Abstract
Extracellular vesicles like exosomes are important therapeutic tactics for treating COVID -19. By utilizing convalescent plasma derived exosomes (CPExo) from COVID-19 recovered persistence could accelerate the treatment strategies in the current state of affairs. Adequate literature has shown that administering the exosome to the in vivo system could be beneficial and could target the pathogens in an effective and precise manner. In this hypothesis we highlight the CPExo instead of convalescent plasma (CP), perhaps to dispense of exosomes are gratified and it's more effectively acquired immune response conferral through antibodies. COVID-19 convalescent plasma has billions of exosomes and it has aptitudes to carry molecular constituents like proteins, lipids, RNA and DNA, etc. Moreover, exosomes are capable of recognizing antigens with adequate sensitivity and specificity. Many of these derivatives could trigger an immune modulation into the cells and act as an epigenetic inheritor response to target pathogens through RNAs. COIVID-19 resistance activated plasma-derived exosomes are either responsible for the effects of plasma beyond the contained immune antibodies or could be inhibitory. The proposed hypothesis suggests that preselecting the plasma-derived antibodies and RNAs merged exosomes would be an optimized therapeutic tactic for COVID-19 patients. We suggest that, the CPExo has a multi-potential effect for treatment efficacy by acting as immunotherapeutic, drug carrier, and diagnostic target with noncoding genetic materials as a biomarker.
Keywords: Exosomes, Convalescent plasma, Immunotherapy, COVID-19, Drug delivery, miRNAs, Diagnosis
Graphical abstract
1. Introduction
COVID –19, is identified as a major pandemic disease in the last 10 over the previous decades of global history which is a serious respiratory inflammation by the SARS-CoV-2 virus [1,2]. As of September 2020, more than 2.6 million confirmed cases of COVID-19 infections were recorded, which included 876,616 mortalities [3]. Several economists stated that this pandemic severely influenced the socioeconomic status of several countries and individuals as well. Plenty of choices are being explored as alternative medicines which included both traditional, allopathic medicines, and vaccines [4]. However, further improvements for controlling these infections are not identified yet. This hypothesis edged to improve COVID – 19 treatments with immunotherapeutic tactics. The history of proposed immunotherapy has been initiated before an era back; to the best of our knowledge in the year 1774, a Parisian physician tested and observed deterioration as the infection worsened by injecting pus into the leg of a patient with advanced breast cancer [5]. The major benefits of immunotherapy include the extended and long term survival rate, precise and target-oriented, more specific, wide adaptability, fewer side effects, and ability to replenish and rejuvenate the body's immune function. While the major afflictions include the negative regulation of immune checkpoint inhibitors, autoimmune diseases that lead to fatality, and hyper progressive disease which often leads to decreased survival rate in patients [6]. Further, immunotherapy combined with convalescent plasma has an efficient way to accelerate modern treatment especially this current pandemic disease also treated, and some of the cases were recovered as well. The mechanisms behind this convalescent plasma immunotherapy were identified as antibody-induced cellular cytotoxicity, phagocytosis activation, neutralizing viral load, and finally enhanced recovery rate [7], [8], (a). According to Cantor et al. [9a], the primary study revealed convalescent plasma therapeutic patients have effectively reduced organ failure than hydroxychloroquine and tocilizumab treatment and survival rate was significantly improved in the convalescent plasma treated patients. (see Table 1, Table 2 ).
Table 1.
Typical methods available for COVID-19 detection.
| Methods | Advantages | Disadvantages | Time for analysis |
|---|---|---|---|
| RT-PCR | Highly sensitive, RNA based detection | Expensive instrument, false negative results | 2 h |
| Loop-mediated isothermal amplification (LAMP) | High specificity and rapid | False-positive results | 30min |
| Immunoassays methods (e.g., ELISA) | Antibody-based detection method and high sensitivity. | Expensive antibody and instability of antibody | 2 h |
| Computed tomography (CT) | Rapid test | Nonspecific | Rapid |
| Next-generation sequencing (NGS) | Gene-based detection, able to understand the genome. | Require technical expertise and time-consuming | 1–2 weeks |
Table 2.
Examples of some repurposing antiviral.
| S. No | Drug | Structure | Target | Mode of Action | Experimental Model | Clinical trials | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Lopinavir-ritonavir | 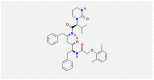 |
Protease enzyme | Prevent viral protein entry | In-silico modeling, In-vitro and humans | Phase 2 clinical trial NCT04372628 | [[45], [46], [47], [48], [49]] |
| Phase 2 clinical trial NCT04276688 | |||||||
| Phase 2 clinical trial-NCT04330690 | |||||||
| 2 | Darunavir | 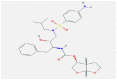 |
Protease enzyme | Prevent viral protein entry | In-silico modeling, In-vitro and humans | Phase 3 clinical trial NCT04252274 | [[50], [51], [52]] |
| Phase 3 clinical trial NCT04303299 | |||||||
| Phase 3 clinical trial NCT04425382 | |||||||
| 3 | Prulifloxacin | 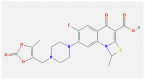 |
Proteases enzyme | Blocks active sites/Disturb viral protein dimer formation of viral protein | In-silico modeling | nonscientific trial | [53] |
| 4 | Tegobuvir | 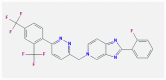 |
Proteases enzyme | Blocks active sites/Disturb viral protein dimer formation of viral protein | In-silico modeling | nonscientific trial | [[53], [54], [55]] |
| 5 | Nelfinavir |  |
Proteases enzyme | Blocks active sites/Disturb viral protein dimer formation of viral protein | In-silico modeling | nonscientific trial | [53,56,57] |
| 6 | Bictegravir |  |
Proteases enzyme | Blocks active sites/Disturb viral protein dimer formation of viral protein | In-silico modeling | nonscientific trial | [50,53] |
| 7 | Azithromycin |  |
Not conclusive (Change in endosomal pH), cytokines | Inhibits viral replication and IL-6 production | In-vitro (host cells), humans | NCT04381962- Phase 3 clinical trial | [[58], [59], [60]] |
| NCT04332107- Phase 3 clinical trial | |||||||
| NCT04334382- Phase 3 clinical trial | |||||||
| 8 | Doxycycline |  |
Cytokines | Inhibits viral replication and IL-6 production | Humans | NCT04371952- Phase 3 clinical trial | [58] |
| NCT04433078- Phase 2 clinical trial | |||||||
| IRCT20200418047121N1-Phase 3 clinical trial | |||||||
| 9 | Tocilizumab |  |
IL-6 receptor protein | Inhibits IL-6 release | Humans | NCT04356937- Phase 3 clinical trial | [52,61] |
| NCT04445272- Phase 2 clinical trial NCT04403685- Phase 2 clinical trial | |||||||
| 10 | Auranofin |  |
Viral RNA | inhibits viral RNA and Cytokines | In-vitro | nonscientific trial | [62] |
| 11 | Ruxolitinib |  |
Janus-kinase 1/2 | Inhibits cytokine storm | In silico modeling, humans | NCT04414098- Phase 2 clinical trial | [[62], [63], [64]] |
| NCT04338958- Phase 2 clinical trial | |||||||
| NCT04362137- Phase 3 clinical trial | |||||||
| 12 | Baricitinib |  |
Janus-kinase 1/2 | Inhibits cytokine storm | In silico, modeling humans | NCT04414098- Phase 2 clinical trial | [62,63,65] |
| NCT04338958- Phase 2 clinical trial | |||||||
| NCT04362137- Phase 3 clinical trial | |||||||
| 13 | Dexamethasone |  |
Inflammatory cells Inhibits | Inhibits release of cytokines | In silico modeling, humans | NCT04325061- Phase 4 clinical trial | [[66], [67], [68]] |
| NCT04395105- Phase 3 clinical trial | |||||||
| NCT04347980- Phase 3 clinical trial |
Nonetheless, COVID-19 treatment using convalescent plasma therapeutic mechanisms is still uncertain [9]. Hence, we outlined hypotheses for the actual mechanism behind the convalescent plasma therapeutic tactics. An Exosomes provide synergistic effect in drug delivery systems and or the spike protein (S protein) of SARS-CoV plays a pivotal role in viral infection and pathogenesis [[10], (a), (b)]. Mechanism of action behind this treatment to target monoclonal antibody to bind with spike protein and internalization of antibody mediated exosomes into endosome then membrane fusion to rely genetic materials for inhibits the viral transformation. It aids the extracellular signaling through non-coding RNAs and has played an integral role in the mechanism of antibody-mediated exosomes perhaps; it contains proteins derived after maternal cells.
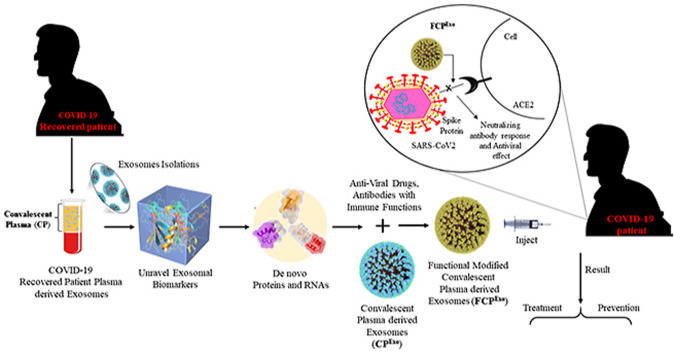
Furthermore, it has provided abundant functions such as membrane transport and fusion protein associated with multivesicular bodies (MVB) and heat shock proteins (HSP) intricate antigen staging [[11], (a), (b)]. We proposed a convalescent plasma-derived exosomes (CPExo) immunotherapeutic approach that could better deal with COVID-19 treatment (Fig. 1 ). This has been the best idea for improving the efficacy of targeted antibody binding and surface trans-membrane fusion in targeted cells.
Fig. 1.
Convalescent plasma-derived exosomes (CPExo) from COVID-19 recovered patients could provide immunotherapy for COVID-19.
Expected biomaterial for drug delivery in the biological system is based on the transfer membrane activity and translocation potential during treatment. Based on these characteristics, we recommended the appropriate biomaterial with size is about less than 100 nm like extracellular vesicles or exosomes. Plasma derived exosomal dimensions are between 60 and 100 nm and it has multi-potent features like optimistic size will be used for drug translocation, and inbound carrier of genetic materials would be best therapeutic biomarkers.
2. Convalescent plasma derived exosomes As A Covid-19 diagnostic tool
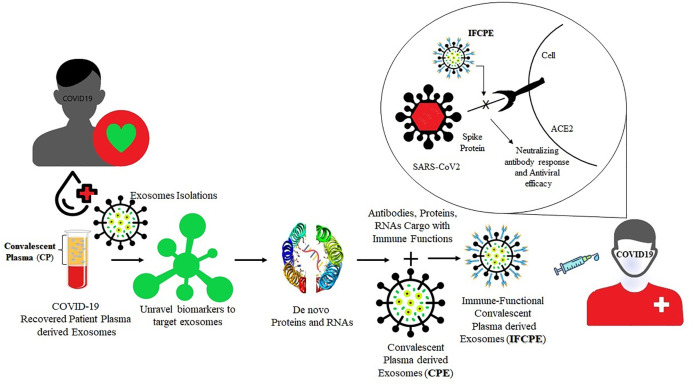
Various molecular techniques are under development or already accessible for the diagnosis and management of COVID-19 patients. Diagnostic testing of COVID-19 is crucial to guide the treatment, disease surveillance, contact tracing, and reopening of the economy. There are advantages and disadvantages for the currently available techniques used for the diagnosis. Presently, Real Time Polymerase Chain Reaction (RT-PCR) tests are considered the gold standard for identifying the presence of SARS-CoV2, as it directly tests to the presence of the virus RNA. Fig. 2 shows the number of techniques/methods available and their advantages and disadvantages [12] (see Fig. 3) (see Fig. 3).
Fig. 2.
Schematic representation showing the potential role of Convalescent plasma derived exosomes (CPExo) from COVID-19 recovered patients could provide novel biomarkers for COVID-19.
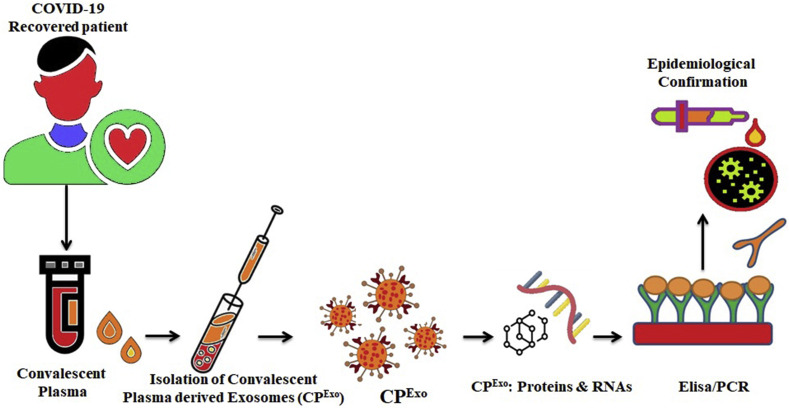
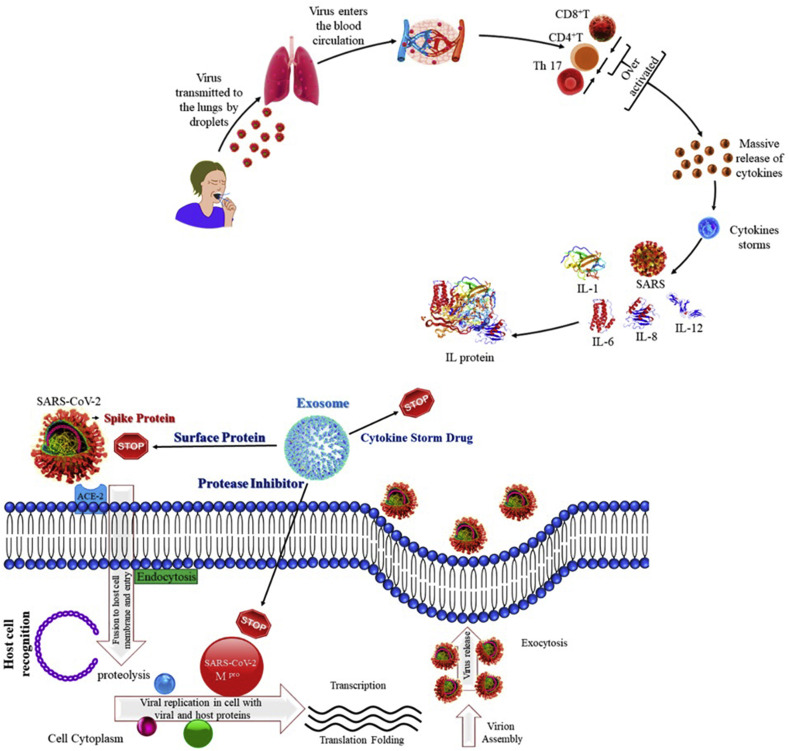
Fig. 3.
Schematic representation showing the potential role of Convalescent plasma-derived exosomes (CPExo) in combating COVID-19 Infection. Synergistic effect of the drug and exosomes may be utilized as an effective approach against the virus and cytokine storm.
SARS-CoV-2 infection can be grouped into three stages: stage 1- an asymptomatic period with or without detectable virus; stage 2-non-severe symptomatic period with the presence of virus; stage 3- severe symptomatic stage with high viral load. For about 50% of COVID-19 infected people only by day 7, the seroconversion takes place and for the rest of the patients by day 13–14 [[13], [14], [15], [16]]. It is reported that the several recovered SARS-CoV2 infected patients exhibited a positive viral RNA load as long as 10–27 days [15,17] and in some cases, it was observed for 37 days after discharge [[18], (a)]. Early screening and accurate diagnosis are undoubtedly an issue for patients with severe COVID-19 in reducing mortality and increasing the recovery rate. Even though there are several methods accessible for the detection of the virus, but these available diagnostic methods have their own limitations. For example, false-negative results occur in the RT-PCR test due to a low level of viral RNA. Currently available PCR-based methods cannot differentiate between the infected virus and the non-infectious nucleic acid of the same virus. Therefore, there is an immediate surge in the development of methods and platforms to diagnose the COVID-19. Recently, the scientific interest with regard to exosomes is immensely elevated for their feasible implications in clinical applications. The origin, number and delivery of circulating exosomes vary under physiological state, advocating their possible as a biomarker of disease. However, the role of exosomes as a diagnostic tool for COVID-19 patients are still scare [[18], (b)].
It is known that many viruses enter the extracellular double-membrane vesicle (EDMV) or exosome path during synthesis and intra-host spreading [19]. Exosomes are lipid-bilayer vesicles that are 30–120 nm in size and cooperate in the various pathological state [20]. Virus-infected cells deliver exosomes, which include viral-derived miRNAs and proteins and also receptors for viruses that allow recipient cells to virus entry [21]. It is reported that the viral particles can be seen within the double-membrane vesicles, where SARS-CoV is cultured in the AT2 cells [14,22]. Studies introduced the probability for the use of the exosomal pathway for transport of the COVID-19 virus [[23], [24], [25]]. The difference in composition of exosomes in healthy and infected patients and exosome stability, easy storage, easy isolation, make exosomes an exceptional biomarker for diagnosis [20]. Analysis of specific exosomal miRNA cargo content could serve as a biomarker to detect the virus from least or non-invasive biological samples of infected patients. The study of the exosomal cargo might give us pivotal data in respect of differential secretion of cargo in SARS-CoV infected cells as compared to uninfected cells. Particular proteins recognized within exosomes separated from COVID-19 -infected cells may serve as a crucial biomarker for the disease.
3. Exosomes in Covid-19 immunotherapy: hypothesis
The global catastrophe COVID -19 pandemic and the urgent need for effective treatment instigated to develop the COVID-19-convalescent plasma therapy (CCP) into viable therapy for patients under the critical stage of infection [26]. However, its clinical efficacy and safety are not fully evidenced but it could be projected to be a potentially effective option for existing prophylaxis of the disease. To date, no studies have focused on evaluating the role and planned participation of the exosomes in the treatment of COVID-19. Indeed, prior viral infections have been encountered using the convalescent plasma as a therapeutic approach [27]. Therefore, studies based on adopting convalescent plasma treatment for infectious diseases should be fortified and the significance of convalescent plasma-derived exosomal (CPExo) immunotherapy for COVID-19 should be investigated under ethical and controlled conditions.
3.1. Evaluation of the hypothesis
3.1.1. Immune cells derived exosomes in immunotherapy
Exosomes, a component of extracellular vesicles are reported as potential mediators in liquid biopsy and are involved in many augmentative cellular biochemical events including immunomodulation. Immune cells can communicate through extracellular vesicle (EV) secretion and uptake [28]. In the past 10 years, immuno-exosomes have been studied in correlation to surface oncology, metabolic reprogramming, autoimmune syndromes, and infectious disease. The immune system of the body is a natural defense mechanism to fight against invasive microbial infections. Recent literature suggested that the exosomal surface proteins are a much-desired target since they play a crucial role in cell-to-cell communications and are involved in immune modulations and cell signalling processes. The exosomes mediate various processes during infections with different types of microorganisms to promote immune responses. Further, the role of exosomal-immune activation has also been explored in the context of auto-immune disorders [29]. Immuno-exosomes are produced by the inward budding of sub-cellular endosomal membranes from immune effector cells [30]. They are delivered extracellularly from a wide range of cell types like dendritic cells (DCs), T and B cells, mast cells, platelets, NK cells, epithelial cells [31]. The extracellular vesicles from antigen-presenting cells (APCs) carry major histocompatibility complex, co-stimulatory molecules like CD54, CD80, and CD86 antigen-presenting cell and they have enriched in exosomal surface proteins tetraspanin like CD63 and CD81 [32,33]. The role of exosome biomarkers in vivo is not known, but it has been shown that exosomes, depending on donor cell origin, can function as a transport vesicle for the movable of wanted biomolecules, activate T cells, and transport antigen between APCs, and can be of importance in tolerance induction and in inhibiting antitumor responses by receptor-binding domain-specific Abs. Exosomes have also been investigated as cell derived tools in immunodominant for cancer-linked infections and transplantation [34]. Thus, we envisage that developing a potential exosomal therapy may exhibit broad-spectrum antiviral activity mediated through the modulation of the host immune responses.
3.1.2. COVID-19 convalescent plasma (CCP) therapy
Plasma is the colourless part of blood that does not contain red blood cells [35]. COVID-19 convalescent plasma (CCP) refers to solvent/detergent-treated plasma or cryo-supernatent antibodies (Abs) rich plasma collected from donors who have recovered from COVID-19 and have likely produced neutralizing antibodies (nAbs) to SARS-CoV-2 infection [36]. It is hypothesised that infusing plasma that has virus-specific antibodies will provide immediate transfer of passive immunity to the recipient and may improve their clinical course and outcomes by accelerating viral clearance and antibody-dependent killing of infected cells. Passive transfer of re-emergence immune protection with convalescent plasma (CP) implicates transfusing the sub-cellular portion of blood from individuals who have recovered from covid-infection to persons who are infected or at risk of infection and decreased viral loads. Immune-plasma donors are presumed to have developed an effective antibody response to the offending pathogen receptor-binding domain (RBD). The use of convalescent plasma (CP) as passive immunisation to treat viral contaminations is not novel [37]. Convalescent Plasma Therapy (CPT) decreased the mortality rate in severe influenza and related SARS-CoV as well. Moreover, it was a proven successful treatment model in the Middle East respiratory syndrome (MERS)-CoV [38] and ebola virus infection [39]. The disadvantage of this treatment-conferred immunity is the short term and mistarget receptor-binding domain (RBD).
4. Exosome As A repurposing antiviral drug delivery carrier
Exosomes have obtained a significant interest as a potential biomarker for drug delivery because it is less likely to be cytotoxic or immunogenic and it contains endogenous cellular components enabling them to overcome biological barriers. Also, the exosome lipid-bilayer may protect the drug from rapid blood clearance and may decrease the cytotoxicity related to off-target drug effects [40,41]. Exosomes are nanosized (30–120 nm) membrane vesicles secreted by all cell types and recognized for their cell-to-cell interactions [42]. Exosome's carry several biologically active molecules such as proteins and miRNA. Exosomes lipid-bilayer composition, high stability, and biocompatibility make them a perfect candidate for drug delivery carrier [41].
The interesting facts about exosomes have attained interest in analysing their potential role as therapeutic interventions in SARS-CoV virus infection. The therapeutic proteins or miRNA present in exosomes could support the depletion of cellular repair, inflammation, alveolar fluid clearance, and other damage triggered to the lung during virus infection [43]. A study by Sengupta et al. [44], reported that mesenchymal stem cell (MSCs) or - or medicinal signaling cells derived exosomes treatment in twenty-four COVID-19 patients resulted in significant improvement by enhancing immunity, down-regulating cytokine storm, and restoring oxygen storage capacity. Possibly, the use of plasma-derived exosomes from COVID-19 recovered patients as a drug carrier for antiviral drugs could prevent the cytokines storm elicited by the immune system. Plasma-derived exosomes could be one of the ideal and competitive nanocarriers for the clinical approved antiviral drugs including Lopinavir-ritonavir and Darunavir Prulifloxacin even with or without being modified.
5. Consequences of the hypothesis and discussion
From our point of view, considering the effects of the exosomes in the convalescent plasma needs to be done sooner rather than later. Recently, phase 2 novel therapeutic that is derived from the soluble and exosomes fraction of human amniotic fluid bionanoparticles as a safe and potentially efficacious therapeutic treatment for respiratory failure induced by COVID-19 infection. The past use of human amniotic products (i.e., membrane and fluid) has previously been FDA-approved as human cells, tissues, and cellular and tissue-based products for tissue injury; and has been used to reduce inflammation and fibrosis in patients with a variety of ailments. Given this, the investigators hypothesize that intravenously administered processed sterile filtered amniotic fluid will reduce inflammation in COVID-19 patients, and improve secondary clinical outcomes [69]. There is no phase 3 clinical trial Food and Drug Administration (FDA) approved and effective medicine for acute respiratory distress syndrome. Looking over prior knowledge of exosomes and especially their role in immune regulation, it seems that plasma during COVID-19 infection, and especially useful in those with the cytokine storm and acute respiratory distress syndrome may be augmentative as likely derived from the overblow over blow innate immune cell response of the macrophage family per Mac 1-type subsets stimulated by TNF-a of the immoderate innate immune response and consequently during still active infection by the viral Ag activated T helper cells 1 and T helper cells 17 derived cytokines like interferon-gamma (INF-g).
With disease evolution towards resolution and beyond, the biosynthesis of the extracellular vesicle response likely converts to reflect the healing positive aspect also in convalescent responses with more cohort from the curative and trophic M2-type macrophages making interleukin-4, interleukin −10, and transforming growth factor-beta (TGF-b), and perhaps later regulatory T helper 2 cells also making interleukin-10 and TGF-b, with interleukin −4, interleukin −13, and interleukin-25. Furthermore, the plasma-derived exosomes will promise the source of unraveling biomarkers. A more relevant example is that exosomes used as vaccines infused with the spike protein of the coronavirus pathogen of SARS pneumonia induce high levels of neutralizing antibodies (nAbs) [[70], (a), (b)].
Thus, convalescent plasma exosomes (CPExo) should be clinical pivotal cell therapy in Covid-19 induced acute respiratory distress syndrome (ARDS) patients. Therefore, it is postulated that activated exosomes from immune stimulated regulatory and suppressor T cells and M2-type macrophages may make a very significant administration to the helpful side-effects of convalescent plasma therapy (CPT). This would be beside and beyond the effects of remaining immunoglobulin M antibodies of the primary immune response and the later developed crucial higher affinity immunoglobulin G anti-COVID 19 antibodies of the acquired T cell-mediated secondary B cell response. In fact, there may be an effective antigen-specific antibody actually on the surface of the immune cells derived exosomes from blood plasma.
The use of COVID-19 convalescent plasma (CCP) for its content of acquired immune antibodies that must consider the role in this therapy of billions of unraveling exosomes in the plasma. Many of these derive from activated immune-modulating cells and likely transfer exosomal cellular RNAs like tRNA, rRNA, mRNA, miRNA, snRNAs, functional ncRNA), and lncRNA that acting epigenetically to also influence the recipient response to the virus. These immune activated plasma exosomes may either be responsible for positive effects of the plasma beyond the contained immune antibodies or could be inhibitory. Pre-selection of plasma with the best neutralizing antibodies (nAbs) and the immune (cell) derived exosomes mimetics would produce the most optimum therapy for very severely affected COVID-19 patients also we would say that exosomal RNA therapeutics will take their place as a viable future COVID-19 drug discovery platform. Exosomes are used to translocate miRNA into viruses and inhibit mRNA gene expressions. It has provided direct detection of viral transformation and controls their transcription. Moreover, miRNAs are acting as regulating factors for posttranscriptional processes by regulating mRNA splicing. By utilizing the exosomal derived snRNAs to target the promoter region of mRNA genes which is responsible for viral transformation. Exosomes are the best biomaterials for theragnostics strategy, which could provide remarkable efficacy for drug delivery, especially critical infectious diseases like COVID-19. In nature, exosomes are carriers of congenital cargos like RNAs, would be the best tool for extracellular signaling and will be great advantages for disease biomarkers identification. As an innate substance of exosome, may tolerate primary immune response such as phagocytosis.
Author statement
Krishnan Anand: Supervision, Conceptualization, Writing – original draft, The supervision, conceptualization, writing- original draft preparation of using convalescent plasma derived exosomal (CPExo) for COVID-19 therapeutic and diagnostic was conceived by Krishnan Anand, manuscript validation, Chithravel Vadivalagan: Funding acquisition, apart from providing funding. Chithravel Vadivalagan provided valuable suggestions and insights that helped strengthen the scientific content, Jitcy Saji Joseph: Validation, manuscript validation, Sachin Kumar Singh: Validation, manuscript validation with help of Anand Krishnan, Sachin Kumar Singh, Monica Gulati: Writing – review & editing, editing the manuscript, Mohd Shahbaaz: helped drawing the schematic diagram, Magda H. Abdellattif: helped drawing the schematic diagram, Parteek Prasher: Writing – review & editing, editing the manuscript, Gaurav Gupta: Writing – review & editing, reviewing the manuscript, Dinesh Kumar Chellappan: Writing – review & editing, reviewing the manuscript, Kamal Dua: Software, helped software validation of manuscript preparation.
Ethics approval
Not applicable.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The author K.A. is grateful to National Research Foundation (NRF), SA for the research funding in the form of NRF/DSI Innovation Post-Doctoral Research Fellowship (grant no. 120677) and The author M. H. A is very thankful for Taif University researcher supporting project Number TURSP/91, Taif University, Taif, Saudi Arabia.
References
- 1.Jeyanathan M., Afkhami S., Smaill F., Miller M.S., Lichty B.D., Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020;20:615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta M., Prasher P., Sharma M., Shastri M.D., Khurana N., Vyas M., Dureja H., Gupta G., Anand K., Satija S. Advanced drug delivery systems can assist in targeting coronavirus disease (COVID-19): a hypothesis. Med. Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.110254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Organization W.H. World Heal. Organ. Geneva; Switzerland: 2020. WHO Coronavirus Disease (COVID-19) Dashboard.https//Covid19.Who.Int/ Accessed. 5. [Google Scholar]
- 4.Mirzaie A., Halaji M., Dehkordi F.S., Ranjbar R., Noorbazargan H. A narrative literature review on traditional medicine options for treatment of corona virus disease 2019 (COVID-19), Complement. Ther. Clin. Pract. 2020 doi: 10.1016/j.ctcp.2020.101214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis I.D. An overview of cancer immunotherapy. Immunol. Cell Biol. 2000;78:179–195. doi: 10.1046/j.1440-1711.2000.00906.x. [DOI] [PubMed] [Google Scholar]
- 6.Tan S., Li D., Zhu X. Cancer immunotherapy: pros, cons and beyond, biomed. Pharma. 2020;124 doi: 10.1016/j.biopha.2020.109821. [DOI] [PubMed] [Google Scholar]
- 7.Van Erp E.A., Luytjes W., Ferwerda G., Van Kasteren P.B. Fc-mediated antibody effector functions during respiratory syncytial virus infection and disease. Front. Immunol. 2019;10:548. doi: 10.3389/fimmu.2019.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Gunn B.M., Yu W.-H., Karim M.M., Brannan J.M., Herbert A.S., Wec A.Z., Halfmann P.J., Fusco M.L., Schendel S.L., Gangavarapu K. A role for Fc function in therapeutic monoclonal antibody-mediated protection against Ebola virus. Cell Host Microbe. 2018;24:221–233. doi: 10.1016/j.chom.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wibmer CK., Ayres F, Hermanus T, Madzivhandila M, Kgagudi P, Oosthuysen B, Lambson BE, de Oliveira T, Vermeulen M, van der Berg K, Rossouw T, Boswell M, Ueckermann V, Meiring S, von Gottberg A, Cohen C, Morris L, Bhiman JN, Moore PL. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat. Med. 2021;27(4):622–625. doi: 10.1038/s41591-021-01285-x. [DOI] [PubMed] [Google Scholar]
- 9.(a) Cantore I., Valente P. Convalescent plasma from COVID 19 patients enhances intensive care unit survival rate. A preliminary report. Transfus. Apher. Sci. 2020 Oct 1;59(5) doi: 10.1016/j.transci.2020.102848. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yiğenoğlu T.N., Hacıbekiroğlu T., Berber İ., Dal M.S., Baştürk A., Namdaroğlu S., Korkmaz S., Ulas T., Dal T., Erkurt M.A. Convalescent plasma therapy in patients with COVID‐19. J. Clin. Apher. 2020;35:367–373. doi: 10.1002/jca.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Salvatori G., Luberto L., Maffei M., Aurisicchio L., Roscilli G., Palombo F., Marra E. SARS-CoV-2 SPIKE PROTEIN: an optimal immunological target for vaccines. J. Transl. Med. 2020;18(1) doi: 10.1186/s12967-020-02392-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lee E., Sandgren K., Duette G., Stylianou V.V., Khanna R., Eden J.S., Blyth E., Gottlieb D., Cunningham A.L., Palmer S. Identification of SARS-CoV-2 nucleocapsid and spike T-cell epitopes for assessing T-cell immunity. J. Virol. 2021 doi: 10.1128/JVI.02002-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Futter C.E., White I.J. Annexins and endocytosis. Traffic. 2007;8:951–958. doi: 10.1111/j.1600-0854.2007.00590.x. [DOI] [PubMed] [Google Scholar]; (b) Gastpar R., Gehrmann M., Bausero M.A., Asea A., Gross C., Schroeder J.A., Multhoff G. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Canc. Res. 2005;65:5238–5247. doi: 10.1158/0008-5472.CAN-04-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rabiee N., Bagherzadeh M., Ghasemi A., Zare H., Ahmadi S., Fatahi Y., Dinarvand R., Rabiee M., Ramakrishna S., Shokouhimehr M. Point-of-use rapid detection of sars-cov-2: nanotechnology-enabled solutions for the COVID-19 pandemic. Int. J. Mol. Sci. 2020;21:5126. doi: 10.3390/ijms21145126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., Bucci E., Piacentini M., Ippolito G., Melino G. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mason R.J. 2020. Pathogenesis of COVID-19 from a Cell Biology Perspective. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korber B., Fischer WM., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., Foley B., Giorgi EE., Bhattacharya T., Parker MD., Partridge DG, Evans CM, Freeman TM, de Silva TI, on behalf of the Sheffield COVID-19 Genomics Group, LaBranche CC, Montefiori DC. Sheffield COVID-19 Genomics Group, CC LaBranche, DC Montefiori. Spike mutation pipeline reveals the emergence of a more transmissible form of SARS-CoV-2. BioRxiv. 2020 [Google Scholar]
- 16.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brünink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 17.Ye G., Pan Z., Pan Y., Deng Q., Chen L., Li J., Li Y., Wang X. Clinical characteristics of severe acute respiratory syndrome coronavirus 2 reactivation. J. Infect. 2020;80:e14–e17. doi: 10.1016/j.jinf.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Marta Z., Patrizia A., Jeness C., Leonardo S., Silvia S.B. Exosomes in cardiovascular diseases. Diagnostics. 2020;10(11):943. doi: 10.3390/diagnostics10110943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Badierah R.A., Uversky V.N., Redwan E.M. Dancing with Trojan horses: an interplay between the extracellular vesicles and viruses. J. Biomol. Struct. Dyn. 2020:1–27. doi: 10.1080/07391102.2020.1756409. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y., Liu Y., Liu H., Tang W.H. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19. doi: 10.1186/s13578-019-0282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassanpour M., Rezaie J., Nouri M., Panahi Y. The role of extracellular vesicles in COVID-19 virus infection. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2020;85 doi: 10.1016/j.meegid.2020.104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian Z., Travanty E.A., Oko L., Edeen K., Berglund A., Wang J., Ito Y., V Holmes K., Mason R.J. Innate immune response of human alveolar type II cells infected with severe acute respiratory syndrome-coronavirus. Am. J. Respir. Cell Mol. Biol. 2013;48:742–748. doi: 10.1165/rcmb.2012-0339OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farkash E.A., Wilson A.M., Jentzen J.M. Ultrastructural evidence for direct renal infection with SARS-CoV-2. J. Am. Soc. Nephrol. 2020;31:1683–1687. doi: 10.1681/ASN.2020040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su H., Yang M., Wan C., Yi L.-X., Tang F., Zhu H.-Y., Yi F., Yang H.-C., Fogo A.B., Nie X., Zhang C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knoops K., Kikkert M., van den Worm S.H.E., Zevenhoven-Dobbe J.C., van der Meer Y., Koster A.J., Mommaas A.M., Snijder E.J. SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLoS Biol. 2008;6:e226. doi: 10.1371/journal.pbio.0060226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Berg K., Vermeulen M., Glatt T.N., Wasserman S., Barrett C.L., Peter J., Brittain D., Louw V.J. COVID-19: convalescent plasma as a potential therapy. SAMJ South African Med. J. 2020;110:1–2. [PubMed] [Google Scholar]
- 27.Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect. Dis. 2020;20:398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan W., Jiang S. Immune cell-derived exosomes in the cancer-immunity cycle. Trends in Cancer. 2020:506–517. doi: 10.1016/j.trecan.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Chitra R., Kb H. The origin and functions of exosomes in cancer. Front. Oncol. 2018;8 doi: 10.3389/fonc.2018.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu R., Gao W., Yao K., Ge J. Roles of exosomes derived from immune cells in cardiovascular diseases. Front. Immunol. 2019;10:648. doi: 10.3389/fimmu.2019.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Admyre C., Bohle B., Johansson S.M., Focke-Tejkl M., Valenta R., Scheynius A., Gabrielsson S. B cell–derived exosomes can present allergen peptides and activate allergen-specific T cells to proliferate and produce TH2-like cytokines. J. Allergy Clin. Immunol. 2007;120:1418–1424. doi: 10.1016/j.jaci.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 32.Anand K., Khan F.I., Singh T., Elumalai P., Balakumar C., Premnath D., Lai D., Chuturgoon A.A., Saravanan M. Green synthesis, experimental and theoretical studies to discover novel binders of exosomal tetraspanin CD81 protein. ACS Omega. 2020;5:17973–17982. doi: 10.1021/acsomega.0c01166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai P., Chen X., Guo L., Wang Y., Liu X., Liu Y., Zhou T., Huang T., Geng S., Luo C. A potent immunomodulatory role of exosomes derived from mesenchymal stromal cells in preventing cGVHD. J. Hematol. Oncol. 2018;11:1–15. doi: 10.1186/s13045-018-0680-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao Q., Zuo B., Lu Z., Gao X., You A., Wu C., Du Z., Yin H. Tumor‐derived exosomes elicit tumor suppression in murine hepatocellular carcinoma models and humans in vitro. Hepatology. 2016;64:456–472. doi: 10.1002/hep.28549. [DOI] [PubMed] [Google Scholar]
- 35.Arabi Y.M., Hajeer A.H., Luke T., Raviprakash K., Balkhy H., Johani S., Al-Dawood A., Al-Qahtani S., Al-Omari A., Al-Hameed F. Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia. Emerg. Infect. Dis. 2016;22:1554. doi: 10.3201/eid2209.151164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rojas M., Rodríguez Y., Monsalve D.M., Acosta-Ampudia Y., Camacho B., Gallo J.E., Rojas-Villarraga A., Ramírez-Santana C., Díaz-Coronado J.C., Manrique R. Convalescent plasma in Covid-19: possible mechanisms of action. Autoimmun. Rev. 2020 doi: 10.1016/j.autrev.2020.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marano G., Vaglio S., Pupella S., Facco G., Catalano L., Liumbruno G.M., Grazzini G. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus. 2016;14:152–157. doi: 10.2450/2015.0131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hung I.F.N., To K.K.W., Lee C.-K., Lee K.-L., Chan K., Yan W.-W., Liu R., Watt C.-L., Chan W.-M., Lai K.-Y. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin. Infect. Dis. 2011;52:447–456. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Griensven J., De Weiggheleire A., Delamou A., Smith P.G., Edwards T., Vandekerckhove P., Bah E.I., Colebunders R., Herve I., Lazaygues C., Haba N., Lynen L. The use of ebola convalescent plasma to treat ebola virus disease in resource-constrained settings: a perspective from the field. Clin. Infect. Dis. an Off. Publ. Infect. Dis. Soc. Am. 2016;62:69–74. doi: 10.1093/cid/civ680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin K., Wang S., Zhao R.C. Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomark. Res. 2019;7:8. doi: 10.1186/s40364-019-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarvar D.P., Shamsasenjan K., Akbarzadehlaleh P. Mesenchymal stem cell-derived exosomes: new opportunity in cell-free therapy. Adv. Pharmaceut. Bull. 2016;6:293. doi: 10.15171/apb.2016.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou Y., Kosaka N., Xiao Z., Ochiya T. Exosomes. Elsevier; 2020. MSC-exosomes in regenerative medicine; pp. 433–465. [Google Scholar]
- 43.Gupta S., Krishnakumar V., Sharma Y., Dinda A.K., Mohanty S. Mesenchymal stem cell derived exosomes: a nano platform for therapeutics and drug delivery in combating COVID-19. Stem Cell Rev. Reports. 2020:1–11. doi: 10.1007/s12015-020-10002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sengupta V., Sengupta S., Lazo A., Woods P., Nolan A., Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cell. Dev. 2020;29(12):747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu X., Wang X.-J. Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicines. J. Genet. Genomics. 2020;47:119–121. doi: 10.1016/j.jgg.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choy K.-T., Wong A.Y.-L., Kaewpreedee P., Sia S.-F., Chen D., Hui K.P.Y., Chu D.K.W., Chan M.C.W., Cheung P.P.-H., Huang X. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir. Res. 2020 doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu K., Cai H., Shen Y., Ni Q., Chen Y., Hu S., Li J., Wang H., Yu L., Huang H. Management of corona virus disease-19 (COVID-19): the Zhejiang experience. J. Zhejiang Univ. Med. Sci. 2020;49 doi: 10.3785/j.issn.1008-9292.2020.02.02. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lim J., Jeon S., Shin H.-Y., Kim M.J., Seong Y.M., Lee W.J., Choe K.-W., Kang Y.M., Lee B., Park S.-J. Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J. Kor. Med. Sci. 2020;35 doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ortega J.T., Serrano M.L., Pujol F.H., Rangel H.R. Unrevealing sequence and structural features of novel coronavirus using in silico approaches: the main protease as molecular target. EXCLI J. 2020;19:400. doi: 10.17179/excli2020-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan R.J., Jha R.K., Amera G.M., Jain M., Singh E., Pathak A., Singh R.P., Muthukumaran J., Singh A.K. Targeting SARS-CoV-2: a systematic drug repurposing approach to identify promising inhibitors against 3C-like proteinase and 2’-O-ribose methyltransferase. J. Biomol. Struct. Dyn. 2020:1–14. doi: 10.1080/07391102.2020.1753577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Meyer S., Bojkova D., Cinatl J., Van Damme E., Buyck C., Van Loock M., Woodfall B., Ciesek S. Lack of antiviral activity of darunavir against SARS-CoV-2. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020;97:7–10. doi: 10.1016/j.ijid.2020.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nicastri E., Petrosillo N., Ascoli Bartoli T., Lepore L., Mondi A., Palmieri F., D'Offizi G., Marchioni L., Murachelli S., Ippolito G., Antinori A. National institute for the infectious diseases “L. Spallanzani,” IRCCS. Recommendations for COVID-19 clinical management. Infect. Dis. Rep. 2020;12:8543. doi: 10.4081/idr.2020.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Y., Zhang J., Wang N., Li H., Shi Y., Guo G., Liu K., Zeng H., Zou Q. 2020. Therapeutic Drugs Targeting 2019-nCoV Main Protease by High-Throughput Screening. [DOI] [Google Scholar]
- 54.Ruan Z., Liu C., Guo Y., He Z., Huang X., Jia X., Yang T. SARS-CoV-2 and SARS-CoV: virtual screening of potential inhibitors targeting RNA-dependent RNA polymerase activity (NSP12) J. Med. Virol. 2020 doi: 10.1002/jmv.26222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Encinar J.A., Menendez J.A. Potential drugs targeting early innate immune evasion of SARS-coronavirus 2 via 2’-O-methylation of viral RNA. Viruses. 2020;12 doi: 10.3390/v12050525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohashi H., Watashi K., Saso W., Shionoya K., Iwanami S., Hirokawa T., Shirai T., Kanaya S., Ito Y., Kim K.S., Nishioka K., Ando S., Ejima K., Koizumi Y., Tanaka T., Aoki S., Kuramochi K., Suzuki T., Maenaka K., Matano T., Muramatsu M., Saijo M., Aihara K., Iwami S., Takeda M., McKeating J.A., Wakita T. Multidrug treatment with nelfinavir and cepharanthine against COVID-19. BioRxiv. 2020:2020. doi: 10.1101/2020.04.14.039925. 04.14.039925. [DOI] [Google Scholar]
- 57.Musarrat F., Chouljenko V., Dahal A., Nabi R., Chouljenko T., Jois S.D., Kousoulas K.G. The anti-HIV drug nelfinavir mesylate (Viracept) is a potent inhibitor of cell fusion caused by the SARSCoV-2 spike (S) glycoprotein warranting further evaluation as an antiviral against COVID-19 infections. J. Med. Virol. 2020;92:2087–2095. doi: 10.1002/jmv.25985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sargiacomo C., Sotgia F., Lisanti M.P. COVID-19 and chronological aging: senolytics and other anti-aging drugs for the treatment or prevention of corona virus infection? Aging (Albany. NY) 2020;12:6511–6517. doi: 10.18632/aging.103001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poschet J.F., Perkett E.A., Timmins G.S., Deretic V. Azithromycin and ciprofloxacin have a chloroquine-like effect on respiratory epithelial cells. BioRxiv. 2020:2020. doi: 10.1101/2020.03.29.008631. 03.29.008631. [DOI] [Google Scholar]
- 60.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Tissot Dupont H., Honoré S., Colson P., Chabrière E., La Scola B., Rolain J.-M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bersanelli M. Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy. 2020;12:269–273. doi: 10.2217/imt-2020-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., Zhang X., Pan A., Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. U.S.A. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rothan H.A., Stone S., Natekar J., Kumari P., Arora K., Kumar M. The FDA-approved gold drug auranofin inhibits novel coronavirus (SARS-COV-2) replication and attenuates inflammation in human cells. Virology. 2020;547:7–11. doi: 10.1016/j.virol.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.La Rosée F., Bremer H.C., Gehrke I., Kehr A., Hochhaus A., Birndt S., Fellhauer M., Henkes M., Kumle B., Russo S.G., La Rosée P. The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19 with severe systemic hyperinflammation. Leukemia. 2020;34:1805–1815. doi: 10.1038/s41375-020-0891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marinho E.M., Batista de Andrade Neto J., Silva J., Rocha da Silva C., Cavalcanti B.C., Marinho E.S., Nobre Júnior H.V. Virtual screening based on molecular docking of possible inhibitors of Covid-19 main protease. Microb. Pathog. 2020;148 doi: 10.1016/j.micpath.2020.104365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cao Y., Wei J., Zou L., Jiang T., Wang G., Chen L., Huang L., Meng F., Huang L., Wang N., Zhou X., Luo H., Mao Z., Chen X., Xie J., Liu J., Cheng H., Zhao J., Huang G., Wang W., Zhou J. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J. Allergy Clin. Immunol. 2020;146:137–146. doi: 10.1016/j.jaci.2020.05.019. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cantini F., Niccoli L., Nannini C., Matarrese D., Di Natale M.E., Lotti P., Aquilini D., Landini G., Cimolato B., Di Pietro M.A., Trezzi M., Stobbione P., Frausini G., Navarra A., Nicastri E., Sotgiu G., Goletti D. Beneficial impact of Baricitinib in COVID-19 moderate pneumonia; multicentre study. J. Infect. 2020;81:647–679. doi: 10.1016/j.jinf.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khan S.U., Htar T. the. Computational Molecular Modelling Approach; 2020. Deciphering the Binding Mechanism of Dexamethasone against SARS-CoV-2 Main Protease. [DOI] [Google Scholar]
- 69.Maria I., Michael M., Bellio A., Sagel Anthony, Saylor Marie, Kapp William, VanOsdol Kathryn, Haskell Gwendolyn, Stewart Danique, Abdullah Zanub, Santos Ivan, Milberg Julian, Arango Alissa, Mitrani Albert, Shapiro George C. Case report: administration of amniotic fluid-derived nanoparticles in three severely ill COVID-19 patients. Front. Med. 2021;8 doi: 10.3389/fmed.2021.583842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.(a) Kuate S., Cinatl J., Doerr H.W., Überla K. Exosomal vaccines containing the S protein of the SARS coronavirus induce high levels of neutralizing antibodies. Virology. 2007;362:26–37. doi: 10.1016/j.virol.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Askenase P.W. COVID‐19 therapy with mesenchymal stromal cells (MSC) and convalescent plasma must consider exosome involvement: do the exosomes in convalescent plasma antagonize the weak immune antibodies? J. Extracell. Vesicles. 2020;10 doi: 10.1002/jev2.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]