondation ARC young researcher grant and CEA.
Author contributions
StM and F.R.H. conceived the study with help from all co-authors. F.R.H. performed the NHEJ, ChIP, Southern and Two-Hybrid assays. K.J. performed the telomere fusions experiments. S.P. and StM performed the genetic screen. D.B. created plasmids for protein purification and mutagenesis. J.D. and X.V. expressed and purified proteins and performed the GST pull-downs with help from SiM and S.Z.-J. P.C. performed molecular modelling, docking and MD simulations. I.C. performed the protein sequence alignements. StM and F.R.H. wrote the manuscript with editorial help from P.C., S.Z.J., J.B.C., I.C., M.H.L.D., D.B. and X.V.
Data availability
All relevant data are available from the authors without restriction. Raw images and quantitative data have been deposited in the “Mendeley repository” [10.17632/8ghyn6n6x8.1].
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Lorraine Symington and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-23035-w.
References
- 1.Wellinger RJ, Zakian VA. Everything you ever wanted to know about Saccharomyces cerevisiae telomeres: beginning to end. Genetics. 2012;191:1073–1105. doi: 10.1534/genetics.111.137851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maciejowski J, de Lange T. Telomeres in cancer: tumour suppression and genome instability. Nat. Rev. Mol. Cell Biol. 2017;18:175–186. doi: 10.1038/nrm.2016.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Lange T. Shelterin-mediated telomere protection. Annu. Rev. Genet. 2018;52:223–247. doi: 10.1146/annurev-genet-032918-021921. [DOI] [PubMed] [Google Scholar]
- 4.Hou H, Cooper JP. Stretching, scrambling, piercing and entangling: challenges for telomeres in mitotic and meiotic chromosome segregation. Differentiation. 2018;100:12–20. doi: 10.1016/j.diff.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Ruis P, Boulton SJ. The end protection problem-an unexpected twist in the tail. Genes Dev. 2021;35:1–21. doi: 10.1101/gad.344044.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribes-Zamora A, Indiviglio SM, Mihalek I, Williams CL, Bertuch AA. TRF2 interaction with Ku heterotetramerization interface gives insight into c-NHEJ prevention at human telomeres. Cell Rep. 2013;5:194–206. doi: 10.1016/j.celrep.2013.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okamoto K, et al. A two-step mechanism for TRF2-mediated chromosome-end protection. Nature. 2013;494:1–5. doi: 10.1038/nature11873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan L, Hildebrand K, Stutz C, Thomä N, Baumann P. Minishelterins separate telomere length regulation and end protection in fission yeast. Genes Dev. 2015;29:1164–1174. doi: 10.1101/gad.261123.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benarroch-Popivker D, et al. TRF2-mediated control of telomere DNA topology as a mechanism for chromosome-end protection. Mol. Cell. 2016;61:274–286. doi: 10.1016/j.molcel.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J-K, et al. Structural basis for shelterin bridge assembly. Mol. Cell. 2017;68:698–714.e5. doi: 10.1016/j.molcel.2017.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rivera T, Haggblom C, Cosconati S, Karlseder J. A balance between elongation and trimming regulates telomere stability in stem cells. Nat. Struct. Mol. Biol. 2017;24:30–39. doi: 10.1038/nsmb.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li JSZ, et al. TZAP: a telomere-associated protein involved in telomere length control. Science. 2017;355:638–641. doi: 10.1126/science.aah6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmutz I, Timashev L, Xie W, Patel DJ, de Lange T. TRF2 binds branched DNA to safeguard telomere integrity. Nat. Struct. Mol. Biol. 2017;24:734–742. doi: 10.1038/nsmb.3451. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong CA, et al. Fission yeast Ccq1 is a modulator of telomerase activity. Nucleic Acids Res. 2018;46:704–716. doi: 10.1093/nar/gkx1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Ly,D, et al. Telomere loop dynamics in chromosome end protection. Mol. Cell. 2018;71:510–525.e6. doi: 10.1016/j.molcel.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 16.Irie H, et al. Telomere-binding proteins Taz1 and Rap1 regulate DSB repair and suppress gross chromosomal rearrangements in fission yeast. PloS Genet. 2019;15:e1008335. doi: 10.1371/journal.pgen.1008335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarek G, et al. CDK phosphorylation of TRF2 controls t-loop dynamics during the cell cycle. Nature. 2019;575:523–527. doi: 10.1038/s41586-019-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guérin TM, et al. Condensin-mediated chromosome folding and internal telomeres drive dicentric severing by cytokinesis. Mol. Cell. 2019;75:131–144.e3. doi: 10.1016/j.molcel.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Lototska L, et al. Human RAP1 specifically protects telomeres of senescent cells from DNA damage. EMBO Rep. 2020;2012:e49076. doi: 10.15252/embr.201949076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scannell DR, et al. Independent sorting-out of thousands of duplicated gene pairs in two yeast species descended from a whole-genome duplication. Proc. Natl Acad. Sci. USA. 2007;104:8397–8402. doi: 10.1073/pnas.0608218104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcand S, Pardo B, Gratias A, Cahun S, Callebaut I. Multiple pathways inhibit NHEJ at telomeres. Genes Dev. 2008;22:1153–1158. doi: 10.1101/gad.455108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi T, et al. Rif1 and Rif2 shape telomere function and architecture through multivalent Rap1 interactions. Cell. 2013;153:1340–1353. doi: 10.1016/j.cell.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Wotton D, Shore D. A novel Rap1p-interacting factor, Rif2p, cooperates with Rif1p to regulate telomere length in Saccharomyces cerevisiae. Genes Dev. 1997;11:748–760. doi: 10.1101/gad.11.6.748. [DOI] [PubMed] [Google Scholar]
- 24.Levy DL, Blackburn EH. Counting of Rif1p and Rif2p on Saccharomyces cerevisiae telomeres regulates telomere length. Mol. Cell. Biol. 2004;24:10857–10867. doi: 10.1128/MCB.24.24.10857-10867.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teixeira MT, Arneric M, Sperisen P, Lingner J. Telomere length homeostasis is achieved via a switch between telomerase- extendible and -nonextendible states. Cell. 2004;117:323–335. doi: 10.1016/S0092-8674(04)00334-4. [DOI] [PubMed] [Google Scholar]
- 26.Viscardi V, Bonetti D, Cartagena-Lirola H, Lucchini G, Longhese MP. MRX-dependent DNA damage response to short telomeres. Mol. Biol. Cell. 2007;18:3047–3058. doi: 10.1091/mbc.e07-03-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGee JS, et al. Reduced Rif2 and lack of Mec1 target short telomeres for elongation rather than double-strand break repair. Nat. Struct. Mol. Biol. 2010;17:1438–1445. doi: 10.1038/nsmb.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaizer H, Connelly CJ, Bettridge K, Viggiani C, Greider CW. Regulation of telomere length requires a conserved N-terminal domain of Rif2 in Saccharomyces cerevisiae. Genetics. 2015;201:573–586. doi: 10.1534/genetics.115.177899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cassani C, et al. Tel1 and Rif2 regulate MRX functions in end-tethering and repair of DNA double-strand breaks. PLoS Biol. 2016;14:e1002387. doi: 10.1371/journal.pbio.1002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hailemariam S, et al. The telomere-binding protein Rif2 and ATP-bound Rad50 have opposing roles in the activation of yeast Tel1ATM kinase. J. Biol. Chem. 2019;294:18846–18852. doi: 10.1074/jbc.RA119.011077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lescasse R, Pobiega S, Callebaut I, Marcand S. End-joining inhibition at telomeres requires the translocase and polySUMO-dependent ubiquitin ligase Uls1. EMBO J. 2013;32:805–815. doi: 10.1038/emboj.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teng SC, Chang J, McCowan B, Zakian VA. Telomerase-independent lengthening of yeast telomeres occurs by an abrupt Rad50p-dependent, Rif-inhibited recombinational process. Mol. Cell. 2000;6:947–952. doi: 10.1016/S1097-2765(05)00094-8. [DOI] [PubMed] [Google Scholar]
- 33.Hirano Y, Fukunaga K, Sugimoto K. Rif1 and Rif2 Inhibit Localization of Tel1 to DNA Ends. Mol. Cell. 2009;33:312–322. doi: 10.1016/j.molcel.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonetti D, et al. Shelterin-like proteins and Yku inhibit nucleolytic processing of Saccharomyces cerevisiae telomeres. PLoS Genet. 2010;6:e1000966. doi: 10.1371/journal.pgen.1000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonetti D, Clerici M, Manfrini N, Lucchini G, Longhese MP. The MRX complex plays multiple functions in resection of Yku- and Rif2-protected DNA ends. PLoS ONE. 2010;5:e14142. doi: 10.1371/journal.pone.0014142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ribeyre C, Shore D. Anticheckpoint pathways at telomeres in yeast. Nat. Struct. Mol. Biol. 2012;19:307–313. doi: 10.1038/nsmb.2225. [DOI] [PubMed] [Google Scholar]
- 37.Bringas, F. R. R. et al. Rap1, Rif2, and the Ku complex work in concert to cap chromosome ends. bioRxiv10.1101/2020.12.30.424824 (2020).
- 38.Chen L, Trujillo K, Ramos W, Sung P, Tomkinson AE. Promotion of Dnl4-catalyzed DNA end-joining by the Rad50/Mre11/Xrs2 and Hdf1/Hdf2 complexes. Mol. Cell. 2001;8:1105–1115. doi: 10.1016/S1097-2765(01)00388-4. [DOI] [PubMed] [Google Scholar]
- 39.Palmbos PL, Daley JM, Wilson TE. Mutations of the Yku80 C terminus and Xrs2 FHA domain specifically block yeast nonhomologous end joining. Mol. Cell. Biol. 2005;25:10782–10790. doi: 10.1128/MCB.25.24.10782-10790.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmbos PL, Wu D, Daley JM, Wilson TE. Recruitment of Saccharomyces cerevisiae Dnl4-Lif1 complex to a double-strand break requires interactions with Yku80 and the Xrs2 FHA domain. Genetics. 2008;180:1809–1819. doi: 10.1534/genetics.108.095539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsuzaki K, Shinohara A, Shinohara M. Forkhead-associated domain of yeast Xrs2, a homolog of human Nbs1, promotes nonhomologous end joining through interaction with a ligase IV partner protein, Lif1. Genetics. 2008;179:213–225. doi: 10.1534/genetics.107.079236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reis CC, Batista S, Ferreira MG. The fission yeast MRN complex tethers dysfunctional telomeres for NHEJ repair. EMBO J. 2012;31:4576–4586. doi: 10.1038/emboj.2012.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deshpande RA, et al. ATP-driven Rad50 conformations regulate DNA tethering, end resection, and ATM checkpoint signaling. EMBO J. 2014;33:482–500. doi: 10.1002/embj.201386100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mojumdar A, et al. Nej1 interacts with Mre11 to regulate tethering and Dna2 binding at DNA double-strand breaks. Cell Rep. 2019;28:1564–1573.e3. doi: 10.1016/j.celrep.2019.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oh J, Symington LS. Role of the Mre11 complex in preserving genome integrity. Genes (Basel) 2018;9:589. doi: 10.3390/genes9120589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cannavo E, Reginato G, Cejka P. Stepwise 5’ DNA end-specific resection of DNA breaks by the Mre11-Rad50-Xrs2 and Sae2 nuclease ensemble. Proc. Natl Acad. Sci. 2019;116:5505–5513. doi: 10.1073/pnas.1820157116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shim EY, Ma J-L, Oum J-H, Yanez Y, Lee SE. The yeast chromatin remodeler RSC complex facilitates end joining repair of DNA double-strand breaks. Mol. Cell. Biol. 2005;25:3934–3944. doi: 10.1128/MCB.25.10.3934-3944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shim EY, et al. Saccharomyces cerevisiae Mre11/Rad50/Xrs2 and Ku proteins regulate association of Exo1 and Dna2 with DNA breaks. EMBO J. 2010;29:3370–3380. doi: 10.1038/emboj.2010.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delamarre A, et al. MRX increases chromatin accessibility at stalled replication forks to promote nascent DNA resection and cohesin loading. Mol. Cell. 2020;77:395–410.e3. doi: 10.1016/j.molcel.2019.10.029. [DOI] [PubMed] [Google Scholar]
- 50.Ritchie KB, Petes TD. The Mre11p/Rad50p/Xrs2p complex and the Tel1p function in a single pathway for telomere maintenance in yeast. Genetics. 2000;155:475–479. doi: 10.1093/genetics/155.1.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee SS, Bohrson C, Pike AM, Wheelan SJ, Greider CW. ATM kinase is required for telomere elongation in mouse and human cells. Cell Rep. 2015;13:1623–1632. doi: 10.1016/j.celrep.2015.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cassani C, et al. The ATP-bound conformation of the Mre11-Rad50 complex is essential for Tel1/ATM activation. Nucleic Acids Res. 2019;47:3550–3567. doi: 10.1093/nar/gkz038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keener R, Connelly CJ, Greider CW. Tel1 activation by the MRX complex is sufficient for telomere length regulation but not for the DNA damage response in Saccharomyces cerevisiae. Genetics. 2019;213:1271–1288. doi: 10.1534/genetics.119.302713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hailemariam S, Kumar S, Burgers PM. Activation of Tel1ATM kinase requires Rad50 ATPase and long nucleosome-free DNA but no DNA ends. J. Biol. Chem. 2019;294:10120–10130. doi: 10.1074/jbc.RA119.008410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang L-L, Wu Z, Zhou J-Q. Tel1 and Rif2 oppositely regulate telomere protection at uncapped telomeres in Saccharomyces cerevisiae. J. Genet. Genomics. 2018;45:467–476. doi: 10.1016/j.jgg.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 56.Guirouilh-Barbat J, Rass E, Plo I, Bertrand P, Lopez BS. Defects in XRCC4 and KU80 differentially affect the joining of distal nonhomologous ends. Proc. Natl Acad. Sci. 2007;104:20902–20907. doi: 10.1073/pnas.0708541104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rass E, et al. Role of Mre11 in chromosomal nonhomologous end joining in mammalian cells. Nat. Struct. Mol. Biol. 2009;16:819–824. doi: 10.1038/nsmb.1641. [DOI] [PubMed] [Google Scholar]
- 58.Park YB, et al. Eukaryotic Rad50 functions as a rod-shaped dimer. Nat. Struct. Mol. Biol. 2017;24:248–257. doi: 10.1038/nsmb.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Syed A, Tainer JA. The MRE11–RAD50–NBS1 complex conducts the orchestration of damage signaling and outcomes to stress in DNA replication and repair. Annu. Rev. Biochem. 2018;87:263–294. doi: 10.1146/annurev-biochem-062917-012415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paull TT. 20 years of Mre11 biology: no end in sight. Mol. Cell. 2018;71:419–427. doi: 10.1016/j.molcel.2018.06.033. [DOI] [PubMed] [Google Scholar]
- 61.Käshammer L, et al. Mechanism of DNA end sensing and processing by the Mre11-Rad50 complex. Mol. Cell. 2019;76:382–394. doi: 10.1016/j.molcel.2019.07.035. [DOI] [PubMed] [Google Scholar]
- 62.Soh, Y.-M., Basquin, J. & Gruber, S. A rod conformation of the Pyrococcus furiosus Rad50 coiled coil. bioRxiv10.1101/2020.06.24.160820 (2020). [DOI] [PubMed]
- 63.Pobiega, S., Alibert, O. & Marcand, S. a new assay capturing chromosome fusions shows a protection trade-off at telomeres and NHEJ vulnerability to low density ionizing radiations. (2021) in revision. [DOI] [PMC free article] [PubMed]
- 64.Seifert FU, Lammens K, Stoehr G, Kessler B, Hopfner K-P. Structural mechanism of ATP-dependent DNA binding and DNA end bridging by eukaryotic Rad50. EMBO J. 2016;35:759–772. doi: 10.15252/embj.201592934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blaszczyk M, et al. Modeling of protein-peptide interactions using the CABS-dock web server for binding site search and flexible docking. Methods. 2016;93:72–83. doi: 10.1016/j.ymeth.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 66.Kironmai KM, Muniyappa K. Alteration of telomeric sequences and senescence caused by mutations in RAD50 of Saccharomyces cerevisiae. Genes Cells. 1997;2:443–455. doi: 10.1046/j.1365-2443.1997.1330331.x. [DOI] [PubMed] [Google Scholar]
- 67.Yu T-Y, Kimble MT, Symington LS. Sae2 antagonizes Rad9 accumulation at DNA double-strand breaks to attenuate checkpoint signaling and facilitate end resection. Proc. Natl Acad. Sci. 2018;115:E11961–E11969. doi: 10.1073/pnas.1816539115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu T-Y, Garcia VE, Symington LS. CDK and Mec1/Tel1-catalyzed phosphorylation of Sae2 regulate different responses to DNA damage. Nucleic Acids Res. 2019;47:11238–11249. doi: 10.1093/nar/gkz814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mattarocci S, et al. Rif1 maintains telomeres and mediates DNA repair by encasing DNA ends. Nat. Struct. Mol. Biol. 2017;24:588–595. doi: 10.1038/nsmb.3420. [DOI] [PubMed] [Google Scholar]
- 70.Kedziora S, et al. Rif1 acts through protein phosphatase 1 but independent of replication timing to suppress telomere extension in budding yeast. Nucleic Acids Res. 2018;46:3993–4003. doi: 10.1093/nar/gky132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shubin, C. B. & Greider, C. W. The Role of Rif1 in telomere length regulation is separable from its role in origin firing. bioRxiv10.1101/2020.04.24.060491 (2020). [DOI] [PMC free article] [PubMed]
- 72.van Zundert GCP, et al. The HADDOCK2.2 Web Server: User-friendly integrative modeling of biomolecular complexes. J. Mol. Biol. 2016;428:720–725. doi: 10.1016/j.jmb.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 73.Webb B, Sali A. Comparative protein structure modeling using MODELLER. Curr. Protoc. Protein Sci. 2016;86:2.9.1–2.9.37. doi: 10.1002/cpps.20. [DOI] [PubMed] [Google Scholar]
- 74.Seifert FU, Lammens K, Hopfner K-P. Structure of the catalytic domain of Mre11 from Chaetomium thermophilum. Acta Crystallogr. F: Struct. Biol. Commun. 2015;71:752–757. doi: 10.1107/S2053230X15007566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Diebold-Durand M-L, et al. Structure of full-length SMC and rearrangements required for chromosome organization. Mol. Cell. 2017;67:334–347.e5. doi: 10.1016/j.molcel.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marko JF, De Los Rios P, Barducci A, Gruber S. DNA-segment-capture model for loop extrusion by structural maintenance of chromosome (SMC) protein complexes. Nucleic Acids Res. 2019;47:6956–6972. doi: 10.1093/nar/gkz497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Myler LR, et al. Single-molecule imaging reveals how Mre11-Rad50-Nbs1 initiates DNA break repair. Mol. Cell. 2017;67:891–898.e4. doi: 10.1016/j.molcel.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alani E, Padmore R, Kleckner N. Analysis of wild-type and rad50 mutants of yeast suggests an intimate relationship between meiotic chromosome synapsis and recombination. Cell. 1990;61:419–436. doi: 10.1016/0092-8674(90)90524-I. [DOI] [PubMed] [Google Scholar]
- 79.Hopfner KP, et al. Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell. 2000;101:789–800. doi: 10.1016/S0092-8674(00)80890-9. [DOI] [PubMed] [Google Scholar]
- 80.Cannavo E, et al. Regulatory control of DNA end resection by Sae2 phosphorylation. Nat. Commun. 2018;9:4016–14. doi: 10.1038/s41467-018-06417-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee K, Zhang Y, Lee SE. Saccharomyces cerevisiae ATM orthologue suppresses break-induced chromosome translocations. Nature. 2008;454:543–546. doi: 10.1038/nature07054. [DOI] [PubMed] [Google Scholar]
- 82.Graf M, et al. Telomere length determines TERRA and R-loop regulation through the cell cycle. Cell. 2017;170:72–85.e14. doi: 10.1016/j.cell.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 83.Poschke H, et al. Rif2 promotes a telomere fold-back structure through Rpd3L recruitment in budding yeast. PLoS Genet. 2012;8:e1002960. doi: 10.1371/journal.pgen.1002960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pasquier E, Wellinger RJ. In vivo chromatin organization on native yeast telomeric regions is independent of a cis-telomere loopback conformation. Epigenetics Chromatin. 2020;13:23–22. doi: 10.1186/s13072-020-00344-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bender CF, et al. Cancer predisposition and hematopoietic failure in Rad50(S/S) mice. Genes Dev. 2002;16:2237–2251. doi: 10.1101/gad.1007902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Remali J, et al. In silico analysis on the functional and structural impact of Rad50 mutations involved in DNA strand break repair. PeerJ. 2020;8:e9197. doi: 10.7717/peerj.9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hohl M, et al. Modeling cancer genomic data in yeast reveals selection against ATM function during tumorigenesis. PLoS Genet. 2020;16:e1008422. doi: 10.1371/journal.pgen.1008422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Davey NE, et al. Attributes of short linear motifs. Mol. Biosyst. 2012;8:268–281. doi: 10.1039/C1MB05231D. [DOI] [PubMed] [Google Scholar]
- 89.Finn RD, Clements J, Eddy SR. HMMER web server: interactive sequence similarity searching. Nucleic Acids Res. 2011;39:W29–W37. doi: 10.1093/nar/gkr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zimmermann L, et al. A completely reimplemented mpi bioinformatics toolkit with a new HHpred server at its core. J. Mol. Biol. 2018;430:2237–2243. doi: 10.1016/j.jmb.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 91.wwPDB consortium. Protein Data Bank: the single global archive for 3D macromolecular structure data. Nucleic Acids Res. 2019;47:D520–D528. doi: 10.1093/nar/gky949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J. Mol. Graph. 1996;14:33–8– 27–8. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 93.Phillips JC, et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Best RB, et al. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone φ, ψ and side-chain χ(1) and χ(2) dihedral angles. J. Chem. Theory Comput. 2012;8:3257–3273. doi: 10.1021/ct300400x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Delano, W. L. The PyMOL Molecular Graphics System. http://www.pymol.org (2002).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All relevant data are available from the authors without restriction. Raw images and quantitative data have been deposited in the “Mendeley repository” [10.17632/8ghyn6n6x8.1].