Abstract
Background
A year after the COVID-19 pandemic started, there are still few scientific reports on COVID-19 in Africa. This study explores the clinical profiles and factors associated with COVID-19 in Cameroon.
Materials and methods
In this prospective cohort study, we followed patients admitted for suspicion of COVID-19 at Djoungolo Hospital between 01st April and 31st July 2020. Patients were categorised by age groups and disease severity: mild (symptomatic without clinical signs of pneumonia), moderate (with clinical signs of pneumonia without respiratory distress) and severe cases (clinical signs of pneumonia and respiratory distress not requiring invasive ventilation). Demographic information and clinical features were summarised. Multivariable analysis was performed to predict risk.
Findings
A total of 313 patients were admitted during the study period; 259 were confirmed cases of COVID-19 by Polymerase Chain Reaction (PCR). Among the confirmed cases, the male group aged 40 to 49 years (13.9%) was predominant. Disease severity ranged from mild (26.2%; n = 68) to moderate (59%; n = 153) to severe (14.7%; n = 38); the case fatality rate was 1% (n = 4). Dysgusia (46%; n = 119) and hyposmia/anosmia (37.8%; n = 98) were common features of COVID-19. Nearly one-third of patients had comorbidities (29%; n = 53), of which hypertension was the most common (18.9%; n = 49). Participation in mass gatherings (Odds Ratio (OR) = 2.37; P = 0.03) and dysgusia (OR = 2.09, P = 0.02) were predictive of diagnosis of COVID-19. Age groups 60 to 69 (OR = 7.41; P = 0.0001), 50 to 59 (OR = 4.09; P = 0.03), 40 to 49 (OR = 4.54; P = 0.01), male gender (OR = 2.53; P = 0.04), diabetes (OR = 4.05; P = 0.01), HIV infection (OR = 5.57; P = 0.03), lung disease (OR = 6.29; P = 0.01), dyspnoea (OR = 3.70; P = 0.008) and fatigue (OR = 3.35; P = 0.02) significantly predicted COVID-19 severity.
Conclusions
Most COVID-19 cases in this study were benign with low fatality. Age (40–70), male gender, HIV infection, lung disease, dyspnoea and fatigue are associated with severe COVID-19. Such findings may guide public health decision-making.
Introduction
In December 2019, COVID-19 was first identified in Wuhan, Capital City of Hubei Province, in China [1]. COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel ribonucleic acid (RNA) betacoronavirus [2]. COVID-19 was declared a public health emergency of international concern on 30th January 2020 by the World Health Organisation (WHO) and it became a pandemic on the 11th March 2020, acknowledging the rapid spread of the disease across continents [3].
In Cameroon, the first case was reported on the 06th March 2020, a traveler who arrived in Cameroon on the 24th February 2020 from France [4]. As a response, Cameroon adopted public health measures which evolved with the trends of the pandemic. On the 17th March 2020, social regulations were enforced in Cameroon: closure of borders, confinement at home from 6PM, and regulations on transportation means (passengers 1m apart) [5]. Towards 13th April 2020, it became compulsory to wear a facial mask [6]. Specialized COVID-19 treatment centres and laboratories were identified for diagnosis and management of COVID-19 in Cameroon.
In Cameroon, the epidemiology of COVID-19 may be peculiar for a number of reasons. First, like most other African countries, Cameroon suffers from the double burden of infectious diseases (e.g. tuberculosis, Human immunodeficiency virus infection and acquired immunodeficiency syndrome (HIV/AIDS) and malaria) and non-communicable diseases (e.g., sickle cell diseases, cancer, cardiovascular diseases, diabetes, and renal diseases amongst others) [7]. In addition, confinement was not the hallmark of mitigation efforts in Cameroon [5]. For these reasons, we expected all these factors to contribute to an escalation of the transmission of COVID-19 in Cameroon. Unfortunately, less than 3.9% of scientific reports on COVID-19 have originated from Africa [8] and very few from Cameroon [9]. To fill this gap, the study sought to describe clinical features of COVID-19 infection and risk factors for patients admitted in the Djoungolo Hospital COVID-19 treatment centre in Cameroon.
Methods
Study setting and design
Cameroon, located at the heart of Central Africa, is a country with a young population of over 25 million with only 2.7% of people aged more than 65 years [10]. The environment is also distinct with high temperatures and meteorological variabilities [11]. Moreover, the sociocultural context is unique with strong beliefs in traditional medicine [12]. The Djoungolo Hospital, located in Yaounde, the Centre Region, was one of the six COVID-19 treatment centres dedicated officially for the management of suspected and confirmed cases of COVID-19 by the Ministry of Public Health and was supported by Medecins Sans Frontieres (MSF). We followed up patients admitted at the Djoungolo Hospital for COVID-19 infection from the 01st April 2020 to the 31st July 2020. At the beginning of the pandemic, COVID-19 patients were discharged from the hospital based on availability of a negative PCR results after 14 days of hospital admission.
Study participants
We considered eligible for this study all patients admitted at the Djoungolo Hospital for suspicion of COVID-19 and/or with a confirmed diagnosis of COVID-19 by PCR according to national protocols proposed by the Ministry of Public Health. The study goals were explained to potential study participants. All eligible patients who gave their consent were included in the study. The standard WHO case definition of COVID-19 was used as a checklist for admission [13]. Suspected cases who ultimately had no laboratory confirmation of infection with SARS-CoV-2 were considered non-cases. Patients who had a positive test for SARS-CoV-2 by PCR, were confirmed cases. Confirmed cases were categorised by age groups as follows: 0 to 17, 18 to 29, 30 to 39, 40 to 49, 50 to 59, 60 to 69 and more than 70 years. COVID-19 cases were also grouped by disease severity as mild (i.e. symptomatic without clinical signs of pneumonia), moderate (with clinical signs of pneumonia without respiratory distress) and severe cases (clinical signs of pneumonia and respiratory distress not requiring invasive ventilation) [14]. Patients who required invasive ventilation were considered critical and had to be referred to specialised centres when possible. We considered mild and moderate severity levels as benign forms of COVID-19.
Data collection
Socio-demographic and clinical events were recorded on a case-report form (CRF) designed exclusively for all suspected or confirmed cases of COVID-19 patients admitted in Djoungolo Hospital. Sociodemographic variables collected included gender, age, religion, profession, residence, risk of exposure (i.e., recent travel, exposure to health facility, exposure to a traditional healer, and any other form of attendance to a gathering), and contact tracing (i.e., contacts traced and tested with corresponding dates). Clinical events were recorded including symptoms and date of onset, comorbidities, patient itinerary (from onset of symptoms to discharge), complications, diagnostic procedure, test results, and treatment provided.
Data management and analysis
Data from the CRFs was entered into a password-protected line list in Excel. A total of 26 patients with more than 80% missing variables were automatically excluded from the analysis. Epidemic curves were constructed with admission dates at the treatment centre. Age sex pyramids were computed for all confirmed cased and for severe COVID-19 cases. Comorbidities and symptoms were summarised as counts and percentages. Length of hospital stay and time-to-hospitalisation were expressed as ranges and medians. We compared eligible patients with a confirmed diagnosis of COVID-19 with non-cases. Multivariate logistic regression was used to determine the associations between the diagnosis of COVID-19 infection and the following variables: age, gender, profession, smoking, symptoms and exposure (travel history and participation in a mass gathering). We compared confirmed severe cases with confirmed non-severe cases. Multivariate logistic regression analysis was performed to determine the association between age, gender, underlying comorbidity, symptoms, and the dependent variable of severity of disease. The glmulti package was used in R for automated model selection and multimodel inference [15]. The fit and plausibility of various models [15] were examined in RStudio, focusing on models which contained none, one, and up to seven (i.e., all) of moderator variables for risk of severity. We used two groups of moderator variables: group 1 for the prediction of COVID-19 diagnosis and group 2 for the prediction of severity of COVID-19. Group 1 included age group, gender, symptoms and exposure. Group 2 included age groups, gender and comorbidities (i.e., HIV infection, diabetes, lung disease and hypertension). We considered models with main effects only. The Akaike information criterion (AIC) [16] was used to choose the most performant model. In other words, the best model was considered to have the lowest AIC with Akaike weights and Kullback-Leibler divergence indicating the highest probability of model accuracy. A p-value <0.05 for any test or model was considered statistically significant. Data analysis was performed with STATA version 13 and RStudio Version 1.3.1073 for modelling.
Ethical oversight
Ethical clearance (Number 2020/09/1294/CE/CNERSH/SP) was sought from the National Ethical Committee of Research for Human Health in Cameroon. Administrative authorisations were also obtained from all authorities concerned. All adult study participants gave a verbal consent to participate in this study and this was documented on the case report forms (CRFs). For participants below the age of 21 years and unstable participants, consent was obtained from parents or other adult witnesses.
Results
Patients characteristics
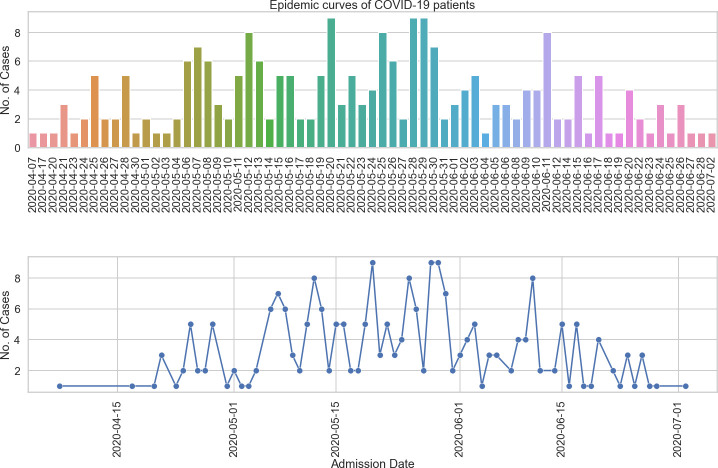
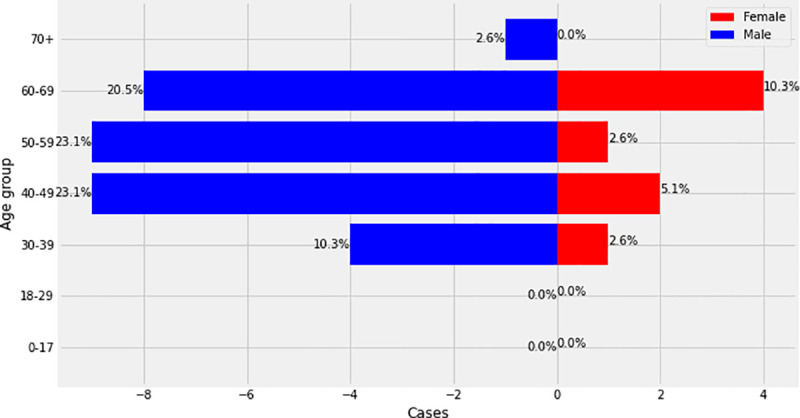
During the study period, a total number of 313 patients were admitted at the Djoungolo Hospital with suspicion for COVID-19, among which 259 (82.7%) were confirmed cases and 54 (17.3%) non-cases (Table 1). Admissions evolved in a sawtooth–like wave, with some peaks in May and June and a major drop in admissions around late June 2020. The number of cases admitted daily ranged from 1 to 9 patients (Fig 1). Among all confirmed cases, there was a male predominance and the most prevalent age category was 40 to 49 years (13.9%) (Figs 2 & 3). In the group of severe patients, there was also a male predominance but the most common age groups were 40 to 49 (23%) and 50 to 59 years (23%).
Table 1. Clinical characteristics of patients admitted for suspicion of COVID-19 at the Djoungolo Hospital.
| Characteristic | Confirmed cases | Non-cases | |||
|---|---|---|---|---|---|
| Mild | Moderate | Severe | Total | ||
| Disease severity | 68 (26.2%) | 153 (59%) | 38 (14.7%) | 259 | 54 |
| Male | 54(33.3%) | 78 (48.2%) | 30 (18.5%) | 162 | 36 |
| Female | 32 (36%) | 49 (55%) | 8 (9%) | 89 | 18 |
| Mean Age | 40 | 45 | 53 | - | 45 |
| Retired | 15 (37.5%) | 16 (40%) | 9 (22.5%) | 40 | 12 |
| Employed (formal) | 16 (27.5%) | 32 (55.2%) | 10 (17.2%) | 58 | 6 |
| Employed (informal) | 30 (31.6%) | 46 (48.4%) | 19 (20%) | 95 | 17 |
| Prisoners | 3 (27.3%) | 8 (72.7%) | 0 | 11 | 8 |
| Healthcare workers | 7 (36.8%) | 12 (63.2%) | 0 | 19 | 6 |
| Students | 13 (56.5%) | 10 (43.5%) | 0 | 23 | 5 |
| Unemployed | 2 (50%) | 2 (50%) | 0 | 4 | 0 |
| Hypertension | 11 (22.4%) | 25 (51%) | 13 (26.5%) | 49 | 9 |
| Diabetes | 3 (20%) | 6 (40%) | 6 (40%) | 15 | 8 |
| HIV infection | 0 | 4 (57.2%) | 3 (42.8%) | 7 | 4 |
| Cardiovascular diseases | 6 (28.6%) | 9 (42.8%) | 6 (28.6%) | 21 | 6 |
| Chronic lung disease | 0 | 3 (50%) | 3 (50%) | 6 | 3 |
| Chronic kidney disease | 0 | 1 (100%) | 0 | 1 | 0 |
| Sickle cell disease | 0 | 1 (100%) | 0 | 1 | 0 |
| Pregnancy | 3 (75%) | 1 (25%) | 0 | 4 | 1 |
| Number of smokers | 3 (33.3%) | 0 | 6 (66.6%) | 9 | 3 |
| Number with a recent travel history (<14 days) | 16 (27.1%) | 11 (18.6%) | 32 (54.2%) | 59 | 10 |
| Patients who participated in a mass gathering | 13 (26%) | 17 (34%) | 20 (40%) | 50 | 6 |
Fig 1. Epidemic curve of COVID-19 cases at the Djoungolo Hospital.
Fig 2. Age/sex pyramid (all cases).
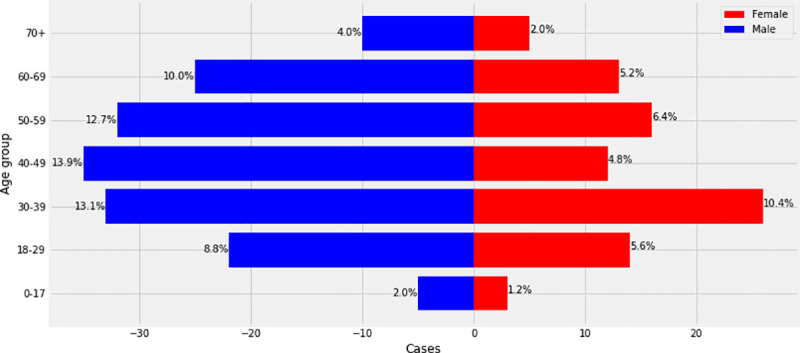
Fig 3. Age/sex pyramid (severe cases).
Clinical profile of confirmed cases
Symptoms and signs
Most confirmed cases presented with a cough (59%; n = 153), fever (51.7%; n = 134), dyspnoea (49.8%; n = 129), fatigue (50.1%; n = 130), and headaches (49.8%; n = 129). Other common symptoms were dysgusia (46%; n = 119), arthralgia or myalgia (43%; n = 112), and hyposmia/anosmia (37.8%; n = 98). Nasal discharge (5.4%; n = 14) and sore throat (8.8%; n = 23) were the least common symptoms.
Severity and comorbidities
Most confirmed cases had benign disease (85.3%; n = 221), some patients had severe disease (14.6%; n = 38) and required a supplemental oxygen supply. Among the severe cases, a few patients became critical and required a transfer to specialised centres (4%; n = 18). However, it is worth mentioning that only 30% of critical patients who required a transfer were finally transferred as a result of overcrowding in sophisticated COVID-19 treatment centres with intensive care units. Four confirmed cases died, yielding a CFR of 1.5%. Twenty-nine percent (n = 75) had at least one comorbidity. The most common comorbidities were hypertension (18.9%; n = 49), other cardiovascular diseases (8.1%; n = 21), diabetes (5.8%: n = 15), and HIV infection (2.7%; n = 7). Three out of the 04 patients (75%) who died had hypertension; one patient had both hypertension and diabetes. The deceased cases were aged 53, 66, 68, and 73 years.
Hospital course
The average length of hospitalisation was 15 days (10–26 days). Length of stay was lowest for male children aged 11 months to 17 years and highest for male patients aged more than 70 years. Time to hospitalisation ranged from 0 to 40 days for some patients, but the majority of patients were admitted 07 to 13 days after symptom onset.
Treatment
All patients received a treatment protocol with oral chloroquine, paracetamol, vitamin C, zinc, amoxicillin combined with clavulanic acid, and azithromycin. Depending on severity and comorbidities, some patients received anticoagulants, corticosteroids or intravenous antibiotics. Fifteen percent of confirmed cases underwent non-invasive ventilation (n = 38).
Risk factors
Factors associated with COVID-19 diagnosis
We compared groups of confirmed (n = 259; 82.7%) cases and non-cases (n = 54; 17.3%). The most important variables for prediction of risk of diagnosis with COVID-19 were chills, fatigue, abdominal pains, myalgia/arthralgia, running nose, dysgusia and participation at a mass gathering. Abdominal pains, (OR = 6.41; P = 0.04: Confidence interval CI = 1.23–118.1), loss of state (OR = 2.09; P = 0.02: CI = 1.1–4.14), myalgia/arthralgia (OR = 2.35; P = 0.01: CI = 1.19–4.84), loss of taste (OR = 2.09; P = 0.02: CI = 1.1–4.14) and participation at a mass gathering (OR = 2.37; P = 0.03: CI = 0.97–6.8) significantly increased the odds of being diagnosed with COVID-19. Chills (OR = 0.46; P = 0.03: CI = 2.6–4.8.06), fatigue (OR = 0.46; P = 0.01; CI = 0.23–0.89) and running nose (OR = 0.26; P = 0.02; CI = 0.09–0.79) decreased the occurrence of a diagnosis of COVID-19in our settings (Table 2).
Table 2. Output for multivariable analysis of factors associated with a diagnosis of COVID-19.
| OR | Std Error | t Value | P value | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|
| Chills | 0.46 | 0.05 | -2.03 | 0.03* | 2.6 | 8.06 |
| Fatigue | 0.46 | 0.04 | -2,37 | 0.01* | 0.23 | 0.89 |
| Abdominal pains | 6.41 | 0.07 | 2.02 | 0.04* | 1.23 | 118.6 |
| Arthralgia/myalgia | 2.35 | 0.04 | 2.47 | 0.01* | 1.19 | 4.84 |
| Running nose | 0.26 | 0.08 | -2.32 | 0.02* | 0.09 | 0.79 |
| Loss of taste | 2.09 | 0.04 | 2.29 | 0.02* | 1.1 | 4.14 |
| Participation in a mass gathering | 2.37 | 0.05 | 2.12 | 0.03* | 0.97 | 6.8 |
*Level of statistical significance.
Factors associated with COVID-19 severity
We compared groups of benign confirmed cases (85.3%; n = 221) and severe cases (14.7%; n = 38). The most important variables for prediction of risk of COVID-19 severity were: diabetes, lung disease, HIV infection, male gender, and age groups 40 to 49, 50 to 59, and 60- to 69. In our settings, age groups 60 to 69, 50 to 59, 40 to, male gender, diabetes, HIV infection and lung disease were significant predictors of COVID-19 severity (Table 3). Dyspnoea and fatigue are warning signs of COVID-19 severity (Table 4).
Table 3. Multivariable analysis of comorbidities associated with COVID-19 severity.
| OR | Std Error | t Value | P value | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|
| Male gender | 2.53 | 0.04 | 1.98 | 0.04* | 1.06 | 6.76 |
| Age group 40 to 49 | 4.54 | 0.05 | 2.37 | 0.01* | 1.52 | 14.63 |
| Age group 50 to 59 | 4.09 | 0.05 | 2.16 | 0.03 * | 1.36 | 13.18 |
| Age group 60 to 69 | 7.41 | 0.06 | 3.56 | 0.0001*** | 2.52 | 23.85 |
| HIV infection | 5.57 | 0.13 | 0.13 | 0.03* | 0.92 | 31.76 |
| Diabetes | 4.05 | 0.09 | 2.53 | 0.01* | 1.12 | 14.15 |
| Lung disease | 6.29 | 0.13 | 0.14 | 0.02* | 0.91 | 44.93 |
*Level of statistical significance.
Table 4. Multivariable analysis of symptoms associated with COVID-19 severity.
| OR | Std error | t value | P- value | 95% Confidence interval | ||
|---|---|---|---|---|---|---|
| Male gender | 2.75 | 0.47 | 2.12 | 0.008* | 1.12 | 7.4 |
| Age group 40 to 49 | 4.79 | 0.60 | 2.58 | 0.009** | 1.49 | 16.63 |
| Age group 50 to 59 | 3.19 | 0.59 | 1.95 | 0.05 | 1.01 | 10.7 |
| Age group 60 to 69 | 6.65 | 0.59 | 3.13 | 0.001** | 2.07 | 22.3 |
| Fatigue | 3.35 | 0.52 | 2.31 | 0.02* | 1.26 | 10.1 |
| Dyspnoea | 3.70 | 0.49 | 2.64 | 0.008** | 1.46 | 10.45 |
| Abdominal pains | 0.31 | 0.8 | 61.42 | 0.15 | 0.04 | 1.27 |
| Muscle pains | 2.25 | 0.59 | 1.93 | 0.05 | 1.00 | 5.23 |
*Level of statistical significance.
Discussion
This study adds to the description of COVID-19 in African hospital settings where there is a huge gap in the literature. Most patients experienced a benign form of COVID-19 infection with low fatality. Mass gathering, loss of taste, abdominal pains and myalgia/arthralgia were predictive of a diagnosis of COVID-19 in suspected cases. Male gender, older age, comorbidities (i.e., diabetes, HIV, and lung disease) and the presence of fatigue and dyspnoea were predictors of COVID-19 severity.
Analysis of COVID-19 severity and mortality varies across the world. Grant et al. (2020) reported findings synthesised from studies conducted across 10 Western countries: United Kingdom UK, the United States of America, Singapore, Italy, Australia, Japan, Korea, and the Netherlands [17]. These authors indicated that 19% of patients hospitalised in these countries required non-invasive ventilation, which is quite close to the value found in our setting [17]. The CFR was relatively low at the Djoungolo Hospital (1%) and this can be partially explained by standard operating procedures for management at Djoungolo Hospital which required transfer of critical cases to more sophisticated treatment centres. However, due to overcrowding in sophisticated centres, some critical patients were managed at the Djoungolo Hospital and this accounts for the fatalities reported. Nonetheless, the CFR obtained in this study was similar to the Cameroonian National CFR estimated at 2% as of the 07th October 2020 [4]. Our CFR was also similar to the average CFR reported in South Korea and Germany, while countries like France and Belgium had relatively high CFR (20 and 16% respectively) [5]. Grant et al. (2020) found a CFR of 7% in their meta-analysis [17]. This variability could be due to the difference of age of confirmed cases. As seen in our study, more than 55% of patients in our study were aged less than 50 and the mean age was 44 years as compared to 49 years as reported by Grant et al. [17].
Clinical features of COVID-19 infection in our setting were slightly different from those reported by studies in high-income countries. The Grant et al. (2020) meta-analysis reported: fever (78% [95% CI 75%-81%] in 138 studies, 21,701 patients; I2 94%), cough (57% [95% CI 54%-60%]; 138 studies, 21,682 patients; I2 94%) and fatigue (31% [95% CI 27%-35%]; 78 studies, 13,385 patients; I2 95%) as the most common symptoms. Fatigue (31%, CI = 27–35%, 31 studies; I2 = 95%), dyspnoea (23%, CI = 19–28%; 94 studies; I2 = 97%) and headaches (13%, CI = 10–16, 65 studies) had smaller proportions than those reported in our study [17]. In the same line with Grant et al. (2020), gastrointestinal manifestations, sore throat, and nasal discharge were also less common in the current study. In our study, ophthalmic manifestations were not prevalent. Differences in population characteristics (i.e., age, race, genetic make-up or immunity) and even settings (climate and environment) might explain the differences noticed in the findings.
Dysgeusia (46%) and hyposmia/anosmia (39%) had higher prevalence in our study when compared with findings reported by Grant et al. (2020) (i.e., hypogusia 25%, hyposmia 4%) [17]. In a systematic review recently published by Mehraeen et al. (2020), 95% of studies had anosmia as a feature of COVID-19 infection [18]. It is worth mentioning that Mehraeen et al. (2020) and Grant et al. (2020) had similar methods as they both included evidence from at least 10 Western countries and conducted their searches from December 2019/January 2020 to April 2020. However, Grant et al. (2020) had no language restrictions and reviewed a wider range of papers. This difference in scope might explain the difference in findings. In general, there is growing evidence that COVID-19 also presents with olfactory as well as gustatory dysfunction, but the pathogenesis is not yet known [18]. This finding could imply that the current case definition of COVID-19 published by the WHO may need to be updated [13].
In our study, 29% of patients suffered from a comorbidity, while Morgan et al. (2020) had twice more patients who had at least 01 comorbidity (40%) in a meta-analysis of COVID-19 and comorbidities [19]. Morgan et al. (2020) synthesized evidence from Western countries including China, South Korea, Australia, and the USA, which have relatively older populations [19]. This difference in ages might explain the higher prevalence of cases with comorbidities in their settings. They also found the commonest comorbidity to be hypertension and the majority of fatal cases had at least 01 comorbidity (74%), in agreement with our findings. They equally observed that diabetes and respiratory illnesses were prevalent in the deceased [19], which is not the case in the current study where hypertension was identified as common in the deceased.
The median length of hospital stay was 15 (10 to 27 days) in this study. In a systematic review of length of hospital stay for COVID-19 patients, Rees et al. (2020) observed that length of hospital stay was generally higher for patients followed up in China. The median range of length of hospital stay ranged from 4 to 53 days in China and 4 to 21 days elsewhere (i.e., Europe, USA and UK) [20]. Most studies included by Rees et al. (2020) were conducted in China (88%) and specifically in Wuhan [20]. Lengthy hospital stays reported by Rees et al. (2020) can be explained by inclusion of studies analysing critically ill and old patients (average 68–69 years) [20]. In our context, long hospital stays were equally observed in patients aged more than 70 years. The speed at which the last negative test for SARS-CoV2 was obtained also explains lengthy stays in our study. With the reduced testing capacity at the beginning of the epidemic in Cameroon, it was a real challenge to obtain PCR results after 14 days. Additionally, length of hospitalization can vary by the culture of clinical practice, as well as by disease severity and logistical constraints, thus complicating comparisons among international settings.
Worldwide, several diagnostic models have been developed for the detection of COVID-19 in suspected cases as well as for the prognosis of COVID-19. Laure et al. (2020) synthesised available evidence on models for COVID-19, The most frequent predictors of COVID-19 diagnosis in suspected cases were vital signs, flu-like signs and symptoms, age, sex, radiological features, laboratory results (electrolytes, white blood cell count, liver enzymes etc), cough or sputum and contacts with confirmed cases of COVID-19 [21]. In the context of this study, we obtained a few similar predictors including flu-like symptoms (myalgia/arthralgia) and exposure. However, most flu-like symptoms such as runny nose, fatigue and chills were not predictive of a diagnosis of COVID-19 in our study. Unfortunately, laboratory and radiological tests could not be systematically performed for patients in our setting.
Elsewhere, increasing age, male gender, comorbidities (e.g., diabetes, lung disease) have also been associated with COVID-19 severity [22–24]. In addition, HIV infection appeared as a significant predictor of severity in this study though not reported in Western settings. In Cameroon, HIV prevalence was estimated at 2.9% in 2019, with 75% of HIV infected people knowing their status and only 62% on treatment [25]. Perhaps this burden might explain our findings. The most significant variable for prediction of COVID-19 severity in the current study was age group 60 to 69 and unexpectedly, people aged more than 70 years were not at increased risk of COVID-19 severity in our study. The small representation of patients aged more than 70 years in our sample might explain this finding. In a meta-analysis conducted by Rahman et al. (2020), male gender, hypertension, diabetes, fatigue or myalgia, and smoking history were found to be risk factors for COVID-19 severity [22]. There are some differences in comparison with our results, which do not include smoking, hypertension as risk factors for severity. Studies included in the meta-analysis were conducted in China, perhaps these differences in population characteristics such as smoking history might account for differences in risk factors for severity. For instance, Rahman et al. (2020) had a proportion of 11% of cigarette smokers compared with 1% in our study population [22]. On the other hand, diabetes and male gender have been consistently reported to potentially increase the risk of suffering from severe form of COVID-19 in other settings [23, 24].
This study also provided evidence that mass gatherings contribute to the spread of COVID-19. Apart from social gatherings, mass gatherings and high density activities in general are associated with generation of income and revenues through formal or informal employment and other businesses [26]. Cameroon faced a dire dilemma between restricting all mass gatherings to curb COVID-19 while suffering from an economic crisis and choosing to let businesses be open and taking the risk of increasing the spread of COVID-19, which is particularly challenging given its developing status. Due to the effects on the economy, on 30th April 2020, the government relaxed social distancing measures [11]. Restrictions were lifted on opening hours for businesses and even transportation. This change might explain, at least in part the rise in admissions at the Djoungolo Hospital from May 2020 to June 2020.
This study adds to the knowledge gap on COVID-19 in African settings. Despite its original content, our study has limitations inherent to the observational design. Only 54 suspected cases had no evidence of infection with SARS-CoV2, which might have impacted the multivariable analysis of risk of COVID-19 infection. The study was conducted at a single centre; thus generalisability of findings nationwide might not be accurate. Additionally, conducting rigorous research in a context of a novel pandemic was challenging and missing data was common. For instance, incubation periods were not calculated as only 07% had required data. Moreover, in our setting, laboratory and radiological diagnostics could not be systematically performed for patients. Some critical cases were referred to more sophisticated centres as required and this might in part explain the low CFR obtained at the Djoungolo Hospital.
Conclusions
In this study, we described clinical features of COVID-19 infection in a typical COVID-19 treatment centre in Cameroon. The Djoungolo Hospital experienced its highest number of admissions from early May to mid-June. Most patients had a benign form of COVID-19 and the CFR was low (1%). There was a broad range of prevalent symptoms in patients, including olfactory symptoms. The majority of patients admitted had no comorbidity and the commonest comorbidity was hypertension, although it did not predict severity. Mass gathering, loss of taste, abdominal pains and myalgia/arthralgia were predictors of COVID-19 diagnosis. Male gender, history of diabetes, history of lung disease, history of HIV infection, the presence of dyspnoea and fatigue were predictors of disease severity. Future studies should aim at presenting clinical and epidemiological features of COVID-19 with larger data sets.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We acknowledge the entire staff of the Djoungolo Hospital as well as representatives of the Ministry of Public Health.
Abbreviations
- AIC
Akaike information criterion
- CFR
Case fatality rate
- CI
Confidence interval
- HIV/AIDS
Human immunodeficiency virus infection and acquired immunodeficiency syndrome
- PCR
Polymerase chain reaction
- OR
odds ratio
- RNA
Ribonucleic acid
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- WHO
World Health Organisation
Data Availability
Medecins Sans Frontieres and the Regional Delegation of Public Health of the Centre both agreed to managed the Djoungolo Hospital. Therefore, data sharing is regulated by both parties. Any author willing to utilise the data for replication of the study will have to make a formal request to the Regional Delegate of Public Health of the Centre Region of Cameroon, Dr Moussi Charlotte or to the Representative Epicentre in Africa, Pr Yap Boum II. Epicentre is the research branch of Medecins Sans Frontieres. As such the anonymised data was not uploaded but is available upon formal request to any of the following: Dr Moussi Charlotte, Regional Delegate, The Regional Delegation of Public Health in Yaounde, Cameroon. E-mail: lotyomgba73@gmail.com; Telephone number: 00237676248182 Epicentre: Pr Yap Boum II, Yaounde, Cameroon. E-mail: yap.boum@epicentre.msf.org; Telephone number: 00237695276842 Cameroon National Ethics Commitee for Research in Human Health (CNERSH): cnethique_minsante@yahoo.fr Epicentre: Dr Fai Karl, Clinical Research Associate at Epicentre, Yaounde, Cameroon.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020. February 15;395(10223):497–506. Available from: https://isaric.tghn.org/protocols/ 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020. February 22;395(10224):565–74. Available from: https://www.ncbi. 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coronavirus (COVID-19) events as they happen. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen
- 4.MINSANTE-COVID-19 –Barrons la route au COVID-19. Available from: http://COVID-19.minsante.cm/
- 5.Head of Government. Government response strategy to the coronavirus pandemic (COVID-19)- Special statement by the Prime Minister Head of Government. 2020 March 24. Available from: https://www.spm.gov.cm/site/sites/default/files/special_statement_of_24_march_2020.pdf
- 6.Coronavirus: le port du masque devient obligatoire dans plusieurs pays d’Afrique centrale. Available from: https://www.lemonde.fr/afrique/article/2020/04/16/coronavirus-le-port-du-masque-devient-obligatoire-dans-plusieurs-pays-d-afrique-centrale_6036755_3212.html
- 7.Young F, Critchley JA, Johnstone LK, Unwin NC. A review of co-morbidity between infectious and chronic disease in Sub Saharan Africa: TB and Diabetes Mellitus, HIV and Metabolic Syndrome, and the impact of globalization. Vol. 5, Globalization and Health. Global Health; 2009. Available from: https://pubmed.ncbi.nlm.nih.gov/19751503/ 10.1186/1744-8603-5-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naidoo AV, Hodkinson P, Lai King L, et al. African authorship on African papers during the COVID-19 pandemic BMJ Global Health 2021;6:e004612. 10.1136/bmjgh-2020-004612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mekolo David et al. Clinical and epidemiological characteristics and outcomes of patients hospitalized for COVID-19 in Douala, Cameroon. Pan African Medical Journal. 2021;38:246. 10.11604/pamj.2021.38.246.28169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Population ages 65 and above (% of total population)—Cameroon | Data [Internet]. Available from:
- 11.Heaney A, Little E, Ng S, Shaman J. Meteorological variability and infectious disease in Central Africa: a review of meteorological data quality. Ann N Y Acad Sci. 2016. October 1;1382(1):31–43. Available from: http://doi.wiley.com/10.1111/nyas.13090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abia WE, Fonchang GN, Kaoke MD, Fomboh R, Ageh MT, Abia EA et al. Interest and perceptions on traditional medicines in Cameroon. IJR. 2015. May.2(5) Available from: https://www.researchgate.net/publication/319312281_Interest_and_perceptions_on_traditional_medicines_in_Cameroon [Google Scholar]
- 13.World Health Organisation (WHO). COVID-19: Surveilllance, case investigation and epidemiological protocols- WHO COVID-19 case definition. Aug 2020 Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-Surveillance_Case_Definition-2020.1
- 14.World Health Organisation. clinical management of COVID-19 Interim guidance. 2020 [Internet]. [cited 2020 Aug 17]. Available from: https://www.who.int/publications/i/item/clinical-management-of-covid-19
- 15.Calcagno V. Model Selection and Multimodel Inference Made Easy. May 25. Available from: https://cran.r-project.org/web/packages/glmulti/glmulti.pdf
- 16.Wagenmakers EJ, Farrell S. AIC model selection using Akaike weights [Internet]. Vol. 11, Psychonomic Bulletin and Review. Psychonomic Society Inc.; 2004. p. 192–6. Available from: https://link.springer.com/article/10.3758/BF03206482 [DOI] [PubMed] [Google Scholar]
- 17.Grant MC, Geoghegan L, Arbyn M, Mohammed Z, McGuinness L, Clarke EL, et al. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): A systematic review and meta-analysis of 148 studies from 9 countries. Hirst JA, editor. PLoS One [Internet]. 2020. June 23;15(6):e0234765. Available from: 10.1371/journal.pone.0234765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehraeen E, Behnezhad F, Salehi MA, Noori T, Harandi H, SeyedAlinaghi SA. Olfactory and gustatory dysfunctions due to the coronavirus disease (COVID-19): a review of current evidence [Internet]. Vol. 1, European Archives of Oto-Rhino-Laryngology. Springer; 2020. p. 3. Available from: 10.1007/s00405-020-06120-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gold MS, Sehayek D, Gabrielli S, Zhang X, McCusker C, Ben-Shoshan M. COVID-19 and comorbidities: a systematic review and meta-analysis. Postgrad Med. 2020. July 14;1–7. Available from: https://www.tandfonline.com/doi/full/10.1080/00325481.2020.1786964 [DOI] [PubMed] [Google Scholar]
- 20.Rees EM, Nightingale ES, Jafari Y, Waterlow N, Clifford S, Group CW, et al. COVID-19 length of hospital stay: a systematic review and data synthesis. medRxiv. 2020. May 17;2020.04.30.20084780. Available from: https://www.medrxiv.org/content/10.1101/2020.04.30.20084780v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laure Wynants, Van Calster Ben, Collins Gary S, Riley Richard D, Heinze Georg, Schuit Ewoud et al. Prediction models for diagnosis and prognosis of covid-19: systematic review and critical appraisal BMJ 2020; 369: m1328 10.1136/bmj.m1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahman A, Sathi NJ. Risk Factors of the Severity of COVID-19: a Meta-Analysis. medRxiv. 2020. May 10;2020.04.30.20086744. Available from: 10.1111/ijcp.13916 [DOI] [PubMed] [Google Scholar]
- 23.Mantovani A, Byrne CD, Zheng MH, Targher G. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: A meta-analysis of observational studies. Nutr Metab Cardiovasc Dis [Internet]. 2020. July 24;30(8):1236. Available from: /pmc/articles/PMC7258796/?report = abstract 10.1016/j.numecd.2020.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galbadage T, Peterson BM, Awada J, Buck AS, Ramirez DA, Wilson J, et al. Systematic Review and Meta-Analysis of Sex-Specific COVID-19 Clinical Outcomes. Front Med. 2020. June 23;7:348. Available from: https://www.frontiersin.org/article/10.3389/fmed.2020.00348/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.PEPFAR. CAMEROON Country Operational Plan COP 2020 Strategic Direction Summary March 23, 2020. Available from: https://www.state.gov/wp-content/uploads/2020/07/COP-2020-Cameroon-SDS-FINAL.pdf
- 26.Ryan BJ, Coppola D, Williams J, Swienton R. COVID-19 Contact tracing solutions for mass gatherings. Disaster Med Public Health Prep. 2020. July 14; 1–7. 10.1017/dmp.2020.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
Medecins Sans Frontieres and the Regional Delegation of Public Health of the Centre both agreed to managed the Djoungolo Hospital. Therefore, data sharing is regulated by both parties. Any author willing to utilise the data for replication of the study will have to make a formal request to the Regional Delegate of Public Health of the Centre Region of Cameroon, Dr Moussi Charlotte or to the Representative Epicentre in Africa, Pr Yap Boum II. Epicentre is the research branch of Medecins Sans Frontieres. As such the anonymised data was not uploaded but is available upon formal request to any of the following: Dr Moussi Charlotte, Regional Delegate, The Regional Delegation of Public Health in Yaounde, Cameroon. E-mail: lotyomgba73@gmail.com; Telephone number: 00237676248182 Epicentre: Pr Yap Boum II, Yaounde, Cameroon. E-mail: yap.boum@epicentre.msf.org; Telephone number: 00237695276842 Cameroon National Ethics Commitee for Research in Human Health (CNERSH): cnethique_minsante@yahoo.fr Epicentre: Dr Fai Karl, Clinical Research Associate at Epicentre, Yaounde, Cameroon.