ABSTRACT
Sodium polystyrene sulfonate (kayexalate) is a cation-exchange resin widely used in the management of hyperkalemia. Gastrointestinal adverse events are uncommon; symptoms are nonspecific, and mucosal injury can range from mild ulceration to bowel perforation. An 81-year-old man was admitted because of decompensation of cirrhosis with acute kidney injury and hyperkalemia, treated with sodium polystyrene sulfonate. During admission, he presented multiple episodes of hematochezia, accompanied by tachycardia and hemoglobin drop. Colonoscopy revealed colonic ulceration, and histopathological findings were compatible with ulceration due to kayexalate injury. Despite rare, the widespread use of sodium polystyrene sulfonate puts a large population at risk of serious complications related to its use.
INTRODUCTION
Hyperkalemia is a common condition found in medical and surgical patients, potentially leading to numerous complications, including life-threatening cardiac arrhythmias. Sodium polystyrene sulfonate (SPS) is an ion-exchange resin commonly used to treat hyperkalemia that acts by binding to potassium ions along the digestive tract, promoting its elimination by exchange with sodium ions.1 Although generally safe, its use has been associated with some gastrointestinal (GI) complications.
CASE REPORT
An 81-year-old man with hypertension, type 2 diabetes, dyslipidemia, nonalcoholic steatohepatitis-related cirrhosis, and ischemic heart disease presented with weakness, dizziness, and vomiting which began 1 hour before. On presentation, he was normotensive and bradycardic (heart rate 43–50 bpm). Physical examination revealed moderate ascites and mild edema of the left foot, with visible inflammatory signs on the first toe.
Laboratory findings showed mild anemia (hemoglobin 11 g/dL), acute kidney injury (urea 162 mg/dL and creatinine 1.99 mg/dL), hyperkalemia (7.6 mEq/L), and elevated C-reactive protein (4.3 mg/dL); diagnostic paracentesis excluded spontaneous bacterial peritonitis. The electrocardiogram revealed a right bundle branch block and atrial fibrillation with bradycardia. He was treated with calcium gluconate/insulin/glucose cocktail and started SPS 15 g 3 times daily; he was also prescribed albumin and empirical antibiotic therapy with ceftriaxone.
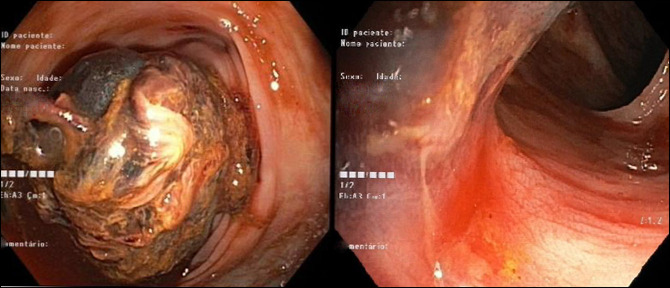
During hospitalization, he initially presented a favorable evolution, with the resolution of the acute kidney injury and hyperkalemia by the third day of hospitalization, when SPS treatment was suspended. However, on the seventh day, he developed massive hematochezia, accompanied by tachycardia (heart rate 120 bpm) and hemoglobin drop (lowest value 7.5 g/dL). He had a total colonoscopy, which revealed moderate amount of bright red blood in the descending colon, originating from a large organized adherent clot obscuring an area of ulcerated mucosa, from which biopsies were taken (Figure 1). Histopathologic examination revealed mucosal ulceration with kayexalate deposition (Figure 2).
Figure 1.
Colonoscopy revealing bright red blood in the descending colon, originating from a large adherent clot obscuring an area of ulcerated mucosa.
Figure 2.
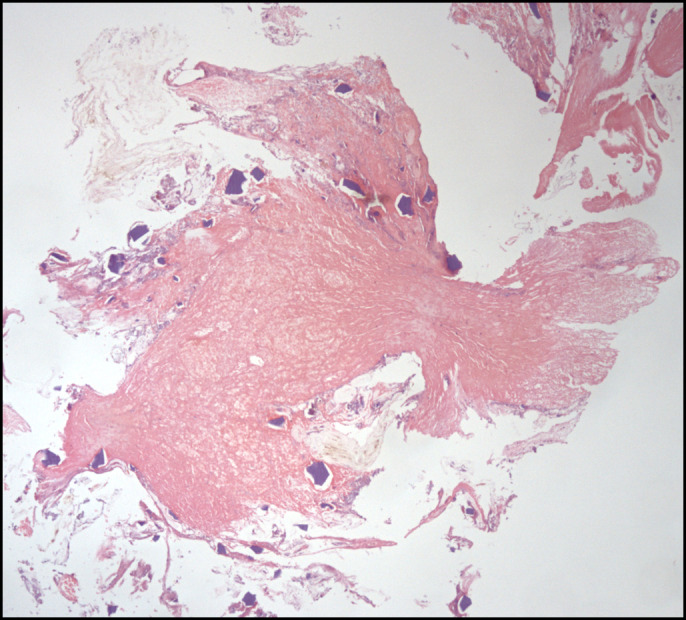
Histology: ulceration area with deposits of kayexalate crystals (hematoxylin and eosin stain, 40× magnification).
After the colonoscopy, no more bleeding occurred. The patient received 1 unit of packed red blood cells and intravenous ferric carboxymaltose, with analytic improvement. Colonoscopy was repeated 1 week later, revealing a large 5-cm ulcer in the descending colon, with evidence of re-epithelization, whose histopathologic examination was similar to the previous one (Figure 3). He was then discharged, clinically asymptomatic.
Figure 3.
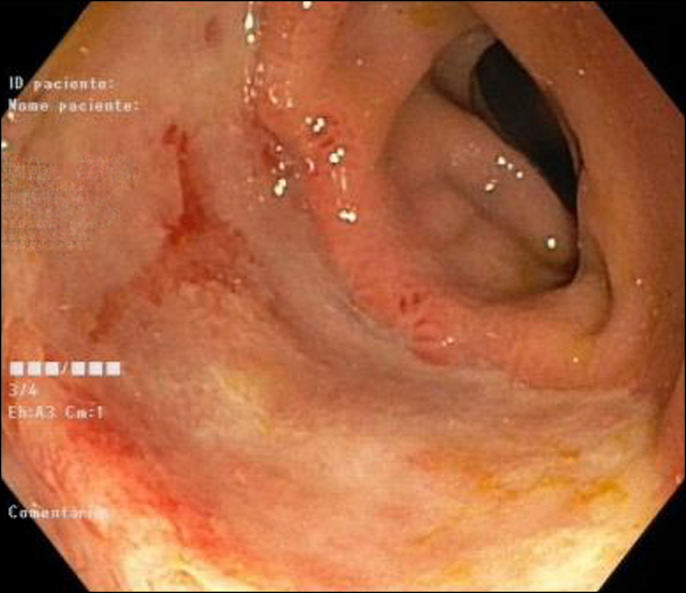
Repeat colonoscopy, with area of ulceration and evidence of re-epithelization.
DISCUSSION
SPS is a cation-exchange resin that has been used widely since its first approval in 1958.2 It can be administered orally or rectally, and it is not an ideal agent to treat acute hyperkalemia because of its delayed onset of action. It was initially administered as a suspension in water; however, as it may also bind to calcium ions, some concerns were raised about potential fecal impaction and decrease in potassium clearance, leading to its administration with sorbitol, a laxative agent.3 In 1987, Lillemoe et al reported, for the first time, an association between colonic necrosis and concomitant use of SPS in sorbitol.4 The authors took this clinical observation to a laboratory model using rats, which were given either sorbitol enemas alone, SPS with sorbitol, or SPS enemas alone. Colonic necrosis was noted in all rats given sorbitol or SPS with sorbitol, but not in the ones without sorbitol. Numerous cases of similar injuries continued to occur, ultimately leading to a recommendation by the Food and Drug Administration in 2009 against the use of SPS in sorbitol.2,5,6 However, even after this recommendation, cases of colonic injuries using SPS formulations without sorbitol have been reported, suggesting that SPS itself may be pathogenic.7,8 Various histopathologic features are associated with SPS-induced GI injury, including mucosal ulceration and erosion, transmural necrosis, inflammatory exudates, pseudomembrane formation, and acute/chronic serositis. The hallmark of SPS toxicity is the identification of kayexalate crystals, visible with standard hematoxylin and eosin staining and showing characteristic polygonal, refractile, basophilic appearance and typical fish scale/mosaic pattern, which displays red color on periodic acid-Schiff and acid-fast stains.9
The mechanism of kayexalate-induced bowel necrosis remains unknown; a possible explanation involves the elevated renin levels seen in patients with renal failure, with subsequent splanchnic vasoconstriction, predisposing the colon to nonocclusive ischemic events, which can progress to transmural ischemia. Our patient had a history of chronic kidney disease, which may have increased the risk of this adverse event. Other risk factors suggested to contribute to SPS-related GI injury include uremia, solid organ transplantation and immunosuppressive therapy, postoperative status, hemodynamic instability, ileus, bowel obstruction, and concomitant administration of opioids.10,11
Symptoms of SPS-related GI injuries may occur from hours up to several days after its administration.12 They are nonspecific and frequently underrecognized, with abdominal pain, nausea and vomiting, lower GI bleeding, and diarrhea being the most common.10 Although the colon remains the most common location, it has been increasingly recognized that such injuries can occur in more proximal locations, up to 30% of cases.13–16 The lesions range from erosive ulcers of the esophagus and stomach to colonic injuries, such as bleeding or perforated ulcers, necrotizing colitis, ischemic colitis, and diverticular perforation.17 Irrespectively of the location, mortality from these complications remains high.
We report an SPS-induced injury in a cirrhotic patient who presented with acute kidney injury and hyperkalemia and developed lower GI bleeding because of colonic ulceration 4 days after treatment withdrawal. The time relation between the onset of symptoms and exposure to SPS, with evidence of kayexalate crystals in biopsy samples, allowed for diagnostic confirmation. We present this case as a reminder to other healthcare providers about the possibility of rare and potentially dangerous complications associated with SPS.
DISCLOSURES
Author contributions: E. Dantas wrote the manuscript, reviewed the literature and is the article guarantor. M. Coelho, C. Sequeira, and I. Santos edited the manuscript. C. Martins, C. Cardoso, R. Freire, and AP Oliveira revised the manuscript for intellectual content. All authors approved the final version to be published.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
Contributor Information
Mariana Coelho, Email: dcoelho.mariana@gmail.com.
Cristiana Sequeira, Email: c.afonso.sequeira@gmail.com.
Inês Santos, Email: inesvcsantos@campus.ul.pt.
Cláudio Martins, Email: cmartins1@campus.ul.pt.
Cláudia Cardoso, Email: claudiamarcal@gmail.com.
Ricardo Freire, Email: rfreire33@gmail.com.
Ana Paula Oliveira, Email: ana.paula@chs.min-saude.pt.
REFERENCES
- 1.Kessler C, Ng J, Valdez K, Xie H, Geiger B. SPS in hyperkalemia. J Hosp Med 2011;3:136–40. [DOI] [PubMed] [Google Scholar]
- 2.Saleh Almulhim A, Hall E, Mershid Al Rehaili B, Saleh Almulhim A. Sodium polystyrene sulfonate induced intestinal necrosis; a case report. Saudi Pharm J 2018;26:771–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng ES, Stringer KM, Pegg SP. Colonic necrosis and perforation following oral sodium polystyrene sulfonate (resonium A/kayexalate) in a burn patient. Burns 2002;28:189–90. [DOI] [PubMed] [Google Scholar]
- 4.Lillemoe KD, Romolo JL, Hamilton SR, Pennington LR, Burdick JF, Williams GM. Intestinal necrosis due to sodium polystyrene (kayexalate) in sorbitol enemas: Clinical and experimental support for the hypothesis. Surgery 1987;101:267–72. [PubMed] [Google Scholar]
- 5.Gerstman BB, Kirkman R, Platt R. Intestinal necrosis associated with postoperative orally administered sodium polystyrene sulfonate in sorbitol. Am J Kidney Dis 1992;20(2):159–61. [DOI] [PubMed] [Google Scholar]
- 6.Rogers FB, Li SC. Acute colonic necrosis associated with sodium polystyrene sulfonate (kayexalate) enemas in a critically ill patient: Case report and review of the literature. J Trauma 2001;51(2):395–7. [DOI] [PubMed] [Google Scholar]
- 7.Albeldawi M, Gaur V, Weber L. Kayexalate-induced colonic ulcer. Gastroenterol Rep (Oxf) 2014;2(3):235–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edhi AI, Cappell MS, Sharma N, Amin M, Patel A. One oral dose of sodium polystyrene sulfonate associated with ischemic colitis and crystal deposition in colonic mucosa. ACG Case Rep J 2018;5:e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romano RC, Thirumala S, Cushman WH, Mounajjed T. Inflammatory pseudotumor containing kayexalate crystals: A case report and review of the literature. Int J Surg Pathol 2014;22(5):464–9.. [DOI] [PubMed] [Google Scholar]
- 10.Harel Z, Harel S, Shah PS, Wald R, Perl J, Bell CM. Gastrointestinal adverse events with sodium polystyrene sulfonate (kayexalate) use: A systematic review. Am J Med 2013;126(3):264.e9–24. [DOI] [PubMed] [Google Scholar]
- 11.Joo M, Bae WK, Kim NH, Han SR. Colonic mucosal necrosis following administration of calcium polystryrene sulfonate (kalimate) in a uremic patient. J Korean Med Sci 2009;24(6):1207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGowan CE, Saha S, Chu G, Resnick MB, Moss SF. Intestinal necrosis due to sodium polystyrene sulfonate (kayexalate) in sorbitol. South Med J 2009;102:493–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abraham SC, Bhagavan BS, Lee LA, Rashid A, Wu TT. Upper gastrointestinal tract injury in patients receiving kayexalate (sodium polystyrene sulfonate) in sorbitol: Clinical, endoscopic, and histopathologic findings. Am J Surg Pathol 2001;25:637–44. [DOI] [PubMed] [Google Scholar]
- 14.Gorospe EC, Lewis JT, Bruining D. Kayexalate-induced esophageal ulcer in a patient with gastroparesis. Clin Gastroenterol Hepatol 2011;10:A28. [DOI] [PubMed] [Google Scholar]
- 15.Hajjar R, Sebajang H, Schwenter F, Mercier F. Sodium polystyrene sulfonate crystals in the gastric wall of a patient with upper gastrointestinal bleeding and gastric perforation: An incidental finding or a pathogenic factor? J Surg Case Rep 2018;2018(6):rjy138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trottier V, Drolet S, Morcos MW. Ileocolic perforation secondary to sodium polystyrene sulfonate in sorbitol use: A case report. Can J Gastroenterol 2009;23(10):689–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bui M, Chou SY, Faubert P, Loarte P, Cohen R. Resin-induced colonic pseudotumor: Rare complication from chronic use of potassium binders in a hemodialysis patient. Case Rep Nephrol 2016;2016:3692086. [DOI] [PMC free article] [PubMed] [Google Scholar]