Epithelial to mesenchymal transition (EMT) is a key signature in both physiological processes (i.e. development, regeneration, wound healing) and in tumor metastasis [1]. While the involvement of transcription factors (e.g. SNAIL, SLUG, ZEB1/2, TWIST1/2) has been extensively explored, the contribution of epigenetic mechanisms has emerged only in the last decades [2]; furthermore, interest is growing in the role of ncRNAs (e.g. miRNAs and lncRNAs) in modulating cell plasticity.
In particular, it was reported that HOTAIR, a well-established predictor of metastasis in different solid tumors, is involved in chromatin modification, acting as a scaffold for the general chromatin modifier PRC2 complex in tumorigenesis [3, 4]; however, the mechanisms conferring specificity to the PRC2 recruitment to genomic loci during EMT was not disclosed. In the last years, we focused on the role of the lncRNA HOTAIR in relation to the EMT master transcriptional factor SNAIL. We reported that SNAIL directly interacts with HOTAIR, thus conferring the site-specificity to the recruitment of PRC2 complex on promoters of epithelial genes upon EMT induction [5]. The central mechanistic role of HOTAIR is represented by its bridging activity that allows the interaction between SNAIL and the catalytic subunit of the PRC2 complex, EZH2. Functionally, HOTAIR depletion was shown to inhibit the SNAIL repressive capacity.
Building on this evidence, we designed an RNA-based dominant negative molecular approach to counteract HOTAIR function in hepatocellular carcinoma (HCC) cells.
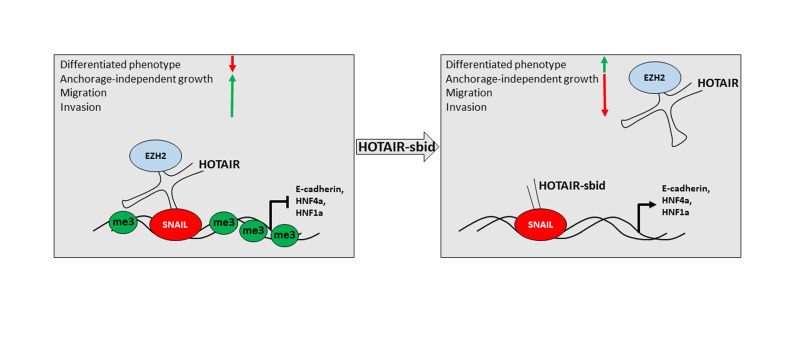
RNA therapeutics represent a growing field of investigation and application. The use of RNA molecules shows several advantages since they show a very low immunogenicity, are able to penetrate the cell/nuclear membrane, and to target the desired gene even if highly expressed, they are cheap and easy to synthesize, and can be chemically modified, in order to increase their stability or to stabilize secondary structures. Moreover, concerning the delivery of these molecules, in recent years, many strategies have been developed for increasing the efficient delivery of RNA therapeutic molecules to specific target cells. Some of these strategies are represented by the use of nanoparticles, lipid nanoparticles, and extracellular vesicles, above all exosomes, properly engineered in order to increase the delivery and the on-target effects. Also other approaches have been proposed, like the conjugation of these nucleic acid molecules with sugars, lipids, peptides, nucleic acids ligands in order to interact with the cell membrane or with the surface receptors (for extensive review, see [6]). Taking advantage of a bioinformatic tool, catRAPID fragments, [7] we predicted a specific HOTAIR domain as the region with the highest affinity of interaction with SNAIL. This sequence, namely HOTAIR-sbid (for SNAIL-binding domain), devoid of the EZH2-binding capacity, was expressed in different contexts (i.e. tumor and TGFβ-induced EMT cells) to test its ability to impair endogenous HOTAIR/SNAIL pro-EMT function. Notably, HOTAIR-sbid was functionally proved to reduce cellular motility, invasiveness, anchorage-independent growth, and responsiveness to TGFβ-induced EMT. Mechanistically, while SNAIL was shown to maintain its ability to bind to its target genes, its repressive function results abolished (Figure 1). Cells not expressing HOTAIR appear, as conceivably expected, not affected by HOTAIR-sbid [8].
Figure 1. Figure 1: Schematic representation of HOTAIR-sbid activity during EMT (me3 represents H3K27me3).
Overall, the described RNA-based dominant negative approach further contributes to the translational applications based on the use of RNA therapeutics. Specifically, this strategy appears conceivably devoid of off-target effects in EMT and tumorigenesis and holds promises of effectiveness when the function of lncRNAs is proved as a determinant.
Footnotes
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest.
REFERENCES
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- McDonald OG, Wu H, Timp W, Doi A, Feinberg AP. Genome-scale epigenetic reprogramming during epithelial-to-mesenchymal transition. Nat Struct Mol Biol. 2011;18:867–74. doi: 10.1038/nsmb.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–76. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, Shi Y, Segal E, Chang HY. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–93. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistelli C, Cicchini C, Santangelo L, Tramontano A, Grassi L, Gonzalez FJ, de Nonno V, Grassi G, Amicone L, Tripodi M. The Snail repressor recruits EZH2 to specific genomic sites through the enrollment of the lncRNA HOTAIR in epithelial-to-mesenchymal transition. Oncogene. 2017;36:942–55. doi: 10.1038/onc.2016.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman J. Tapping the RNA world for therapeutics. Nat Struct Mol Biol. 2018;25:357–64. doi: 10.1038/s41594-018-0054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo D, Blanco M, Armaos A, Buness A, Avner P, Guttman M, Cerase A, Tartaglia GG. Quantitative predictions of protein interactions with long noncoding RNAs. Nat Methods. 2016;14:5–6. doi: 10.1038/nmeth.4100. [DOI] [PubMed] [Google Scholar]
- Battistelli C, Garbo S, Riccioni V, Montaldo C, Santangelo L, Vandelli A, Strippoli R, Tartaglia GG, Tripodi M, Cicchini C. Design and Functional Validation of a Mutant Variant of the LncRNA HOTAIR to Counteract Snail Function in Epithelial-to-Mesenchymal Transition. . Cancer Res. 2021;81:103–13. doi: 10.1158/0008-5472.CAN-20-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]