Abstract
Introduction:
Liver fibrosis (LF) is the excessive deposition of extracellular matrix (ECM), produced by overactivated hepatic stellate cells, following prolonged transforming growth factor-β (TGF-β) stimulation. The ability of mesenchymal stem cells (MSCs) to improve LF has been reported. However, the mechanisms of MSCs to ameliorate LF through suppressing TGF-β and α-smooth muscle actin (α-SMA) remains unclear.
Aim:
To investigate the effects of MSCs treatment on suppressing TGF-β levels and decreasing α-SMA expression in an LF model.
Methods:
In this study, wenty-four male Wistar rats were injected intraperitoneal (IP) with carbon tetrachloride (CCL4), twice weekly, for eight weeks, to induce LF. Rats were randomly assigned to six groups: Sham, Control, Sham-lo, Sham-hi, and MSC-treated groups, at doses of 1 x 106 (T1) and 2x106 (T2) cells. TGF-β levels were analyzed by enzyme-linked immunosorbent assay (ELISA), whereas α-SMA expression was determined by immunohistochemistry staining.
Results:
MSCs decreased the expression of TGF-β in T1 and T2 groups on day 3 and 14. The T2 group showed lower TGF-β levels than that in the T1 group. This finding was in line with the observed decrease in α-SMA expression and the number of collagen.
Conclusion:
MSCs treatment ameliorated LF by suppressing TGF-β production, leading to decreased α-SMA expression in a CCL4-induced LF animal model.
Keywords: Liver Fibrosis, Mesenchymal Stem Cells, transforming growth factor-beta, alpha-smooth muscle actin
1. INTRODUCTION
Liver fibrosis (LF) is the excessive deposition of extracellular matrix (ECM), with scar tissue formation, encapsulating several areas of liver injury, particularly the central vein and portal areas (1). End-stage chronic progressive fibrosis results in cirrhosis hepatic disease (CHD), which accounts for approximately 55% of the 1.4 million liver-disease-related deaths reported each year, worldwide (2). Currently, liver transplantation has been the most effective therapy for patients with advanced liver diseases, including CHD; however, liver donors' limited availability and the low survival rates among patients who ever receive liver transplants remains a serious problem (3). Therefore, novel, alternative LF treatments that can be used in place of transplantation must be explored.
In recent years, mesenchymal stem cells (MSCs) have been studied as part of the therapeutic paradigms for regenerative therapies. MSCs are described as multipotent, stromal progenitor cells that express the various surface markers including CD105, CD90, CD73 and CD44, and lack of other surface marker expression such as CD31, CD45, CD43, CD14, CD11b, jmajor histocompatibility complex (MHC) class II molecule, or co-stimulatory proteins (CD80, CD86, and CD40) (4, 5). MSCs can regenerate damaged tissue through differentiation into various cell lineages, including hepatocytes, and the trans-differentiation or fusion with local hepatocytes (6). Our previous study demonstrated that the intravenous delivery of MSCs resulted in the migration of MSCs to injury sites, including liver injury (7). MSCs also have immunosuppressive properties, driving the transition from inflammation to proliferation, which accelerates the healing process (8) MSCs release anti-inflammatory cytokines, such as prostaglandin E2 (PGE2), transforming growth factor-β (TGF-β), indoleamine 2,3-dioxygenase (IDO), and interleukin (IL)-10, to control the inflammation (9-11). Therefore, MSCs play a pivotal role suppressing of inflammation, including controlling the prolongation of inflammation during LF.
Furthermore, the prolonged stimulation of inflammatory cytokines following continuous exposure to hepatotoxic substances, such as carbon tetrachloride (CCl4) could activate hepatic stellate cells (HSCs) (12). HSCs are the primary ECM-producing fibroblast cells, which are activated by chronic liver injury to differentiate into myofibroblasts (MFs), which secrete large amounts of ECM proteins, particularly collagen (I, III, and IV), fibronectin, and proteoglycans. MFs are characterized by a-smooth muscle actin (α-SMA) expression, in addition to being spindle or stellate-shaped and lacking epithelial or endothelial markers. Several studies have reported that the release of a wide range of inflammatory mediators, such as IL-13, TGF-β, and IL-17, during chronic inflammation may contribute to fibrosis formation; however, TGF-β is the central factor associated with the fibrosis pathway in LF (13, 14). Furthermore, MSCs treatment has been reported to improve LF, but the mechanism remains unclear. Thus, this study aims to investigate the effects of MSCs treatment on suppressing TGF-β levels and decreasing α-SMA expression in an LF model.
2. AIM
This study aimed to investigate the effects of MSCs treatment on suppressing TGF-β levels and decreasing α-SMA expression in an LF model.
3. MATERIAL AND METHODS
CCl4-induced liver fibrosis model
The Ethics Committee approved all procedures performed in this study of Sultan Agung Islamic University. The animals were obtained and certified healthy by the Agricultural and Fishery Service of Salatiga under no. 524.3/0211/421. Thirty, healthy, male Wistar rats (5–6 weeks), weighing 250–300 g were fed ad libitum and housed in plastic cages with mesh wire covers, at a room temperature of 24°C, with a 12-h light-dark cycle (laboratory standard). All rats were intra-peritoneally (IP) injected with 0.1 mL/kg CCl4 (Sigma-Aldrich), dissolved in olive oil (1:1), twice weekly, for eight weeks, to induce LF (15). Sirius red staining was used to validate LF.
Isolation, culture, and characterization of human MSCs
Human umbilical cords were obtained from full-term births, after cesarean section delivery, with informed consent and approved by the Ethics Committee of Sultan Agung Islamic University. Human umbilical cord-MSCs isolation was performed, as previously described (16). Briefly, after all blood vessels were removed, the Wharton’s jelly was separated, washed twice in phosphate-buffered saline (PBS, Gibco, Thermo Fisher Scientific, NJ, USA), and minced into small pieces. The Wharton’s jelly was placed in a T25 culture flask and grown in 3 mL Dulbecco’s modified Eagle medium (DMEM, Gibco, Thermo Fisher Scientific, NJ, USA), supplemented with 10% fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific, NJ, USA), 100 mg/ml penicillin-streptomycin (Penstrep, Gibco, Thermo Fisher Scientific, NJ, USA) and 0.25 μg/ml amphotericin B (Gibco, Thermo Fisher Scientific, NJ, USA). These cells were incubated in a humidified atmosphere, containing 5% CO2, at 37°C, and the medium was replaced in three-day intervals. After cells reached 80% confluence, the cells were passaged, and cells from the fourth passage were used for the following treatments.
Before the treatment, MSCs were subjected to flow cytometer analysis, including the detection of MSC-specific surface markers. Briefly, under dark conditions and at ambient temperatures, MSCs were incubated with monoclonal antibodies against MSC-specific surface marker including, CD90-FITC, CD105-PerCP, CD73-APC, and PE-human MSCs Negative Cocktail (Lin), containing CD34, CD19, CD11b, CD19, CD45, and HLA-DR monoclonal antibodies for 30 minutes. The cells were washed twice with BD Pharmingen™ Stain Buffer (BD Biosciences, Franklin Lakes, NJ, USA) and analyzed using a BD Accuri 6 plus flow cytometer (BD Biosciences, San Jose, CA, USA).
A standard osteogenic assay was performed to validate the differentiation capacity of MSCs. After MSCs reached 95% confluence, MessenCult Osteogenic medium (Stemcell technologies, VN, Canada) was added to induce osteogenic differentiation. An Alizarin red staining kit (Sigma Aldrich, USA) was used to observe calcium deposition in the cultures.
Administration of MSCs
CCl4-induced-LF rats model were randomly assigned into six groups: sham, control (C), sham-low-MSC (1×106 MSCs, Sham-lo), sham-high-MSC (2×106, sham-hi), low-MSCs (1×106 MSCs, T1), and high-MSCs (2×106 MSCs, T2) treatment groups. All groups were replicated 5 times. MSCs were administered in 0.1 mL saline via tail vein injection.
Collagen histology analysis
Liver tissue samples were immediately fixed in cold 4% neutral buffer formalin, at 4°C, and processed for paraffin embedding. Before staining, sections were deparaffinized and rehydrated, using xylol and alcohol. The slides were incubated with a 0.1% Sirius Red solution, dissolved in aqueous saturated picric acid, for 1 hour, washed in 0.5% hydrogen chloride, dehydrated, and mounted with DPX mounting medium. Collagen and non-collagen components were red-stained and orange-stained, respectively.
Immunohistochemical examinations of α-SMA
Paraffin-embedded liver slides were deparaffinized using xylene and alcohol. After rehydration, slides were incubated with a primary monoclonal antibody for α-SMA (1:100, Abcam, Cambridge, MA, United States), followed by biotinylated secondary antibody. The detection was examined using streptavidin peroxidase, and the expression intensity of α-SMA was semi-quantified using ImageJ software (17).
Enzyme-linked immunosorbent assays
Blood was collected under general anesthesia, 3 and 14 days after treatment, from the periorbital venous plexus using hematocrit capillary tubes. Blood samples were centrifuged at 2,000 rpm for 10 min to obtain the blood serum. TGF-β concentrations were analyzed using enzyme-linked immunosorbent assay (ELISA) kits, based on the manufacturer’s instructions (Fine Test, Wuhan, China). Serum samples were assessed in duplicate, and the absorbance was read at 450 nm on a Bio-Rad microplate reader. TGF-β concentrations were determined using standard curves, generated by the plate-reader software.
Statistical analysis
The data were analyzed by one-way analysis of variance (ANOVA), using SPSS ver.24 (The IBM SPSS®). The values are presented as the mean ± SD. Differences were considered significant at p < 0.05.
4. RESULTS
Characteristics of MSCs, based on cell morphology, immunophenotypic profile, and osteogenic differentiation capacity
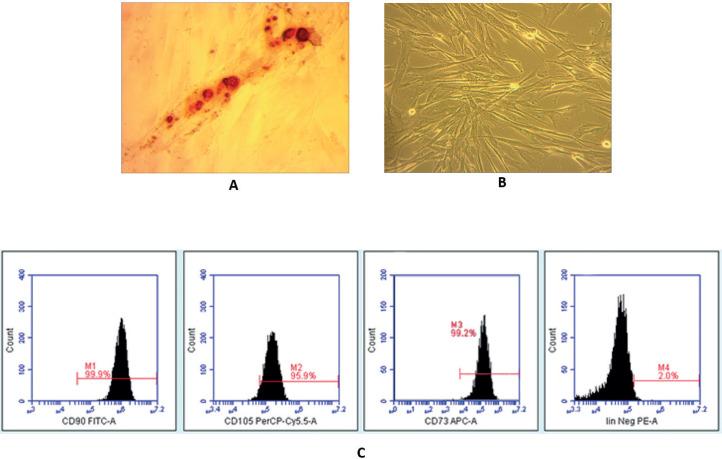
To characterize MSCs, we evaluated cell morphology, the expression of surface markers, and the in vitro differentiation potential, as indicated by the International Society for Stem Cell Therapy (4).. We used MSCs isolated from umbilical cords after the fourth passage, which presented as typical monolayers of spindle-shaped, fibroblast-like cells, with the capability to adhere to the plastic flask (Figure 1a). The umbilical cord-derived cells showed an immunophenotype consistent with the accepted definition of MSCs, namely CD44+, CD73+, CD90+, and CD105+, combined with the negative detection of hematopoietic lineage markers, suggesting that these MSCs were not contaminated with HSCs or progenitor cells (Figure 1b). To confirm the in vitro differentiation potential of MSCs, we used osteogenic differentiation media to demonstrate that these MSCs can differentiate into osteogenic cells, characterized by the deposition of calcium (Figure 1c).
Figure 1. Characterization of isolated MSCs. (a) Morphological characterization. After the fourth passage, MSCs appeared as homogeneous, spindle-shaped, fibroblast-like cells (200× magnification). (b) Expression of immunophenotypic surface markers. Graphs display the phenotype of MSCs in culture, after the fourth passage; CD90 (99.9%), CD105 (95.9%), CD73 (99.2%), and Lin (2.0%). (c) In vitro osteogenic differentiation test. MSCs were able to differentiate towards osteogenic lineages, which appeared as red color in most MSC populations, following Alizarin Red staining (200× magnification).
MSCs suppress the release of TGF-β to inactivate HSCs
Recent studies have reported that prolonged inflammation can induce macrophage type-2 (M2) cells to release several growth factors, particularly TGF-β, as robust mediators for the activation and differentiation of HSCs into MFs, which produce ECM (12, 18). On the other hand, recent studies have reported that MSCs play pivotal roles in immune cell suppression, including M2 macrophage cells, under prolonged inflammatory conditions. Therefore, to determine the therapeutic potential of MSCs to suppress TGF-β release in an experimental model of CCl4-induced LF in rats, we assessed the concentration of TGF-β following MSC administration, using ELISA.
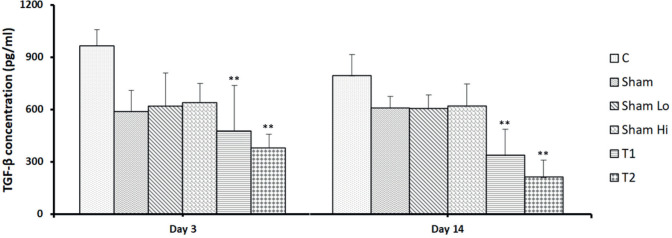
As shown in Figure 2, the concentration of TGF-β significantly decreased 3 days after treatment with various doses of MSCs, and a higher dose of MSCs resulted in lower TGF-β levels compared with the lower dose of MSCs (respectively, 380.3±79.0 and 477.3±261.7 pg/mL, p<0.05). TGF-β levels must remain low for longer than 3 days to achieve optimum liver regeneration. In this study, decreased TGF-β levels were also detected 14 days following MSCs administration in both high- and low-dose treatment groups (respectively, 213.3±96.9 pg/mL and 338.3±149,5 pg/mL, p<0.05).
Figure 2. MSC suppresses the TGF-β concentration in a rat experimental model of CCl4- induced LF. TGF-β levels were quantified by ELISA 3 and 14 days after MSC treatment. Bars represent the mean ±SD. *p<0.05. The TGF-β level on day 3, the higher dose of MSCs showed a lower level of TGF-β than the low dose. TGF-β on day 14, the higher dose of MSCs showed a lower level of TGF-β than the low dose.
MSCs decrease the α-SMA expression in MFs, to reduce collagen formation during liver fibrosis
The expression of α-SMA can be used as a marker of HSC activity, and these are the primary cell type responsible for inducing fibrogenesis in CCl4-induced LF (19). The prolonged activation of Kupffer cells and other macrophages, due to hepatocyte damage, can result in the release of TGF-β, which activate HSCs to differentiate into MFs. To determine the therapeutic potential of MSCs to decrease the HSC activity in a rat experimental model of CCl4-induced LF, we assessed the expression of α-SMA using an immunohistochemical staining method.
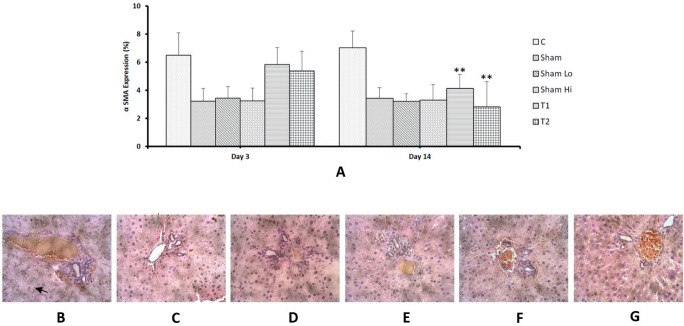
As shown in Figure 3a (upper panel), a significant decrease in percentage area showing α-SMA expression was observed, 14 days after MSCs administration, and the high-dose MSCs treatment resulted in the reduced expression of α-SMA compared with the low-dose MSCs treatment (respectively, 2.8±1.8 % and 4.1±1.0 %, p<0.05). The α-SMA-positive cells appeared as small, spindle-shaped cell bodies, with multiple cytoplasmic processes, as shown in Figure 3b-e (lower panel).
Figure 3. Expression of α-SMA. (a) No significant change in α-SMA expression was observed 3 days following MSC administration and a significant decrease occurred after 14 days. Bars represent the mean ± SD. * p < 0.05. Sham (b), Sham-lo (c), Sham-high and control group (d) showed α-SMA positive cells, characterized as spindle-shaped cells with brown cytoplasmic staining (black arrow) in-between hepatocytes, around the central veins, and in the connective tissue septa, between the hepatic lobules (magnification 200´). The high-dose MSC treatment (e) showed lower α-SMA expression than the low-dose MSC treatment (f).
MSCs alleviate large amounts of collagen fibers in liver fibrosis
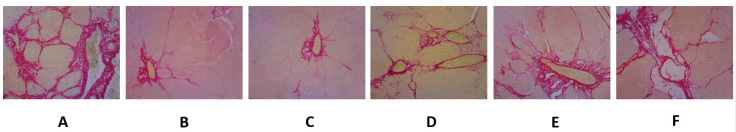
We used the CCl4-induced LF experimental animal model because this model represents the model that most closely resembles human liver cirrhosis (20). MSCs alleviate large amounts of collagens by inactivating and inducing the apoptosis of active HSCs, in addition to suppressing profibrotic genes and increasing anti-fibrotic hepatic genes in the LF animal model, as shown Figure 4c, d. High-dose MSCs treatment reduced collagen fiber numbers by a larger amount than low-dose MSCs treatment (Figure 4d).
Figure 4. MSC administration alleviates large amounts of collagen fibers in LF, detected by Sirius Red staining. CCl4-induced liver fibrosis, as shown in sham (a), sham-lo (b), sham-hi (c) and control groups (d); however, MSC treatments reduce the large amounts of collagen fibers, and the high-dose MSC treatment (e) reduced the numbers collagen fibers to a greater extent than the low-dose MSC treatment (f).
5. DISCUSSION
Prolonged inflammation is a common hallmark of fibrosis, represented by the release of a wide range of growth factors and inflammatory mediators, such as TGF-β, IL-17, and IL-13, to stimulate organ fibrosis, including LF (13). However, TGF-β is the most well-known mediators that initiate the complex pathways involved in LF (21). The prolonged expression of TGF-β consistently results in the activation and differentiation of HSCs into active MFs, characterized by α-SMA expression, and resulting in LF formation (22). Furthermore, our previous study demonstrated that MSCs can migrate to liver injury sites (13), to regenerate liver damage and restore liver structure and function following LF (23). In the same time, MSCs can suppress inflammation by releasing anti-inflammatory cytokines, particularly IL-10 (11). However, the mechanism through which MSCs improve and restore LF by controlling TGF-β and α-SMA expression remains unclear. Therefore, this study investigated the role played by MSCs in the amelioration of LF by suppressing the release of TGF-β release, leading to decreased α-SMA expression, 3 and 14 days after treatment.
We used CCl4 as a hepatotoxic chemical to induce LF in an experimental animal model because the CCl4 model represents the model that most closely resembles human liver cirrhosis (20). After 8 weeks of CCl4 injections, we successfully established a rat model of LF (24). To confirm the LF in this study we used Sirius Red staining to identify collagen fibers, and we found a significant increase in the percentage of collagen fibers in several areas, indicating the successful induction of LF in all study groups (Figure 4a, b). LF is characterized by chronic inflammation and the massive recruitment of Th2 lymphocytes, which induce the polarization of macrophage type 1 (M1) into macrophage type 2 (M2) cells, through the release of IL-13 and IL-4 (25). Active M2 and Th2 cells release TGF-β to activate quiescent HSCs (inactivate HSCs), which differentiate into MFs (active HSC) and produce large amounts of ECM (26). Therefore, to analyze the role played by MSCs in the inactivation of HSCs, we explored the levels of TGF-β following MSCs administration.
Here, we present evidence that MSCs can suppress the release of TGF-β in an LF model animal. We believe this discovery is both novel and important discovery because it demonstrates mechanistically how fully developed TGF-β-dependent fibrosis can be disrupted by MSCs treatment. In this study, using a CCl4-induced LF animal model, we showed that MSCs can decrease TGF-β levels, released by Kupffer cells and M2 macrophages. We suggest that MSCs regulate TGF-β release through immunomodulatory mechanisms, by releasing several anti-inflammatory cytokines, particularly IL-10. The binding of IL-10 with receptors on Kupffer cells and M2 macrophages might activate tyrosine kinase 2 and Janus tyrosine kinase 1 (JAK1), which phosphorylate IL-10Ra and signal transducer and activator of transcription 3 (STAT3). The phosphorylated STAT3 translocates into the nucleus, where it binds the promoters of various IL-10 target genes, particularly the suppressor of cytokine signaling 3 (SOCS3) whose expression has been correlated with the decreased expression of tumor necrosis factor (TNF)-a, IL-1b, and TGF-β (27) (Figure 2). These findings were confirmed by our previous study, which reported that MSCs can prevent peritoneal fibrosis by releasing IL-10 (28). Furthermore, other studies have also reported that IL-10 plays a crucial role in the inhibition of fibrosis-related inflammation (27). TNF-a-exposed MSCs can release IL-10 to control inflammatory cytokine release (11). To analyze the effects of decreased levels of TGF-β following MSCs treatment on HSCs activity, we assessed α-SMA expression.
LF was evidenced by the significant increase in the percentage area percentage of collagen fibers produced by active HSCs, which represent the primary cell type responsible for fibrogenesis. Activated HSCs are characterized by α-SMA expression (20). The prolonged release of TGF-β associated with CCl4-induced hepatocyte damage can activate quiescent HSCs to differentiate into the star-shaped stellate cells or into MF-like cells, which synthesize large quantities of ECM components, including collagen, proteoglycan, and adhesive glycoproteins (21). Our findings revealed that LF was also resolved after MSCs administration, associated with a significant decrease in the area of α-SMA-positive cells, which significantly decreased compared with the control group, similar to the observed decrease in TGF-β levels (Figure 4c, d). We suggest that MSCs release IL-10, to downregulate profibrotic genes and upregulate anti-fibrotic hepatic genes (29). Moreover, IL-10-released by MSCs may act as a receptor-binding competitor for TGF-β in quiescent HSCs; thus, IL-10 may play an inhibitory role in process HSC transition from the quiescent state to the activated state, in addition to inducing the apoptosis of active HSCs (30). These findings were confirmed by another study, which reported that MSCs can reduce the expression levels of collagen type I and α-SMA (31) (Figure 5).
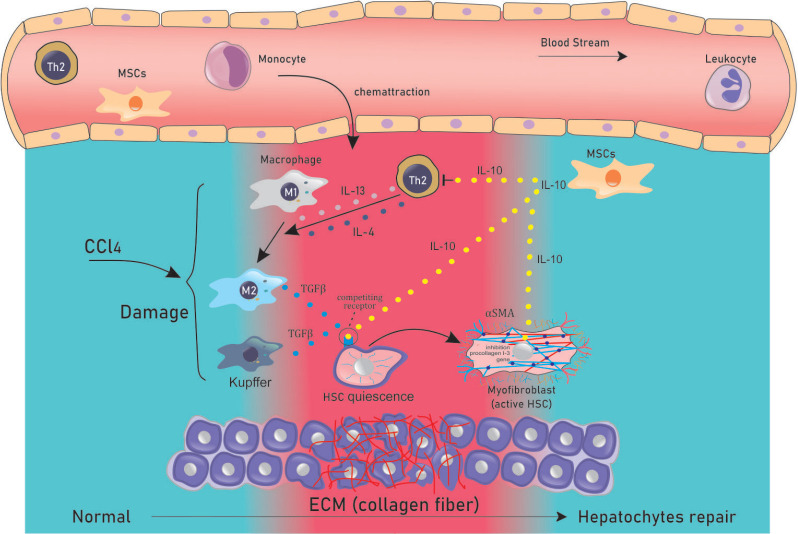
Figure 5. MSCs release IL-10 to suppress TGF-β release and decrease α-SMA expression. During a prolonged inflammatory response, recruited Th2-lymphocytes release of IL-13 and IL-4 to induce the polarization of macrophage type 1 (M1) into macrophage type 2 (M2). Activated M2 and Th2 cells release TGF-β, to activate quiescent HSCs, which differentiate into MFs (active HSCs), which are characterized by α-SMA expression, resulting in the massive production of ECM. MSCs supress the release of TGF-β from Kupffer and M2 cells by releasing IL-10. The binding of IL-10 to receptors on target cells may activate the promoters of the suppressor of cytokine signaling 3 (SOCS3), leading to the decreased expression of TGF-β. IL-10-releasing MSCs inhibit the activation of HSCs by competing against TGF-β for receptor binding, inducing apoptosis in the activated HSCs. Furthermore, IL-10 also downregulates the expression of profibrotic genes and up-regulates anti-fibrotic hepatic genes.
In summary, the administration of MSCs attenuated LF 14 days after MSCs administration by reducing TGF-β concentrations and α-SMA expression. However, whether IL-10 was the primary actor associated with the reductions in TGF-β concentration and α-SMA expression) was not measured and represent a limitation of this study.
6. CONCLUSION
In conclusion, our results showed that MSCs treatment ameliorated LF by reducing TGF-β production, leading to decreased α-SMA expression in an LF animal model.
Acknowledgments:
We thank the SCCR Laboratory of Sultan Agung Islamic University for finishing this study.
Author‘s contribution: A.P and D.H.: Conception, design, and manuscript writing; K.W: provision of study material; B.T.J : administrative support; A.M.M and R: provision of study material, data analysis and interpretation; D.H:
conception and design; provision of study material and data analysis. All authors read and approved the final manuscript.
Conflicts of interest:
There are no conflicts of interest.
Financial support and sponsorship:
This work was carried out with the support of the “Stem Cell and Cancer Research (SCCR) Laboratory”. The funding bodies have played role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
REFERENCES
- 1.Guo J, Friedman SL. Hepatic fibrogenesis. Semin Liver Dis. 2007;27(4):413–426. doi: 10.1055/s-2007-991517. [DOI] [PubMed] [Google Scholar]
- 2.Poynard T, Yuen MF, Ratziu V, et al. Viral hepatitis C. Lancet. 2003;362(9401):2095–2100. doi: 10.1016/s0140-6736(03)15109-4. [DOI] [PubMed] [Google Scholar]
- 3.Freeman RB, Jr, Steffick DE, Guidinger MK, et al. Liver and intestine transplantation in the United States, 1997-2006. Am J Transplant. 2008;8(4 Pt 2):958–976. doi: 10.1111/j.1600-6143.2008.02174.x. [DOI] [PubMed] [Google Scholar]
- 4.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 5.Lv FJ, Tuan RS, Cheung KM, et al. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32(6):1408–1419. doi: 10.1002/stem.1681. [DOI] [PubMed] [Google Scholar]
- 6.Hao NB, Li CZ, Lü MH, et al. SDF-1/CXCR4 Axis Promotes MSCs to Repair Liver Injury Partially through Trans-Differentiation and Fusion with Hepatocytes. Stem Cells Int. 2015;2015:960387. doi: 10.1155/2015/960387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Putra A, Rosdiana I, Darlan DM, et al. Intravenous Administration is the Best Route of Mesenchymal Stem Cells Migration in Improving Liver Function Enzyme of Acute Liver Failure. Folia Med (Plovdiv) 2020;62(1):52–58. doi: 10.3897/folmed..e47712. [DOI] [PubMed] [Google Scholar]
- 8.Li H, Shen S, Fu H, et al. Immunomodulatory Functions of Mesenchymal Stem Cells in Tissue Engineering. Stem Cells Int. 2019;9671206 doi: 10.1155/2019/9671206. Published 2019 Jan 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikhsan R, Putra A, Munir D, et al. Mesenchymal Stem Cells Induce Regulatory T-cell Population in Human SLE. BJMS. 2020;19(4):743–748. doi: 10.3329/bjms.v19i4.46635. [DOI] [Google Scholar]
- 10.Ling W, Zhang J, Yuan Z, et al. Mesenchymal stem cells use IDO to regulate immunity in tumor microenvironment. Cancer Res. 2014;74(5):1576–1587. doi: 10.1158/0008-5472.CAN-13-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sungkar T, Putra A, Lindarto D, Sembiring RJ. Intravenous Umbilical Cord-derived Mesenchymal Stem Cells Transplantation Regulates Hyaluronic Acid and Interleukin-10 Secretion Producing Low-grade Liver Fibrosis in Experimental Rat. Med Arch. 2020;74(3):177–182. doi: 10.5455/medarh.2020.74.177-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guyot C, Lepreux S, Combe C, et al. Hepatic fibrosis and cirrhosis: the (myo)fibroblastic cell subpopulations involved. Int J Biochem Cell Biol. 2006;38(2):135–151. doi: 10.1016/j.biocel.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 13.Borthwick LA, Barron L, Hart KM, et al. Macrophages are critical to the maintenance of IL-13-dependent lung inflammation and fibrosis. Mucosal Immunol. 2016;9(1):38–55. doi: 10.1038/mi.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-βeta as major players and therapeutic targets. J Cell Mol Med. 2006;10(1):76–99. doi: 10.1111/j.1582-4934.2006.tb00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iredale JP, Benyon RC, Pickering J, et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102(3):538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chai NL, Zhang XB, Chen SW, et al. Umbilical cord-derived mesenchymal stem cells alleviate liver fibrosis in rats. World J Gastroenterol. 2016;22(26):6036–6048. doi: 10.3748/wjg.v22.i26.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Yu Q, Xu CB. A convenient method for quantifying collagen fibers in atherosclerotic lesions by ImageJ software. Int J Clin Exp Med. 2017;10(10):14927–14935. [Google Scholar]
- 18.Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12(6):325–338. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- 19.Cheung PY, Zhang Q, Zhang YO, et al. Effect of WeiJia on carbon tetrachloride induced chronic liver injury. World J Gastroenterol. 2006;12(12):1912–1917. doi: 10.3748/wjg.v12.i12.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geerts AM, Vanheule E, Praet M, et al. Comparison of three research models of portal hypertension in mice: macroscopic, histological and portal pressure evaluation. Int J Exp Pathol. 2008;89(4):251–263. doi: 10.1111/j.1365-2613.2008.00597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Györfi AH, Matei AE, Distler JHW. Targeting TGF-β signaling for the treatment of fibrosis. Matrix Biol. 2018;68-69:8–27. doi: 10.1016/j.matbio.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 22.Nakerakanti S, Trojanowska M. The Role of TGF-β Receptors in Fibrosis. Open Rheumatol J. 2012;6:156–162. doi: 10.2174/1874312901206010156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sungkar T, Putra A, Lindarto D, et al. The effect of mesenchymal stem cells for the reduction of liver fibrosis through platelet derived growth factor regulation in rats. Biochem. Cell. Arch. 2019;19(2):4749–4753. [Google Scholar]
- 24.Dong S, Chen QL, Song YN, et al. Mechanisms of CCl4-induced liver fibrosis with combined transcriptomic and proteomic analysis. J Toxicol Sci. 2016;41(4):561–572. doi: 10.2131/jts.41.561. [DOI] [PubMed] [Google Scholar]
- 25.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Braga TT, Agudelo JS, Camara NO. Macrophages During the Fibrotic Process: M2 as Friend and Foe. Front Immunol. 2015;6:602. doi: 10.3389/fimmu.2015.00602. Published 2015 Nov 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sziksz E, Pap D, Lippai R, et al. Fibrosis Related Inflammatory Mediators: Role of the IL-10 Cytokine Family. Mediators Inflamm. 2015;2015:764641. doi: 10.1155/2015/764641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muhar AM, Putra A, Warli SM, et al. Hypoxia-Mesenchymal Stem Cells Inhibit Intra-Peritoneal Adhesions Formation by Upregulation of the IL-10 Expression. Open Access Maced J Med Sci. 2019;7(23):3937–3943. doi: 10.3889/oamjms.2019.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ali G, Masoud MS. Bone marrow cells ameliorate liver fibrosis and express albumin after transplantation in CCl4-induced fibrotic liver. Saudi J Gastroenterol. 2012;18(4):263–267. doi: 10.4103/1319-3767.98433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai LJ, Li HY, Guan LX, et al. The therapeutic potential of bone marrow-derived mesenchymal stem cells on hepatic cirrhosis. Stem Cell Res. 2009;2(1):16–25. doi: 10.1016/j.scr.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Shao CH, Chen SL, Dong TF, et al. Transplantation of bone marrow-derived mesenchymal stem cells after regional hepatic irradiation ameliorates thioacetamide-induced liver fibrosis in rats. J Surg Res. 2014;186(1):408–416. doi: 10.1016/j.jss.2013.08.016. [DOI] [PubMed] [Google Scholar]