Abstract
Background:
Hiatal hernias are often repaired concurrently with bariatric surgery to reduce risk of gastroesophageal reflux disease–related complications.
Objectives:
To examine the association between concurrent hiatal hernia repair (HHR) and bariatric outcomes. Setting: A 2010–2017 U.S. commercial insurance claims data set.
Methods:
We conducted a retrospective cohort study. We identified adults who underwent sleeve gastrectomy (SG) or Roux-en-Y gastric bypass (RYGB) alone or had bariatric surgery concurrently with HHR. We matched patients with and without HHR and followed patients up to 3 years for incident abdominal operative interventions, bariatric revisions/conversions, and endoscopy. Time to first event for each outcome was compared using multivariable Cox proportional hazards modeling.
Results:
We matched 1546 SG patients with HHR to 3170 SG patients without HHR, and we matched 457 RYGB patients with HHR to 1156 RYGB patients without HHR. A total of 73% had a full year of postoperative enrollment. Patients who underwent concurrent SG and HHR were more likely to have additional abdominal operations (adjusted hazard ratio [aHR], 2.1; 95% CI, 1.5–3.1) and endoscopies (aHR, 1.5; 95% CI, 1.2–1.8) but not bariatric revisions/conversions (aHR, 1.7; 95% CI, .6–4.6) by 1 year after surgery, a pattern maintained at 3 years of follow-up. Among RYGB patients, concurrent HHR was associated only with an increased risk of endoscopy (aHR, 1.4; 95% CI, 1.1–1.8)) at 1 year of follow-up, persisting at 3 years.
Conclusions:
Concurrent SG/HHR was associated with increased risk of some subsequent operative and nonoperative interventions, a pattern that was not consistently observed for RYGB. Additional studies could examine whether changes to concurrent HHR technique could reduce risk.
Keywords: Hiatal hernia repair, Sleeve gastrectomy, Gastric bypass
Hiatal hernias are common among bariatric surgery candidates, affecting 20%–50% of individuals with severe obesity [1,2], and resulting gastroesophageal reflux disease (GERD) [3] may increase risk for reinterventions [4,5]. Most surgeons therefore electively repair hiatal hernias concurrently with bariatric surgery [6], although scientific study of this practice is limited mostly to case series of patients undergoing concurrent bariatric procedures with hiatal hernia repair (HHR) [7] with no control groups. Several recent larger studies used more rigorous methods; however, postoperative follow-up was limited in duration [8,9].
We used a nationwide health insurance claims data set to compare rates of postbariatric operative and endoscopic interventions among matched cohorts of patients who did and did not undergo contemporaneous HHR. Despite the potential longer-term benefits of HHR in reducing GERD-related complications, addition of a second complex surgical procedure alongside a bariatric surgery may increase risk for early complications, such as leaks, bleeding, and infection, and for later complications due to formation of adhesions or other subacute complications. We explored whether reintervention risk is higher or lower after concurrent HHR plus bariatric surgery and if it differs between sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB).
Methods
Study design and data source
We conducted a retrospective cohort study using Optum’s deidentified Clinformatics Data Mart Database, which includes 2010–2017 inpatient, outpatient, and pharmacy claims for approximately 33 million members of a large national health plan as well as enrollment and demographic information. The study was approved by the Harvard Pilgrim Institutional Review Board.
Study populations
We used Current Procedural Terminology (CPT) and International Classification of Diseases (ICD-9, ICD-10) procedure codes to identify members ages 18–64 years who underwent a primary laparoscopic SG or RYGB between January 1, 2010, and June 7, 2017 (detailed methods previously published) [10].
To identify patients who had concurrent HHR, we selected those with at least 1 inpatient or 2 outpatient hiatal hernia diagnoses (eTable 1) in the 6 months before or on the day of bariatric surgery who also had a CPT code (eTable 1) for HHR on the day of surgery. We excluded patients with preoperative diagnosis for hiatal hernia but no repair coded on the surgical date because it is possible that some of these individuals had a repair that was not billed to insurance [11,12]. Our comparison population comprised patients who had neither any hiatal hernia diagnoses nor an HHR code associated with their index surgeries.
We required patients to be enrolled at least 6 months preoperatively and 1 month postoperatively. After surgery, up to 3 years of follow-up data were examined. Because SG and RYGB confer different risks of operative reintervention [10], we separately compared patients who did and did not have contemporaneous HHR within each bariatric surgical category, that is, SGalone versus SGHHR and RYGBalone versus RYGBHHR.
Outcome measures
Our primary outcome was subsequent abdominal operation for presumed complication, based on a previously-curated list of procedures (eTable 2), including codes for lysis of adhesions, omental flaps, and more generic codes such as “CPT 22999 (Abdomen Surgery Procedure)” [10].
We also examined bariatric revision/conversion as a standalone outcome (eTable 2) because worsened GERD, particularly after SG, could lead to surgical revision or conversion to a different bariatric procedure [10]. Finally, we separately assessed postsurgery endoscopy (diagnostic and/or therapeutic) because patients with GERD may be more likely to require invasive workup (eTable 2).
Covariates
Demographic measures included age group, sex, and United States region. We classified patients as residing in predominantly white, black, or mixed race neighborhoods based on geocoding, with a superseding Hispanic or Asian categorization based on the E-tech System (Ethnic Technologies, Hackensack, NJ) [13,14]. We measured neighborhood poverty [15] using 2008–2012 American Community Survey [16] data, which defined patients as living in low-income neighborhoods when ≥10% of their census tract were below the poverty line. We characterized timing of procedure in 2-year blocks between 2010 and 2017.
We categorized body mass index (BMI) using the most recently coded presurgery diagnosis: 30–39.9, 40–49.9, 50–59.9, or ≥60 kg/m2; nonspecific obesity (when only a generic obesity code such as ICD-9 278.01 was available); or missing (<1%) [10].
We used Johns Hopkins Adjusted Clinical Groups System software to calculate a summary measure of 6-month baseline morbidity based on age, sex, and diagnoses in patients’ claims [17]. We classified patients with scores ≥3 as having higher morbidity and flagged diagnosis groups such as cardiovascular disease, mental illness, and hypertension. We classified baseline GERD as “no GERD” (no diagnoses), “possible GERD” (single outpatient diagnosis), or “likely GERD” (≥2 outpatient diagnoses or 1 inpatient diagnosis). Patients were separately characterized as filling medications for GERD (proton pump inhibitor or H2 blocker) or not. Finally, we categorized history of tobacco use based on codes in the 6 months before surgery (eTable 3).
Matching strategy
To address potential confounding by indication, we matched patients with HHR to controls based on factors likely associated with both hiatal hernia and reintervention. For each comparison (SGonly versus SGHHR and RYGBonly versus RYGBHHR), we balanced groups using coarsened exact matching (CEM) [18]. Unlike traditional approaches that match individuals based on specific characteristics, CEM creates population weights that balance groups within strata of selected variables, akin to stratified randomization in a trial. We balanced groups on variables with standardized differences ≥|.1| at baseline: preoperative GERD diagnosis and prescriptions, age group, sex, baseline BMI category, Adjusted Clinical Groups score, calendar year group of surgery, and U.S. region [19].
Analytic approach
For each analysis (SGonly versus SGHHR and RYGBonly versus RYGBHHR), we compared baseline characteristics before and after matching [19], with standardized difference <|.1|used to deem groups well balanced [19].
We plotted time to first operative reintervention, revision, or endoscopy using separate CEM-weighted Kaplan-Meier curves with 95% CIs. We used the survival proportions underlying these curves to estimate the prevalence of outcomes over follow-up, accounting for dropout. We also identified the most commonly coded procedures and their associated diagnoses for each cohort.
To compare cumulative risk between those with and without HHR, we specified Cox proportional hazards models for each outcome. We adjusted models for matching covariates plus any variables with a postmatch standardized difference exceeding |.1|. Patients without a qualifying event were censored at the time of incident gastrointestinal malignancy (to avoid counting surgeries for incident cancer), death, the end of our data set (June 30, 2017), or disenrollment (loss to follow-up). Patients in the SGalone and RYGBalone groups were also censored if they “crossed over” into the HHR exposure category based on an incident hiatal hernia diagnosis or HHR.
Sensitivity analyses
Because interventions such as bariatric revision or conversion are rare immediately after an index procedure, we performed additional analyses extending follow-up to 3 years. We considered these as exploratory because of the small sample sizes remaining in our cohorts, particularly for RYGB.
Results
Study population
Preoperative hiatal hernia diagnosis and contemporaneous HHR were common in both SG and RYGB populations (eFig. 1). Of 8627 SG patients, 3275 (38%) had a presurgery hiatal hernia diagnosis. Among 4496 RYGB patients, hiatal hernia was diagnosed preoperatively in 1206 (27%; χ2 P < .001 comparing hiatal hernia prevalence for SG versus RYGB). Similarly, contemporaneous HHR was more common among SG (1765 [20%]) than RYGB (591 [13%]) patients with a diagnosed hernia (χ2 P = .02).
Our final SG cohort included 1546 patients with contemporaneous HHR (SGHHR) and 3170 SGonly patients (eFig. 1). The matched groups were well balanced on sex (83.3% were women), age (mean, 43.3 [SD, 10.3] years), BMI category (19.7% <40 kg/m2, 75.8% ≥40 kg/m2, 4.5% with nonspecific or missing BMI), measured co-morbidity burden, and sociodemographic characteristics (Table 1). The matched population of RYGB patients included 457 patients with contemporaneous HHR (RYGBHHR), and 1156 RYGBonly patients (eFig. 1). Among RYGB patients, 87.5% were women, mean age was 44.2 (SD, 10.5) years, 15.8% had a BMI <40 kg/m2, 74.2% had a BMI ≥40 kg/m2, and 10.1% had nonspecific or missing BMI (Table 2). The percentage of patients, by exposure category, with 1 and 3 years of follow-up, were SGonly: 72.8% and 40.5%; SGHHR: 74.1% and 43.4%; RYGBonly: 73.9% and 37.0%; and RYGBHHR: 70.1% and 35.9% (eTable 4).
Table 1.
Baseline characteristics of SG patients with and without hiatal hernia repair
| Variables | Unmatched cohorts* | Matched cohorts* | ||||
|---|---|---|---|---|---|---|
| SG + hiatal hernia repair (n = 1765) | SGonly (n = 5352) | Standardized difference† | SG + hiatal hernia repair (n = 1546) | SGonly (n = 3170) | Standardized differenc† | |
| Age group, n (%) | .16 | .00 | ||||
| 18–29 yr | 570 (32.3) | 2077 (38.8) | 518 (33.5) | 1062 (33.5) | ||
| 30–39 yr | 602 (34.1) | 1764 (33.0) | 550 (35.6) | 1128 (35.6) | ||
| 40–49 yr | 466 (26.4) | 1191 (22.3) | 403 (26.1) | 826 (26.1) | ||
| 50–64 yr | 127 (7.2) | 320 (6.0) | 75 (4.9) | 154 (4.9) | ||
| Female sex, n (%) | 1415 (80.2) | 3949 (73.8) | .15 | 1288 (83.3) | 2641 (83.3) | .00 |
| Predominantly white neighborhood (≥75%), n (%)‡ | 815 (46.2) | 2622 (49.0) | .07 | 707 (45.7) | 1532 (48.3) | .08 |
| Neighborhood below poverty line, n(%)‡,¶ | .04 | .04 | ||||
| Less poor (<10%) | 798 (45.2) | 2321 (43.4) | 685 (44.3) | 1394 (44.0) | ||
| More poor (≥10%) | 816 (46.2) | 2557 (47.8) | 728 (47.1) | 1473 (46.5) | ||
| Missing | 151 (8.6) | 474 (8.9) | 133 (8.6) | 303 (9.6) | ||
| Region of United States, n (%) | .29 | .00 | ||||
| West | 290 (16.4) | 1053 (19.7) | 231 (14.9) | 474 (14.9) | ||
| South | 1037 (58.8) | 2555 (47.7) | 959 (62.0) | 1966 (62.0) | ||
| Midwest | 217 (12.3) | 1138 (21.3) | 199 (12.9) | 408 (12.9) | ||
| Northeast | 221 (12.5) | 595 (11.1) | 157 (10.2) | 322 (10.2) | ||
| Year of surgery, n (%) | .24 | .00 | ||||
| 2010–2011 | 156 (8.8) | 611 (11.4) | 113 (7.3) | 232 (7.3) | ||
| 2012–2013 | 504 (28.6) | 1240 (23.2) | 442 (28.6) | 906 (28.6) | ||
| 2014–2015 | 701 (39.7) | 1881 (35.1) | 628 (40.6) | 1288 (40.6) | ||
| 2016–2017 | 404 (22.9) | 1620 (30.3) | 363 (23.5) | 744 (23.5) | ||
| BMI category, n (%), kg/m2 | .30 | .00 | ||||
| 30–39.9 | 371 (21.0) | 855 (16.0) | 304 (19.7) | 623 (19.7) | ||
| 40–49.9 | 980 (55.5) | 2830 (52.9) | 924 (59.8) | 1895 (59.8) | ||
| 50–59.9 | 251 (14.2) | 912 (17.0) | 217 (14.0) | 445 (14.0) | ||
| ≥60 | 44 (2.5) | 297 (5.5) | 31 (2.0) | 64 (2.0) | ||
| Nonspecific obesity | 111 (6.3) | 431 (8.1) | 67 (4.3) | 137 (4.3) | ||
| Missing | 8 (.5) | 27 (.5) | 3 (.2) | 6 (.2) | ||
| GERD, n (%)‡ | .61 | .00 | ||||
| No diagnosis | 392 (22.2) | 2532 (47.3) | 350 (22.6) | 718 (22.6) | ||
| 1 inpatient diagnosis | 214 (12.1) | 817 (15.3) | 154 (10.0) | 316 (10.0) | ||
| 2+ outpatient diagnoses | 1159 (65.7) | 2003 (37.4) | 1042 (67.4) | 2137 (67.4) | ||
| Filled prescription for GERD medication, n (%)‡ | 686 (38.9) | 1652 (30.9) | .17 | 566 (36.6) | 1161 (36.6) | .00 |
| ACG score ≥3, n (%)‡,|| | 344 (19.5) | 783 (14.6) | .13 | 287 (18.6) | 521 (16.5) | .06 |
| Hypertension, n (%)‡ | 778 (44.1) | 2309 (43.1) | .02 | 646 (41.8) | 1351 (42.6) | − .02 |
| T2D, n (%)‡ | 535 (30.3) | 1680 (31.4) | −.02 | 445 (28.8) | 968 (30.5) | −.04 |
| Cardiovascular disease, n (%)‡ | 141 (8.0) | 335 (6.3) | .07 | 123 (8.0) | 187 (5.9) | .08 |
| Psychiatric illness, n (%)‡ | 708 (40.1) | 2231 (41.7) | − .03 | 635 (41.1) | 1348 (42.5) | − .03 |
| Tobacco/smoking history, n (%)‡ | 310 (17.6) | 932 (17.4) | .00 | 267 (17.3) | 588 (18.6) | − .03 |
| Surgery in inpatient setting, n (%) | 1397 (79.2) | 3739 (69.9) | .21 | 1225 (79.2) | 2335 (73.7) | .13 |
BMI = body mass index; GERD = gastroesophageal reflux disease; ACG = Adjusted Clinical Groups; T2D = type 2 diabetes.
We conducted coarsened exact matching on preoperative GERD diagnosis, GERD medication use, age group, sex, baseline BMI category, ACG score, calendar year group of surgery, and United States region. Matched cohorts reflect weighted samples post matching.
Standardized differences are the difference in means between the intervention and control groups divided by the SD of the difference in means. Lower absolute values indicate greater similarity between groups, and values <.2 indicate minimal differences between groups.
For complete descriptions of how we constructed baseline variables, please refer to the Methods section.
Neighborhoods with less poverty were those where <10% of households were below the poverty line; more poverty were those where ≥ 10% of households were below the poverty line.
Johns Hopkins Adjusted Clinical Groups System.
Table 2.
Baseline characteristics of RYGB patients with and without hiatal hernia repair
| Variables | Unmatched cohorts* | Matched cohorts* | ||||
|---|---|---|---|---|---|---|
| RYGB + hiatal hernia repair (n = 591) | RYGBonly (n = 3290) | Standardized differenc† | RYGB + hiatal hernia repair (n = 457) | RYGBonly (n = 1156) | Standardized differenc† | |
| Age group, n (%) | .16 | .00 | ||||
| 18–29 yr | 164 (27.7) | 1132(34.4) | 136 (29.8) | 344 (29.8) | ||
| 30–39 yr | 189 (32.0) | 1097 (33.3) | 158 (34.6) | 400 (34.6) | ||
| 40–49 yr | 178 (30.1) | 829 (25.2) | 133 (29.1) | 336 (29.1) | ||
| 50–64 yr | 60 (10.2) | 232 (7.1) | 30 (6.6) | 76 (6.6) | ||
| Female sex, n (%) | 478 (80.9) | 2438 (74.1) | .16 | 400 (87.5) | 1012 (87.5) | .00 |
| Predominantly white neighborhood (≥75%), n (%)‡ | 288 (48.7) | 1688 (51.3) | .11 | 215 (47.0) | 603 (52.1) | .13 |
| Neighborhood below poverty line, n (%)‡,¶ | .05 | .12 | ||||
| Less poor (<10%) | 246 (41.6) | 1426 (43.3) | 178 (38.9) | 507 (43.9) | ||
| More poor (≥10%) | 301 (50.9) | 1627 (49.5) | 243 (53.2) | 580 (50.2) | ||
| Missing | 44 (7.4) | 237 (7.2) | 36 (7.9) | 69 (5.9) | ||
| Region of United States, n (%) | .21 | .00 | ||||
| West | 139 (23.5) | 859 (26.1) | 105 (23.0) | 266 (23.0) | ||
| South | 292 (49.4) | 1277 (38.8) | 253 (55.4) | 640 (55.4) | ||
| Midwest | 111 (18.8) | 814 (24.7) | 80(17.5) | 202 (17.5) | ||
| Northeast | 49 (8.3) | 324 (9.8) | 19 (4.2) | 48 (4.2) | ||
| Year of surgery, n (%) | .19 | .00 | ||||
| 2010–2011 | 169 (28.6) | 1150 (35.0) | 139 (30.4) | 352 (30.4) | ||
| 2012–2013 | 188 (31.8) | 832 (25.3) | 144 (31.5) | 364 (31.5) | ||
| 2014–2015 | 147 (24.9) | 741 (22.5) | 110 (24.1) | 278 (24.1) | ||
| 2016–2017 | 87 (14.7) | 567 (17.2) | 64 (14.0) | 162 (14.0) | ||
| BMI category, n (%), kg/m2 | .34 | .00 | ||||
| 30–39.9 | 117 (19.8) | 405 (12.3) | 72 (15.8) | 182 (15.8) | ||
| 40–49.9 | 298 (50.4) | 1462 (44.4) | 259 (56.7) | 655 (56.7) | ||
| 50–59.9 | 97 (16.4) | 691 (21.0) | 75 (16.4) | 190 (16.4) | ||
| ≥60 | 13 (2.2) | 165 (5.0) | 5(1.1) | 13(1.1) | ||
| Nonspecific obesity | 64 (10.8) | 529 (16.1) | 46(10.1) | 116 (10.1) | ||
| Missing | 2 (.3) | 38 (1.2) | ||||
| GERD, n (%)‡ | .60 | .00 | ||||
| No diagnosis | 121 (20.5) | 1511 (45.9) | 102 (22.3) | 258 (22.3) | ||
| 1 inpatient diagnosis | 68 (11.5) | 383 (11.6) | 32 (7.0) | 81 (7.0) | ||
| 2+ outpatient diagnoses | 402 (68.0) | 1396 (42.4) | 323 (70.7) | 817 (70.7) | ||
| Filled prescription for GERD | 280 (47.4) | 1092 (33.2) | .29 | 200 (43.8) | 506 (43.8) | .00 |
| medication, n (%)‡ | ||||||
| ACG score ≥3, n (%)‡,|| | 119 (20.1) | 563 (17.1) | .08 | 84(18.4) | 229 (19.8) | −.04 |
| Hypertension, n (%)‡ | 300 (50.8) | 1538 (46.7) | .08 | 225 (49.2) | 541 (46.8) | .05 |
| T2D, n (%)‡ | 234 (39.6) | 1418 (43.1) | −.07 | 167 (36.5) | 492 (42.6) | −.12 |
| Cardiovascular disease, n (%)‡ | 45 (7.6) | 237 (7.2) | .02 | 29 (6.3) | 72 (6.2) | .01 |
| Psychiatric illness, n (%)‡ | 208 (35.2) | 1295 (39.4) | − .09 | 156 (34.1) | 494 (42.7) | −.18 |
| Tobacco/smoking history, n (%)‡ | 97 (16.4) | 552 (16.8) | − .01 | 75 (16.4) | 186 (16.1) | .01 |
| Surgery in inpatient setting, n (%) | 561 (94.9) | 2735 (83.1) | .38 | 434 (95.0) | 1005 (87.0) | .28 |
RYGB = Roux-en-Y gastric bypass; BMI = body mass index; GERD = gastroesophageal reflux disease; ACG = Adjusted Clinical Groups; T2D = type 2 diabetes.
We conducted coarsened exact matching on preoperative GERD diagnosis, GERD medication use, age group, sex, baseline BMI category, neighborhood race/ethnicity, calendar year group of surgery, and United States region.
Standardized differences are the difference in means between the intervention and control groups divided by the SD of the difference in means. Lower absolute values indicate greater similarity between groups, and values <.2 indicate minimal differences between groups.
For complete descriptions of how we constructed baseline variables, please refer to the Methods section.
Neighborhoods with less poverty were those where <10% of households were below the poverty line; more poverty were those where ≥ 10% of households were below the poverty line.
Johns Hopkins Adjusted Clinical Groups System.
Primary outcome: operative intervention for presumed complications
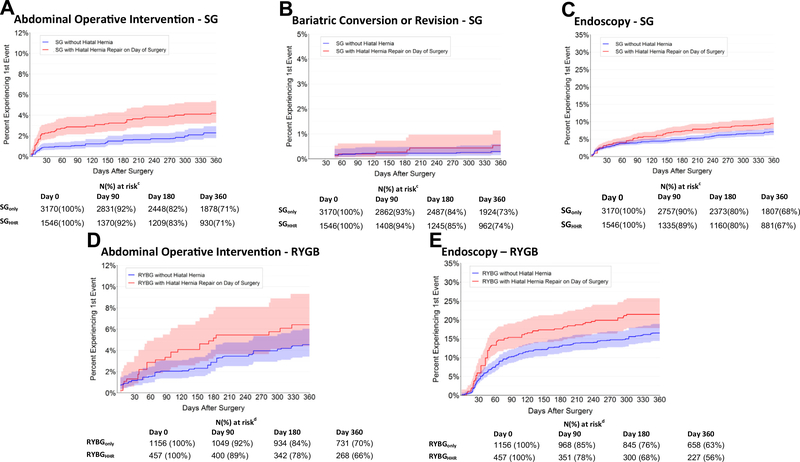
The estimated prevalence of additional abdominal operations by 1 year was 4.2% (95% CI, 3.3%–5.4%) among SGHHR patients and 2.3% (95% CI, 1.8%–2.9%) among SGonly patients, with adjusted hazard ratio (aHR) of 2.1 (95% CI, 1.5–3.1; P < .001) (Table 3; Fig. 1). The excess risk of abdominal operations for SGHHR patients appeared early after the index surgery. By 30 days, the estimated prevalence of operative reintervention in SGHHR patients was 2.3% (95% CI, 1.6–3.1%) versus .9% (.6–1.3%) for SGonly (Table 3; Fig. 1). Omental flaps (probably indicative of leaks) represented 46% of these early operations for SGHHR, while among SGonly patients, the most common procedure by postoperative day 30 was lysis of adhesions (24%) (eTable 5; eTable 6). By the end of postoperative year 1, the top 3 abdominal operations among SGHHR patients were omental flap (27%), lysis of adhesions (17%), and nonspecific laparoscopic procedure (12%). Among SGonly patients, they were lysis of adhesions (36%), nonspecific laparoscopic procedure (21%), and omental flap (7%).
Table 3.
Results from 1-yr Cox proportional hazards models* comparing matched cohorts of SG and RYGB patients with and without hiatal hernia repair
| 1-Year adjusted hazard ratio (95% CI) P value | Cumulative incidence of outcome (95% CI) for SG and RYGB in the early (90 d) and later (1 yr) postoperative period† |
||||
|---|---|---|---|---|---|
| 90 Days after index procedure | 1 Year after index procedure | ||||
| Sleeve gastrectomy | SGonly (n = 2864; 93% of cohort remains enrolled), % | SGHHR(n = 1411; 94% of cohort remains enrolled), %‡ | SGonly (n 5 1926; 73% of cohort remains enrolled), % | SGHHR (n 5 968; 74% of cohort remains enrolled), %‡ | |
| Abdominal operative intervention§ | 2.14 (1.49–3.05) <.001 | 1.10 (.8–1.5) | 2.90 (2.1–3.8) | 2.30(1.8–2.9) | 4.20 (3.3–5.4) |
| Bariatric revision/conversion¶ | 1.66 (.60–4.56).326 | .20 (.1,−.5) | .20 (.1,−.6) | .30 (.1,−.6) | .50 (.3–1.1) |
| Endoscopy|| | 1.45 (1.16–1.81) .001 | 3.90 (3.3–4.7) | 5.30 (4.4–6.7) | 7.10(6.2–8.1) | 9.50 (8.1–11.3) |
| Roux-en-Y gastric bypass | |||||
| RYGBonly (n = 1070; 94% of cohort remains enrolled), % | RYGBHHR (n 5 412; 92% of cohort remains enrolled), %‡ | RYGBonly (n 5 767; 74% of cohort remains enrolled), % | RYGBHHR (n 5 286; 70% of cohort remains enrolled), %‡ | ||
| Abdominal operative intervention§ | 1.49 (.91–2.46).108 | 2.00 (1.4–3.0) | 3.30 (2.0–5.5) | 4.50 (3.4–6.0) | 6.40 (4.4–9.3) |
| Endoscopy|| | 1.40 (1.09–1.82) .010 | 10.10(8.5–12.1) | 15.30 (12.3–19.1) | 16.50 (14.4–18.9) | 21.40 (17.8–25.7) |
SG = sleeve gastrectomy; RYGB = Roux-en-Y gastric bypass; HHR = hiatal hernia repair; TD2 = type 2 diabetes; VSG = vertical sleeve gastrectomy.
Models were adjusted for all matched covariates, plus in SG models a covariate indicating whether the index procedure was performed in an inpatient setting, and for RYGB models covariates indicating T2D status, neighborhood education and poverty levels, and setting of surgery (inpatient). The selection of additional variables for adjustment was based on which covariates had a standardized difference ≥.1 after performing coarsened exact matching.
From adjusted Kaplan-Meier plots at days 90, 180, and 360 relative to index procedure.
SG 1 HHR and RYGB 1 HHR indicates patients who had codes for contemporaneous hiatal hernia repair on the date of their index sleeve gastrectomy or Roux-en-Y gastric bypass procedures, respectively.
Abdominal operative intervention: outcome: category includes operations such as lysis of adhesions, abscess drainage, omental flaps, and more generic codes, such as exploratory laparotomy, or nonspecific abdominal operation codes. Complete code list can be found in eTable 1 in Appendix 1.
Bariatric conversion/revision: represents subsequent bariatric procedure codes (e.g., RYGB after index VSG) or repeat gastrectomy codes for initial SG patients.
Endoscopy category includes any endoscopic procedure for diagnosis or treatment on the upper gastrointestinal tract. Note that models were not built for revision/conversion outcome among RYGB patients because there were too few qualifying events at the 1-yr follow-up mark.
Fig. 1.
Kaplan-Meier plots of reinterventions up to 1 year after SG or RYGB by hiatal hernia repair. SG = sleeve gastrectomy; RYGB = Roux-en-Y gastric bypass.
Among RYGB patients, there was no detectable difference in risk of additional abdominal operations at 1 year according to HHR status (aHR, 1.5; 95% CI, .9–2.5; P = .11) (Table 3; Fig. 1). By 30 days, the estimated prevalence of operative reintervention in RYGBHHR patients was 1.3% (95% CI, .6–2.9%) versus 1.2% (95% CI, .7–2.0%) for RYGBonly (Table 3; Fig. 1). Operative reintervention codes differed among RYGB compared with SG (eTable 5, eTable 6), with nonspecific laparoscopy (33%) and gastropexy (21%) the most common early (0–30 days) procedures among RYGB patients. By the end of year 1, nonspecific laparoscopy (27% of RYGBHHR operations and 18% of RYGBonly operations) and lysis of adhesions (15% of RYGBHHR operations and 22% of RYGBonly operations) remained the 2 most common procedure subcategories.
Bariatric conversion or revisional procedures
Bariatric conversion or revision events were rare, with a 1-year estimated prevalence of .5% (95% CI, .3–1.1%) among SGHHR and .3% (95% CI, .1%–.6%) among SGonly (Fig. 1) and no detected difference by HHR status (Cox aHR, 1.7; 95% CI, .6–4.6; P = .33) (Table 3). We did not conduct these analyses among RYGB patients because there were only 2 qualifying events in the first postoperative year.
Endoscopy
SGHHR patients had an increased risk of endoscopy compared with SGonly patients (aHR, 1.5; 95% CI, 1.2–1.8; P < .001) (Table 3; Fig. 1) at 1 year, with an estimated prevalence of 9.5 (95% CI, 8.1–11.3%) versus 7.1% (95% CI, 6.2–8.1%), respectively. Risk of endoscopy by 1 year was similarly higher among RYGBHHR patients compared with RYGBonly patients (aHR, 1.4; 95% CI, 1.1–1.8; P = .01) (Table 3; Fig. 1), with an estimated 21.4% (95% CI, 17.8–25.7%) of RYGBHHR patients undergoing endoscopy by 1 year after surgery versus 16.5% (95% CI, 14.4–18.9%) of RYGBonly patients.
Sensitivity analyses
A total of 42% of SG patients were enrolled at 3 years, at which point risk of abdominal operative intervention among SGHHR patients remained elevated compared with SGonly patients (aHR, 1.9; 95% CI, 1.4–2.6; P < .001) (eTable 7; eFig. 2). The magnitude and direction of the estimates for risk of endoscopy (aHR, 1.3; 95% CI, 1.1–1.6; P = .004) and revision/conversion (aHR, 1.3; 95% CI, .5–3.1; P = .61) among SGHHR versus SGonly patients at 3 years mirrored our 1-year results.
A total of 36% of RYGB patients remained enrolled at 3 years, at which point there was no increased risk of subsequent abdominal operation among RYGBHHR patients compared with RYGBonly (aHR, 1.0; 95% CI, .7–1.6; P = .88) (eTable 7; eFig. 3). However, RYGB patients with HHR remained more likely to receive endoscopic interventions (3-year aHR, 1.3; 95% CI, 1.0–1.6; P = .046). There were still insufficient revision/conversion events among the RYGB cohort (n = 5 total events) at 3 years to permit modeling.
Discussion
In this cohort study, we observed a higher 1- and 3-year risk of additional abdominal operations among patients undergoing combined SGHHR compared with matched SGonly patients. These differences were driven by early postoperative risk of additional operation among SGHHR patients. We did not detect a difference in reoperation risk between RYGB patients with and without HHR. Bariatric revisions and conversions were rare, and risk of this outcome did not differ by HHR status. For both SG and RYGB, contemporaneous HHR was associated with increased risk of subsequent endoscopy.
Reoperation after bariatric surgery is an important source of morbidity [20,21] and cost [22,23] that surgeons, health systems, and patients seek to avoid. Prior research has shown that SG is associated with a lower risk of reoperation compared with RYGB, with a relative difference of approximately 30% by 3–5 years [10,24–26]. However, our data suggest that patients who undergo SG along with a higher complexity procedure like HHR may have increased risk of reintervention, particularly during the early postoperative period when reoperation is typically prompted by leaks, bleeding, and infection [20,27,28]. Omental flaps (a plurality of procedure codes in the early postoperative period for SGHHR) may be indicative of leaks and may have been more common for SGHHR because of the dissection of the hiatus resulting in an injury to the stomach or the esophagus or a devascularization injury. Although risk of subsequent operative intervention did not differ for RYGB patients by concurrent HHR status, this does not suggest that hiatal hernia patients should be triaged to RYGB because the overall risk of operative reintervention remains higher for RYGB, as noted above.
Among both SG and RYGB patients, we observed a higher risk of endoscopy among those with concurrent HHR, generally emerging after the 90-day postsurgery mark (Fig. 1). Although higher rates of endoscopy could signify GERD symptoms in the HHR groups, our finding that bariatric conversions/revisions were very rare (and did not differ by HHR status) out to 3 years after surgery is reassuring, particularly among SG patients, that GERD symptoms (if present) were not leading to numerous conversions.
We are aware of 3 recent short-term studies comparing bariatric outcomes according to concurrent HHR status. A 2018 analysis found no difference in 30-day adverse event rates in bariatric surgery patients with and without concurrent paraesophageal hernia repair (PEH) status. [29]. Similarly, a 2019 Metabolic and Bariatric Surgery Association Quality Improvement Program (MBSAQIP) registry study found no increased risk of 30-day adverse events associated with concurrent PEH repair (1.1% event rate without PEH and 1.2% with PEH) [8]. These articles are difficult to compare with our study because they included conditions such as pneumonia, venous thromboembolism, and cardiovascular events in addition to reoperation and complications such as bleeding and anastomotic leaks. Shada et al. separately examined operative reintervention (finding no difference in 30-day rates [1.5%] regardless of PEH status); however, this analysis grouped RYGB and SG patients together, unlike in our study [29]. Another study in the MBSAQIP registry observed higher rates of 30-day reoperation (1.1% versus .8%), readmission, and overall morbidity among SG patients with HHR compared with propensity-matched SGonly patients [9]. We observed higher 30-day reoperation rates among SGHHR patients (2.3%) than either MBSAQIP-based article, possibly because insurance claims data may more completely capture adverse events outside the hospital system of the index procedure. Based on our review of the literature, ours is the first study to assess differences in risk up to 3 years by HHR status separately for SG versus RYGB patients.
Limitations of our study include the observational, nonrandomized design, which precludes causal inference. Because we used claims data, there is potential for unmeasured confounding by provider and patient characteristics. SG patients were more likely to have diagnoses for hiatal hernias and to undergo contemporaneous repair compared with RYGB patients. This should not have biased our results because we did not directly compare the SG and RYGB groups. However, providers who only offer SG may more aggressively screen for hernias and/or perform HHR because SG is associated with greater GERD risk. Our SG analyses could be biased from the null if such practice styles correlate with a greater likelihood of subsequent operations and interventions.
Although our cohorts were well matched on measured characteristics, we lacked data about hernia severity that may have both promoted a decision to repair a hiatal hernia and a decreased threshold for reintervening after the procedure. The nature of our data set also limited our ability to assess clinical improvements after contemporaneous hernia repair or differences by surgical approach. Finally, the cohorts had attrition over the follow-up period, as is typical with insurance claims-based studies.
Because insurance claims cannot provide clinical insight into improvement or worsening in GERD symptoms, we do not know whether leaving hiatal hernias unrepaired at the time of bariatric surgery would improve outcomes. Even if pairing SG with HHR leads to higher early complication rates, the absolute risk difference is relatively small; therefore, this approach may still be preferable to nonrepair or a multistage operation. Although we identified a group of hiatal hernia patients who had bariatric surgery without apparent HHR, our study does not allow assessment of this management approach because we excluded these patients from analyses. We felt that such “nonrepaired” patients likely differed from those undergoing HHR in prognostically significant ways (e.g., potentially had smaller hiatal hernia, had no symptoms, or were misdiagnosed preoperatively). Additionally, coding for HHR with bariatric surgery appears to have declined over time, leading to risk of misclassification for these “nonrepaired” patients. Among patients with presurgery hiatal hernia diagnoses, 20% did not have concurrent HHR coded in the early years of our data compared with 43% by 2016–2017. Some insurers have recently stopped reimbursing for contemporaneous HHR with bariatric surgery [11,12]; therefore, it is possible that some patients in this excluded “nonrepaired” hernia group actually did undergo HHR that was not billed to insurance, particularly in the latter years under study.
Conclusions
In this cohort study of bariatric procedures with concurrent HHR, we observed a higher risk of additional abdominal operations for SG patients and a higher risk of endoscopy for both SG and RYGB. Our findings support a need for additional studies with clinical data sets to better understand the risks and benefits associated with HHR, how they may differ between SG and RYGB, and how differences in technical approach might decrease complication risk.
Supplementary Material
Acknowledgments
Funding Information: The Bariatric CHOICE (Comparative Health Outcomes Using Insurance Claims to Study Effectiveness) project was supported through a grant from NIH/NIDDK (R01 DK112750; Lewis PI).
Role of Funder
The Bariatric CHOICE (Comparative Health Outcomes Using Insurance Claims to Study Effectiveness) project was supported through a grant from NIH/NIDDK (R01DK112750; Lewis PI) Additionally, Drs. Wharam and Ross-Degnan were supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award No. P30DK092924.
Disclosures
Dr. Arterburn reports grants from NIH during the conduct of the study and grants from PCORI, nonfinancial support from IFSO Latin American Chapter, and nonfinancial support from World Congress for Interventional Therapy for Diabetes outside of the submitted work. Dr. Dimick reports an equity ownership in ArborMetrix outside of the submitted work. Dr. Zhang, Dr. Ross-Degnan, Dr. Wharam, Dr. Lewis, Ms. Callaway, Ms. Argetsinger, and Ms. Wallace report grants from NIDDK during the conduct of the study.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.1016/j.soard.2020.08.035.
References
- [1].Bakhos CT, Patel SP, Petrov RV, Abbas AES. Management of paraesophageal hernia in the morbidly obese patient. Thorac Surg Clin 2019;29(4):379–86. [DOI] [PubMed] [Google Scholar]
- [2].Che F, Nguyen B, Cohen A, Nguyen NT. Prevalence of hiatal hernia in the morbidly obese. Surg Obes Relat Dis 2013;9(6):920–4. [DOI] [PubMed] [Google Scholar]
- [3].Callaway JP, Vaezi MF. Hiatal and paraesophageal hernias. Clin Gastroenterol Hepatol 2018;16(6):810–3. [DOI] [PubMed] [Google Scholar]
- [4].DuPree CE, Blair K, Steele SR, Martin MJ. Laparoscopic sleeve gastrectomy in patients with preexisting gastroesophageal reflux disease: a national analysis. JAMA Surg 2014;149(4):328–34. [DOI] [PubMed] [Google Scholar]
- [5].Obeid T, Krishnan A, Abdalla G, Schweitzer M, Magnuson T, Steele KE. GERD is associated with higher long-term reoperation rates after bariatric surgery. J Gastrointest Surg 2016;20(1):119–24:discussion 124. [DOI] [PubMed] [Google Scholar]
- [6].Gagner M, Deitel M, Erickson AL, Crosby RD. Survey on laparoscopic sleeve gastrectomy (LSG) at the Fourth International Consensus Summit on Sleeve Gastrectomy. Obes Surg 2013;23(12):2013–7. [DOI] [PubMed] [Google Scholar]
- [7].Mahawar KK, Carr WR, Jennings N, Balupuri S, Small PK. Simultaneous sleeve gastrectomy and hiatus hernia repair: a systematic review. Obes Surg 2015;25(1):159–66. [DOI] [PubMed] [Google Scholar]
- [8].Hefler J, Dang J, Mocanu V, Switzer N, Birch DW, Karmali S. Concurrent bariatric surgery and paraesophageal hernia repair: an analysis of the Metabolic and Bariatric Surgery Association Quality Improvement Program (MBSAQIP) database. Surg Obes Relat Dis 2019;15(10):1746–54. [DOI] [PubMed] [Google Scholar]
- [9].Janik MR, Ibikunle C, Aryaie AH. Safety of concurrent sleeve gastrectomy and hiatal hernia repair: a propensity score-matched analysis of the MBSAQIP registry. Surg Obes Relat Dis 2020;16(3):365–71. [DOI] [PubMed] [Google Scholar]
- [10].Lewis KH, Arterburn DE, Callaway K, et al. Risk of operative and nonoperative interventions up to 4 years after Roux-en-Y gastric bypass vs vertical sleeve gastrectomy in a nationwide US commercial insurance claims database. JAMA Netw Open 2019;2(12):e1917603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Horizon Blue Cross Blue Shield of New Jersey [homepage on the Internet]. Bariatric surgery billed with hiatal hernia repair. c2015. [cited 2019 Oct 28]. Available from: https://www.horizonblue.com/providers/policies-procedures/policies/reimbursementpolicies-guidelines/bariatric-surgery-billed.
- [12].Premera Blue Cross [homepage on the Internet]. Medical policy – 7.01.516 bariatric surgery. c2020. [cited 2019 Oct 28]. Available from: https://www.premera.com/medicalpolicies/7.01.516.pdf.
- [13].Ethnic Technologies [homepage on the Internet]. Ethnic technologies e-tech. c2016. [cited 2018 Oct 24]. Available from: http://www.ethnictechnologies.com/product/e-tech/.
- [14].Fiscella K, Fremont AM. Use of geocoding and surname analysis to estimate race and ethnicity. Health Serv Res 2006;41(4 Pt 1):1482–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures–the public health disparities geocoding project. Am J Public Health 2003;93(10):1655–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].United States Census Bureau [homepage on the Internet]. American community survey (ACS). c2019. [cited 2019 Dec 6]. Available from : https://www.census.gov/programs-surveys/acs.
- [17].ACG [homepage on the Internet]. The Johns Hopkins ACG system. [cited 2018 Jun 2]. Available from: https://www.hopkinsacg.org/advantage/.
- [18].Iacus SM, King G, Porro G. Causal inference without balance checking: coarsened exact matching. Polit Anal 2012;20(1):1–24. [Google Scholar]
- [19].Yang DDJ. A unified approach to measuring the effect size between two groups using SAS. [serial on the Internet]. SAS Global Forum 2012. [cited 2017 May 12]. Available from: https://www.lerner.ccf.org/qhs/software/lib/stddiff.pdf.
- [20].Melissas J, Stavroulakis K, Tzikoulis V, et al. Sleeve gastrectomy vs Roux-en-Y gastric bypass. Data from IFSO-European Chapter Center of Excellence Program. Obes Surg 2017;27(4):847–55. [DOI] [PubMed] [Google Scholar]
- [21].Hjorth S, Naslund I, Andersson-Assarsson JC, et al. Reoperations after bariatric surgery in 26 years of follow-up of the Swedish Obese Subjects study. JAMA Surg 2019;154(4):319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rios-Diaz AJ, Metcalfe D, Devin CL, Berger A, Palazzo F. Six-month readmissions after bariatric surgery: results of a nationwide analysis. Surgery 2019;166(5):926–33. [DOI] [PubMed] [Google Scholar]
- [23].Shah N, Greenberg JA, Leverson G, Funk LM. Predictors of high cost after bariatric surgery: a single institution review. Surgery 2016;160(4):877–84. 10.1016/j.surg.2016.06.038. [DOI] [PubMed] [Google Scholar]
- [24].Zak Y, Petrusa E, Gee DW. Laparoscopic Roux-en-Y gastric bypass patients have an increased lifetime risk of repeat operations when compared to laparoscopic sleeve gastrectomy patients. Surg Endosc 2016;30(5):1833–8. [DOI] [PubMed] [Google Scholar]
- [25].Hutter MM, Schirmer BD, Jones DB, et al. First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg 2011;254(3):410–20:discussion 420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Courcoulas A, Coley RY, Clark JM, et al. Interventions and operations 5 years after bariatric surgery in a cohort from the US National Patient-Centered Clinical Research Network Bariatric study. JAMA Surg 2020;155(3):194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Young MT, Gebhart A, Phelan MJ, Nguyen NT. Use and outcomes of laparoscopic sleeve gastrectomy vs laparoscopic gastric bypass: analysis of the American College of Surgeons NSQIP. J Am Coll Surg 2015;220(5):880–5. [DOI] [PubMed] [Google Scholar]
- [28].Helmio M, Victorzon M, Ovaska J, et al. SLEEVEPASS: a randomized prospective multicenter study comparing laparoscopic sleeve gastrectomy and gastric bypass in the treatment of morbid obesity: preliminary results. Surg Endosc 2012;26(9):2521–6. [DOI] [PubMed] [Google Scholar]
- [29].Shada AL, Stem M, Funk LM, Greenberg JA, Lidor AO. Concurrent bariatric surgery and paraesophageal hernia repair: comparison of sleeve gastrectomy and Roux-en-Y gastric bypass. Surg Obes Relat Dis 2018;14(1):8–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.