Abstract
Background:
Thyroid dysfunction is associated with cardiovascular diseases. However, the role of thyroid function in lipid metabolism remains partly unknown. The present study aimed to investigate the causal association between thyroid function and serum lipid metabolism via a genetic analysis termed Mendelian randomization (MR).
Methods:
The MR approach uses a genetic variant as the instrumental variable in epidemiological studies to mimic a randomized controlled trial. A two-sample MR was performed to assess the causal association, using summary statistics from the Atrial Fibrillation Genetics Consortium (n = 537,409) and the Global Lipids Genetics Consortium (n = 188,577). The clinical measures of thyroid function include thyrotropin (TSH), free triiodothyronine (FT3) and free thyroxine (FT4) levels, FT3:FT4 ratio and concentration of thyroid peroxidase antibodies (TPOAb). The serum lipid metabolism traits include total cholesterol (TC) and triglycerides, high-density lipoprotein, and low-density lipoprotein (LDL) levels. The MR estimate and MR inverse variance-weighted method were used to assess the association between thyroid function and serum lipid metabolism.
Results:
The results demonstrated that increased TSH levels were significantly associated with higher TC (β = 0.052, P = 0.002) and LDL (β = 0.041, P = 0.018) levels. In addition, the FT3:FT4 ratio was significantly associated with TC (β = 0.240, P = 0.033) and LDL (β = 0.025, P = 0.027) levels. However, no significant differences were observed between genetically predicted FT4 and TPOAb and serum lipids.
Conclusion:
Taken together, the results of the present study suggest an association between thyroid function and serum lipid metabolism, highlighting the importance of the pituitary-thyroid-cardiac axis in dyslipidemia susceptibility.
Keywords: Dyslipidemia, Mendelian randomization Analysis, Lipids, Thyroid hormones
Introduction
Cardiovascular diseases (CVDs) account for one-half of all non-communicable disease-associated deaths worldwide,[1] while the epidemic of obesity, dyslipidemia, and metabolic syndrome, which are major risk factors of CVDs, shows no signs of remission.[2] The thyroid gland is an important endocrine organ that is implicated in the occurrence and development of CVDs.[3] However, the causal association between thyroid hormones and serum lipid levels remains unclear.
The clinical measures of thyroid function include thyrotropin (TSH), free triiodothyronine (FT3) and free thyroxine (FT4) levels, FT3:FT4 ratio and concentration of thyroid peroxidase antibodies (TPOAb). TSH promotes the synthesis and release of FT4. Subsequently, FT4, which affects TSH production by the pituitary gland through a feedback loop, is converted into the active FT3 hormone in thyroid and peripheral tissues, and the FT3:FT4 ratio reflects the proportion of this conversion.[4] As such, the two-way feedback regulation of the hypothalamus-pituitary-thyroid (HPT) axis results in relatively complex effects of thyroid hormones. Given that thyroid peroxidase plays a key role in the synthesis of thyroid hormones, TPOAb, which is a marker of autoimmune thyroid disease, was selected as the thyroid function trait in the present study.[5]
Increasing evidence suggest that thyroid dysfunction is associated with the development of cardiometabolic disease.[3] Observational studies have reported that thyroid hormones, such as TSH and FT4, are associated with glycolipid metabolism and increased risk of CVDs.[3,6] It has been reported that levothyroxine is beneficial for decreasing cholesterol levels and cardiac morbidity and mortality rates.[7] Thus, the use of thyroid hormone analogs in patients at high risk of CVD has been investigated.[7,8] Identifying the causal association between thyroid hormones and serum lipids is important, given that observational studies are susceptible to confounding or reverse causation bias.
The Mendelian randomization (MR) approach uses a genetic variant as the instrumental variable (IV) in epidemiological studies to mimic a randomized controlled trial, where genetic alleles are randomly assorted at conception.[9] Thus, it is less likely to be affected by confounding or reverse causation,[9] and has been extensively applied to investigate the causal effect of exposure on disease.[10,11]
The present study performed a two-sample MR analysis to determine whether FT4 levels, TSH levels, FT3:FT4 ratio and concentration of TPOAb have causal associations with total cholesterol (TC) and triglycerides (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL), using summary data from genome-wide association studies (GWASs).
Methods
Ethical approval
This study is based on publicly available summary level data. All studies included in the analyses received ethics approval from a relevant Institutional Review Board, and all participants had provided informed consent.
Study design
The MR approach must satisfy the following assumptions:[12] The genetic variant selected as the IV must be associated with thyroid function traits; the genetic variant must not be associated with any confounders; and the genetic variant must be associated with serum lipid traits and pathways only associated with thyroid function [Figure 1]. The second and third assumptions are known as independence from pleiotropy. Subsequently, the causal effect of thyroid function on serum lipids was assessed.
Figure 1.
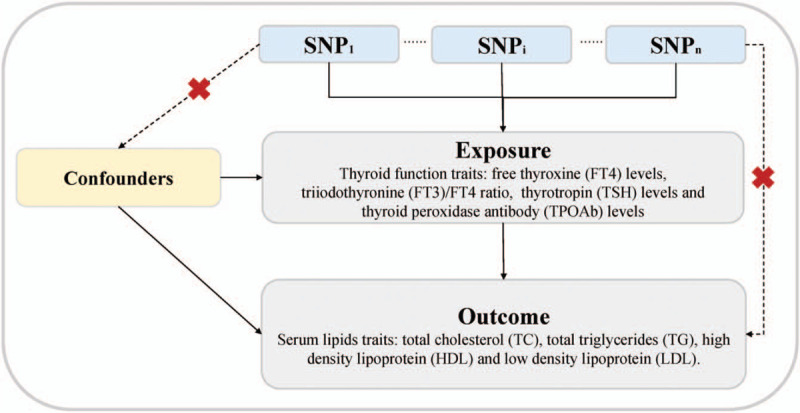
Principles of Mendelian randomization (MR) analysis for thyroid function and serum lipids traits and assumptions that need to be met to obtain unbiased estimates of causal effects. Broken lines represent potential pleiotropic or direct causal effects between variables that would violate MR assumptions. Three assumptions of MR: (1) Genetic variants must be associated with thyroid traits. (2) Genetic variants must not be associated with confounders. (3) Genetic variants must influence the serum lipids outcomes only through thyroid traits and not through any alternative pathways. FT3: Free triiodothyronine; FT4: Free thyroxine; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; SNP: Single nucleotide polymorphism; TC: Total cholesterol; TG: Total triglyceride; TPOAb: Thyroid peroxidase antibodies; TSH: Thyrotropin.
Selection and validation of IVs
As presented in Table 1, the clinical measures of thyroid function include FT4 and TSH levels within the reference range, concentration of TPOAb, and FT3:FT4 ratio from the Atrial Fibrillation Consortium (AFGen 2018 GWAS dataset, n = 537,409 European-descent individuals).[5,13–20] A total of 115 single-nucleotide polymorphisms (SNPs; P < 5 × 10–8) from previously published summary-level GWASs were used as the IVs for thyroid function traits [Supplementary Table 1].
Table 1.
Description of thyroid function traits.
| Thyroid function traits | Consortium or study | Sample size | Population | Year |
| FT4 | BHS, CHS, HBCS, KORA, NBS, Rotterdam Study, SardiNIA, SHIP/SHIP-Trend, TwinsUK, ASKLEPIOS, CARLA, EFSOCH, Health2006, SardiNIA2 | 26,089 | Europeans | 2018 |
| TSH | BHS, CHS, HBCS, KORA, NBS, Rotterdam Study, SardiNIA, SHIP/SHIP-Trend, TwinsUK, ValBorbera, ASKLEPIOS, CARLA, EFSOCH, Health2006, SardiNIA2 | 27,916 | Europeans | 2018 |
| TPOAb | ARIC, BHS, CHS, HBCS, KORA, NBS, RS, SardiNIA, SHIP, TwinsUK, Val Borbera, ASKLEPIOS, CARLA, EFSOCH, Health2006, SardiNIA2 | 18,297 | Trans-ethnic | 2015 |
| FT3:FT4 ratio | Weston Area T3/T4 Study (WATTS) cohort, Exeter Family Study of Childhood Health (EFSOCH), Invecchiare in Chianti (InCHIANTI), Depression and Thyroid Disease (DEPTH) | 3065 | Trans-Ethnics | 2008 |
ASKLEPIOS: The Asklepios Study; BHS: The 1994/1995 Busselton health survey; CHS: Cardiovascular Health Study; EFSOCH, Exeter family of childhood health; FT3: Free triiodothyronine; FT4: Free thyroxine; HBCS: Helsinki Birth Cohort Study; NBS: The Nijmegen Biomedical Study; SHIP: Study of Health in Pomerania; SIBLOS: The SIBLing Osteoporosis Study; TPOAb: Thyroid peroxidase antibodies; TSH: Thyrotropin.
Serum lipid traits and data sources
PubMed was searched for GWASs of serum lipid traits. Summary-level data were extracted from the Global Lipids Genetics Consortium (n = 188,577) for lipids, including HDL, LDL, TC, and TG.[21] Details on the GWASs are presented in Table 2. A summary of the genetic datasets are presented in Supplementary Tables 3 to 6.
Table 2.
Description of lipid metabolism traits.
| Lipid metabolism traits | Consortium or study | Sample size | Population | Year |
| HDL (mg/dL) | GLGC | 188,578 | Trans-ethnic | 2013 |
| LDL (mg/dL) | GLGC | 188,578 | Trans-ethnic | 2013 |
| TC (mg/dL) | GLGC | 188,578 | Trans-ethnic | 2013 |
| TG (mg/dL) | GLGC | 188,578 | Trans-ethnic | 2013 |
GLGC: The Global Lipids Genetics; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; TC: Total cholesterol; TG: Total triglyceride.
Linkage disequilibrium (LD) assessment and pleiotropy assessment
To determine whether the SNPs selected in the present study met the second and third assumptions, the genetic association with each thyroid function trait was assessed, and LD between all SNPs for the same thyroid function trait was measured.[22] In addition, independent genetic variants were selected for each thyroid function trait.[9] The variant with the lowest P value was selected for association with each thyroid function trait if genetic variants were in LD to ensure the SNPs did not violate the second assumption. All SNPs for the same thyroid function trait exhibited strong associations (F-statistic >10, the strength of the instrument), thus meeting the first assumption. MR-Egger regression analysis was performed to assess the presence of pleiotropic effects on serum lipid outcomes.[12] The effects of SNPs on each metabolite were plotted against its effect upon outcome, and an intercept distinct from the origin was used to assess pleiotropic effects.
MR analysis
The causal effect of each metabolite on outcome was analyzed using the inverse variance-weighted (IVW) method, which provides a combined estimate of the causal estimate from each SNP.[9] IVW is equivalent to a two-stage least squares or allele score analysis using individual-level data, and is thus considered a conventional alterative to MR.[23] Data are presented as standard deviation of the biomarkers. Association analyses were performed for four primary hypotheses (FT4, TSH, TPOAb, and FT3:FT4 ratio).
To control for false-positive findings due to multiple testing, we used Bonferroni correction adjusted for the number of primary exposures and outcomes in this study, and P < 0.003 (= 0.05/16) was considered to indicate statistical significance.
Furthermore, complementary approaches, such as simple median method, weighted median method, and MR-Egger for multiple genetic variants were performed to assess the causal effect.[23–26] Detailed information on these MR methods have been previously described.[23–26] MR-Egger regression analysis was performed to assess the pleiotropic effects.[12] The effect of the IV on exposure was plotted against its effect on the outcome, and an intercept distinct from the origin was used to assess the pleiotropic effects. The slope of the MR-Egger regression provides pleiotropy-corrected causal estimates.[12] Median weighted and MR-Egger methods are highly sensitive for MR investigations with multiple genetic variants.[23,24,26] Power calculations for MR were performed using mRnd (http://cnsgenomics.com/shiny/mRnd).
Analyses were performed using R version 3.2.3 (RStudio, PBC, Boston, Massachusetts, USA). P < 0.003 (= 0.05/16) following Bonferroni correction was considered to indicate statistical significance. P < 0.05 above the Bonferroni corrected threshold was considered to indicate statistical significance.
Results
Validation of selected SNPs and IVs
The characteristics of the selected SNPs for thyroid function are presented in Table 1 and Supplementary Table 1. All SNPs for thyroid function exhibited strong associations (F-statistic >21.83, the strength of the instrument), thus meeting the first assumption. The present study investigated whether any of the selected SNPs were influenced by LD and pleiotropy to assess the second and third assumptions. None of the SNPs were demonstrated to be in LD with each other in the same thyroid function trait (identified as r2 > 0.2). With regards to the association between thyroid function and serum lipid metabolism, intercepts from MR-Egger regression demonstrated that the results were not influenced by pleiotropy [Supplementary Table 2].
Thyroid function and lipid traits
Genetically predicted FT4 levels and TPOAb concentration within the normal range were not associated with any lipid traits following Bonferroni correction [Table 3]. However, genetically increased TSH levels were significantly associated with higher LDL (β = 0.041, P = 0.018) and TC (β = 0.052, P = 0.002) levels. In addition, a genetically higher FT3:FT4 ratio was significantly associated with higher LDL (β = 0.170, P = 0.037) and TC (β = 0.160, P = 0.043) levels [Table 3]. Effect estimates were broadly consistent between the IVW method and the complementary approaches (simple median, weighted median, and MR-Egger) in two-sample MR [Supplementary Table 2].
Table 3.
Mendelian randomization analysis of lipid metabolism traits.
| FT4 | TSH | TPOAb | FT3:FT4 ratio | |||||
| Items | β ± SE | P | β ± SE | P | β ± SE | P | β ± SE | P |
| HDL (mg/dL) | −0.013 ± 0.026 | 0.602 | −0.016 ± 0.016 | 0.331 | −0.472 ± 0.496 | 0.342 | 0.003 ± 0.067 | 0.970 |
| LDL (mg/dL) | −0.059 ± 0.044 | 0.174 | 0.041 ± 0.018 | 0.018 | −0.649 ± 0.371 | 0.080 | 0.170 ± 0.082 | 0.037 |
| TC (mg/dL) | −0.041 ± 0.037 | 0.276 | 0.052 ± 0.017 | 0.002 | −0.930 ± 0.627 | 0.138 | 0.160 ± 0.079 | 0.043 |
| TG (mg/dL) | 0.009 ± 0.035 | 0.801 | 0.008 ± 0.015 | 0.610 | 0.116 ± 0.222 | 0.602 | 0.003 ± 0.064 | 0.969 |
Results are presented as β ± SE. Causal effects were estimated using instrumental variables. FT3: Free triiodothyronine; FT4: Free thyroxine; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; SE: Standard error; TC: Total cholesterol; TG: Total triglyceride; TPOAb: Thyroid peroxidase antibodies; TSH: Thyrotropin.
MR power calculation assumes a statistical confidence level of 0.05 and an r2 value equaling genus heritability. The effect size presented in Supplementary Table 1 suggests statistical power >80% for the association between TSH and HF, but <80% for other significant associations. In addition, the associations in sensitivity were consistent between the simple median and weighted median methods [Supplementary Table 2]. The MR-Egger intercepts for each outcome were centered at the origin, with a confidence interval including the null, suggesting no strong unbalanced horizontal pleiotropy [Supplementary Table 2].
Discussion
The results of the present study provide novel insight into the associations between TSH and FT3:FT4 ratio and LDL and TC, respectively, highlighting the importance of the pituitary-thyroid-cardiac axis in lipid metabolism susceptibility. In addition, no significant causal associations were observed between other thyroid function traits and lipid traits.
Previous studies have reported that thyroid metabolism is closely associated with lipid and glucose metabolism.[27,28] Notably, it has been demonstrated that TSH has a direct effect on HMG-CoA expression.[29] In the present study, genetically increased TSH levels within the normal range were significantly associated with higher TC and LDL levels. Despite discrepancy between a previous observational study,[28] the MR results for TSH in the present study are consistent with previous findings.[30]In vitro studies have suggested that thyroid hormones regulate LDL catabolism by their effects on lipid metabolizing enzymes and LDL receptor expression in the liver.[31,32] It has been reported that thyroid hormones regulate hepatic lipid metabolism in a cell autonomous manner.[33] TSH binds to its receptor and modulates activated protein kinase via cyclic adenosine monophosphate/protein kinase A to change its inhibitory effect on the peroxisome proliferator-activated receptor-γ signaling pathway, which triggers sterol regulatory element-binding protein 1c activity in the liver and induces gene expression related to adipogenesis.[33] Thyroid hormones can induce reverse cholesterol transport and thyroid hormones analogs may be used as therapeutic alternatives for the treatment of lipid-associated hepatic pathologies, both directly and indirectly.[34]
The FT3:FT4 ratio reflects the conversion rate of FT4 to FT3.[11] The results of the present study demonstrated that genetically increased FT3:FT4 ratio was associated with higher TC and LDL levels. Part of the association between thyroid function and dyslipidemia may be mediated by intricate sensing and feedback systems that function at the physiological, metabolic, molecular, and transcriptional levels in the liver.[35] It is possible that some control points in the signaling pathways, which regulate TC and TG levels within the liver and serum, are modulated by thyroid hormones, and may thus be used as potential drug targets for patients with dyslipidemia.[34] Recently, it was reported the treatment, management, and especially prevention actions in early life might restrain the increase risks of metabolic disorders.[36] Our MR analysis suggests prevention of higher TSH and FT3:FT4 ratio within normal range might also help to regulate TC and LDL levels.
However, the present study did not identify an association between thyroid function and TG levels. These results are consistent with previous findings, which have demonstrated that treatment with L-thyroxine decreases TC and LDL levels in euthyroid patients, but has no effect on HDL or TG levels.[37] Further MR studies with more genetic instruments are required to elucidate the causality.
Hyperlipidemia is a traditional risk factor for coronary heart disease (CHD). However, the causality between thyroid function and CHD remains equivocal. Population studies on the association between thyroid dysfunction and CHD morbidity and mortality rates are conflicting, whereby not all studies have demonstrated a positive association.[3,6,38] These inconsistencies may be due to differences in the populations, as well as the duration of follow-up.
The present study has several strengths. First, the causal effect of thyroid function on serum lipids was systematically assessed. A common limitation in previous observational studies is the heterogeneity among individual studies that use different TSH cut-offs, different confounding factors for adjustment and different disease definitions. Second, the present study used data from large GWASs and implemented the MR design, which prevent reverse causation and confounding bias.
However, several limitations merit consideration. First, MR has stringent core assumptions. Although SNPs were selected at the level of genome-wide significance, and F statistics suggested a strong genetic association with thyroid function, the results may have been affected by weak instrument bias. Second, sex-specific associations with lipids were not investigated; thus, the potential of positive estimates in sex-specific analyses cannot be ruled out. The estimates for these outcomes are likely to be conservative as some associations may be sex-specific. Third, the influence of genetic determinants may have been affected by compensatory developmental processes, such as canalization. However, the GWASs for all exposures were performed in individuals with normal thyroid function. Given the discrepancy between the observational and MR instrumental findings for FT4, further studies are required, particularly downstream of FT4, along the HPT-cardiac axis. Determining the mechanistic inferences downstream of FT4 was limited, as there was only one instrument for the FT3:FT4 ratio and no instruments for FT3, although this hormone, as opposed to FT4, is transported into cardiomyocytes and is considered biologically relevant.[3] Given that every HPT axis setpoint differs, the TSH levels within the reference range are unable to exclude a disease thyroid state as driving the instrumental associations with diseases in the present study. In addition, it is impossible to assess the causal association between thyroglobulin and lipid metabolism as there are currently no GWAS data on thyroglobulin antibody. Furthermore, an alternative direct causal pathway for all MR analyses cannot be ruled out, particularly for thyroid function index determined by both thyroid function and multiple genetic variants.
In conclusion, the results of the present study demonstrated that genetically increased TSH levels were associated with higher TC and LDL levels, highlighting the importance of the pituitary-thyroid-cardiac axis in lipid metabolism.
Funding
This work is supported by the National Natural Science Foundation of China (No. 81825003, 91957123), the Peking University Start-up Grant (BMU2018YJ002), High-performance Computing Platform of Peking University and Beijing Technology and Business University Grant (No.88442Y0033), Thyroid Hormone Replacement for Subclinical Hypothyroidism and Dyslipidemia in Patients with Atherosclerotic Cardiovascular Diseases (NCT03606824).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Wang JJ, Zhuang ZH, Shao CL, Yu CQ, Wang WY, Zhang K, Meng XB, Gao J, Tian J, Zheng JL, Huang T, Tang YD. Assessment of causal association between thyroid function and lipid metabolism: a Mendelian randomization study. Chin Med J 2021;134:1064–1069. doi: 10.1097/CM9.0000000000001505
Supplemental digital content is available for this article.
References
- 1.Benziger CP, Roth GA, Moran AE. The global burden of disease study and the preventable burden of NCD. Glob Heart 2016; 11:393–397. doi: 10.1016/j.gheart.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 2.Eckel RH, Blaha MJ. Cardiometabolic medicine: a call for a new subspeciality training track in internal medicine. Am J Med 2019; 132:788–790. doi: 10.1016/j.amjmed.2019.02.027. [DOI] [PubMed] [Google Scholar]
- 3.Grais IM, Sowers JR. Thyroid and the heart. Am J Med 2014; 127:691–698. doi: 10.1016/j.amjmed.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X, Zhou Y, Zhou M, Yin Q, Wang S. Diagnostic values of free triiodothyronine and free thyroxine and the ratio of free triiodothyronine to free thyroxine in thyrotoxicosis. Int J Endocrinol 2018; 2018:4836736.doi: 10.1155/2018/4836736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medici M, Porcu E, Pistis G, Teumer A, Brown SJ, Jensen RA, et al. Identification of novel genetic Loci associated with thyroid peroxidase antibodies and clinical thyroid disease. PLoS Genet 2014; 10:e1004123.doi: 10.1371/journal.pgen.1004123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selmer C, Olesen JB, Hansen ML, von Kappelgaard LM, Madsen JC, Hansen PR, et al. Subclinical and overt thyroid dysfunction and risk of all-cause mortality and cardiovascular events: a large population study. J Clin Endocrinol Metab 2014; 99:2372–2382. doi: 10.1210/jc.2013-4184. [DOI] [PubMed] [Google Scholar]
- 7.Brenta G, Danzi S, Klein I. Potential therapeutic applications of thyroid hormone analogs. Nat Clin Pract Endocrinol Metab 2007; 3:632–640. doi: 10.1038/ncpendmet0590. [DOI] [PubMed] [Google Scholar]
- 8.Larsson SC, Allara E, Mason AM, Michaelsson K, Burgess S. Thyroid function and dysfunction in relation to 16 cardiovascular diseases. Circ Genom Precis Med 2019; 12:e002468.doi: 10.1161/CIRCGEN.118.002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med 2008; 27:1133–1163. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 10.Zhang YM, Zhou XJ, Shi SF, Liu LJ, Lyu JC, Zhang H. Homocysteine and IgA nephropathy: observational and Mendelian randomization analyses. Chin Med J 2020; 133:277–284. doi: 10.1097/CM9.0000000000000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellervik C, Roselli C, Christophersen IE, Alonso A, Pietzner M, Sitlani CM, et al. Assessment of the relationship between genetic determinants of thyroid function and atrial fibrillation: a Mendelian randomization study. JAMA Cardiol 2019; 4:144–152. doi: 10.1001/jamacardio.2018.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 2015; 44:512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porcu E, Medici M, Pistis G, Volpato CB, Wilson SG, Cappola AR, et al. A meta-analysis of thyroid-related traits reveals novel loci and gender-specific differences in the regulation of thyroid function. PLoS Genet 2013; 9:e1003266.doi: 10.1371/journal.pgen.1003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor PN, Porcu E, Chew S, Campbell PJ, Traglia M, Brown SJ, et al. Whole-genome sequence-based analysis of thyroid function. Nat Commun 2015; 6:5681.doi: 10.1038/ncomms6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panicker V, Cluett C, Shields B, Murray A, Parnell KS, Perry JR, et al. A common variation in deiodinase 1 gene DIO1 is associated with the relative levels of free thyroxine and triiodothyronine. J Clin Endocrinol Metab 2008; 93:3075–3081. doi: 10.1210/jc.2008-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eriksson N, Tung JY, Kiefer AK, Hinds DA, Francke U, Mountain JL, et al. Novel associations for hypothyroidism include known autoimmune risk loci. PLoS One 2012; 7:e34442.doi: 10.1371/journal.pone.0034442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pickrell JK, Berisa T, Liu JZ, Segurel L, Tung JY, Hinds DA. Detection and interpretation of shared genetic influences on 42 human traits. Nat Genet 2016; 48:709–717. doi: 10.1038/ng.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schultheiss UT, Teumer A, Medici M, Li Y, Daya N, Chaker L, et al. A genetic risk score for thyroid peroxidase antibodies associates with clinical thyroid disease in community-based populations. J Clin Endocrinol Metab 2015; 100:E799–E807. doi: 10.1210/jc.2014-4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gudmundsson J, Sulem P, Gudbjartsson DF, Jonasson JG, Masson G, He H, et al. Discovery of common variants associated with low TSH levels and thyroid cancer risk. Nat Genet 2012; 44:319–322. doi: 10.1038/ng.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teumer A, Chaker L, Groeneweg S, Li Y, Di Munno C, Barbieri C, et al. Genome-wide analyses identify a role for SLC17A4 and AADAT in thyroid hormone regulation. Nat Commun 2018; 9:4455.doi: 10.1038/s41467-018-06356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet 2013; 45:1274–1283. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genomes Project C, Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, et al. An integrated map of genetic variation from 1,092 human genomes. Nature 2012; 491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 2013; 37:658–665. doi: 10.1002/gepi.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 2016; 40:304–314. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol 2017; 46:1985–1998. doi: 10.1093/ije/dyx102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yavorska OO, Burgess S. Mendelian randomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol 2017; 46:1734–1739. doi: 10.1093/ije/dyx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roef GL, Rietzschel ER, Van Daele CM, Taes YE, De Buyzere ML, Gillebert TC, et al. Triiodothyronine and free thyroxine levels are differentially associated with metabolic profile and adiposity-related cardiovascular risk markers in euthyroid middle-aged subjects. Thyroid 2014; 24:223–231. doi: 10.1089/thy.2013.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takamura N, Akilzhanova A, Hayashida N, Kadota K, Yamasaki H, Usa T, et al. Thyroid function is associated with carotid intima-media thickness in euthyroid subjects. Atherosclerosis 2009; 204:e77–e81. doi: 10.1016/j.atherosclerosis.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 29.Tian L, Song Y, Xing M, Zhang W, Ning G, Li X, et al. A novel role for thyroid-stimulating hormone: up-regulation of hepatic 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase expression through the cyclic adenosine monophosphate/protein kinase A/cyclic adenosine monophosphate-responsive element binding protein pathway. Hepatology 2010; 52:1401–1409. doi: 10.1002/hep.23800. [DOI] [PubMed] [Google Scholar]
- 30.Asvold BO, Vatten LJ, Nilsen TI, Bjoro T. The association between TSH within the reference range and serum lipid concentrations in a population-based study. The HUNT study. Eur J Endocrinol 2007; 156:181–186. doi: 10.1530/eje.1.02333. [DOI] [PubMed] [Google Scholar]
- 31.Staels B, Van Tol A, Chan L, Will H, Verhoeven G, Auwerx J. Alterations in thyroid status modulate apolipoprotein, hepatic triglyceride lipase, and low density lipoprotein receptor in rats. Endocrinology 1990; 127:1144–1152. doi: 10.1210/endo-127-3-1144. [DOI] [PubMed] [Google Scholar]
- 32.Salter AM, Hayashi R, al-Seeni M, Brown NF, Bruce J, Sorensen O, et al. Effects of hypothyroidism and high-fat feeding on mRNA concentrations for the low-density-lipoprotein receptor and on acyl-CoA: cholesterol acyltransferase activities in rat liver. Biochem J 1991; 276:825–832. doi: 10.1042/bj2760825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan F, Wang Q, Lu M, Chen W, Song Y, Jing F, et al. Thyrotropin increases hepatic triglyceride content through upregulation of SREBP-1c activity. J Hepatol 2014; 61:1358–1364. doi: 10.1016/j.jhep.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 34.Sinha RA, Singh BK, Yen PM. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat Rev Endocrinol 2018; 14:259–269. doi: 10.1038/nrendo.2018.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beukhof CM, Massolt ET, Visser TJ, Korevaar TIM, Medici M, de Herder WW, et al. Effects of thyrotropin on peripheral thyroid hormone metabolism and serum lipids. Thyroid 2018; 28:168–174. doi: 10.1089/thy.2017.0330. [DOI] [PubMed] [Google Scholar]
- 36.Zhou LY, Deng MQ, Zhang Q, Xiao XH. Early-life nutrition and metabolic disorders in later life: a new perspective on energy metabolism. Chin Med J (Engl) 2020; 133:1961–1970. doi: 10.1097/CM9.0000000000000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michalopoulou G, Alevizaki M, Piperingos G, Mitsibounas D, Mantzos E, Adamopoulos P, et al. High serum cholesterol levels in persons with ’high-normal’ TSH levels: should one extend the definition of subclinical hypothyroidism? Eur J Endocrinol 1998; 138:141–145. doi: 10.1530/eje.0.1380141. [DOI] [PubMed] [Google Scholar]
- 38.Boekholdt SM, Titan SM, Wiersinga WM, Chatterjee K, Basart DC, Luben R, et al. Initial thyroid status and cardiovascular risk factors: the EPIC-Norfolk prospective population study. Clin Endocrinol (Oxf) 2010; 72:404–410. doi: 10.1111/j.1365-2265.2009.03640.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


