Abstract
Background
Over 66 million people worldwide have been diagnosed with COVID-19. Therefore, understanding their clinical evolution beyond hospital discharge is essential not only from an individual standpoint, but from a populational level.
Objectives
Our primary aim was to assess the impact of COVID-19 on health-related quality of life (HRQoL) 3 months after hospital discharge. Additionally, we screened for anxiety and depression and assessed important clinical outcomes.
Methods
This was a single-center cohort study performed in Sao Paulo (Brazil), in which participants were contacted by telephone to answer a short survey. EQ-5D-3L was used to assess HRQoL and clinical data from patients’ index admission were retrieved from medical records.
Results
We contacted 251 participants (59.8% males, mean age 53 years old), 69.7% of which had presented with severe COVID-19. At 3 months of follow-up, 6 patients had died, 51 (20.3%) had visited the emergency department again and 17 (6.8%) had been readmitted to hospital. Seventy patients (27.9%) persisted with increased dyspnoea and 81 had a positive screening for anxiety/depression. Similarly, patients reported an overall worsening of EQ-5D-3L single summary index at 3 months compared to before the onset of COVID-19 symptoms (0.8012 (0.7368 – 1.0) vs. 1.0(0.7368 – 1.0), p < 0.001). This affected all 5 domains, but especially pain/discomfort and anxiety/depression. Only female sex and intensive care requirement were independently associated with worsening of HRQoL.
Conclusion
Patients hospitalized for COVID-19 frequently face persistent clinical and mental health problems up to 3 months following hospital discharge, with significant impact on patients’ HRQoL.
Keywords: COVID-19, Quality of life, Critical care
1. Introduction
The coronavirus disease 19 (COVID-19), caused by the recently discovered beta coronavirus SARS-CoV-2 [1], can present with a wide range of symptoms. While a significant proportion of infected patients are believed to remain asymptomatic, most develop flu-like symptoms such as fever, rhinorrhea, cough or dyspnoea. Those most severely affected by the disease may progress to a hyperinflammatory and hypercoagulable state resulting in a myriad of life-threatening complications, such as acute respiratory distress syndrome, deep vein thrombosis and pulmonary embolism, stroke, acute coronary syndrome, and acute kidney failure, among others [2, 3]. Since the first cases were reported in China in December 2019, the disease has spread to virtually every country, with over 66 million people being infected and 1,524,994 deaths worldwide. Brazil, in particular, has been severely hit by the pandemic, having registered over 6,5 million COVID-19 cases and 175,964 casualties as of December 5th, 2020 (https://coronavirus.jhu.edu/map.html).
Thanks to an unparalleled collective effort of the medical and scientific communities, today we have gathered extensive information and managed to build up solid knowledge on risk factors, clinical presentation, diagnosis and treatment of the acute disease. However, the long-term outcomes of COVID-19 survivors are less well understood. Given that the pandemic affected such a tremendous number of patients, understanding their clinical evolution beyond hospital discharge is essential not only from an individual standpoint, but from a populational level.
A few small studies carried out in the US, China, and Europe have reported impaired mental health and health-related quality of life, as well as persistent respiratory symptoms up to 60 days post-discharge [[4], [5], [6], [7]]. However, while communicating important data, these studies still provide limited insight onto the potential long-term impacts of COVID-19 on healthcare systems, either because they fail to provide objective assessments or because comparisons are made with the general population and not with the patient's baseline symptoms and performance status.
Having said that, the primary aims of this study were to assess the impact of COVID-19 on health-related quality of life 3 months after hospital discharge and to investigate individual characteristics and disease severity markers associated with worsening in health-related quality of life at 3 months after discharge in adults admitted for COVID-19 to a secondary hospital. Secondarily, we sought to screen for anxiety and depressive symptoms and assess important clinical outcomes (mortality, readmission, dyspnoea intensity and need for home oxygen supplementation or dialysis) over this 3-month period follow-up.
2. Methods
2.1. Study design and population
This was a single-center cohort study carried out at Hospital Municipal Dr. Moysés Deutsch (Sao Paulo, Brazil) from March to August 2020. Hospital Municipal Dr. Moysés Deutsch is a public university hospital located at one of the most underprivileged neighborhoods in Sao Paulo, the largest city in Brazil. Under normal conditions, this hospital is the only reference for secondary medical care for almost 1 million people. In April 2020, with escalating numbers of COVID-19 cases in Sao Paulo, it was turned into one of the largest reference centers for COVID-19 in the city, having treated over 3500 patients until October 2020.
In the beginning of the pandemic, a few of the frontline attending physicians were relocated to different non-clinical posts because they were considered to belong to high-risk groups. In this context, some were given the task to contact all discharged patients by telephone at 1 month and 3 months following hospital discharge in order to assist with continuous improvement of institutional guidelines. A few months later, we received ethical approval by the Institutional Review Board (CAAE 36201020.3.0000.0086) to use the resulting dataset for the purpose of this study.
Eligible patients were adults (≥18 years-old) admitted between March 16th and May 8th, 2020 with microbiological confirmation of SARS-CoV-2 infection by RT-PCR of nasal/pharyngeal swabs or tracheal aspirate, and who survived to hospital discharge. Since too many patients had missed the one-month follow-up call, we decided to use only the data regarding the endpoint of 3 months.
Given the time gap between the beginning of data collection by the institutional team and the IRB approval for using this data for research purposes, many patients were not consented at the time they answered the telephone interview. Therefore, the researchers attempted to contact all eligible patients by telephone once again in order to explain the aims of the study, read our informed consent and ask whether they would be willing to participate.
Consent was given verbally over the phone and recorded by the investigators. When patients were unable to consent due to cognitive or physical impairment, or eventually because they had died following hospital discharge, their caretakers and proxies were authorized to respond on the patients’ behalf. A waiver of consent was granted for those who could not be reached by the investigators despite multiple attempts, but patients who declined to participate were excluded from the study.
2.2. There was no sample size calculation
2.2.1. Instruments and data collection
Participants (patients or caretakers) were required to answer a short survey which included patient demographic characteristics (age, gender, self-reported race and level of education), important clinical outcomes (mortality, readmissions, residual dyspnoea severity, need for home oxygen supplementation and haemodyalisis), healthcare support following discharge (scheduled outpatient visits and rehabilitation programs), assessment of health-related quality of life and screening for anxiety and depressive symptoms.
Health-related quality of life was assessed by the 3-level version of the EQ-5D questionnaire (EQ-5D-3L), which comprises 5 dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. The participant describes the severity levels of each dimension in a categorical scale without arithmetic properties from 1 to 3, where 1 indicates no problem, 2 indicates some problem and 3 indicates extreme problem. Therefore, 243 different qualitative combinations of health statuses are possible. Each combination can be translated into a single summary index value that ranges from −0.1755 (worse than dead) to 1.000 (perfect HRQL) and it is weighted according to Brazilian values and preferences regarding health outcomes [8]. Following formal request to the EuroQol Group, the researchers were provided with two versions of the EQ-5D-3L scale translated to Portuguese and validated in the Brazilian population [8]: one dedicated to phone interviews with patients and the other specifically for interviews with patients' proxies, which were applied as suitable. Patients and caretakers were first asked to answer their versions of the EQ-5D-3L scale considering the patients' perceived health status at 3 months following discharge. Then, they were asked to answer the same questionnaire considering patients’ perceived health status immediately before the onset of COVID-19 symptoms.
Dyspnoea severity was classified according to the Modified Medical Research Council Scale (mMRC). Based on this scale, patients were asked to describe the intensity of dyspnoea not only on the day of the phone interview, but also how it was before the onset of COVID-19 symptoms. Similarly, patients receiving home-oxygen or on dialysis were questioned whether they had already been receiving such treatments before the onset of COVID-19.
Finally, screening for depression and anxiety symptoms were assessed by the Patient Health Questionnaire – 4 (PHQ-4), which represents the sum of Patient Health Questionnaire – 2 (PHQ-2) and Generalized Anxiety Disorder – 2 (GAD-2). These two questionnaires have been validated in the Brazilian population [9, 10]. Patients were asked to answer the PHQ-4 questionnaire considering how they were feeling over the past 2 weeks; then, they were asked whether they regarded their mental health to be worse, equal or better compared to how they remembered feeling before the onset of COVID-19 symptoms. When the phone interview was carried with the caretaker/proxy, this part of the survey was not performed.
In order to investigate factors related with disease severity, we reviewed patients’ medical records from the index admission. Thus, we collected data on comorbidities, smoking status, level of respiratory support at admission and highest respiratory support required during hospital stay, as well as the percentage of lung parenchyma involvement on chest CT up to two days following hospital admission. We also reviewed whether the patients had needed hemodialysis, vasopressors or ICU admission and recorded their length of hospital stay. Based on the information collected from medical records, we categorized patients mild or severe COVID-19 at admission following the recommendations of the Chinese Center for Disease Control and Prevention [11]. Hence, patients who required no oxygen supplementation and had lung infiltrates taking up less than 50% of lung parenchyma at admission were considered to have presented to hospital with mild disease; conversely, those needing any type of respiratory support or having extensive lung infiltrates (≥50%) were considered to have severe COVID-19 at admission.
2.3. Statistical analysis
Data were presented as mean ± standard deviation, median [interquartile range (IQR)] and absolute and relative frequencies for continuous normally distributed, continuous non-normally distributed and categorical variables, respectively.
Health status’ single summary index prior to admission and at 3 months following discharge were compared using Wilcoxon matched-pairs signed-rank test. Additionally, severity levels in each EQ-5D-3L dimensions were dichotomized as “no problems” (level 1) and “any problems” (levels 2 and 3). The absolute and relative frequencies of participants reporting problems prior to admission and at 3 months following discharge were described, together with the change in these frequencies. Finally, the EQ-5D-3L health state at 3 months following discharge was deemed worse than the health state prior to admission if it was worse in at least one dimension and it was no better in any other dimension.
Individual characteristics and markers of COVID-19 severity were compared between participants with and without worsening of health-status and with or without worsening in each dimension of the EQ5D-3L using Student t-test, Wilcoxon ranksum test, chi-square test and Fisher exact test for normally distributed continuous variables, non-normally distributed continuous variables, categorical variables and categorical variables with small cell counts, respectively. To investigate the individual characteristics and markers of COVID-19 severity with worsening of health status 3 months following discharge we performed logistic regression considering all variables that reached statistical significance in univariate analysis and subject-matter knowledge. Model's discrimination and calibration were assessed by the area under the receiver operating characteristic curve and Hosmer-Lemeshow goodness of fit test, respectively.
Prior to running the multivariable logistic model we assessed collinearity between COVID-19 in-hospital severity markers: requirement of intensive care, requirement of invasive mechanical ventilation, length of hospital stay, disease severity at admission and incident requirement of hemodialysis. Variance inflation factor (VIF) was performed after linear regression in which worsening of health status was the dependent variable and all COVID-19 severity markers were independent variables. We considered requirement of intensive care (VIF = 4.46), invasive mechanical ventilation (VIF = 4.88) and length of hospital stay (VIF = 2.97) collinear variables and we opted to include intensive care requirement in the multivariable logistic model. No collinearity was found for disease severity at admission (VIF = 1.09) or incident requirement of hemodialysis (VIF = 1.64). STATA version 14.2 was used for all statistical analyses and a p < 0.05 was considered statistically significant.
Finally, we investigated the changes in health status during follow-up based on the health status prior to admission. As the distribution of EQ5D-3L single summary index prior to admission was skewed, we first dichotomized the sample in two groups, according to the median of the summary index prior to admission. The groups with better and poorer health status prior to admission were composed of participants with EQ5D-3L summary index = 1.000 and < 1.000, respectively. Then, we used mixed-effects restricted maximum likelihood model to assess the association of these baseline health status groups with changes in the summary index during the follow-up period. This model included the following terms: baseline health status group, time, interaction of baseline health status group with time, and variables of adjustment (age, sex and number of comorbidities). The group comprised of patients with EQ5D-3L summary index = 1.000 was considered as the reference. The estimate for the interaction of baseline health status group with time reflects the change in the EQ5D-3L summary index during the follow-up period based on the baseline health status group.
3. Results
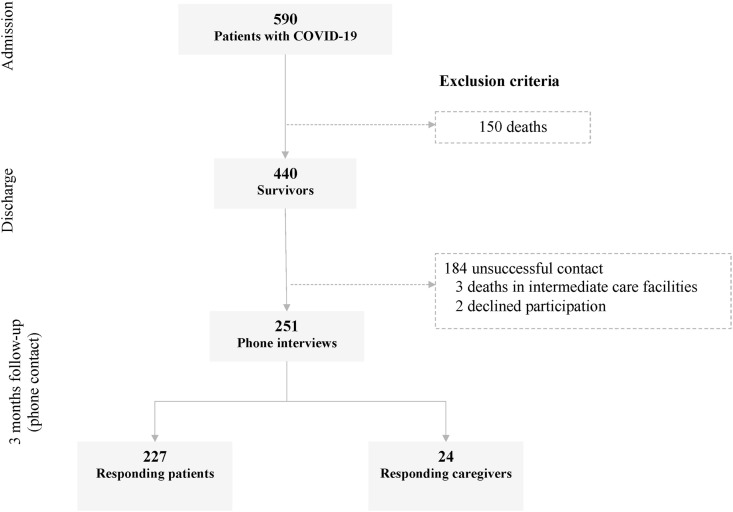
Between March 16th and May 8th, 590 adult patients were admitted for COVID-19, of which 440 survived to hospital discharge. After multiple attempts, we were unable to reach 184 (41,8%), 3 patients had died shortly after being transferred to intermediate care facilities, and 2 patients declined to participate in the study. The telephone interviews were conducted with 227 patients and 24 caretakers (Fig. 1 ).
Fig. 1.
Sample flow chart. The figure displays a visual guide of the eligible patients admitted at the secondary medical care hospital for treatment for COVID-19 and the final sample that survived and accepted to participate in the cohort study.
Eventually, 251 patients were included, whose demographics and severity of acute COVID-19 episode are described in Table 1 . The majority (59.8%) were males, their mean age was 53 years old and most had at least one comorbidity, particularly hypertension (51.8%) and diabetes (33.1%). At admission, 163 (69.7%) had presented with severe COVID-19 and 42 (16.3%) had required intensive care treatment at some point throughout hospital stay. The median length of hospital stay was 5 days, although it ranged from 1 to 72 days.
Table 1.
Characteristics of participants and comparison between participants with and without worsening of health status at 3 months following discharge (n = 251).
| Total sample | With worsening of health-statusa n = 96 | Without worsening of health-statusa n = 143 | p | |
|---|---|---|---|---|
| Sociodemographic characteristics and conditions related to higher risk of COVID-19 severity | ||||
| Age (years), mean ± SD | 53.6 ± 14.9 | 52.2 ± 14.0 | 53.4 ± 14.9 | 0.539c |
| Female, n (%) | 101 (40.2) | 53 (55.2) | 42 (29.4) | <0.001d |
| Self-reported race, n (%)a | 0.716e | |||
| Black | 48 (20.4) | 21 (23.1) | 26 (18.8) | |
| White | 67 (28.5) | 22 (24.2) | 42 (30.4) | |
| Brown | 113 (48.1) | 45 (49.5) | 66 (47.8) | |
| Other | 7 (3.0) | 3 (3.3) | 4 (2.9) | |
| Education less than 8 years, n (%)a | 82 (33.6) | 62 (66.0) | 98 (69.0) | 0.623d |
| Obesity, n (%) | 62 (24.7) | 25 (26.0) | 34 (23.8) | 0.690d |
| Diabetes, n (%) | 83 (33.1) | 29 (30.2) | 46 (32.2) | 0.749d |
| Hypertension, n (%) | 130 (51.8) | 53 (55.2) | 68 (47.6) | 0.246d |
| Chronic obstructive pulmonary disease, n (%) | 13 (5.2) | 5 (5.2) | 7 (4.9) | 1.000e |
| Asthma, n (%) | 12 (4.8) | 5 (5.2) | 7 (4.9) | 1.000e |
| Neoplasia, n (%) | 7 (2.8) | 4 (4.2) | 1 (0.7) | 0.161e |
| Previous history of coronary artery disease, n (%) | 17 (6.8) | 7 (7.3) | 6 (6.3) | 0.796e |
| Previous history of stroke, n (%) | 5 (2.0) | 0 | 4 (2.8) | 0.151e |
| Heart failure, n (%) | 12 (4.8) | 4 (4.2) | 7 (4.9) | 1.000e |
| Chronic renal disease, n (%) | 10 (4.0) | 5 (5.2) | 5 (3.5) | 0.528e |
| Number of comorbidities, median (IQR) | 1 (0–2) | 2 (1–2.5) | 1 (0–2) | 0.522f |
| Smoking status at admission, n (%) | 0.725e | |||
| Current smoker | 9 (3.6) | 2 (2.1) | 6 (4.2) | |
| Previous smoker | 56 (22.3) | 21 (21.9) | 32 (22.4) | |
| COVID-19 severity during index hospitalization | ||||
| Highest respiratory support required, n (%)a | 0.008e | |||
| No need for oxygen supplementation | 60 (23.9) | 25 (26.0) | 34 (23.8) | |
| Nasal catheter oxygenation | 109 (43.4) | 36 (37.5) | 69 (48.2) | |
| Oxygen mask | 47 (18.7) | 14 (14.6) | 29 (20.3) | |
| Non-invasive mechanical ventilation | 1 (0.4) | 0 (0) | 1 (0.7) | |
| Invasive mechanical ventilation | 34 (13.6) | 21 (21.9) | 10 (7.0) | |
| Lung involvement > 50% on chest CT, n (%)a | 85 (42.3) | 36 (46.8) | 45 (39.8) | 0.343d |
| Severe COVID-19b at admission, n (%)a | 163 (69.7) | 66 (71.7) | 85 (65.4) | 0.317d |
| Intensive care, n (%) | 42 (16.3) | 24 (25.0) | 15 (10.5) | 0.003d |
| Hemodialysis in patients who have never received | 9 (3.7) | 6 (6.3) | 2 (1.4) | 0.064e |
| hemodialysis before, n (%)a | 4 (1.7) | 3 (2.1) | 1 (1.0) | 0.650e |
| Length of hospital stay (days), median (IQR) | 5 (3–10) | 13.5 (5–26.5) | 4 (2–8) | 0.014f |
CT: computed tomography; IQR: interquartile range; SD: standard deviation.
Missing values: 12 for worsening of health-status; 16 for self-reported race, 7 for education, 50 for lung involvement on chest CT, 17 for severe COVID-19 at admission, 5 for hemodialysis in patients who have never received hemodialysis before.
Severe COVID-19 defined in the first 48 h after admission as requirement of any type of respiratory support or having extensive lung infiltrates on chest CT (≥50%).
Student T-test.
Chi-square test.
Fisher exact test.
Wilcoxon ranksum test.
3.1. Health-related quality of life
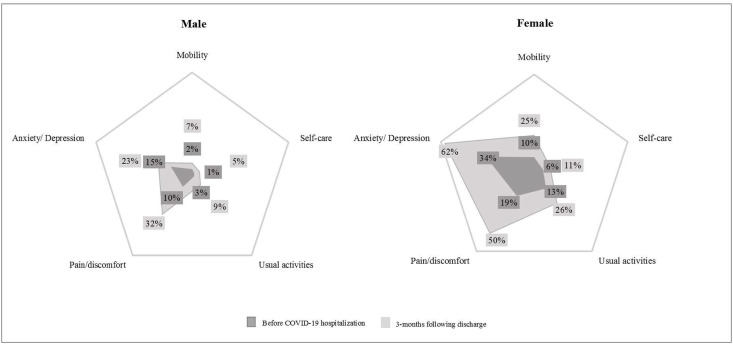
When enquired after health-related quality of life, patients reported an overall worsening of EQ-5D-3L single summary index at 3 months compared to before the onset of COVID-19 symptoms (0.8012 (0.7368 – 1.0) vs. 1.0 (0.8012 – 1.0), respectively, p < 0.001). As presented in Table 2 , this decrease in quality of life affected all 5 domains, although pain/discomfort and anxiety/depression were the ones most commonly impaired. Fig. 2 depicts the proportion of individuals with some problem in each dimension of the EQ-5D-3L before the onset of COVID-19 symptoms and at 3 months following discharge according to sex. Worsening of health status in Anxiety/Depression, Pain/discomfort and Usual activities were higher among females. Additionally, 81 patients scored 3 or higher on PHQ-4, of which 35 (43.2%) considered their current mood to be worse than before the onset of COVID-19 symptoms.
Table 2.
Description of health status within EQ-5D dimensions pre-admission and at the 3-months assessment after discharge from index hospitalization.
| Mobility |
Self-care |
Usual activities |
Pain/discomfort |
Anxiety/depression |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-admission | 3-months assessment | Pre-admission | 3-months assessment | Pre-admission | 3-months assessment | Pre-admission | 3-months assessment | Pre-admission | 3-months assessment | |
| Level 1, n (%) | 229 (94.2) | 209 (86.0) | 235 (96.7) | 225 (92.6) | 227 (93.4) | 205 (84.4) | 209 (86.0) | 147 (60.5) | 189 (77.8) | 158 (65.6) |
| Level 2, n (%) | 12 (4.9) | 28 (11.5) | 5 (2.1) | 8 (3.3) | 6 (2.5) | 22 (9.1) | 33 (13.6) | 85 (35.0) | 42 (17.3) | 60 (24.9) |
| Level 3, n (%) | 2 (0.8) | 6 (2.5) | 3 (1.2) | 10 (4.1) | 10 (4.1) | 16 (6.5) | 1 (0.4) | 11 (4.5) | 12 (4.9) | 23 (9.5) |
| Total | 243 | 243 | 243 | 243 | 243 | 243 | 243 | 243 | 243 | 241 |
| Reporting some problems,a n (%) | 14 (5.7) | 34 (14.0) | 8 (3.3) | 18 (7.4) | 16 (6.6) | 38 (15.6) | 34 (14.0) | 96 (39.5) | 54 (22.2) | 83 (34.4) |
| Change in numbers reporting problems | +20 | +10 | +22 | +62 | +29 | |||||
| % change reporting problems | +243% | +225% | +238% | +282% | +154% | |||||
Some problems = levels 2 + 3.
Fig. 2.
Dimensions of quality of life pre and post COVID-19 infection. The figure presents the percentual of individuals with some problem (2 or more points in EQ-5D questionnaire) in each dimension of quality of life before the onset of COVID-19 symptoms and at 3-months following discharge according to sex. Graphics range: [0%–65%].
Participants with worsening of health status at 3 months following discharge were predominantly female, more frequently had required mechanical ventilation and intensive care and had longer length of hospital stay than participants without worsening of health status at 3 months following discharge (Table 1). Factors associated with worsening in each EQ5D-3L dimension in univariate analysis were the following: i. mobility: gender, diabetes, hypertension, number of comorbidities, highest respiratory support required, intensive care, new onset hemodialysis, and length of hospital stay (Supplementary Table S1); ii. self-care: age, hypertension, number of comorbidities, intensive care, new onset hemodialysis, and length of hospital stay (Supplementary Table S2); iii. usual activities: age, heart failure, number of comorbidities, new onset hemodialysis, and length of hospital stay (Supplementary Table S3); iv. pain/discomfort: gender, highest respiratory support required, intensive care, and new onset hemodialysis (Supplementary Table S4); v. anxiety/depression: gender and length of hospital stay (Supplementary Table S5).
Table 3 shows the factors independently associated with worsening of health status based on a multivariable logistic regression model including age, sex, education, number of comorbidities, smoking status, requirement of intensive care, COVID-19 severity at admission and requirement of hemodialysis during the index admission due to COVID-19 severity. Only female sex and intensive care requirement were independently associated with worsening of health status at 3 months following discharge. Although the model was calibrated (Hosmer-Lemeshow goodness of fit test p-value was 0.264) it did not present good discrimination (area under the receiver operating characteristic curve = 0.68).
Table 3.
Factors associated with worsening of health status 3 months following discharge (n = 219).
| Odds ratio (95% CI) | p | |
|---|---|---|
| Sex (female) | 3.38 (1.85–6.19) | <0.001 |
| Age | 0.99 (0.96–1.01) | 0.257 |
| Schooling >8 years | 0.81 (0.42–1.58) | 0.535 |
| Number of chronic conditions | 0.95 (0.69–1.29) | 0.978 |
| Smoking status | ||
| Current smoker | 0.35 (0.06–2.12) | 0.255 |
| Previous smoker | 1.33 (0.63–2.80) | 0.450 |
| Severity of COVID-19 at admission | 1.02 (0.52–1.97) | 0.963 |
| Hemodialysis | 2.33 (0.38–14.76) | 0.507 |
| Intensive care requirement | 2.78 (1.17–6.62) | 0.021 |
Finally, 84 (34.5%) participants had an EQ-5D-3L summary index below 1.000 prior to admission. Poorer health status prior to admission was associated with more significant decline in health status at 3 months after discharge, even after adjusting for age, gender and number of comorbidities (Table 4 ).
Table 4.
Changes in health status summary index during 3 months of follow-up based on health status summary index prior to admission (n = 241).
| βa(95% CI) | p | |
|---|---|---|
| Baseline EQ5D-3L summary index < 1.000b | −0.07 (−0.13; −0.01) | 0.024 |
| Time | −0.03 (−0.07; 0.00) | 0.078 |
| Time*baseline EQ5D-3L summary index < 1.000 | −0.07 (−0.13; −0.01) | 0.030 |
Mixed-effects restricted maximum likelihood model adjusted for age, sex, and number of comorbidities.
Reference: EQ5D-3L summary index = 1.000 (better health status).
3.2. Additional clinical outcomes
At 3 months of follow-up, 6 patients had died, all of which passed away within the first month they had left the hospital. Fifty-one patients (20.3%) reported having sought care at the emergency department at least once since being discharged and 17 (6.8%) had been readmitted to hospital.
Seventy patients (27.9%) reported feeling more breathless and had increased their mMRC score by at least 1 unit compared to baseline status (before the onset of COVID-19 symptoms). At 3-months, their median (IQR) dyspnoea severity was 1 [1,2] and 18 of them presented with mMRC scores equal or higher than 2.
Of the 9 patients who had required in-hospital hemodialysis because of acute kidney injury, 3 remained on renal replacement therapy at the time of the phone interview. Similarly, 6 patients who did not require oxygen supplementation before COVID-19 were still on home-oxygen therapy at 3 months following hospital discharge.
4. Discussion
This was the first study to assess quality of life and hard clinical outcomes in Brazilian patients following hospitalization due to COVID-19. Moreover, even though previous studies had already reported persistent symptoms and reduced quality of life in patients recovering from COVID-19, to our knowledge, this was the first that allowed adjustment of such findings by the patients’ baseline clinical and health status. Thus, our findings regarding dyspnoea intensity, use of home-oxygen therapy, renal replacement therapy, quality of life and mental health can be more easily interpreted and give a clearer notion of the physical and emotional burden of the disease in the long term.
Our data confirms that, unfortunately, the toll of COVID-19 extends beyond hospital discharge. Of the 251 patients that we have managed to contact 3 months after of hospital discharge, 2.4% had died and 7.1% had been readmitted, most within the first month they had left the hospital. We believe these numbers are possibly underestimated given that 41.8% of the patients discharged alive were lost to follow-up. In keeping with this notion, several cohort studies [7, 12, 13], including two cohort studies supported by integrated healthcare system records [12, 13], reported mortality and readmission rates that were effectively twice to three times higher than what we found in our cohort. While the population analyzed in such studies was significantly older than ours and, therefore, a direct comparison is challenging, this discrepancy should be noted and considered in light of our high rate of loss to follow-up. Still, our data raises attention to how debilitating this condition can be even after patients have supposably recovered from its acute phase.
Furthermore, it is noteworthy that, at 3 months, more than a quarter of our cohort reported feeling persistently more breathless than before the onset of COVID-19 symptoms. Previous studies had already identified dyspnoea as a frequent symptom among patients recovering from COVID-19 up to 6 months following hospital discharge [14]. Nonetheless, such studies usually fail to report whether dyspnoea developed after COVID-19 or if those patients already presented with some degree of breathlessness before falling acutely ill. Given that frailty, chronic cardiovascular and pulmonary diseases are risk factors for severe COVID-19 [15, 16], it is important to try to discriminate between chronic and new or worsened dyspnoea. In our cohort, even though only about 9% had mMRC ≥2, which is similar to the findings reported by a French study (5), a total of 70 patients had increased their mMRC scores by at least one point, which is the minimal clinically important difference for this scale [17, 18]. In addition, and not surprisingly, 6 patients who had never required supplemental oxygen before COVID-19 were still on home-oxygen therapy at 3 months following discharge.
At first glance, having 6 new patients on supplemental oxygen (and 3 on hemodialysis) might seem pretty small numbers, as they represent only 2.4% of our cohort. Indeed, considering our study sample was comprised predominantly of severe COVID-19 patients, these numbers seem reasonable. However, if we analyze them from a populational perspective and keep in mind that 749.947 patients were hospitalized for acute severe respiratory syndrome in Brazil between February and September (excluding influenza-confirmed cases) [19], this could suggest thousands of new patients may have needed long-term oxygen therapy and/or long-term renal replacement therapy in Brazil alone. Of course, our cohort was not designed to investigate this question nor had the power to do so, but we believe our data provides a glimpse into the potential long-term impacts of COVID-19 on our healthcare system and should, therefore, stimulate dedicated studies in the field.
Finally, our study leaves no room for doubt with regard to the effects of COVID-19 on health-related quality of life and mental health. A few previous reports objectively assessed HRQL among COVID-19 survivors [14, 20] and found lower EQ-5D scores among those who required intensive care admission compared with those treated in wards, particularly regarding the pain/discomfort domain. Our study not only corroborates their findings, but also expands the knowledge in the area.
In our study, ICU admission was also an independent predictor of worsening HRQL. However, the novelty of our findings lies on the fact that the present study was the first to compare patients’ HRQL and mental health over time. Given that many patients admitted for COVID-19 are frail and/or have chronic clinical conditions, it is essential to understand whether reduced quality of life following hospital discharge is a result of the disease itself or if the patients have merely returned to their baseline status. By asking patients to respond to the EQ-5D-3L questionnaire according to both their current status and their perceived status before the onset of COVID-19 symptoms, we have managed to demonstrate that the disease in fact worsens all 5 domains, although especially pain/discomfort and anxiety/depression. Additionally, we were able to demonstrate that poorer health status prior to hospitalization was associated with higher decline in EQ5D-3L scores after 3 months of follow-up. In keeping with this finding, 35.5% of our study sample had a positive screening for anxiety and depression according to the PHQ-4 score and 20.2% reported feeling their mental health was worse than before they developed COVID-19. Although the instrument which we employed is not intended for diagnosis, but rather for screening of psychiatric diseases, our prevalence of positive screening for anxiety and depression in similar to what was identified in previous studies using validated diagnostic tools [21].
It is true that the impairment in quality of life and mental health may have been, at least in part, due to the social and economic changes imposed by the SARS-CoV-2 pandemic, as recently demonstrated in a Brazilian study looking mainly at individuals who had never had COVID-19 [22]. Similarly, a French survey investigating mental health in high-risk groups during lockdown associated unemployment with higher rates of psychological distress [23] and a populational Chinese study found that pain/discomfort and anxiety/depression were the worst rated domains in the EQ-5D-3L questionnaire among participants who had not had the disease [24].
The fact that in our study female sex was a strong and independent predictor of worsened health-related quality of life at 3 months of follow-up also raises the possibility that social factors may have played a role. Data from all over the world suggests that women's mental health have been disproportionately impacted by the SARS-CoV-2 pandemic, even if they have not had COVID-19 [[24], [25], [26]]. Nevertheless, the strong association between the severity of the acute episode of COVID-19 and HRQL at 3 months follow-up in our study emphasizes the role of the disease itself, possibly on top of several social and financial issues contributing to augment its burden.
Our study has several limitations. First, this was a single center study focusing on a socially vulnerable population, so our results may not be generalizable to patients of higher social strata with access to premium healthcare insurances. Second, we have been unable to reach more than half of the patients discharged alive, which has been a common problem among studies following patients with COVID-19 and is inherently associated with selection bias. Third, because participants were asked to report health-related quality of life and mental health symptoms prior to COVID-19 in the third month following discharge, measurement bias cannot be ruled out. Forth, we did not have a socially and demographically matched control group who had not had COVID-19 in order to completely set apart the impact of the disease itself from the social and economic burdens of the SARS-Cov-2 pandemic on the overall health-related quality of life and mental health. Fifth, the low sample size precluded the investigation of factors associated with worsening in each EQ5D-3L dimension using multiple regression and adjusting for known confounders. Factors associated with worsening in each dimension can differ and larger studies assessing health-related quality of life after COVID-19 hospitalizations could clarify this issue. Finally, follow-up data was self-reported and we were unable to perform objective pulmonary imaging or functional assessments during follow-up. In that sense, it is possible that some patients who reported persistent dyspnoea did not actually have pulmonary sequelae, but rather suffered from mood disorders, deconditioning or chronic fatigue, which can all manifest with exercise intolerance. Specifically, chronic fatigue has been described as a common complication of “long COVID” [27, 28], but was not investigated in our cohort.
In conclusion, we have demonstrated that a large proportion of patients hospitalized for COVID-19 face persistent clinical and mental health conditions as a consequence of the disease up to 3 moths following hospital discharge, with significant impacts on patient health-related quality of life and possibly resulting on increased long-term demand to the healthcare system.
CRediT authorship contribution statement
Beatriz Costa Todt: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. Claudia Szlejf: Conceptualization, Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Etienne Duim: Methodology, Formal analysis, Writing – review & editing. Alana O.M. Linhares: Investigation, Writing – review & editing. Diogo Kogiso: Conceptualization, Writing – review & editing. Gabriela Varela: Investigation, Writing – review & editing. Bruna A. Campos: Investigation, Writing – review & editing. Cristina Mara Baghelli Fonseca: Investigation, Writing – review & editing. Leonardo E. Polesso: Investigation, Writing – review & editing. Ingra N.S. Bordon: Investigation, Writing – review & editing. Bruno T. Cabral: Investigation, Writing – review & editing. Victor L.P. Amorim: Investigation, Writing – review & editing. Felipe M.T. Piza: Resources, Writing – review & editing. Luiza Helena Degani-Costa: Conceptualization, Methodology, Formal analysis, Writing – original draft, Writing – review & editing, Supervision, Project administration.
Declaration of competing interest
The authors have no conflict of interest to declare.
Acknowledgements
This study was not funded by any institutions and the authors did not receive any grants for this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmed.2021.106453.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. J. Am. Med. Assoc. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. 10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.George P.M., Barratt S.L., Condliffe R., Desai S.R., Devaraj A., Forrest I., et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax. 2020;75(11):1009–1016. doi: 10.1136/thoraxjnl-2020-215314. [DOI] [PubMed] [Google Scholar]
- 5.Carvalho-Schneider C., Laurent E., Lemaignen A., Beaufils E., Bourbao-Tournois C., Laribi S., et al. Follow-up of adults with non-critical COVID-19 two months after symptoms’ onset. Clin. Microbiol. Infect. 2021;27(2):258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen K.Y., Li T., Gong F.H., Zhang J.S., Li X.K. Predictors of health-related quality of life and influencing factors for COVID-19 patients, a follow-up at one month. Front. Psychiatr. 2020;11:668. doi: 10.3389/fpsyt.2020.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chopra V., Flanders S.A., O’Malley M., Malani A.N., Prescott H.C. Sixty-day outcomes among patients hospitalized with COVID-19. Ann. Intern. Med. 2021;174(4):576–578. doi: 10.7326/M20-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santos M., Cintra M.A., Monteiro A.L., Santos B., Gusmao-Filho F., Andrade M.V., et al. Brazilian valuation of EQ-5D-3L health states: results from a saturation study. Med. Decis. Making. 2016;36(2):253–263. doi: 10.1177/0272989X15613521. [DOI] [PubMed] [Google Scholar]
- 9.de Lima Osorio F., Vilela Mendes A., Crippa J.A., Loureiro S.R. Study of the discriminative validity of the PHQ-9 and PHQ-2 in a sample of Brazilian women in the context of primary health care. Psychiatr. Care. 2009;45(3):216–227. doi: 10.1111/j.1744-6163.2009.00224.x. [DOI] [PubMed] [Google Scholar]
- 10.Hughes A.J., Dunn K.M., Chaffee T., Bhattarai J.J., Beier M. Diagnostic and clinical utility of the GAD-2 for screening anxiety symptoms in individuals with multiple sclerosis. Arch. Phys. Med. Rehabil. 2018;99(10):2045–2049. doi: 10.1016/j.apmr.2018.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. J. Am. Med. Assoc. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly J.P., Wang X.Q., Iwashyna T.J. Prescott HC. Readmission and death after initial hospital discharge among patients with COVID-19 in a large multihospital system. J. Am. Med. Assoc. 2021;325(3):304–306. doi: 10.1001/jama.2020.21465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Islam N., Lewington S., Kharbanda R.K., Davies J., Varnai K.A., Lacey B. Sixty-day consequences of COVID-19 in patients discharged from hospital: an electronic health records study. Eur. J. Publ. Health. 2021;31(2):280–282. doi: 10.1093/eurpub/ckab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. 10270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorjee K., Kim H., Bonomo E., Dolma R. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: a comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. PloS One. 2020;15(12) doi: 10.1371/journal.pone.0243191. e0243191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aliberti M.J.R., Szlejf C., Avelino-Silva V.I., Suemoto C.K., Apolinario D., Dias M.B., et al. COVID-19 is not over and age is not enough: using frailty for prognostication in hospitalized patients. J. Am. Geriatr. Soc. 2021 doi: 10.1111/jgs.17146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones P.W., Beeh K.M., Chapman K.R., Decramer M., Mahler D.A., Wedzicha J.A. Minimal clinically important differences in pharmacological trials. Am. J. Respir. Crit. Care Med. 2014;189(3):250–255. doi: 10.1164/rccm.201310-1863PP. [DOI] [PubMed] [Google Scholar]
- 18.Mahler D.A., Witek T.J., Jr. The MCID of the transition dyspnea index is a total score of one unit. COPD. 2005;2(1):99–103. doi: 10.1081/copd-200050666. [DOI] [PubMed] [Google Scholar]
- 19.BOLETIM EPIDEMIOLÓGICO ESPECIAL Doença pelo Coronavírus COVID-19-Semana Epidemiológica 39 (20 a 26/09/2020) Brasiliana: Ministério da Saúde/Secretaria de Vigilância em Saúde. 2020 [Google Scholar]
- 20.Garrigues E., Janvier P., Kherabi Y., Le Bot A., Hamon A., Gouze H., et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J. Infect. 2020;81(6):e4–e6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazza M.G., De Lorenzo R., Conte C., Poletti S., Vai B., Bollettini I., et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav. Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teotonio I., Hecht M., Castro L.C., Gandolfi L., Pratesi R., Nakano E.Y., et al. Repercussion of COVID-19 pandemic on Brazilians' quality of life: a nationwide cross-sectional study. Int. J. Environ. Res. Publ. Health. 2020;22 doi: 10.3390/ijerph17228554. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaix B.D., G, Guillemassé A., Brouard B., Bibault J.E. Psychological distress during the COVID-19 pandemic in France: a national assessment of at-risk populations. General Psychiatr. 2020;33 doi: 10.1136/gpsych-2020-100349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ping W., Zheng J., Niu X., Guo C., Zhang J., Yang H., et al. Evaluation of health-related quality of life using EQ-5D in China during the COVID-19 pandemic. PloS One. 2020;15(6) doi: 10.1371/journal.pone.0234850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eurofound . 2020. Living, Working and COVID-19 Dataset. Dublin. [Google Scholar]
- 26.Souza A.S., GFA, Praciano G.A.F. Women's mental health in times of COVID-19. Rev. Bras. Saúde Materno Infant. 2020;20(3) [Google Scholar]
- 27.Townsend L., Dyer A.H., Jones K., Dunne J., Mooney A., Gaffney F., et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PloS One. 2020;15(11) doi: 10.1371/journal.pone.0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Townsend L., Dowds J., O’Brien K., Sheill G., Dyer A.H., O’Kelly B., et al. Persistent poor health post-COVID-19 is not associated with respiratory complications or initial disease severity. Ann Am Thorac Soc. 2021 doi: 10.1513/AnnalsATS.202009-1175OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.