Abstract
Background
The clinical features and outcomes of mechanically ventilated patients with COVID-19 infection who develop a pneumothorax has not been rigorously described or compared to those who do not develop a pneumothorax.
Purpose
To determine the incidence, clinical characteristics, and outcomes of critically ill patients with COVID-19 infection who developed pneumothorax. In addition, we compared the clinical characteristics and outcomes of mechanically ventilated patients who developed a pneumothorax with those who did not develop a pneumothorax.
Methods
This study was a multicenter retrospective analysis of all adult critically ill patients with COVID-19 infection who were admitted to intensive care units in 4 tertiary care centers in the United States.
Results
A total of 842 critically ill patients with COVID-19 infection were analyzed, out of which 594 (71%) were mechanically ventilated. The overall incidence of pneumothorax was 85/842 (10%), and 80/594 (13%) in those who were mechanically ventilated. As compared to mechanically ventilated patients in the non-pneumothorax group, mechanically ventilated patients in the pneumothorax group had worse respiratory parameters at the time of intubation (mean PaO2:FiO2 ratio 105 vs 150, P<0.001 and static respiratory system compliance: 30ml/cmH2O vs 39ml/cmH2O, P = 0.01) and significantly higher in-hospital mortality (63% vs 49%, P = 0.04).
Conclusion
The overall incidence of pneumothorax in mechanically ventilated patients with COVID-19 infection was 13%. Mechanically ventilated patients with COVID-19 infection who developed pneumothorax had worse gas exchange and respiratory mechanics at the time of intubation and had a higher mortality compared to those who did not develop pneumothorax.
Keywords: Pneumothorax, Pneumomediastinum, Barotrauma, 2, SARS-CoV-2, Coronavirus disease 2019, COVID-19, Incidence, Mortality
1. Introduction
Pneumothorax is a common complication of mechanical ventilation during critical illness [1], and is an independent risk factor for mortality in this setting [[2], [3], [4]]. Pneumothorax is known to complicate diffuse pulmonary infections such as Pneumocystis jirovecii pneumonia and is associated with increased mortality in patients with Human Immunodeficiency Virus infection [3,5]. Pneumothorax and other forms of barotrauma are common in patients with acute respiratory failure caused by two coronaviruses: Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS) [6,7]. Currently, the Coronavirus Disease 2019 (COVID-19) pandemic has been a major cause of respiratory failure and death worldwide. Several case reports and series have described the occurrence of pneumothorax and pneumomediastinum in critically ill patients with COVID-19 pneumonia [[8], [9], [10], [11], [12], [13]]. Although a few recent studies have described the clinical features and outcomes of COVID-19 patients who developed barotrauma, including pneumothorax [14,15], the clinical features and outcomes of these patients have not been systematically compared to mechanically ventilated patients with COVID-19 who did not develop pneumothorax. In this multicenter analysis, we described the incidence, clinical characteristics, and outcomes of critically ill patients with COVID-19 who developed pneumothorax during their intensive care unit (ICU) stay. We also compared the clinical characteristics and outcomes of mechanically ventilated patients who developed a pneumothorax with those of mechanically ventilated patients who did not develop a pneumothorax.
Materials and Methods: This study was a retrospective analysis of all adult critically ill patients with COVID-19 who were admitted to the ICU in 4 US tertiary care centers (Albany Medical Center, Albany, NY; Stony Brook University Hospital, Stony Brook, NY; Westchester Medical Center, Valhalla, NY and Froedtert Hospital, Milwaukee, WI) between the dates of March 1st and July 31st, 2020. This study was approved by the Institutional Review Board of the respective institutions. Patients with COVID-19 were identified by using institutional databases, and patients with pneumothorax were identified by ICD-10 billing Code (J93.9 or J93.83) or comprehensive chart review depending on the institution. COVID-19 infection was diagnosed via real-time reverse transcription-polymerase chain reaction from a nasopharyngeal swab. Patients who developed an iatrogenic pneumothorax (e.g., from central venous catheter insertion) were excluded from the pneumothorax group and were included in the non-pneumothorax group. We compared the clinical characteristics and outcomes of mechanically ventilated patients who developed pneumothorax (cases) with those who did not develop pneumothorax (controls). We selected control cases randomly from the cohort of mechanically ventilated patients without pneumothorax in a 1:2 (case: control) ratio by using a random number generator.
Patient data collected included: a) Demographics: age, sex, race/ethnicity; b) Comorbidities: coronary arterial disease, chronic pulmonary disease, diabetes mellitus, hypertension and cancer; c) Admission laboratory parameters: ferritin, procalcitonin, C-reactive protein (CRP), and D-dimer levels; d) Level of respiratory support: high flow nasal canula, non-invasive positive pressure ventilation, invasive mechanical ventilation and extracorporeal membrane oxygenation (ECMO); e) Radiographic findings of first pneumothorax: laterality of pneumothorax, presence of tension pneumothorax and presence of pneumomediastinum; g) Respiratory parameters before occurrence of pneumothorax: Pao2/Fio2 (PF) ratio, positive end expiratory pressure (PEEP), plateau pressure, peak pressure if plateau pressure was not available, driving pressure (plateau pressure minus PEEP), tidal volume and tidal volume per kilogram of ideal body weight, FIO2, static respiratory compliance (tidal volume/driving pressure) or dynamic respiratory compliance (tidal volume/(Peak pressure-PEEP); h) COVID-19 treatment received: Remdesivir, therapeutic anti-coagulation, corticosteroids, and convalescent plasma; and i) Outcome: in-hospital mortality, ventilator-free days at day 28, ICU-free days at day 28 and hospital-free days at day 28.
We performed analyses to determine a) the incidence of pneumothorax in critically ill patients with COVID-19 infection; b) the clinical characteristics and outcomes of critically ill patients with COVID-19 infection who developed pneumothorax; and c) the in-hospital mortality, ventilator-free days at day 28, ICU-free days at day 28 and hospital-free days at day 28 of mechanically ventilated patients with COVID-19 infection who developed a pneumothorax compared with those of mechanically ventilated patients who did not develop a pneumothorax.
1.1. Statistical analysis
Continuous variables were represented as mean, median and standard deviation. Statistical inference for continuous variables was done by Mann-Whitney non-parametric test with significance accepted at p<0.05. Categorical data were presented as frequencies and percentages with inference by Pearson's chi-square test or Fisher's exact test if the expected value in any cell was less than five. Kaplan-Meier Survival curves were plotted to compare the in-hospital survival of COVID-19 patients with and without pneumothorax. Log rank test was used to assess the difference between survival curves and chi-square test was used to calculate difference of survival between two groups at 120 days. Analysis was performed using Minitab (v.19.2020.1) and R (v.3.6.1) statistical software.
2. Results
A total of 842 ICU patients with COVID-19 infection were admitted to the ICU at the 4 participating institutions during the study period. Of these, 594 (71%) required mechanical ventilation. A total of 85/842 (10%) patients developed a pneumothorax, out of which 2 patients developed a pneumothorax after a central line insertion. After excluding these 2 iatrogenic pneumothoraxes, the overall incidence of pneumothorax in critically ill patients was 83/842 (10%) and in mechanically ventilated patients it was 80/594 (13%). The vast majority of patients who developed a pneumothorax were on mechanical ventilation (80/83, 96%). Of the three patients who developed a pneumothorax without mechanical ventilation, one patient was receiving non-invasive positive pressure ventilation, one patient was on high flow oxygen therapy, and one patient was receiving ECMO without mechanical ventilation. Remainder of the manuscript exclusively focuses on mechanically ventilated patients.
2.1. Clinical characteristics of mechanically ventilated patients with pneumothorax
The baseline clinical characteristics of mechanically ventilated patients who developed a pneumothorax are shown in Table 1 . The mean age of the cohort was 58 ± 16 years, and 74% of the patients were male. Chronic pulmonary disease was present in 11% of patients. Most patients (80%) received systemic corticosteroids for the treatment of COVID-19 pneumonia, which was the most commonly administered pharmacotherapy.
Table 1.
Clinical characteristics of mechanically ventilated patients with pneumothorax (N = 80).
| Variables | Values |
|---|---|
| Age-Year; Mean (SD) | 58 (16) |
| Sex- N (%) | |
| Male | 59 (74%) |
| Female | 21 (26%) |
| Ethnicities- N* (%) | |
| White | 32 (51%) |
| Black | 5 (8%) |
| Hispanic | 23 (36%) |
| Asian | 3 (5%) |
| Comorbidities- N (%) | |
| ≥1 comorbidity | 44 (55%) |
| Chronic pulmonary Disease | 9 (11%) |
| Diabetes Mellitus | 24 (30%) |
| Coronary Artery Disease | 6 (8%) |
| Hypertension | 23 (29%) |
| Cancer | 1 (1%) |
| Laboratory Values at admission; Mean (SD) | |
| Ferritin (ng/mL) | 2259 (2543) |
| C-reactive protein (mg/dL) | 19 (13) |
| D-Dimer (mg/L) | 6 (12) |
| Procalcitonin (ng/ml) | 1.3 (1.9) |
| Treatment received N (%) | |
| Corticosteroid | 64 (80%) |
| Convalescent Plasma | 30 (38%) |
| Therapeutic anti-Coagulation | 30 (38%) |
* Ethinicity was missing in 17 patients.
In the 80 mechanically ventilated COVID-19 patients who developed pneumothorax, the majority (60/80, 75%) of the patients had one episode of pneumothorax, however 20 patients had more than one pneumothorax. Out of these 20 patients with multiple pneumothoraces, 17 were contralateral and 3 were ipsilateral compared to the initial pneumothorax. In terms of the initial pneumothorax of each patient, the majority were right-sided (40/80, 50%), followed by left-sided (23/80, 29%) and bilateral (17/80, 21%). Clinical evidence of a tension pneumothorax was seen in 26/80 (32%) cases. Concurrent pneumomediastinum was seen in 24/80 (30%) of cases. The majority 71/80 (89%) of the patients received tube thoracostomy for the management of their pneumothorax, whereas the remainder 9/80 (11%) did not undergo any intervention.
In the 80 patients who were mechanically ventilated and developed a pneumothorax, the pneumothorax occurred at a mean of 10 ± 12 days after initiation of mechanical ventilation. Table 2 describes the ventilator parameters recorded prior to detection of a pneumothorax. The PaO2/FiO2 (PF) was reduced (Mean = 117 ± 77), and static respiratory system compliance was low (Median = 24 ± 15 ml/cmH2O). The mean tidal volume (TV) per kilogram (kg) of ideal body weight (IBW) recorded before the occurrence of pneumothorax was higher than the 6 ml/kg recommended for ARDS patients (Mean = 6.9 ± 1.2) [16]. Mean plateau pressure was 29 ± 7 cmH2O and driving pressure was 18 ± 6 cmH2O.
Table 2.
Respiratory parameters at the time of occurrence of first pneumothorax in mechanically ventilated patients (N=80).
| Ventilation information | Value |
|---|---|
| Respiratory support- N (%) | |
| Mechanical ventilation | 80 (100%) |
| ECMO | 6 (7%) |
| Ventilator parameters; Mean (SD) | |
| Pao2/FIO2 | 117 (77) |
| Tidal volume | 451 (74) |
| TV/Kg of IBW | 6.9 (1.2) |
| FiO2 | 83 (22) |
| PEEP | 11 (4) |
| Peak pressure cm H2O (N = 59) | 34 (7) |
| Plateau pressure cm H2O (N = 18) | 29 (7) |
| Driving pressure cm H2O (N = 18) | 18 (6) |
| Static Compliance ml/cm H2O (N = 18) | 24 (15) |
| Dynamic Compliance ml/cm H2O (N = 59) | 23 (11) |
| Blood Gas; Median (SD) | |
| PH | 7.3 (0.1) |
| PaO2 | 87 (73) |
| PCO2 | 61 (23) |
ECMO: Extracorporeal membrane oxygenation; CPAP: continuous positive airway pressure; HFNC: high flow nasal canula; Pao2: Partial Pressure of oxygen; FIO2: Fraction of Inspired Oxygen; TV: Tidal Volume; IBW: Ideal body weight; PEEP: Positive end-expiratory pressure; PCO2: Partial Pressure of Carbon Dioxide.
2.2. parison of mechanically ventilated patients with pneumothorax and without pneumothorax (case and control)
There were 80 mechanically ventilated COVID-19 patients who developed pneumothorax. We randomly selected 160 mechanically ventilated COVID-19 patients without pneumothorax to serve as controls. The baseline characteristics of the two groups are listed in Table 3 . There was no significant difference in age, gender and COVID-specific treatment received between two groups.
Table 3.
Clinical characteristics of mechanically ventilated patients with pneumothorax and without pneumothorax group.
| Variables | Pneumothorax (N) = 80 | Non- Pneumothorax (N) = 160 | P value* |
|---|---|---|---|
| Age-Year; Mean (SD) | 58 (16) | 61 (16) | 0.08 |
| Sex- N (%) | 59 (74%) | 101 (63%) | 0.10 |
| Male | |||
| Female | 21 (26%) | 59 (37%) | |
| Ethnicities- N*** (%) | 0.002** | ||
| White | 32 (51%) | 54 (45%) | |
| Black | 5 (8%) | 34 (29%) | |
| Hispanic | 23 (36%) | 22 (18%) | |
| Asian | 3 (5%) | 9 (8%) | |
| BMI Mean (SD) | 30 (8) | 31 (9) | 0.50 |
| Comorbidities- N (%) | |||
| One Comorbidity at least | 44 (55%) | 106 (66%) | 0.12 |
| Chronic pulmonary Disease | 9 (11%) | 26 (16%) | 0.40 |
| Diabetes Mellitus | 24 (30%) | 72 (45%) | 0.04 |
| Coronary Artery Disease | 6 (7.5%) | 28 (17.5%) | 0.04 |
| Hypertension | 23 (28.8%) | 39 (24.4%) | 0.50 |
| Cancer | 1 (1.3%) | 7 (4.4%) | 0.27** |
| Treatment- N (%) | |||
| Corticosteroid | 64 (80%) | 115 (71.9%) | 0.23 |
| Convalescent Plasma | 30 (37.5%) | 52 (32.5%) | 0.53 |
| Therapeutic anti-Coagulation | 30 (37.5%) | 59 (36.9%) | >0.99 |
* continuous variables p value is from Mann-Whitney test; categorical variables p value from chi-square or Fisher's exact test (**) if any cell expected values is less than five.
*** Ethnicity was not reported in 17 patients in pneumothorax group and 41 patients in non-pneumothorax group.
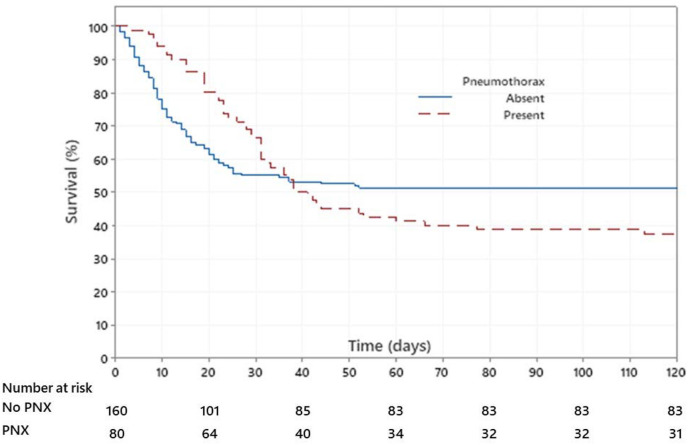
Fig. 1 displays the Kaplan-Meier Survival curve of in-hospital survival of COVID-19 patients with pneumothorax and without pneumothorax. In this analysis, we assumed that those patients who were discharged were alive at 120 days after the hospital admission. Survival curves are not statistically different (log rank test; P = 0.55), although survival at 120 days was statistically higher in non-pneumothorax group as compared to pneumothorax group (chi-square test; p = 0.04). Overall, mechanically ventilated patients with pneumothorax had significantly higher in-hospital mortality as compared to patients without pneumothorax (63% vs 49%, P = 0.04). Odds of in-hospital death were increased nearly two-fold (OR 1.75; CI: 1.01 to 3.03) in those who had a pneumothorax.
Fig. 1.
Kaplan-Meir Survival curve of in-hospital survival of patients with COVID-19 infection. In this analysis, we assumed that those patients who were discharged were alive at 120 days after the hospital admission. Red dotted curve shows in-hospital survival of patients with pneumothorax and blue curve represent in-hospital survival of patients without pneumothorax. Survival curves are not statistically different (log rank test; P = 0.55), although survival at 120 days was statistically higher in non-pneumothorax group as compared to pneumothorax group (chi-square test; p = 0.04). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
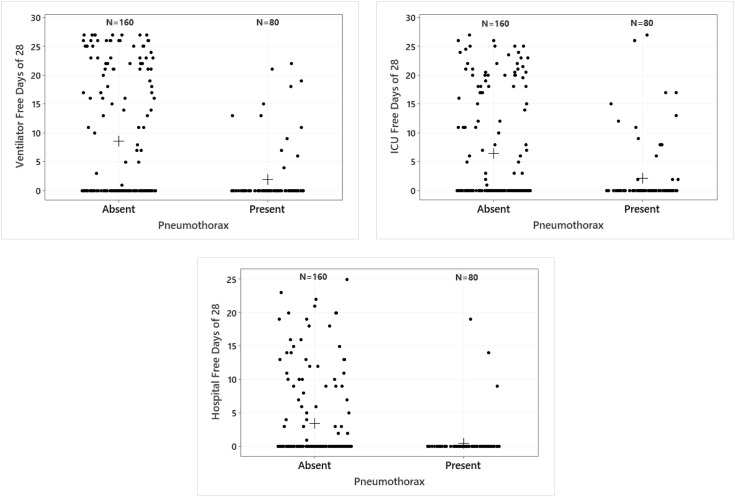
Mechanically ventilated patients who developed a pneumothorax had fewer mean ventilator-free days at day 28 (2 vs 9, P<0.001), ICU free days at day 28 (2 vs 6, P<0.001) and hospital free days at day 28 (1 vs 3, P<0.001) (Fig. 2 and Table 4 ).
Fig. 2.
a-2c Comparison of ventilator, ICU, and hospital free days at day 28. Dots are individual patients and crosses are the means. Medians are zero for both pneumothorax and non-pneumothorax group. Ventilator (2a), ICU (2b) and hospital (2c) free days at day 28, are higher for those without a pneumothorax as compared to those with pneumothorax (Mann Whitney test; p<0.001).
Table 4.
Outcome of mechanically ventilated patients with pneumothorax and without pneumothorax.
| Outcome | Pneumothorax (N) = 80 | Non-Pneumothorax (N) = 160 | P value |
|---|---|---|---|
| Mortality N (%) | 50 (62.5%) | 78 (48.8%) | 0.04 |
| Number of days on ventilator; Mean, SD | 31 (23) | 12 (13) | <0.001 |
| Number of ICU Days; Mean, SD | 31 (22) | 15 (13) | <0.001 |
| Hospital Length of Stay; Mean, SD | 42 (28) | 21 (17) | <0.001 |
| 28 -ventilator free days; Mean, SD | 2 (5) | 9 (11) | <0.001 |
| 28 -ICU free days; Mean, SD | 2 (6) | 6 (9) | <0.001 |
| 28 -Hospital free days; Mean, SD | 1 (3) | 3 (6) | <0.001 |
ICU: Intensive care unit * mortality p value from chi-square test; others from Mann-Whitney test
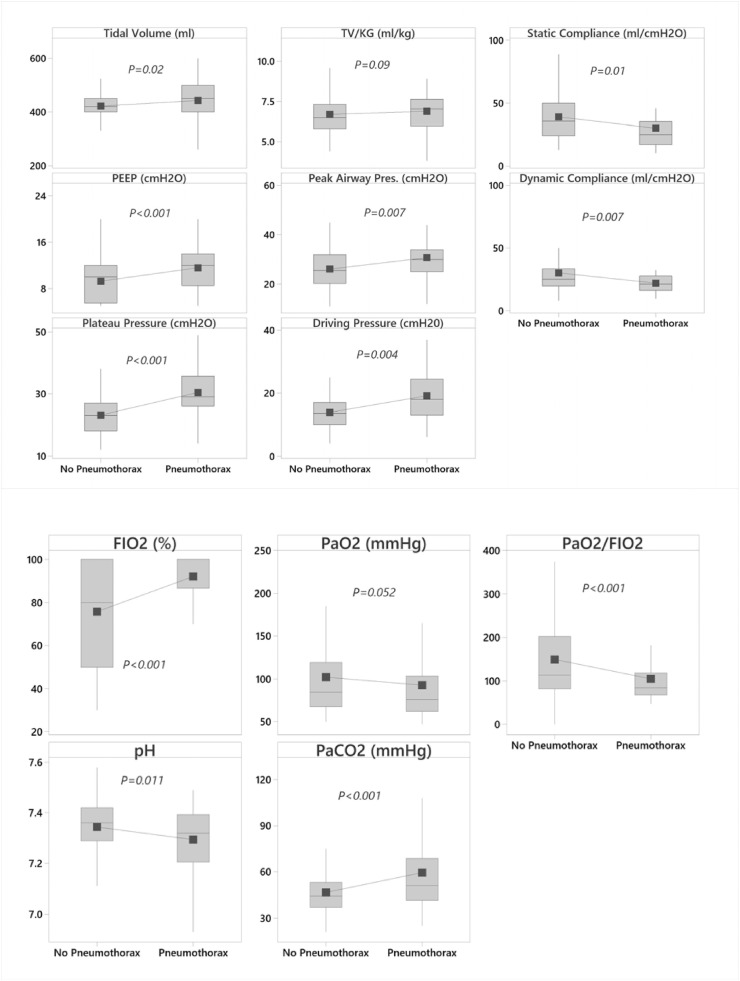
Patients in the pneumothorax group had inferior gas exchange and respiratory system mechanics compared to the non-pneumothorax group as measured by the mean PF ratio (105 vs 150, P<0.001), static respiratory system compliance (30 vs 39 ml/cm H2O, P = 0.01), plateau pressure (30 vs 23 cm H20, P<0.001), driving pressure (19 vs 14 cm H20, P = 0.004) and peak pressure (31 vs 26 cm H2O,P = 0.007) measured at the time of intubation (Fig. 3 ). There was trend towards use of higher tidal volume per Kg of IBW in patients with pneumothorax as compared to non-pneumothorax (6.9 vs 6.7 ml/kg of IBW, p = 0.09).
Fig. 3.
Whisker plot comparing the respiratory parameters (Ventilator parameters-3a and blood gas values -3b) of pneumothorax and non-pneumothorax at the time of intubation. Grey shaded rectangular box is the interquartile range (IQR, the 25th to the 75th percentile), the horizontal line within the box is the median, the solid square symbol is the mean.
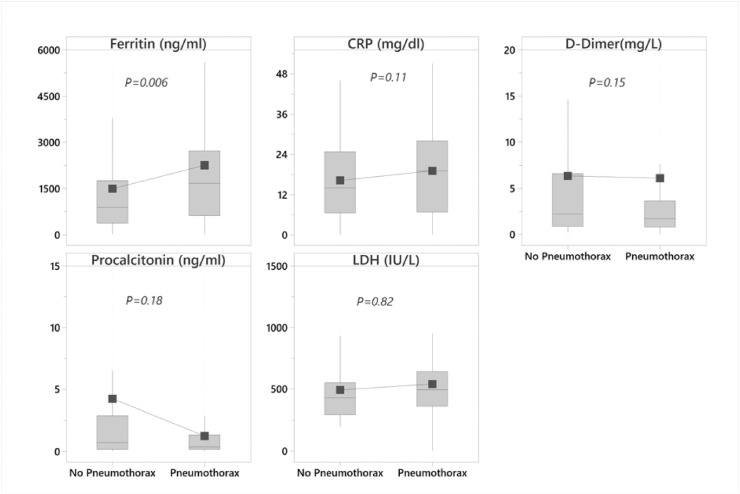
Patients in pneumothorax group have significantly higher mean Ferritin levels at the time of admission as compared to those without pneumothorax (2259 vs 1496 ng/ml, P = 0.006) (Fig. 4 ). There was no significant difference in the level of other inflammatory markers at the time of admission.
Fig. 4.
Whisker plot comparing the laboratory values of pneumothorax and non-pneumothorax at the time of admission. Grey shaded rectangular box is the interquartile range (IQR, the 25th to the 75th percentile), the horizontal line within the box is the median, the solid square symbol is the mean.
3. Discussion
In our analysis of 842 critically ill patients with COVID-19, the incidence of pneumothorax was 10%. Pneumothoraces were almost exclusively found in patients who were mechanically ventilated where the rate of this complication was over 13%. Mechanically ventilated patients who developed a pneumothorax as compared to mechanically ventilated patients without a pneumothorax had greater severity of lung disease as reflected by poorer oxygenation, lower respiratory system compliance, and higher plateau pressure at the time of intubation. Mechanically ventilated patients with a pneumothorax had a higher in-hospital mortality than mechanically ventilated patients who did not develop a pneumothorax (63% vs 49%, P = 0.04).
The present study is the largest to date describing the incidence, patient characteristics, and outcomes of pneumothorax complicating critical illness due to COVID-19 infection. Earlier small studies from China reported an incidence of pneumothorax of approximately 1% in all patients infected with COVID-19 [17,18]. A large multicenter study involving 71,904 COVID-19 patients evaluated across 61 emergency departments (ED) in Spain reported an overall pneumothorax incidence at presentation of 0.56% [19]. In a subsequent study from the United States, the incidence of barotrauma (presence of pneumothorax or pneumomediastinum) was found to be 15% in mechanically ventilated patients with COVID-19 infection [9], which is similar to what was observed in our analysis. Previous reports in patients with severe acute respiratory distress syndrome (SARS) coronavirus infection found that the overall incidence of pneumothorax was 1.7% (6/356) [7] and was higher 12% (5/41) in those who were mechanically ventilated.
There are limited published data describing the outcomes of patients with COVID-19 who developed pneumothorax. Recently, a large series from the United Kingdom reported 60 cases of pneumothorax and 11 cases of pneumomediastinum in all patients with COVID-19 infection. Overall in-hospital mortality was 37% as opposed to 63% in our cohort [14] However, there is concern for selection bias in the UK study as cases were collected based on the authors’ recall and collaboration via internet media platforms such as Twitter. Moreover, fewer than half (44%) of the patients in that study were mechanically ventilated and one third of the patients were not intubated. A recent study compared the outcome of non-intubated patients with COVID-19 infection, who presented to the Emergency Department with a pneumothorax to those without a pneumothorax. Patients with pneumothorax were found to have a 4-fold increase in risk of death as compared to patients who did not have pneumothorax [19]. In an analysis based on thoracic imaging, McGuiness and colleagues identified 89 patients with barotrauma among 601 mechanically ventilated patients with COVID-19 infection [15] for an overall barotrauma incidence of 15%. Among the 89 patients who sustained barotrauma, there were 62 occurrences of a pneumothorax and 45 occurrences of a pneumomediastinum. In that study, barotrauma was associated with increased risk of death (OR = 2.2, P = 0.03). Similar to the McGuiness study, we found the odds of death to be 1.8 times higher in mechanically ventilated patients who developed pneumothorax as compared to those who did not.
It is likely that pneumothorax is a marker of more severe COVID-19 induced lung disease. This conjecture is supported by our findings that patients with pneumothorax had inferior gas exchange and respiratory system compliance than their counterparts without pneumothorax. Recent radiological studies have shown that COVID-19 infection is associated with architectural distortion of lung parenchyma with cyst formation [21,22], which may predispose the lung to the development of pneumothorax. An additional cause of pneumothorax in patients COVID-19 infection likely occurs because of barotrauma induced from excessive positive pressure ventilation imposed on a lung that is already structurally vulnerable. This is a known occurrence in mechanically ventilated patients with other forms of ARDS. Support for this cause of pneumothorax in our cohort includes that patients who developed pneumothoraces were also receiving higher-than-recommended TV at the time of intubation as well as before the occurrence of pneumothorax. Such iatrogenic lung injury from suboptimal ventilator management may have played a role in the development of pneumothorax in some patients.
Our study had several limitations. First, neither COVID-19 management nor ventilator management were standardized among the 4 participating centers. Second, the radiographic data were not analyzed in detail. Third, this is a retrospective study, so we were not able to record ventilator parameters in real time. Finally, the COVID-19 pandemic appears to have changed significantly over time in terms of available therapy and outcomes [23,24]. It is possible that our results, including the severity of lung disease and mortality, cannot be extrapolated to future critically ill COVID-19 infected patients. Nevertheless, we suspect that our comparison between patients with and without a pneumothorax will remain valid.
In summary, we found that 13% of critically ill patients with COVID-19 infection who were mechanically ventilated developed a pneumothorax. Patients who developed pneumothorax had evidence of severe lung injury with poor gas exchange and low respiratory system compliance. Those who developed pneumothorax appear to have recieved higher tidal volumes per kilogram of IBW as compared to those who did not develop a pneumothorax and these tidal volumes were above those recommended for ARDS. Hospital length of stay and mortality was higher in those who developed pneumothorax compared to those who did not. These results suggest that the development of a pneumothorax in a mechanically ventilated COVID-19 infected patient is a poor prognostic sign. Although we did not establish causation, these results also suggest that strict attention to accepted ventilatory strategies for ARDS may be important in minimizing the likelihood of barotrauma and poor outcome in these patients.
4. Future directions
There is a need for a prospective study examining the development of pneumothorax in mechanically ventilated patients with COVID-19 pneumonia. With careful attention to clinical, physiological and radiographic parameters, such a study may improve our ability to identify mechanically ventilated COVID-19 patients at high of development of a pneumothorax. Furthermore, such a study may provide insights on how to optimally manage mechanical ventilation in these patients.
Funding
None.
CRediT authorship contribution statement
Amit Chopra: Writing – original draft, is the guarantor of the paper and takes responsibility for the integrity of the work, from inception to published article, All authors contributed to the writing of the manuscript. Ali Hani Al-Tarbsheh: All authors contributed to the writing of the manuscript. Nidhi J. Shah: Writing – original draft, were involved in data collection, All authors contributed to the writing of the manuscript, AHA: were involved in data collection, All authors contributed to the writing of the manuscript. Hamid Yaqoob: Writing – original draft, were involved in data collection, All authors contributed to the writing of the manuscript. Kurt Hu: Writing – original draft, were involved in data collection, All authors contributed to the writing of the manuscript. Paul J. Feustel: Writing – original draft, was involved in performing the statistical analysis, All authors contributed to the writing of the manuscript. Ronaldo Ortiz-Pacheco: Writing – original draft, were involved in data collection, All authors contributed to the writing of the manuscript. Kinner M. Patel: Writing – original draft, were involved in data collection, All authors contributed to the writing of the manuscript. Jozef Oweis: Writing – original draft, were involved in data collection, All authors contributed to the writing of the manuscript. Natalya Kozlova: were involved in data collection, All authors contributed to the writing of the manuscript. Spyridon Zouridis: Writing – original draft, were involved in data collection, All authors contributed to the writing of the manuscript. Sahar Ahmad: All authors contributed to the writing of the manuscript. Oleg Epelbaum: All authors contributed to the writing of the manuscript. Woon H. Chong: Writing – original draft, were involved in data collection, All authors contributed to the writing of the manuscript. John T. Huggins: All authors contributed to the writing of the manuscript. Biplab K. Saha: All authors contributed to the writing of the manuscript. Edward Conuel: All authors contributed to the writing of the manuscript. Hau Chieng: Writing – original draft, were involved in data collection, All authors contributed to the writing of the manuscript. Jeanette Mullins: Writing – original draft, were involved in data collection, All authors contributed to the writing of the manuscript. Divyansh Bajaj: Writing – original draft, were involved in data collection, All authors contributed to the writing of the manuscript.
Declaration of competing interest
MAJ: received institution grant support from Mallinckrodt pharmaceuticals. JH: Consultant/Advisory Boards: IBIOS [IPF]; Roche/Genentech [IPF (Nintedanib)]; Boehringer Ingelheim [IPF (Pirfenidone)]. PJF: Scientific advisor with shares in Penrose TherapeuTx, LLC. The remaining authors have no disclosures or any potential conflicts of interest.
References
- 1.Yarmus L., Feller-Kopman D. Pneumothorax in the critically ill patient. Chest. 2012;141(4):1098–1105. doi: 10.1378/chest.11-1691. [DOI] [PubMed] [Google Scholar]
- 2.Esteban A., Anzueto A., Frutos F., et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. J. Am. Med. Assoc. 2002;287(3):345–355. doi: 10.1001/jama.287.3.345. [DOI] [PubMed] [Google Scholar]
- 3.Bedos J.P., Dumoulin J.L., Gachot B., et al. Pneumocystis carinii pneumonia requiring intensive care management: survival and prognostic study in 110 patients with human immunodeficiency virus. Crit. Care Med. 1999;27(6):1109–1115. doi: 10.1097/00003246-199906000-00030. [DOI] [PubMed] [Google Scholar]
- 4.Gattinoni L., Bombino M., Pelosi P., et al. Lung structure and function in different stages of severe adult respiratory distress syndrome. J. Am. Med. Assoc. 1994;271(22):1772–1779. [PubMed] [Google Scholar]
- 5.Rivero A., Perez-Camacho I., Lozano F., et al. Etiology of spontaneous pneumothorax in 105 HIV-infected patients without highly active antiretroviral therapy. Eur. J. Radiol. 2009;71(2):264–268. doi: 10.1016/j.ejrad.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Das K.M., Lee E.Y., Al Jawder S.E., et al. Acute Middle East respiratory syndrome coronavirus: temporal lung changes observed on the chest radiographs of 55 patients. AJR Am. J. Roentgenol. 2015;205(3):W267–W274. doi: 10.2214/AJR.15.14445. [DOI] [PubMed] [Google Scholar]
- 7.Sihoe A.D., Wong R.H., Lee A.T., et al. Severe acute respiratory syndrome complicated by spontaneous pneumothorax. Chest. 2004;125(6):2345–2351. doi: 10.1378/chest.125.6.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.do Lago V.C., Cezare T.J., Fortaleza C., Okoshi M.P., Baldi B.G., Tanni S.E. Does COVID-19 increase the risk for spontaneous pneumothorax? Am. J. Med. Sci. 2020;360(6):735–737. doi: 10.1016/j.amjms.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flower L., Carter J.L., Rosales Lopez J., Henry A.M. Tension pneumothorax in a patient with COVID-19. BMJ Case Rep. 2020;13(5) doi: 10.1136/bcr-2020-235861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janssen M.L., van Manen M.J.G., Cretier S.E., Braunstahl G.J. Pneumothorax in patients with prior or current COVID-19 pneumonia. Respir Med Case Rep. 2020;31 doi: 10.1016/j.rmcr.2020.101187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong N., Gao C., Xu M.S., Xie Y.L., Zhou C.Y. Spontaneous pneumomediastinum in an elderly COVID-19 patient: a case report. World J Clin Cases. 2020;8(16):3573–3577. doi: 10.12998/wjcc.v8.i16.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manna S., Maron S.Z., Cedillo M.A., et al. Spontaneous subcutaneous emphysema and pneumomediastinum in non-intubated patients with COVID-19. Clin. Imag. 2020;67:207–213. doi: 10.1016/j.clinimag.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou C., Gao C., Xie Y., Xu M. COVID-19 with spontaneous pneumomediastinum. Lancet Infect. Dis. 2020;20(4):510. doi: 10.1016/S1473-3099(20)30156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinelli A.W., Ingle T., Newman J., et al. COVID-19 and pneumothorax: a multicentre retrospective case series. Eur. Respir. J. 2020;56(5) doi: 10.1183/13993003.02697-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGuinness G., Zhan C., Rosenberg N., et al. Increased incidence of barotrauma in patients with COVID-19 on invasive mechanical ventilation. Radiology. 2020;297(2):E252–E262. doi: 10.1148/radiol.2020202352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan E., Del Sorbo L., Goligher E.C., et al. An official American thoracic society/European society of intensive care medicine/society of critical care medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2017;195(9):1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 17.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang F., Shi S., Zhu J., Shi J., Dai K., Chen X. Analysis of 92 deceased patients with COVID-19. J. Med. Virol. 2020;92(11):2511–2515. doi: 10.1002/jmv.25891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miro O., Llorens P., Jimenez S., et al. Frequency, risk factors, clinical characteristics and outcomes of spontaneous pneumothorax in patients with Covid-19: a case-control, emergency medicine-based multicenter study. Chest. 2020;159(3):1241–1255. doi: 10.1016/j.chest.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong M., Yang H., Li X., Shen J., Xu X., Lv D. Evolution of chest CT manifestations of COVID-19: a longitudinal study. J. Thorac. Dis. 2020;12(9):4892–4907. doi: 10.21037/jtd-20-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu K., Zeng Y., Xie P., et al. COVID-19 with cystic features on computed tomography: a case report. Medicine. 2020;99(18) doi: 10.1097/MD.0000000000020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhimraj A., Morgan R.L., Shumaker A.H., et al. Clinical Infectious Diseases : an Official Publication of the Infectious Diseases Society of America. 2020. Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dyer O. Covid-19: Remdesivir has little or no impact on survival, WHO trial shows. BMJ. 2020;371 doi: 10.1136/bmj.m4057. [DOI] [PubMed] [Google Scholar]