Abstract
BACKGROUND:
Intraoperative hypotension (IOH) occurs frequently during surgery and may be associated with organ ischemia; however, few multicenter studies report data regarding its associations with adverse postoperative outcomes across varying hemodynamic thresholds. Additionally, no study has evaluated the association between IOH exposure and adverse outcomes among patients by various age groups.
METHODS:
A multicenter retrospective cohort study was conducted between 2008 and 2017 using intraoperative blood pressure data from the US electronic health records database to examine postoperative outcomes. IOH was assessed in 368,222 noncardiac surgical procedures using 5 methods: (a) absolute maximum decrease in mean arterial pressure (MAP) during surgery, (b) time under each absolute threshold, (c) total area under each threshold, (d) time-weighted average MAP under each threshold, and (e) cumulative time under the prespecified relative MAP thresholds. MAP thresholds were defined by absolute limits (≤75, ≤65, ≤55 mm Hg) and by relative limits (20% and 40% lower than baseline). The primary outcome was major adverse cardiac or cerebrovascular events; secondary outcomes were all-cause 30- and 90-day mortality, 30-day acute myocardial injury, and 30-day acute ischemic stroke. Residual confounding was minimized by controlling for observable patient and surgical factors. In addition, we stratified patients into age subgroups (18–40, 41–50, 51–60, 61–70, 71–80, >80) to investigate how the association between hypotension and the likelihood of major adverse cardiac or cerebrovascular events and acute kidney injury differs in these age subgroups.
RESULTS:
IOH was common with at least 1 reading of MAP ≤75 mm Hg occurring in 39.5% (145,743) of cases; ≤65 mm Hg in 19.3% (70,938) of cases, and ≤55 mm Hg in 7.5% (27,473) of cases. IOH was significantly associated with the primary outcome for all age groups. For an absolute maximum decrease, the estimated odds of a major adverse cardiac or cerebrovascular events in the 30-day postsurgery was increased by 12% (95% confidence interval [CI], 11-14) for ≤75 mm Hg; 17.0% (95% CI, 15-19) for ≤65 mm Hg; and by 26.0% (95% CI, 22-29) for ≤55 mm Hg.
CONCLUSIONS:
IOH during noncardiac surgery is common and associated with increased 30-day major adverse cardiac or cerebrovascular events. This observation is magnified with increasing hypotension severity. The potentially avoidable nature of the hazard, and the extent of the exposed population, makes hypotension in the operating room a serious public health issue that should not be ignored for any age group.
KEY POINTS.
Question: Is there an association between intraoperative hypotension and postoperative major adverse cardiac or cerebrovascular events (MACCE)?
Findings: This multicenter retrospective analysis of 368,222 surgical procedures demonstrated that regardless of how hypotension exposure was defined (ie, mean arterial pressure [MAP] ≤75, ≤65, ≤55 mm Hg), intraoperative hypotension is significantly associated with MACCE in a “dose-dependent” manner.
Meaning: Intraoperative hypotension is a potentially avoidable hazard that appears to be associated with clinically significant morbidity regardless of age group; it remains to be demonstrated whether avoidance of hypotension reduces the incidence of MACCE, and if so, whether the way normotension is maintained matters.
More than 300 million noncardiac surgeries are performed worldwide annually.1 Intraoperative hypotension (IOH) occurs frequently during noncardiac surgery2,3 and may be associated with organ ischemia and mortality.4–8 Therefore, any relationship between hypotension and adverse outcomes would be of clinical importance because of the potentially modifiable nature of the hazard.
Several groups have documented a significant association between IOH and adverse postoperative outcomes.4,6–11 A recent systematic review evaluating 42 studies for an association between IOH and adverse outcomes after noncardiac surgery reported that end-organ injury occurs when the mean arterial pressure (MAP) is <80 mm Hg for prolonged periods (≥10 minutes) and <70 mm Hg for shorter durations, although no high-quality studies were found to demonstrate specific organ injury until a threshold of MAP <65 mm Hg.5 Additionally, the risk of end-organ dysfunction increases with progressively lower arterial blood pressure (BP).5,11–15 According to Walsh et al,7 as little as 1 minute of exposure to MAP <55 mm Hg is associated with myocardial injury, any cardiac complication, and kidney injury after noncardiac surgery.7 However, currently, there are few large, multicenter studies demonstrating an association between IOH with multiple thresholds and a wide range of outcomes.4,11
Furthermore, a recent study found a strong relationship between IOH exposure and noncardiac surgery–related acute kidney injury (AKI) in patients <60 years of age.16 However, another study found no independent association between varying age and postoperative AKI, without evaluating IOH exposure.17 Therefore, we wanted to understand how the association between IOH and the likelihood of major adverse cardiac or cerebrovascular events (MACCE) and AKI differs among various age groups.
The goal of this multicenter retrospective cohort study is to assess the association between IOH and the incidence of postoperative adverse events (AE) across multiple hemodynamic thresholds in patients undergoing noncardiac surgery. We hypothesized that IOH would be associated with postoperative 30-day MACCE, defined as a composite measure of all-cause mortality, acute myocardial infarction (AMI), or acute ischemic stroke (AIS), and that this association would be magnified with increasing hypotension severity.
METHODS
Data Source
Data were obtained from the Optum (Optum, Eden Prairie, MN) deidentified electronic health records (EHR) database, which standardizes/integrates US EHRs from >2000 hospitals and 7000 clinics. The data are sourced from both ambulatory and inpatient settings, and cover diagnosis/procedure codes, clinical observations (ie, vital signs), medications, and laboratory results. This study was determined exempt from Institutional Review Board (IRB) review by Western IRB (Puyallup, WA). A statistical analysis plan, submitted to Western IRB, was made before accessing the data. This article adheres to Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Study Cohort
A total of 621,482 noncardiac/noncesarian surgeries with ≥10 minutes of intraoperative MAP recordings at an interval of ≤5 minutes, allowing for less than or equal to two 5- to 10-minute gaps between MAP readings, were identified between January 1, 2008 and December 31, 2017 based on data availability. Both a-line and cuff readings were included. There were no patient age restrictions. All MAP values were calculated using the formula: [(2 × diastolic BP {DBP}) + systolic BP (SBP)]/3. Artifactual data were removed using previously published criteria: an invalid MAP value was defined as (a) SBP ≥300 or SBP ≤20 mm Hg, (b) SBP ≤DBP +5 mm Hg, or (c) DBP ≤5 mm Hg or DBP ≥225 mm Hg; abrupt changes were removed defined by SBP change ≥80 mm Hg within 1 minute in either direction or abrupt SBP changes ≥40 mm Hg within 2 minutes in both directions.8 The list of noncardiac surgeries were derived from procedures in the Center for Disease Control’s National Health care Safety Network Surgical Site Infection monitoring program, and surgeries were identified using the International Classification of Diseases (ICD) 9/10, and Healthcare Common Procedure Coding System codes.18,19
Patient procedures were excluded as follows. All patients were required to have ≥1 year of presurgical history and ≥1 touchpoint (or death) recorded in the year postindex surgery to establish baseline status and subsequent outcomes. For patients with multiple procedures within 30 days of each other, the last surgery was utilized as index procedure. In cases where qualifying surgeries were >30 days apart, all qualifying procedures were included for a patient. Procedures were excluded when (1) there were no MAP readings available in the 6 months presurgery; (2) admission and discharge were on the same day; (3) there was an invalid MAP value during the operative time; or (4) there was an invalid date of death (eg, death date/mo does not match date/mo of last follow-up visit).
Potential Confounding Variables
Patient characteristics including age, sex, race, geographic area, education level, and income were directly captured from the database. Comorbidities and procedures were identified in the year presurgery using ICD-9/10 and Current Procedural Terminology (CPT) codes (Supplemental Digital Content, Table 1, http://links.lww.com/AA/D218). Preexisting comorbidity was assessed using the Deyo adaptation of the Charlson Comorbidity Index.20 The use of antihypertensive agents in the year presurgery was captured from patient medication history. To adjust for patient status immediately before surgery and to determine new-onset outcomes, the following conditions were identified in the 7 days before index procedure (using appropriate ICD-9/10 codes): sepsis/systemic inflammatory response syndrome (SIRS; with/without acute organ damage), delirium, electrolyte disorders, AMI, ischemic stroke, and new-onset AKI. Preexisting dialysis or continuous renal replacement therapy (CRRT) was identified over 1 year before the index procedure via ICD-9/10 codes.
The use of antihypertensive agents in the 24 hours presurgery and at the care site from which the patient was admitted (ie, home, skilled nursing facility) were directly captured from patient records. Total surgery time was estimated from the duration of high-frequency MAPs with an interval of ≤5 minutes between readings. Time of surgery (day/night—defined as after 6 pm local time) and date (weekend/weekday) were included in the models to adjust for the association between surgical (or facility) factors on the likelihood of an AE. See Tables 1 and 2 for the full list of potential confounding variables utilized and variables used to assess new onset outcomes. A sensitivity analysis to assess the magnitude of an unobserved or unaccounted confounding effect for the absolute maximum decrease (AMD) exposure was performed by calculating E values, defined as the effect required to reduce the observed odds ratio (OR) for an outcome to 1.0 (Supplemental Digital Content, Table 2, http://links.lww.com/AA/D218).21
Table 1.
Patient Characteristics
| Patient Characteristics | Overall | IOH ≤55 mm Hg | IOH >55 mm Hg | IOH ≤65 mm Hg | IOH >65 mm Hg | IOH ≤75 mm Hg | IOH >75 mm Hg |
|---|---|---|---|---|---|---|---|
| (n = 368,222) | (n = 27,473) | (n = 340,749) | (n = 70,938) | (n = 297,284) | (n = 145,743) | (n = 222,479) | |
| Sex | |||||||
| Male | 141,528 (38%) | 10,402 (38%) | 131,126 (38%) | 25,874 (36%) | 115,654 (39%) | 51,725 (35%) | 89,803 (40%) |
| Female | 226,694 (62%) | 17,071 (62%) | 209,623 (62%) | 45,064 (64%) | 181,630 (61%) | 94,018 (65%) | 132,676 (60%) |
| Race | |||||||
| White | 302,248 (82%) | 22,555 (82%) | 279,693 (82%) | 58,948 (83%) | 243,300 (82%) | 122,331 (84%) | 179,917 (81%) |
| Asian | 2625 (1%) | 456 (2%) | 2169 (1%) | 955 (1%) | 1670 (1%) | 1440 (1%) | 1185 (1%) |
| Black | 33,880 (9%) | 1953 (7%) | 31,927(9%) | 4909 (7%) | 28,971 (10%) | 10,018 (7%) | 23,862 (11%) |
| Other/unknown | 29,469 (8%) | 2509 (9%) | 26,960 (8%) | 6126 (9%) | 23,343 (8%) | 11,954 (8%) | 17,515 (8%) |
| Age (y) | |||||||
| <40 | 44,106 (12%) | 4264 (16%) | 42,364 (12%) | 9265 (13%) | 37,773 (13%) | 18,525 (13%) | 25,581 (12%) |
| 40–49 | 45,011 (12%) | 2647 (10%) | 39,842 (12%) | 7238 (10%) | 34,481 (12%) | 15,952 (11%) | 29,059 (13%) |
| 50–59 | 75,057 (20%) | 4963 (18%) | 70,094 (21%) | 12,990 (18%) | 62,067 (21%) | 27,126 (19%) | 47,931 (22%) |
| 60–69 | 94,843 (26%) | 6869 (25%) | 87,974 (26%) | 18,486 (26%) | 76,357 (26%) | 38,107 (26%) | 56,736 (26%) |
| 70–79 | 70,304 (19%) | 5379 (20%) | 64,925 (19%) | 14,462 (20%) | 55,842 (19%) | 29,413 (20%) | 40,891 (18%) |
| 80+ | 38,901 (11%) | 3351 (12%) | 35,550 (10%) | 8497 (12%) | 30,404 (10%) | 16,620 (11%) | 22,281 (10%) |
| Zip-3 level median household income ($)a | |||||||
| Median (25th, 75th percentile) | 39,237 (35,981, 44,234) | 40,732 (39,005, 44,234) | 39,155 (35,981, 44,234) | 40,550 (37,614, 44,234) | 39,035 (35,981, 44,234) | 39,816 (36,555, 44,234) | 39,035 (35,981, 44,376) |
| Division | |||||||
| East North Central | 128,631 (35%) | 11,242 (41%) | 117,389 (34%) | 25,739 (36%) | 102,892 (35%) | 50,317 (35%) | 78,314 (35%) |
| East South Central | 8189 (2%) | 287 (1%) | 7902 (2%) | 1192 (2%) | 6997 (2%) | 3123 (2%) | 5066 (2%) |
| Mountain | 12,592 (3%) | 1049 (4%) | 11,543 (3%) | 2969 (4%) | 9623 (3%) | 5960 (4%) | 6632 (3%) |
| Middle Atlantic | 949 (0%) | 172 (1%) | 777 (0%) | 343 (0%) | 606 (0%) | 519 (0%) | 430 (0%) |
| New England | 4711 (1%) | 719 (3%) | 3992 (1%) | 1803 (3%) | 2908 (1%) | 2984 (2%) | 1727 (1%) |
| Pacific | 18,563 (5%) | 7493 (27%) | 11,070 (3%) | 13,798 (19%) | 4765 (2%) | 16,891 (12%) | 1672 (1%) |
| South Atlantic/West South Central | 139,566 (38%) | 3568 (13%) | 135,998 (40%) | 15,589 (22%) | 123,977 (42%) | 44,431 (30%) | 95,135 (43%) |
| West North Central | 46,214 (13%) | 2417 (9%) | 43,797 (13%) | 7987 (11%) | 38,227 (13%) | 18,178 (12%) | 28,036 (13%) |
| Other/unknown | 8807 (2%) | 526 (2%) | 8281 (2%) | 1518 (2%) | 7289 (2%) | 3340 (2%) | 5467 (2%) |
| Charlson Comorbidity Index | |||||||
| 0 | 159,261 (43%) | 10,471 (38%) | 148,790 (44%) | 28,443 (40%) | 130,818 (44%) | 62,327 (43%) | 96,934 (44%) |
| 1 | 66,154 (18%) | 4746 (17%) | 61,408 (18%) | 12,676 (18%) | 53,478 (18%) | 25,959 (18%) | 40,195 (18%) |
| 2 | 55,131 (15%) | 4306 (16%) | 50,825 (15%) | 10,634 (15%) | 44,497 (15%) | 21,243 (15%) | 33,888 (15%) |
| 3 | 30,371 (8%) | 2454 (9%) | 27,917 (8%) | 6069 (9%) | 24,302 (8%) | 12,042 (8%) | 18,329 (8%) |
| 4+ | 57,305 (16%) | 5496 (20%) | 51,809 (15%) | 13,116 (18%) | 44,189 (15%) | 24,172 (17%) | 33,133 (15%) |
| Surgery types (5 most common)b | |||||||
| Knee prosthesis | 68,281 (19%) | 2978 (11%) | 65,303 (19%) | 11,342 (16%) | 56,939 (19%) | 27,844 (19%) | 40,437 (18%) |
| Hip prosthesis | 38,160 (10%) | 3286 (12%) | 34,874 (10%) | 9122 (13%) | 29,038 (10%) | 18,671 (13%) | 19,489 (9%) |
| Open reduction of fracture | 20,584 (6%) | 1742 (6%) | 18,842 (6%) | 4064 (6%) | 16,520 (6%) | 8080 (6%) | 12,504 (6%) |
| Spinal fusion | 20,508 (6%) | 1228 (4%) | 19,280 (6%) | 3047 (4%) | 17,461 (6%) | 6380 (4%) | 14,128 (6%) |
| Gallbladder | 20,122 (5%) | 832 (3%) | 19,290 (6%) | 2462 (3%) | 17,660 (6%) | 6083 (4%) | 14,039 (6%) |
| Year of surgery | |||||||
| 2008–2011c | 19,472 (5%) | 1079 (4%) | 18,393 (5%) | 3115 (4%) | 16,357 (6%) | 7240 (5%) | 12,232 (6%) |
| 2012–2013 | 68,211 (19%) | 2664 (10%) | 65,547 (19%) | 8967 (13%) | 59,244 (20%) | 23,005 (16%) | 45,206 (20%) |
| 2014–2015 | 134,398 (37%) | 11,468 (42%) | 122,930 (36%) | 27,106 (38%) | 107,292 (36%) | 53,797 (37%) | 80,601 (36%) |
| 2016–2017 | 146,141 (40%) | 12,262 (45%) | 133,879 (39%) | 31,750 (45%) | 114,391 (38%) | 61,701 (42%) | 84,440 (38%) |
Due to rounding, categories will not always add to 100%.
Abbreviation: IOH, intraoperative hypotension.
aThe zip-3 level median household income is generated by Optum labs. The first 3 digits of the zip code usually designate a sectional center facility and are used in this case to map salary territories at a higher level.
bSurgery types were not used for model adjustment but were used to evaluate the cumulative incidence of intraoperative hypotension for common surgeries.
cFour years were combined due to small sample size in 2008 and 2009.
Table 2.
Patient Comorbidities and Additional Characteristics
| Patient Characteristics | Overall | IOH ≤55 mm Hg | IOH >55 mm Hg | IOH ≤65 mm Hg | IOH >65 mm Hg | IOH ≤75 mm Hg | IOH >75 mm Hg |
|---|---|---|---|---|---|---|---|
| (n = 368,222) | (n = 27,473) | (n = 340,749) | (n = 70,938) | (n = 297,284) | (n = 145,743) | (n = 222,479) | |
| Comorbidities | |||||||
| Myocardial infarction | 25,919 (7%) | 2483 (9%) | 23,436 (7%) | 5995 (8%) | 19,924 (7%) | 11,382 (8%) | 14,537 (7%) |
| Stroke | 30,113 (8%) | 2800 (10%) | 27,313 (8%) | 6756 (10%) | 23,357 (8%) | 12,795 (9%) | 17,318 (8%) |
| COPD | 92,002 (25%) | 7474 (27%) | 84,528 (25%) | 18,983 (27%) | 73,019 (25%) | 37,783 (26%) | 54,219 (24%) |
| Heart failure | 32,334 (9%) | 3429 (12%) | 28,905 (8%) | 8139 (11%) | 24,195 (8%) | 14,864 (10%) | 17,470 (8%) |
| Valvular heart disease | 35,129 (10%) | 3439 (13%) | 31,690 (9%) | 8148 (11%) | 26,981 (9%) | 15,194 (10%) | 19,935 (9%) |
| Pulmonary circulatory disorder | 11,730 (3%) | 1297 (5%) | 10,433 (3%) | 2887 (4%) | 8843 (3%) | 5308 (4%) | 6422 (3%) |
| Peripheral vascular disease | 40,749 (11%) | 4008 (15%) | 36,741 (11%) | 9774 (14%) | 30,975 (10%) | 18,134 (12%) | 22,615 (10%) |
| Hypertension | 230,482 (63%) | 16,880 (61%) | 213,602 (63%) | 43,905 (62%) | 186,577 (63%) | 88,770 (61%) | 141,712 (64%) |
| Paralysis | 6030 (2%) | 749 (3%) | 5281 (2%) | 1552 (2%) | 4478 (2%) | 2613 (2%) | 3417 (2%) |
| Diabetes | 92,799 (25%) | 7085 (26%) | 85,714 (25%) | 17,995 (25%) | 74,804 (25%) | 36,233 (25%) | 56,566 (25%) |
| Hypothyroidism | 61,713 (17%) | 4911 (18%) | 56,802 (17%) | 12,712 (18%) | 49,001 (16%) | 25,830 (18%) | 35,883 (16%) |
| Renal disease | 65,433 (18%) | 6490 (24%) | 58,943 (17%) | 15,297 (22%) | 50,136 (17%) | 28,087 (19%) | 37,346 (17%) |
| Liver disease | 28,790 (8%) | 2588 (9%) | 26,202 (8%) | 5997 (8%) | 22,793 (8%) | 11,216 (8%) | 17,574 (8%) |
| Lymphoma | 2924 (1%) | 280 (1%) | 2644 (1%) | 645 (1%) | 2279 (1%) | 1219 (1%) | 1705 (1%) |
| Solid tumor | 69,582 (19%) | 5960 (22%) | 63,622 (19%) | 13,997 (20%) | 55,585 (19%) | 26,640 (18%) | 42,942 (19%) |
| RA/connective tissue disease | 17,174 (5%) | 1249 (5%) | 15,925 (5%) | 3317 (5%) | 13,857 (5%) | 6814 (5%) | 10,360 (5%) |
| Coagulopathy | 7951 (2%) | 1082 (4%) | 6869 (2%) | 2233 (3%) | 5718 (2%) | 3767 (3%) | 4184 (2%) |
| Obesity | 106,905 (29%) | 7513 (27%) | 99,392 (29%) | 19,041 (27%) | 87,864 (30%) | 38,953 (27%) | 67,952 (31%) |
| Anemia | 128,775 (35%) | 11,336 (41%) | 117,439 (34%) | 27,844 (39%) | 100,931 (34%) | 54,641 (37%) | 74,134 (33%) |
| Alcohol abuse | 13,536 (4%) | 1218 (4%) | 12,318 (4%) | 2838 (4%) | 10,698 (4%) | 5212 (4%) | 8324 (4%) |
| Drug abuse | 17,453 (5%) | 1612 (6%) | 15,841 (5%) | 3768 (5%) | 13,685 (5%) | 6884 (5%) | 10,569 (5%) |
| Smoking | 69,048 (19%) | 5018 (18%) | 64,030 (19%) | 13,270 (19%) | 55,778 (19%) | 27,063 (19%) | 41,985 (19%) |
| Depression | 88,996 (24%) | 7150 (26%) | 81,846 (24%) | 17,672 (25%) | 71,324 (24%) | 35,654 (24%) | 53,342 (24%) |
| Sleep apnea | 56,316 (15%) | 4459 (16%) | 51,857 (15%) | 10,839 (15%) | 45,477 (15%) | 21,521 (15%) | 34,795 (16%) |
| Dementia | 7765 (2%) | 692 (3%) | 7073 (2%) | 1703 (2%) | 6062 (2%) | 3291 (2%) | 4474 (2%) |
| Home oxygen | 7610 (2%) | 924 (3%) | 6686 (2%) | 2083 (3%) | 5527 (2%) | 3645 (3%) | 3965 (2%) |
| Dialysis | 6149 (2%) | 908 (3%) | 5241 (2%) | 1914 (3%) | 4235 (1%) | 3076 (2%) | 3073 (1%) |
| Coronary artery bypass graft | 1609 (0%) | 221 (1%) | 1388 (0%) | 486 (1%) | 1123 (0%) | 779 (1%) | 830 (0%) |
| Percutaneous coronary intervention | 2167 (1%) | 220 (1%) | 1947 (1%) | 525 (1%) | 1642 (1%) | 988 (1%) | 1179 (1%) |
| Antihypertensive medication (year presurgery) | 296,965 (81%) | 20,607 (75%) | 276,358 (81%) | 54,298 (77%) | 242,667 (82%) | 113,150 (78%) | 183,815 (83%) |
| Antihypertensive medication (24 h presurgery) | 151,229 (41%) | 7938 (29%) | 143,291 (42%) | 21,778 (31%) | 129,451 (44%) | 50,048 (34%) | 101,181 (45%) |
| Within 7 d before surgery | |||||||
| Acute myocardial infarctiona | 3275 (1%) | 529 (2%) | 2746 (1%) | 1046 (1%) | 2229 (1%) | 1693 (1%) | 1582 (1%) |
| Acute ischemic strokea | 4269 (1%) | 431 (2%) | 3838 (1%) | 997 (1%) | 3272 (1%) | 1814 (1%) | 2455 (1%) |
| Acute kidney injury | 22,570 (6%) | 2825 (10%) | 19,745 (6%) | 6132 (9%) | 16,438 (6%) | 10,586 (7%) | 11,984 (5%) |
| Delirium | 6064 (2%) | 685 (2%) | 5379 (2%) | 1520 (2%) | 4544 (2%) | 2685 (2%) | 3379 (2%) |
| Electrolyte disorder | 58,010 (16%) | 6365 (23%) | 51,645 (15%) | 14,261 (20%) | 43,749 (15%) | 25,448 (17%) | 32,562 (15%) |
| Sepsis | 12,925 (4%) | 2293 (8%) | 10,632 (3%) | 4593 (6%) | 8332 (3%) | 7141 (5%) | 5784 (3%) |
| Median surgery length (min) | |||||||
| Median (25th, 75th percentile) | 30 (16, 60) | 97 (50, 158) | 29 (15, 55) | 70 (31, 127) | 25 (15, 49) | 45 (22, 92) | 25 (15, 45) |
| Date of surgery | |||||||
| Weekend | 61,132 (17%) | 5166 (19%) | 55,966 (16%) | 12,463 (18%) | 48,669 (16%) | 24,600 (17%) | 36,532 (16%) |
| Night | 23,562 (6%) | 1935 (7%) | 21,627 (6%) | 4579 (6%) | 18,983 (6%) | 9132 (6%) | 14,430 (6%) |
| Admitted from: | |||||||
| Home | 287,335 (78%) | 21,509 (78%) | 265,826 (78%) | 56,034 (79%) | 231,301 (78%) | 115,199 (79%) | 172,136 (77%) |
| Inpatient | 61,603 (17%) | 4779 (17%) | 56,824 (17%) | 11,876 (17%) | 49,727 (17%) | 23,711 (16%) | 37,892 (17%) |
| Skilled nursing facility | 6981 (2%) | 501 (2%) | 6480 (2%) | 1206 (2%) | 5775 (2%) | 2465 (2%) | 4516 (2%) |
| Unknown | 12,303 (3%) | 684 (2%) | 11,619 (3%) | 1822 (3%) | 10,481 (4%) | 4368 (3%) | 7935 (4%) |
Due to rounding, categories will not always add to 100%.
aThese variables were not used for model adjustment but were used to assess patient status to determine new onset outcomes.
Abbreviations: COPD, chronic obstructive pulmonary disease; IOH, intraoperative hypotension; RA, rheumatoid arthritis.
Primary and Secondary Outcomes
The primary outcome was 30-day MACCE, defined by the composite measure of all-cause mortality, AMI, or AIS1,22,23 (Supplemental Digital Content, Table 3, http://links.lww.com/AA/D218, for the incidence of outcomes). Death was captured from the Social Security Index while AMI and AIS were identified from ICD-9, and the corresponding ICD-10 codes identified utilizing an ICD-9 to ICD-10 medical code crosswalk24,25 and published literature. AMI was also captured with Clinical Classifications Software diagnosis code 100.
The following secondary outcomes were evaluated: all-cause 30- and 90-day mortality, 30-day AMI, and 30-day AIS. In the 7 days following surgery, AKI was determined by either (1) using ICD-9/10 codes for AKI or (2) evaluating if the highest postoperative creatinine (within 7 days) was >1.5-fold, or >0.3 mg/dL greater than the most recent preoperative value (urine output was not utilized). The rates of new-onset intermittent hemodialysis or CRRT, delirium, and sepsis/SIRS were assessed using ICD-9/10 or CPT codes. We determined hospital-free days in the first 30 days postsurgery using inpatient EHR; patients dying in the hospital had this variable coded as zero. Primary/secondary outcomes were not limited to in-hospital events.
Determining MAP Thresholds and Exposures
The closest available MAP value to surgery was selected as baseline based on a previous study (Supplemental Digital Content, Figure 1, http://links.lww.com/AA/D218, for distribution of baseline MAPs).26,27 Five thresholds were defined/chosen based on a review of published literature: 3 absolute (≤75, ≤65, ≤55 mm Hg) and 2 relative thresholds (20% and 40% lower than baseline).5 We selected metrics previously identified as the most consistent across different BP measures, thresholds, and outcomes:8,28 (a) AMD (maximum drop below specified MAP threshold) during surgery, (b) time spent under each absolute threshold value, (c) total area under each threshold-time plot, (d) time-weighted average MAP (TWA-MAP) under each threshold, and (e) the cumulative time under the prespecified relative MAP thresholds. Supplemental Digital Content, Figure 2, http://links.lww.com/AA/D218, summarizes how each hypotensive exposure was determined. The AMD exposure was chosen for the main figure for secondary outcomes because it is the easiest exposure to conceptualize/interpret and is commonly used to measure IOH exposure in the literature.5
IOH for the relevant threshold was defined by at least 1 MAP measurement below the threshold. All of the exposures and metrics were chosen a priori as primary exposures/metrics given the variability in IOH assessment in the published literature.2 A systematic review revealed that 140 different IOH definitions exist in terms of what threshold defines IOH and event duration. Depending on the applied definition, the incidence of IOH can vary significantly, between 5% and 99%.2
Statistical Analysis
We used χ2 tests to assess unadjusted associations between patients with hypotensive events at each threshold against those with no event (Tables 1 and 2). The incidence of hypotension was reported by surgery type.
Patients who had any record of an outcome (within 7 days for AIS, AMI, AKI, delirium, or sepsis; within 1 year for dialysis or CRRT) or a procedure within the 30 days presurgery were excluded from the corresponding outcome analyses. We performed a sensitivity analysis for MACCE using a 30-day exclusion for AMI and AIS. Model covariates were defined a priori based on clinical and operative factors that might affect the estimated odds of MACCE (Tables 1 and 2). Multivariable logistic regression models were used to estimate the independent association of hypotension with primary/secondary outcomes. To assess variability across the range of IOH exposures, we plotted the mean event rate across 20 BP bins and examined the results for substantive nonlinear trends. No significant nonlinear trends were identified, and therefore, we modeled hypotensive exposures as linear predictors with each unit being a 5-mm Hg increment. Model strength was evaluated by randomly dividing the data into 80/20 testing/validation sets and evaluating the resulting c statistics from the validation datasets to assess model fit.29
For nondeath outcomes, death could potentially be a competing risk. Therefore, we confirmed results using Fine-Gray competing risk models for models in which mortality was not an outcome.30 Poisson regression was used to examine the relationship between hypotensive exposures and hospital-free days in the perioperative period. Given that we examined 3 MAP thresholds for the primary analysis, the Bonferroni correction was used to adjust for multiple comparisons with a P value ≤.016 considered statistically significant.
Furthermore, to understand how the association between hypotension and the likelihood of MACCE or AKI differs among selected patient subgroups, we conducted conditional multivariable logistic regression stratified by key patient characteristics (sex, age, Charlson Comorbidity Index (CCI), surgery length, surgery type, year, time of day, and day of week), as shown in Supplemental Digital Content, Table 4, http://links.lww.com/AA/D218. Corresponding interaction P values for AMD and TWA-MAP for MACCE/AKI outcomes are provided in Supplemental Digital Content, Table 5, http://links.lww.com/AA/D218.
As a previous study found that the association between IOH and outcome may differ by age,16 we performed a post hoc analysis to evaluate the interaction between IOH and age for the MACCE/AKI outcomes (AMD/TWA exposures) at absolute MAP thresholds. In another analysis, we stratified patients by age groups (18–40, 41–50, 51–60, 61–70, 71–80, >80) and investigated the association between IOH and MACCE/AKI outcomes (AMD/TWA exposures). Since we examined 3 MAP thresholds and 6 age groups, the Bonferroni correction was used to adjust for multiple comparisons, with a P value ≤.0028 considered statistically significant; results were reported with 99.72% confidence intervals (CI) in Supplemental Digital Content, Table 7, http://links.lww.com/AA/D218. We utilized a linear regression model to examine the trend of the odds of MACCE and AKI outcomes by age group.
All analyses were performed in SAS version 9.4 (SAS Institute Inc, Cary, NC), with all statistical tests being 2 sided. Secondary end point variable analyses were considered exploratory and thus not subjected to multiple comparison correction.
Sample Size Considerations
A previous review of 10,581,621 hospitalizations in the United States (2004–2013) found that MACCE occurred in 3.0% of major noncardiac surgeries.1 We hypothesized at least a 3% difference in the rate of MACCE, which would require a sample size of 999 for a 95% CI and 90% power. Therefore, in our study with 621,482 procedures, we anticipate to distinguish a 0.2% difference in the primary outcome with 90% power at a 95% CI.
RESULTS
Study Cohort and Patient Characteristics
A total of 368,222 surgeries (330,827 distinct patients) met our inclusion/exclusion criteria (Figure 1).
Figure 1.
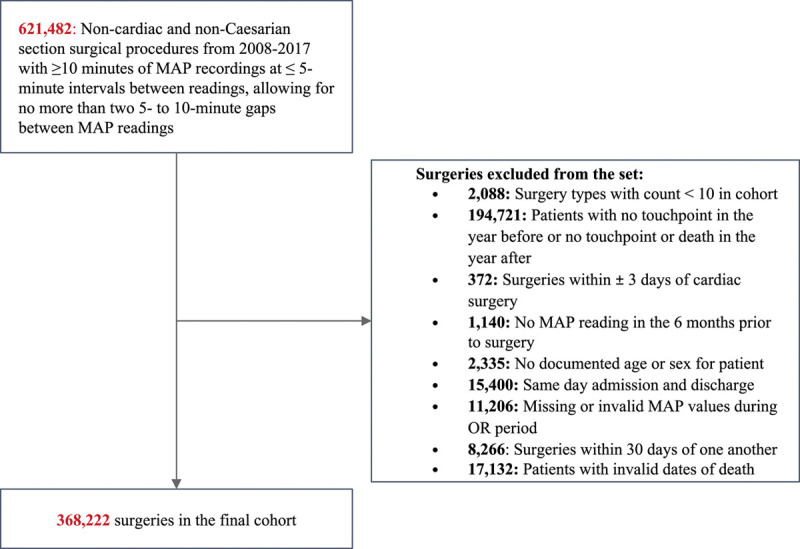
Attrition diagram. MAP indicates mean arterial pressure; OR, operating room.
The mean (±standard deviation [SD]) age of the patients was 60 ± 16.33 years (range, 1–89). A total of 98.5% (n = 363,439) of patients were ≥18 years of age. Of the study cohort, 82% were White (n = 302,248) and 62% were women (n = 226,694). The overall incidence of MACCE in the study population was 2.7%. The 30-day mortality rate was 1.7% and 90-day mortality was 2.5% (Supplemental Digital Content, Table 3, http://links.lww.com/AA/D218, for incidence of outcomes). Descriptive statistics comparing patients with hypotensive events to those with no event and the overall cohort are shown in Tables 1 and 2.
Association Between IOH and Outcomes
Overall, 19.3% of patients experienced a hypotensive event defined as at least 1 MAP ≤65 mm Hg reading. Supplemental Digital Content, Figure 3, http://links.lww.com/AA/D218, describes the incidence of IOH across the most common noncardiac surgeries performed during the study period.
The adjusted ORs with 95% CIs for the regression models with all exposure methods are depicted in Figure 2A, B. For AMD, every 5-mm Hg decrease in MAP ≤65 mm Hg is associated with an estimated 17% increase in odds of MACCE (95% CI, 15-19; Figure 2A) and in MAP ≤55 mm Hg, it is associated with an estimated 26% increase in the odds of MACCE (95% CI, 22-29).
Figure 2.
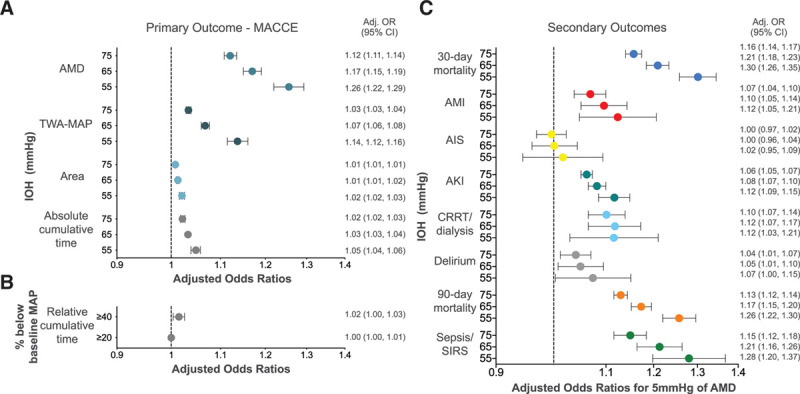
Adjusted ORs for the likelihood of adverse clinical events (A) for the primary outcome using all absolute exposure methods and (B) for the primary outcome using relative cumulative time (C) for the secondary outcomes for the AMD for each 5 mm Hg under IOH MAP thresholds. A, The adjusted ORs with 95% CIs for the regression models for MACCE for the following IOH exposures: AMD in MAP during surgery (maximum drop below specified MAP threshold), time spent under each absolute threshold value, total area under each threshold-time plot, TWA-MAP under each threshold during the study period are shown. B, The adjusted ORs with 95% CIs for the regression models for MACCE for the IOH exposure of cumulative time under the prespecified relative MAP thresholds during the study period are shown. C, The adjusted ORs with 95% CIs for the regression models for secondary outcomes with AMD in MAP during the study period are shown. Adj. indicates adjusted; AIS, acute ischemic stroke; AKI, acute kidney injury; AMD, absolute maximum decrease; AMI, acute myocardial infarction; CI, confidence interval; CRRT, continuous renal replacement therapy; IOH, intraoperative hypotension; MACCE, major adverse cardiac or cerebrovascular events; MAP, mean arterial pressure; OR, odds ratio; SIRS, systemic inflammatory response syndrome; TWA, time-weighted average.
The post hoc analysis revealed a significant interaction between IOH and age when evaluated for MACCE/AKI outcomes for MAP thresholds ≤75, ≤65, ≤55 mm Hg for AMD (P < .001, all thresholds) and TWA (P < .001 for ≤55 and ≤65-mm Hg thresholds; ≤75-mm Hg threshold: P < .008, MACCE; P < .005, AKI). A subgroup analysis showed an association between IOH and MACCE for all age groups when evaluated by AMD (Figure 3) and TWA-MAP.
Figure 3.
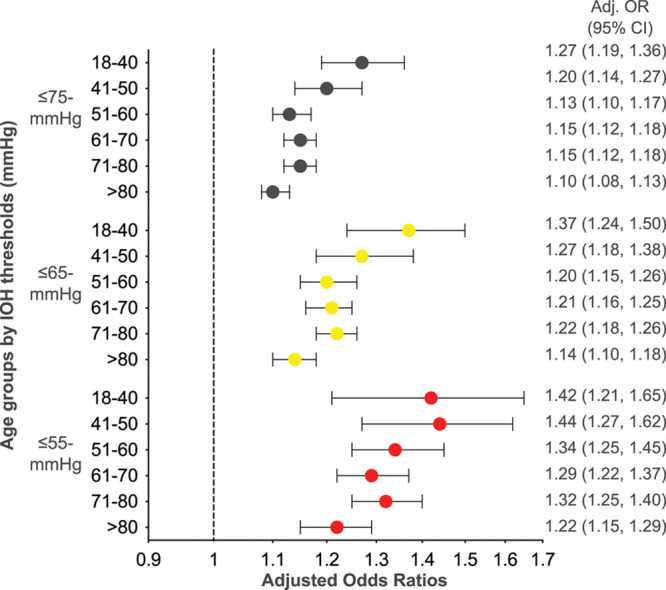
Adjusted ORs for the likelihood of MACCE for each 5 mm Hg under IOH MAP thresholds, by age group. The adjusted ORs with 95% CIs for the regression models with AMD in MAP during the study period are shown. ORs are plotted on the log-OR scale but labeled on the OR scale. Of note due to rounding some of the CIs appear to be asymmetrical or not visible. N for each age group by threshold: MAP ≤75 mm Hg: 18–40, N = 16,390; 41–50, N = 16,757; 51–60, N = 28,724; 61–70, N = 38,108; 71–80, N = 27,767; >80, N = 14,641; MAP ≤65 mm Hg: 18–40, N = 7466; 41–50, N = 7676; 51–60, N = 13,783; 61–70, N = 18,483; 71–80, N = 13,651; >80, N = 7553); MAP ≤55 mm Hg: 18–40, N = 2931; 41–50, N = 2843; 51–60, N = 5217; 61–70, N = 6866; 71–80, N = 5093; >80, N = 2994). Adj. indicates adjusted; AMD, absolute maximum decrease; CI, confidence interval; IOH, intraoperative hypotension; MACCE, major adverse cardiac or cerebrovascular events; MAP, mean arterial pressure; OR, odds ratio.
Descriptive statistics for the subgroup analysis and adjusted ORs, stratified by age group, for the association between IOH and MACCE/AKI outcomes evaluated by AMD and TWA-MAP metrics, are provided in Supplemental Digital Content, Tables 6–7, http://links.lww.com/AA/D218. The estimated odds for association of AMD-IOH with MACCE among adults age 18–40 was higher than for the oldest adults >80 years for both MAP ≤75-mm Hg (18–40 years: OR = 1.27, [99.72% CI, 1.16-1.39]; >80 years: OR = 1.10, [99.72% CI, 1.07-1.14]) and MAP ≤65-mm Hg thresholds (18–40 years: OR = 1.37, [99.72% CI, 1.18-1.58]; >80 years: OR = 1.14, [99.72% CI, 1.07-1.21]). Linear regression models demonstrated that the association between IOH and MACCE decreased as age increased (≤75 mm Hg P = .024, ≤65 mm Hg P = .020, ≤55 mm Hg P = .010); however, no association was found for AKI (≤75 mm Hg P = .387, ≤65 mm Hg P = .137, ≤55 mm Hg P = .095). Thus, when adjusted for covariates, the risk associated with IOH among the oldest patients (>80 years) is less than for patients 18–40 years, indicating that other factors likely affect MACCE events among older patients.
AMD below all MAP thresholds examined was associated with all secondary outcomes except for AIS (Figure 2C). Specifically, AMD below all hypotensive thresholds was correlated with 30- and 90-day mortality, perioperative AMI, AKI, CRRT, postoperative delirium, and sepsis/SIRS (Figure 2C). A sensitivity analysis incorporating 30-day exclusion criteria for AMI/AIS provided similar results (data not shown). E value results evaluating unmeasured confounders required to reduce the OR to 1.0 are located in Supplemental Digital Content, Table 2, http://links.lww.com/AA/D218. Calculated E values for AMD-MAP for significant primary and secondary outcomes ranged from 1.92 to 1.24 (MACCE ≤55-mm Hg threshold to delirium ≤75-mm Hg threshold).
Sensitivity analyses showed similar results using competing risk models for nonmortal secondary end points (data not shown) or using different IOH measurements including TWA-MAP, total area under each threshold-time plot, the time spent under each absolute threshold value, and cumulative time under the prespecified relative MAP thresholds (Supplemental Digital Content, Figure 4, http://links.lww.com/AA/D218).
For all absolute MAP thresholds, and under the 40% below baseline relative MAP threshold, IOH was associated with MACCE; P < .001 (Table 3). There was no significant association between time under the relative threshold of 20% below baseline MAP and MACCE (Table 3). Across all thresholds, AMD-IOH was associated with fewer hospital-free days (MAP ≤75 mm Hg, incidence rate ratio [IRR] 0.99 [95% CI, 0.99-0.99]; MAP ≤65 mm Hg, IRR 0.99 [95% CI, 0.98-0.99]; MAP ≤55 mm Hg, IRR 0.97 [95% CI, 0.97-0.98]).
Table 3.
ORs for IOH for Risk of MACCE
| IOH Threshold | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤55 mm Hg | ≤65 mm Hg | ≤75 mm Hg | ≥20% Below Baseline | ≥40% Below Baseline | ||||||
| Mean time under threshold (min) (SD) | 12 (24) | 22 (34) | 32 (44) | 32 (44) | 17 (27) | |||||
| Mean lowest MAP under threshold (SD) | 47 (10) | 55 (10) | 63 (10) | 67 (13) | 53 (10) | |||||
| Exposure Measures OR and P Values for MACCE | ||||||||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| AMD below specified MAP threshold (for 5 mm Hg) | 1.26 (1.22-1.29) | <.001 | 1.17 (1.15-1.19) | <.001 | 1.12 (1.11-1.14) | <.001 | ||||
| Area (per 5 mm Hg × 5 min) | 1.02 (1.02-1.03) | <.001 | 1.01 (1.01-1.02) | <.001 | 1.01 (1.01-1.01) | <.001 | ||||
| Cumulative time (per 5 min) | 1.05 (1.04-1.06) | <.001 | 1.03 (1.03-1.04) | <.001 | 1.02 (1.02-1.03) | <.001 | 1.00 (1.00-1.01) | .99 | 1.02 (1.00-1.03) | .01 |
| TWA-MAP (per 5 mm Hg) | 1.14 (1.12-1.16) | <.001 | 1.07 (1.06-1.08) | <.001 | 1.03 (1.03-1.04) | <.001 | ||||
Grey fields mean not applicable for this IOH threshold.
Abbreviations: AMD, absolute maximum decrease (below specified MAP threshold); CI, confidence interval; IOH, intraoperative hypotension; MACCE, major adverse cardiac or cerebrovascular events; MAP, mean arterial pressure; OR, odds ratio; SD, standard deviation; TWA, time-weighted average.
DISCUSSION
We report an association between hypotension during noncardiac surgery and increased occurrence of major clinical AEs, namely death, myocardial infarction, and stroke. We studied 368,222 noncardiac surgical procedures from the Optum EHR database, making this the largest multicenter retrospective cohort study of IOH outcomes to date, and analyzing a range of outcomes across multiple hemodynamic thresholds and definitions. The association between IOH and MACCE was assessed using 5 different methods, and all 5 methods demonstrated a consistent and significant association between IOH and 30-day MACCE (Table 3). There does not appear to be a safe duration of time for exposure to MAP ≤75 mm Hg. We also examined the associations between IOH and MACCE for varying age groups, which is novel to this study.
Our findings demonstrate that there is a progressive increased association for each absolute MAP threshold examined (≤75, ≤65, ≤55 mm Hg) for MACCE and 30- and 90-day mortality. Furthermore, with the exception of stroke, the estimated odds for the association of AMD thresholds were greater than unity for an increase in all secondary outcomes (only delirium at MAP ≤55-mm Hg threshold had a lower 95% CI value of 1.0). The apparent lack of a statistically significant association with AIS in our analysis is likely reflective of the low incidence (0.68%) in our population. Our study demonstrated an increased association between hypotensive events and delirium, which is in contrast to a previous study that examined a specific hypotensive anesthesia protocol where no significant association between IOH and delirium or cardiac/renal complication was found.31 One study showed no causality between IOH and 1-year mortality, except in the elderly; however, this is a longer timeframe than the 30- and 90-day mortality defined here.32 Our findings are consistent with a recent systematic review that investigated the association between IOH and postoperative outcomes, but which cautioned about interstudy differing patient characteristics and exposure definitions. The authors found that prolonged exposure (≥10 minutes) to MAP <80 mm Hg and for shorter durations, <70 mm Hg, was associated with mildly increased risk of end-organ injury.5 Increased time with MAP <65 mm Hg or any exposure to MAP <55 mm Hg was significantly associated with moderately- or highly-elevated postoperative risk.5 Previous analyses support our observation that even short periods of IOH below MAP thresholds of 55–65 mm Hg are associated with significant postoperative AEs. Futier et al33 recently reported results from a randomized trial that found that maintaining SBP within 10% of the reference value may prevent postoperative organ damage compared to standard care (treating SBP <80 mm Hg or <40% of the reference value). Taken together, our study and these data suggest that IOH has an important, and avoidable, association with postoperative 30-day MACCE.
We also show that IOH exposure is associated with postoperative MACCE and AKI across various age groups. In fact, higher adjusted ORs in the younger patient groups suggest that the estimated odds of AE associations of IOH exposure are as least as high as in older patients. Specifically, patients 18–40 years had a significantly higher estimated odds for MACCE associated with IOH exposure than patients >80 years of age. Tang et al16 also reported a strong relationship between IOH exposure and noncardiac surgery–related AKI in patients <60 years old. Mathis et al17 explored the relationship between varying age and AKI and found no independent association without evaluating exposure to IOH. To our knowledge, this is the first study that evaluated the association between IOH exposure and MACCE in various age groups. There may be 2 main reasons for lower ORs in older patients: (1) we controlled for various risk factors that are more likely to affect older patients (ie, Charlson Comorbidity Index), which may have led to a slightly less incremental risk, and (2) older patients may tolerate lower perfusion pressures better due to multiple, transient, brief episodes of ischemia (ischemic preconditioning) over their lifespan compared to younger patients.34 Overall, our findings clearly show that IOH matters in all patients, old or young, and, therefore, should not be ignored in any age group.
We also characterized IOH exposure by cumulative time beneath relative MAP thresholds and found an association between MACCE and time spent with MAP ≥40% below the baseline; however, the relative thresholds were less sensitive in detecting an association with MACCE compared to absolute thresholds. The relationship of IOH with postoperative outcomes regarding relative decreases from baseline BP in chronically hypertensive patients is debated among anesthesiologists.35 Salmasi et al8 found that IOH (MAP <65 mm Hg) was associated with myocardial or kidney injury in noncardiac surgery patients, and also performed a detailed comparison between absolute (MAP <65 mm Hg) and relative (decrease of >25% below preoperative baseline) thresholds and demonstrated no advantage to using relative over absolute thresholds. Absolute thresholds provide multiple advantages: (1) baseline BP measurements are frequently unknown (in a study by Monk et al,4 31% of patients did not have a baseline BP measurement recorded); (2) absolute thresholds are easier to incorporate into a decision-making framework that may not have access to preoperative BP measurements for individual patients; and (3) preoperative readings are often elevated due to patients experiencing anxiety presurgery. This may explain our finding that a reduction of 40% below baseline is more likely to represent a true effect, while a 20% decrease from the preoperative readings might be caused by a temporary increase in BP immediately presurgery.
This study has several limitations. While the dataset contains data from centers throughout the United States, it is relatively under-representative of the geographical West and Northeast regions. Additionally, race distribution does not reflect US national demographics. We were limited to estimating surgical duration by using the time period in which high-frequency MAP readings were available. Therefore, we conducted sensitivity analyses to confirm the consistency of our findings across different surgery durations. Although we only included MAP readings with intervals of <5 minutes, allowing a maximum of 2 gaps of 5–10 minutes between MAP readings, we did not lose significant fidelity of measurement. Furthermore, a single preoperative MAP reading might not be representative of the true patient baseline BP. We captured outcomes using administrative codes, which may not represent the true number of events. However, we removed patients with an immediate prior history of key outcomes to increase the likelihood of capturing new-onset AEs; moreover, we provided a sensitivity analysis with a longer timeline for the AMI and AIS outcomes. Due to database limitations, we could not capture and verify the cause of death. The postoperative use of antihypertensives was not evaluated. Specific diagnosis criteria for health conditions may be variable across/within institutions. The risk of individual site biases in reporting was likely mitigated by our sample size, and our event rate for the primary outcome is consistent with previous publications in noncardiac surgery. We utilized a Bonferroni correction of P ≤ .0028 and 99.72% CI for the likelihood of MACCE and AKI with hypotension stratified by age which corresponded to our 3 MAP thresholds and 6 age groups, but did not take into account the number of outcomes or the number of exposures evaluated (ie, AMD and TWA). Finally, this is an observational, retrospective study, for which exists the risk of residual confounding and unobservable bias, although we have attempted to control for observable patient and surgical factors and provided E values for unobserved confounding (provides the strength of association that an unmeasured confounder must have to reduce the observed association to unity). Calculated E values for MACCE were 1.83–1.49 (≤55–≤75-mm Hg threshold), indicating that an unmeasured confounder with this association strength would be required to change our conclusion. This study can only comment on the association between IOH and outcomes, which highlights the need for further prospective research to investigate the associations between reducing patient exposure to IOH and AEs.
In conclusion, we report an association between IOH and increased MACCE. The relationship is “dose-dependent,” with no apparent safe period of hypotension. Given the potentially avoidable nature of the hazard and the extent of the exposed population, we believe hypotension in the operating room is a serious public health issue, and should not be ignored in any age group. We suggest there is an urgent and currently unmet need for prospective interventional studies focused on its prevention.
ACKNOWLEDGMENTS
The authors thank Sibyl H. Munson, PhD, Halit O. Yapici, MD, MPH, MBA, and Francie Moehring, PhD, at Boston Strategic Partners, Boston, MA (supported by Edwards Lifesciences) for editorial support.
DISCLOSURES
Name: Anne Gregory, MD, MSc, FRCPC.
Contribution: This author helped design the study, supervise the data collection, analyze the data, and prepare the manuscript.
Conflicts of Interest: None.
Name: Wolf H. Stapelfeldt, MD.
Contribution: This author helped design the study, supervise the data collection, analyze the data, and prepare the manuscript.
Conflicts of Interest: W. H. Stapelfeldt received consulting fees from Edwards Lifesciences.
Name: Ashish K. Khanna, MD, FCCP, FCCM.
Contribution: This author helped design the study, supervise the data collection, analyze the data, and prepare the manuscript.
Conflicts of Interest: A. K. Khanna received consulting fees from Edwards Lifesciences and is supported by a NIH/NCATS Wake Forest University CTSI KL2 award TR001421 for a pilot trial of continuous portable postoperative hemodynamic and saturation monitoring on the hospital ward.
Name: Nathan J. Smischney, MD, MSc.
Contribution: This author helped design the study, supervise the data collection, analyze the data, and prepare the manuscript.
Conflicts of Interest: N. J. Smischney received consulting fees from Edwards Lifesciences.
Name: Isabel J. Boero, MD, MS.
Contribution: This author helped design the study, supervise the data collection, analyze the data, and prepare the manuscript.
Conflicts of Interest: I. J. Boero is an employee of Boston Consulting Group, who received funds from Edwards Lifesciences to perform the research.
Name: Qinyu Chen, MS.
Contribution: This author helped analyze the data and prepare the manuscript.
Conflicts of Interest: Q. Chen is an employee of Boston Consulting Group, who received funds from Edwards Lifesciences to perform the research.
Name: Mitali Stevens, PharmD, BCPS.
Contribution: This author helped analyze the data and prepare the manuscript.
Conflicts of Interest: M. Stevens is an employee of Edwards Lifesciences.
Name: Andrew D. Shaw, MB, FRCPC.
Contribution: This author helped design the study, supervise the data collection, analyze the data, and prepare the manuscript.
Conflicts of Interest: A. D. Shaw received consulting fees from Edwards Lifesciences.
This manuscript was handled by: Tong J. Gan, MD.
Supplementary Material
GLOSSARY
- AE
- adverse event
- AIS
- acute ischemic stroke
- AKI
- acute kidney injury
- AMD
- absolute maximum decrease
- AMI
- acute myocardial infarction
- BP
- blood pressure
- CCI
- Charlson Comorbidity Index
- CI
- confidence interval
- CPT
- Current Procedural Terminology
- CRRT
- continuous renal replacement therapy
- DBP
- diastolic BP
- EHR
- electronic health records
- HR
- hazard ratio
- ICD
- International Classification of Diseases
- IOH
- intraoperative hypotension
- IRB
- = institutional review board
- IRR
- = incidence rate ratio;
- MACCE
- major adverse cardiac or cerebrovascular events
- MAP
- mean arterial pressure
- OR
- odds ratio
- SBP
- systolic BP
- SD
- standard deviation
- SIRS
- sepsis/systemic inflammatory response syndrome
- STROBE
- = Strengthening the Reporting of Observational Studies in Epidemiology
- TWA
- time-weighted average
Funding: Funding for this research was provided by Edwards Lifesciences.
Conflicts of Interest: See Disclosures at the end of the article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website.
Reprints will not be available from the authors.
REFERENCES
- 1.Smilowitz NR, Gupta N, Ramakrishna H, Guo Y, Berger JS, Bangalore S. Perioperative major adverse cardiovascular and cerebrovascular events associated with noncardiac surgery. JAMA Cardiol. 2017;2:181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bijker JB, van Klei WA, Kappen TH, van Wolfswinkel L, Moons KG, Kalkman CJ. Incidence of intraoperative hypotension as a function of the chosen definition: literature definitions applied to a retrospective cohort using automated data collection. Anesthesiology. 2007;107:213–220. [DOI] [PubMed] [Google Scholar]
- 3.Seeling M, Papkalla N, Radtke F, Franck M, Spies C. Incidence of intraoperative hypotension during anaesthesia varies greatly with the chosen definition: 1AP1-5. Eur J Anaesthesiol. 2008;25:8.17892613 [Google Scholar]
- 4.Monk TG, Bronsert MR, Henderson WG, et al. Association between intraoperative hypotension and hypertension and 30-day postoperative mortality in noncardiac surgery. Anesthesiology. 2015;123:307–319. [DOI] [PubMed] [Google Scholar]
- 5.Wesselink EM, Kappen TH, Torn HM, Slooter AJC, van Klei WA. Intraoperative hypotension and the risk of postoperative adverse outcomes: a systematic review. Br J Anaesth. 2018;121:706–721. [DOI] [PubMed] [Google Scholar]
- 6.Mascha EJ, Yang D, Weiss S, Sessler DI. Intraoperative mean arterial pressure variability and 30-day mortality in patients having noncardiac surgery. Anesthesiology. 2015;123:79–91. [DOI] [PubMed] [Google Scholar]
- 7.Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119:507–515. [DOI] [PubMed] [Google Scholar]
- 8.Salmasi V, Maheshwari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 2017;126:47–65. [DOI] [PubMed] [Google Scholar]
- 9.Bijker JB, Persoon S, Peelen LM, et al. Intraoperative hypotension and perioperative ischemic stroke after general surgery: a nested case-control study. Anesthesiology. 2012;116:658–664. [DOI] [PubMed] [Google Scholar]
- 10.Sun LY, Wijeysundera DN, Tait GA, Beattie WS. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123:515–523. [DOI] [PubMed] [Google Scholar]
- 11.Stapelfeldt WH, Yuan H, Dryden JK, et al. The SLUScore: a novel method for detecting hazardous hypotension in adult patients undergoing noncardiac surgical procedures. Anesth Analg. 2017;124:1135–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brinkman R, HayGlass KT, Mutch WA, Funk DJ. Acute kidney injury in patients undergoing open abdominal aortic aneurysm repair: a pilot observational trial. J Cardiothorac Vasc Anesth. 2015;29:1212–1219. [DOI] [PubMed] [Google Scholar]
- 13.Sirivatanauksorn Y, Parakonthun T, Premasathian N, et al. Renal dysfunction after orthotopic liver transplantation. Transplant Proc. 2014;46:818–821. [DOI] [PubMed] [Google Scholar]
- 14.Hallqvist L, Mårtensson J, Granath F, Sahlén A, Bell M. Intraoperative hypotension is associated with myocardial damage in noncardiac surgery: an observational study. Eur J Anaesthesiol. 2016;33:450–456. [DOI] [PubMed] [Google Scholar]
- 15.Sessler DI, Meyhoff CS, Zimmerman NM, et al. Period-dependent associations between hypotension during and for four days after noncardiac surgery and a composite of myocardial infarction and death: a substudy of the POISE-2 trial. Anesthesiology. 2018;128:317–327. [DOI] [PubMed] [Google Scholar]
- 16.Tang Y, Zhu C, Liu J, et al. Association of intraoperative hypotension with acute kidney injury after noncardiac surgery in patients younger than 60 years old. Kidney Blood Press Res. 2019;44:211–221. [DOI] [PubMed] [Google Scholar]
- 17.Mathis MR, Naik BI, Freundlich RE, et al. ; Multicenter Perioperative Outcomes Group Investigators. Preoperative risk and the association between hypotension and postoperative acute kidney injury. Anesthesiology. 2020;132:461–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. 2019 NHSN Operative Procedure Code Mappings 2019. Available at: https://www.cdc.gov/nhsn/xls/icd10-pcspcm-nhsn-opc.xlsx. Accessed May 8, 2019.
- 19.Centers for Disease Control and Prevention. ICD-10-PCS & CPT Codes - Guidance for HPRO & KPRO Procedure Details. 2019. Available at: https://www.cdc.gov/nhsn/xls/guidance-for-hpro-kpro-procedure-details. Accessed May 8, 2019.
- 20.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 21.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167:268–274. [DOI] [PubMed] [Google Scholar]
- 22.Xia J, Xu J, Li B, et al. Association between glycemic variability and major adverse cardiovascular and cerebrovascular events (MACCE) in patients with acute coronary syndrome during 30-day follow-up. Clin Chim Acta. 2017;466:162–166. [DOI] [PubMed] [Google Scholar]
- 23.Ham SY, Song SW, Nam SB, Park SJ, Kim S, Song Y. Effects of chronic statin use on 30-day major adverse cardiac and cerebrovascular events after thoracic endovascular aortic repair. J Cardiovasc Surg (Torino). 2018;59:836–843. [DOI] [PubMed] [Google Scholar]
- 24.ICD10data.com. Convert ICD-9-CM Codes to ICD-10-CM/PCS. Available at: https://www.icd10data.com/Convert. Published 2018. Accessed March 1, 2019.
- 25.National Cancer Institute SEER. ICD-9-CM to ICD-10-CM Based on FY2017 ICD-10-CM codes. 2016.
- 26.Smischney NJ, Hoskote SS, Gallo de Moraes A, et al. Ketamine/propofol admixture (ketofol) at induction in the critically ill against etomidate (KEEP PACE trial): study protocol for a randomized controlled trial. Trials. 2015;16:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smischney NJ, Nicholson WT, Brown DR, et al. Ketamine/propofol admixture vs etomidate for intubation in the critically ill: KEEP PACE randomized clinical trial. J Trauma Acute Care Surg. 2019;87:883–891. [DOI] [PubMed] [Google Scholar]
- 28.Vernooij LM, van Klei WA, Machina M, Pasma W, Beattie WS, Peelen LM. Different methods of modelling intraoperative hypotension and their association with postoperative complications in patients undergoing non-cardiac surgery. Br J Anaesth. 2018;120:1080–1089. [DOI] [PubMed] [Google Scholar]
- 29.Lever J, Krzywinski M, Altman N. Model selection and overfitting. Nat Methods. 2016;13:703. [Google Scholar]
- 30.Khanna AK, Maheshwari K, Mao G, et al. Association between mean arterial pressure and acute kidney injury and a composite of myocardial injury and mortality in postoperative critically ill patients: a retrospective cohort analysis. Crit Care Med. 2019;47:910–917. [DOI] [PubMed] [Google Scholar]
- 31.Williams-Russo P, Sharrock NE, Mattis S, et al. Randomized trial of hypotensive epidural anesthesia in older adults. Anesthesiology. 1999;91:926–935. [DOI] [PubMed] [Google Scholar]
- 32.Bijker JB, van Klei WA, Vergouwe Y, et al. Intraoperative hypotension and 1-year mortality after noncardiac surgery. Anesthesiology. 2009;111:1217–1226. [DOI] [PubMed] [Google Scholar]
- 33.Futier E, Lefrant J-Y, Guinot P-G, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA. 2017;318:1346–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gassanov N, Nia AM, Caglayan E, Er F. Remote ischemic preconditioning and renoprotection: from myth to a novel therapeutic option? J Am Soc Nephrol. 2014;25:216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu X, Jiang Z, Ying J, Han Y, Chen Z. Optimal blood pressure decreases acute kidney injury after gastrointestinal surgery in elderly hypertensive patients: a randomized study: optimal blood pressure reduces acute kidney injury. J Clin Anesth. 2017;43:77–83. [DOI] [PubMed] [Google Scholar]


