ABSTRACT
Vaccinating premature and low birthweight (LBW) infants according to chronological age has been found safe and effective. Although these infants are susceptible to infections, vaccinations are often delayed. We estimated vaccination coverage (VC) in preterm and LBW infants compared to term infants in a cohort study (2016 Israel birth cohort, n = 181,543) using the National Immunization Registry. Vaccinations included Hepatitis B, Diphtheria-Tetanus-acellular Pertussis-IPV-Haemophilus influenzae B, Oral Polio Bivalent, Rotavirus, Pneumococcal Conjugate, Measles-Mumps-Rubella-Varicella and Hepatitis A. Inclusion criteria: (1) born in Israel; (2) having a unique identifier (allowing data matching); and (3) surviving to 24 months. VC at 24 months and timeliness of vaccine doses were evaluated according to infants’ birthweight (BW) and gestational age (GA). Preterm infants (GA < 37 weeks) comprised 7.0% (n = 12,264); LBW infants (BW< 2500 g) were 7.7% (n = 13,950); BW was 1500–2499 g in 6.8%, 1000–1499 g in 0.6% and below 1000 g in 0.3%. Compared to normal birthweight (NBW) infants (BW≥2500 g), LBW infants showed delayed initiation of vaccinations. Odds ratio (OR) for delay: DTaP-IPV-Hib 1 OR = 1.26 [95%CI 1.19–1.33]; Rota 1, OR = 1.22 [95%CI 1.16–1.29]. Vaccination delay rates were higher among smaller new-borns (below 1000 g). At 24 months there was no significant difference regarding vaccination status. This national cohort VC analysis focused on preterm/LBW infants. Vaccinating preterm and LBW infants according to the recommended schedule induces protection against life-threatening infectious diseases. Vaccination initiation among LBW infants showed considerable delay. Health practitioners and parents should cooperate to improve timely vaccination initiation.
KEYWORDS: Vaccination, vaccination coverage, low birthweight, preterm infants, public health
Introduction
Preterm birth, defined as birth before 37 weeks of gestation, and low birthweight (LBW) of less than 2500 g, are associated with substantial morbidity and mortality. Globally, the rates of preterm birth and LBW are estimated as 10% and 14.6% of all livebirths, respectively, varying between countries and population groups (in Israel, 7% and 7.7%, respectively).1,2
The immune system of preterm infants is immature compared to term infants. Maternal-fetal antibody transmission occurs mainly in the third trimester of pregnancy, including against vaccine-preventable infections. Preterm infants have lower antibody levels against infectious agents with an earlier decay than full-term infants. Also, the innate immune system has yet to mature in preterm infants, increasing infection risk.3–6 Thus, preterm and LBW infants are more susceptible to infection with a higher disease severity. Hospitalization and complication rates associated with rotavirus acute gastroenteritis and with pertussis (whooping cough) have been reported as higher in preterm and LBW infants vs. full-term infants.7–12
A fundamental component of the prevention of infectious diseases is childhood vaccinations. Continuous monitoring of vaccination coverage rates is part of health indicator evaluation and may also be utilized to detect risk groups and to assess the equity of health-care systems.13–17 Health organizations, including the American Academy of Pediatrics and the World Health Organization (WHO), recommend that preterm and LBW infants should be routinely vaccinated according to schedule at the chronological age specified for each vaccine dose (with the exception of HBV vaccine in infants born weighing <2000 g). Infants still hospitalized and medically stable at the age of 2 months should receive all age-appropriate inactivated vaccinations. Routine childhood vaccinations have been shown to be safe, immunogenic and effective for preterm and LBW infants, providing protection against Vaccine-Preventable-Diseases (VPD).18–25 Yet, considerable gaps in routine vaccination timeliness and completeness have been evident when comparing LBW and preterm infants to term infants. Vaccination delays tend to be greater when the preterm infants are born earlier and with lower birthweights. Medical conditions associated with preterm birth such as chronic lung disease and health provider change have also been associated with immunization non-completion.26–32 Targeted preterm vaccination intervention programs have reported favorable results in Ireland and Italy.33,34 Delay of vaccination in preterm and LBW infants may most likely stem from inappropriate knowledge and perceptions of both parents and health-care providers regarding the safety and efficacy of timely routine childhood vaccinations.35,36
Data validity of vaccination coverage rates is essential for planning and evaluation. Vaccination coverage estimates based on periodical surveys and cumulative data have limited validity in reflecting the actual uptake rates.37–39 The national immunization registry in Israel provides a framework for vaccination coverage assessment in a national cohort.40,41 Routine childhood vaccinations are offered without charge to all children at community-based child-health clinics for young children (birth to 6 y) and by school health services for schoolchildren (6 to 15 y).42 The Israeli immunization registry incorporates all routine childhood vaccinations into one database.43 While Israel’s childhood vaccination coverage upholds the WHO up-to-date (UTD) targets adequately, registry data show a high prevalence of vaccine delays.41 Previous studies evaluating vaccination coverage in preterm infants included samples that are often quite limited in size and data collected from parents’ self-reporting, health-care provider records or incomplete databases.26–29,33,34,44,45 National registry data are more appropriate to estimate vaccination coverage in relatively small groups that can be under-represented in population sample surveys.
The study aims are to assess routine vaccination coverage rates in a national birth cohort, to estimate vaccination completeness and timeliness in preterm and LBW infants compared to term infants and to explore risk markers for lacking completeness and timeliness.
Methods
Routine childhood vaccinations are included in the preventive services of Israel’s National Health Insurance Law. Vaccines are offered in community-based clinics from birth to 6 y.46 Routine vaccines are provided free of charge to all resident children through the national health insurance law. The eligibility for preventive health services including routine vaccinations is for all children nationally, regardless of legal residency status.44 Vaccination receipt is not mandatory; the overall vaccination coverage rates reported are over 95%.47 The study is designed as a historical prospective study of a national annual cohort.
Study population
The population registration law in Israel requires reporting all births to the Ministry of Interior; infants receive personal identification numbers (ID). The national population (2016) was 8.6 million with 181,000 livebirths nationally. The study cohort included all infants born in Israel from January 1, 2016 to December 31, 2016. The infants’ follow-up period lasted until age 24 months. Inclusion criteria were: (1) born in Israel; (2) having a unique identifier (allowing data matching); and (3) surviving to 24 months. Infants born abroad (different schedules), lacking unique identifier or not surviving to 24 months were excluded.
Data assembly (demographics)
Demographic data of infants born in 2016 were retrieved from the national newborn registry. Variables were: (1) child: date of birth, gender, ethnicity, birth order, gestational age at birth, birthweight; (2) mother: age, birth country, marital status and socio-economic (SE) rank of residence locality. The SE rank used was an ordinal scale of 1 (lowest) to 10 (highest).48 The main investigated variables were birthweight and gestational age. Birthweight was evaluated as a continuous variable (grams) and an ordinal variable in the following categories: (1) Normal Birthweight (NBW) 2500 g; (2) Low Birthweight (LBW) <2500 g. The LBW group was stratified: 2000–2499 g, 1500–1999 g; 1000–1499 g and <1000 g. Gestational age (GA) was assessed as a continuous and ordinal variable using groups stratified according to GA variable in the following categories: <28 weeks, 28–31 weeks, 32–36 weeks and GA ≥37 weeks.41
Data assembly (vaccinations)
The routine vaccination schedule (0–2 y, 2016) included: Hepatitis B vaccine (HBV) at birth, 1 and 6 months, Diphtheria-Tetanus-acellular Pertussis-Polio-Haemophilus influenzae type B vaccine (DTaP-IPV-Hib) at 2, 4, 6 and 12 months, Polio Oral bivalent (bOPV) at 6 and 18 months, Rotavirus vaccine (Rota) at 2,4 and 6 months, Pneumococcal conjugate vaccine (PCV) at 2, 4 and 12 months, Measles-Mumps-Rubella-Varicella (MMR/MMRV) at 12 months and Hepatitis A vaccine (HAV) at 18 and 24 months. The vaccination schedule for preterm infants is the same, according to chronological age with the exception of HBV which requires a minimum weight of 2000 g. RSV vaccinations are not provided in the community clinics nor do they appear in the registry and are therefore not included in the current study. They are provided free of charge through government-sponsored health maintenance organizations, to infants meeting a list of criteria. The criteria include preterm infants considered at risk and other infants with severe chronic lung disease.49 The participants’ unique identifiers (personal ID, date of birth) were crossed against the immunization database for data on vaccine doses including dates.
Study outcomes
The primary outcomes were set as up-to-date and age-appropriate vaccination status as previously described.41 Up-to-date vaccination status was defined as the rate of vaccination at 24 months of age. Age-appropriate vaccination status was defined as timeliness administration of the individual vaccine doses.
Up-to-date vaccination status was assessed at 24 months (including HBV3, DTaP-IPV-Hib 4, PCV3 and MMR/MMRV1). Series completion was defined as receiving all required doses of a vaccine at the time of assessment (e.g. 4 doses of DTaP-IPV-Hib 4 at 24 months).
Timeliness was assessed by examining the vaccination timing for each vaccine dose. Vaccine doses were defined valid according to the Ministry of Health guidelines for minimum ages and intervals between doses. Doses administered more than 1 month after the recommended age were considered delayed. Vaccination status was grouped into: a) Vaccinated age-appropriate, b. Delayed less than 6 months, divided into two sub-groups (mild: delayed 1–3 months, moderate: delayed 3–5 months), c. Delayed 6 months and above (severe delay) and d. Unvaccinated (at 24 months).41
Statistical analysis
Data analysis was performed with IBM SPSS Statistics for Macintosh, Version 25.0. Armonk, NY: IBM Corp. Categorical variables are presented as rate and proportion; continuous variables as mean ± Standard Deviation (SD) and median. An initial analysis found a strong correlation between birthweight and gestational age (r = 0.621, Figure 1). All further vaccination status data analysis was performed using birthweight categories. Immunization coverage represented by the cumulative proportion of vaccinated was retrieved (according to age in days) and plotted on an inverse Kaplan–Meier curve, separately for each weight group. Analysis of delay in vaccination was performed using Cox Proportional Hazards model. Time to vaccination was grouped into five weight categories. Not vaccinated by end of follow-up (age 24 months) were considered censored observations. Results are presented as hazard ratio (HR) with a 95% Confidence Interval (CI). A univariate analysis was performed for NBW compared to all LBW infants for each vaccine. Comparison results are presented as odds ratio (OR) and a 95% CI. Unadjusted OR for any delay is presented in a forest plot. Additional stratification of the birthweight categories was performed (2000 g and 1500 g as dichotomous cutoff points). Unadjusted OR for any delay in these additional categories is presented in a forest plot as well. A multiple regression analysis model was performed for general variables associated with a child’s vaccination status being age appropriate. Associations between the variables and vaccination status are presented as OR and a 95% CI. P-values less than 0.05 were considered significant for all, and all P values were two-sided.
Figure 1.
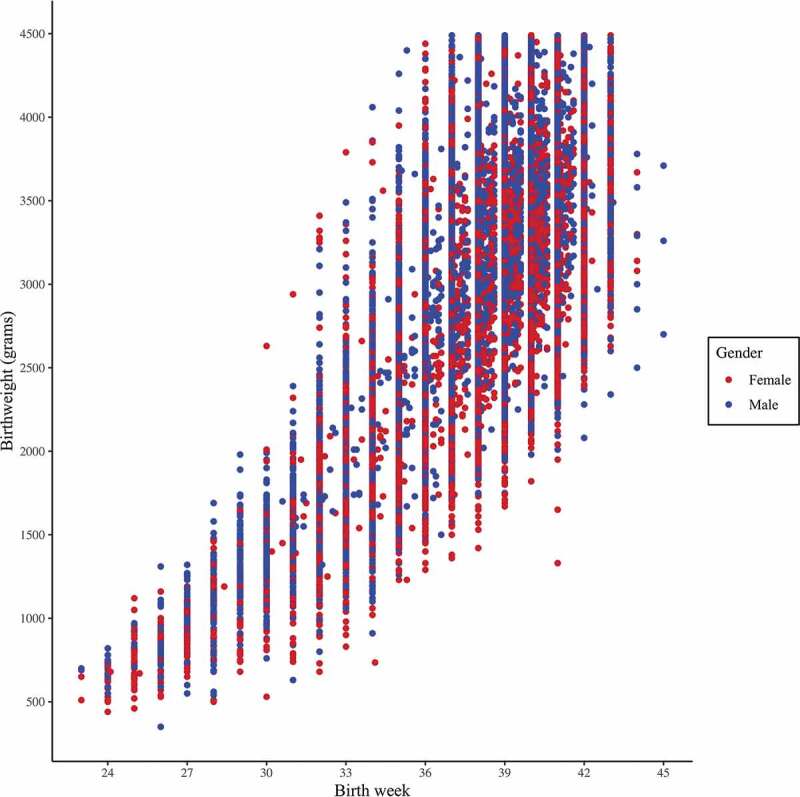
Scatter plot for birth week and birthweight
Note: A strong correlation was found between birth week and birthweight, with a Pearson Correlation coefficient r = 0.621.
Results
Study population characteristics
Demographic and general characteristics for the national birth cohort born in 2016 (n = 181,543) are presented in Table 1. Infants born with LBW (birthweight below 2500 g) comprised 7.7% of the national cohort and gestational age of less than 37 weeks was reported in 7% of the cohort. Further stratification of birthweight categories (2500 g, 2000–2499 g, 1500–1999 g, 1000–1499 g, <1000 g) and gestational age at birth categories (GA ≥37 weeks, 32–36 weeks, 28–31 weeks, <28 weeks) into sub-groups is presented in Table 1.
Table 1.
General characteristics of the 2016 birth cohort, Israel
| Characteristics | Total (n = 181,543) |
|---|---|
| Gender, No. (%) | |
| Male | 93,434 (51.5) |
| Female | 88,109 (48.5) |
| Birthweight – categorical, No. (%) | |
| 2500 g | 167,647 (92.3) |
| 2000–2499 g | 9,661 (5.3) |
| 1500–1999 g | 2,629 (1.4) |
| 1000–1499 g | 1025 (0.6) |
| 1000 g | 580 (0.3) |
| Birthweight – continuous (gram) | |
| Mean [SD] | 3200.0 [530.0] |
| Median [IQR] | 3240.0 [2920.0–3550.0] |
| Gestational age at birth (weeks) | |
| Mean [SD] | 39.0 [1.9] |
| Median [IQR] | 39.0 [38.0–40.0] |
| Gestational age at birth – categorical, No. (%) | |
| 37 weeks | 168,835 (93.0) |
| 32–36 weeks | 11,074 (6.1) |
| 28–31 weeks | 1,089 (0.6) |
| 28 weeks | 545 (0.3) |
| Birth order – categorical, No. (%) | |
| First born | 51,870 (28.6) |
| Second born | 49,060 (27.0) |
| Third born | 35,845 (19.7) |
| Fourth born and above | 44.768 (24.7) |
| Number of children – categorical, No. (%) | |
| Singleton | 173,737 (95.7) |
| Multiple | 7,806 (4.3) |
| Ethnicity, No. (%) | |
| Jews & Others | 140,233 (77.2) |
| Arabs | 41,310 (22.8) |
| Socioeconomic rank (1–10) | |
| Rank 1–3 | 77,451 (42.7%) |
| Rank 4–7 | 73,748 (40.6%) |
| Rank 8–10 | 30,344 (16.7%) |
| Month of birth, categorical, No. (%) | |
| January–March | 44,618 (24.6) |
| April–June | 42,856 (23.6) |
| July–September | 47,752 (26.3) |
| October–December | 46,317 (25.5) |
| Length of hospital stay, categorical, No. (%) | |
| 1–6 d | 171, 313 (94.4) |
| 7–29 d | 8,097 (4.5%) |
| 30 d or more | 2133 (1.2%) |
Abbreviations: SD, standard deviation; IQR, interquartile range.
Infants who did not survive to the age of 2 y (n = 633) were excluded from the study population, 373 (59%) and 260 (41%) of them were born at LBW and NBW, respectively. The general characteristics were compared between the groups of LBW infants and NBW infants. The two groups differed as to gender (males were 46.4% of LBW and 51.9% of NBW, p < .001) and in singleton birth rate (65.8% of LBW and 98.1% of NBW, p < .001). Parents’ characteristics did not show a significant difference between LBW and NBW infants. Mean parents’ age was 30.4 ± 5.7 and 33.3 ± 6.3 y among mothers and fathers, respectively. Most parents were born in Israel (88.7% of mothers and 90.0% of fathers). Mothers had 14.0 ± 2.4 y and fathers 13.6 ± 2.5 y of education. Most mothers were married (92.3%). The mean length of hospital stay afterbirth was considerably longer in the LBW infants than in the NBW infants (mean hospital stay 13.720.4 d in LBW infants vs. 3.19.1 d in NBW infants, respectively, p < .001; median 5.0[3.0–15.0] and 3.0[2.0–3.0], respectively).
Vaccination timeliness
The cumulative proportion of vaccine uptake according to age (in days) for the first dose of DTaP-IPV-Hib vaccine among infants in the five birthweight categories is presented in Figure 2. Notably, the rate of delayed vaccine receipt increased with decreasing infant birthweight. Compared to NBW infants, the age-appropriate vaccination rates did not differ regarding the 2000–2499 g birthweight group (HR 1.01 [95% CI 0.98–1.03]). However, the rates showed a significant gradient decline in infants with birthweight under 2000 g. For the three birthweight categories (1500–1999 g, 1000–1499 g, below 1000 g). The hazard ratios were HR 0.85 [95% CI 0.82–0.88], HR 0.67 [95% CI 0.63–0.72] and HR 0.45 [95% CI 0.40–0.50], respectively. Notably, infants born below 1000 g reached 90% DTaP-IPV-Hib 1 uptake at 167 d compared to 89–115 d in all other weight categories.
Figure 2.
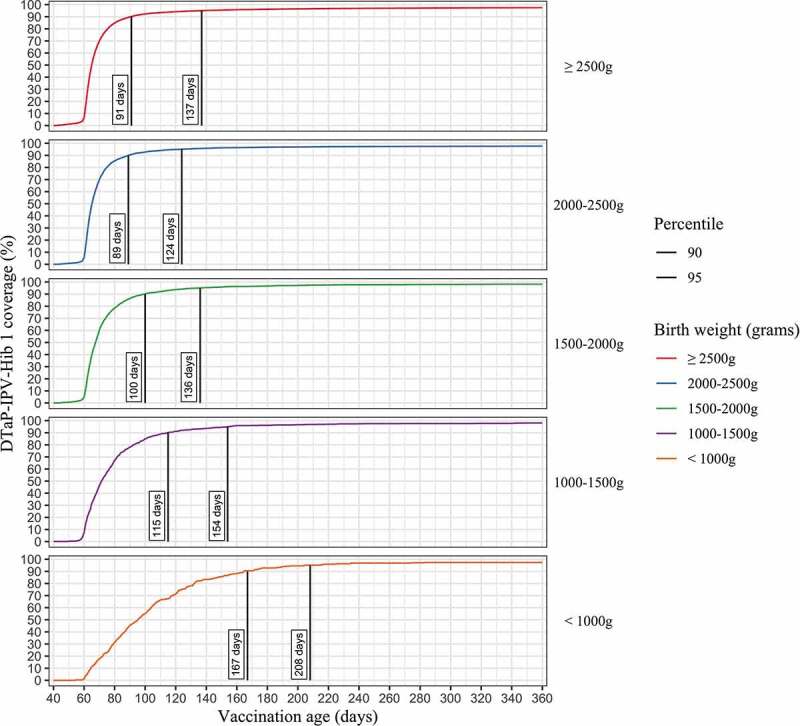
Cumulative proportion of first dose of DTaP-IPV-Hib vaccine uptake by child’s age in days in NBW and LBW children born in 2016 and followed up to 24 months, Israel
Note: The curves show the cumulative proportion of children vaccinated for first dose of DTaP-IPV-Hib over time. The vertical lines represent the day at which vaccination uptake reached 90% and 95% within each age group.
Figure 3 presents the distribution of vaccination status, regarding the first vaccine doses for the DTaP-IPV-Hib vaccine, pneumococcal vaccine and rotavirus vaccine (evaluated at age 24 months). All first vaccine doses showed a pattern of delay in vaccination initiation among LBW infants, especially in the lower weight categories. Notably, less than 50% of ELBW infants were vaccinated as age-appropriate with nearly 50% not vaccinated with rotavirus vaccine.
Figure 3.
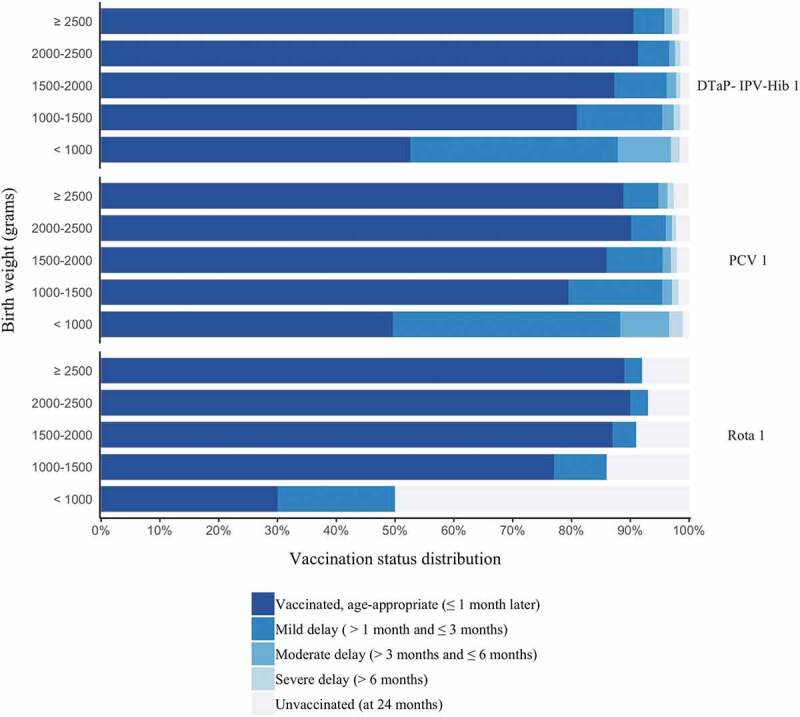
Distribution of vaccination timing by defined categories (status at the age of 24 months) for selected vaccine doses, in children born in Israel, 2016
Abbreviations: DTaP-IPV-Hib 1, diphtheria, tetanus, acellular pertussis, polio, Haemophilus influenzae B vaccine, first dose.
Aiming to evaluate the timeliness of all routine vaccine doses scheduled in the first 24 months, we compared NBW to LBW infants in regard to age-appropriate uptake of the specific vaccine dose (Figure 4). The crude OR for risk of vaccination delay among LBW infants declined with later vaccine doses (overall and within each series). Lower birthweight categories had a progressively higher risk of delay, with the same pattern of risk decline over time described above.
Figure 4.
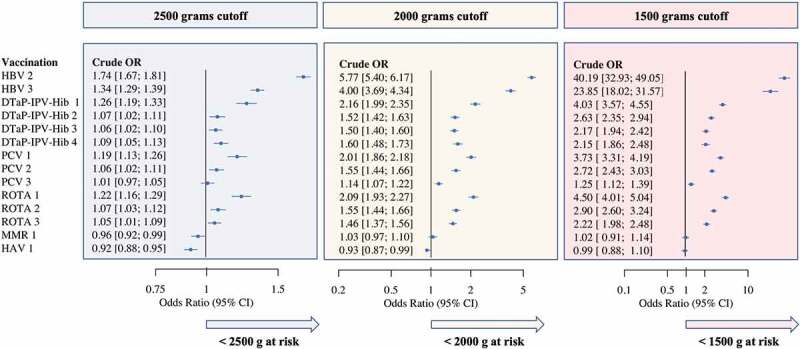
Forest plot presenting OR for risk of vaccination delay among NBW and LBW infants
Note: In early infancy LBW children are at a higher risk of vaccination delay (vaccine dose administered more than 30 d after required age), compared to NBW weight. The risk for later doses within each series is generally lower as doses progress. In some cases, vaccinations administered at older ages and not limited by earlier doses from a series show an advantage among LBW infants. Abbreviations: NBW, normal birthweight (≥2500 g); LBW, low birthweight (<2500 g).
Up-to-date vaccination status at 24 months
By 24 months, LBW infants had caught-up to NBW infants, concerning the UTD vaccination status. HBV3 was UTD in 94.5% of LBW infants and 93.4%% of NBW infants. DTaP-IPV-Hib4 was UTD in 89.3% of LBW infants and 87.7% of NBW infants. PCV 3 was UTD in 90.7% of LBW infants and 89.9% of NBW infants. MMRV1 was UTD in 94.3% of LBW infants and 94% of NBW infants.
The completion rate for HBV1-3, DTaP-IPV-Hib 1–4 and PCV 1–3 at 24 months in LBW and NBW infants was 87.3% and 85.7%, respectively.
Hospital length of stay and initiation of routine vaccination
At age 60 d, 450 infants were still hospitalized and were therefore eligible for DTaP-IPV-Hib first dose by the time of discharge. The birthweight distribution of the 450 infants was: 73 (16.2%) were NBW, 11 (2.4%) 2000–2500 g, 18 (4.0%) 1500–1999 g, 128 (28.4%) 1000–1499 g, 220 (48.9%) below 1000 g.
Only one-third (34.7%) were vaccinated before hospital discharge, with no significant difference between birthweight groups. Some hospitals had as few as two infants with long hospitalizations with the most being 47. A noticeable difference was noted at the vaccination rate in different hospitals. Out of 28 hospitals, nine (32.1%) vaccinated at least 50% of these infants before discharge. Other hospitals vaccinated anywhere between 0/17 and 13/36 (36.1%) of these infants. Infants hospitalized for 60 d or more and not receiving their first DTaP-IPV-Hib vaccination by discharge were less likely to have completed four vaccinations (i.e. DTaP-IPV-Hib 4) by 24 months (OR 1.35 [95% CI 0.75–2.44]).
Regression model
Several regressions were performed, with no difference found in UTD status with regard to birthweight or GA. Following the univariate analysis, a multiple logistic regression model was performed with the variables found to be significantly associated with timely initiation (defined as the cutoff for “timely” being 30 d following the appropriate age for vaccination). The variables included in the final model were gender, mother family status, ethnicity, birthweight/GA, birth order and socioeconomic rank. The independent variables significantly associated with non-timely vaccination initiation were male gender, non-married mother, Jewish ethnicity, non-first born, low socioeconomic rank and birthweight/GA. Birthweight and GA had a very strong association with vaccination delay (Table 2).
Table 2.
Multiple logistic regression model, dependent variable – DTaP-IPV-Hib 1 – age-appropriate vaccination status.a
| Variables | Birthweight – main independent variable Adjusted OR [95% CI] |
Gestational age – main independent variable Adjusted OR [95% CI] |
|---|---|---|
| Gender | ||
| Male | 1.06 [1.02–1.10] * | 1.06 [1.03–1.10] * |
| Female | reference | reference |
| Mother family status | ||
| Married | reference | reference |
| Other | 1.54 [1.44–1.65] * | 1.54 [1.44–1.65] * |
| Ethnicity | ||
| Jew & others | 3.27 [3.07–3.48] * | 3.30 [3.10–3.52] * |
| Arab | reference | reference |
| Birthweight | ||
| 2500 g | reference | Na |
| 2000–2500 g | 0.98 [0.90–1.07] | Na |
| 1500–2000 g | 1.56 [1.36–1.78] * | Na |
| 1000–1500 g | 3.34 [2.81–3.96] * | Na |
| 1000 g | 17.03 [13.58–21.35] * | Na |
| Gestational age at birth | ||
| 37 weeks | Na | reference |
| 32–36 weeks | Na | 1.10 [1.02–1.19] * |
| 28–32 weeks | Na | 3.62 [3.08–4.27] * |
| 28 weeks | Na | 21.77 [16.98–27.91] * |
| Birth order | ||
| First born | reference | reference |
| Second born | 1.75 [1.65–1.85] * | 1.77 [1.67–1.87] * |
| Third born | 2.02 [1.90–2.14] * | 2.02 [1.91–2.15] * |
| Fourth born and above | 3.22 [3.04–3.40] * | 3.25 [3.08–3.44] * |
| Socioeconomic rank | ||
| Rank 1–3 | 1.30 [1.23–1.37] * | 1.31 [1.24–1.39] * |
| Rank 4–7 | 0.56 [0.53–0.59] * | 0.56 [0.53–0.59] * |
| Rank 8–10 | reference | reference |
| Month of birth | ||
| January–March | reference | reference |
| April–June | 0.91 [0.87–0.96] * | 0.91 [0.87–0.96] * |
| July–September | 1.11 [1.06–1.17] * | 1.11 [1.06–1.17] * |
| October–December | 0.93 [0.89–0.98] * | 0.93 [0.88–0.98] * |
*p-value <0.001.
aThe OR in the regression is defined as the rate of delayed initiation of the DTaP-IPV-Hib 1 vaccine.
Discussion
This study is the first to assess routine vaccination completeness and timeliness in a full annual national cohort (n = 181,543), among preterm infants compared to term infants, in Israel. The study showed that LBW infants were significantly more likely to receive the first dose of DTaP-Hib-IPV in delay (21.9% of VLBW and 53.7% of ELBW were delayed beyond 1 month from the recommended schedule), compared to 90% of term infants who received the first dose on time. Besides this initial delay in providing protection, all future doses in the series were subsequently delayed. The same delay pattern is evident in the other early infancy vaccines (PCV13, Rota and HBV). By the time the children reach 12 months of age, the delay gap has decreased and their UTD coverage rates are very similar to NBW infants. UTD vaccination coverage rates at age 24 months are comparable. Assessment of the association between birthweight and vaccination timeliness showed that vaccination delay rates increased with decreasing birthweight. In this study, we used the definitions for vaccination timeliness (for timely vaccine doses, mild, moderate and severe delay) described in our previous studies. In the multiple regression model, the independent variables associated with vaccination delay were male gender, Jewish ethnicity, increasing child’s birth order and low socioeconomic status, as previously described.41,50
Several studies have documented vaccination delay among preterm infants. While some studies examined the age-appropriate vaccination status, others reported only the UTD status. Most studies found that LBW infants have not achieved a catch-up of UTD status at the age of 24 months. Langkamp et al. demonstrated that VLBW infants were significantly less likely to be vaccinated for UTD.26 Nestander et al. showed immunization completion to be significantly decreased at 24 months in LBW infants.30 Hofstetter et al. found preterm infants less likely to have completed all doses of the “7-vaccine series” by age the age of 19 months.51
The finding that preterm infants have completed catch up at 24 months in Israel may be attributed to the organization of provision of vaccination and accessibility. Childhood vaccinations are provided free of charge at community-based maternal and child-health clinics country-wide. The public health nurses are well practiced in planning catch-up vaccination schedules for multiple indications. It also seems that while early beliefs about LBW infant frailty may explain vaccination delay, this misconception wanes over time.
Despite international guidelines, the actual practice varied markedly between hospitals, with only one-third of infants hospitalized at 2 months of age vaccinated before discharge. These already fragile infants remain unprotected for longer periods, while also having an increased risk of not completing their vaccinations by 24 months. Vaccination delay diminishes the young preterm protection against multiple pathogens. We have shown in a previous study that the rate of pertussis among LBW infants is significantly higher than in term infants.52
No child received Rotavirus vaccine while hospitalized. The practice in Israeli Neonatal Intensive Care Units is not to vaccinate Rotavirus in hospitalized infants.20
This study has several strengths. Beyond being a large cohort, it has the advantage of comprising a full birth cohort, minimizing selection bias and enabling the results to represent the whole population. The comprehensive Israeli immunization registry enabled the inclusion of a full birth cohort. This provided a natural control group, strengthening validity, showing that delay is not only absolute but also compared to NBW infants. Clear definitions for timeliness enabled stratification according to degrees of delay, highlighting that most of the delay for LBW infants occurs in the first months of their life.
There are several limitations to this study. Only children with an ID number and born in Israel were included, excluding a small part of the birth cohort from the study. Data on preterm infants’ health status and medical diagnoses were unavailable and should be included in future studies.
In conclusion, our large national cohort study showed a significant delay in preterm infant vaccination initiation. For the small group of infants hospitalized at age 2 months, a clear policy should be implemented requiring the provision of vaccinations before discharge. Implementation of this policy may require training of health providers. Nevertheless, despite the delayed initiation, by the age of 24 months, the preterm/low birthweight group showed adequate catch-up with the vaccination schedule in Israel. It is our belief that the national system of comprehensive and dedicated child-health services supported this achievement.
Acknowledgments
The authors wish to acknowledge the support of Ziona Haklai MA, Head of Health Information Division, Israel Ministry of Health, Hanna Shoob MPH, and Zoey Gotlieb, Jerusalem District Health Office, Nesia Cohen, Computing Division, Ministry of Health.
Funding Statement
This study was financially supported by the Office of the Chief Scientist, the Israel Ministry of Health, [grant number MOHIG 010 2017]. The funding sources had no role in the design or conduct of the study; collection, management, analysis and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author contributions
Both authors either participated in the design, implementation or analysis, and interpretation of the study, as well as the development of this manuscript. Both authors had full access to the data and granted their final approval of the paper before submission.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, Landoulsi S, Jampathong N, Kongwattanakul K, Laopaiboon M, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Heal. 2019;7:e37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blencowe H, Krasevec J, de Onis M, Black RE, An X, Stevens GA, Borghi E, Hayashi C, Estevez D, Cegolon L, et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: a systematic analysis. Lancet Glob Heal. 2019;7:e849–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Berg JP, Westerbeek EAM, FRM VDK, Berbers GAM, Van Elburg RM.. Transplacental transport of IgG antibodies to preterm infants: A review of the literature. Early Hum Dev. 2011;87:67–72. [DOI] [PubMed] [Google Scholar]
- 4.Zinkernagel RM. Maternal antibodies, childhood infections, and autoimmune diseases. N Engl J Med. 2001;345:1331–35. [DOI] [PubMed] [Google Scholar]
- 5.Sharma AA, Jen R, Butler A, Lavoie PM. The developing human preterm neonatal immune system: A case for more research in this area. Clin Immunol. 2012;145:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melville JM, Moss TJM. The immune consequences of preterm birth. Front Neurosci. 2013;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dennehy PH, Cortese MM, Begue RE, Jaeger JL, Roberts NE, Zhang R, Rhodes P, Gentsch J, Ward R, Bernstein DI, et al. A case-control study to determine risk factors for hospitalization for rotavirus gastroenteritis in U.S. children. Pediatr Infect Dis J [Internet]. 2006;25:1123–31. Available from http://www.ncbi.nlm.nih.gov/pubmed/17133157. [DOI] [PubMed] [Google Scholar]
- 8.Newman RD, Grupp-Phelan J, Shay DK, Davis RL. Perinatal risk factors for infant hospitalization with viral gastroenteritis. Pediatrics. 1999;103:e3. [Internet] 10.1542/peds.103.1.e3 [DOI] [PubMed] [Google Scholar]
- 9.ØR R, Laake I, Vestrheim D, Flem E, Moster D, Bergsaker MAR, Storsæter J. Risk of pertussis in relation to degree of prematurity in children less than 2 years of age. Pediatr Infect Dis J. 2017;36:e151–6. [DOI] [PubMed] [Google Scholar]
- 10.Marshall H, Clarke M, Rasiah K, Richmond P, Buttery J, Reynolds G, Andrews R, Nissen M, Wood N, Mcintyre P. Predictors of disease severity in children hospitalized for pertussis during an epidemic. Pediatr Infect Dis J. 2015;34:339–45. [DOI] [PubMed] [Google Scholar]
- 11.Janagaraj PD, Gurusamy PSR, Webby R. Current antenatal pertussis vaccination guidelines miss preterm infants: an epidemiological study from the Northern Territory. Aust New Zeal J Obstet Gynaecol [Internet]. 2018. [[cited 2018 November1]]. doi: 10.1111/ajo.12896. [DOI] [PubMed] [Google Scholar]
- 12.Dahl RM, Curns AT, Tate JE, Parashar UD. Effect of rotavirus vaccination on acute diarrheal hospitalizations among low and very low birth weight US infants, 2001–2015. Pediatr Infect Dis J [Internet]. 2018;37:817–22. http://insights.ovid.com/crossref?an=00006454-201808000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plotkin PSA, Orenstein WA, Offit PA, Short A. History of vaccination. Plotkin’s vaccines. Elsevier; 2017. 1. [Google Scholar]
- 14.WHO, UNICEF, World Bank . State of the world’s vaccines and immunization 3rd ed. Geneva: World Helath Organization; 2009. [Google Scholar]
- 15.Frieden TR. The future of public health. N Engl J Med [Internet]. 2015. [cited 2018 November3];18:1748–54. https://www.nejm.org/doi/pdf/10.1056/NEJMsa1511248. [DOI] [PubMed] [Google Scholar]
- 16.Wagstaff A, Eozenou P, Neelsen S, Smitz M. The health equity and financial protection indicators database 2019. Washington (DC): World Bank; 2019. [DOI] [PubMed] [Google Scholar]
- 17.Healthy people 2020 [Internet]. Washington (DC U.S.) Dep. Heal. Hum. Serv. Off. Dis. Prev. Heal. Promot. [cited 2020 July20]; Available from: https://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases [Google Scholar]
- 18.Doherty M, Schmidt-Ott R, Santos JI, Stanberry LR, Hofstetter AM, Rosenthal SL, Cunningham AL. Vaccination of special populations: protecting the vulnerable. Vaccine [Internet]. 2016;34:6681–90. doi: 10.1016/j.vaccine.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Ezeanolue E, Harriman K, Hunter P, Kroger A, Pellegrini C. General best practice guidelines for immunization. Best practices guidance of the advisory committee on immunization practices (ACIP) [Internet]. [cited 2020 July20]; Available from: www.cdc.gov/vaccines/hcp/acip-recs/general-recs/downloads/general-recs.pdf
- 20.American Academy of Pediatrics . Red book online: immunization in preterm and low birth weight infants. [Internet]. 2018. [cited 2020 July20]; Available from: https://redbook.solutions.aap.org/chapter.aspx?sectionid=189639976&bookid=2205
- 21.Gagneur A, Pinquier D, Quach C. Immunization of preterm infants. Hum Vaccines Immunother. 2015;11:2556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirmani KI, Lofthus G, Pichichero ME, Voloshen T, Angio CTD. Seven-year follow-up of vaccine response in extremely premature infants. Pediatrics. 2002;109:498–504. [DOI] [PubMed] [Google Scholar]
- 23.Verma C, Faridi MMA, Narang M, Kaur IR. Anti-HBs Titers Following Pentavalent Immunization (DTwP-HBV-Hib) in Term Normal Weight. Indian Pediatr. 2018;55:395–99. [PubMed] [Google Scholar]
- 24.Omeñaca F, Vázquez L, Garcia-Corbeira P, Mesaros N, Hanssens L, Dolhain J, Gómez IP, Liese J, Knuf M. Immunization of preterm infants with GSK’s hexavalent combined diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliovirus-Haemophilus influenzae type b conjugate vaccine: A review of safety and immunogenicity. Vaccine [Internet]. 2018. [cited 2018 November1];36:986–96. Available from https://www.sciencedirect.com/science/article/pii/S0264410X1830029X?via%3Dihub [DOI] [PubMed] [Google Scholar]
- 25.Bonhoeffer J, Siegrist CA, Heath PT. Immunisation of premature infants. Arch Dis Child. 2006;91:929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langkamp DL, Hoshaw-Woodard S, Boye ME, Lemeshow S. Delays in receipt of immunizations in low-birth-weight children: a nationally representative sample. Arch Pediatr Adolesc Med [Internet]. 2001;155:167–72. http://www.ncbi.nlm.nih.gov/pubmed/11177092. [DOI] [PubMed] [Google Scholar]
- 27.Tozzi AE, Piga S, Corchia C, Di Lallo D, Carnielli V, Chiandotto V, Fertz MC, Miniaci S, Rusconi F, Cuttini M. Timeliness of routine immunization in a population-based Italian cohort of very preterm infants: results of the ACTION follow-up project. Vaccine [Internet]. 2014;32:793–99. doi: 10.1016/j.vaccine.2013.12.044. [DOI] [PubMed] [Google Scholar]
- 28.Ochoa TJ, Zea-Vera A, Bautista R, Davila C, Salazar JA, Bazán C, López L, Ecker L. Vaccine schedule compliance among very low birth weight infants in Lima, Peru. Vaccine [Internet]. 2015;33:354–58. doi: 10.1016/j.vaccine.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batra JS, Eriksen EM, Zangwill KM, Lee M, Marcy SM, Ward JI. Evaluation of vaccine coverage for low birth weight infants during the first year of life in a large managed care population. Pediatrics [Internet]. 2009;123:951–58. doi: 10.1542/peds.2008-0231. [DOI] [PubMed] [Google Scholar]
- 30.Nestander M, Dintaman J, Susi A, Gorman G, Hisle-Gorman E. Immunization completion in infants born at low birth weight. J Pediatric Infect Dis Soc. 2017;7:e58–e64. [DOI] [PubMed] [Google Scholar]
- 31.Nestander M, Dintaman J, Susi A, Gorman G, Hisle-Gorman E. Risk factors impacting incomplete immunization in low birth weight and preterm infants. Pediatrics [Internet]. 2018;141:758–758. http://pediatrics.aappublications.org/content/141/1_MeetingAbstract/758?utm_source=TrendMD&utm_medium=TrendMD&utm_campaign=Pediatrics_TrendMD_0. [Google Scholar]
- 32.Davis RL, Rubanowice D, Shinefield HR, Lewis N, Gu D, Black SB, DeStefano F, Gargiullo P, Mullooly JP, Thompson RS, et al. Immunization levels among premature and low-birth-weight infants and risk factors for delayed up-to-date immunization status. J Am Med Assoc. 1999;282:547–53. [DOI] [PubMed] [Google Scholar]
- 33.McCrossan P, McCafferty C, Murphy C, Murphy J. Retrospective review of administration of childhood primary vaccination schedule in an Irish tertiary neonatal intensive care unit. Public Health [Internet]. 2015;129:896–98. doi: 10.1016/j.puhe.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Laforgia N, Mauro AD, Bianchi FP, Mauro FD, Zizzi A, Capozza M, Intini S, Gallone MS, Tafuri S, Are pre-terms born timely and right immunized? Results of an Italian cohort study. Hum Vaccines Immunother. 2018;1–5. [Internet]. doi: 10.1080/21645515.2018.1428509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fredrickson DD, Davis TC, Arnold CL, Kennen EM, Humiston SG, Cross JT, Bocchini JA. Childhood immunization refusal: provider and parent perceptions. Fam Med. 2004;36:431–39. [PubMed] [Google Scholar]
- 36.Langkamp D, Langhough R. Primary care physicians’ knowledge about diphtheria-tetanus-pertussis immunizations in preterm infants. Pediatrics [Internet]. 1992;89:52–55. Available from http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed2&NEWS=N&AN=1992049395 [PubMed] [Google Scholar]
- 37.Lim SS, Stein DB, Charrow A, Murray CJL. Tracking progress towards universal childhood immunisation and the impact of global initiatives: a systematic analysis of three-dose diphtheria, tetanus, and pertussis immunisation coverage. Lancet (London, England) [Internet]. 2008. [cited 2018 November6];372:2031–46. Available from http://www.ncbi.nlm.nih.gov/pubmed/19070738 [DOI] [PubMed] [Google Scholar]
- 38.Murray CJL, Shengelia B, Gupta N, Moussavi S, Tandon A, Thieren M. Validity of reported vaccination coverage in 45 countries. Lancet (London, England) [Internet]. 2003. [cited 2018 November6];362:1022–27. Available from http://www.ncbi.nlm.nih.gov/pubmed/14522532 [DOI] [PubMed] [Google Scholar]
- 39.Modi RN, King C, Bar-Zeev N, Colbourn T. Caregiver recall in childhood vaccination surveys: systematic review of recall quality and use in low- and middle-income settings. Vaccine [Internet]. 2018. [cited 2018 November6];36:4161–70. Available from https://www.sciencedirect.com/science/article/pii/S0264410X18307552 [DOI] [PubMed] [Google Scholar]
- 40.Stein-Zamir C, Mph M, Zentner G, Fracp MB, Tallen-Gozani E, Grotto I, Phd MM. The Israel national immunization registry. IMAJ. 2010;12:296–300. [PubMed] [Google Scholar]
- 41.Stein-Zamir C, Israeli A. Age-appropriate versus up-to-date coverage of routine childhood vaccinations among young children in Israel. Hum Vaccines Immunother. 2017;13:2102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubin L, Belmaker I, Somekh E, Urkin J, Rudolf M, Honovich M, Bilenko N, Grossman Z. Maternal and child health in Israel: building lives. Lancet [Internet]. 2017;389:2514–30. doi: 10.1016/S0140-6736(17)30929-7. [DOI] [PubMed] [Google Scholar]
- 43.Stein-Zamir C, Israeli A, Grotto I. Immunization registry as a digital assessment tool during outbreaks. Clin Microbiol Infect [Internet]. 2020. [cited 2020 September27] Available from https://linkinghub.elsevier.com/retrieve/pii/S1198743X20305383. [DOI] [PubMed] [Google Scholar]
- 44.Rouers EDM, Berbers GAM, JAP VD, Sanders EAM, Bruijning-Verhagen P. Timeliness of immunisations in preterm infants in the Netherlands. Vaccine [Internet]. 2019;37:5862–67. doi: 10.1016/j.vaccine.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Nakatudde I, Rujumba J, Namiiro F, Sam A, Mugalu J, Musoke P. Vaccination timeliness and associated factors among preterm infants at a tertiary hospital in Uganda. PLoS One [Internet]. 2019;14:e0221902. doi: 10.1371/journal.pone.0221902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zimmerman DR, Verbov G, Edelstein N, Stein-Zamir C. Preventive health services for young children in Israel: historical development and current challenges. Isr J Health Policy Res. 2019;8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization (WHO) . Israel: WHO and UNICEF estimates of immunization coverage: 2018 revision [Internet]. [cited 2020 July20]; Available from: http://www.who.int/immunization/monitoring_surveillance/data/isr.pdf
- 48.Israel Central Bureau of Statistics . Characterization and classification of geographical units by the socio-economic level of the population 2015 [Internet]. [cited 2020 July20]; Available from: https://www.cbs.gov.il/en/mediarelease/Pages/2018/Characterization-and-Classification-of-Geographical-Units-by-the-Socio-Economic-Level-of-the-Population-2015.aspx
- 49.Ginsberg GM, Somekh E, Schlesinger Y. Should we use Palivizumab immunoprophylaxis for infants against respiratory syncytial virus? - A cost-utility analysis. Isr J Health Policy Res [Internet]. 2018. [cited 2020 September27];7. Available from: https://pubmed.ncbi.nlm.nih.gov/30554570/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stein-Zamir C, Israeli A. Timeliness and completeness of routine childhood vaccinations in young children residing in a district with recurrent vaccine-preventable disease outbreaks, Jerusalem, Israel. Eurosurveillance. 2019;24:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hofstetter AM, Jacobson EN, deHart MP, Englund JA. Early Childhood Vaccination Status of Preterm Infants. Pediatrics [Internet]. 2019;144:e20183520. https://pediatrics.aappublications.org/content/early/2019/08/05/peds.2018-3520?sso=1&sso_redirect_count=2&nfstatus=401&nftoken=00000000-0000-0000-0000-000000000000&nfstatusdescription=ERROR%3ANolocaltoken&nfstatus=401&nftoken=00000000-0000-0000-0000-00. [DOI] [PubMed] [Google Scholar]
- 52.Stein C, Bardugo D, Shoob H. Pertussis in infants under one year old: risk markers and vaccination status — A case-control study. Vaccine [Internet]. 2015;33:2073–78. doi: 10.1016/j.vaccine.2015.02.050. [DOI] [PubMed] [Google Scholar]


