ABSTRACT
To date, there is no consensus regarding palivizumab prophylaxis for respiratory syncytial virus infection. The purpose of this study is to assess the effectiveness of palivizumab prophylaxis to prevent respiratory syncytial virus-related infection consultations and hospitalizations in high-risk children <2 y. We studied children <2 y of age with risk factors who had indication of palivizumab prophylaxis over eight epidemic seasons (2011–2012 to 2018–2019) in Navarra, Spain. Children positives for respiratory syncytial virus by reverse-transcription polymerase chain reaction were compared to negative testers. Palivizumab was indicated in 1,214 children <2 y of age with risk factors during 2011–2012 to 2018–2019 seasons. A total of 142 high-risk children tested for respiratory syncytial virus were included in the study. From the 35 respiratory syncytial virus-positive confirmed cases, 20 (57%) had received palivizumab versus 82 (77%) from the 107 negative controls. The effectiveness of prophylactic palivizumab was 70% (95% CI, 19%-90%) in preventing confirmed clinical infection and 82% (95% CI, 29%-96%) in preventing hospitalized cases. Our results show that palivizumab is notably effective for preventing laboratory-confirmed cases of respiratory syncytial virus and hospitalization in high-risk children <2 y of age. For children who have received palivizumab, the risk of getting sick remains high; thus, other preventive measures are necessary.
KEYWORDS: Respiratory syncytial virus, palivizumab, effectiveness, test-negative design, monoclonal antibody
Introduction
Lower respiratory tract infection caused by the respiratory syncytial virus (RSV) is the leading cause of hospitalization among children <1 y of age.1 The RSV may cause severe respiratory infections and death, mainly in children <6 months with serious comorbidities.2,3 To date, no vaccine against RSV is available,4 nor there is any specific treatment,5 thus, the clinical management of patients is limited to support measures.6
Prophylactic palivizumab immunization (Synagis®, AbbVie Deutschland GmbH & Co. KG, Knollstrasse, Germany) is recommended for children <2 y with risk factors to prevent RSV-related lower respiratory tract infections.7 Palivizumab is a monoclonal antibody (IgG1) against an epitope in the II antigenic site of the F protein of RSV. It prevents virus-cell membrane fusion and syncytium formation; this way limiting virus spread.8
The IMpact clinical trial showed that palivizumab prophylaxis reduced the rate of hospitalization due to RSV to 5.8% (55% relative reduction) in comparison to the placebo group. Efficacy was higher in preterm infants <35 weeks without bronchopulmonary dysplasia (78% relative reduction in RSV hospitalizations), than infants with bronchopulmonary dysplasia (39% relative reduction).9 In a subsequent trial, prophylactic palivizumab immunization in children with congenital heart disease was associated with 45% relative reduction in RSV hospitalizations.10
In 2014, the American Academy of Pediatrics updated the indications for prophylactic palivizumab immunization.11 In Spain, indications were reviewed in 201512 and 2019.13 Currently, palivizumab is recommended in children born prematurely or presenting bronchopulmonary dysplasia, congenital respiratory malformations, congenital heart and vascular anomalies, cystic fibrosis, neuromuscular disorders, Down’s syndrome, immunodeficiency, congenital and metabolic disorders, and a history of low birth weight.
It is recommended to start prophylactic palivizumab immunization at the beginning of the RSV season with the administration of a monthly dose with up to five doses during the epidemic season. Indications between countries remain controversial.
Rapid antigen tests for RSV were used for diagnostic confirmation in both clinical trials,9,10 but this test has shown a modest sensitivity (81%) for the detection of RSV as compared with molecular techniques, such as reverse-transcription polymerase-chain-reaction (RT-PCR).14,15 Measurement biases due to lack of sensitivity of the diagnostic test used could influence the results of these clinical trials, reducing the estimated efficacy of palivizumab.16
Besides under ideal clinical trial conditions, very few effectiveness studies on palivizumab have been carried out in real-life conditions.17 The purpose of the current study was to estimate the effectiveness of palivizumab in preventing consultations and hospitalizations due to RT-PCR-confirmed RSV in high-risk children <2 y of age.
Materials and methods
The study was carried out in a tertiary university hospital in the North of Spain throughout eight epidemiological seasons spanning 2011–2012 to 2018–2019.18 The study population was composed of children <2 y of age born prematurely or with comorbidities leading to high-risk of RSV complications that were monitored at the hospital and had an indication of prophylactic palivizumab immunization. Palivizumab was only administered at hospital and all doses were registered.
Following routine clinical practice for detection of RSV, a nasopharyngeal swab was collected from children who attended consultation with respiratory symptoms. The presence of RSV and influenza virus in a sample was determined by reverse-transcription polymerase-chain-reaction (RT-PCR) (RealCycler FLURSV®, Progenie Molecular). Subgroup identification (A or B) was performed for positive RSV samples (RSV RT-PCR kit 1.0 RealStar®, Altona Diagnostics). When immediate analysis was not possible, swab samples were kept at 4–8 ºC.
A test-negative case–control design was performed that compared RSV-positive (cases) and RSV-negative children (controls). The study included patients tested between October 1 and April 30 of each season. Only subjects who had indication for palivizumab prophylaxis and who were swabbed within a maximum of 7 days following the beginning of the symptoms were included in the study.
Palivizumab immunization dates and doses for cases and controls were obtained in the hospital’s registry of administered drugs. Compliance was considered adequate when the number of administered doses corresponded with the recommended ones, based on the date of initiation of prophylactic immunization over the course of RSV epidemic period. The appropriate dosing interval was considered to be 28–30 days.8
Fisher’s exact test and the Chi-square test were used for comparing proportions. Cases and controls for the same calendar month were compared. Only months for which there were confirmed cases of RSV were considered. Exact logistic regression was used to calculate the odds ratio (OR) adjusted by age group (0–5 months, 6–11 months, 12–23 months) and by type of healthcare (outpatient or hospitalization), with 95% confidence interval (CI). P values <.05 were considered significant. The effectiveness of prophylactic immunization was calculated as percentages based on the following equation (1 – OR) x 100.
The main analysis included all children that fulfilled the inclusion criteria. In separate analyses, we also estimated the effect of palivizumab in different subgroups of patients. Children who have received palivizumab in the previous 30 days were compared with those who had not received palivizumab; cases of each RSV subgroup were compared with the RSV-negative controls; and hospitalized positive cases were compared with hospitalized RSV-negative controls.
The study was approved by the Navarre’s Clinical Research Ethics Committee (160629 Pyto 2016/42).
Results
Prophylactic palivizumab immunization was indicated in 1,214 children <2 years of age with risk factors during the 2011–2012 to 2018–2019 seasons. One hundred and forty-two of this group of children attended consultation due to acute respiratory infection and were tested for detection of RSV by RT-PCR, and were thus included in the study. Thirty-five children (25%) were RSV-positive cases and 107 were RSV-negative controls (Figure 1). RSV subgroup A was identified in 17 cases and subgroup B in 16 cases. The subgroup could not be determined for two cases.
Figure 1.
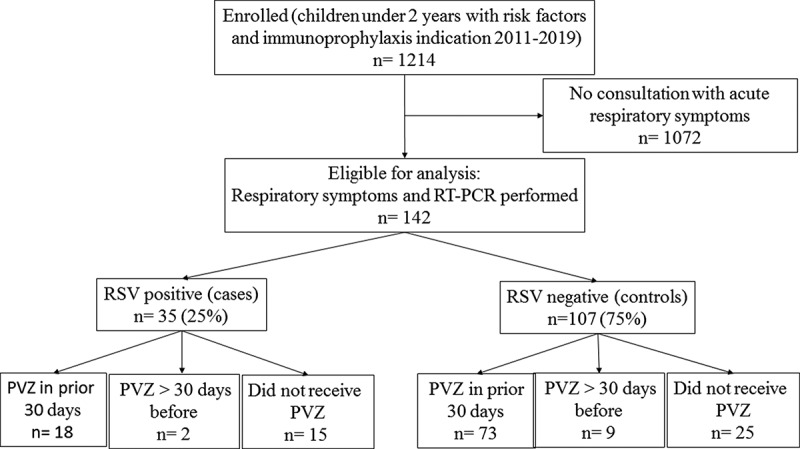
Flow diagram for study participants
Abbreviations: PVZ, palivizumab; RT-PCR, reverse transcription-polymerase chain reaction.
Fifty-four percent of the children included in this study who attended consultation for respiratory symptoms and tested for RSV required hospitalization.
No differences were observed between cases and controls regarding sex, season, or hospitalizations (Table 1). The number of cases of >12 months was higher than that of controls (31% vs. 12%; P = .0082). Palivizumab compliance was 72% among all study patients; 57% for cases and 77% for controls (P = .0261), among children <2 years with risk factors included in the indication of immunoprophylaxis.
Table 1.
Characteristics of cases and controls
| Total N: 142 n (%) |
RSV-positive cases N: 35 n (%) |
RSV-negative controls N: 107 n (%) |
P-value | |
|---|---|---|---|---|
| Sex | 0.3385 | |||
| Male | 75 (53) | 21 (60) | 54 (50) | |
| Female | 67 (47) | 14 (40) | 53 (50) | |
| Age, months | ||||
| 0–5 | 83 (58) | 16 (46) | 67 (63) | Reference |
| 6–11 | 35 (25) | 8 (23) | 27 (25) | 0.6590 |
| 12–17 | 12 (8) | 7 (20) | 5 (5) | 0.0073 |
| 18–23 | 12 (8) | 4 (11) | 8 (7) | 0.2707 |
| Season | 0.4013 | |||
| 2011–12 | 18 (13) | 3 (9) | 15 (14) | |
| 2012–13 | 4 (3) | 2 (5) | 2 (2) | |
| 2013–14 | 9 (6) | 3 (9) | 6 (6) | |
| 2014–15 | 26 (18) | 5 (14) | 21 (20) | |
| 2015–16 | 16 (11) | 5 (14) | 11 (10) | |
| 2016–17 | 36 (25) | 7 (20) | 29 (27) | |
| 2017–18 | 16 (11) | 7 (20) | 9 (8) | |
| 2018–19 | 17 (12) | 3 (9) | 14 (13) | |
| Doses of Palivizumab | 0.1645 | |||
| 0 | 40 (28) | 15 (43) | 25 (23) | |
| 1 | 13 (9) | 2 (6) | 11 (10) | |
| 2 | 35 (25) | 7 (20) | 28 (26) | |
| ≥3 | 54 (38) | 11 (31) | 43 (41) | |
| Palivizumab within 30 d | 0.0722 | |||
| Yes | 91 (64) | 18 (51) | 73 (68) | |
| No | 51 (36) | 17 (49) | 34 (32) | |
| Any dose of palivizumab | 0.0261 | |||
| Yes | 102 (72) | 20 (57) | 82 (77) | |
| No | 40 (28) | 15 (43) | 25 (23) | |
| Healthcare setting | 0.4373 | |||
| Hospitalization | 76 (54) | 21 (60) | 55 (51) | |
| Outpatient consultation | 66 (46) | 14 (40) | 52 (49) |
Of the 91 children who had received palivizumab prophylaxis, 90% (18/20) of cases and 89% (73/82) of controls had received the last dosage within 30 days of attended consultation.
Two out of 35 cases (6%) developed RSV infection after the first palivizumab dosage. We detected 20 children (14%) who had received palivizumab and were later confirmed to be RSV-positive, from which 18 had received the last dosage within the 30 previous days. Table 2 shows the characteristics of these RSV cases categorized based on whether they had received prophylactic immunization. None of the groups showed a statistically significant difference based on prophylactic immunization status.
Table 2.
Characteristics of cases by prophylactic immunization status
| Palivizumab N: 20 n (%) |
No Palivizumab N: 15 n (%) |
P value | |
|---|---|---|---|
| Sex | 0.7282 | ||
| Male | 11 (55) | 10 (67) | |
| Female | 9 (45) | 5 (33) | |
| Age, months | 0.3008 | ||
| 0–5 | 7 (35) | 9 (60) | |
| 6–11 | 6 (30) | 2 (13) | |
| 12–23 | 7 (35) | 4 (27) | |
| Season | 0.1516 | ||
| 2011–12 | 2 (10) | 1 (7) | |
| 2012–13 | 1 (5) | 1 (7) | |
| 2013–14 | 0 (0) | 3 (20) | |
| 2014–15 | 3 (15) | 2 (13) | |
| 2015–16 | 3 (15) | 2 (13) | |
| 2016–17 | 3 (15) | 4 (27) | |
| 2017–18 | 7 (35) | 0 (0) | |
| 2018–19 | 1 (5) | 2 (13) | |
| RSV subgroup | 0.2911 | ||
| A | 12 (60) | 5 (33) | |
| B | 7 (35) | 9 (60) | |
| Unknown | 1 (5) | 1 (7) | |
| Healthcare setting | 0.1632 | ||
| Hospital admission | 10 (50) | 11 (73) | |
| Outpatient consultation | 10 (50) | 4 (27) |
The comparison between confirmed cases and negative controls shows that the effectiveness of palivizumab prophylaxis in preventing confirmed RSV cases was 70% (95% CI, 19%–90%) and 82% (95% CI, 29%–96%) for preventing hospitalization (Table 3). In the analyses per RSV subgroup, effectiveness was 87% (95% CI, −181%–87%) for subgroup A and 79% (95% CI, 12%–96%) for subgroup B, although statistical significance was achieved only for subgroup B. Effectiveness was similar for subjects who received any dosage of palivizumab or who had received the last dosage within the last 30 days.
Table 3.
Effectiveness of prophylactic immunization with palivizumab in preventing laboratory-confirmed cases of respiratory syncytial virus
| Analysis |
Cases Treated/Total |
Controls Treated/Total |
Effectiveness* |
95% CI |
P value |
|---|---|---|---|---|---|
| All patients | |||||
| Any dose of palivizumab vs. none | 20/35 | 82/107 | 70% | 19 to 90 | 0.0148 |
| Palivizumab dose in prior 30 d vs. none | 18/33 | 73/98 | 68% | 13 to 89 | 0.0238 |
| Hospitalized patients | |||||
| Any dose of palivizumab vs. none | 10/21 | 42/55 | 82% | 29 to 96 | 0.0102 |
| Palivizumab dose in prior 30 d vs. none | 9/20 | 36/49 | 79% | 16 to 96 | 0.0234 |
| RSV subgroup A | |||||
| Any dose of palivizumab vs. none | 12/17 | 65/86 | 87% | −181 to 87 | 0.6472 |
| Palivizumab dose in prior 30 d vs. none | 10/15 | 57/78 | 38% | −192 to 86 | 0.6882 |
| RSV subgroup B | |||||
| Any dose of palivizumab vs. none | 7/16 | 56/79 | 79% | 12 to 96 | 0.0307 |
| Palivizumab dose in prior 30 d vs. none | 7/16 | 48/71 | 75% | −10 to 95 | 0.0695 |
*Exact logistic regression analysis adjusted by age group (0–5, 6–11, 12–23 months) and healthcare setting (outpatient or hospitalization).
CI, confidence interval
Discussion
The results of this study indicate that palivizumab is significantly effective to prevent laboratory-confirmed RSV cases (70%) and hospital admissions (82%) in children <2 y at high-risk. Fifty-one and 60% of the included controls and cases, respectively, required hospitalization, consistent with the fact that the study was carried out with high-risk children.19,20
RSV infection is a frequent condition and this study was carried out including high-risk children. Thus, despite the high effectiveness and compliance of prophylactic palivizumab immunization, in a large number of cases (n = 20) immunization failed to prevent RSV disease. This is in line with the results communicated by other authors.21
Palivizumab accumulation due to repeated doses may associate with a greater protective effect, although it has been suggested that extended use of palivizumab in high-risk patients may lead to the selection of RSV-resistant variants among the general population.22
Prophylactic palivizumab immunization failure to prevent disease has been associated with inappropriate dosing intervals, exposure to high concentrations of the virus, child’s frailty, or coinfection with other respiratory pathogens.23 On the other hand, variable degree of protection and serum concentrations has been reported among patients in clinical studies. Palivizumab dosing by body weight in children, in a five-dose monthly regimen (not in abbreviated three-dose schedule), provides prolonged protection.24 Some authors recommend an interval of 16–24 days between the administration of the first and the second dosage to achieve an adequate level of protection.25 Our results show that most failures (18/20) occurred in children who had received two or more doses (Table 1). This may be explained because the RSV epidemic usually came when most of the children following the immunoprophylaxis had received more than one dose of palivizumab.18 When the analysis was restricted to children who had followed the recommended dosages and administration intervals, no statistically significant changes in the estimate of effectiveness were observed. Another study has suggested that palivizumab protection could extend beyond 30 days of the last dose.26
The IMpact-RSV trial with palivizumab was carried out in the cases confirmed using rapid antigen detection tests.9 Rapid antigen detection tests are less sensitive than RT-PCR for RSV detection.14 Using less sensitive tests may have contributed to the lower efficacy estimate generated in the IMpact study.
Anderson et al. communicated an effectiveness of 58% in preventing hospital admissions using information on prophylactic palivizumab immunization provided by the parents, which may have led to a classification error as indicated by the author.17
In our study, the confirmed cases and negative control design imply recruitment before the physician and the parents knew the etiological diagnosis. This limits the occurrence of a selection bias and allows better comparisons between cases and controls. This design is currently being used for assessing the effectiveness of vaccines and has shown good validity in comparison with clinical trials.27
RSV has two antigenic subgroups, A and B, that can co-circulate during the different seasons and with different patterns of dominance.28 In our study, it was not possible to determine the effectiveness differences for cases between these two subgroups due to the small sample size available for sub-analyses. Differences in effectiveness by subgroup would be possible as, while both have the F protein (the target of palivizumab), subgroup variations exist.15
Compliance with prophylactic immunization was 77% in the control group, which is within the 61% to 100% range described in a review by Frogel et al.29 Worse compliance was observed for the group of cases (57% vs. 77%; p = .0261), but no statistically significant differences were observed in the percentage of hospitalizations between cases and controls (60% vs. 51%), in contrast to what has been reported elsewhere.30 Some authors have shown that home administration of palivizumab may generate better results as it increases compliance and reduces environmental exposures within the hospital of high-risk children.31 This may be improved by detecting the factors associated with poor adherence,32 and educational interventions.33 Nirsevimab, a new monoclonal antibody with an extended half-life for RSV prophylaxis in healthy preterm infants, is being developed to protect infants for an entire RSV season with a single intramuscular dose. This competitive advantage over palivizumab could achieve optimal compliance and reduce the costs of multi-dose administration, furthermore, it could be a valuable option for RSV prophylaxis in healthy infants.34
Several limitations have to be considered in the interpretation of our results. Although eight epidemic seasons were included, the strength of the study was limited, mainly because most children complied with the indication. The results presented here refer to a single region and hospital and further studies should be designed to include other places. We cannot rule out some residual confusion as certain factors such as social parameters, the use of healthcare services and the seriousness of comorbidities, which were not considered. Specific comorbidities were not included in the regression model to avoid categories with very small number of cases; however, all participants had conditions with indication of palivizumab.
The test-negative design that compared confirmed cases and negative controls allows for good comparability between these groups as the same healthcare procedures were used and there were no differences in the recruiting process.35 This design has been successfully used to assess the effectiveness of vaccines.27,36
In conclusion, palivizumab demonstrated a high, although not complete, effectiveness in preventing laboratory-confirmed RSV cases and hospitalization in high-risk children <2 years of age. Since the risk of infection in children who have received prophylactic palivizumab immunization remains high, other complementary environmental control measures are recommended as hand hygiene, limit the contact with persons with respiratory symptoms, and reduction in the number of visits during the epidemic period.11
Due to the limited effectiveness of palivizumab, efforts should be put toward developing more effective treatments and vaccines against RSV. Improve adherence through educational measures may help reduce slightly the development of cases and hospitalization.
Funding Statement
RJ and JC received research grants from Instituto de Salud Carlos III through the European Regional Development Fund during the conduct of the study (JR19/00044; INT19/00028).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Langley GF, Anderson LJ.. Epidemiology and prevention of respiratory syncytial virus infections among infants and young children. Pediatr Infect Dis J. 2011;30(6):510–17. doi: 10.1097/INF.0b013e3182184ae7. [DOI] [PubMed] [Google Scholar]
- 2.Scheltema NM, Gentile A, Lucion F, Nokes DJ, Munywoki PK, Madhi SA, Groome MJ, Cohen C, Moyes J, Thorburn K, et al. Global respiratory syncytial virus-associated mortality in young children (RSV GOLD): a retrospective case series. Lancet Glob Health. 2017;5(10):e984–e991. doi: 10.1016/S2214-109X(17)30344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byington CL, Wilkes J, Korgenski K, Sheng X. Respiratory syncytial virus-associated mortality in hospitalized infants and young children. Pediatrics. 2015;135(1):e24–31. doi: 10.1542/peds.2014-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazur NI, Higgins D, Nunes MC, Melero JA, Langedijk AC, Horsley N, Buchholz UJ, Openshaw PJ, McLellan JS, Englund JA, et al.Respiratory Syncytial Virus Network (ReSVINET) Foundation. The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect Dis. 2018;18(10):e295–e311. doi: 10.1016/S1473-3099(18)30292-5. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhuri S, Symons JA, Deval J. Innovation and trends in the development and approval of antiviral medicines: 1987-2017 and beyond. Antiviral Res. 2018;155:76‐88. doi: 10.1016/j.antiviral.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazur NI, Martinón-Torres F, Baraldi E, Fauroux B, Greenough A, Heikkinen T, Manzoni P, Mejias A, Nair H, Papadopoulos NG, et al. Respiratory Syncytial Virus Network (ReSViNET). Lower respiratory tract infection caused by respiratory syncytial virus: current management and new therapeutics. Lancet Respir Med. 2015. November;3(11):888–900. doi: 10.1016/S2213-2600(15)00255-6. [DOI] [PubMed] [Google Scholar]
- 7.Luna MS, Manzoni P, Paes B, Baraldi E, Cossey V, Kugelman A, Chawla R, Dotta A, Rodríguez Fernández R, et al. Expert consensus on palivizumab use for respiratory syncytial virus in developed countries. Paediatr Respir Rev. 2018. December 18:pii: S1526-0542(18)30139–8. doi: 10.1016/j.prrv.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Resch B. Product review on the monoclonal antibody palivizumab for prevention of respiratory syncytial virus infection. Hum Vaccin Immunother. 2017;13(9):2138–49. doi: 10.1080/21645515.2017.1337614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The IMpact-RSV Study Group . Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102(3 Pt 1):531–37. doi: 10.1542/peds.102.3.531. [DOI] [PubMed] [Google Scholar]
- 10.Feltes TF, Cabalka AK, Meer HC, Piazza FM, Carlin DA, Top FH, Connor EM, Sondheimer HM, Cardiac Synagis Study Group . Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143(4):532–40. doi: 10.1067/s0022-3476(03)00454-2. [DOI] [PubMed] [Google Scholar]
- 11.American Academy of Pediatrics Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee . Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134(2):415–20. doi: 10.1542/peds.2014-1666. [DOI] [PubMed] [Google Scholar]
- 12.Figueras Aloy J, Carbonell Estrany X; Comité de Estándares de la SENeo . Update of recommendations on the use of palivizumab as prophylaxis in RSV infections. An Pediatr (Barc). 2015;82(3):199.e1-2. doi: 10.1016/j.anpedi.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Sánchez Luna M, Pérez Muñuzuri A, Leante Castellanos JL, Ruiz Campillo CW, Sanz López E, Benavente Fernández I, Sánchez Redondo MD, Rite Gracia S. en representación de la Comisión de Estándares de la Sociedad Española de Neonatología. [An update of the recommendations of the Spanish Neonatology Society for the use of palivizumab as prophylaxis for severe infections due to syncytial respiratory virus in high-risk infants]. An Pediatr (Barc). 2019;91(5):348–50. doi: 10.1016/j.anpedi.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Chartrand C, Tremblay N, Renaud C, Papenburg J. Diagnostic accuracy of rapid antigen detection tests for respiratory syncytial virus infection: systematic review and meta-analysis. J Clin Microbiol. 2015;53(12):3738–49. doi: 10.1128/JCM.01816-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffiths C, Drews SJ, Marchant DJ. Respiratory syncytial virus: infection, detection, and new options for prevention and treatment. Clin Microbiol Rev. 2017;30(1):277–319. doi: 10.1128/CMR.00010-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mansournia MA, Higgins JP, Sterne JA, Hernán MA. Biases in randomized trials: a conversation between trialists and epidemiologists. Epidemiology. 2017;28(1):54–59. doi: 10.1097/EDE.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson EJ, Carosone-Link P, Yogev R, Yi J, Simões EAF. Effectiveness of palivizumab in high-risk infants and children: a propensity score weighted regression analysis. Pediatr Infect Dis J. 2017;36(8):699–704. doi: 10.1097/INF.0000000000001533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viguria N, Martínez-Baz I, Moreno-Galarraga L, Sierrasesúmaga L, Salcedo B, Castilla J. Respiratory syncytial virus hospitalization in children in northern Spain. PLoS One. 2018;13(11):e0206474. doi: 10.1371/journal.pone.0206474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haerskjold A, Kristensen K, Kamper-Jørgensen M, Nybo Andersen AM, Ravn H, Graff Stensballe L. Risk factors for hospitalization for respiratory syncytial virus infection: a population-based cohort study of Danish children. Pediatr Infect Dis J. 2016;35(1):61–65. doi: 10.1097/INF.0000000000000924. [DOI] [PubMed] [Google Scholar]
- 20.Lim A, Butt ML, Dix J, Elliott L, Paes B. Respiratory syncytial virus (RSV) infection in children with medical complexity. Eur J Clin Microbiol Infect Dis. 2019;38(1):171–76. doi: 10.1007/s10096-018-3409-1. [DOI] [PubMed] [Google Scholar]
- 21.Boivin G, Caouette G, Frenette L, Carbonneau J, Ouakki M, De Serres G. Human respiratory syncytial virus and other viral infections in infants receiving palivizumab. J Clin Virol. 2008;42(1):52–57. doi: 10.1016/j.jcv.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gimferrer L, Campins M, Codina MG, Martín MDC, Fuentes F, Esperalba J, Bruguera A, Vilca LM, Armadans L, Pumarola T, et al. Molecular epidemiology and molecular characterization of respiratory syncytial viruses at a tertiary care university hospital in Catalonia (Spain) during the 2013-2014 season. J Clin Virol. 2015;66:27–32. doi: 10.1016/j.jcv.2015.02.01. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira DB, Iwane MK, Prill MM, Weinberg GA, Williams JV, Griffin MR, Szilagyi PG, Edwards KM, Staat MA, Hall CB, et al. Molecular characterization of respiratory syncytial viruses infecting children reported to have received palivizumab prophylactic immunization. J Clin Virol. 2015;65:26–31. doi: 10.1016/j.jcv.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robbie GJ, Zhao L, Mondick J, Losonsky G, Roskos LK. Population pharmacokinetics of palivizumab, a humanized anti-respiratory syncytial virus monoclonal antibody, in adults and children. Antimicrob Agents Chemother. 2012;56(9):4927–36. doi: 10.1128/AAC.06446-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu SY, Bonaparte J, Pyati S. Palivizumab use in very premature infants in the neonatal intensive care unit. Pediatrics. 2004;114(5):e554–e556. doi: 10.1542/peds.2004-0226. [DOI] [PubMed] [Google Scholar]
- 26.Winterstein AG, Hampp C, Saidi A. Effectiveness of palivizumab prophylaxis in infants and children in Florida. Pharmacoepidemiol Drug Saf. 2012;21(1):53–60. doi: 10.1002/pds.2246. [DOI] [PubMed] [Google Scholar]
- 27.De Serres G, Skowronski DM, Wu XW, Ambrose C. The test-negative design: validity, accuracy and precision of vaccine efficacy estimates compared to the gold standard of randomised placebo-controlled clinical trials. Euro Surveill. 2013;18:37. doi: 10.1371/journal.pone.0163586. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Fernandez R, Tapia LI, Yang CF, Torres JP, Chavez-Bueno S, Garcia C, Jaramillo LM, Moore-Clingenpeel M, Jafri HS, Peeples ME, et al. Respiratory syncytial virus genotypes, host immune profiles, and disease severity in young children hospitalized with bronchiolitis. J Infect Dis. 2017;217(1):24–34. doi: 10.1093/infdis/jix543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frogel MP, Stewart DL, Hoopes M, Fernandes AW, Mahadevia PJ. A systematic review of compliance with palivizumab administration for RSV immunoprophylaxis. J Manag Care Pharm. 2010;16(1):46–58. doi: 10.18553/jmcp.2010.16.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart DL, Ryan KJ, Seare JG, Pinsky B, Becker L, Frogel M. Association of RSV-related hospitalization and non-compliance with palivizumab among commercially insured infants: a retrospective claims analysis. BMC Infect Dis. 2013;13:334. doi: 10.1186/1471-2334-13-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hand IL, Noble L, Geiss D, Shotkin A. Respiratory syncytial virus prophylactic immunization in an urban population: a comparison of delivery strategies and outcomes. Pediatr Infect Dis J. 2008;27(2):175–76. doi: 10.1097/INF.0b013e318159832b. [DOI] [PubMed] [Google Scholar]
- 32.Wong SK, Li A, Lanctôt KL, Paes B. Adherence and outcomes: a systematic review of palivizumab utilization. Expert Rev Respir Med. 2018;12(1):27–42. doi: 10.1080/17476348.2018.1401926. [DOI] [PubMed] [Google Scholar]
- 33.Bernard L, Lecomte B, Pereira B, Proux A, Boyer A, Sautou V. Optimisation de la prévention de la bronchiolite à VRS chez les nouveaux-nés à risque et les prématurés: mesure de l’impact d’une intervention éducative ciblée [Impact of a targeted educational intervention on respiratory syncytial virus bronchiolitis prevention in full-term and preterm infants]. Arch Pediatr. 2015;22(2):146–53. doi: 10.1016/j.arcped.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 34.Griffin MP, Yuan Y, Takas T, Domachowske JB, Madhi SA, Manzoni P, Simões EAF, Esser MT, Khan AA, Dubovsky F, et al.; Nirsevimab Study Group . Single-dose nirsevimab for prevention of RSV in preterm infants. N Engl J Med. 2020;383(5):415–25. doi: 10.1056/NEJMoa1913556. [DOI] [PubMed] [Google Scholar]
- 35.Bond HS, Sullivan SG, Cowling BJ. Regression approaches in the test-negative study design for assessment of influenza vaccine effectiveness. Epidemiol Infect. 2016;144(8):1601–11. doi: 10.1017/S095026881500309X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization . Evaluation of influenza vaccine effectiveness: a guide to the design and interpretation of observational studies. Geneva; 2017. [accesed 2020 Sept 29]. https://apps.who.int/iris/bitstream/handle/10665/255203/9789241512121-eng.pdf;jsessionid=BF8E8B943503324D3B7CF47325EABD46?sequence=1 [Google Scholar]


