ABSTRACT
The therapeutic effects of off-label oral vancomycin in pediatric and adult primary sclerosing cholangitis (PSC)-inflammatory bowel disease, more commonly PSC-ulcerative colitis (UC), indicate the translational relevance of disease-associated microbiome findings. This is the first report on longitudinal salivary and fecal microbiome changes in a pediatric PSC-UC patient over the first 90 days of vancomycin therapy. Increase in bacterial diversity and abundance changes in Fusobacterium, Haemophilus, and Neisseria were observed. Our findings highlight the importance of longitudinal microbiome sampling in PSC-UC and serve as a nidus for larger-scale observations toward advancing microbial therapeutics for PSC.
INTRODUCTION
Tan et al1 reported that oral vancomycin induces clinical and mucosal remission of colitis in children with primary sclerosing cholangitis-ulcerative colitis (PSC-UC). Distinct microbiome profiles in adult PSC-UC patients have been described.2 Fecal and salivary microbiomes of pediatric patients are considered potential biomarkers for PSC-inflammatory bowel disease (IBD).3 The importance of longitudinal microbiome sampling in patients with IBD for translational research purposes has been emphasized.4 However, such longitudinal studies in patients with PSC or PSC-IBD have not been performed in either children or adults.
CASE REPORT
A 7-year-old Hispanic white boy presented with weight loss, bloody diarrhea, and abnormal liver enzymes in Puerto Rico. He was diagnosed with Crohn's colitis by endoscopy. Hepatic imaging through magnetic resonance cholangiopancreatography was normal, but liver biopsy revealed small duct PSC. Initial treatments were prednisone, ursodiol, and azathioprine. One year after diagnosis, he developed pancreatitis and his azathioprine was changed to methotrexate. At age 9, he moved to Houston, Texas. He never achieved steroid-free remission, and continued to have mild colitis symptoms. Endoscopy was more consistent with mild pancolitic UC. Prednisone wean was initiated before endoscopy. After endoscopy, balsalazide (750 mg 3 times daily) was started, while ursodiol (10 mg/kg) continued and methotrexate stopped. He flared 1 month later and was started on budesonide (9 mg daily). Fecal calprotectin (FC) and serum gamma-glutamyl transferase (GGT) levels remained elevated after 5 weeks of budesonide therapy. The decision was made to switch him to off-label vancomycin (125 mg 4 times daily) with the goal of optimizing colitis and possibly PSC control (Figure 1). Ursodiol was discontinued per his hepatologist's recommendation.
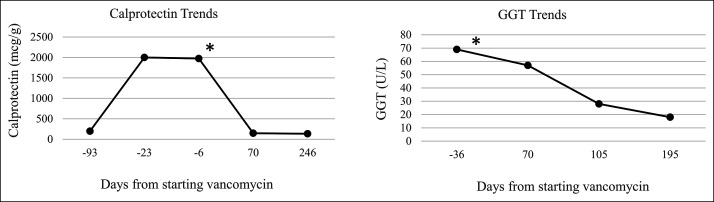
Figure 1.
Fecal calprotectin and serum gamma-glutamyl transferase GGT changes our patient (*indicates vancomycin start).
He was recruited into our Institutional Review Board-approved study (H-43759) after written consent. We prospectively performed longitudinal microbiome analyses over a 90-day period of vancomycin treatment. Clinical response to vancomycin was assessed by pediatric UC activity index.5 Colitis activity was also monitored by FC, and biochemical PSC activity was monitored by GGT.
Clinical colitis resolved within 90 days (pediatric UC activity index 25 [day 0] to 5 [day 90]). FC normalized 70 days after vancomycin (Figure 1) when budesonide was discontinued. His serum GGT declined after vancomycin initiation and reached normal levels by 195 days (Figure 1). He continued vancomycin and balsalazide therapy and has remained in clinical remission. He was monitored with FC levels, which remained normal. No follow-up endoscopy has been performed to date because of high copay mandated by the patient's insurance.
Stool and saliva were collected at 4 timepoints: 0 (before treatment with vancomycin), 2, 7, and 90 days after starting vancomycin. OMNIgene ORAL saliva and OMNIgene GUT stool collection kits were used.6,7 Stool and saliva samples were processed for high-throughput sequencing of the bacterial 16S rRNA gene at the Alkek Center for Metagenomics and Microbiome Research at Baylor College of Medicine. A rise in microbiome diversity (a combined measure of microbiome [bacteriome in this case] abundance and richness) in stool and saliva was observed (Figure 2). Vancomycin affected the fecal microbiome faster than the salivary (Figure 2). Near consistent abundance changes occurred in Fusbacterium (decrease), Haemophilus (decrease), and Veilonella (increase) (Figure 3). In saliva, the abundance of Neisseria consistently declined (Figure 4).
Figure 2.
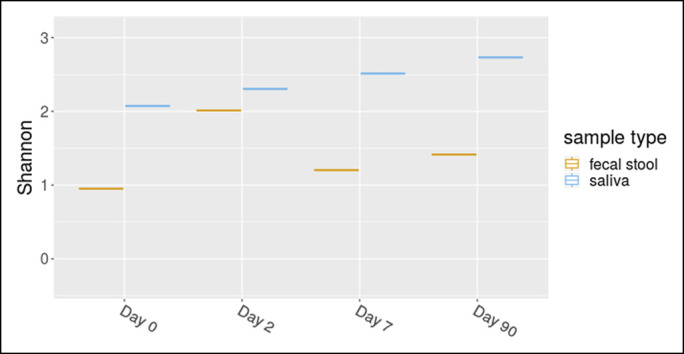
Shannon Diversity Index of stool and saliva samples from our patient separated out by day. Day 1 indicates start of vancomycin.
Figure 3.
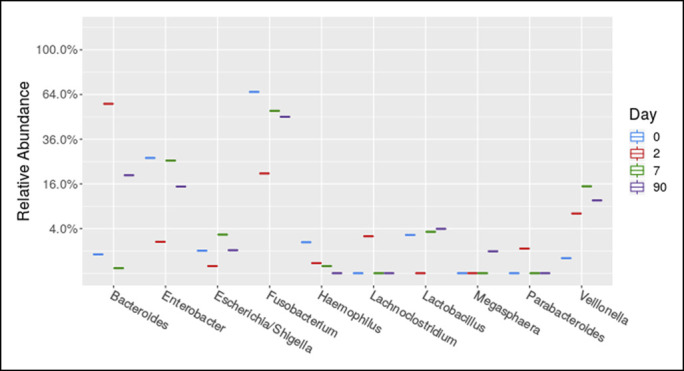
Top 10 genera changes in the stool of our patient separated out by day. Day 1 indicates start of vancomycin.
Figure 4.
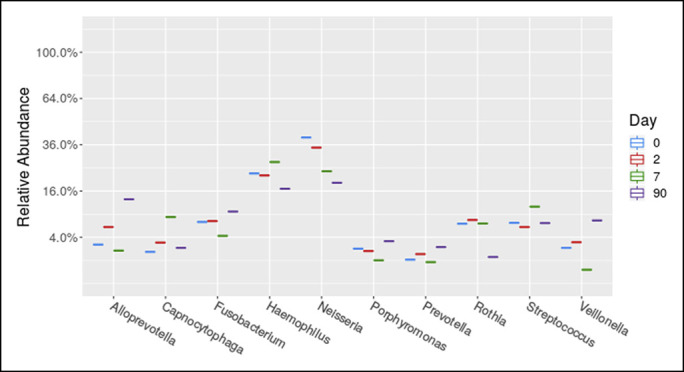
Top 10 genera in the saliva of our patient separated out by day. Day 1 indicates start of vancomycin.
DISCUSSION
PSC-UC represents a subtype of UC in pediatric patients associated with a less aggressive (ie, reduced progression to colectomy) course of colitis compared with non–PSC-UC.8 There appears to be a frequent disconnect between the clinical and objective (ie, biochemical: FC; and endoscopic) severity of colitis within this group compared with non–PSC-UC.9 The clinically “silent” colitis can be rather refractory to current conventional treatments.1 This may be one reason for the significantly increased colorectal cancer rate in PSC-UC over non–PSC-UC.10 Because of these clinical characteristics, novel modes of colitis control, such as vancomycin therapy, are important to investigate.
There is limited literature on the gut microbial effects of oral vancomycin.11–13 The unique findings in our case were unlikely to be related to the weaning of steroids, as the patient was still taking budesonide on days 2 and 7 after starting vancomycin, but diversity already increased.14 The withdrawal of ursodiol was also unlikely to lead to our findings since the salivary microbiome in PSC patients on and off ursodiol therapy was not different in previous reports.3 Therefore, we speculate that vancomycin therapy may have induced dampening of mucosal inflammation by modulating epithelial biology and/or bacterial composition and thereby indirectly led to the secondary rise of bacterial diversity.15 Microbiome changes on day 2 differed from those on day 90 after vancomycin initiation. This highlights the importance of longitudinal sampling. Since vancomycin is used in a chronic fashion for PSC-UC, we focused more on the consistent/longitudinal microbiome changes in our patient.
Fusobacterium and Haemophilus have been observed to be more common in PSC fecal microbiomes, and Neisseria has been found to be elevated in the PSC bile.16–18 Interestingly, the addition of vancomycin decreased these genera in our patient. Veillonella has been found to be increased in the stool,2 bile, and duodenal biopsies of adult PSC patients.18 However, Veillonella is commonly resistant to vancomycin,19 which may explain the increase in its abundance we observed after treatment. Consequently, our observations implicate Fusobacterium, Haemophilus, and Neisseria, but not Veillonella as potential targets of long-term vancomycin therapy in PSC-UC.
In conclusion, this is the first report on longitudinal microbiome analysis with respect to vancomycin treatment in PSC-UC. Microbiome changes have recently been recognized to be highly personalized in IBD,20 which points to the significance of longitudinal observations in single cases, such as ours. The findings will serve as a nidus for larger cohort and extended length longitudinal observations on vancomycin in PSC-UC.
DISCLOSURES
Author contributions: All authors contributed equally to this manuscript. R. Kellermayer is the article guarantor.
Financial disclosure: R. Kellermayer was supported in part by philanthropic funds from the Wagner Family led Gutsy Kids Fund and by the Klaasmeyer family funds for PSC research. He was also supported in part by the PROKIIDs Network of the Crohn's and Colitis Foundation.
Informed consent was obtained for this case report.
Contributor Information
Savini Lanka Britto, Email: sbritto@bcm.edu.
Kristi Louise Hoffman, Email: kristi.hoffman@bcm.edu.
Mary Elizabeth Tessier, Email: metessie@bcm.edu.
Joseph Petrosino, Email: jpetrosi@bcm.edu.
Tamir Miloh, Email: txm760@med.miami.edu.
REFERENCES
- 1.Tan LZ, Reilly CR, Steward-Harrison LC, Balouch F, Muir R, Lewindon PJ. Oral vancomycin induces clinical and mucosal remission of colitis in children with primary sclerosing cholangitis-ulcerative colitis. Gut. 2019;68(8):1533–5. [DOI] [PubMed] [Google Scholar]
- 2.Kummen M, Holm K, Anmarkrud JA, et al. The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut. 2017;66(4):611–9. [DOI] [PubMed] [Google Scholar]
- 3.Iwasawa K, Suda W, Tsunoda T, et al. Dysbiosis of the salivary microbiota in pediatric-onset primary sclerosing cholangitis and its potential as a biomarker. Sci Rep. 2018;8(1):5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lloyd-Price J, Arze C, Ananthakrishnan AN, et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569(7758):655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner D, Hyams J, Markowitz J, et al. Appraisal of the pediatric ulcerative colitis activity index (PUCAI). Inflamm Bowel Dis. 2009;15(8):1218–23. [DOI] [PubMed] [Google Scholar]
- 6.Speicher DJ, Johnson NW. Comparison of salivary collection and processing methods for quantitative HHV-8 detection. Oral Dis. 2014;20(7):720–8. [DOI] [PubMed] [Google Scholar]
- 7.Song SJ, Amir A, Metcalf JL, et al. Preservation methods differ in fecal microbiome stability, affecting suitability for field studies. mSystems. 2016;1(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiau H, Ihekweazu FD, Amin M, Fofanova T, Miloh T, Kellermayer R. Unique inflammatory bowel disease phenotype of pediatric primary sclerosing cholangitis: A single-center study. J Pediatr Gastroenterol Nutr. 2017;65(4):404–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricciuto A, Hansen BE, Ngo B, et al. Primary sclerosing cholangitis in children with inflammatory bowel diseases is associated with milder clinical activity but more frequent subclinical inflammation and growth impairment. Clin Gastroenterol Hepatol. 2019;18(7):1509–17. [DOI] [PubMed] [Google Scholar]
- 10.Wyatt A, Kellermayer R. Right-sided colonic juvenile polyp [polyps] in paediatric primary sclerosing cholangitis patients. J Crohns Colitis. 2018;12(11):1375–6. [DOI] [PubMed] [Google Scholar]
- 11.Vrieze A, Out C, Fuentes S, et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J Hepatol. 2014;60(4):824–31. [DOI] [PubMed] [Google Scholar]
- 12.Contijoch EJ, Britton GJ, Yang C, et al. Gut microbiota density influences host physiology and is shaped by host and microbial factors. Elife. 2019;22:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isaac S, Scher JU, Djukovic A, et al. Short- and long-term effects of oral vancomycin on the human intestinal microbiota. J Antimicrob Chemother. 2017;72(1):128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wills ES, Jonkers DM, Savelkoul PH, Masclee AA, Pierik MJ, Penders J. Fecal microbial composition of ulcerative colitis and Crohn's disease patients in remission and subsequent exacerbation. PLoS One. 2014;9(3):e90981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King DW, Smith MA. Proliferative responses observed following vancomycin treatment in renal proximal tubule epithelial cells. Toxicol In Vitro. 2004;18(6):797–803. [DOI] [PubMed] [Google Scholar]
- 16.Sabino J, Vieira-Silva S, Machiels K, et al. Primary sclerosing cholangitis is characterised by intestinal dysbiosis independent from IBD. Gut. 2016;65(10):1681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bajer L, Kverka M, Kostovcik M, et al. Distinct gut microbiota profiles in patients with primary sclerosing cholangitis and ulcerative colitis. World J Gastroenterol. 2017;23(25):4548–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liwinski T, Zenouzi R, John C, et al. Alterations of the bile microbiome in primary sclerosing cholangitis. Gut. 2020;69(4):665–72. [DOI] [PubMed] [Google Scholar]
- 19.Perez-Jacoiste Asin MA, Fernandez-Ruiz M, Serrano-Navarro I, Prieto-Rodriguez S, Aguado JM. Polymicrobial endocarditis involving Veillonella parvula in an intravenous drug user: Case report and literature review of Veillonella endocarditis. Infection. 2013;41(2):591–4. [DOI] [PubMed] [Google Scholar]
- 20.Schirmer M, Franzosa EA, Lloyd-Price J, et al. Dynamics of metatranscription in the inflammatory bowel disease gut microbiome. Nat Microbiol. 2018;3(3):337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]