Abstract
Background:
In patients with T2DM, the therapeutic effects of conservative treatment are quite limited, and there is a need for additional therapeutic procedures to achieve the desired satisfactory and solid effect. Low-level laser therapy (LLLT) has an anti-inflammatory effect, and is used to heal lesions. This mechanism is realized through inhibition of lipopolysaccharides (LPS), so it can be used in the treatment of periodontal disease in patients with diabetes.
Objective:
The aim of this study is to assess the effect of level laser therapy (LLLT) on serum IL-6 values in patients with periodontitis and T2DM.
Methods:
Patients at age between 35-60 years old, with chronic periodontitis (CH) where the clinical loss of attachment (CAL) was ≥4 mm therefore covering at least 50 % of affected teeth. In this study we included 80 patients, divided into two groups: 40 patients with type 2 diabetes mellitus (2TDM) treated with conservative periodontal treatment supplemented with laser therapy (LLLT), group A, and 40 patients with 2TDM, conservatively treated without LLLT. therapy i.e. group B. The laser light was applied to the gingiva in separate quadrants in 5 sessions for the next five days in a row. Blood samples were taken from all subjects at the first treatment, then in 6 weeks and 3 months after treatment, and interleukin 6 (IL-6) levels were measured. The blood samples in the test tubes remained for about 30 minutes and were then distributed in a biochemical laboratory, where they were centrifuged at 6,000 rpm for 10 minutes. The serum was separated from the test tube and transferred to the eppendorph. All serum samples were stored at -80 ° C until complete analysis and determination of IL-6, according to the standardized methodology.
Results:
In group A, on the first examination serum IL-6 levels varies in the interval 11.54 ± 1.11 pg / mL, after 6 weeks of therapy the values range between 11.26 ± 0.77 pg / mL, and after 3 months of therapy levels oscillate at intervals of 11.02 ± 0.67 pg / mL. In group B the findings are similar. At the first examination, the serum IL-6 values were 11.56 ± 0.81 pg / mL, after 6 weeks of therapy ranged from 11.59 ± 0.71 pg / mL, and after 3 months of therapy levels were recorded at intervals. 11.41 ± 0.78 pg /mL. The serum IL-6 value after 6 weeks of therapy in patients in group B for Z = -2.04 and p <0.05 (p = 0.04) was significantly higher than in patients in group A, while after 3 months of therapy in patients in group B for Z = -2.42 and p <0.05 (p = 0.02) is significantly higher than the value in patients in group A.
Conclusion:
LLLT resulted in significantly reduced serum IL-6 values in patients with periodontitis and T2DM after 6 weeks and 3 months of therapy in which conservative treatment was supplemented with LLLT.
Keywords: low power laser radiation (LLLT), interleukin 6 (IL-6), serum, periodontitis, type 2 diabetes mellitus (T2DM)
1. BACKGROUND
According to the data from the Center for control and prevention of diabetes, which treats people with diabetes in the United States, diabetes affects about 25.6 million people, or 11.3% of the country’s population (1). The disease gradually and quietly but systematically attacks the organs and tissues, causing micro and macro circulatory disorders, which affect the health status of the individuals. These changes also occur locally, soft and hard structures in the oral cavity (2-3). It has been clinically proven that type 2 diabetes mellitus (T2DM) is a risk factor for periodontitis, sometimes defined as a moderate association (4), sometimes as an increased risk of initiation and progression of periodontal disease (5) or very often the case is presence of both diseases in the same patient (6-7). In conditions of chronic disease and systemic disorder, the periodontium becomes a barrier which is easy to overcome, which allows the penetration of certain harmful pathogens first into the gingival tissue and then into the remaining periodontal structures. This condition results in a host response, activation of enzymes, and release of pro-inflammatory cytokines, including IL-6. IL-6 is responsible for regulating the immune and inflammatory tissue response and participates in the acute phase of the inflammatory response where it acts together with TNF-α (8). Through regulatory mechanisms, the production of TNF-α (9) is impaired, hence its effect on diabetes is indisputable. It has been shown to have a potential synergistic effect on fibroblasts, and is a stimulator of alveolar bone destruction by stimulating osteoclasts (10). In patients with T2DM, the therapeutic effects of conservative treatment are quite limited, and there is a need for additional therapeutic procedures to achieve the desired satisfactory and solid effect. Low-level laser therapy (LLLT) has very wide use in medicine, and is used to heal lesions. This mechanism is realized through inhibition of lipopolysaccharides (LPS), so it can be used in the treatment of periodontal disease in patients with diabetes (11).
The role and effect of lasers on fibroblasts and osteoblasts have been investigated and proven in hyperglycemic conditions (12-13). At the systemic level, the impact of LLLT on the secretion of pro-inflammatory mediators TNF-α and IL-6 from endothelial cell cultures has been investigated. According to some research, the link between these components is due to the structural placement of endothelial cells on the walls of blood vessels and the initial contact with blood rich in glucose (14).
LLLT as an addition to conservative treatment minimized the effects of 5-FU on the periodontium (15-16), the diode laser provided significant improvements in clinical parameters, confirming that lasers have a positive effect along with non-surgical periodontal therapy (17). The researchers suggest that LLLT reduces gingivitis and contributes to better therapeutic results when LLLT is used in conjunction with basic periodontal therapy, opposing to the classical conservative treatment only (18).
2. OBJECTIVE
The aim of this study was to evaluate the effect of LLLT on serum IL-6 values in patients with periodontitis and T2DM.
3. MATERIAL AND METHODS
Study design
Patients in this study were selected by the Department of Periodontology and Oral Diseases at the University of Kosovo, University Dental Clinical Center in Pristina, aged 35-60, with chronic periodontitis (CH) where the clinical attachment loss (CAL) was ≥ 4 mm covering at least 50% of affected teeth. The research was approved by the Ethics Commission of the Faculty of Dentistry in Skopje(01/434/17).
The selected patients in this study were informed about the motive and course of the study. Only volunteers who agreed to be part of this research took part in the study, and written consent was submitted, signed by hand. The study included survey of 80 patients, who were divided into two groups:
patients with type 2 Diabetes mellitus where conservative periodontal treatment was supplemented with laser therapy in 40 patients (group A);
patients with type 2 Diabetes mellitus where conservative periodontal treatment has been performed without applied laser therapy, which also counted 40 patients (group B).
All patients in both groups regulated hyper-glycaemia with oral antidiabetic drugs (Glucophage XR tablets of 750 mgr. 2x daily, manufacturer Merck Sante, France).
Certain criteria were used in selection of the patients, respectively proposed by the World Health Organization as criteria for inclusion and exclusion in the study.
Criteria for exclusion from the study are: a) use of antibiotics in the previous 4 months; b) pregnancy; c) patients - smokers; d) malignant diseases; e) use of immunosuppressive drugs; f) medications that may affect periodontal status; and g) Fentoin, cyclosporine, calcium channel blockers, etc.
Criteria for inclusion in the study are: a) diagnosed with diabetes mellitus type 2; b) regulation of diabetes with oral antidiabetics; and c) diagnosed periodontitis with depth of periodontal pockets ≥ 4 mm in at least 50% of affected teeth.
After determined diagnosis in all patients who were part of the study, conservative treatment (removal of hard and soft deposits) of periodontal pockets was performed. After the initial measurements and after the determining of the clinical parameters, non-surgical treatment of the periodontal pockets was performed in all participants in the study. Periodontal pockets were irrigated with 1% chlorhexidine solution (three times for 5 minutes). Scaling and root planning was carried out in 5 sessions, in separate quadrants each session. The supra-gingival tartar was removed by ultrasound, and the treatment was performed with Grace’s curette, model Hu-Friedy, Chicago, IL, USA by the same therapist.
All patients were instructed to maintain daily oral hygiene: tooth brushing, use of dental floss, Listerine solution use. In the first group of respondents, conservative treatment was supplemented with laser therapy. For this purpose, low level laser therapy (LLLT), laser light (660 nm, 10 mW, 8 min/day, in contact with the gingiva) was applied; model: (Hager & Werken LASER HF ” confort “ Vo23-17, Duisburg, Germany) for the next five days in a row. Blood was taken from all patients during the first treatment, and after in the 6th week an 3rd month of treatment.
Collecting serum
Samples of venous blood from the cubital vein were taken from each patient. Collected blood samples were transferred into a test tube with an anticoagulant (pre-fabricated). The blood samples remained in the test tubes for about 30 minutes and were then distributed in a biochemical laboratory, where they were centrifuged at 6,000 rpm for 10 minutes. The serum was separated from the test tube and transferred to the eppendorph tube. All serum samples were stored at -80 ° C until complete analysis and measurement of IL-6 levels.
Determining IL-6 levels in serum
In the test procedure, the reagents are prepared firstly. Namely, first, before use, all reagents should be brought to room temperature (10-25 oC). The standard reagent is prepared 15 minutes before starting with work. The concentaration of the solution is 1000 pg/mL. Then 7 tubes are prepared containing 1.0 μL dilution for standard and are used to make a double dilution series according to the picture shown below. Each tube is vigorously mixed before the next transfer. 7 tubes with dissolved standard are obtained with the following concentrations: 1000 pg/mL, 500 pg/mL, 250 pg/mL, 125 pg/mL, 62,5 pg/mL, 31,25 pg/mL, 250 pg/mL, 15,625 pg/mL and 0 pg/mL.
Rinsing buffer - diluted with 30 mL of concentrated rinse buffer in 750 mL rinse buffer with deionized or distilled water.
Biotinylated Detection Ab - The exact amount needed (100μL / well) is calculated before the experiment begins. Before use, centrifuge the tube with the solution, and dilute with the concentrated Biotinylated Detection Ab to the working concentration using the Biotinylated Detection Ab Diluent (1: 100).
Concentrated HRP Conjugate - Before the start of the experiment, the exact amount needed (100μL / well) is calculated. Before use, the tube is centrifuged with the solution, and diluted with the concentrated HRP Conjugate to working concentration needed using the Concentrated HRP Conjugate Diluent (1: 100).
Test protocol
We added 100μL standard or samples to the appropriate well. Reference Standard and Sample diluent is added to standard wells. The solutions are added at the bottom of the microplate. Gently mix and cover with protective foil. Then incubate for 90 minutes at 37°. The liquid is then removed from each well. Immediately afterwards, 100 μL of the Biotinylated Detection Ab working solution is added to each well. Cover the microplate with protective foil, gently touch the tile to ensure thorough mixing. Incubate for 1 hour at 37° C.
Each well is aspirated and rinsed, repeating the process three times. Each well is rinsed with a rinsing buffer (approximately 350μL). After the last rinse, remove the remaining rinse buffer by aspiration or decantation. Then 100 ml of HRP Conjugate working solution is added to each well. Cover with foil and incubate for 30 minutes at 37 ° C. Each well is aspirated and rinsed, repeating the process five times. Each well is flushed with a wash buffer (approximately 350μL).
After the last rinse, remove the remaining rinsing buffer by aspiration or decantation. Add 90 μL of Substrate Solution to each well, cover the plate with foil and incubate in the dark for about 15-25 minutes at 37 ° C. Add 50 μL of Stop Solution to each well. The color immediately turns yellow. The optical density (OD value) of each well is determined simultaneously, using a 450 nm microplate tile reader, and at the end we calculate the results.
Statistical processing and data analysis
The statistical processing is performed in the statistical program Statistica 7.1 for Windows. The data is displayed as a table and graphically.
In the analysis of the data we used: a) The differences in the analyzed parameters in the first examination, after 6 weeks of therapy and after 3 months of therapy were tested using Friedman ANOVA Chi Sqr. / p; b) Differences in relations: first examination and after 6 weeks of therapy; first examination, after 3 months of therapy; after 6 weeks of therapy and after 3 months of therapy, they were tested using T-test for Dependent Samples (t / p), Wilcoxon Matched Pairs Test (Z / p) depending on the data distribution; c) The differences in the analyzed parameters between group A and group B were tested with T-test, independent, by groups (t / p) and Mann-Whitney U Test (Z / p), depending on the data distribution.
4. RESULTS
Figure 1 shows the descriptive statistics of serum IL-6 in group A. At the first examination IL-6 in serum varies in the interval 11.54 ± 1.11 pg / ml, after 6 weeks of therapy the values range between 11.26 ± 0.77 pg / mL and after 3 months of therapy they oscillate in the interval 11, 02 ± 0.67 pg / mL.
Graph 1. View the values of IL-6 in serum at different time intervals in group A.
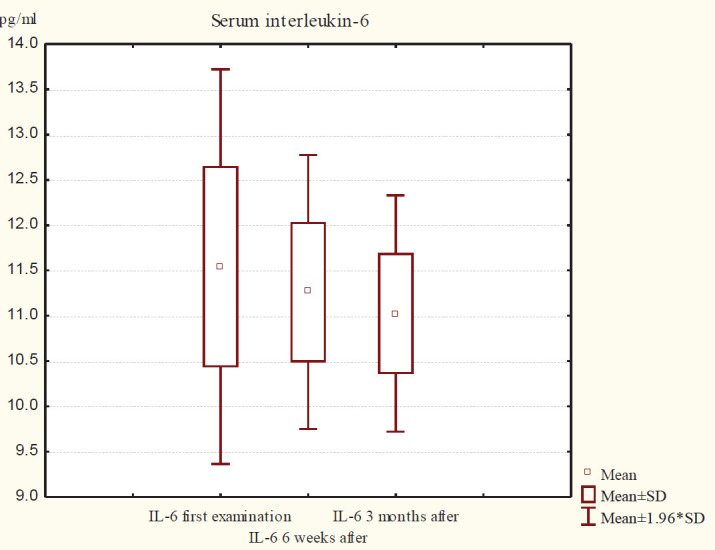
Among the IL-6 serum values in group A (first examination, six weeks, and three months) for Friedman ANOVA Chi Sqr. (N = 40, df = 2) = 13.11 and p <0.01 (p = 0.001) there is a significant difference. In group B, IL-6 in serum (first examination and six weeks and three months after therapy) for Friedman ANOVA Chi Sqr. (N = 40, df = 2) = 10.11 and p <0.01 (p = 0.006) there is a significant difference (Table 1).
Table 1. Described the attributes of the data.
| IL-6 in serum | Average Rank | Sum of Ranks | Mean | Std.Dv. |
|---|---|---|---|---|
| Group A | ||||
| First examination | 2.33 | 93.00 | 11.54 | 1.11 |
| After 6 weeks | 2.13 | 85.00 | 11.26 | 0.77 |
| After 3 months | 1.55 | 62.00 | 11.02 | 0.67 |
| Group B | ||||
| First examination | 1.96 | 78.50 | 11.56 | 0.81 |
| After 6 weeks | 2.36 | 94.50 | 11.59 | 0.71 |
| After 3 months | 1.68 | 67.00 | 11.41 | 0.78 |
Figure 2 shows the descriptive statistics of serum IL-6 in group B. At the first examination, IL-6 serum values ranged from 11.56 ± 0.81 pg / mL, after 6 weeks of therapy ranged from 11.59 ± 0.71 pg / mL, and after 3 months of therapy were recorded at intervals. 11.41 ± 0.78 pg /mL.
Graph 2. View the values of IL-6 in serum at different time intervals in group B.
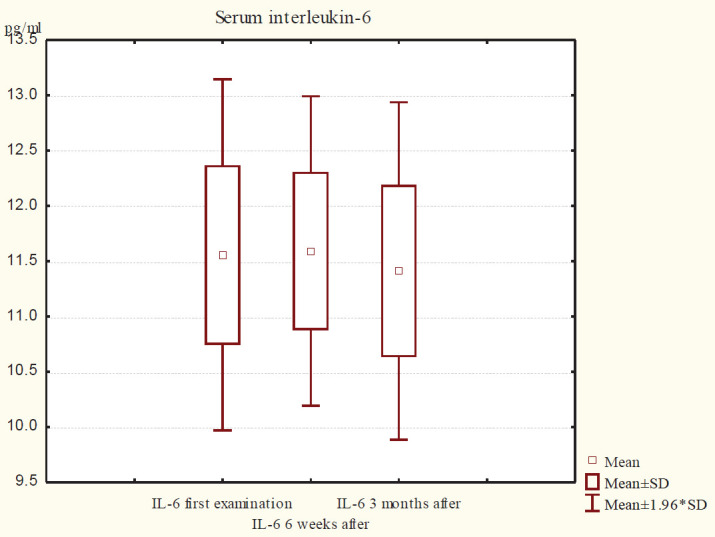
Table 2. Intergroup differences between serum IL-6 values in groups A and B at different time intervals after therapy
In group A, the serum IL-6 value after 6 weeks of therapy for t = 1.80 and p> 0.05 (p = 0.08) was significantly lower than the value at first examination, after 3 months for Z = 3,61 and p <0.001 (p = 0.000) is significantly lower than the value after 6 weeks of therapy, and after 3 months of therapy is significantly lower than the value at the first examination, ie. Z = 3.18 and p <0.01 (p = 0.001).
In group B, after 6 weeks of therapy for Z = 0.53 and p> 0.05 (p = 0.60) the values for IL-6 were insignificantly higher than the values at first examination. Comparison made between 6 weeks and 3 months of IL-6, after 3 months of therapy for Z = 4.30 and p <0.001 (p = 0.000) is significantly lower than the value after 6 weeks, while after 3 months of therapy for Z = 0.99 and p > 0.05 (p = 0.32) values of IL-6 are slightly lower than those at first examination (Table 2).
The serum IL-6 value after 6 weeks of therapy in patients in group B for Z = -2.04 and p <0.05 (p = 0.04) was significantly higher than in patients in group A, while again after 3 months of therapy in patients in group B for Z = -2.42 and p <0.05 (p = 0.02) is significantly higher than in patients with group A (Table 3).
Table 3. Differences in serum IL-6 values after 6 weeks and 3 months of treatment between groups A and B.
| IL-6 in serum | N | T | Z / t | p-level |
|---|---|---|---|---|
| group A | ||||
| First examination and after 6 weeks | 40 | / | t=1.80 | 0.08 |
| After 6 weeks and 3 months | 40 | 141.50 | 3.61 | 0.0003 |
| First examination and after 3 months | 40 | 151.00 | 3.18 | 0.001 |
| group B | ||||
| First examination and after 6 weeks | 40 | 316.50 | 0.53 | 0.60 |
| After 6 weeks and 3 months | 40 | 90.00 | 4.30 | 0.000 |
| First examination and after 3 months | 40 | 239.50 | 0.99 | 0.32 |
5. DISCUSSION
Between chronic periodontitis and diabetes, a two-way relationship has been proven. On the one hand, destruction of the supporting apparatus of the teeth is more advanced in patients with T2DM (19, 20), while on the other hand CH may worsen glycemic control in patients with T2DM (21). This two way street is thought to be due to the presence of pro-inflammatory mediators, such as TNF-α and IL-6. It is believed that their presence is a consequence of constant microbiological stimulation or as an response of the host. In circulation, pro-inflammatory mediators come in contact with the insulin receptors, disrupting insulin function and signalization (20).
In addition to IL-10, the study of the effect of IL-6 is quite complicated and completely unclear (22, 23). The role of IL-6 is crucial because it is involved in osteoclastic activity and has a strong effect on Th-17 cells (24). In addition to this exceptional activity, it simultaneously stimulates the production of IL-1 α, which contributes to the stimulation of the anti-inflammatory process (25). There is varying information about the association between these diseases. Khosravi (26) says there is insufficient evidence to support a link between elevated IL-6 levels and alveolar destruction in periodontal disease in individuals with hyperglycaemia. While Javed et al. (27) reported that cytokines in GCF in patients with and without T2D are regulated by the intensity of periodontal infection, while the role of T2DM is quite secondary.
In group A, ie. in patients with T2DM where conservative periodontal treatment with LLLT application has been performed, the value of IL-6, TNF-α in serum after 6 weeks (11.26 ± 0.77 pg / mL, and after 3 months of therapy is significantly lower than the values of the first examination. At the first examination IL-6 in serum varies in the interval 11.54 ± 1.11 pg / mL, after 6 weeks of therapy the values range between and after 3 months of therapy they oscillate in the interval 11.02 ± 0.67 pg / mL.
Quantitative analyzes have shown that serum IL-6 values after 3 months of treatment are significantly lower than the value after 6 weeks of therapy in patients with 2TDM whose conservative therapy was supplemented with LLLT.
In group B, ie. in patients with T2DM where conservative periodontal treatment was performed without LLLT application, IL-6 values after 6 weeks (11.59 ± 0.71 pg / mL) were insignificantly higher, while after 3 months (11.41 ±) 0.78 pg / mL.), they are insignificantly lower than the value at first examination (11.56 ± 0.81 pg / mL). Serum IL-6 values are significantly lower after 3 months of therapy than 6 weeks after therapy.
The results indicate that serum values of IL-6 were corrected 6 and 3 months after treatment in both groups, with a significant difference in group A p <0.01 (p = 0.001) and in group B p <0.01 (p = 0.006). Statistical analysis showed that in the second group, IL-6 values were significantly higher than those in group A patients after 6 weeks and 3 months of therapy. Regarding the quantification of values of IL-6 in serum after 6 weeks of therapy in patients in group B for Z = -2.04 and p <0.05 (p = 0.04) is significantly higher than the value in patients with group A. After 3 months of treatment, differences between the two groups showed that the values after treatment between groups A and B for IL-6 in serum in group B were higher, ie. for Z = -2.42 and p <0.05 (p = 0.02) in relation to the value in patients of group A. Intergroup differences at all time intervals showed better results in group A, confirming the effectiveness of LLLT in the treatment of periodontal disease in patients with 2TDM.
The use of lasers in the treatment of periodontitis dates back not long ago, but in the beginning the recommendations for the use of LLLT in the treatment of many diseases including 2TDM and periodontitis for many years were based only on vague assumptions, conclusions, reports or pilot clinical trials (28). However, experience showed that LLLT was applied to wound healing (29), against inflammation (30, 31), pain relief (32-33), swelling reduction (34-35), according to specific guidelines.
In recent years, the interest in the use of laser therapy has increased gradually, although there is still heterogeneity in research data and findings, the justification for their application has been unequivocally confirmed (36). Our findings agree with the findings of Boschi (14, 37, 38). Identical to the values obtained after applying LLLT (In GaAlP, 660 nm) in the study, the values of IL-6 and TNF-α 37 were significantly reduced.
Complexed progression of periodontal disease, with advanced inflammatory and destructive processes, as well as inadequate therapeutic effect are the basic features in patients with periodontitis in which T2DM (39-40) is diagnosed. These results are due to the presence of perio-pathogens that secrete endotoxin, which can increase the amount of many pro-inflammatory markers, including IL-6 (41-42).
In this study, in the group of patients treated conservatively with LLLT - therapy applied as adjuvant, we received 6 weeks and 3 months of reduced IL-6 values in serum. We believe that the results are due to the numerous positive properties of LLLT that are reflected on the examined pro-inflammatory mediator.
6. CONCLUSION
The low power laser radiation LLLT resulted in significantly reduced serum IL-6 values in patients with periodontitis and T2DM after 6 weeks and 3 months of therapy.
Author’s contribution:
All authors were involved in all steps of preparation this article. Final proofreading was made by the first author.
Conflicts of Interest:
None declared.
Financial support and sponsorchip:
No specific funding was received for this study.
REFERENCES
- 1.Centers for Disease Control and Prevention. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. National diabetes fact sheet: national estiamtes and general information on diabetes and prediabetes in the United States, 2011. [Google Scholar]
- 2.Loe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329–334. [PubMed] [Google Scholar]
- 3.National Institute of Diabetes and Digestive and Kidney Diseases. National Institutes of Health US Department of Health and Human Services; 2012. Feb, Prevent Diabetes Problems: Keep your mouth healthy. NIH Publication No. 12-4280. [Google Scholar]
- 4.Kinane D, Bouchard P. Periodontal diseases and health: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35:333–337. doi: 10.1111/j.1600-051X.2008.01278.x. [DOI] [PubMed] [Google Scholar]
- 5.Khader YS, Dauod AS, El-Qaderi SS, Alkafajei A, Batayha WQ. Periodontal status of diabetics compared with nondiabetics: a meta-analysis. J Diabetes Complications. 2006;20:59–68. doi: 10.1016/j.jdiacomp.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Mealey BL. Diabetes and periodontal disease: Two sides of a coin. Compend Contin Educ Dent. 2000;21:943–946. 948, 950. [PubMed] [Google Scholar]
- 7.Lappin DF, Robertson D, Hodge P, Treagus D, Awang RA, Ramage G, Nile CJ. The Influence of Glycated Hemoglobin on the Cross Susceptibility Between Type 1 Diabetes Mellitus and Periodontal Disease. J Periodontol. 2015 Nov;86(11):1249–1259. doi: 10.1902/jop.2015.150149. [DOI] [PubMed] [Google Scholar]
- 8.Deon D, Ahmed S, Tai K, Scaletta N, Herrero C, Lee IH, Krause A, Ivashkiv LB. Cross-talk between IL-1 and IL-6 signalling pathways in rheumatoid arthritis. J Immunol. 2001;167:5395–5403. doi: 10.4049/jimmunol.167.9.5395. Lasers Med Sci 2016: 31: 825–831. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen BK. IL-6 signalling in exercise and disease. Biochem Soc Trans. 35:1295–1297. doi: 10.1042/BST0351295. [DOI] [PubMed] [Google Scholar]
- 10.Kinane DF, Bartold PM. Clinical relevance of the host responses of periodontitis. Periodontology. 2000;43:178–193. doi: 10.1111/j.1600-0757.2006.00169.x. 2007. [DOI] [PubMed] [Google Scholar]
- 11.Lee KD, Chiang MH, Chen PH, Ho ML, Lee HZ, Lee HE, Wang YH. The effect of low-level laser irradiation on hyperglycemia-induced inflammation in human gingival fibroblasts. Lasers Med Sci. 2019 Jul;34(5):913–920. doi: 10.1007/s10103-018-2675-6. [DOI] [PubMed] [Google Scholar]
- 12.Kwon H, Lim W, Kim J, Jeon S, Kim S, Karna S, Cha H, Kim O, Choi H. Effect of 635 nm irradiation on high glucose-boosted inflammatory responses in LPS-induced MC3T3-E1 cells. Lasers Med Sci. 2013;28:717–724. doi: 10.1007/s10103-012-1122-3. [DOI] [PubMed] [Google Scholar]
- 13.Cornejo-Garrido J, Becerril-Chávez F, Carlín-Vargas G, OrdoñezRodríguez JM, Abrajan-González Mdel C, de la Cruz-Ramírez R, Ordaz-Pichardo C. Antihyperglycemic effect of laser acupuncture treatment at BL20 in diabetic rats. Acupunct Med Dec. 2014;32:486–494. doi: 10.1136/acupmed-2014-010573. [DOI] [PubMed] [Google Scholar]
- 14.Góralczyk K, Szymańska J, Szot K, Fisz J, Rość D. Low-level laser irradiation effect on endothelial cells under conditions of hyperglycemia. Lasers Med Sci. 2016;31:825–831. doi: 10.1007/s10103-016-1880-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theodoro LH, Longo M, Novaes VCN, Miessi DMJ, Ferro-Alves ML, Ervolino E, de Almeida JM, Garcia VG. Low-level laser and antimicrobial photodynamic therapy on experimental periodontitis in rats submitted to chemotherapy by 5-fluorouracil. Support Care Cancer. 2017 Oct;25(10):3261–3271. doi: 10.1007/s00520-017-3738-0. [DOI] [PubMed] [Google Scholar]
- 16.Theodoro LH, Longo M, Ervolino E, Duque C, Ferro-Alves ML, Assem NZ, Louzada LM, Garcia VG . Effect of low-level laser therapy as an adjuvant in the treatment of periodontitis induced in rats subjected to 5-fluorouracil chemotherapy. J Periodontal Res. 2016 Oct;51(5):669–680. doi: 10.1111/jre.12347. [DOI] [PubMed] [Google Scholar]
- 17.Saglam M, Kantarci A, Dundar N, Hakki SS. Clinical and biochemical effects of diode laser as an adjunct to nonsurgical treatment of chronic periodontitis: a randomized, controlled clinical trial. Lasers Med Sci. 2014 Jan;29(1):37–46. doi: 10.1007/s10103-012-1230-0. [DOI] [PubMed] [Google Scholar]
- 18.Abellán R, Gómez C, Oteo MD, Scuzzo G, Palma JC. Short- and Medium-Term Effects of Low-Level Laser Therapy on Periodontal Status in Lingual Orthodontic Patients. Photomed Laser Surg. 2016 Jul;34(7):284–290. doi: 10.1089/pho.2015.4024. [DOI] [PubMed] [Google Scholar]
- 19.Hong M, Kim HY, Seok H, Yeo CD, Kim YS, Song JY, et al. Prevalence and risk factors of periodontitis among adults with or without diabetes mellitus. Korean J Intern Med. 2016;31(5):910–919. doi: 10.3904/kjim.2016.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santos Tunes R, Foss-Freitas MC, Nogueira-Filho GR. Impact of periodontitis on the diabetes-related inflammatory status. J Can Dent Assoc. 2010;76:a35. [PubMed] [Google Scholar]
- 21.Khanuja PK, Narula SC, Rajput R, Sharma RK, Tewari S. Association of periodontal disease with glycemic control in patients with type 2 diabetes in Indian population. Front Med. 2017;11(1):110–119. doi: 10.1007/s11684-016-0484-5. [DOI] [PubMed] [Google Scholar]
- 22.Pirim Gorgun E, Toker H, Korkmaz EM, Poyraz O. IL-6 and IL-10 gene polymorphisms in patients with aggressive periodontitis: effects on GCF, serum and clinic parameters. Braz Oral Res. 2017;31:e12. doi: 10.1590/1807-3107BOR-2017.vol31.0012. [DOI] [PubMed] [Google Scholar]
- 23.Kishimoto T. Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res Ther. 2006;8(Suppl 2):S2. doi: 10.1186/ar1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosokawa Y, Shindo S, Hosokawa I, Ozaki K, Matsuo T. IL-6 trans-signaling enhances CCL20 production from IL-1beta-stimulated human periodontal ligament cells. Inflammation. 2014;37(2):381–386. doi: 10.1007/s10753-013-9750-8. [DOI] [PubMed] [Google Scholar]
- 25.Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994;83(1):113–118. [PubMed] [Google Scholar]
- 26.Khosravi R, Ka K, Huang T, Khalili S, Nguyen BH, Nicolau B, et al. Tumor necrosis factor- alpha and interleukin-6: potential interorgan inflammatory mediators contributing to destructive periodontal disease in obesity or metabolic syndrome. Mediators Inflamm. 2013;2013:728987. doi: 10.1155/2013/728987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Javed F, Al-Askar M, Al-Hezaimi K. Cytokine profile in the gingival crevicular fluid of periodontitis patients with and without type 2 diabetes: a literature review. J Periodontol. 2012;83(2):156–161. doi: 10.1902/jop.2011.110207. [DOI] [PubMed] [Google Scholar]
- 28.National Pressure Ulcer Advisory Panel; European Pressure Ulcer Advisory Panel. Pan Pacific Pressure Injury Alliance In Prevention and Treatment of Pressure Ulcers: Quick Reference Guide. In: Haesler E, editor. Perth, Australia: Cambridge Media; 2014. [Google Scholar]
- 29.Arany PR. Craniofacial wound healing with photobiomodulation therapy: new insights and current challenges. J Dent Res. 2016;95(9):977–984. doi: 10.1177/0022034516648939. [DOI] [PubMed] [Google Scholar]
- 30.Bortone F, Santos HA, Albertini R, Pesquero JB, Costa MS, Silva JA., Jr (2008) Low level laser therapy modulates kinin receptors mRNA expression in the subplantar muscle of rat paw subjected to carrageenan-induced inflammation. Int Immunopharmacol. 8(2):206–210. doi: 10.1016/j.intimp.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Obradovic R, Kesic L, Mihailovic D, Antic S, Jovanovic G, Petrovic A, Pesevska S . A histological evaluation of a lowlevel laser therapy as an adjunct to periodontal therapy in patients with diabetes mellitus. Lasers Med Sci. 2013;28(1):19–24. doi: 10.1007/s10103-012-1058-7. [DOI] [PubMed] [Google Scholar]
- 32.Hagiwara S, Iwasaka H, Okuda K, Noguchi T. GaAlAs (830 nm) low-level laser enhances peripheral endogenous opioid analgesia in rats. Lasers Surg Med. 2007;39:797–802. doi: 10.1002/lsm.20583. [DOI] [PubMed] [Google Scholar]
- 33.Peševska S, Nakova M, Ivanovski K, Angelov N, Kesic L, Obradovic R, Mindova S, Nares S. Dentinal hypersensitivity following scaling and root planing: comparison of low-level laser and topical fluoride treatment. Lasers Med Sci. 2010;25:647–650. doi: 10.1007/s10103-009-0685-0. [DOI] [PubMed] [Google Scholar]
- 34.Bayat M, Abdi S, Javadieh F, Mohsenifar Z, Rashid MR. The effects of low-level laser therapy on bone in diabetic and nondiabetic rats. Photomed Laser Surg. 2009;27:703–708. doi: 10.1089/pho.2008.2351. [DOI] [PubMed] [Google Scholar]
- 35.Obradović R, Kesić LJ, Peševska S. Influence of low-level laser therapy on biomaterial osseointegration: a mini-review. Lasers Med Sci. 2009;24:447–451. doi: 10.1007/s10103-008-0573-z. [DOI] [PubMed] [Google Scholar]
- 36.Machado RS, Viana S, Sbruzzi G. Low-level laser therapy in the treatment of pressure ulcers: systematic review. Lasers Med Sci. 2017;32:937–944. doi: 10.1007/s10103-017-2150-9. [DOI] [PubMed] [Google Scholar]
- 37.Boschi ES, Leite CE, Saciura VC, Caberlon E, Lunardelli A, Bitencourt S, Melo DA, Oliveira JR. Anti-inflammatory effects of low-level laser therapy (660 nm) in the early phase in carrageenan-induced pleurisy in rat. Lasers Surg Med. 2008;40(7):500–508. doi: 10.1002/lsm.20658. [DOI] [PubMed] [Google Scholar]
- 38.Taradaj J, Shay B, Dymarek R, Sopel M, Walewicz K, Beeckman D, Schoonhoven L, Gefen A, Rosińczuk J. Effect of Laser Therapy on Expression of Angio- And Fibrogenic Factors, and Cytokine Concentrations During the Healing Process of Human Pressure Ulcers. Int J Med S. 2018;15(11):1105–1112. doi: 10.7150/ijms.25651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salvi GE, Beck JD, Offenbacher S. PGE2, IL-1 beta, and TNF-alpha responses in diabetics as modifiers of periodontal disease expression. Annals of Periodontology. 1998;3(1):40–50. doi: 10.1902/annals.1998.3.1.40. [DOI] [PubMed] [Google Scholar]
- 40.Graves DT, Cochran D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. Journal of Periodontology. 2003;74(3):391–401. doi: 10.1902/jop.2003.74.3.391. [DOI] [PubMed] [Google Scholar]
- 41.Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, de Nardin E. Periodontal infections contribute to elevated systemic C-reactive protien level. Journal of Periodontology. 2001;72(9):1221–1227. doi: 10.1902/jop.2000.72.9.1221. [DOI] [PubMed] [Google Scholar]
- 42.Loos BG, Craandijk J, Hoek FJ, Wertheim-Van Dillen PME, van Dder Velden U. Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. Journal of Periodontology. 2000;71(10):1528–1534. doi: 10.1902/jop.2000.71.10.1528. [DOI] [PubMed] [Google Scholar]


