Abstract
Metabolic homeostasis in mammals is tightly regulated by the complementary actions of insulin and glucagon. The secretion of these hormones from pancreatic β-cells and α-cells, respectively, is controlled by metabolic, endocrine, and paracrine regulatory mechanisms and is essential for the control of blood levels of glucose. The deregulation of these mechanisms leads to various pathologies, most notably type 2 diabetes, which is driven by the combined lesions of impaired insulin action and a loss of the normal insulin secretion response to glucose. Glucose stimulates insulin secretion from β-cells in a bi-modal fashion, and new insights about the underlying mechanisms, particularly relating to the second or amplifying phase of this secretory response, have been recently gained. Other recent work highlights the importance of α-cell-produced proglucagon-derived peptides, incretin hormones from the gastrointestinal tract and other dietary components, including certain amino acids and fatty acids, in priming and potentiation of the β-cell glucose response. These advances provide a new perspective for the understanding of the β-cell failure that triggers type 2 diabetes.
The pancreatic islets of Langerhans were discovered in 1869 by Paul Langerhans as a 21-year-old medical student1. Each of the several million islets in a human pancreas (range of 1 to 15 million2-5) harbours its own vasculature and comprises approximately 2,000 endocrine hormone-producing cells. These hormone-producing cells span five different cell types distinguished by their primary endocrine hormone products: α-cells (glucagon), β-cells (insulin and islet amyloid polypeptide (IAPP), also known as amylin), δ-cells (somatostatin), γ-cells or pancreatic polypeptide cells, and rare ε-cells (ghrelin) (FIG. 1a).
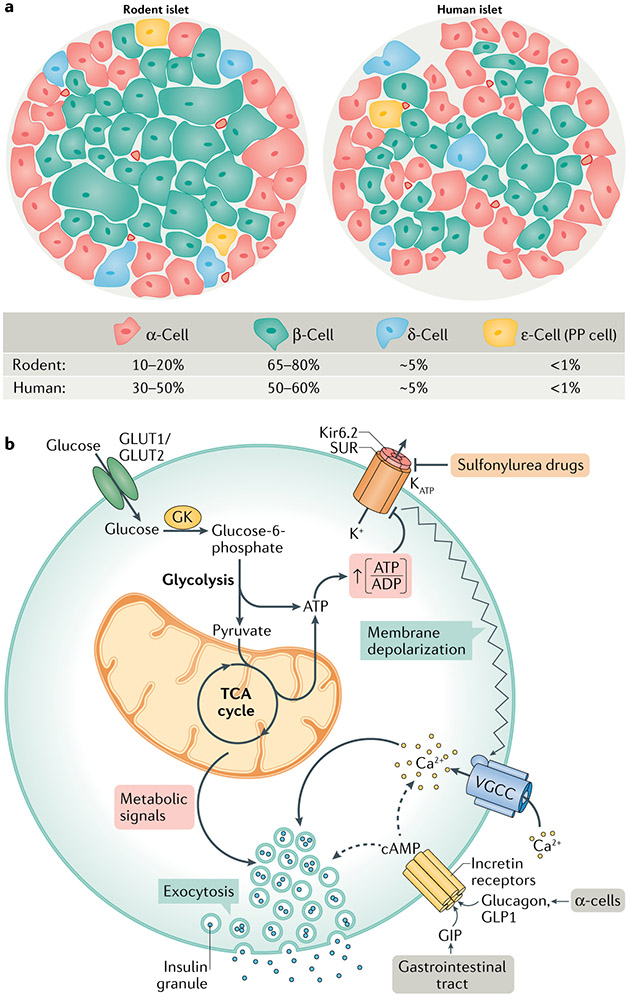
Fig. 1 ∣. Islet cell architecture and fundamental signalling pathways of GSIS.
a ∣ Comparison of the cellular composition of rodent and human islets. Rodent islets are made up of 10–20% of α-cells, found predominantly on the outer mantle, and 65–80% of β-cells comprising their inner core. δ-cells, γ-cells (also known as or pancreatic polypeptide (PP) cells) and ε-cells are found scattered throughout the islet. Human islets contain a higher percentage of α-cells, which are found throughout the islet, and a slightly lower percentage of β-cells than rodents. b ∣ Glucose-stimulated insulin secretion (GSIS) is mediated by a triggering pathway (solid arrows) and amplification pathways (dashed arrows). Glucose uptake into β-cells occurs via GLUT1 (human) or GLUT2 (rodent) transporters; glucose uptake is not rate limiting for GSIS, but rather the rate of β-cell glucose metabolism is controlled by glucokinase (GK), which determines the entry of glucose into the glycolytic pathway, followed by its oxidation via the tricarboxylic acid (TCA) cycle and subsequent generation of ATP. Glucose metabolism initiates the triggering pathway of GSIS via an elevation in the ATP to ADP ratio, resulting in the closure of KATP channels, membrane depolarization and subsequent opening of voltage-gated calcium channels (VGCC). The resulting increase in intracellular calcium drives the triggering phase of insulin granule exocytosis. The importance of GK and the KATP channel for regulating first-phase insulin secretion is clearly illustrated by the functional impact of human genetic mutations in these proteins. Human GK mutations that lower its Km result in a form of persistent hyperinsulinism and hypoglycaemia caused by a lower glucose threshold for the activation of β-cell glucose metabolism and insulin secretion152,153. Other GK mutations cause partial or complete loss of enzyme activity, resulting in insulin insufficiency and in a form of maturity onset diabetes of the young (MODY2)152,153. Islet cell KATP channels are composed of an octamer of four potassium channel subunits (Kir6.2) and four sulfonylurea receptor 1 (SUR1) regulatory subunits, which bind ATP to cause channel closure, plasma membrane depolarization and subsequent activation of insulin secretion. KATP channel closure can also be induced by sulfonylurea drugs, which are used as anti-diabetic medications. Mutations that force the chronic closure of the channel result in hypoglycaemia due to insulin hypersecretion154. Conversely, gain-of-function mutations that prevent closure of the channel by ATP cause syndromes of insulin insufficiency and neonatal diabetes mellitus155,156. The amplification pathways are dependent upon membrane depolarization initiated by the triggering pathway. Multiple metabolic signalling mechanisms contribute to the amplification of insulin secretion, as detailed in subsequent figures. cAMP, cyclic AMP; GIP, gastric inhibitory polypeptide; GLP1, glucagon-like peptide 1.
The hormones produced by the islets of Langerhans have important roles in the regulation of metabolic fuel homeostasis. Insulin is a key anabolic hormone responsible for promoting the storage of metabolic fuels and is opposed by the catabolic effects of glucagon to promote the mobilization and oxidation of metabolic stores. The metabolic effects of islet hormones align with factors that affect their secretion under different physiological circumstances. Insulin secretion is potently activated by a post-prandial increase in glucose concentrations — referred to as glucose-stimulated insulin secretion (GSIS) — potentiated by the effects of amino acids and fatty acids and of hormones produced in α-cells and the gastrointestinal tract. Glucagon secretion is suppressed by elevations in glucose and insulin but also stimulated by amino acids and fatty acids, whose levels rise with fasting. Somatostatin functions as a paracrine suppressor of both glucagon and insulin secretion6,7. IAPP suppresses glucagon secretion, delays gastric emptying and enhances satiety (leading to its consideration as a therapeutic agent for obesity and diabetes)8. Ghrelin stimulates appetite, although this effect is mediated mainly by its production in the stomach, while pancreatic polypeptide is an appetite suppressant and stimulant for pancreatic secretion of digestive enzymes.
A critical function of the pancreatic islets is to control blood glucose levels given that the brain and central nervous system are reliant on glucose as their major metabolic fuel. Proper function of the pancreatic islets is required for metabolic homeostasis, and their dysfunction manifests in diabetes. Type 1 diabetes (T1D) involves the autoimmune destruction of pancreatic islet β-cells, resulting in complete or near-complete insulin deficiency. Type 2 diabetes (T2D) is a disease of complex aetiology, often associated with obesity, that involves insulin resistance, impaired control of hepatic glucose production and islet dysfunction that includes loss of normal GSIS, inadequate insulin delivery to offset insulin resistance and glucagon hypersecretion.
The core purpose of this article is to review the latest research on the cellular and molecular mechanisms controlling β-cell insulin secretion and how these functions intertwine with the secretory activity of α-cells. This will include emergent information about signalling mechanisms activated by nutrient and peptide secretagogues as well as the role of intra-islet paracrine communication networks. We will also assess how newly emergent signalling mechanisms in α-cells and β-cells are altered during the development of T2D.
Overview of insulin secretion
The primary physiologic stimulus for insulin secretion is the increase in circulating glucose concentration that occurs in the post-prandial state. Exposure of the β-cell to stimulatory glucose not only has an intrinsic activating effect on insulin secretion but is also required for the concomitant action of several agents that potentiate GSIS. The direct stimulation of insulin secretion by glucose involves a ‘triggering’ pathway and an ‘amplifying’ pathway9. The triggering pathway is activated by biochemical signals described more than 35 years ago10,11, involving the metabolism of glucose to generate ATP, the closure of ATP-sensitive potassium (KATP) channels to cause membrane depolarization and the consequent activation of voltage-gated Ca2+ channels (FIG. 1b). The resultant sharp rise in intracellular Ca2+ levels during the triggering phase activates the exocytosis of a ‘readily releasable’ pool of insulin secretory granules localized to the plasma membrane by a tetrameric complex of proteins that facilitate the formation of a membrane fusion pore through which insulin is released to the cell exterior12. The triggering pathway is largely responsible for what is referred to as the ‘first phase’ of insulin secretion, involving a sharp peak in insulin secretion followed by a decline occurring in the first 10–20 minutes after a glucose stimulus. The amplifying pathway involves a secondary set of stimuli that allow insulin secretion to continue at a lower but sustained rate during the entire post-absorptive phase of a meal, spanning several hours — a period also referred to as the ‘second phase’ of insulin secretion. The amplifying pathway is activated even in the presence of maximal levels of intracellular Ca2+ and is driven largely by KATP channel-independent mechanisms. Whereas the triggering pathway causes the release of about 1% of a pool of plasma membrane-associated ‘primed’ or readily releasable insulin-containing granules, the amplifying phase continues to cause the release of primed granules but also invokes the recruitment of granules from a cell-internal storage pool to the cell surface, releasable pool12 (FiG. 1b). Below, we provide a brief background on the mechanisms of the well-understood triggering phase and then delve more deeply into emergent work on the factors particularly relevant to the amplifying phase.
Glucose metabolism triggers GSIS
Islet β-cells are equipped with glucose-sensing machinery that ensures the release of exactly the right amount of insulin, with exactly the right dynamics, to maintain blood glucose levels within a range of euglycaemic values. The rate of glucose metabolism within β-cells is a primary determinant of the magnitude of the insulin secretion response due to the production of various coupling factors that are tied to the rate of glucose utilization.
A tight linkage between changes in extracellular glucose concentrations and changes in the rate of glucose metabolism in islet β-cells is mediated by permissive rates of glucose transport into the cell and by the kinetic features of hexokinase IV (also known as glucokinase), an enzyme that converts intracellular glucose to glucose-6-phosphate. Both rodent and human β-cells have an excess capacity for glucose uptake relative to β-cell metabolic rate, meaning that glucose transport is not rate limiting for GSIS13-15. Glucokinase is expressed in both rodent and human β-cells and has a lower affinity for glucose than the other three members of the hexokinase gene family, with a Km of approximately 8 mM as compared with the sub-millimolar range for the other family members16,17. Glucokinase is approximately half as large as the other hexokinases, lacking the N-terminal domain that mediates product inhibition by glucose-6-phosphate. These kinetic and regulatory features allow glucokinase activity to change sharply as β-cells are exposed to increasing glucose, with this change in activity exactly mirroring (and regulating) changes in β-cell glucose metabolism and insulin secretion16.
Once phosphorylated, glucose is metabolized to the level of pyruvate. In β-cells, pyruvate is utilized primarily for metabolism in the mitochondrial tricarboxylic acid (TCA) cycle, entering the cycle via an approximately even split between its conversion to acetyl-CoA via the oxidative enzyme pyruvate dehydrogenase (PDH) and its conversion to oxaloacetate via the anaplerotic enzyme pyruvate carboxylase (PC)18-21. The conversion of pyruvate to lactate is limited in β-cells due to low levels of expression of lactate dehydrogenase, one of several ‘disallowed genes’ thought to be repressed in β-cells to facilitate the funnelling of glucose to mitochondrial metabolism22. β-cells also have substantial reducing equivalent shuttle activity, including glycerol phosphate and malate–aspartate shuttles that convert cytosolic NADH to mitochondrial FADH and NADH, fuelling ATP production that contributes to KATP-channel closure23,24.
Metabolic amplifiers of GSIS
Whereas the KATP channel-dependent pathway is critical for activating the triggering phase of GSIS, significant amounts of insulin are secreted in response to glucose under conditions where the KATP channel is neutralized by pharmacologic9,25 or molecular26,27 tools. In particular, the treatment of islets with diazoxide, a KATP channel opener, in conjunction with high concentrations of potassium, causes membrane depolarization and Ca2+ influx. Despite the membrane depolarization and Ca2+ current induced by these conditions, glucose has an additional robust effect to stimulate insulin secretion25. In this article, we refer to this KATP channel-independent glucose-sensing mechanism as the amplifying phase of GSIS. The KATP channel-independent/amplifying pathway is active over the full period of glucose stimulation, which in the postprandial state can involve increases in circulating glucose for several hours and is estimated to account for as much as 60–70% of insulin secreted in response to sustained glucose stimulation9. Therefore, investigation of the biochemical mechanisms of the amplifying phase has intensified in recent years.
Several emergent models for explaining the sustained amplifying phase of GSIS have emerged and will be reviewed in the following subsections.
The role of anaplerotic metabolism and cytosolic NADPH.
Carbon isotope tracing by NMR and mass spectrometry consistently demonstrate that the anaplerotic flux of pyruvate to oxaloacetate via PC is strongly responsive to changes in extracellular glucose in islet β-cells18-21. The fraction of pyruvate that enters the TCA cycle through PC is higher in β-cells than in other tissues such as the heart28, supporting a potential signalling role of this pathway for insulin secretion. Indeed, a panel of subclones derived from the INS-1 rat insulinoma cell line with different GSIS responses29 show strong correlation of GSIS with anaplerotic but not with oxidative metabolism of pyruvate20. The NADPH:NADP ratio achieved during glucose stimulation is also tightly correlated with the capacity for GSIS across this set of variably glucose-responsive cell lines30, as discussed further below. In addition, phenylacetic acid, an inhibitor of PC, causes a decrease in anaplerotic metabolism of pyruvate that correlates exactly with reduced GSIS in the robustly glucose-responsive INS-1-derived 832/13 cell line20 and phenylacetic acid also inhibits GSIS in primary rat islets31-33. Together, these findings provide evidence for the importance of anaplerotic metabolic pathways in GSIS. Anaplerotic metabolism of pyruvate and other substrates is most active in the postprandial state when metabolic fuels are abundant. Periods of active anaplerotic metabolism cause TCA cycle intermediates to accumulate and exit the mitochondria via organic acid carriers to engage in cytosolic metabolic reactions34,35 (FIG. 2). For example, malate and citrate/isocitrate exit the mitochondria via the malate carrier or citrate/isocitrate carrier, respectively, with malate being recycled to pyruvate via malic enzyme (ME) or citrate being reconverted to pyruvate via a sequence of reactions catalysed by ATP-citrate lyase (ACLY), malate dehydrogenase and ME. siRNA-mediated suppression of the cytosolic or mitochondrial isoforms of ME impairs GSIS in the 832/13 cell line36,37. This cell line was derived by clonal selection from the rat insulinoma INS-1 cell line on the basis of its robust GSIS response29, thereby providing a useful system for investigating the mechanisms of cellular insulin secretion, storage and synthesis. However, the robust suppression of cytosolic ME activity has no effect in primary rat islets37. Moreover, a strain of mice with genetic deficiency in cytosolic ME expression has normal GSIS37. Importantly, knockdown of the mitochondrial citrate/isocitrate carrier to disrupt egress of citrate or isocitrate from the mitochondria to the cytosol causes impairment of GSIS both in 832/13 cells and in primary rat islets38. Once in the cytosol, citrate and isocitrate can be metabolized back to pyruvate via ACLY-malate dehydrogenase-ME or can be converted to α-ketoglutarate and glutamate through the cytosolic NADP-linked isoform of isocitrate dehydrogenase (IDH1) (FIG. 2). siRNA-mediated knockdown of ACLY has no effect on GSIS in 832/13 cells or rat islets39, whereas knockdown of IDH1 causes strong inhibition of the glucose response30,40. Other groups have reported modest inhibition of GSIS in response to the suppression of ACLY activity36,41 or that knockdown of IDH1 increases rather than decreases GSIS42; however, in those studies, data are only provided for the 832/13 cell line and not for primary islets. Moreover, other work discussed below has demonstrated direct effects of isocitrate and IDH1-derived NADPH on insulin secretory granule exocytosis43. A potential role for ACLY in generating lipid messengers for insulin secretion has also been debated, as discussed further below. Overall, whereas there are some discrepancies among different labs with regard to the role of certain enzymes in anaplerotic signalling and insulin secretion, data from primary islet preparations and genetic mutant strains of mice are probably the most reliable, and these indicate that anaplerotic metabolism through ACLY and ME are of lesser significance relative to the use of citrate and isocitrate in the cytosolic IDH1 reaction in control of GSIS.
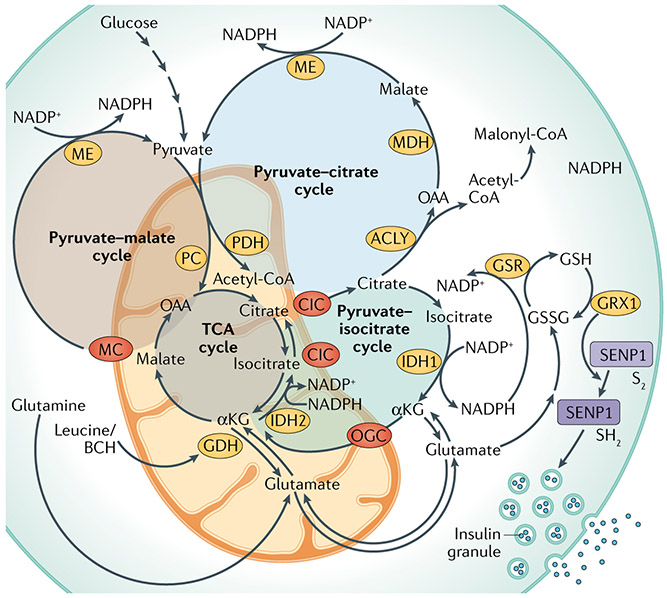
Fig. 2 ∣. Pyruvate cycling pathways implicated in the regulation of GSIS.
In the post-prandial state, increased anaplerotic metabolism of pyruvate and other fuels leads to the accumulation of tricarboxylic acid (TCA) cycle intermediates, which leave the mitochondria to engage in cytosolic reactions that create signals for the amplifying/second phase of glucose-stimulated insulin secretion (GSIS). This includes (1) the pyruvate–malate cycle (highlighted by the brown circle and involving malic enzyme (ME) and the malate carrier (MC)), (2) the pyruvate–citrate cycle (highlighted by the light blue circle and involving ATP citrate lyase (ACLY), malate dehydrogenase (MDH), the citrate/isocitrate carrier (CIC) and ME), and (3) the pyruvate–isocitrate cycle (highlighted by the green circle and involving the cytosolic NADP-linked isoform of isocitrate dehydrogenase (IDH1), the mitochondrial NADPH-linked isoform of isocitrate dehydrogenase (IDH2) and CIC). Note that the pyruvate–isocitrate pathway is a full cycle that includes reductive TCA flux, involving the carboxylation of α-ketoglutarate (αKG) to isocitrate in the mitochondria by IDH2. In β-cells, this reductive TCA cycle flux ensures that αKG generated by IDH1 is used to regenerate citrate and isocitrate to sustain IDH1 activity. NADPH produced by IDH1 in the cytosol connects to insulin granule exocytosis via glutathione reductase (GSR) to produce reduced glutathione (GSH), leading to the activation of glutaredoxin (GRX1), which mediates the reduction (S2 to SH2 isoform transition) and activation of sentrin/SUMO-specific protease 1 (SENP1). SENP1 then acts as a deSUMOylase that removes SUMO peptides from secretory granule-trafficking proteins to enhance exocytosis. The activity of the pyruvate–isocitrate cycle is maintained by the supply of glutamate, which can be converted to αKG by glutamate dehydrogenase (GDH). The supply of glutamate can be achieved either through the activity of the malate–aspartate shuttle or by the reversible activity of GDH, which is regulated by leucine and its analogue 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH). Recent findings show that the effect of glutamine plus leucine/BCH co-treatment to stimulate insulin secretion is explained, in part, by the isocitrate cycle catalysed by IDH1 and IDH2. Throughout the figure, metabolic enzymes are shown in yellow ovals, whereas mitochondrial organic acid transporters are shown in red ovals. GSSG, glutathione disulfide; OGC, 2-oxoglutarate carrier; PDH, pyruvate dehydrogenase; PC, pyruvate carboxylase.
Recent studies using patch-clamped human and rodent β-cells provide firm support for a particular role of IDH1 in the regulation of GSIS43. In this approach, glucose and other metabolic fuels are pre-infused to the interior of patch-clamped β-cells to measure their ability to amplify insulin granule exocytosis. Using this method, β-cells from non-diabetic human donors exhibit a strong potentiation of exocytosis by 10 mM glucose compared with 1 mM glucose, which respectively represent stimulatory and non-stimulatory doses. Importantly, this response to 10 mM glucose is absent in β-cells from donors with T2D43. In this experimental protocol, the patch-clamped cells are subjected to direct membrane depolarization and the effect of 10 mM glucose to stimulate exocytosis occurs without a change in Ca2+ current, thus aligning with our definition of a KATP channel-independent/amplifying signal for insulin secretion43. The pre-treatment of normal human β-cells with the substrate of the IDH1 reaction (isocitrate) or with a cofactor that it produces (NADPH) but not with the IDH1 reaction product (α-ketoglutarate) amplifies insulin granule exocytosis to a similar extent as stimulatory glucose. In human β-cells treated with an IDH1 siRNA, the potentiation of insulin granule exocytosis by glucose and isocitrate is lost, whereas the effect of NADPH to stimulate exocytosis remains. These experiments suggest that NADPH generated in the IDH1 reaction is a cytosolic coupling factor for GSIS43. Supporting this, siRNA-mediated knockdown of IDH1 in 832/13 cells lowers the NADPH:NADP ratio30. Importantly, isocitrate and NADPH also potentiate insulin granule exocytosis in glucose-insensitive β-cells from patients with T2D43. This suggests that β-cells from patients with T2D have a metabolic deficiency upstream of IDH1 that limits flux through this reaction, as discussed further below.
Recently, a new pathway that sustains flux through the IDH1 reaction during the fuel stimulation of β-cells has been uncovered through the application of stable isotope flux methods44. The work evolved from studies showing that insulin secretion stimulated by treatment with glutamine together with an analogue of leucine, 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH) is impaired in β-cells with siRNA-mediated suppression of IDH1 (REF.40). At low glucose levels, glutamine alone does not stimulate insulin secretion, but it becomes a potent insulin secretagogue in the presence of the glutamate dehydrogenase (GDH) activators leucine or BCH. The finding that knockdown of IDH1 expression impairs insulin secretion induced by co-treatment with glutamine and BCH was originally interpreted to mean that BCH activates the entry of glutamine into the TCA cycle via its conversion — through glutamate — to α-ketoglutarate by GDH; α-ketoglutarate would then be metabolized by oxidative (clockwise) TCA cycle flux to contribute to the formation of citrate and isocitrate to support the activity of IDH1. However, recent studies have demonstrated that glutamine is also efficiently converted to citrate via reductive (counter-clockwise) TCA cycle flux, involving the carboxylation of α-ketoglutarate to isocitrate in the mitochondria by a separate IDH isoform —the NADPH-dependent IDH2 (REF.44). The metabolism of glutamine via reductive TCA cycle flux is used to generate biomass during cancer cell growth, including de novo lipogenesis from acetyl-CoA generated by citrate cleavage45-49. In β-cells, reductive TCA cycle flux contributes to a metabolic cycle in which glutamine/glutamate or α-ketoglutarate generated by IDH1 are used to regenerate citrate and isocitrate via reductive flux through IDH2 (FIG. 2; for details of NADPH–insulin granule secretion coupling see subsection Connections of metabolic mediators to insulin granule exocytosis). Supporting this model, the suppression of IDH2 activity in 832/13 cells or in primary rat islets, either by specific siRNA duplexes or with a small molecule inhibitor, AGI6780 (REFS50,51), impairs insulin secretion in response to glutamine–BCH co-treatment or glucose stimulation; this is accompanied by a suppression of reductive conversion of glutamine to citrate44. Moreover, the acute inhibition of IDH2 in mice suppresses the normal GSIS response44. Of particular note, the suppression of IDH2 by pharmacologic or molecular methods results in a robust decrease in total cell NADPH and in the NADPH:NADP ratio in β-cells, even though IDH2 is an NADPH-consuming enzyme. This is accompanied by suppression of de novo lipogenesis from glucose and, given that fatty acid synthase is a cytosolic NADPH-requiring enzyme44, is consistent with depletion of the cytoplasmic NADPH pool. These data align fully with a model in which the effect of IDH2 suppression to hamper cellular NADPH production (and insulin secretion) is mediated by a reduced production of citrate and isocitrate from reductive TCA cycle flux, resulting in less substrate for NADPH production in the cytosol via IDH1.
An alternate — but not mutually exclusive — model of signalling by glutamine/glutamate during GSIS posits a role for glutamate in insulin granule exocytosis (see subsection Connections of metabolic mediators to insulin granule exocytosis).
The role of pentose monophosphate shunt pathway and its nucleotide products.
Other recent studies have focused on products of the pentose monophosphate shunt pathway as potential regulators of GSIS. The inhibition of the NADPH-generating enzymes of the pathway, namely glucose-6-phosphate dehydrogenase (G6PDH)52 or 6-phosphogluconate dehydrogenase (6PGDH)53, results in impaired GSIS. In the first case, the effect could be explained by impaired NADPH generation52 whereas, in the latter, the negative impact on GSIS was attributed to the accumulation of intermediate metabolites of the pathway, leading to the activation of extracellular-regulated-kinase (ERK); ERK is known to promote insulin transcription in response to acute signals but its sustained activation may lead to β-cell dysfunction and apoptosis53.
An alternate explanation for the importance of sustained pentose monophosphate shunt pathway in GSIS is the potential involvement of nucleotides derived from the pathway. Several metabolomics studies reported glucose-induced changes in levels of nucleotide pathway intermediates in β-cells52,54,55 but did not pursue underlying mechanisms. A more recent study identified a sharp decrease in inosine monophosphate and an increase in adenylosuccinate (S-AMP) in glucose-stimulated 832/13 cells, suggesting that the enzyme linking the two metabolites — adenylosuccinate synthase — could play a regulatory role in β-cell glucose sensing56. Indeed, pharmacologic or molecular suppression of adenylosuccinate synthase lowered S-AMP levels and caused impairment of GSIS (FIG. 3). Furthermore, the infusion of S-AMP into patch-clamped 832/13 cells or β-cells from healthy individuals stimulated insulin granule exocytosis with the same potency as glucose, and S-AMP also enhanced insulin granule exocytosis in glucose-insensitive β-cells from patients with T2D56. This direct effect of S-AMP to augment insulin secretion provides an alternative explanation for studies showing impaired GSIS in response to suppression of the pentose shunt. If S-AMP is in fact the main signalling intermediate generated in the pentose shunt, this would imply that NADPH generated in the pathway may be less impactful for GSIS than NADPH produced by IDH1. If so, the unique signalling properties of NADPH generated in the IDH1 reaction remain to be explained. The possibilities to be explored include a higher rate of NADPH production by IDH1 or, potentially, a specific subcellular localization of the enzyme to facilitate the use of the NADPH that it produces for the activation of exocytosis.
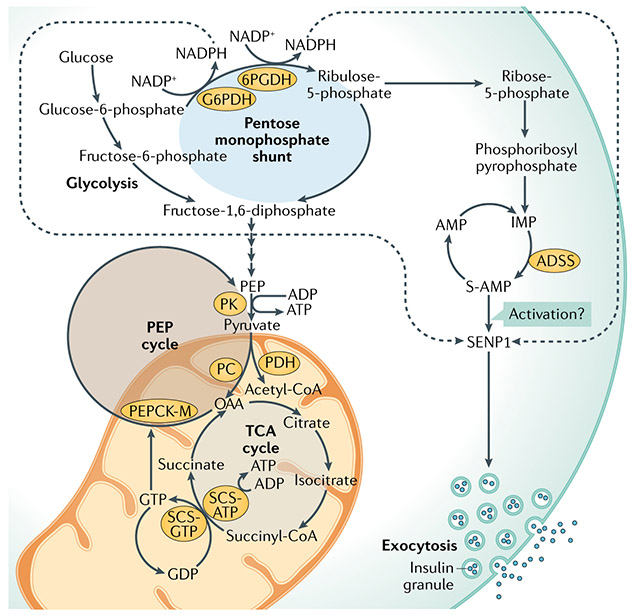
Fig. 3 ∣. Pentose monophosphate shunt, phosphoenolpyruvate cycle and their nucleotide metabolites in GSIS.
As highlighted in the article, the regulation of glucose-stimulated insulin secretion (GSIS) may involve the synthesis of nucleotides other than ATP, specifically adenylosuccinate (S-AMP) generated by the pentose monophosphate shunt or GTP produced in the tricarboxylic acid (TCA) cycle by the GTP-forming isoform of the TCA cycle enzyme succinyl-CoA synthetase (SCS-GTP). Glucose metabolism via the pentose monophosphate shunt generates ribose-5-phosphate and inosine monophosphate (IMP), which is converted to S-AMP via adenylosuccinate synthetase (ADSS). The inhibition of ADSS to block S-AMP formation inhibits GSIS, while infusion of S-AMP into patch-clamped human β-cells amplifies insulin granule exocytosis. NADPH generated in the first two reactions of the pentose monophosphate shunt catalysed by glucose-6-phosphate dehydrogenase (G6PDH) and 6-phosphogluconate dehydrogenase (6PGDH) may synergize with NADPH generated by IDH1 to enhance insulin granule exocytosis via the activation of sentrin/SUMO-specific protease 1 (SENP1) (FIG. 2). The effects of S-AMP on granule exocytosis are attenuated in β-cells with the suppression of SENP1 but the mechanisms by which S-AMP may interact with SENP1 remain unknown (question mark). Alternatively, succinyl-CoA can be converted to succinate in the TCA cycle by SCS-GTP. The manipulation of SCS-GTP expression affects GSIS via the modulation of GTP levels, required for activity of the mitochondrial isoform of phosphoenolpyruvate carboxykinase (PEPCK-M). The synthesis of phosphoenolpyruvate (PEP) by this PEPCK-catalysed PEP cycle produces ATP for the regulation of the KATP channel. OAA, oxaloacetate; PK, pyruvate kinase; PDH, pyruvate dehydrogenase; PC, pyruvate carboxylase.
The role of GTP and the phosphoenolpyruvate cycle.
An alternate nucleotide signalling pathway for GSIS has been proposed involving mitochondrial GTP generated via the GTP-forming isoform of the TCA cycle enzyme succinyl-CoA synthetase (SCS-GTP). In addition to this isoform, mitochondria also harbour a second, ATP-forming isoform of SCS (SCS-ATP). The knockdown of SCS-GTP but not of SCS-ATP causes impairment of GSIS, whereas the overexpression of SCS-GTP enhances GSIS yet the overexpression of SCS-ATP impairs it57,58. In this model, GTP generated by SCS-GTP during glucose stimulation is utilized by the mitochondrial isoform of phosphoenolpyruvate carboxykinase (PEPCK-M) to synthesize phosphoenolpyruvate (PEP) from oxaloacetate57,58 (FIG. 3). Although the synthesis of a new molecule of PEP from pyruvate via PC and PEPCK-M requires two moles of nucleotide triphosphate (one ATP and one GTP), the very high free energy of PEP is suggested to allow the β-cell to continue to synthesize ATP as a direct product of the pyruvate kinase reaction, even at maximum mitochondrial phosphorylation potential57. Interestingly, metabolic flux studies revealed an approximate doubling of PEPCK-M flux when comparing cells expressing SCS-GTP to those expressing SCS-ATP However, no differences in oxidative flux of glucose to the TCA cycle through PDH or in anaplerotic flux via PC were found between the two groups of cells. The source of oxaloacetate for driving the increase in mitochondrial PEPCK-M flux in the absence of changes in PC or PDH flux is currently unexplained. Also of note, glucose stimulation of 832/13 cells caused no changes in whole-cell GTP levels56, although it is possible that intra-mitochondrial changes of GTP still occur and are relevant for the amplification of GSIS, as described here.
The role of free fatty acids and the glycerolipid/free fatty acid cycle.
Free fatty acids (FFAs) potentiate GSIS in a glucose-dependent manner. In addition, the depletion of islet lipid stores causes complete ablation of GSIS, whereas restoring FFA in lipid-depleted islets rescues insulin secretion59,60. The potentiating effect of FFAs on GSIS is influenced by chain length and degree of saturation, with long-chain, unsaturated fatty acids such as palmitate and stearate (C16:0; C18:0) being the most potent secretagogues for insulin61. Mechanistically, FFAs were proposed to act via FFA receptor 1 (FFAR1, also known as G protein-coupled receptor 40), which is expressed at high levels in β-cells62,63, to regulate insulin granule exocytosis (FIG. 4).
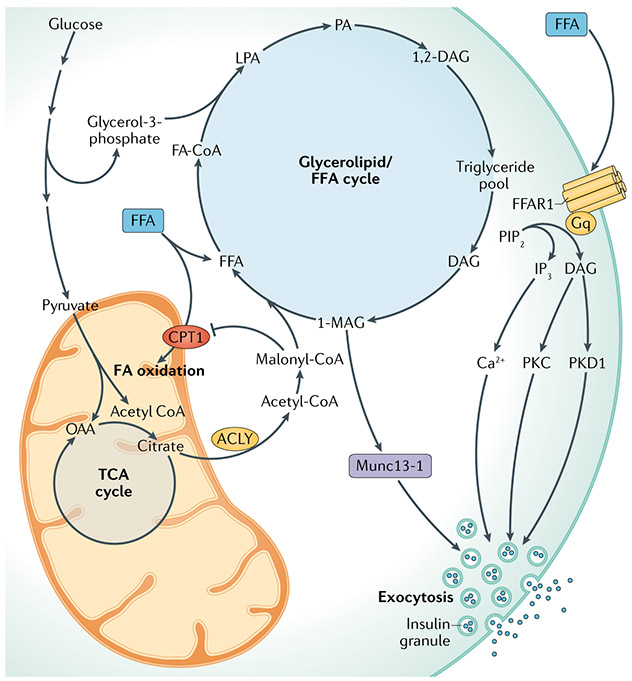
Fig. 4 ∣. Glycerolipid/FFA cycle for the amplification of GSIS.
This model holds that the increase in malonyl-CoA levels induced by stimulatory glucose inhibits carnitine palmitoyl transferase 1 (CPT1) and fatty acid (FA) oxidation, contributing to lipid synthesis and diversion of free fatty acids (FFAs) away from oxidation and towards esterification with glycerol-3-phosphate to form glycerolipids. Glycerolipids are used to form triglycerides, which are then hydrolysed to form diacylglycerols (DAG) and further to monoacylglycerols (MAG), including 1-MAG, which binds the insulin granule trafficking protein Munc13-1 to enhance insulin granule exocytosis. FAs also potentiate glucose-stimulated insulin secretion (GSIS) by interaction with the plasma membrane FFA receptor 1 (FFAR1) to activate the metabolism of phosphoinositide bisphosphate (PIP2) to inositol triphosphate (IP3) and DAG to stimulate insulin secretion via mobilization of Ca2+ and activation of protein kinase C (PKC) and protein kinase D (PKD1), respectively. 1,2-DAG, 1,2-diacylglycerol; ACLY, ATP citrate lyase; FA-CoA, fatty acyl-CoA; LPA, lysophosphatidic acid; OAA, oxaloacetate; PA, phosphatidic acid; TCA, tricarboxylic acid.
FFAs bind to FFAR1 to activate the phospholipase C (PLC)-mediated hydrolysis of phosphatidylinositol 4,5-bisphosphate to form diacylglycerol (DAG) and inositol trisphosphate. DAG activates protein kinase C, whereas inositol trisphosphate mobilizes Ca2+ from endoplasmic reticulum (ER) stores to potentiate GSIS. FFA–FFAR1 signalling also causes DAG-mediated activation of protein kinase D1 to promote cortical F-actin remodelling and recruitment of secretory granules to the readily releasable pool64 (Supplementary Box 1).
Notably, siRNA-mediated knockdown or complete knockout of FFAR1 results in partial but not complete suppression of FFA-mediated potentiation of GSIS62, suggesting that receptor-independent metabolic mechanisms also contribute to the response. In exploring this, a model has emerged that posits the role of a glycerolipid/FFA (GL/FFA) cycle — a pathway that couples glucose and lipid metabolism — in GSIS. In this model, flux through ACLY generates acetyl-CoA, which is then converted to malonyl-CoA, a known inhibitor of carnitine palmitoyl transferase 1 (CPT1) and FFA oxidation65,66. Reduced oxidation expands the FFA pool and promotes FFA esterification with glycerol phosphate to form tria-cylglycerides (TGs), comprising the lipogenic arm of the cycle. In the lipolytic arm of the cycle, TGs are hydrolysed by: adipose triglyceride lipase catalysing hydrolysis of TGs to DAG; hormone-sensitive lipase, which forms monoacylglycerols (MAGs) from DAG; and DAG lipase, which also forms MAGs67,68 (FIG. 4). Importantly, among the MAG species produced is 1-monoacylglycerol (1-MAG), which activates insulin granule exocytosis (for mechanistic details see the subsection Connections of metabolic mediators to insulin granule exocytosis)68. In addition, the fatty acids generated in the lipolytic arm of this fatty acid cycle could leave the β-cell to activate FFAR1/GPR40.
Whereas glucose clearly increases malonyl-CoA levels to inhibit fatty acid oxidation in β-cells65,66,69, this action may not regulate GSIS per se. Thus, the pharmacologic inhibition of acetyl-CoA carboxylase or ACLY or siRNA-mediated knockdown of ACLY in 832/13 cells or primary rat islets has little to no impact on GSIS39,70. Similarly, the overexpression of malonyl-CoA carboxylase to lower malonyl-CoA levels or the siRNA-mediated suppression of fatty acid synthase to inhibit the lipogenic arm of the GL/FFA cycle in 832/13 cells or rat islets is without effect on GSIS39,66,69, and mice with whole-body knockout of fatty acid synthase have normal insulin secretion71. It has been argued that some of these experiments were conducted in β-cell lines or primary islets cultured in the absence of exogenous FFA and in the presence of lipid-depleted serum and that a minimal fatty acid supply is needed to ‘prime’ lipogenesis in the GL/FFA cycle to allow it to contribute to GSIS67. This argument seems untenable given the fact that robust GSIS was demonstrated in cell culture experiments in which lipids were absent from the culture media (ranging from fourfold to 12-fold in four separate publications37,39,66,69). However, it should be noted that the overexpression of malonyl-CoA carboxylase does impair insulin secretion in the presence of fatty acids, but not in their absence66, and that siRNA-mediated knockdown of long chain acyl synthases 3 or 4, which catalyse the formation of long-chain acyl-CoA species that can contribute to TG synthesis — results in impaired insulin secretion72. Overall, the findings to date do not support a requirement for lipid cycling in the GSIS response per se, but are consistent with a combinatorial mechanism of FFA potentiation of GSIS, involving both FFAR1 signalling and the activity of the GL/FFA cycle resulting in the generation of GSIS-potentiating metabolites.
Connections of metabolic mediators to GSIS.
Ultimately, the several metabolic mediators of insulin secretion described above must make a biochemical connection to the insulin granule exocytotic machinery in order to control insulin release. Our understanding of the mechanisms controlling insulin granule exocytosis is built upon pioneering work on molecular mechanisms of synaptic vesicle exocytosis in neuronal cells73. For a summary of key regulators of insulin secretory granule trafficking, please see Supplementary Box 1.
The isocitrate–IDH1/IDH2 pathway described above as an important contributor to amplifying signals for GSIS links to the secretory machinery via a pathway involving the NADPH-driven reduction of glutathione (GSH) by GSH reductase43,74 (FIG. 2). Reduced GSH activates glutaredoxin, which in turn reduces cysteine disulfides in the sentrin/SUMO-specific protease 1 (SENP1) enzyme to activate its function43 (FIG. 2). Small ubiquitin modifier (SUMO) proteins are attached to other proteins (SUMOylation) via a series of reactions ending with the transfer of SUMO peptides from the E2 SUMO conjugating enzyme UBC9 to a target protein lysine residue75. SENP1 localizes with membrane-associated secretory granules in β-cells and functions as a deSUMOylase enzyme. Stimulatory glucose increases SENP1 activity and triggers the deSUMOylation of several proteins implicated in granule trafficking, including the Ca2+-sensing protein synaptotagmin VII76. Other proteins known to be SUMOylated in β-cells include syntaxin 1A, synapsin 1a, RIM1α, Kv2.1 and tomosyn, all of which play roles in insulin granule exocytosis or in Ca2+ sensing77. In line with the importance of SENP1-driven deSUMOylation in insulin granule trafficking, the overexpression of one of the three SUMO peptides, SUMO1, or of the SUMO conjugating enzyme UBC9, impairs insulin secretion from mouse β-cells75-77. Multiple lines of evidence support a critical role of SENP1 in insulin secretion generally and as a component regulated by IDH1 flux specifically The knockout of SENP1 in mice results in glucose intolerance due to insulin insufficiency and causes a loss of amplification of the insulin granule exocytotic response to glucose or GSH43. Moreover, siRNA-mediated knockdown of SENP1 in human β-cells blocks amplification of insulin granule exocytosis in response to glucose, NADPH or GSH. Finally, recent work has shown that the suppression of IDH2 activity to blunt reductive TCA cycle flux sharply lowers cellular NADPH levels and increases SUMOylation of proteins in a β-cell secretory granule-enriched subcellular fraction44. Taken together, these findings connect a metabolic cycle involving IDH1 and IDH2 to NADPH, GSH, glutaredoxin and SENP1 in the regulation of insulin granule exocytosis43,44,74 (FIG. 2). The specific secretory granule trafficking proteins that are targeted by this pathway to regulate insulin secretion remain to be fully characterized.
Interestingly, coupling factors generated by the pentose monophosphate shunt and nucleotide biosynthesis pathways may also signal via SENP1. Thus, the knockdown of SENP1 prior to patch clamp analysis reduces S-AMP-mediated amplification of insulin exocytosis from human β-cells by approximately 50%56 (FIG. 3). These findings suggest that S-AMP may regulate SENP1 activity but, if so, the mechanism is unknown. Alternatively, S-AMP is an intermediate in the synthesis of adenine nucleotides, through which it may contribute to the maintenance of robust pools of ATP, NADH and NADPH for signalling. Consistent with this idea, the glucose-induced increase in S-AMP is linked to parallel increases in NADPH and NADH levels56. Thus, it remains possible that the IDH1 and pentose shunt pathways are coordinated to facilitate both S-AMP and NADPH-mediated signalling for insulin granule exocytosis.
Glutamate, introduced earlier as an intermediate in the isocitrate-IDH1/IDH2 pathway for the generation of cytosolic NADPH, may also regulate insulin secretion via a direct effect on secretory granules. This mechanism involves glutamate transport into insulin secretory granules by vesicular glutamate transporters78-80 in a manner dependent on cyclic AMP (cAMP), which is important for various aspects of insulin granule exocytosis (Supplementary Box 2). The knockdown of vesicular glutamate transporters with siRNA or global knockout in mice do not affect GSIS but do reduce insulin secretion in response to co-stimulation with glucose and agents that increase β-cell cAMP levels. The specific mechanism by which glutamate triggers granule exocytosis has not been elucidated but appears to involve the activity of a proton pump vacuolar ATPase and the chloride transport protein CIC3 to regulate granule electrical potential, facilitating the recruitment and fusion of secretory granules to the plasma membrane78,79.
The supply of glutamate for GSIS can be achieved either through the activity of the malate–aspartate shuttle78 or through the activity of GDH in mitochondria, which catalyses the bidirectional conversion of α-ketoglutarate and glutamate80. Support for the latter model comes from experiments with β-cell-specific GDH-knockout mice, which exhibit reduced GSIS and abrogation of glucose-induced increases in glutamate levels80. We note that impairment of GSIS in response to GDH knockout could be consistent with dual roles of glutamate in insulin secretion: as a substrate for reductive TCA cycle metabolism to sustain IDH1 activity and as a direct regulator of insulin granule exocytosis.
A specific mechanism for the stimulation of insulin granule exocytosis has also emerged for the GL/FFA pathway, which involves the generation of MAGs. Whereas both 1-MAG and 2-MAG are increased by glucose stimulation of islet cells, only 1-MAG consistently correlates with enhanced insulin secretion68. Levels of 1-MAG in β-cells are regulated by α/β-hydrolase domain-6-accessible monoacylglycerol (ABHD6), a plasma membrane-associated MAG lipase. The overexpression of ABHD6 impairs GSIS while lowering 1-MAG levels, whereas molecular or pharmacologic suppression of ABHD6 activity enhances GSIS while increasing 1-MAG levels. Mechanistically, 1-MAG binds to Munc13-1 (a protein that orchestrates membrane fusion events in various cell types) to activate its vesicle-priming function68 (FIG. 3). 1-MAG is reported to have a higher binding affinity for Munc13-1 than DAG, the more well-known PLC-derived regulator of Munc13-1. Munc13-1 translocation to the plasma membrane is stimulated by glucose, an effect amplified by the inhibition of ABHD6. Taken together, these data establish a solid connection between the generation of 1-MAG and the regulation of insulin secretion, at least under conditions where fatty acid supply is not limiting for the operation of the GL/FFA cycle.
Mechanistic synergy of multiple pathways regulating GSIS amplification.
A reasonable question to ask here is why do so many metabolic signalling pathways exist for the control of insulin secretion? Do they each serve a specific purpose or do they ultimately converge to ensure a robust insulin secretion response to nutrient ingestion? Although some of the pathways appear well-suited for a specific function, such as the GL/FFA pathway as a natural conduit for the potentiation of GSIS by fatty acids, there is growing evidence of congruency among these pathways. One example already mentioned is the requirement of SENP1 for both the IDH1 and pentose shunt/S-AMP pathways, although their potential additivity remains to be explored. Furthermore, recent studies show that the inhibition of IDH2 not only lowers cellular NADPH levels as it attenuates insulin secretion but also leads to reduced de novo lipogenesis from glucose44, thus potentially connecting reductive TCA cycle metabolism to GL/FFA signalling. Finally, the generation of PEP by the SCS-GTP-PEPCK-M pathway may potentiate the other signalling mechanisms by allowing the β-cell to continue to synthesize ATP, thereby contributing to KATP-channel closure and insulin secretion, even when β-cells reach a maximal mitochondrial oxidative phosphorylation potential57. Overall, the potential for crosstalk and synergy among the emergent pathways deserves further investigation.
Role of paracrine signalling in islets
In addition to the multiple metabolic signalling mechanisms described above, insulin secretion from islet β-cells is tightly regulated by crosstalk with other islet endocrine cells, especially α-cells and δ-cells. These signals from adjoining islet cells, referred to as ‘paracrine signals’, govern β-cell function through signalling cascades that typically converge on cAMP levels in β-cells (Supplementary Box 2).
Paracrine effects of α-cells on insulin secretion.
New studies from several laboratories have revealed a previously unappreciated role for α-cells in the regulation of β-cell insulin secretion mediated by proglucagon-derived peptides81-83. Although known for producing glucagon, α-cells also produce glucagon-like peptide 1 (GLP1) as an alternate peptide product of the proglucagon gene. The administration of either glucagon or GLP1 to isolated islets ex vivo or to mice or humans in vivo potently stimulates insulin secretion in a glucose-dependent manner. Deciphering the relative contributions of glucagon versus GLP1 generated by the α-cell in regulation of insulin secretion is an ongoing line of investigation.
Important findings have emerged in studies with transgenic mice in which the GLP1 receptor (GLP1R) or the glucagon receptor (GCGR) were knocked out in β-cells, individually and in combination, or in which knockout of the proglucagon gene in α-cells eliminated the secretion of glucagon and GLP1. Islets lacking the production of proglucagon-derived peptides have impaired insulin secretion responses to glucose and amino acids that are restored by treatment with glucagon or GLP1. These effects of proglucagon products are mainly mediated by GLP1R, as knockout of this receptor or its antagonism with exendin 9 causes strong impairment of insulin secretion by glucose and amino acids. Similarly, the loss of GLP1R impairs insulin secretion in response to graded increases in glucagon in mouse islets exposed to high glucose concentrations. By contrast, knockout of the β-cell GCGR has little effect on glucose-stimulated or amino acid-stimulated insulin secretion. Helping to explain these observations is the fact that both glucagon and GLP1 effectively activate GLP1R, whereas GLP1 is a very weak agonist of GCGR. α-cell to β-cell signalling is mediated by changes in β-cell cAMP levels (see Supplementary Box 2 for details of the role of cAMP in insulin secretion) as evidenced by the findings that dual antagonism of GLP1R and GCGR sharply lowers cAMP levels and that treatment of islets from proglucagon-knockout mice with the adenylate cyclase activator forskolin rescues GSIS81. Importantly, α-cell to β-cell signalling has also been shown to be active in human islets, where the antagonism of GLP1R or GCGR produces similar reductions in insulin secretion to those observed in preclinical models81. Interestingly, human islets contain a larger proportion of α-cells, have significantly higher GSIS than rodent islets (FiG. 1a) and, at the same time, exhibit a greater reduction in insulin secretion when exposed to exendin 9 to inhibit GLP1R-mediated α-cell to β-cell communication81.
Given that proglucagon gene products play an essential role in the regulation of insulin secretion, how does this relate to the canonical roles of glucagon in hepatic glucose production or ketogenesis (the synthesis of ketone bodies from fatty acids)? Glucagon clearly increases blood glucose levels when administered in the fasted state and stimulates insulin secretion through cAMP in a glucose-dependent manner, although this effect is minimized at low fasting glucose levels. The administration of glucagon in the fed state, when higher glucose levels enable signalling in β-cells, results in the potent stimulation of insulin secretion and in the lowering of blood glucose levels84. Consistent with this model, mice with impaired α-cell to β-cell communication are glucose intolerant due to insufficient insulin secretion81,83,84.
β-cell paracrine factors regulating islet function and glucose homeostasis.
In general, insulin and glucagon secretion are reciprocally regulated, with insulin falling and glucagon rising in concert with the decline in glucose levels in the fasted state and the converse response occurring upon the ingestion of carbohydrate-containing meals. This ensures the appropriate assimilation and usage of glucose depending on its availability. Opposite to its signalling in β-cells, glucose metabolism in α-cells has been proposed to generate intrinsic signals that inhibit rather than stimulate glucagon secretion. The proposed mechanisms for this intrinsic signalling are discussed in BOX 1.
Box 1 ∣. Metabolic control of glucagon secretion.
Glucose has been proposed to regulate glucagon secretion in a manner opposite to its effects on insulin secretion, such that low glucose enhances glucagon secretion while glucose levels found in the postprandial state suppress glucagon release. Rodent α-cells express glucose transporter GLUT1 rather than GLUT2 (REF.157); however, similar to β-cells, the glucose metabolic rate in α-cells appears to be controlled by glucokinase rather than by glucose transport157,158. The glycolytic flux is estimated to be comparable in α-cells and β-cells157, but α-cells have been reported to mainly metabolize glucose to lactate anaerobically, whereas β-cells metabolize glucose mainly by aerobic glycolysis and mitochondrial metabolism21. Accordingly, the increase in ATP:ADP ratio induced by glucose metabolism in β-cells, a primary factor for glucose-stimulated insulin secretion, is less evident in α-cells159. Nevertheless, glucagon-secreting α-cells express the KATP channel and many of the same granule trafficking and exocytosis regulatory proteins as found in β-cells160. These similarities made it historically difficult to reconcile the opposite secretory responses of the two cell types to low and high glucose until more recent studies identified key electrophysiological differences. At low glucose (1 mM), KATP channel activity in α-cells is about 1% of that in β-cells, meaning that α-cells are already electrically active (more depolarized) at low glucose levels, causing glucagon secretion160,161. The exposure of α-cells to postprandial glucose levels inhibits the small residual KATP channel activity, causing depolarization to an extent not obtained in β-cells, resulting in the inactivation of voltage-dependent Na+ channels and in a reduced amplitude of the α-cell action potential. These events suppress Ca2+ currents and glucagon exocytosis, offsetting the effects of closure of residual KATP channels on voltage-gated Ca2+ channels.
If α-cells have an intrinsic ability to respond to glucose, there are circumstances where this mode of regulation is silenced. For example, long-term fasting, which causes chronic lowering of blood glucose levels, causes a gradual decline in glucagon levels to reach those found in the postprandial state162. Recent studies bring fresh reminders of the fact that a primary role of glucagon is to regulate amino acid levels162 and that amino acids in turn are key regulators of glucagon release163,164. A resurgence of interest in this area was triggered by studies that tested glucagon receptor antagonists as agents for the lowering of blood glucose in type 2 diabetes. Metabolomics analyses revealed that amino acids were the metabolites most strongly affected by glucagon receptor knockout or antagonism in mice, with particular increases in the glucagon secretagogues glutamine and alanine165,166. In addition, glutamine appears to mediate the α-cell hyperplasia observed in response to the suppression of glucagon receptor activity via an mTOR-mediated mechanism165,166. Thus, the potent effect of glucagon to regulate amino acid utilization is mirrored by an equally potent effect of amino acids to regulate α-cell mass and function.
Another interesting observation is that amino acids and free fatty acids (FFA) stimulate both glucagon and insulin secretion, which may seem puzzling in the context of the reciprocal regulation of insulin and glucagon levels in the physiological setting. A unifying model160, is as follows: after ingestion of a mixed meal containing glucose, lipids and protein (amino acids), all of these nutrients stimulate insulin secretion from the β-cell, which serves as a paracrine inhibitory signal for α-cell glucagon secretion. Meanwhile, the increase in glucose may also cause an intrinsic suppression of glucagon release. This combination of paracrine-mediated and intrinsic inhibition by glucose supersedes the stimulatory effects of amino acids and FFA on glucagon secretion under these conditions. Moreover, FFA levels decline significantly in the postprandial compared with the fasted state. The paracrine effect of insulin to suppress glucagon secretion may be mediated in part by insulin stimulation of somatostatin secretion from the islet δ-cell, as knockout of the insulin receptor in mouse δ-cells attenuates insulin-mediated suppression of glucagon secretion167. In the transition from the fed to the fasted state, the decrease in glucose levels reduces insulin and somatostatin release to relieve the paracrine suppression of glucagon secretion. Meanwhile, the intrinsic effect of low glucose to stimulate glucagon secretion is amplified by the increase in FFA and amino acid levels that occurs with fasting.
The regulation of glucagon secretion by an intrinsic α-cell glucose-sensing mechanism has been challenged by other recent findings. Thus, it has been documented that isolated α-cells (in the absence of β-cells) exhibit a modest increase in glucagon secretion in response to stimulatory glucose85. This finding suggests that the inhibition of glucagon secretion from α-cells in whole islets is largely due to paracrine factors originating in other endocrine cell types rather than directly mediated by glucose. Consistent with this idea, the transition from low to high glucose concentrations increases glucagon secretion from intact islets for a period of ~3 minutes, followed by rapid suppression to levels below those observed at low glucose81. These data support a model in which glucose exerts a transient stimulatory effect on α-cell glucagon secretion that is rapidly overcome by inhibitory paracrine mechanisms.
Insulin has long been viewed as one of the negative paracrine regulators of glucagon secretion (FiG. 5). This was revealed in a classical study in which the administration of an insulin-neutralizing antibody during perfusion of the rat pancreas with 5.5 mM glucose caused a sharp increase in glucagon secretion86. Similarly, dogs in which β-cells were ablated showed elevations in glucagon levels that were normalized by insulin infusion87. Consistent with these findings, α-cell-specific knockout of the insulin receptor in mice causes hyperglucagonaemia and hyperglycaemia88. Other products released from β-cells during insulin secretion, including zinc and GABA, are reported to dampen glucagon secretion by a paracrine mechanism89,90, although the physiological significance of zinc-mediated suppression has been questioned91. These data demonstrate that the activation of β-cells generates an inhibitory paracrine signal on α-cells to suppress glucagon secretion. Deciphering the relative roles of direct, glucose metabolism-mediated versus indirect, β-cell-mediated effects in the control of glucagon secretion is an ongoing line of investigation.
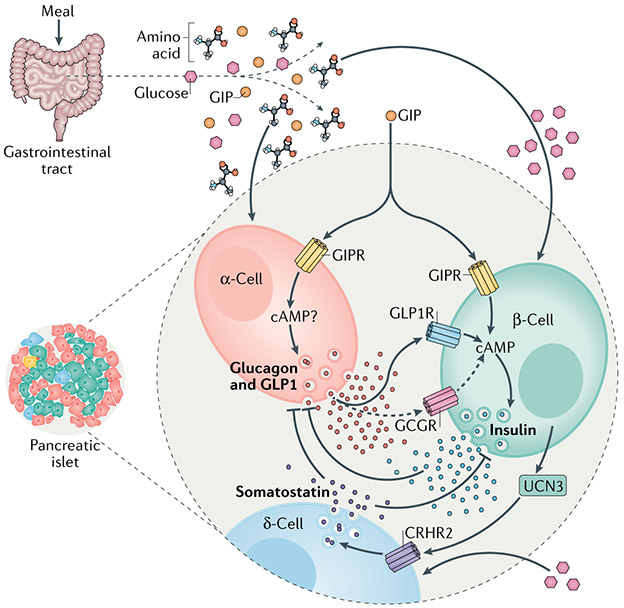
Fig. 5 ∣. Other hormones in control of insulin secretion.
Nutrient ingestion increases the circulating concentrations of glucose, amino acids and the incretin hormones produced by the gastrointestinal tract: glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP1). Agonism of either the GIP receptor (GIPR) or GLP1 receptor (GLP1R) potentiates glucose-stimulated insulin secretion (GSIS) through a cyclic AMP (cAMP)-dependent mechanism. GIP also potentiates amino acid-stimulated glucagon secretion in α-cells, which occurs through as-of-yet undefined mechanisms. The secretion of glucagon generally opposes the action of insulin and insulin negatively regulates glucagon secretion. However, there is now evidence that endocrine activity of α-cells is important for regulating glucose-stimulated insulin secretion in β-cells. Together with glucagon, α-cells also produce GLP1 through alternative processesing of the proglucagon peptide precursor, and both hormones increase the level of cAMP in β-cells to regulate insulin secretion in response to a meal. This is mostly mediated by binding of these hormones to GLP1R and to a lesser extent (dashed arrows) to the glucagon receptor (GCGR). Thus, the incretin action of GIP to stimulate insulin secretion includes both direct effects on β-cells and indirect actions on α-cells, mediated by the paracrine stimulatory effects of proglucagon-derived peptides. Urocortin 3 (UCN3) produced by β-cells initiates a separate mechanism of paracrine interaction within the islet. UCN3 enhances δ-cell activity to increase somatostatin secretion through the corticotropin-releasing hormone receptor(CRHR2). The elevation in somatostatin secretion inhibits the secretory activity of β-cells, completing a negative feedback loop.
Another emergent paracrine factor for the regulation of insulin secretion is urocortin 3 (UCN3)92. Islets from (Ucn3-knockout mice have markedly enhanced GSIS after glucose exposure, which can be suppressed by exogenous UCN3, suggesting that the peptide has inhibitory actions on β-cells. However, the receptor for UCN3, the type 2 corticotropin-releasing hormone receptor (CRHR2), is exclusively expressed in δ-cells within the islet92,93, pointing instead to an indirect mechanism (FiG. 5). Exogenous UCN3 stimulates somatostatin secretion from δ-cells, while (Ucn3-knockout mice have decreased somatostatin content and secretion as well as fewer δ-cells. The enhancement in somatostatin secretion induced by UCN3 would be expected to increase the inhibitory tone on α-cells and β-cells. In support of this, a specific CRHR2 antagonist, astressin 2B, reduces somatostatin secretion and enhances insulin secretion in mouse islets. Still to be explored is the impact of modulating UCN3 signalling on α-cells or glucagon secretion. Interestingly, UCN3 protein levels are decreased in mouse models of hyperglycaemia and in diabetic macaques and humans, indicating that the reduction in UCN3 during diabetes may reflect an adaptive response to maximize insulin release in the face of peripheral insulin resistance. In addition, continuous glucose monitoring experiments demonstrate that the loss of UCN3-mediated buffering effects on islet hormone secretion may increase the volatility in blood glucose levels92. Furthermore, β-cells lacking UCN3 have been identified in human islets derived from young and adult donors as well as in islets from patients with T1D94. These cells have been characterized as ‘immature’ β-cells based on their expression of insulin but not of the GLUT2 glucose transporter and on their failure to respond to glucose or depolarization.
Mechanisms of β-cell failure in T2D
The triggering factor for the transition from obese and insulin-resistant states to T2D is β-cell failure, involving both a loss of β-cell mass and a deterioration of β-cell function. Our evolving understanding of the mechanisms that control islet hormone secretion has led to the development of T2D therapeutic agents that work either wholly or in part through their actions on pancreatic islet cells, as summarized in BOX 2. It has also led to better understanding of the mechanisms underlying the development of β-cell dysfunction in T2D, as summarized below.
Box 2 ∣. Type 2 diabetes therapies targeting the pancreatic islets.
Several current therapies for type 2 diabetes (T2D) target islet cell function, including analogues of glucagon-like peptide 1 (GLP1; also known as incretin mimetics) and sulfonylurea drugs that promote the closure of the KATP channel. Both these drug classes work by stimulating insulin secretion but with different long-term outcomes. Sulfonylureas, such as glyburide, have a limited therapeutic window (30–40% loss of efficacy within 5 years in patients with T2D) relative to other T2D drugs168. Because sulfonylureas bind the SUR1 subunit of the KATP channel to stimulate insulin secretion at all glucose concentrations, in contrast to GLp1-based drugs, which work in a glucose-dependent manner, they have up to a sixfold higher risk of causing hypoglycaemia. GLp1-based drugs have an additional benefit of causing weight loss in some patients, perhaps contributing to the sustained efficacy of these agents. Other commonly used medicines, such as metformin or thiazolidinediones such as pioglitazone, indirectly enhance islet health through alleviating metabolic stress on the β-cell by relieving peripheral insulin resistance.
The clinical success of GLp1 receptor (GLp1R) agonists has spurred the development of next-generation, combinatorial incretin-based therapeutics. Colloquially termed multi-receptor agonists, these therapeutics comprise combinations of GLp1, glucagon and gastric inhibitory polypeptide (GIP) merged in a single peptide sequence, causing simultaneous activation of multiple receptors169. The most efficacious molecules combine GLP1R agonism with either GIP or glucagon receptor activation170-173. Tirzepatide, a GIP–GLP1 receptor co-agonist, demonstrated superiority over a GLP1R monoagonist in phase IIb clinical trials173 and is currently under investigation in phase III trials. However, the mechanism by which the inclusion of GIP receptor agonism produces improvements in glycaemic control and weight loss compared with GLP1R monoagonism remains unclear. One possibility is that such co-agonism in β-cells produces an additive or synergistic effect on insulin secretion and glycaemic control, as supported by co-infusion studies of incretin peptides in humans174,175. Further complexity comes from the finding that improvements in glycaemia and insulin secretion may be invoked through non-β-cell mechanisms by GLP1 (REF.176) but not GIP177. Thus, as multi-receptor agonists continue to progress, understanding the unique versus redundant roles of their components in β-cell signalling is an important area for further study.
Other emergent therapies for T2D targeting islet-independent mechanisms may also impinge unexpectedly on islet function. One such approach involves the inhibition of sodium-glucose cotransporter 2 (SGLT2) activity in the proximal tubule of the kidney, which is known to inhibit glucose re-uptake to facilitate the excretion of glucose in urine, thereby contributing to the control of hyperglycaemia. Whereas SGLT2 inhibitors (SGLT2i) have moderate effects to lower glucose levels in patients with T2D, they remain of high interest because of their striking effects to reduce the risk for incident cardiovascular events and death178 by mechanisms that remain incompletely defined. A potential liability of these drugs was introduced with the report that they increase circulating glucagon levels179, although this is an area of considerable disagreement and lack of concordance among different groups180. A recent study suggests that variable findings may be explained by a high degree of heterogeneity in glucagon secretion responses to SGLT2i, correlating with SGLT2 protein expression in α-cells181. However, it remains unclear if SGLT2i increase glucagon secretion via a direct effect on α-cell SGLT2 activity as opposed to via indirect actions related to the effect of these drugs to lower circulating glucose levels or somatostatin secretion from adjoining δ-cells167,180.
Genetics and β-cell failure.
Genetics plays an important role in T2D as illustrated by the high rates of concordance of the disease in twins. The majority of genetic variants associated with T2D are linked to disturbances in pancreatic islet function but each genomic polymorphism contributes only a very small amount of disease risk, and causal variants have been difficult to identify95. Further complicating matters, many T2D-associated genomic polymorphisms are not located within specific genes but rather in intergenic regions that contribute to the control of expression of a subset of islet-specific genes96-98. Some studies have combined chromatin accessibility data, single-cell RNA-sequencing and chromatin immunoprecipitation data to define differentially expressed genes and associated cis-regulatory control networks in islet cells as well as the associations of these networks with insulin secretion99-101.
Some candidate genes have emerged from these approaches. A common finding in single-cell RNA sequencing studies is a decline in the transcript encoding insulin in β-cells of patients with T2D102,103. It remains unclear if decreased insulin transcript levels result in decreased insulin production in islets from patients with T2D given that insulin content is more directly controlled by the translation of the pro-insulin transcript than by transcription. Other candidates include CDK5 regulatory subunit-associated protein-like 1 (CDKAL1), which functions to modify tRNALys to regulate codon recognition and pro-insulin processing104,105; the β-cell secretory granule zinc transporter (SLC30A8)95; and the melatonin receptor MTNR1B106. The overexpression of MTNR1B in islets or the treatment of islets with melatonin lowers cAMP levels and impairs insulin secretion106. In addition, the expression of the Delta-like homologue (DLK1) is upregulated in T2D compared with control human β-cells102,107,108, and knockout of Dlk1 in mice results in increased insulin secretion107. The essential granule-trafficking gene STX1A has been reported to be downregulated in β-cells in patients with T2D102,109 as has FXYD2, which encodes a Na+/K+-ATPase. Curiously, mice lacking the Fxyd2 gene are actually more glucose tolerant due to increases in pancreatic (β-cell mass and circulating insulin levels110. Furthermore, counterintuitively, the expression of glycerol phosphate dehydrogenase 2 (GPD2), a gene involved in NADH metabolism and ATP production in mitochondria, is upregulated rather than downregulated in (β-cells from patients with T2D103. Other metabolic genes dysregulated in T2D emerged from studies involving laser capture of β-cells from partial pancreatectomy samples or from whole islets obtained from organ donors. These studies used microarray analysis rather than single-cell RNA sequencing111. The glycolytic enzyme aldolase B was found to be upregulated in partial pancreatectomy but not in organ donor samples and subsequently shown to be negatively associated with insulin secretion using a small cohort of partial pancreatectomy specimens112. Expression of a β-cell-specific isoform of glucose-6-phosphatase (G6PC2) was found to be downregulated in both partial pancreatectomy and organ donor samples. Comprehensive mouse genetics studies have also identified genes that regulate islet cell function, but their relevance to human T2D remains to be defined99,113.
Although all of these studies provide interesting clues regarding insulin secretion defects in T2D, functional studies, usually conducted in murine models, have in some instances provided opposite effects to those that would be anticipated (for example, results with FXYD2, and an effect of G6PC2 knockdown to enhance rather than impair insulin secretion and glucose homeostasis114) or inconsistent results across different laboratories, as has been a particular issue for studies involving the manipulation of SLC30A8 (REF.95). Certainly, no single genetic variant has emerged for which a universal mechanistic contribution to β-cell dysfunction of T2D can be demonstrated.
β-cell de-differentiation and β-cell failure.
Several recent studies suggest that loss of β-cell mass and function in T2D occurs as a result of gradual de-differentiation of (β-cells to endocrine progenitor-like cells, as recently reviewed115,116. This concept first emerged with the demonstration that (β-cell-specific knockout of the transcription factor FoxO1 in mice causes gradual loss of insulin expression and activates endocrine progenitor genes such as those encoding chromogranin A, Neurogenin 3, Oct4 and Nanog117. Similarly, mice expressing an ATP-insensitive, gain-of-function mutant allele of the KATP channel subunit Kir6.2 develop hypoinsulinism and diabetes and show loss of mature β-cells and appearance of markers of endocrine progenitor-like cells118. Interestingly, the lowering of blood glucose by insulin therapy in this model silences the expression of Neurogenin 3 and re-activates β-cell insulin expression. The role of de-differentiation in the development of human β-cell dysfunction is less well-studied and not yet clear. One study used immunocytochemical staining of synaptophysin as the primary indicator of de-differentiation, leading to an estimate of 32% de-differentiated β-cells in patients with T2D compared with 9% in controls119. A different group used chromogranin A as the primary marker of de-differentiation, resulting in the identification of smaller numbers of de-differentiated β-cells in both T2D and non-diabetic tissue samples120, although the two studies agreed in reporting an increase in the number of cells lacking the expression of islet hormones in T2D-derived samples119,120. The use of additional validated endocrine progenitor cell markers such as aldehyde dehydrogenase 1A3 (REFS119,121) could be helpful for the interpretation of future studies of this nature. One single-cell RNA sequencing study of islets from T2D and non-diabetic donors found no evidence of upregulated endocrine progenitor genes in β-cells or other islet cell types102, whereas another reported that islets from T2D donors have expression signatures resembling those of islets of paediatric patients, suggestive of de-differentiation122. Further studies are certainly warranted in this emergent area.
Metabolic mediators of β-cell failure.
There is evidence that some of the emergent metabolic signalling pathways discussed earlier may be involved in the development of islet cell dysfunction in T2D. Obesity and overnutrition result in the chronic exposure of islets to elevated levels of multiple nutrients, including glucose, fatty acids and amino acids123. The chronic exposure of 832/13 cells to elevated lipid levels induces fatty acid oxidation124,125 and leads to the impairment of GSIS and a parallel increase in anaplerotic pyruvate metabolism at basal glucose levels, resulting in elimination of the normal glucose-induced increment in pyruvate cycling125,126. A membrane-permeant ester of malate, dimethyl malate, restores GSIS in glucose-unresponsive islets from a rodent model of T2D, the Zucker Diabetic Fatty rat, or in 832/13 cells exposed chronically to elevated lipid levels125. Malate can be converted to pyruvate to provide an anaplerotic substrate for citrate and isocitrate synthesis, which upon export from the mitochondria could contribute to cytosolic NADPH production via IDH1 (REF.43) (FIG. 2). Alternatively, dimethyl malate could provide a substrate for mitochondrial GTP production in the SCS-GTP reaction and for the PEPCK-M-mediated production of PEP57,58 (FIG. 3). Lipids may also be metabolized to pro-inflammatory metabolites by 12-lipoxygenase, the knockout of which was shown to be protective against the loss of β-cell mass and function in mice127.
Perturbation of the reductive cycle formed by the concerted actions of IDH2 and IDH1 may also contribute to insulin secretory defects in T2D, as suggested by the robust exocytotic response to isocitrate or NADPH but not to glucose in patch-clamped β-cells from donors with T2D43. The potency of isocitrate and NADPH to enhance exocytosis in glucose-insensitive T2D β-cells suggests a defect in reductive cycling for the replenishment of citrate and isocitrate. Consistent with this idea, global knockout of Idh2 in mice results in decreased insulin secretion in both the fasted and fed states128. Additionally, IDH2 mRNA and protein expression are decreased in two diabetic mouse strains with β-cell dysfunction129,130. Finally, the inhibition of IDH2 results in the suppression of insulin secretion in isolated islets and in mice44. All of these data are consistent with a model in which the interruption of IDH2-catalysed reductive TCA cycle flux results in impaired GSIS (FIG. 2).
Roles of β-cell replication and death in β-cell failure.
In addition to impairment of GSIS, loss of β-cell mass is evident in rodent and human forms of T2D. β-cell mass represents a balance between β-cell replication and β-cell death. Human β-cell replication appears to occur primarily in the immediate postnatal period, declining to a very low steady-state level by around 2 years of age131. Certain conditions, including obesity and pregnancy, are thought to increase β-cell numbers132. In rodents, this increase in mass has been ascribed to the replication of pre-existing β-cells and/or to neogenesis of β-cells from pancreatic ductal cells, although cellular lineage tracing in mice showed that neogenesis of ductal cells is a rare event133. Evidence for these pathways in humans is more equivocal134. Importantly, both major forms of diabetes involve the deterioration of β-cell mass. This suggests that the capacity for β-cell regeneration in humans cannot compensate for the gradual decline in β-cell numbers in T2D or for the more abrupt loss of β-cells occurring in T1D due to autoimmune attack. Several recent reviews on the mechanisms of islet β-cell regeneration are available134-136.
Deregulated protein SUMOylation may contribute to β-cell failure in T2D. Mice with inducible, β-cell-specific knockout of the SUMO-conjugating enzyme UBC9 exhibit loss of both β-cell function and mass, whereas UBC9-overexpressing mice have impaired GSIS77. The effects of UBC9 overexpression are consistent with the effects of overexpression of SUMO1 or knockout of SENP1 on insulin secretion43,76. Ubc9-knockout mice also have impaired insulin secretion but, in this case, linked to a gradual loss of β-cell mass due to increased oxidative stress. An important SUMOylated substrate of UBC9 is the transcription factor NRF2, which controls the expression of antioxidant genes encoding, for example, superoxide dismutase and GSH peroxidase, thereby preventing oxidative stress and cell death. SUMOylation of NRF2 stabilizes the protein and facilitates its translocation to the nucleus for engagement with its target genes. In line with this, Ubc9-knockout mice exhibit lower levels of expression of multiple antioxidant genes, increased levels of reactive oxygen species and greater sensitivity of β-cells for destruction by toxins such as streptozotocin relative to wild-type mice, whereas UBC9-overexpressing mice have the opposite phenotypes77. In addition, the overexpression of SUMO1 in mouse islets causes SUMOylation of GLP1R, resulting in impairment of its trafficking to the plasma membrane and in reduced GLP1 signalling137. In support of the regulation of GLP1 signalling by SENP1, inhibition of the GLP1-degrading enzyme, which enhances insulin secretion and improves glucose tolerance in wild-type mice, is without these effects in mice with β-cell-specific knockout of SENP1 (REF.138). Thus, UBC9 and SENP1 influence β-cell survival on the one hand and β-cell secretory function on the other, suggesting that a balance must be maintained among these factors in order to maintain β-cell health and metabolic homeostasis. Further studies of the potential role of these signalling networks in the pathogenesis of T2D are needed.
Another pathway that can exert deleterious effects on both β-cell function and survival is ER stress, which is driven by the effects of chronic exposure of islets to elevated levels of nutrients, leading to constitutive increases in hormone synthesis and secretion. The PKR-like ER-associated kinase (PERK) links ER stress to changes in protein translation in mammalian cells by phosphorylation and inhibition of eukaryotic translation initiation factor 2a (eIF2a). Humans and mice lacking PERK have profound β-cell dysfunction and have severe diabetes139, whereas mice lacking a PERK phosphorylation site in eIF2a have reduced β-cell mass and diabetes due to insulin deficiency140,141. As insulin biosynthetic demand increases with obesity and overnutrition to compensate for insulin resistance in peripheral tissues, this eventually overloads the protein folding capacity of the ER, leading to activation of the unfolded protein response (UPR), which in turn leads to activation of PERK and inhibition of protein translation, ultimately contributing to insulinopenia. Consistent with these findings, the chronic exposure of islets to elevated glucose and lipids to create ‘glucolipotoxicity’, impairs insulin secretion and activates β-cell apoptosis123.
In addition to insulin, β-cells express IAPP/amylin, which, when overexpressed, may have unique damaging effects that contribute to β-cell dysfunction and destruction. Early studies on this protein were focused on its capacity to form extracellular amyloid fibrils, which were ascribed β-cell cytotoxic effects analogous to the proposed role of amyloid fibrils in neurodegenerative diseases8,142,143. In addition, high rates of IAPP synthesis induced by chronic nutrient stimulation of islet cells may lead to the formation of intracellular aggregates of the protein, contributing to ER stress144. The propensity of the human and non-human primate forms of IAPP to form amyloid fibrils and intracellular aggregates is due to a hydrophobic pocket of amino acids clustered in residues 20–29 of the protein, which are lacking in rodent IAPP. This sequence difference helps to explain why early studies with transgenic mice overexpressing rodent amylin revealed no pathogenic effect, whereas later studies of human IAPP overexpression in rats found increased rates of β-cell apoptosis, diminished first phase insulin secretion and decreased β-cell mass, leading to glucose intolerance and diabetes145.
Interestingly, transgenic rats expressing human IAPP exhibit increased expression of genes involved in glycolytic glucose metabolism, apparently activated by the upregulation of the hypoxia-activated transcription factor HIF1α146. This includes the known HIF1α target genes encoding lactate dehydrogenase C, pyruvate kinase M2 and 6-phosphofructo-2-kinase/fructose 2,6 biphosphatase 3 (PFKFB3). The activation of the glycolytic programme in response to human IAPP overexpression in islets is accompanied by the downregulation of transcripts encoding the anaplerotic enzyme PC and several TCA cycle enzymes, consistent with decreased energy charge in these islets, as reflected by increases in the AMP to ATP and ADP to ATP ratios146. These experiments provide a potential link between disturbed proteostasis and dysregulation of β-cell metabolism.
Impaired α-cell to β-cell signalling in T2D.
In T2D, the loss of normal control of insulin secretion is accompanied by elevations in glucagon levels. This is partially explained by reduced paracrine suppression of glucagon secretion as the release of insulin and somatostatin begins to wane. In addition, obesity and insulin resistance are associated with increased levels of multiple amino acids, including two known glucagon secretagogues, arginine and alanine147,148. However, given the insulinotropic actions of glucagon and other proglucagon-derived peptides outlined above, how can T2D present with impaired insulin secretion in the face of hyperglucagonaemia? One hypothesis is that the activity of G protein-coupled receptors (GPCRs), such as GLP1R, GCGR or the glucose-dependent insulinotropic polypeptide (GIP) receptor, in β-cells is downregulated following the development of β-cell dysfunction. cAMP signalling derived from GPCR activity sets β-cell tone81, defined as the level of insulin secretion in response to a set concentration of glucose. Therefore, a decrease in GPCR activity could diminish the insulin secretion response to stimulatory glucose in β-cells. GIP is a meal-induced incretin hormone produced in the gastrointestinal tract that serves as an additional potentiator of GSIS149,150. A decrease in the insulinotropic actions of the GIP receptor has been proposed to occur with progressive metabolic stress, as even pharmacological levels of GIP fail to stimulate insulin secretion in people with T2D151. However, the same studies show preserved GLP1R activity, the primary receptor for glucagon, in response to exogenous GLP1. The insulinotropic activity of glucagon on the GLP1R in healthy people versus in patients with T2D has not been tested. Although underlying mechanisms will require further study, what is clear is that the hyperglucagonaemia present in T2D fails to drive insulin to levels sufficient to overcome the hepatic actions of glucagon to stimulate endogenous glucose production and peripheral insulin resistance, ultimately leading to hyperglycaemia.
Conclusions and perspectives
A great deal has been learnt about the mechanisms underlying key functions of α-cells and β-cells since the discovery of the pancreatic islets more than 150 years ago. This article has attempted to update the reader on several new mechanisms by which glucose, amino acids and fatty acids participate in the regulation of insulin and glucagon secretion. This includes the metabolism of each of these nutrients to generate specific intermediates or cofactors that eventually regulate the exocytotic machinery to control hormone secretion. Several of the newly described pathways appear to converge upon a specific post-translational modification event, SUMOylation, as a mediator of insulin secretion. Moreover, there is evidence that some of the emerging signalling pathways may be compromised or modified to contribute to islet dysfunction of T2D, although this area requires further investigation. Finally, recent years have seen rapid emergence of new information about paracrine signalling networks involving islet α-cells, β-cells and δ-cells in control of β-cell function and reciprocal regulation of insulin and glucagon secretion. Important areas for future study include assessment of the possible additivity of the multiple metabolic pathways described herein for the regulation of insulin secretion as well as deeper investigation of the metabolic mechanisms that regulate glucagon secretion. We hope that the advances summarized here will provide fresh ideas for investigators working in the field of pancreatic islet biology, ultimately helping in the search for new therapeutic targets that can restore islet cell health and function in T2D.
Supplementary Material
Insulin resistance.
Referring to a condition in which the effect of insulin to promote the uptake and storage of glucose and other metabolic fuels is blunted; a major contributing metabolic lesion in type 2 diabetes.
Secretagogues.
Referring to metabolites and hormones that stimulate hormone secretion. Mainly used in this article as a general term for agents that stimulate insulin secretion from β-cells.
Stimulatory glucose.
Referring to glucose levels above those typically found in fasting mammals (~4 mM); insulin secretion increases in a linear fashion as glucose levels increase above those found in the fasted state.
Euglycaemic values.
Referring to the tight control of glucose levels achieved in humans without diabetes or without insulin resistance, ranging from 3.9 mM in the fasted state to 5.5 mM in the fed state.
Km.
Also known as the Michaelis constant, it is the concentration of a substrate (glucose in the case of glucokinase) that permits an enzyme to achieve half of its maximal activity (Vmax).
Anaplerotic enzyme.
From the Greek meaning ‘to fill up’, referring to an enzyme that contributes substrate to the tricarboxylic acid (TCA) cycle that is used for a non-oxidative purpose, for example, the exit of TCA cycle intermediates from the mitochondria to participate in cytosolic metabolism.
Reducing equivalent shuttle.
Referring to a series of reactions by which cytosolic NADH is converted to mitochondrial NADH and FADH, involving the metabolism of organic acids in the cytosol to their reduced forms, followed by transport into the mitochondria for re-oxidation by FAD-linked or NAD-linked enzymes. Examples are the malate–aspartate shuttle and the glycerol phosphate shuttle.
Stable isotope flux methods.
Use of stable isotope-labelled substrates (for example, 13C-glucose or 13C-glutamine) to discern their metabolic fates by analysing the labelling of their metabolic by-products by mass spectrometry or NMR.
Reductive (counter-clockwise) TCA cycle flux.
Referring to the metabolism of substrates (for example, glutamine) in the TCA cycle in a ‘counter-clockwise’ fashion, requiring their reductive rather than oxidative metabolism.
Pentose monophosphate shunt pathway.
One of the major pathways of glucose-6-phosphate in mammalian cells; it produces ribose-5-phosphate for nucleotide synthesis and is a source of NADPH via its first two enzymatic steps, catalysed by glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase.
Glutathione.
(GSH). A tripeptide (composed of cysteine, glycine and glutamate) with a major role in the regulation of the cellular redox state via its intra-conversion between its oxidized and reduced forms.
Proglucagon-derived peptides.
Referring to peptides produced by the processing of the proglucagon precursor, including glucagon, GLP1, GLP2, oxyntomodulin, glicentin, major proglucagon fragment and glicentin-related pancreatic peptide.
mTOR.
Standing for ‘mammalian target of rapamycin’; a protein kinase that serves important signalling roles for the regulation of protein synthesis, insulin action and intermediary metabolism.
Pancreatic ductal cells.
These cells form the epithelial lining of structures that deliver pancreatic enzymes to the duodenum. in response to specific challenges in rodents, these cells can differentiate to form new insulin-producing β-cells (‘neogenesis’).
Streptozotocin.
A glucosamine-nitrosourea compound that is particularly toxic to β-cells and is used as an agent for creating experimental type 1 diabetes in animal models.
ER stress.
Occurs when the capacity of the endoplasmic reticulum (ER) to fold proteins becomes saturated.
Unfolded protein response.
A group of signal transduction pathways that are activated by ER stress.
Acknowledgements
Work cited in this review from the authors’ laboratories was supported by NIH grants DK046492-25 (to C.B.N.) and RO1 DK123075 (to J.E.C.), a career development award 1-18-JDF-017 from the American Diabetes Association (to J.E.C.), and a Borden Scholar award (to J.E.C.). The authors are grateful for the contributions of collaborators and members of their labs to the cited work, including Drs. Mette Jensen, Guofang Zhang, Megan Capozzi and Kimberly El.
Footnotes
Competing interests
C.B.N. is a member of the Eli Lilly Global Diabetes Advisory Board. J.E.C. has a sponsored research agreement with Eli Lilly that has supported a portion of his work referenced in this article.
Peer review information
Nature Reviews Molecular Cell Biology thanks M. Prentki, D. Accili and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at https://doi.org/10.1038/s41580-020-00317-7.
References
- 1.Sakula A Paul Langerhans (1847–1888): a centenary tribute. J. R. Soc. Med 81, 414–415 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dolensek J, Rupnik MS & Stozer A Structural similarities and differences between the human and the mouse pancreas. Islets 7, e1024405 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ionescu-Tirgoviste C et al. A 3D map of the islet routes throughout the healthy human pancreas. Sci. Rep 5, 14634 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olehnik SK, Fowler JL, Avramovich G & Hara M Quantitative analysis of intra- and inter-individual variability of human beta-cell mass. Sci. Rep 7, 16398 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito K, Takahashi T, Yaginuma N & Iwama N Islet morphometry in the diabetic pancreas of man. Tohoku J. Exp. Med 125, 185–197 (1978). [DOI] [PubMed] [Google Scholar]
- 6.Brazeau P et al. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 179, 77–79 (1973). [DOI] [PubMed] [Google Scholar]
- 7.Rorsman P & Huising MO The somatostatin-secreting pancreatic delta-cell in health and disease. Nat. Rev. Endocrinol 14, 404–414 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hay DL, Chen S, Lutz TA, Parkes DG & Roth JD Amylin: pharmacology, physiology, and clinical potential. Pharmacol. Rev 67, 564–600 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Henquin JC Regulation of insulin secretion: a matter of phase control and amplitude modulation. Diabetologia 52, 739–751 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Ashcroft FM, Harrison DE & Ashcroft SJ Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature 312, 446–448 (1984). [DOI] [PubMed] [Google Scholar]
- 11.Cook DL & Hales CN Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature 311, 271–273 (1984).Together with Ashcroft, F. M. et al., these two original papers established the role of KATP channels in GSIS.
- 12.Gaisano HY Recent new insights into the role of SNARE and associated proteins in insulin granule exocytosis. Diabetes Obes. Metab 19, 115–123 (2017). [DOI] [PubMed] [Google Scholar]
- 13.De Vos A et al. Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. J. Clin. Invest 96, 2489–2495 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson JH, Newgard CB, Milburn JL, Lodish HF & Thorens B The high Km glucose transporter of islets of Langerhans is functionally similar to the low affinity transporter of liver and has an identical primary sequence. J. Biol. Chem 265, 6548–6551 (1990). [PubMed] [Google Scholar]
- 15.Thorens B, Sarkar HK, Kaback HR & Lodish HF Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell 55, 281–290 (1988). [DOI] [PubMed] [Google Scholar]
- 16.Matschinsky FM Regulation of pancreatic beta-cell glucokinase: from basics to therapeutics. Diabetes 51 (Suppl. 3), S394–S404 (2002).A comprehensive review of studies establishing glucokinase as a key regulator of β-cell glucose metabolism and GSIS.
- 17.Wilson JE Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J. Exp. Biol 206, 2049–2057 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Alves TC et al. Integrated, step-wise, mass-isotopomeric flux analysis of the TCA cycle. Cell Metab. 22, 936–947 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan A, Ling ZC & Landau BR Quantifying the carboxylation of pyruvate in pancreatic islets. J. Biol. Chem 271, 2539–2542 (1996). [DOI] [PubMed] [Google Scholar]
- 20.Lu D et al. 13C NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS). Proc. Natl Acad. Sci. USA 99, 2708–2713 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuit F et al. Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in beta cells. J. Biol. Chem 272, 18572–18579 (1997). [DOI] [PubMed] [Google Scholar]
- 22.Schuit F et al. beta-cell-specific gene repression: a mechanism to protect against inappropriate or maladjusted insulin secretion? Diabetes 61, 969–975 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eto K et al. Role of NADH shuttle system in glucose-induced activation of mitochondrial metabolism and insulin secretion. Science 283, 981–985 (1999). [DOI] [PubMed] [Google Scholar]
- 24.MacDonald MJ High content of mitochondrial glycerol-3-phosphate dehydrogenase in pancreatic islets and its inhibition by diazoxide. J. Biol. Chem 256, 8287–8290 (1981). [PubMed] [Google Scholar]
- 25.Gembal M, Detimary P, Gilon P, Gao ZY & Henquin JC Mechanisms by which glucose can control insulin release independently from its action on adenosine triphosphate-sensitive K+ channels in mouse B cells. J. Clin. Invest 91, 871–880 (1993).A paper that established the existence of KATP channel-independent pathways for the regulation of GSIS.
- 26.Miki T et al. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc. Natl Acad. Sci. USA 95, 10402–10406 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Remedi MS et al. Diet-induced glucose intolerance in mice with decreased beta-cell ATP-sensitive K+channels. Diabetes 53, 3159–3167 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Malloy CR, Sherry AD & Jeffrey FM Evaluation of carbon flux and substrate selection through alternate pathways involving the citric acid cycle of the heart by 13C NMR spectroscopy. J. Biol. Chem 263, 6964–6971 (1988). [PubMed] [Google Scholar]
- 29.Hohmeier HE et al. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49, 424–430 (2000). [DOI] [PubMed] [Google Scholar]
- 30.Ronnebaum SM et al. A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretion. J. Biol. Chem 281, 30593–30602 (2006).A paper describing a unique role for IDH1 in the control of GSIS.
- 31.Attali V et al. Regulation of insulin secretion and proinsulin biosynthesis by succinate. Endocrinology 147, 5110–5118 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Farfari S, Schulz V, Corkey B & Prentki M Glucose-regulated anaplerosis and cataplerosis in pancreatic beta-cells: possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes 49, 718–726 (2000). [DOI] [PubMed] [Google Scholar]
- 33.Fransson U, Rosengren AH, Schuit FC, Renstrom E & Mulder H Anaplerosis via pyruvate carboxylase is required for the fuel-induced rise in the ATP:ADP ratio in rat pancreatic islets. Diabetologia 49, 1578–1586 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Jensen MV et al. Metabolic cycling in control of glucose-stimulated insulin secretion. Am. J. Physiol. Endocrinol. Metab 295, E1287–E1297 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacDonald MJ et al. Perspective: emerging evidence for signaling roles of mitochondrial anaplerotic products in insulin secretion. Am. J. Physiol. Endocrinol. Metab 288, E1–E15 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Pongratz RL, Kibbey RG, Shulman GI & Cline GW Cytosolic and mitochondrial malic enzyme isoforms differentially control insulin secretion. J. Biol. Chem 282, 200–207 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Ronnebaum SM et al. Silencing of cytosolic or mitochondrial isoforms of malic enzyme has no effect on glucose-stimulated insulin secretion from rodent islets. J. Biol. Chem 283, 28909–28917 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joseph JW et al. The mitochondrial citrate/isocitrate carrier plays a regulatory role in glucose-stimulated insulin secretion. J. Biol. Chem 281, 35624–35632 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Joseph JW et al. Normal flux through ATP-citrate lyase or fatty acid synthase is not required for glucose-stimulated insulin secretion. J. Biol. Chem 282, 31592–31600 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Odegaard ML et al. The mitochondrial 2-oxoglutarate carrier is part of a metabolic pathway that mediates glucose- and glutamine-stimulated insulin secretion. J. Biol. Chem 285, 16530–16537 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guay C, Madiraju SR, Aumais A, Joly E & Prentki M A role for ATP-citrate lyase, malic enzyme, and pyruvate/citrate cycling in glucose-induced insulin secretion. J. Biol. Chem 282, 35657–35665 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Guay C et al. A role for cytosolic isocitrate dehydrogenase as a negative regulator of glucose signaling for insulin secretion in pancreatic b-cells. PLoS ONE 8, e77097 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferdaoussi M et al. Isocitrate-to-SENP1 signaling amplifies insulin secretion and rescues dysfunctional beta cells. J. Clin. Invest 125, 3847–3860 (2015).A paper that connects cytosolic NADPH production by IDH1 to downstream signalling events that enhance insulin secretion.
- 44.Zhang GF et al. Reductive TCA cycle metabolism fuels glutamine- and glucose-stimulated insulin secretion. Cell Metab. 10.1016/j.cmet.2020.11.020 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holleran AL, Briscoe DA, Fiskum G & Kelleher JK Glutamine metabolism in AS-30D hepatoma cells. Evidence for its conversion into lipids via reductive carboxylation. Mol. Cell Biochem 152, 95–101 (1995). [DOI] [PubMed] [Google Scholar]
- 46.Jiang L et al. Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature 532, 255–258 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Metallo CM et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 481, 380–384 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mullen AR et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 481, 385–388 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wise DR et al. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc. Natl Acad. Sci. USA 108, 19611–19616 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Urban DJ et al. Assessing inhibitors of mutant isocitrate dehydrogenase using a suite of pre-clinical discovery assays. Sci. Rep 7, 12758 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang F et al. Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation. Science 340, 622–626 (2013). [DOI] [PubMed] [Google Scholar]
- 52.Spegel P et al. Time-resolved metabolomics analysis of beta-cells implicates the pentose phosphate pathway in the control of insulin release. Biochem. J 450, 595–605 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Goehring I et al. Identification of an intracellular metabolic signature impairing beta cell function in the rat beta cell line INS-1E and human islets. Diabetologia 54, 2584–2594 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Huang M & Joseph JW Assessment of the metabolic pathways associated with glucose-stimulated biphasic insulin secretion. Endocrinology 155, 1653–1666 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Lorenz MA, El Azzouny MA, Kennedy RT & Burant CF Metabolome response to glucose in the beta-cell line INS-1 832/13. J. Biol. Chem 288, 10923–10935 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gooding JR et al. Adenylosuccinate is an insulin secretagogue derived from glucose-induced purine metabolism. Cell Rep. 13, 157–167 (2015).A paper establishing a novel role for S-AMP in regulation of insulin secretion.
- 57.Jesinkey SR et al. Mitochondrial GTP links nutrient sensing to beta cell health, mitochondrial morphology, and insulin secretion independent of OxPhos. Cell Rep. 28, 759–772.e10 (2019).A paper presenting evidence for an alternate nucleotide signalling pathway involving the GTP-forming isoform of succinyl CoA synthetase (SCS-GTP).
- 58.Kibbey RG et al. Mitochondrial GTP regulates glucose-stimulated insulin secretion. Cell Metab. 5, 253–264 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koyama K et al. beta-cell function in normal rats made chronically hyperleptinemic by adenovirus-leptin gene therapy. Diabetes 46, 1276–1280 (1997). [DOI] [PubMed] [Google Scholar]
- 60.Stein DT et al. Essentiality of circulating fatty acids for glucose-stimulated insulin secretion in the fasted rat. J. Clin. Invest 97, 2728–2735 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stein DT et al. The insulinotropic potency of fatty acids is influenced profoundly by their chain length and degree of saturation. J. Clin. Invest 100, 398–403 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Briscoe CP et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J. Biol. Chem 278, 11303–11311 (2003). [DOI] [PubMed] [Google Scholar]
- 63.Mancini AD & Poitout V The fatty acid receptor FFA1/GPR40 a decade later: how much do we know? Trends Endocrinol. Metab 24, 398–407 (2013). [DOI] [PubMed] [Google Scholar]
- 64.Ferdaoussi M et al. G protein-coupled receptor (GPR)40-dependent potentiation of insulin secretion in mouse islets is mediated by protein kinase D1. Diabetologia 55, 2682–2692 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Corkey BE et al. A role for malonyl-CoA in glucose-stimulated insulin secretion from clonal pancreatic beta-cells. J. Biol. Chem 264, 21608–21612 (1989). [PubMed] [Google Scholar]
- 66.Mulder H et al. Overexpression of a modified human malonyl-CoA decarboxylase blocks the glucose-induced increase in malonyl-CoA level but has no impact on insulin secretion in INS-1-derived (832/13) beta-cells. J. Biol. Chem 276, 6479–6484 (2001). [DOI] [PubMed] [Google Scholar]
- 67.Prentki M, Corkey BE & Madiraju SRM Lipid-associated metabolic signalling networks in pancreatic beta cell function. Diabetologia 63, 10–20 (2020). [DOI] [PubMed] [Google Scholar]
- 68.Zhao S et al. alpha/beta-hydrolase domain-6-accessible monoacylglycerol controls glucose-stimulated insulin secretion. Cell Metab. 19, 993–1007 (2014).A paper providing evidence for a glycerolipid cycle mechanism for the enhancement of insulin secretion via the activation of Munc13-1.
- 69.Antinozzi PA, Segall L, Prentki M, McGarry JD & Newgard CB Molecular or pharmacologic perturbation of the link between glucose and lipid metabolism is without effect on glucose-stimulated insulin secretion. A re-evaluation of the long-chain acyl-CoA hypothesis. J. Biol. Chem 273, 16146–16154 (1998). [DOI] [PubMed] [Google Scholar]
- 70.Ronnebaum SM et al. Chronic suppression of acetyl-CoA carboxylase 1 in beta-cells impairs insulin secretion via inhibition of glucose rather than lipid metabolism. J. Biol. Chem 283, 14248–14256 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chakravarthy MV et al. Inactivation of hypothalamic FAS protects mice from diet-induced obesity and inflammation. J. Lipid Res 50, 630–640 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ansari IH et al. Characterization of Acyl-CoA synthetase isoforms in pancreatic beta cells: Gene silencing shows participation of ACSL3 and ACSL4 in insulin secretion. Arch. Biochem. Biophys 618, 32–43 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sudhof TC The molecular machinery of neurotransmitter release (Nobel lecture). Angew. Chem. Int. Ed. Engl 53, 12696–12717 (2014). [DOI] [PubMed] [Google Scholar]
- 74.Ivarsson R et al. Redox control of exocytosis: regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes 54, 2132–2142 (2005). [DOI] [PubMed] [Google Scholar]
- 75.Ferdaoussi M & MacDonald PE Toward connecting metabolism to the exocytotic site. Trends Cell Biol. 27, 163–171 (2017).An informative review about protein SUMOylation in the regulation of islet hormone secretion.
- 76.Dai XQ et al. SUMOylation regulates insulin exocytosis downstream of secretory granule docking in rodents and humans. Diabetes 60, 838–847 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.He X et al. Both conditional ablation and overexpression of E2 SUMO-conjugating enzyme (UBC9) in mouse pancreatic beta cells result in impaired beta cell function. Diabetologia 61, 881–895 (2018). [DOI] [PubMed] [Google Scholar]
- 78.Gheni G et al. Glutamate acts as a key signal linking glucose metabolism to incretin/cAMP action to amplify insulin secretion. Cell Rep. 9, 661–673 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maechler P & Wollheim CB Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature 402, 685–689 (1999). [DOI] [PubMed] [Google Scholar]
- 80.Vetterli L et al. Delineation of glutamate pathways and secretory responses in pancreatic islets with beta-cell-specific abrogation of the glutamate dehydrogenase. Mol. Biol. Cell 23, 3851–3862 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Capozzi ME et al. β Cell tone is defined by proglucagon peptides through cAMP signaling. JCI Insight 4, e126742 (2019).A comprehensive dataset showing the importance of proglucagon peptides for enabling normal regulation of insulin secretion.
- 82.Svendsen B et al. Insulin secretion depends on intra-islet glucagon signaling. Cell Rep. 25, 1127–1134 e1122 (2018). [DOI] [PubMed] [Google Scholar]
- 83.Zhu L et al. Intra-islet glucagon signaling is critical for maintaining glucose homeostasis. JCI Insight 5, e127994 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Capozzi ME et al. Glucagon lowers glycemia when β-cells are active. JCI Insight 5, e129954 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Le Marchand SJ & Piston DW Glucose suppression of glucagon secretion: metabolic and calcium responses from alpha-cells in intact mouse pancreatic islets. J. Biol. Chem 285, 14389–14398 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maruyama H, Hisatomi A, Orci L, Grodsky GM & Unger RH Insulin within islets is a physiologic glucagon release inhibitor. J. Clin. Invest 74, 2296–2299 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Muller WA, Faloona GR & Unger RH The effect of experimental insulin deficiency on glucagon secretion. J. Clin. Invest 50, 1992–1999 (1971).A classic paper defining a key role of insulin to regulate glucagon secretion.
- 88.Kawamori D et al. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab. 9, 350–361 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Egefjord L, Petersen AB & Rungby J Zinc, alpha cells and glucagon secretion. Curr. Diabetes Rev 6, 52–57 (2010). [DOI] [PubMed] [Google Scholar]
- 90.Li C et al. Regulation of glucagon secretion in normal and diabetic human islets by gamma-hydroxybutyrate and glycine. J. Biol. Chem 288, 3938–3951 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hardy AB, Serino AS, Wijesekara N, Chimienti F & Wheeler MB Regulation of glucagon secretion by zinc: lessons from the β cell-specific Znt8 knockout mouse model. Diabetes Obes. Metab. 13, 112–117 (2011). [DOI] [PubMed] [Google Scholar]
- 92.van der Meulen T et al. Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion. Nat. Med 21, 769–776 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.DiGruccio MR et al. Comprehensive alpha, beta and delta cell transcriptomes reveal that ghrelin selectively activates delta cells and promotes somatostatin release from pancreatic islets. Mol. Metab 5, 449–458 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van der Meulen T et al. Virgin beta cells persist throughout life at a neogenic niche within pancreatic islets. Cell Metab. 25, 911–926.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krentz NAJ & Gloyn AL Insights into pancreatic islet cell dysfunction from type 2 diabetes mellitus genetics. Nat. Rev. Endocrinol 16, 202–212 (2020).A comprehensive review of the genetics of T2D, with a focus on genes related to islet dysfunction.
- 96.Parker SC et al. Chromatin stretch enhancer states drive cell-specific gene regulation and harbor human disease risk variants. Proc. Natl Acad. Sci. USA 110, 17921–17926 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pasquali L et al. Pancreatic islet enhancer clusters enriched in type 2 diabetes risk-associated variants. Nat. Genet 46, 136–143 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thurner M et al. Integration of human pancreatic islet genomic data refines regulatory mechanisms at type 2 diabetes susceptibility loci. eLife 7, e31977 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Attie AD, Churchill GA & Nadeau JH How mice are indispensable for understanding obesity and diabetes genetics. Curr. Opin. Endocrinol. Diabetes Obes 24, 83–91 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Haldeman JM et al. Creation of versatile cloning platforms for transgene expression and dCas9-based epigenome editing. Nucleic Acids Res. 47, e23 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vinuela A et al. Influence of genetic variants on gene expression in human pancreatic islets – implications for type 2 diabetes. bioRxiv 10.1101/655670 (2019). [DOI] [Google Scholar]
- 102.Lawlor N et al. Single-cell transcriptomes identify human islet cell signatures and reveal cell-type-specific expression changes in type 2 diabetes. Genome Res. 27, 208–222 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Segerstolpe A et al. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab. 24, 593–607 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kirchhoff K et al. Polymorphisms in the TCF7L2, CDKAL1 and SLC30A8 genes are associated with impaired proinsulin conversion. Diabetologia 51, 597–601 (2008). [DOI] [PubMed] [Google Scholar]
- 105.Stancakova A et al. Association of 18 confirmed susceptibility loci for type 2 diabetes with indices of insulin release, proinsulin conversion, and insulin sensitivity in 5,327 nondiabetic Finnish men. Diabetes 58, 2129–2136 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tuomi T et al. Increased melatonin signaling is a risk factor for type 2 diabetes. Cell Metab. 23, 1067–1077 (2016). [DOI] [PubMed] [Google Scholar]
- 107.Abdallah BM, Ditzel N, Laborda J, Karsenty G & Kassem M DLK1 regulates whole-body glucose metabolism: a negative feedback regulation of the osteocalcin-insulin loop. Diabetes 64, 3069–3080 (2015). [DOI] [PubMed] [Google Scholar]
- 108.Chacon MR et al. Human serum levels of fetal antigen 1 (FA1/Dlk1) increase with obesity, are negatively associated with insulin sensitivity and modulate inflammation in vitro. Int. J. Obes 32, 1122–1129 (2008). [DOI] [PubMed] [Google Scholar]
- 109.Ostenson CG, Gaisano H, Sheu L, Tibell A & Bartfai T Impaired gene and protein expression of exocytotic soluble N-ethylmaleimide attachment protein receptor complex proteins in pancreatic islets of type 2 diabetic patients. Diabetes 55, 435–440 (2006). [DOI] [PubMed] [Google Scholar]
- 110.Arystarkhova E et al. Hyperplasia of pancreatic beta cells and improved glucose tolerance in mice deficient in the FXYD2 subunit of Na,K-ATPase. J. Biol. Chem 288, 7077–7085 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Solimena M et al. Systems biology of the IMIDIA biobank from organ donors and pancreatectomised patients defines a novel transcriptomic signature of islets from individuals with type 2 diabetes. Diabetologia 61, 641–657 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gerst F et al. The expression of Aldolase B in islets is negatively associated with insulin secretion in humans. J. Clin. Endocrinol. Metab 103, 4373–4383 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Keller MP et al. Gene loci associated with insulin secretion in islets from non-diabetic mice. J. Clin. Invest 129, 4419–4432 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bosma KJ et al. Pancreatic islet beta cell-specific deletion of G6pc2 reduces fasting blood glucose. J. Mol. Endocrinol 64, 235–248 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Accili D et al. When beta-cells fail: lessons from dedifferentiation. Diabetes Obes. Metab 18, 117–122 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hunter CS & Stein RW Evidence for loss in identity, de-differentiation, and trans-differentiation of islet beta-cells in type 2 diabetes. Front. Genet 8, 35 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Talchai C, Xuan S, Lin HV, Sussel L & Accili D Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell 150, 1223–1234 (2012).Pioneering paper introducing β-cell de-differentiation as a possible mechanism underlying β-cell failure in T2D.
- 118.Wang Z, York NW, Nichols CG & Remedi MS Pancreatic beta cell dedifferentiation in diabetes and redifferentiation following insulin therapy. Cell Metab. 19, 872–882 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cinti F et al. Evidence of beta-cell dedifferentiation in human type 2 diabetes. J. Clin. Endocrinol. Metab 101, 1044–1054 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Butler AE et al. β-cell deficit in obese type 2 diabetes, a minor role of β-cell dedifferentiation and degranulation. J. Clin. Endocrinol. Metab 101, 523–532 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sun J et al. β-cell dedifferentiation in patients with T2D with adequate glucose control and nondiabetic chronic pancreatitis. J. Clin. Endocrinol. Metab 104, 83–94 (2019). [DOI] [PubMed] [Google Scholar]
- 122.Wang YJ et al. Single-cell transcriptomics of the human endocrine pancreas. Diabetes 65, 3028–3038 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Poitout V et al. Glucolipotoxicity of the pancreatic beta cell. Biochim. Biophys. Acta 1801, 289–298 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Assimacopoulos-Jeannet F et al. Fatty acids rapidly induce the carnitine palmitoyltransferase I gene in the pancreatic beta-cell line INS-1. J. Biol. Chem 272, 1659–1664 (1997). [DOI] [PubMed] [Google Scholar]
- 125.Boucher A et al. Biochemical mechanism of lipid-induced impairment of glucose-stimulated insulin secretion and reversal with a malate analogue. J. Biol. Chem 279, 27263–27271 (2004). [DOI] [PubMed] [Google Scholar]
- 126.Jensen MV et al. Compensatory responses to pyruvate carboxylase suppression in islet beta-cells. Preservation of glucose-stimulated insulin secretion. J. Biol. Chem 281, 22342–22351 (2006). [DOI] [PubMed] [Google Scholar]
- 127.Imai Y, Dobrian AD, Morris MA, Taylor-Fishwick DA & Nadler JL Lipids and immunoinflammatory pathways of beta cell destruction. Diabetologia 59, 673–678 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee SJ, Kim SH, Park KM, Lee JH & Park JW Increased obesity resistance and insulin sensitivity in mice lacking the isocitrate dehydrogenase 2 gene. Free Radic. Biol. Med 99, 179–188 (2016). [DOI] [PubMed] [Google Scholar]
- 129.Haythorne E et al. Diabetes causes marked inhibition of mitochondrial metabolism in pancreatic beta-cells. Nat. Commun 10, 2474 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lu H, Koshkin V, Allister EM, Gyulkhandanyan AV & Wheeler MB Molecular and metabolic evidence for mitochondrial defects associated with beta-cell dysfunction in a mouse model of type 2 diabetes. Diabetes 59, 448–459 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Basile G, Kulkarni RN & Morgan NG How, when, and where do human beta-cells regenerate? Curr. Diab. Rep 19, 48 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Genevay M, Pontes H & Meda P Beta cell adaptation in pregnancy: a major difference between humans and rodents? Diabetologia 53, 2089–2092 (2010). [DOI] [PubMed] [Google Scholar]
- 133.Dor Y, Brown J, Martinez OI & Melton DA Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429, 41–46 (2004). [DOI] [PubMed] [Google Scholar]
- 134.Aguayo-Mazzucato C & Bonner-Weir S Pancreatic beta cell regeneration as a possible therapy for diabetes. Cell Metab. 27, 57–67 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Granger A & Kushner JA Cellular origins of beta-cell regeneration: a legacy view of historical controversies. J. Intern. Med 266, 325–338 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Karakose E, Ackeifi C, Wang P & Stewart AF Advances in drug discovery for human beta cell regeneration. Diabetologia 61, 1693–1699 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rajan S, Torres J, Thompson MS & Philipson LH SUMO downregulates GLP-1-stimulated caMp generation and insulin secretion. Am. J. Physiol. Endocrinol. Metab 302, E714–E723 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ferdaoussi M et al. Improved glucose tolerance with DPPIV inhibition requires beta-cell SENP1 amplification of glucose-stimulated insulin secretion. Physiol. Rep 8, e14420 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Harding HP et al. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol. Cell 7, 1153–1163 (2001). [DOI] [PubMed] [Google Scholar]
- 140.Scheuner D et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell 7, 1165–1176 (2001). [DOI] [PubMed] [Google Scholar]
- 141.Scheuner D et al. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat. Med 11, 757–764 (2005). [DOI] [PubMed] [Google Scholar]
- 142.Hull RL, Westermark GT, Westermark P & Kahn SE Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J. Clin. Endocrinol. Metab 89, 3629–3643 (2004). [DOI] [PubMed] [Google Scholar]
- 143.Zraika S et al. Toxic oligomers and islet beta cell death: guilty by association or convicted by circumstantial evidence? Diabetologia 53, 1046–1056 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mukherjee A, Morales-Scheihing D, Butler PC & Soto C Type 2 diabetes as a protein misfolding disease. Trends Mol. Med 21, 439–449 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Matveyenko AV & Butler PC Beta-cell deficit due to increased apoptosis in the human islet amyloid polypeptide transgenic (HIP) rat recapitulates the metabolic defects present in type 2 diabetes. Diabetes 55, 2106–2114 (2006). [DOI] [PubMed] [Google Scholar]
- 146.Montemurro C et al. IAPP toxicity activates HIF1alpha/PFKFB3 signaling delaying beta-cell loss at the expense of beta-cell function. Nat. Commun 10, 2679 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cheng S et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 125, 2222–2231 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Newgard CB et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 9, 311–326 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Campbell JE & Drucker DJ Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 17, 819–837 (2013). [DOI] [PubMed] [Google Scholar]
- 150.El K & Campbell JE The role of GIP in alpha-cells and glucagon secretion. Peptides 125, 170213 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nauck MA et al. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J. Clin. Invest 91, 301–307 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Matschinsky FM & Wilson DF The central role of glucokinase in glucose homeostasis: a perspective 50 years after demonstrating the presence of the enzyme in islets of langerhans. Front. Physiol 10, 148 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Osbak KK et al. Update on mutations in glucokinase (GCK), which cause maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemic hypoglycemia. Hum. Mutat 30, 1512–1526 (2009). [DOI] [PubMed] [Google Scholar]
- 154.Stanley CA Perspective on the genetics and diagnosis of congenital hyperinsulinism disorders. J. Clin. Endocrinol. Metab 101, 815–826 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pipatpolkai T, Usher S, Stansfeld PJ & Ashcroft FM New insights into KATP channel gene mutations and neonatal diabetes mellitus. Nat. Rev. Endocrinol 16, 378–393 (2020). [DOI] [PubMed] [Google Scholar]
- 156.Shimomura K & Maejima Y KATP channel mutations and neonatal diabetes. Intern. Med 56, 2387–2393 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Heimberg H, De Vos A, Pipeleers D, Thorens B & Schuit F Differences in glucose transporter gene expression between rat pancreatic alpha- and beta-cells are correlated to differences in glucose transport but not in glucose utilization. J. Biol. Chem 270, 8971–8975 (1995). [DOI] [PubMed] [Google Scholar]
- 158.Heimberg H et al. The glucose sensor protein glucokinase is expressed in glucagon-producing alpha-cells. Proc. Natl Acad. Sci. USA 93, 7036–7041 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Detimary P et al. The changes in adenine nucleotides measured in glucose-stimulated rodent islets occur in beta cells but not in alpha cells and are also observed in human islets. J. Biol. Chem 273, 33905–33908 (1998). [DOI] [PubMed] [Google Scholar]
- 160.Walker JN et al. Regulation of glucagon secretion by glucose: paracrine, intrinsic or both? Diabetes Obes. Metab 13, 95–105 (2011).A comprehensive review about the mechanisms regulating glucagon secretion.
- 161.Zhang Q et al. Role of KATP channels in glucose-regulated glucagon secretion and impaired counterregulation in type 2 diabetes. Cell Metab. 18, 871–882 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Marliss EB, Aoki TT, Unger RH, Soeldner JS & Cahill GF Jr. Glucagon levels and metabolic effects in fasting man. J. Clin. Invest 49, 2256–2270 (1970). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dean ED A primary role for alpha-cells as amino acid sensors. Diabetes 69, 542–549 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Finan B, Capozzi ME & Campbell JE Repositioning glucagon action in the physiology and pharmacology of diabetes. Diabetes 69, 532–541 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dean ED et al. Interrupted glucagon signaling reveals hepatic alpha cell axis and role for L-glutamine in alpha cell proliferation. Cell Metab. 25, 1362–1373.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Solloway MJ et al. Glucagon couples hepatic amino acid catabolism to mTOR-dependent regulation of alpha-cell mass. Cell Rep. 12, 495–510 (2015).A pioneering paper demonstrating a link between glucagon receptor activity, amino acid homeostasis, and α-cell mass and function.
- 167.Vergari E et al. Insulin inhibits glucagon release by SGLT2-induced stimulation of somatostatin secretion. Nat. Commun 10, 139 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Genuth S Should sulfonylureas remain an acceptable first-line add-on to metformin therapy in patients with type 2 diabetes? No, it’s time to move on! Diabetes Care 38, 170–175 (2015). [DOI] [PubMed] [Google Scholar]
- 169.Capozzi ME, DiMarchi RD, Tschop MH, Finan B & Campbell JE Targeting the incretin/glucagon system with triagonists to treat diabetes. Endocr. Rev 39, 719–738 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Day JW et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat. Chem. Biol 5, 749–757 (2009). [DOI] [PubMed] [Google Scholar]
- 171.Finan B et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat. Med 21, 27–36 (2015). [DOI] [PubMed] [Google Scholar]
- 172.Frias JP et al. The sustained effects of a dual GIP/GLP-1 receptor agonist, NNC0090-2746, in patients with type 2 diabetes. Cell Metab. 26, 343–352.e2 (2017). [DOI] [PubMed] [Google Scholar]
- 173.Frias JP et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 392, 2180–2193 (2018). [DOI] [PubMed] [Google Scholar]
- 174.Elahi D et al. The insulinotropic actions of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (7-37) in normal and diabetic subjects. Regul. Pept 51, 63–74 (1994). [DOI] [PubMed] [Google Scholar]
- 175.Nauck MA, Bartels E, Orskov C, Ebert R & Creutzfeldt W Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7-36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. J. Clin. Endocrinol. Metab 76, 912–917 (1993). [DOI] [PubMed] [Google Scholar]
- 176.Smith EP et al. The role of beta cell glucagon-like peptide-1 signaling in glucose regulation and response to diabetes drugs. Cell Metab. 19, 1050–1057 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Campbell JE et al. TCF1 links GIPR signaling to the control of beta cell function and survival. Nat. Med 22, 84–90 (2016). [DOI] [PubMed] [Google Scholar]
- 178.Zinman B et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med 373, 2117–2128 (2015). [DOI] [PubMed] [Google Scholar]
- 179.Ferrannini E et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J. Clin. Invest 124, 499–508 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hodson DJ & Rorsman P A variation on the theme: SGLT2 inhibition and glucagon secretion in human islets. Diabetes 69, 864–866 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Saponaro C et al. Interindividual heterogeneity of SGLT2 expression and function in human pancreatic islets. Diabetes 69, 902–914 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.