Stroke is the second leading cause of death globally and accounts for an estimated 5.5 million deaths each year worldwide.[1] Revascularization of the carotid artery is one of the key procedures to reduce the incidence of stroke. Chronic total occlusion (TO) and near-total occlusion (NO) of the carotid artery are rare. Treatment for TO and NO is controversial with potential complications, including periprocedural stroke, intracranial hemorrhage, and carotid-cavernous fistula (CCF).[2] There have been attempts to implement carotid artery endarterectomy (CEA) and endovascular treatment, but the success rates were far from satisfactory. For patients with TO, the success rate of revascularization is around 40% and 60%, respectively, for CEA and endovascular technique.[3] For patients with NO, the morbidity and mortality rates are 3% and 9%, respectively.
We attempted hybrid procedures to improve the revascularization rate. Issues with the suitability of these procedures were addressed by considering intraprocedural complications, 30-day morbidity and mortality, and long-term morbidity and mortality.
NO was diagnosed as a decrease in partial luminal diameter or virtual luminal collapse beyond prominent carotid bulb stenosis. The diagnostic criteria for NO include two of the following four criteria with digital subtraction angiography (DSA): (1) significantly delayed arrival of contrast filling the internal carotid artery (ICA) and its branches; (2) evidence of collaterals; (3) obvious diameter reduction in the distal ICA compared with the contralateral ICA; (4) obvious diameter reduction in the distal ICA compared with the distal external carotid artery.[4] TO is defined as disappearance of the arterial lumen beyond the bifurcation of the common carotid artery (CCA). Enrolled patients were symptomatic before admission; symptomatic referred to hypoperfusion or embolic cerebral infarction in the responsible area or recurrent transient ischemic attack with medical treatment during the last 3 months or longer period of time.
We retrospectively collected patients’ medical data from the database of Peking University Third Hospital. The success rate of revascularization was analyzed. The primary endpoints of the study were intraprocedural and 30-day complications, especially new-onset stroke. The secondary endpoints were long-term patency of the ICA and stroke recurrence.
For patients with NO, in a hybrid catheterization suite equipped with C-arm fluoroscopy equipment (GE Innova 4100-Q, GE healthcare, CA, USA), bifurcation plaques were removed using routine CEA procedures under general anesthesia. The distal end of the endothelium was fixed to the ICA wall using 7–0 prolene sutures. In some cases, the endothelium was too vulnerable to fix, usually in patients with “string” signs; thus, it was left for endovascular stenting to complete revascularization.
Selective CCA angiography was performed through femoral artery access. In cases of ICA residual stenosis (>30%), intimal discontinuity, and intimal laceration (dissection), a 7-mm-diameter WALLSTENT® (Boston Scientific, Marlborough, MA, USA) was applied to cover the initial ICA segment. Since plaques were removed, no embolic protection device nor percutaneous transluminal angioplasty (PTA) balloon was needed.
For patients with TO, bifurcation plaques were removed as a routine CEA procedure as far as the operator was able to reach. If there was no arterial backflow, a 3-F Fogarty® embolectomy catheter (Edwards Lifesciences LLC, Irvine, CA, USA) was inserted first to remove the thrombus from the ICA. With arterial backflow in the ICA, a 6-F sheath (RADIFOCUS® INTRODUCER II, TERUMO, Tokyo, Japan) was advanced into the initial segment. The hemostatic valve of the sheath was half-closed to maintain some ICA backflow to reduce the possibility of thrombosis or embolization of the ICA and its branches. In cases of tandem carotid artery stenosis, 4.5-mm-diameter stents (NEUROFORM EZ®, Stryker Neurovascular, Fremont, CA, USA; Enterprise®, Cordis Neurovascular, Miami, FL, USA) were used to cover the stenotic area post-dilatation with PTA balloons. If necessary, in cases of rigid stenosis, a 4.0 to 4.5-mm-diameter Wingspan® stent system (Stryker Neurovascular, Fremont, CA, USA) was used. For C1, a 7 mm × 40 mm WALLSTENT® was deployed. The carotid artery was reconstructed using continuous 7–0 prolene sutures. Final selective CCA angiography was performed using a femoral access catheter. Follow-up was performed at the outpatient department at 1, 3, 6, and 12 months for the first year and annually thereafter.
Statistical analysis was performed using SPSS software (version 18.0; IBM Corporation, Somers, NY, USA). In examining the differences, enumeration data and measurement data were analyzed using Fisher's exact test (n < 40) and the unpaired Student's t test, respectively. The patency rate was calculated using the Kaplan–Meier method. A P value of <0.05 was considered statistically significant.
A total of seven patients with NO and nine patients with TO were enrolled in the study. The average ages of patients with NO and TO were 63.85 ± 7.90 years and 67.00 ± 6.28 years, respectively. Five of seven patients with NO underwent brain computed tomography perfusion (CTP) or emission computed tomography (ECT) before admission. Four of seven patients had cerebral hypoperfusion responsive to ipsilateral ICA occlusive lesions.
All patients with TO experienced cerebral ischemic stroke before admission. Using CTP or ECT imaging, seven of nine patients displayed ipsilateral cerebral hypoperfusion in correspondence with the involved ICA. Typically, CTP demonstrated ipsilateral prolonged time of peak (TMax), main transit time, and time to peak; decreased cerebral blood flow; and increased cerebral blood volume [Supplementary Table 1].
The hybrid surgery courses of all patients with NO and eight of nine patients with TO were uneventful, both during surgery and 30 days after surgery. However, there were two failed cases, either because of poor outflow (for NO) or CCF emergency (for TO). Patients recovered uneventfully after surgery. Technical success was observed in 14 of 16 patients (87.5%), of which 6 of 7 patients had NO (85.7%) and 8 of 9 patients had TO (88.9%). The difference in success rate between the two series was not statistically significant (x2 = 0.036, P = 0.849). Details of hybrid procedures for patients with NO and patients with TO are summarized in Supplementary Table 2.
For patients with NO, the WALLSTENT® was used in two of seven patients to maintain ICA patency. For patients with TO, besides CEA, endovascular procedures were performed in seven of nine patients. Considering their dependence for endovascular procedures, the difference between patients with NO and patients with TO was statistically significant (x2 = 3.874, P = 0.005). In some cases, CEA with angioplasty using PTA balloons (one of seven patients) or CEA with thrombectomy using Fogarty® only (one of seven patients) was sufficient to revascularize the occluded ICA. No reperfusion complications occurred after surgery in either patient group.
The median follow-up time was 19.5 ± 4.0 months. During follow up, patient No. 2 in the TO group had CCA restenosis around the bifurcation 6 months after surgery without any symptoms. Restenosis was corrected by implantation of another WALLSTENT®. Patient No. 4 in the TO group suffered stroke 23 months after surgery. C2 was found to have severe stenosis by DSA. Restenosis was corrected using a PTA balloon alone.
The ICA of patient No. 4 in the NO group was completely occluded 4 months after surgery, but the patient was asymptomatic; thus, no further measures were taken. Only one death occurred 4 months after surgery because of advanced colon cancer. The overall primary patency rate of the ICA by hybrid surgery for all patients with NO and TO according to the Kaplan-Meier survival analysis is shown in Figure 1. The 2-year patency rate was 70% for all patients. The difference in patency rate between patients with TO and patients with NO was not significant (X2 = 0.205, P = 0.65) [Supplementary Figure 1].
Figure 1.
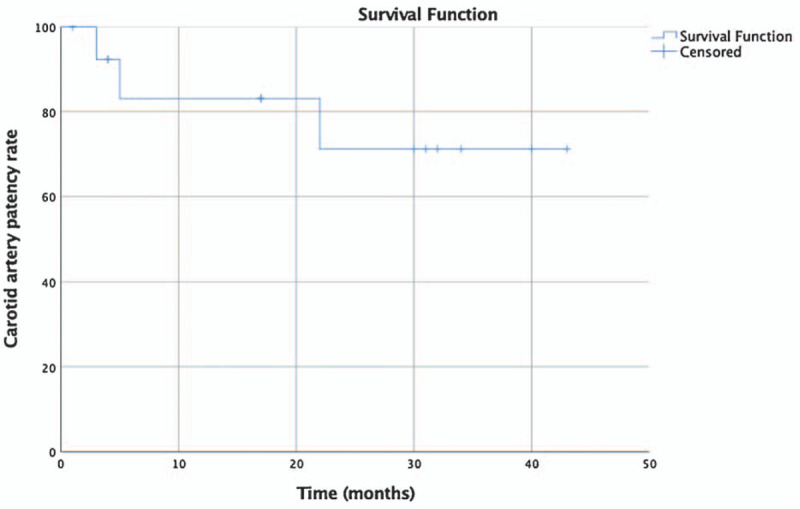
The primary internal carotid artery patency rate of all patients treated with hybrid procedures.
The natural history of ICA NO or TO is poorly understood. In fact, the clinical status of patients varies, from asymptomatic patients to patients with sequelae of major stroke. To simplify the complex question, we selectively enrolled patients with NO and patients with TO in this study. First, all patients were symptomatic, which was related to cerebral hypoperfusion. Second, symptoms and carotid artery occlusive lesions lasted for longer than 3 months, and there was a big difference between acute and chronic cases. Third, to ensure a beneficial effect of the procedures, especially for patients with TO, CTP was used as a prerequisite imaging examination to clarify cerebral hypoperfusion status in the ICA.
For some patients with NO, the distal intima was too fragile to be anchored or the relatively healthy intima was anatomically too high to be reached. ICA outflow was therefore endangered. From another point of view, using the endovascular technique, this difficult situation was easy to overcome by stenting. Two patients in the NO series benefited from this technique. If no endovascular technique was available, surgery was deemed to have failed. Removal of bifurcation plaques avoided the difficult but essential endovascular technique of passing a guidewire through the almost occluded ICA and possible plaque escape, which can lead to thrombotic stroke.
For patients with TO, revascularization was more challenging compared with patients with NO. Tandem lesions were commonly observed; in such cases, C2–C4 segments were usually involved. The endovascular technique was a better choice for revascularization of these segments.
Two failed cases did give some lessons to us. Failure to revascularization of one NO case was because of poor intracranial outflow. In our experience, we prefer occlusive ICA cases with good outflow in C4–C6 segments. TO failure was because of CCF when trying to pass through C4. This was the only complication observed during surgery. CCF was not uncommon when performing endovascular maneuvers in patients with TO; its incidence was approximately 8%.[5] Much attention should be paid when exploring occluded segments, and if CCF occurs, the ICA should be sutured immediately.
ICA restenosis occurred in two patients with TO in this study. One case of stenosis was located at the bifurcation, while the other was at the C2 segment, causing stroke. The bifurcation area was subject to hemodynamic changes in wall stress, and it was prone to arteriosclerosis and inflammation, leading to restenosis. In future practices, we would introduce the C1 stent through the femoral artery and locate it as a routine carotid artery stenting procedure to fully cover the bifurcation area and avoid restenosis. The C2 segment of the ICA, the so-called petrous segment, is the route by which the ICA travels through the osseous canal to the intracranial space. Stenosis here might be successfully stented using stents with stronger radial forces.
In summary, hybrid surgery is a new treatment option for complex carotid artery occlusive disease. The revascularization rate was as high as 87.5% in this study, which is a better result compared with other reports. The complication rate was only 6.25% intraoperatively and 30 days after surgery. However, indications for patients with ICA NO and TO are still controversial and should be evaluated on a case-by-case basis.
Some limitations of the present study should be highlighted. First, the sample size of the study was limited. Second, the study was non-randomized and did not include a control group to compare our approach with widely used medical treatments. Third, the study adopted a retrospective design, and treatment strategies were not perfectly unified.
In conclusion, our study indicates that hybrid surgery may provide a better strategy for indicated patients according to our criteria with a high operational success rate and a fairly low incidence of perioperative complications. The preliminary findings of this retrospective study should be confirmed in larger, randomized controlled trials in the future.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s)/patient's guardians has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the article. The patients/patient's guardians understand that their names and initials will not be published and due efforts will be made to conceal the identity of the patient, although anonymity cannot be guaranteed.
Conflicts of Interest
None.
Supplementary Material
Footnotes
How to cite this article: Wang CM, Han JT, Jia ZC, Yang GX, Li X. Hybrid surgery for symptomatic chronic near-total or total occlusion of the internal carotid artery. Chin Med J 2021;134:1104–1106. doi: 10.1097/CM9.0000000000001373
Supplemental digital content is available for this article.
References
- 1.Kaji R. Global burden of neurological diseases highlights stroke. Nat Rev Neurol 2019; 15:371–372. doi: 10.1038/s41582-019-0208-y. [DOI] [PubMed] [Google Scholar]
- 2.Lin MS, Lin LC, Li HY, Lin CH, Chao CC, Hsu CN, et al. Procedural safety and potential vascular complication of endovascular recanalization for chronic cervical internal carotid artery occlusion. Circ Cardiovasc Interv 2008; 1:119–125. doi: 10.1161/CIRCINTERVENTIONS.108.772350. [DOI] [PubMed] [Google Scholar]
- 3.Chen YH, Leong WS, Lin MS, Huang CC, Hung CS, Li HY, et al. Predictors for successful endovascular intervention in chronic carotid artery total occlusion. JACC Cardiovasc Interv 2016; 9:1825–1832. doi: 10.1016/j.jcin.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Fox AJ, Eliasziw M, Rothwell PM, Schmidt MH, Warlow CP, Barnett HJ, et al. Identification, prognosis, and management of patients with carotid artery near occlusion. AJNR Am J Neuroradiol 2005; 26:2086–2094. [PMC free article] [PubMed] [Google Scholar]
- 5.Yeh CF, Chen YH, Lin MS, Huang CC, Hung CS, Meng SW, et al. Carotid-cavernous fistula after endovascular intervention for chronic carotid artery total occlusion. Catheter Cardiovasc Interv 2018; 91:735–741. doi: 10.1002/ccd.27392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


