Abstract
Objective
To determine the proportion of patients with neuropathic pain who achieve a clinically meaningful improvement in their pain with the use of different pharmacologic and nonpharmacologic treatments.
Data sources
MEDLINE, EMBASE, the Cochrane Library, and a gray literature search.
Study selection
Randomized controlled trials that reported a responder analysis of adults with neuropathic pain—specifically diabetic neuropathy, postherpetic neuralgia, or trigeminal neuralgia—treated with any of the following 8 treatments: exercise, acupuncture, serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), topical rubefacients, opioids, anticonvulsant medications, and topical lidocaine.
Synthesis
A total of 67 randomized controlled trials were included. There was moderate certainty of evidence that anticonvulsant medications (risk ratio of 1.54; 95% CI 1.45 to 1.63; number needed to treat [NNT] of 7) and SNRIs (risk ratio of 1.45; 95% CI 1.33 to 1.59; NNT = 7) might provide a clinically meaningful benefit to patients with neuropathic pain. There was low certainty of evidence for a clinically meaningful benefit for rubefacients (ie, capsaicin; NNT = 7) and opioids (NNT = 8), and very low certainty of evidence for TCAs. Very low-quality evidence demonstrated that acupuncture was ineffective. All drug classes, except TCAs, had a greater likelihood of deriving a clinically meaningful benefit than having withdrawals due to adverse events (number needed to harm between 12 and 15). No trials met the inclusion criteria for exercise or lidocaine, nor were any trials identified for trigeminal neuralgia.
Conclusion
There is moderate certainty of evidence that anticonvulsant medications and SNRIs provide a clinically meaningful reduction in pain in those with neuropathic pain, with lower certainty of evidence for rubefacients and opioids, and very low certainty of evidence for TCAs. Owing to low-quality evidence for many interventions, future high-quality trials that report responder analyses will be important to strengthen understanding of the relative benefits and harms of treatments in patients with neuropathic pain.
Résumé
Objectif
Déterminer la proportion de patients souffrant de douleur neuropathique qui obtiennent une amélioration cliniquement significative de leur douleur grâce à l’utilisation de divers traitements pharmacologiques et non pharmacologiques.
Sources d’information
MEDLINE, EMBASE, la Bibliothèque Cochrane et une recherche documentaire dans la littérature grise.
Sélection des études
Les études randomisées contrôlées qui présentaient une analyse des réponses d’adultes souffrant de douleur neuropathique, plus spécifiquement de neuropathie diabétique, de névralgie post-zostérienne ou de névralgie du trijumeau, traités avec l’un des 8 traitements suivants : exercice, acupuncture, inhibiteurs de la recapture de la sérotonine-norépinéphrine (IRSN), antidépresseurs tricycliques (ATC), rubéfiants topiques, opioïdes, anticonvulsivants et lidocaïne topique.
Synthèse
La revue portait sur un total de 67 études randomisées contrôlées. Des données probantes de certitude modérée étayaient le fait que les anticonvulsivants (risque relatif de 1,54; IC à 95 % de 1,45 à 1,63; nombre de sujets à traiter [NST] de 7) et les IRSN (risque relatif de 1,45; IC à 95 % de 1,33 à 1,59; NST = 7) pouvaient procurer des bienfaits cliniquement significatifs aux patients souffrant de douleur neuropathique. Des données factuelles de faible certitude indiquaient des bienfaits cliniquement significatifs produits par les rubéfiants (p. ex. capsaïcine; NST = 7) et les opioïdes (NST = 8), et des données de très faible certitude étayaient l’usage des ATC. Des données de très faible qualité démontraient que l’acupuncture était inefficace. Dans toutes les classes de médicaments, sauf les ATC, il y avait une plus grande probabilité d’obtenir des bienfaits cliniquement significatifs que de causer la cessation du médicament en raison d’événements indésirables (nombre nécessaire pour nuire entre 12 et 15). Aucune étude portant sur l’exercice ou la lidocaïne ne répondait aux critères d’inclusion, et aucune étude n’a été cernée concernant la névralgie du trijumeau.
Conclusion
Des données probantes de certitude modérée étayent le fait que les anticonvulsivants et les IRSN procurent une réduction cliniquement significative de la douleur chez les personnes souffrant de douleur neuropathique, et des données factuelles de plus faible certitude appuient les rubéfiants et les opioïdes, tandis que des données de très faible qualité proposent les ATC. En raison des données de faible qualité concernant de nombreuses interventions, il sera important d’effectuer à l’avenir des études de grande qualité qui rapportent l’analyse des répondeurs afin de mieux comprendre les bienfaits et les préjudices relatifs des traitements pour les patients souffrant de douleur neuropathique.
Neuropathic pain, caused by damage or dysfunction to the somatosensory system, affects approximately 7% to 8% of the general population.1-4 Neuropathic pain is typically chronic in nature, characterized by paroxysmal episodes, and has been associated with a large burden of disease. Greater negative effects on sleep, quality of life, anxiety and depression symptoms, and health care use are seen with neuropathic pain compared with other chronic pain conditions.5-7
In an attempt to minimize pain and burden, a variety of interventions are used for the treatment of patients with neuropathic pain conditions. The purpose of this set of systematic reviews was to assess the benefit and harms of the pharmacologic and nonpharmacologic therapies used in the management of neuropathic pain, specifically diabetic neuropathy, postherpetic neuralgia, and trigeminal neuralgia in adults. Similar to our systematic reviews of osteoarthritis8 and chronic low back pain,9 we included only randomized controlled trials (RCTs) that reported a responder analysis—the proportion of patients who achieved a clinically meaningful improvement in pain.10 This systematic review is the third in a series of reviews that will provide evidence for a guideline on the treatment of common chronic pain conditions in primary care.
METHODS
We performed 8 individual systematic reviews following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses).11
All reviews included RCTs of adults with chronic (> 3 months) neuropathic pain, specifically diabetic neuropathy, postherpetic neuralgia, and trigeminal neuralgia. These 3 conditions were chosen through consultation with primary care physicians, as they are commonly seen and treated in primary care. We performed individual systematic reviews on each of the following interventions: exercise, acupuncture, serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), topical rubefacients, opioids, anticonvulsant medications, and topical lidocaine. Studies of pharmacologic interventions were required to have a placebo comparator. Exercise or acupuncture could include sham or nonactive comparators, including education, no intervention, or wait lists.
Active agents were allowed in the comparator arm if the active agent was also in the intervention arm. At least 1 dichotomous outcome reporting how many patients achieved a clinically meaningful response (eg, a 30% reduction in pain) had to be reported for the RCT to be included. We excluded studies performed in pregnant women or in populations with acute pain conditions, studies with an active comparator, or studies reporting continuous outcomes only.
Search strategy
Two authors (D.P., J.T.) created a comprehensive search strategy (Appendix 1, available from CFPlus*) to find English publications with no date restrictions, which was performed on May 6, 2020, in 3 medical databases: MEDLINE, EMBASE, and Cochrane Library. Additionally, a gray literature search was performed using Cochrane systematic reviews and clinical trial registries.12,13
Outcomes
The primary outcome was the proportion of patients who achieved a clinically meaningful response to treatment, generally defined as the number of patients who achieved at least a 30% improvement in their pain, or in their pain and function.14 When multiple responder outcome data were reported, we used a hierarchy to prioritize outcomes (Appendix 2, available from CFPlus*; Table 1). Secondary outcomes included serious adverse events, withdrawals due to adverse events (Table 2), and individual adverse events related to each intervention.
Table 1.
Overall proportion of patients with a clinically meaningful response to treatment
| INTERVENTION TYPE | GRADE CERTAINTY OF EVIDENCE | NO. OF RCTs | INTERVENTION EVENT RATE, % (n/N) | CONTROL EVENT RATE, % (n/N) | OUTCOME MEASURED TIME FRAME | RISK RATIO (95% CI) | NUMBER NEEDED TO TREAT | P VALUE |
|---|---|---|---|---|---|---|---|---|
| Acupuncture | Very low | 3 | 22 (27/121) | 13 (16/126) | 8 to 10 wk | 1.81 (0.55 to 5.98) | NSS | NA |
| Anticonvulsant medications* | Moderate | 40 | 46 (2698/5837) | 30 (1120/3738) | 2 to 16 wk | 1.54 (1.45 to 1.63) | 7 | NA |
| • Gabapentin | 10 | 43 (678/1578) | 25 (246/974) | 2 to 16 wk | 1.60 (1.42 to 1.81) | 6 | .17 | |
| • Pregabalin | 27 | 48 (1747/3650) | 31 (758/2419) | 5 to 15 wk | 1.56 (1.45 to 1.67) | 7 | .17 | |
| • Oxcarbazepine | 3 | 43 (170/395) | 33 (79/236) | 16 wk | 1.22 (0.98 to 1.52) | NSS | .17 | |
| • Topiramate | 1 | 48 (103/214) | 34 (37/109) | 12 wk | 1.42 (1.05 to 1.91) | 8 | .17 | |
| Opioids | Low | 6 | 49 (289/593) | 36 (198/556) | 5 to 12 wk | 1.37 (1.19 to 1.57) | 8 | NA |
| Rubefacients* | Low | 10 | 49 (635/1303) | 34 (350/1041) | 6 to 52 wk | 1.40 (1.26 to 1.55) | 7 | NA |
| • Frequent application creams or low-dose patches | 5 | 37 (106/285) | 25 (62/249) | 6 to 8 wk | 1.56 (1.20 to 2.03) | 9 | .35 | |
| • Less frequent application (high-potency patches) | 5 | 52 (529/1018) | 36 (288/792) | 12 to 52 wk | 1.36 (1.22 to 1.52) | 7 | .35 | |
| SNRIs* | Moderate | 8 | 57 (995/1759) | 41 (405/987) | 6 to 13 wk | 1.45 (1.33 to 1.59) | 7 | NA |
| • Duloxetine | 6 | 59 (759/1279) | 42 (344/817) | 12 to 13 wk | 1.48 (1.34 to 1.62) | 6 | .48 | |
| • Venlafaxine or desvenlafaxine | 2 | 49 (236/480) | 36 (61/170) | 6 to 12 wk | 1.35 (1.08 to 1.69) | 8 | .48 | |
| TCAs | Very low | 2 | 78 (66/85) | 26 (22/85) | 6 to 8 wk | 3.00 (2.05 to 4.38), fixed-effects model | 2 | NA |
| 2.35 (0.79 to 6.95), random-effects model | NSS |
GRADE—Grading of Recommendations Assessment, Development and Evaluation; NA—not applicable; NSS—not statistically significant; RCT—randomized controlled trial; SNRI—serotonin-norepinephrine reuptake inhibitor; TCA—tricyclic antidepressant.
For rubefacients, SNRIs, and anticonvulsant medications, no statistically significant difference was found between individual drug types.
Table 2.
Withdrawals due to adverse events
| INTERVENTION TYPE | NO. OF RCTs | INTERVENTION EVENT RATE, % (n/N) | CONTROL EVENT RATE, % (n/N) | RISK RATIO (95% CI) | NUMBER NEEDED TO HARM |
|---|---|---|---|---|---|
| Acupuncture | 1 | 7 (2/28) | 3 (1/31) | 2.21 (0.21 to 23.11) | NSS |
| Anticonvulsant medications | |||||
| • Gabapentin | 8 | 13 (184/1470) | 8 (72/911) | 1.47 (1.13 to 1.91) | 22 |
| • Oxcarbazepine | 3 | 26 (102/395) | 7 (16/234) | 3.82 (2.28 to 6.39) | 6 |
| • Pregabalin | 24 | 11 (399/3701) | 5 (105/2240) | 2.15 (1.74 to 2.65) | 17 |
| • Topiramate | 1 | 24 (52/214) | 8 (9/109) | 2.94 (1.51 to 5.75) | 7 |
| Opioids | 6 | 14 (84/593) | 6 (31/556) | 2.55 (1.73 to 3.76) | 12 |
| SNRIs | 7 | 13 (207/1655) | 5 (42/879) | 2.48 (1.78 to 3.45) | 13 |
| Rubefacients | 3 | 6 (36/599) | 2 (8/428) | 3.31 (1.56 to 7.01) | 25 |
NSS—not statistically significant, RCT—randomized controlled trial, SNRI—serotonin-norepinephrine reuptake inhibitor.
Data collection and analysis
Selection of trials and data extraction. Each systematic review had at least 2 authors independently screen titles and abstracts for inclusion. After initial screening, each study included had 2 authors independently perform a full-text review. After determining RCTs for inclusion, 2 authors independently extracted data according to MECIR (Methodological Expectations of Cochrane Intervention Reviews).15 During the process, all disagreements were resolved through consensus or through consulting a third author.
Risk of bias assessment. We used the Cochrane Collaboration’s risk-of-bias tool16 to assess all trials included for potential sources of bias. Two independent authors reviewed each study and rated the 7 quality domains as low risk, high risk, or unclear risk of bias. Disagreements were resolved through consensus. Because of the subjectivity in measuring pain, we chose to split the blinding of participants and study personnel into 2 separate categories. The GRADE (Grading of Recommendations Assessment, Development and Evaluation) tool was used to report on the overall certainty of evidence.17
SYNTHESIS
Data synthesis
Using RevMan 5 software,18 we performed a meta-analysis for each intervention using dichotomous outcome data for the primary outcome. Both fixed- and random-effects analyses were completed. If trials included relatively similar populations and interventions and both the effect estimates and confidence intervals were comparable between fixed- and random-effects analyses, we concluded it was unlikely that small studies were disproportionately influencing the result and we reported a fixed-effects method for the primary analysis. If results of the analyses differed substantially, we reported a random-effects method to capture uncertainty derived from heterogeneity between trials.19 We chose data that came from the longest available time point for the meta-analysis. Meta-analyses were performed on withdrawals due to adverse events and individual adverse events.
We determined a priori to create subgroups within the primary meta-analysis to explore potential sources of heterogeneity. These subgroups included funding source (industry or public funding), duration of outcome reported (≤ 4 weeks, > 4 to < 12 weeks, and ≥ 12 weeks), sample size (≤ 150 or > 150 participants), type of comparator (for nonpharmacologic treatments, sham versus nonsham comparators), type of neuropathic pain (diabetic neuropathy, postherpetic neuralgia, and trigeminal neuralgia), and risk of bias (higher or lower than median Cochrane risk-of-bias score). When studies reported multiple time points, we chose the midpoint, when available, between 4 weeks or less and more than 4 to less than 12 weeks. For 12 weeks or more, we chose the time point closest to 12 weeks. We made a post hoc decision to perform subgroup analysis for each specific pharmacologic agent for anticonvulsant medications and SNRIs. In addition, we completed a subgroup analysis of frequency of application for rubefacients. Subgroup analyses were performed if at least 2 RCTs were included. An exception was made for the subgroup analysis of risk of bias, which we required to have a minimum of 4 RCTs to perform. Publication bias was analyzed using funnel plots only for interventions that included at least 8 RCTs.15
Results
A total of 33 584 unique records were retrieved from 8 searches. After screening titles and abstracts, 489 publications were selected for full-text review, with a total of 67 trials meeting our inclusion criteria (Appendix 1*).
Of the 8 included interventions, we identified adequate data to perform meta-analyses on 6 interventions: anticonvulsant medications, SNRIs, rubefacients, opioids, TCAs, and acupuncture. We did not identify any RCTs reporting responder analyses for topical lidocaine or exercise. We also did not identify any trials reporting responder analyses for the treatment of trigeminal neuralgia, including carbamazepine. All analyses reported are fixed-effects results unless indicated otherwise. All meta-analyses, adverse event data, and tables can be found in Appendix 2.*
Treatments ordered by level of certainty of evidence and relative risk
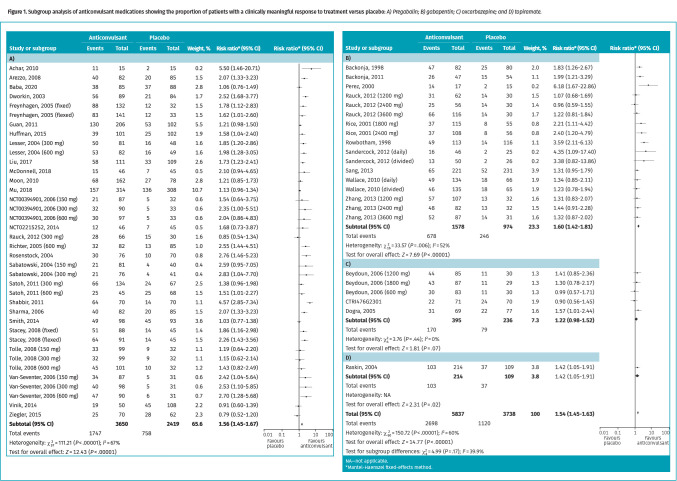
Anticonvulsant medications. Forty RCTs (Table A2 in Appendix 2*) with 9575 patients followed for 2 to 16 weeks were included. Many studies (90%) compared gabapentin or pregabalin with placebo, with a small proportion studying topiramate and oxcarbazepine. Forty-six percent of patients receiving an anticonvulsant medication and 30% of patients receiving placebo attained a clinically meaningful response to treatment (risk ratio [RR] of 1.54; 95% CI 1.45 to 1.63; number needed to treat [NNT] of 7) (Figure 1). No difference in efficacy was found between specific agents (Table A8 and Figure A2.5 in Appendix 2*). No publicly funded trials were identified.
Figure 1.
Subgroup analysis of anticonvulsant medications showing the proportion of patients with a clinically meaningful response to treatment versus placebo: A) Pregabalin; B) gabapentin; C) oxcarbazepine; and D) topiramate.
Subgroup analysis of data based on time points found a statistically significant benefit with anticonvulsant medication data reported at all time points: 4 weeks or less (6 RCTs; RR = 2.26; 95% CI 1.78 to 2.87; NNT = 4), greater than 4 weeks to less than 12 weeks (20 RCTs; RR = 1.56; 95% CI 1.44 to 1.68; NNT = 7), and 12 weeks or more (14 RCTs; RR = 1.42; 95% CI 1.30 to 1.55; NNT = 8).
Subgroup analysis based on neuropathic pain type found a greater statistically significant benefit in patients with postherpetic neuralgia (RR = 1.81; 95% CI 1.62 to 2.01) than in patients with diabetic neuropathy (RR = 1.42; 95% CI 1.32 to 1.53); however, both patient populations saw a statistically significant improvement. Similarly, a greater statistically significant benefit was seen in smaller (≤ 150 participants) trials (P = .03) and those at higher risk of bias (P = .01) than in larger trials and those at lower risk of bias.
Meta-analysis on withdrawals due to adverse events found that oxcarbazepine reported the largest difference in withdrawals (RR = 3.82; 95% CI 2.28 to 6.39; number needed to harm [NNH] of 6), followed by topiramate (RR = 2.94; 95% CI 1.51 to 5.75; NNH = 7), pregabalin (RR = 2.15; 95% CI 1.74 to 2.65; NNH = 17), and gabapentin (RR = 1.47; 95% CI 1.13 to 1.91; NNH = 22).
Meta-analysis of adverse events occurring in more than 10% of patients that were statistically significantly greater than placebo included dizziness (gabapentin, oxcarbazepine, and pregabalin), somnolence (gabapentin, oxcarbazepine, and pregabalin), nausea (oxcarbazepine), and headache (oxcarbazepine). The meta-analysis can be found in Appendix 2 (Table A13).*
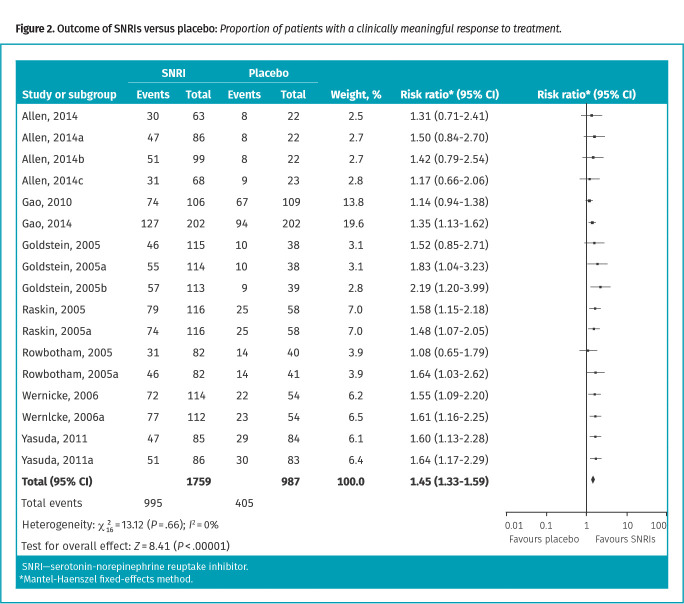
Serotonin-norepinephrine reuptake inhibitors. Eight RCTs (Table A2 in Appendix 2*) with 2746 patients followed for 6 to 13 weeks were included. Fifty-seven percent of patients receiving an SNRI and 41% of patients receiving a placebo attained a clinically meaningful response to treatment (RR = 1.45; 95% CI 1.33 to 1.59; NNT = 7; Table A4 and Figure A9.1 in Appendix 2*). Most studies (73%) involved duloxetine (Figure 2).
Figure 2.
Outcome of SNRIs versus placebo: Proportion of patients with a clinically meaningful response to treatment.
Subgroup analysis of data based on time points found no statistically significant benefit for SNRIs at more than 4 weeks to less than 12 weeks (1 RCT; RR = 1.36; 95% CI 0.97 to 1.91), but found a benefit at more than 12 weeks (7 RCTs; RR = 1.46; 95% CI 1.34 to 1.60; NNT = 7). No trials reported outcomes at 4 weeks or less.
Subgroup analysis by specific medication found no statistically significant difference between duloxetine (59% vs 42%; RR = 1.48; 95% CI 1.34 to 1.62; NNT = 6) or venlafaxine or desvenlafaxine (49% vs 36%; RR = 1.35; 95% CI 1.08 to 1.69; NNT = 8) for a greater significant benefit than placebo (P = .48). Similarly, no difference was seen between smaller and larger trials (P = .48) and in trials at higher and lower risk of bias (P = .12). All trials were industry funded and conducted in patients with diabetic neuropathy.
A meta-analysis found that withdrawals due to adverse events occurred in 13% of patients taking SNRIs, compared with 5% taking placebo (RR = 2.48; 95% CI 1.78 to 3.45; NNH = 13). Meta-analyzed adverse events occurring in more than 10% of patients that were statistically significantly greater than placebo included dizziness, nausea, and somnolence (Table A13 in Appendix 2*).
Rubefacients. Ten RCTs (Table A2 in Appendix 2*) with 2344 patients followed for 6 to 52 weeks were included, all involving capsaicin. Forty-nine percent of patients receiving rubefacients and 34% of patients receiving control attained a clinically meaningful response to treatment (RR = 1.40; 95% CI 1.26 to 1.55; NNT = 7; Table A4 and Figure A8.1 from Appendix 2*).
Subgroup analysis of data based on time points found no statistically significant benefit for rubefacients at 4 weeks or less (2 RCTs; RR = 1.60; 95% CI 0.93 to 2.75); however, a benefit was found from greater than 4 weeks to less than 12 weeks (8 RCTs; RR = 1.37; 95% CI 1.20 to 1.56; NNT = 10), and at 12 weeks or greater (5 RCTs; RR = 1.36; 95% CI 1.22 to 1.52; NNT = 7).
Subgroup analysis of data based on study size found a statistically significant difference between subgroups, with more benefit being seen in studies with 150 patients or fewer (P = .02). No statistically significant difference was found in trials enrolling patients with diabetic neuropathy compared with trials enrolling patients with postherpetic neuralgia (P = .48), in those using low-concentration creams and patches compared with high-potency (concentration of 8%) patches (P = .35), or in trials at lower compared with higher risk of bias (P = .08). All trials were industry funded.
A meta-analysis found withdrawals due to adverse events occurring in 6% of patients using rubefacients, compared with 2% using control (RR = 3.31; 95% CI 1.56 to 7.01; NNH = 25). Meta-analyzed adverse events occurring in more than 10% of patients that were statistically significantly greater than placebo included application site pain, reaction (unspecified), burning, stinging, or erythema (Table A13 from Appendix 2*).
Opioids. Six RCTs (Table A2 in Appendix 2*) with 1149 patients followed for 5 to 12 weeks were included. Forty-nine percent of patients receiving opioids (tramadol, oxycodone, buprenorphine, and tapentadol) and 36% of patients receiving placebo attained a clinically meaningful response to treatment (RR = 1.37; 95% CI 1.19 to 1.57; NNT = 8; Table A4 and Figure A7.1 in Appendix 2*). Four trials were conducted in patients with diabetic neuropathy and 2 trials were conducted in a mixed population.
Subgroup analysis of data based on time points found no statistically significant benefit for opioids at 4 weeks or less (1 RCT; RR = 1.14; 95% CI 0.61 to 2.13); however, benefit was found from more than 4 weeks to less than 12 weeks (3 RCTs; RR = 1.45; 95% CI 1.19 to 1.76; NNT = 7) and 12 weeks or more (3 RCTs; RR = 1.30; 95% CI 1.07 to 1.58; NNT = 10).
No statistically significant difference in efficacy was found between public- and industry-funded trials (P = .06), between trials at higher and lower risk of bias (P = .88), or between smaller and larger trials (P = .07).
A meta-analysis found withdrawals due to adverse events occurring in 14% of patients taking opioids, compared with 6% taking placebo (RR = 2.55; 95% CI 1.73 to 3.76; NNH = 12). Meta-analyzed adverse events occurring in more than 10% of patients included somnolence or fatigue, pruritus, nausea, vomiting, constipation, and dizziness (Table A13 in Appendix 2*).
Tricyclic antidepressants. Two RCTs Table A2 in Appendix 2* with 170 patients followed for 6 to 8 weeks were included, both involving amitriptyline. Seventy-eight percent of patients receiving TCAs and 26% of patients receiving placebo attained a clinically meaningful response to treatment, with RRs varying substantially based on choice of analytic model. In the fixed-effects model, TCAs had 3 times more relative benefit than placebo (RR = 3.00; 95% CI 2.05 to 4.38; NNT = 2); however, in the random-effects model, TCAs were not superior to placebo (RR = 2.35; 95% CI 0.79 to 6.95; Table A4 and Figures A10.1 to A10.2 in Appendix 2*).
Both trials were small, 12 weeks or less in duration, had unclear sources of funding, were heterogeneous (I2 = 88%), and had additional indicators of poorer quality, including unclear descriptions of randomization, allocation concealment, and blinding. Reporting of adverse events was minimal and not combinable in meta-analysis.
Acupuncture. Three RCTs Table A2 in Appendix 2* with 247 patients followed for 8 to 10 weeks were included. A random-effects model was used, finding that 22% of patients receiving acupuncture and 13% of patients receiving control attained a clinically meaningful response to treatment (RR = 1.81; 95% CI 0.55 to 5.98; Table A4 and Figure A1.1 from Appendix 2*). This difference was not statistically significant.
All studies were publicly funded, small, 12 weeks or less in duration, and heterogeneous (I2 = 73%). Withdrawals due to adverse events were reported by 1 trial, finding no statistically significant difference between groups. No other adverse events were reported.
Treatments with no identified RCTs
No RCTs met our inclusion criteria for either exercise or lidocaine.
Quality assessment
Assessments for risk of bias are in Appendix 2 (Figures A20.1 to A20.6).* Following the GRADE process, all rationale to determine certainty of evidence is in Appendix 2 (Table A15).* Anticonvulsant medications and SNRIs were considered “moderate” for evidence quality, while opioids and rubefacients were considered “low,” and acupuncture and TCAs were considered “very low.”
Heterogeneity of trials (reported by I2 statistic) for overall efficacy ranged from 0% to 88% (0% for SNRIs, 17% for opioids, 21% for rubefacients, 60% for anticonvulsant medications, 73% for acupuncture, and 88% for TCAs). Heterogeneity might be due in part to the lower quality of trials, the inclusion of a number of neuropathic pain types or different patient populations, and variance in the delivery of the intervention (eg, acupuncture, electroacupuncture, and auricular acupuncture).
DISCUSSION
This systematic review of 67 RCTs evaluated the efficacy of interventions commonly used in primary care for the management of neuropathic pain, specifically painful diabetic neuropathy and postherpetic neuralgia. We found moderate certainty of evidence that anticonvulsant medications and SNRIs provide a clinically meaningful benefit to patients with neuropathic pain. There was low certainty of evidence for a clinically meaningful benefit for capsaicin and opioids, and a very low certainty of evidence for TCAs. For acupuncture, very low-quality evidence suggested this treatment was not efficacious overall.
Compared with placebo, a greater proportion of patients receiving pharmacotherapy discontinued treatment owing to adverse events. With the exception of TCAs, for which this outcome could not be analyzed as neither of the TCA studies provided evaluable adverse event data, all drug classes had a greater likelihood of deriving a clinically meaningful benefit than being discontinued because of adverse events. However, subgroup analysis of anticonvulsant medications showed NNHs for discontinuation similar to NNTs for benefit for both topiramate and oxcarbazepine. In addition, each drug class had several adverse events with associated NNHs of 10 or less, most notably for opioids (systemic adverse events) and capsaicin (local adverse events).
The duration of trials generally ranged from 4 to 12 weeks, with few trials having follow-up beyond 3 months. Only a small proportion of trials looked at outcomes in the early treatment period (< 4 weeks). Although one could predict, using pharmacokinetic principles, that an effect should be seen early on (eg, 1-2 weeks), we do not know of any studies that can identify the onset of drug effect with certainty. Follow-up at 3 months is likely to provide an understanding of efficacy; however, there is uncertainty to what extent this duration is able to provide an understanding of long-term net benefit.
Based on point estimates derived from our meta-analyses, TCAs appeared to have the highest magnitude of benefit when using a fixed-effects model, but there was no statistically significant benefit when using a random-effects model. In addition, the very low quality of evidence (with deductions in each domain of quality assessment) further diminishes confidence in these findings. While our exclusion criteria aimed to refine the evidence, it narrowed the included studies to a small number of low-quality RCTs with evaluable efficacy data for TCAs compared with placebo, limiting the certainty of efficacy for TCAs. An earlier systematic review of amitriptyline for neuropathic pain by Moore et al,20 which included any type of neuropathic pain, had similar challenges with inclusion of acceptable RCTs with useful efficacy data and rated the evidence for efficacy and adverse outcomes for amitriptyline as very low quality. If broader inclusion criteria (eg, greater allowance for study design flaws and variations in study outcome reporting) of that and other systematic reviews is considered, TCAs appear to be slightly less efficacious (NNT = 4-6 compared with NNT = 2 in our systematic review) and based on a larger, but arguably not better quality, evidence base.20,21
The finding that opioids were associated with a comparable degree of benefit was unexpected. It should be noted that in addition to being supported only by low certainty of evidence and having numerous adverse events, with NNHs below NNTs, there was obvious heterogeneity in the types and doses of opioids used in the included trials. This, along with the potential risk of developing opioid use disorder with longer-term use,22 makes clinical application challenging.
Numerous systematic reviews evaluating treatments for broad or specific neuropathic pain types exist, some of which evaluate multiple different pharmacologic and nonpharmacologic interventions.21,23-25 Our systematic review is novel in using a synthesis of multiple interventions for neuropathic pain typically treated in primary care, reporting outcomes through responder analysis to allow for clinical application, and including robust reporting and meta-analysis of adverse events.
Strengths and limitations
A strength of this review is its scope, weaving together 8 discrete systematic reviews of differing interventions for neuropathic pain. Through meta-analysis of subgroups of individual drugs, we were able to identify class similarities within SNRIs (duloxetine and venlafaxine or desvenlafaxine) and anticonvulsant medications (gabapentin and pregabalin). With populations, interventions, and comparators all inherently having some degree of heterogeneity, limitations include the uncertain generalizability to individual patients and our decision to combine potentially heterogeneous interventions into 1 intervention category. In particular, the acupuncture analysis included several intervention methods and both sham and nonsham controls. Additionally, by choosing only RCTs that reported a responder analysis, we excluded a proportion of the literature that reported continuous outcomes. By focusing on dichotomous outcomes, it allowed us to combine trials using different pain measures by using counts of responders without losing clinical meaning. Changes on a pain scale, or their combination into standard mean differences, are challenging to interpret and do not translate easily in a patient conversation. We prioritized the outcome of pain over function as it is usually the presenting condition in primary care settings, and functional improvement is rarely presented dichotomously in trials. Although broader applications of efficacy to quality of life would be a desirable outcome measure, attempts by previous reviews to meaningfully analyze quality of life outcomes for neuropathic pain interventions have not been successful because of lack of consistent and complete reporting.23,25 The decision to focus our inclusion of studies on painful diabetic neuropathy, postherpetic neuralgia, and trigeminal neuralgia was based on author consensus that these are the most commonly seen and treated conditions in primary care practice. While these neuropathic pain subsets are also the most commonly studied,26 limiting the study to these conditions might limit generalization of the results to other neuropathic pain types such as HIV, multiple sclerosis, and chemotherapy-induced neuropathies.
Future research
Given that we found few RCTs that met our inclusion criteria for exercise, lidocaine, TCAs, carbamazepine, and non-pharmacologic interventions, future RCTs would be valuable additions to the literature. Contemporary RCTs evaluating responder analyses in TCAs for both benefit and harm would allow for some degree of certainty to be applied to the decision to use (or not use) this commonly prescribed drug class, as would be the case for lidocaine, which is currently suggested as an option in several practice guidelines, particularly for postherpetic neuralgia.24,27,28 Currently, no nonpharmacologic interventions can be applied to the management of neuropathic pain with any degree of certainty. Future trials with carefully chosen control groups will be valuable to determine if the pathophysiological aspects of neuropathic pain lend themselves to nondrug approaches to care as is the case in other types of chronic pain.
Conclusion
There is moderate certainty of evidence that anticonvulsant medications and SNRIs provide a clinically meaningful reduction in pain in those with neuropathic pain. There is low certainty of evidence to support rubefacients and opioids providing benefit, and very low certainty of evidence to support TCAs. There is also very low certainty of evidence suggesting acupuncture is not helpful. Although patients receiving drug interventions were less likely to discontinue medications owing to adverse events than to derive benefit, medications were generally associated with multiple adverse events having NNHs close to their NNTs for benefit. Future high-quality pragmatic trials, ideally rooted in primary care and reporting a responder analysis, will be important to providing a better understanding of the relative benefits and harms of interventions for patients with neuropathic pain.
Supplementary Material
Editor’s key points
▸ Chronic neuropathic pain is associated with a large burden of disease and has negative effects on sleep, quality of life, anxiety and depression symptoms, and health care use. Even though there are many interventions for neuropathic pain, the benefits and harms of these interventions need to be more concretely defined to better help patients with neuropathic pain.
▸ There is moderate certainty of evidence that anticonvulsant medications and serotonin-norepinephrine reuptake inhibitors provide a clinically meaningful reduction in pain. There is low certainty of evidence to support use of rubefacients (capsaicin) and opioids, and very low certainty of evidence to support use of tricyclic antidepressants. Very low-quality evidence suggests that acupuncture is not efficacious overall.
▸ Findings of this systematic review were used to develop a clinical decision aid (page 347). This systematic review is one in a series that will inform guidelines on pain treatment in primary care.
Points de repère du rédacteur
▸ La douleur neuropathique chronique est associée à un lourd fardeau de morbidité, et elle a des effets négatifs sur le sommeil, la qualité de vie, l’anxiété et les symptômes de dépression, de même que sur l’utilisation des soins de santé. Même s’il existe de nombreuses interventions pour traiter la douleur neuropathique, il est nécessaire de définir plus concrètement les bienfaits et les préjudices de ces interventions pour aider les patients souffrant de douleur neuropathique.
▸ Des données probantes de certitude modérée étayent le fait que les anticonvulsivants et les inhibiteurs de la recapture de la sérotonine-norépinéphrine procurent une réduction cliniquement significative de la douleur. Des données factuelles de faible certitude appuient l’utilisation des rubéfiants (capsaïcine) et des opioïdes, et des données de très faible certitude soutiennent le recours aux antidépresseurs tricycliques. Des données de très faible qualité donnent à croire que l’acupuncture n’est généralement pas efficace.
▸ Les constatations de cette revue systématique ont servi à élaborer une aide à la décision clinique (page e111). Cette revue systématique compte parmi une série de plusieurs revues qui éclaireront des lignes directrices sur le traitement de la douleur en soins primaires.
Footnotes
The comprehensive search strategy (Appendix 1), the hierarchy of responder outcomes, primary and subgroup meta-analyses, trial funding data, adverse event data, and assessments for risk of bias (Appendix 2) are available at www.cfp.ca. Go to the full text of the article online and click on the CFPlus tab.
Contributors
All authors were part of the Evidence Review Team and contributed to preparing the manuscript for submission.
Competing interests
None declared
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
References
- 1.Mick G, Serpell M, Baron R, Mayoral V, Hans G, Mendez I, et al. Localised neuropathic pain in the primary care setting: a cross-sectional study of prevalence, clinical characteristics, treatment patterns, quality of life and sleep performance. Curr Med Res Opin 2021;37(2):293-302. Epub 2020 Nov 23. Erratum in: Curr Med Res Opin 2021:1. [DOI] [PubMed] [Google Scholar]
- 2.Torrance N, Smith BH, Bennett MI, Lee AJ.. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain 2006;7(4):281-9. [DOI] [PubMed] [Google Scholar]
- 3.Bouhassira D, Lantéri-Minet M, Attal N, Laurent B, Touboul C.. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 2008;136(3):380-7. Epub 2007 Sep 20. [DOI] [PubMed] [Google Scholar]
- 4.Van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N.. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain 2014;155(4):654-62. Epub 2013 Nov 26. Erratum in: Pain 2014;155(9):1907. [DOI] [PubMed] [Google Scholar]
- 5.Poliakov I, Toth C.. The impact of pain in patients with polyneuropathy. Eur J Pain 2011;15(10):1015-22. Epub 2011 May 19. [DOI] [PubMed] [Google Scholar]
- 6.Smith BH, Torrance N.. Epidemiology of neuropathic pain and its impact on quality of life. Curr Pain Headache Rep 2012;16(3):191-8. [DOI] [PubMed] [Google Scholar]
- 7.Attal N, Lanteri-Minet M, Laurent B, Fermanian J, Bouhassira D.. The specific disease burden of neuropathic pain: results of a French nationwide survey. Pain 2011;152(12):2836-43. Epub 2011 Oct 20. [DOI] [PubMed] [Google Scholar]
- 8.Ton J, Perry D, Thomas B, Allan GM, Lindblad AJ, McCormack J, et al. PEER umbrella systematic review of systematic reviews. Management of osteoarthritis in primary care. Can Fam Physician 2020;66:e89-98. Available from: https://www.cfp.ca/content/cfp/66/3/e89.full.pdf. Accessed 2021 Apr 19. [PMC free article] [PubMed] [Google Scholar]
- 9.Kolber MR, Ton J, Thomas B, Kirkwood J, Moe S, Dugré N, et al. PEER systematic review of randomized controlled trials. Management of chronic low back pain in primary care. Can Fam Physician 2021;67:e20-30. Available from: https://www.cfp.ca/content/cfp/67/1/e20.full.pdf. Accessed 2021 Apr 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore A, Derry S, Eccleston C, Kalso E.. Expect analgesic failure; pursue analgesic success. BMJ 2013;346:f2690. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med 2009;6(7):e1000097. Epub 2009 Jul 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institutes of Health . U.S. National Library of Medicine. Clinicaltrials.gov. Bethesda, MD: National Institutes of Health; 2021. Available from: clinicaltrials.gov. Accessed 2020 May 6. [Google Scholar]
- 13.World Health Organization . International Clinical Trials Registry Platform (ICTRP). Geneva, Switz: World Health Organization; 2021. Available from: https://www.who.int/ictrp/en/. Accessed 2021 Apr 6. [Google Scholar]
- 14.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008;9(2):105-21. Epub 2007 Dec 11. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Lasserson T, Chandler J, Tovey D, Thomas J, Flemyng E, et al. Methodological Expectations of Cochrane Intervention Reviews (MECIR). London, UK: The Cochrane Collaboration; 2020. Available from: https://community.cochrane.org/sites/default/files/uploads/Version%20March%202020%20Final%20Online%20version.pdf. Accessed 2021 Apr 6. [Google Scholar]
- 16.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. rating the quality of evidence. J Clin Epidemiol 2011;64(4):401-6. Epub 2011 Jan 5. [DOI] [PubMed] [Google Scholar]
- 18.Review Manager (RevMan), version 5.3 [software]. Copenhagen, Den: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [Google Scholar]
- 19.10.4.4.1 comparing fixed and random-effects estimates. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0. The Cochrane Collaboration; 2011. Available from: https://handbook-5-1.cochrane.org/chapter_10/10_4_4_1_comparing_fixed_and_random_effects_estimates.htm. Accessed 2021 Apr 7. [Google Scholar]
- 20.Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ.. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst Rev 2015;(7):CD008242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015;14(2):162-73. Epub 2015 Jan 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moe S, Kirkwood J, Allan GM.. Incidence of iatrogenic opioid use disorder. Can Fam Physician 2019;65:724 (Eng), e431-2 (Fr). [PMC free article] [PubMed] [Google Scholar]
- 23.Dy SM, Bennett WL, Sharma R, Zhang A, Waldfogel JM, Nesbit SA, et al. Preventing complications and treating symptoms of diabetic peripheral neuropathy: comparative effectiveness reviews no. 187. Rockville, MD: Agency for Healthcare Research and Quality; 2017. Available from: https://www.ncbi.nlm.nih.gov/books/NBK442335/. Accessed 2021 Apr 6. [PubMed] [Google Scholar]
- 24.Moisset X, Bouhassira D, Avez Couturier J, Alchaar H, Conradi S, Delmotte MH, et al. Pharmacological and non-pharmacological treatments for neuropathic pain: systematic review and French recommendations. Rev Neurol (Paris) 2020;176(5):325-52. Epub 2020 Apr 7. [DOI] [PubMed] [Google Scholar]
- 25.Waldfogel JM, Nesbit SA, Dy SM, Sharma R, Zhang A, Wilson LM, et al. Pharmacotherapy for diabetic peripheral neuropathy pain and quality of life: a systematic review. Neurology 2017;88(20):1958-67. Epub 2017 Mar 24. Erratum in: Neurology 2017;89(8):875. [DOI] [PubMed] [Google Scholar]
- 26.Finnerup NB, Haroutounian S, Baron R, Dworkin RH, Gilron I, Haanpaa M, et al. Neuropathic pain clinical trials: factors associated with decreases in estimated drug efficacy. Pain 2018;159(11):2339-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moulin D, Boulanger A, Clark AJ, Clarke H, Dao T, Finley GA, et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag 2014;19(6):328-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haanpää M, Rice ASC, Rowbotham MC.. Pain clinical updates: treating herpes zoster and postherpetic neuralgia. Washington, DC: International Association for the Study of Pain; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.