Abstract
Berthella californica (W. H. Dall, 1900) is a widespread species of heterobranch sea slug distributed across the North Pacific Ocean, from Korea and Japan to the Galapagos Islands. Two distinct morphotypes are observed in B. californica, which differ in external coloration, egg-mass morphology and geographic distribution (with the exception of a small range overlap in Southern California). Molecular and morphological data obtained in this study reveals that these two morphotypes constitute distinct species. The name B. californica (type locality: San Pedro, California) is retained for the southern morphotype, whereas the name Berthella chacei (J. Q. Burch, 1944) (type locality: Crescent City, California) is resurrected for the northern morphotype. Moreover, molecular phylogenetic analyses recovered B. californica as sister to Berthellina, in a well-supported clade separate from Berthella, suggesting that the classification of B. californica may need additional revision.
INTRODUCTION
Several species of cold-water heterobranch sea slugs have been considered to have transpacific ranges, with populations often ranging from Japan and Korea to the southwestern United States, Mexico or even Central America (Behrens & Hermosillo, 2005; Camacho-García et al., 2005; Chichvarkhin, 2016; Nakano, 2018). Integrative molecular and morphological studies of such species have shown that some of them are complexes of two or more pseudocryptic species, with distinct eastern and western taxa (Cooke et al., 2014; Lindsay & Valdés, 2016), whereas others are truly transpacific (Ekimova et al., 2015; Ekimova et al., 2016). A third scenario includes cryptic complexes of two allopatric sister species in which the boundary between their ranges is not east–west, but rather north–south along the eastern Pacific, so the northern species is transpacific (Lindsay et al., 2016). Allopatric speciation caused by ice sheet formation during glacial maxima followed by dispersal during glacial minima has been suggested as a possible explanation for this pattern (Lindsay et al., 2016). The complex evolutionary dynamics of the highly productive and diverse North Pacific Ocean are dominated by glacial cycles (Wilson, 2006; Kelly & Palumbi, 2010; Marko et al., 2010; Clarke & Crame, 2010) and speciation is strongly influenced by climate change (Reid, 1990; Wares, 2001; Grant & Bowen, 2006). Thus, understanding the biological diversity of the region as well as its patterns of speciation and gene flow have important consequences for understanding the past and future of this important region.
Berthella californica (Dall, 1900) is a nominal species of side-gilled sea slug that occurs from the Sea of Japan (East Sea) to the Galapagos Islands (Gosliner & Bertsch, 1988; Behrens & Hermosillo, 2005; Camacho-García et al., 2005; Jung 2014; Nakano, 2018; Chichvarkhin, 2016; Manning, 2016; Valdés, 2019). Berthella californica is characterized by having a white marginal band around the notum, and numerous white spots on the white to brown mantle (Gosliner & Bertsch, 1988; Valdés, 2019). Field observations and photographic records suggest that B. californica has two morphotypes that differ in some aspects of their external morphology and biology. One of these morphotypes, referred to here as the southern morphotype (Fig. 1A–D), is found from Southern California to the Galapagos Islands, and has uniformly sized and distributed white spots on its smooth, whitish cream to brown mantle (Camacho-García et al., 2005; Manning, 2016; Valdés, 2019). The dorsal white spots are usually absent from the foot, rhinophores and oral veil. Most specimens possess a longitudinal white dorsal stripe on each rhinophore (Fig. 1A–D). Animals of the southern morphotype lay greenish brown egg masses in the shape of a high, delicate ribbon (Fig. 1C; Gardner, 1999). This morphotype occurs subtidally to 87 m depth (García-Méndez & Camacho-García, 2016; Manning, 2016; C. Hoover, personal observation) and reaches lengths of at least 127 mm (Fig. 1D). The other morphotype of B. californica, referred to here as the northern morphotype (Fig. 1E–H), is found from the Sea of Japan (East Sea) across the North Pacific to central California (Gosliner, 1996; Chichvarkhin, 2016; Nakano, 2018), intertidally to 100 m depth (MacFarland, 1966; Goddard, 1984, 2010; Gosliner, 1996). It reaches lengths of about 70 mm (A. Chichvarkhin, personal observation) and has white spots of variable size covering its milky white to cream mantle, as well as the foot, rhinophores and oral veil (MacFarland, 1966; Lamb & Handby, 2005). The white spots on the mantle are on tubercles and the rest of the mantle is typically smooth. The northern morphotype lays a white egg mass (Fig. 1H; MacFarland, 1966: pl. 5, fig. 5; Hildering & Miller, 2007; Chichvarkhin, 2016: fig. 3H; Nakano 2018: 152) that is a relatively low, wavy, coiled ribbon. Online photographic records suggest that the ranges of the northern and southern morphotypes of B. californica overlap subtidally in the Southern California Bight (SCB), with both forms recorded from San Miguel Island (e.g. Wight, 2002, 2004; Lee (undated): image 213) to San Diego (e.g. Lee (undated): image 225; Girouard, 2005; Flax, 2017; Gao, 2017: image 26). The online photographic records also suggest that in the SCB the southern morphotype is by far the most prevalent, while the northern morphotype is rare. Both papillate and smooth forms of the northern morphotype have been observed in the SCB (e.g. Lee (undated): images 201, 202).
Figure 1.
Live animals and egg masses. A–D.Berthella californica. A. Specimen from Ventura, California (CPIC 1328). B. Specimen from Ventura, California (CPIC 1761). C. Two specimens with egg mass from San Diego County, California. D. Specimen from La Jolla, California. E–H.Berthella chacei. E. Specimen from Kamchatka, Russia. F. Specimen from Duxbury Reef, California (CASIZ 182207). G. Specimen from Sonyang-myeon, South Korea (CPIC 2119). H. Two specimens with egg mass from Point Lobos, California. Image credits: A, B, H, C. Hoover; C, K. Lee; D, J.R. Lance; E, A. Chichvarkhin; F, T. Gosliner; G, D.-W. Jung.
Figure 5.
SEMs of the radular teeth and jaw elements of the specimen of Berthella californica from Ventura, California (CPIC 1328). A. Innermost teeth. B. Mid-lateral teeth. C. Outer teeth. D. Jaw elements.
Figure 3.
A. Bayesian consensus tree of the concatenated 16S rRNA, COI, 18S rRNA and histone H3 dataset. Posterior probabilities from the Bayesian analysis and bootstrap values from the ML analysis are presented above and below each branch, respectively. B,C. Results of the ABDG analysis showing the distribution of pairwise p-distances (K2 model) between Berthella californica and B. chacei for COI (B) and 16S rRNA (C).
In this study, we examine the DNA sequence divergence between the northern and southern morphotypes of B. californica, using two mitochondrial genes (16S rRNA and cytochrome c oxidase subunit I (COI)) and two nuclear genes (18S rRNA and histone H3). We also compare the external and internal anatomy of these two morphotypes; the characters considered include the morphology of the shell, jaw elements, radular teeth and reproductive system. The molecular and morphological data are integrated to determine whether B. californica is a species complex. Finally, the phylogenetic position of B. californica is discussed, as well as the potential need to introduce a new genus name for this species.
MATERIAL AND METHODS
Source of specimens
A total of 14 specimens and tissue samples of specimens identified as Berthella californica (Table 1) and covering most of its known range (Fig. 2) were obtained for this study. All specimens and tissue samples were preserved in 95% ethanol. Eleven whole specimens were collected in the field and deposited at the California State Polytechnic University, Pomona Invertebrate Collection (CPIC); one tissue sample was obtained from the Natural History Museum of Los Angeles County (LACM); two tissue samples were obtained from the Invertebrate Zoology collection at the California Academy of Sciences (CASIZ) in San Francisco; and three tissue samples were obtained from DeNovo Research Marine Biodiversity Laboratory, Daejeon, Republic of Korea. Information and photographs of type specimens was obtained from the CASIZ and the National Museum of Natural History, Smithsonian Institution, Washington, D.C. (USNM) collections. DNA sequences for 14 additional specimens were downloaded from GenBank (Table 1).
Table 1.
List of specimens sequenced for this study including their final species name, locality, collection date, museum voucher nos, isolate nos and GenBank acc. nos.
| Species name | Locality | Date | Voucher no. | Isolate no. | GenBank acc. no. | |||
|---|---|---|---|---|---|---|---|---|
| 16S rRNA | COI | Histone H3 | 18S rRNA | |||||
| Pleurobranchea meckelii | Spain (Mediterranean) | — | EED-Phy-441 | — | FJ917439 | FJ917499 | EF133470 | FJ917449 |
| P. hilli | Western Australia | — | EED-Phy-659 | — | FJ917438 | FJ917497 | — | FJ917462 |
| P. peroni | Australia | — | — | — | EF489331 | DQ237993 | — | AY427494 |
| P. reticulatus | Guinea | — | EED-Phy-770 | — | — | FJ917498 | — | FJ917464 |
| P. membranaceus | France (Mediterranean) | — | EED-Phy-769 | — | FJ917437 | FJ917496 | — | FJ917460 |
| Berthella plumula | France (Atlantic) | — | EED-Phy-387 | — | FJ917435 | FJ917493 | — | — |
| B. plumula | Ballyhenry Is., Northern Ireland, UK | 4 Aug. 2013 | CASIZ193034 | SG53 | MK542742 | MK542770 | MK542803 | — |
| B. medietas | Victoria, Australia | — | EED-Phy-893 | — | FJ917433 | FJ917491 | — | FJ917455 |
| B. platei | Chile | — | EED-Phy-663 | — | FJ917434 | FJ917492 | — | FJ917446 |
| B. martensi | Islas Secas, Golfo de Chiriquí, Panama | — | MZUCR6982 | — | HM162592 | HM162683 | HM162498 | MF958319 |
| B. martensi | Western Australia | — | EED-Phy-658 | — | FJ917431 | FJ917489 | — | FJ917457 |
| Berthellina citrina | New South Wales, Australia | — | EED-Phy-895 | — | FJ917436 | FJ917494 | — | FJ917448 |
| Berthellina edwardsi | Ghana | — | ZSM Mol 20070249 | — | — | FJ917495 | — | FJ917458 |
| B. californica | Isla Jicarón, Veraguas Province, Panama | 9 May 2003 | LACM153333 | SG3 | MN566881 | — | MN566899 | — |
| B. californica | Isla Jicarón, Veraguas Province, Panama | — | CPIC01400 | JG40 | MN566882 | — | MN566900 | — |
| B. californica | Neptune’s Reef, Ventura, California, USA | 8 Nov. 2014 | CPIC01328 | SG1 | MK542745 | MK542773 | MK542805 | MN566890 |
| B. californica | County Line, Ventura Co., California, USA | 4 Feb. 2016 | CPIC01761 | SG37 | — | — | MN566901 | — |
| B. chacei | Middle Cove, Cape Arago, Oregon, USA | 18 Jun. 2015 | CPIC01409 | SG12A | MN566883 | MN566895 | MN566902 | MN566891 |
| B. chacei | Middle Cove, Cape Arago, Oregon, USA | 18 Jun. 2015 | CPIC01409 | SG12B | MN566884 | MN566896 | MN566903 | MN566892 |
| B. chacei | Whiskey Creek, Oregon, USA | 16 Jun. 2015 | CPIC01407 | SG13 | MN566885 | MN566897 | MN566904 | — |
| B. chacei* | Canada (Pacific) | — | EED-Phy-975 | — | FJ917432 | FJ917490 | — | FJ917445 |
| B. chacei | Vladivostok, Russia | 1 Jul. 2012 | CPIC01327 | SG2 | MN566886 | — | MN566905 | — |
| B. chacei | Sonyang-myeon, Gangwon-do, South Korea | 22 Jul. 2012 | CPIC02119 | SG95 | MN566887 | MN566898 | MN566906 | — |
| B. chacei | Hyeonnam-myeon, Gangwon-do, South Korea | 12 May 2018 | DNOPPC01 | BC1 | — | — | MN566907 | — |
| B. chacei | Hyeonnam-myeon, Gangwon-do, South Korea | 13 May 2018 | DNOPPC02 | BC2 | — | — | MN566908 | — |
| B. chacei | Hyeonnam-myeon, Gangwon-do, South Korea | 13 May 2018 | DNOPPC03 | BC3 | — | — | MN566909 | — |
| B. chacei | Duxbury Reef, Marin Co., California, USA | 30 Dec. 2009 | CASIZ182207 | SG38 | MN566888 | — | MN566910 | MN566893 |
| B. chacei | Pigeon Pt., San Mateo Co., California, USA | 18 Feb. 2008 | CASIZ182036 | SG42 | MN566889 | — | MN566911 | MN566894 |
Asterisks denote sequences obtained from GenBank. For institutional abbreviations see Material and Methods.
Figure 2.
Map of the localities sampled for this study color coded by geographic region and morphotype (including final species names) of specimens collected. Type localities of the different species names indicated by shaded boxes.
DNA extraction, amplification and sequencing
DNA was extracted from all the specimens using a hot Chelex protocol. First, a small fragment (~1–3 mg) of tissue was removed from the foot of the animal and cut into smaller pieces using a sterile razor blade. The pieces of tissue were then placed in a 1.75-ml tube along with 1.0 ml Tris-EDTA (TE) buffer (10 mM Tris, 1.0 mM ethylenediaminetetraacetic acid, pH 8.0) and placed on a rotator for at least 20 min to rehydrate and loosen the tissue. The solution was then centrifuged for 3 min at 21130 × g and 975.0 μl of the supernatant was removed afterwards without disturbing the pellet. Next, 175.0 μl 10% (w/v) Chelex 100 (previously prepared using TE buffer; US Standard 100–200 mesh, sodium form, Bio-Rad) was added to the tube containing the tissue. The samples were then placed in a water bath at 56 °C for at least 20 min and then in a heating block at 100 °C for exactly 8 min. After the heating stages, the samples were centrifuged for 3 min at 21130 × g, and the supernatant was used for PCR.
Sea-slug-specific 18S rRNA primers (18A1seq and 1800seq; Vonnemann et al. 2005), universal histone H3 primers (Colgan et al., 1998), universal COI primers (Folmer et al., 1994) and Berthella-specific 16S rRNA primers (developed for this study) were used to amplify the genes of interest for all the specimens. The PCR master mix for each sample was prepared using 37.25 μl deionized water, 5.00 μl Dream Taq PCR buffer (Fischer Scientific), 2.5 μl 10 mg ml-1 bovine serum albumin (BSA), 1.00 μl 40 mM deoxynucleotide triphosphates, 1.00 μl 10 μM primer 1, 1.00 μl 10 μM primer 2, 0.25 μl 5 mg ml-1 Dream Taq (Fischer Scientific) and 2.00 μl extracted DNA. The reaction conditions for the 18S rRNA gene were as follows: an initial denaturation for 4 min at 95 °C; 38 cycles of (1) denaturation for 30 s at 94 °C, (2) annealing for 30 s at 52.5 °C and (3) elongation for 2.5 min at 72 °C; and a final elongation for 10 min at 72 °C. The reaction conditions for the 16S rRNA and histone H3 genes were as follows: an initial denaturation for 2 min at 94 °C; 30 cycles of (1) denaturation for 30 s at 94 °C, (2) annealing for 30 s at 50 °C and (3) elongation for 1 min at 68 °C; and a final elongation for 7 min at 68 °C. Reaction conditions for COI were: an initial denaturation for 3 min at 95 °C; 35 cycles of (1) denaturation for 45 s at 94 °C, (2) annealing for 45 s at 45 °C, and (3) elongation for 2 min at 72 °C; and a final elongation for 10 min at 72 °C. An agarose gel electrophoresis with ethidium bromide was run using the PCR products. The resulting gel was viewed under UV light and the samples producing bands of appropriate size (about 1980 bp for 18S rRNA, 375 bp for histone H3, 495 bp for 16S rRNA and 695 bp for COI) were purified using a GeneJET PCR purification kit (Thermo Scientific), following the manufacturer’s protocol. DNA concentration in purified PCR products was quantified using a NanoDrop 1000 spectrophotometer (Thermo Scientific). The primers were diluted to 4.0 μM and the PCR products were diluted to between 5 and 30 ng μl-1 before being sent out to Source BioScience Inc. for Sanger sequencing. The sequences obtained for each gene were edited, assembled and extracted using the programme Geneious Pro R11 (Kearse et al., 2012).
Phylogenetic analyses
The sequences generated by us and those obtained from GenBank were aligned using the programme MUSCLE v. 3.8.4 (Edgar, 2004) featured in Geneious. The alignments were trimmed at both 5′ and 3′ends. For the 16S rRNA and 18S rRNA alignments, hypervariable regions, which were difficult to align, were identified and removed from the analyses using the programme Gblocks v. 0.91b (Castresana, 2000). Two Gblocks parameters were defined as follows for the 16S rRNA and 18S rRNA alignments, respectively: minimum number of sequences for a conserved position, 12 and 10; and minimum number of sequences for a flanking position, 18 and 15. The following parameters were the same for both genes: maximum number of contiguous non-conserved positions, 8; minimum length of a block, 10; and allowed gap positions, none. The 18S rRNA sequences generated by us contained internal regions not present in the sequences downloaded from GenBank; these internal regions were removed prior to analysis. After the trimming and removal of internal regions, final fragment lengths were as follows: 1048 bp for 18S rRNA, 328 bp for histone H3, 387 bp for 16S rRNA and 593 bp for COI. The alignments were concatenated in Geneious. The programme jModelTest 2 (Posada, 2008) was used to determine the best-fit model of evolution for each gene sequence under the Akaike information criterion (Akaike, 1974): GTR (16S rRNA), HKY + I (COI), GTR + I (histone H3) and GTR + I + G (concatenated dataset of all four genes). Bayesian and maximum likelihood (ML) phylogenetic analyses were conducted on the same concatenated dataset of all four genes. The Bayesian analysis was run in MrBayes v. 3.2.6 (Ronquist & Huelsenbeck, 2003), partitioned by gene, with the gene specific models obtained with jModelTest; the analysis was conducted with two runs of six chains for 10 million generations, with a sampling interval of 1,000 generations and 25% burn-in. The ML analysis was conducted in RAxML v. 8.0 (Stamatakis, 2014) using the bootstrap + consensus option and the GTR + I + G model with 10,000 bootstrap replicates. Branches were considered to be strongly supported if Bayesian posterior probabilities (PP) ≥ 0.95 (Alfaro & Holder, 2006) and ML bootstrap support (BS) values ≥ 70% (Hillis & Bull, 1993). In addition to specimens of B. californica, other representatives of the family Pleurobranchidae (species of Berthella de Blainville, 1824, Pleurobranchus Cuvier, 1804 and Berthellina Gardiner, 1936) were included for comparison (Table 1). The genus Pleurobranchaea Leue, 1813 has been shown to be sister to all other Pleurobranchomorpha (Göbbeler & Klussmann-Kolb, 2010), so Pleurobranchaea meckeli (de Blainville, 1825) was used as the outgroup in the phylogenetic analyses.
Species delimitation analysis
An automatic barcode gap discovery (ABGD) analysis was conducted to determine the number of species present in the dataset, based on the gap in the distribution of pairwise p-distances among sequences. Two analyses were run, one with the 16S rRNA gene and another with the COI DNA sequence data for B. californica. The programme MEGA 7.0.16 (Kumar et al., 2016) was used to calculate pairwise p-distances for the data set using the Kimura 2-parameter (K2) and Tamura–Nei (TN) models. The p-distance matrix was then analysed using the ABGD webtool (http://wwwabi.snv.jussieu.fr/public/abgd/abgdweb.html) (Puillandre et al., 2012) with default settings (X = 1.5, P = 0.001–0.1).
Morphological analyses
Photographs of the animals were obtained after collection of the specimens in the field. Images of one to three specimens from each clade recovered in the molecular analyses were compared to each other for similarities and differences in external morphology. Also, one to three specimens of each species recovered in the species delimitation analysis were examined morphologically to confirm the ABGD hypotheses of species limits. Morphological studies were based on the shell, jaw and radula, and were done using a Leica EZ4D stereo microscope. For each specimen studied, we first separated the shell from the body and extracted the buccal mass. The buccal mass and the shell were placed in a 10% NaOH solution for c. 45 min to dissolve excess tissue. The jaw was then removed and placed in deionized water for c. 5 min to remove any residual NaOH before being mounted on a scanning electron microscopy (SEM) stub. The radula and the shell were left in the NaOH solution for up to 3 days to dissolve any remaining tissue and then placed in deionized water for 5 min, and then mounted on SEM stubs. These structures were then sputter coated with gold. Images were obtained using a Jeol JSM-6010 SEM at the California State Polytechnic University, Pomona. The reproductive system of each specimen was also dissected and drawn using a Nikon SMZ-100 dissecting microscope equipped with a camera lucida.
RESULTS
Phylogenetic analyses
Both Bayesian and ML analyses produced trees with similar topologies (Fig. 3A). The traditional classification of Pleurobranchidae is generally supported in these results. The genera Pleurobranchus (PP = 0.97, BS = 77), Berthella (PP = 0.97, BS = 84) and Berthellina (PP = 1, BS = 100) are each well supported as being monophyletic. However, representatives of Berthella californica do not form a clade with other Berthella species; instead they constitute a monophyletic group (PP = 1, BS = 92) that is sister to the clade comprising the two species of Berthellina sequenced by us (PP = 0.97, BS = 70). Our analyses showed B. californica to consist of two main clades. One clade comprises all the specimens of the southern morphotype (PP = 1, BS = 100) and the other all the specimens of the northern morphotype (PP = 1, BS = 99; Fig. 3A).
DNA-sequence-based species delimitation
The ABGD analyses of both the 16S rRNA and COI DNA sequence data showed that for both the K2 and TN models the specimens identified as B. californica represented two putative species and that these species corresponded to the two clades recovered in the phylogenetic analyses, consisting respectively of specimens belonging to the northern and southern morphotypes (Fig. 3B, C; Table 2).. The appropriate names assigned to these species are discussed below, in the Systematics Descriptions and the Discussion.
Table 2.
Results of the ABGD species delimitation (K2 and TN models) based on DNA sequence data for COI and 16S rRNA.
| Species | COI | 16S rRNA |
|---|---|---|
| Berthella californica | SG1, —, — | SG1, SG3, JG40 |
| B. chacei | —, SG12A, SG12B, SG13,—, —, SG95 , — | SG2, SG12A, SG12B, SG13, SG38, SG42, SG95, EED-Phy-975 |
Species recovered are organized in columns with the final names presented in the text. Isolate numbers are arranged by the species to which they belong; missing data are indicated by dashes.
Morphological analyses
There were consistent differences in several aspects of the external as well as the internal morphology, including jaw elements, radular teeth and reproductive systems of the two putative species delimited in the ABGD analyses. These differences are discussed below.
SYSTEMATIC DESCRIPTIONS
Family PLEUROBRANCHIDAE J. E. Gray, 1827
Genus Berthella de Blainville, 1824
Type species: Bulla plumula Montagu, 1803; by monotypy.
Remarks: The phylogenetic analyses recovered a well-supported clade containing all animals identified as Berthella californica. This clade is not nested within, nor is it sister to other species of Berthella (including the type species of the genus); instead it is sister to the genus Berthellina, represented by Berthellina citrina and Berthellina edwardsii in this study. These results are consistent with previous analyses using different markers (Göbbeler & Klussmann-Kolb, 2010).
These results are also consistent with the observation that Berthella californica possesses some morphological characteristics that are not present in other species of Berthella. For example, the gill rachis of B. californica is tuberculate (Bergh, 1897; present study) whereas in other species of Berthella it is smooth (see Burn, 1962; Macnae, 1962). Lance (1966) transferred P. californicus to Berthella based on Dall’s original description of the gill rachis of this species as being smooth. Dall disregarded or was unaware of Bergh’s (1897) description of the gill rachis of the type material of P. californicus, which was indicated as being tuberculate. Gosliner & Bertsch (1988) described the gill rachis of the northern morphotype B. californica as smooth, overlooking MacFarland’s (1966, p. 89) description of it as tuberculate. Specimens identified as B. californica and examined in this study all had tuberculate gill rachises, suggesting that Dall’s (1900) and Gosliner & Bertsch’s (1988) descriptions of the gill rachis of B. californica were inaccurate. Therefore, the transfer of P. californicus to Berthella by Lance (1966) was apparently done based on an incorrect assumption.
Although our results and those of Göbbeler & Klussmann-Kolb (2010) suggest B. californica is sister to Berthellina rather than Berthella or Pleurobranchus, Berthellina and B. californica are morphologically quite dissimilar. Berthellina is known to have smooth yellow to orange-red bodies, a highly reduced shell and elongate/serrated radular teeth (Burn, 1962), whereas none of these characters are present in B. californica. A thorough review of other genera in Pleurobranchidae (Willan, 1987) indicates there are no available genus-level names appropriate for B. californica and consequently a new genus name probably needs to be introduced for this species. However, we refrain from doing so here because we recognize that a more comprehensive dataset of pleurobranchids is necessary before sound conclusions can be drawn.
Berthella californica (W. H. Dall, 1900)
(Figs 1A–D, 4A, B, 5, 6A, 7A–D)
Pleurobranchus californicus W. H. Dall, 1900: 92–93 (San Pedro, California; two syntypes: one shell, USNM 107893, San Pedro Bay, California, leg. I. S. Oldroyd; one shell CASIZ 064302, White’s Point, San Pedro, California, leg. I. S. Oldroyd).
Other material examined: One specimen (preserved length 30 mm), Neptune’s Reef, Ventura, California, USA, 8 November 2014, (CPIC 1328). One specimen (preserved length 34 m), County Line, Ventura, California, USA, 2 April 2016, (CPIC 1761).
External description: Figure 1A, B, D. Body elongate-oval. Mantle oval, partly covering lateral sides of foot but not the posterior portion. Mantle creamy white to beige to brown covered with numerous small uniformly-sized and distributed white specks. Mantle with white marginal band. Oral veil broad, trapezoidal, off white with white band along anterior border. Rhinophores rolled, emerging between mantle and oral veil, fused together at base. Rhinophores off-white with white dorsal longitudinal band, more pronounced near tips. Foot off-white with white marginal band. In both specimens examined, gill with 22 pinnae and gill rachis with 1 tubercle at base of each pinna.
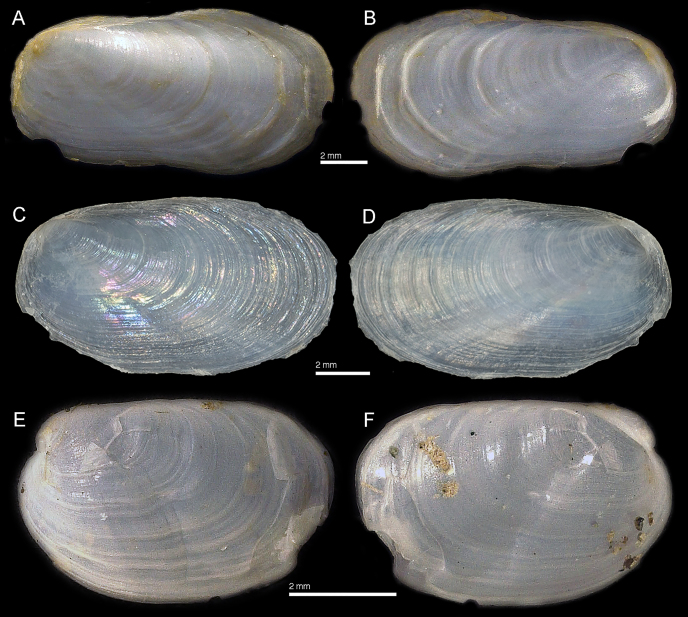
Shell: Figure 4A, B. Oval, elongate, slightly elevated near protoconch, anterior margin regularly curved. Shell with distinct growth lines. Shell measurements for the Ventura specimen CPIC 1761: protoconch diameter c. 773.33 μm, shell length c. 16.6 mm, shell width c. 10.9 mm.
Figure 4.
SEMs of the shells of Berthella californica and B. chacei. A,B. Specimen of B. californica from Ventura, California (CPIC 1761),. A. Dorsal view of the teleoconch. B. Detail of protoconch. C,D. Specimen of B. chacei from Cape Arago, Oregon (CPIC 1409). C. Dorsal view of the teleoconch. D. Detail of protoconch.
Radula and jaw: Figure 5A–D. Radular formula: 72 × 168.0.168. Innermost teeth simple, hook shaped with base of main cusp concave. Inner lateral teeth simple, hook shaped with rounded tips. Outer teeth long, slender with pointed tips, decreasing in size toward outer margin in Ventura (California) specimen CPIC 01328. Jaw elements with central cusp bearing none to 2 small denticles on each side in all material examined. Denticulation not symmetrical. Some jaw elements completely smooth in Ventura, (California) specimen CPIC 01761.
Reproductive system: Figure 6A. Androdiaulic. Ampulla slender, curved but not convoluted, branching into 2 separate ducts proximally near female gland mass. One branch entering the female gland mass, the other branch joining seminal receptacle. Vas deferens emerging near base of ampulla, turning into a prominent prostate, thickening distally toward penis. Penis hook-shaped with pyriform base. Penial gland elongate, uniformly tubular, emerging near genital opening. Bursa copulatrix oval. Seminal receptacle elongate, convoluted, somewhat wider at blind distal end. Vaginal tract joining seminal receptacle about halfway to bursa copulatrix. Vaginal canal convoluted distally toward vaginal orifice, near base of penis.
Figure 6.
Reproductive system of Berthella californica and B. chacei. A. Specimen of B. californica from Ventura, California (CPIC 1328). B. Specimen of B. chacei from Sonyang-myeon, South Korea (CPIC 2119).
Geographic range: Ventura County, southern California to the Pacific Coast of Panama (present study). Also reported from Baja California, Mexico, Cocos Island, Costa Rica and the Galapagos Islands in the eastern Pacific Ocean (Behrens & Hermosillo, 2005; Camacho-García et al., 2005; García-Méndez & Camacho-García, 2016).
Biology: The specimens collected in Ventura, California were found at a depth of c. 25 m, on the vertical surface of a reef about 1 mile off-shore. One specimen from Cocos Island, Costa Rica was found at a depth of c. 86 m on red algae and rocks (García-Méndez & Camacho-García, 2016). The egg mass of this species (Gardner, 1999; Fig. 1C) is a delicate, loosely-coiled ribbon containing eggs similar in colour to the mantle of the adult that laid it (present study).
Remarks: Berthella californica was originally described by Dall (1900) as Pleurobranchus californicus based on two specimens obtained from unspecified depth by Ida S. Oldroyd in San Pedro, Los Angeles County, California. No illustrations were provided. The external coloration was described as “waxen white” and the dorsum as “apparently smooth, or rather like the skin of an orange, not tuberculate, but, under a glass, showing obsolete distant pustules.” The gill was described as “short, its stem finely granular, not tuberculate” and the shell as “rather long and narrow, sub-rectangular, longitudinally obsoletely striate on the left side, obscurely obsoletely punctate near the anterior edge, and covered with a very thin periostracum which reflects nacreous tinges of color” and “white and thin, with a small spiral nucleus; the left margin somewhat recurved, the central part moderately convex.” Oldroyd (1927: 52) confirmed that “the type” of P. californicus was at the Oldroyd Collection at Stanford University (# 307). According to MacFarland (1966: 82) the shell of one specimen “was evidently returned to Mrs. Oldroyd” but “the animal in alcohol was sent to Bergh for anatomical study.”
Bergh (1897) examined one of the syntypes of P. californicus (sent by W. H. Dall), describing its internal anatomy and illustrating the radular teeth and jaw elements. Bergh (1897: 380) transferred this species to the genus Oscaniella based on the presence of the following: a “double row of nodules on the gill rachis,” a “caudal gland, the simple genital opening” and the “rather large shell lying at the middle of the body,” which “covers most of it the intestinal mass.” However, Bergh (1897) noted that O. californica had smooth jaw elements while in other species of Oscaniella the jaw elements were denticulated. It appears that R. Bergh sent the specimen (or the remaining parts) back to W. H. Dall, who deposited them in the USNM collections. Today, only the shell of the syntype remains (USNM 107893), but according to E. Strong (personal communication) the notes associated with this shell read “animal in alc.” and “types in alc.” indicating that the specimen was once in ethanol. Additionally, as given in Dall’s original description, there were two specimens in the type lot, the other now being CASIZ 064302.
MacFarland (1966) transferred californica from Oscaniella back to Pleurobranchus and reproduced both the original description by Dall (1900) as well as the anatomical account by Bergh (1897). MacFarland (1966) also examined a specimen lot deposited at the Stanford University Paleontological type collection (# 6272), containing two shells and several labels. One of the labels in I. S. Oldroyd’s handwriting reads: “Pleurobranchus californicus Dall, Type, Whites Point”. Another label in a different handwriting reads: “Pleurobranchus californicus Dall, San Diego, Cal.; C. W. Gripp, San Diego” and on the reverse “large one is the type.” MacFarland (1966) noted that these two shells belonged to two different species and argued that because they are different, they could not be syntypes. Thus, MacFarland (1966) declared the largest to be the “holotype” of P. californicus. Evidently, MacFarland (1966) was assuming that these two shells were part of the original type series. However, this shell cannot be the holotype since it was not designated in the original description (ICZN 1999: Article 72.1). These two specimens are now deposited at the CASIZ collection (E. Kools, personal communication). It is likely that at some point in the past, the C. W. Gripp specimen from San Diego (now CASIZ 064303) was added to the tray holding Dall’s syntype (originally no. 307, now CASIZ 064302). Thus, the C. W. Gripp specimen from San Diego (CASIZ 064303) has no type status. MacFarland (1966) illustrated the shell of the syntype (CASIZ 064302), which agrees with the shells of other specimens here identified as B. californica.
Lance (1966) examined several specimens collected from Monterey Bay to the San Diego area, California. Following the accepted taxonomy at the time (Odhner, 1939; Burn, 1962; Macnae, 1962), Lance (1966) transferred P. californicus to Berthella based on Dall’s (1900) description of the absence of tubercles on the gill rachis, the presence of hook-shaped radular teeth and a shell longer than half the length of the body. Additionally, Lance (1966) differentiated B. californica from Pleurobranchus digueti Rochebrune, 1895 (also reported from San Pedro by Oldroyd, 1927) and considered it a valid species of Pleurobranchus (see Goodheart et al., 2015).
In this study we examined specimens collected across the range of B. californica and its synonyms. The ABGD analysis of the 16S rRNA and COI DNA sequences recovered the specimens of the northern and southern morphotypes as distinct species (Table 2); northern and southern morphotypes also formed reciprocally monophyletic clades in the phylogenetic analyses (Fig. 3). The shells of specimens of the southern morphotype are elongate, with the nucleus located in a posterodorsal position, slightly elevated vs the rest of the teleochonch. These characteristics are consistent with those of the syntypes of B. californica here examined (Fig. 7A–D) and therefore we confidently assign this name to the southern species. Although Bergh (1897) illustrated the jaw elements of the type specimen of B. californica with no denticles,, our SEMs of the jaw elements (Fig. 6D) show that the denticles on each side of the central cusp may be present or absent and, if the former, are very small and number no more than 2. This suggests that the denticles might have been missed by Bergh (1897), who used a light microscope. Characters of the radular teeth of the type specimen of B. californica, as described by Bergh (1897), also agree with the material here examined, further confirming our conclusions.
Figure 7.
Type specimens. A,B. Shell of a syntype of Pleurobranchus californicus (CASIZ 064302); dorsal view (A), ventral view (B). C,D. Shell of a syntype of P. californicus (USNM 107893); dorsal view (C), ventral view (D). E,F. Shell of a syntype of P. chacei (CASIZ 065576); dorsal view (E), ventral view (F).
Berthella chacei (J. Q. Burch, 1944)
(Figs 1E–H, 4C, D, 6B, 7E, F, 8)
Pleurobranchus chacei J. Q. Burch, 1944: 17–18, text fig. (p. 17) (in tide pools and under rocks, Crescent City, California; one shell, CASIZ 65576, leg. E. P. Chace, 26 June 1933).
Pleurobranchus californicus denticulatus MacFarland, 1966: 84–89, pl. 5, figs 1–5, pl. 13, figs 25–34, pl. 16, fig. 12 (Point Pinos (Great Tide Pool) and Point Lobos, Monterrey Bay, California; syntypes: one specimen, USNM 575223, Point Pinos, leg. G. E. MacGintie, 21 Jul 1931; one specimen and four microscope slides, CASIZ 21630, Point Pinos, leg. G.E. MacGintie; one specimen, CASIZ 21664, Point Lobos, 1894).
Other material examined: Two specimens (preserved length 20 mm): low rocky intertidal, Cape Arago, Oregon, USA, 18 June 2015, (CPIC 1409). One specimen (preserved length 12 mm): low rocky intertidal, Whiskey Creek, Oregon, USA, 16 Jun 2015, (CPIC 1407). Two specimens (preserved length 18 mm): Port Susan, Sonyang-myeon, Yangyang-gun, Gangwon-do, South Korea, 1 Mar 2011, (CPIC 2119, CPIC 2120).
External description: Figure 1E–H. Body elongate-oval. Mantle oval, covering most of foot, translucent white to creamy white, bearing variably-sized white tubercles. Mantle with opaque white marginal band. Oral veil broad, trapezoidal, same colour as mantle, with opaque white band along anterior border, bearing variably-sized opaque white spots throughout. Rhinophores rolled, emerging between the mantle and oral veil, fused together at base, same color as mantle, with scattered small opaque white specks. Foot same colour as mantle with scattered small opaque white specks. Gill bipinnate, with 18 pinnae in the 20 mm-long specimens from Cape Arago, Oregon (CPIC 1409), 19 pinnae in the 12 mm-long specimen from Whiskey Creek, Oregon (CPIC 1407) and 23 pinnae in one of the 18 mm-long specimens from South Korea (CPIC 2119). Gill rachis with 1 tubercle at base of each pinna in all material examined.
Shell: Figure 4C, D. Oval, semi-quadrangular, conspicuously elevated near protoconch, slightly flattened near anterior margin. Shell with distinct growth lines. Shell measurements for the Oregon specimen CPIC 01409: protoconch diameter c. 550 μm, shell length c. 15 mm, shell width c. 9.75 mm.
Radula and jaw: Figure 8A–D. Radular formula: 54 × 70.0.70. Innermost teeth simple hooks. Inner lateral teeth simple hooks, increasing in size toward outer margin, quickly becoming uniform. Outer teeth less hook-shaped. About 15 outer teeth decreasing in size progressively in one South Korean specimen (CPIC 02119). Jaw elements with central cusp bearing 1–3 denticles on each side in other South Korean specimen (CPIC 02110). Denticulation not symmetrical.
Figure 8.
SEMs of the radular teeth and jaw elements of the specimen of Berthella chacei from Sonyang-myeon, South Korea (CPIC 2119). A. Innermost teeth. B. Mid-lateral teeth. C. Outer teeth. D. Jaw elements.
Reproductive system: Figure 6B. Androdiaulic. Ampulla curved but not convoluted, narrowing proximally, branching into two ducts, one duct leading to female gland mass, other duct connecting to seminal receptacle. Vas deferens emerging from female gland mass near base of ampulla, thickening toward prostate, curving back toward base past prostate, leading to conical penis. Penial gland tubular, emerging near base of penis, curved but not convoluted, thickening toward distal blind end. Seminal receptacle with long, convoluted stalk leading to bursa copulatrix. Bursa copulatrix about three times as large as seminal receptacle. Vaginal tract joining stalk of seminal receptacle near base of bursa copulatrix, with three curves about halfway to vaginal orifice. Vaginal orifice immediately ventral to penis.
Geographic range: Known from the Sea of Japan (East Sea) to San Diego, California (Martynov, 1988; Behrens & Hermosillo, 2005; this study).
Biology: The diet of B. chacei (as B. californica) has been confirmed through field observations, laboratory feeding trials and the examination of gut contents. It consists of plakinid sponges, including Oscarella carmela (see Goddard, 2007). The egg mass is a white, coiled, wavy ribbon (MacFarland, 1966; Hildering & Miller, 2007; Chichvarkhin, 2016; Nakano 2018; this study) (Fig. 1H). Planktotrophic veliger larvae, with eyespots and shells averaging 153 μm long, hatched after an embryonic period of 18 days at 11–14 °C (Goddard, 1984).
Remarks: J. Q. Burch (1944) described Pleurobranchus chacei based on five shells obtained from Emery and Elsie Chace and collected in Crescent City, California. Based on the collectors’ notes, J. Q. Burch (1944: 17) mentioned the live animals were white, and had been “laughingly described as looking like Diadora [sic.] aspera [= Diodora aspera (Rathke, 1833)] which had taken off its shell and gone for a walk in its skin.” The shell was described as “an internal plate so fragile that we never succeeded in getting really good material” and “squarish oblong,” “thin and membraneous, semi-transparent,” “somewhat iridescent,” with the concentric sculpture consisting “entirely of elongated depressions,” “of small pits on the surface, and the background of tiny pits of varying size and shape.” J. Q. Burch (1944) noted some differences between his material and the original description of P. californicus but did not elaborate.
Years later, MacFarland (1966: 84) commented: “a specimen of Pleurobranchus was taken by Mr. and Mrs. E. P. Chace at Crescent City, California, and was described briefly by Burch (1944) and given the new name of Pleurobranchus chacei. Based upon the meager description, the present writer expressed the opinion to Mr. Burch that the shell in question was more probably Pleurobranchus californicus and was so given by him in the minutes of the Conchological Club of Southern California, No. 37, 1944, pp. 17–18.” Additionally, MacFarland (1966: 84) indicated: “in 1946, careful comparison of the shell of Pl. chacei sent to the California Academy of Sciences by Mr. Burch, as the type, was made with the two shells of the Oldroyd collection. It closely resembles the larger of the two on the type tray of Pl. californicus Dall. From this comparison it is evident that Pl. chacei is a synonym of Pl. californicus Dall.”
At the same time, MacFarland (1966) described the new subspecies Pleurobranchus californicus denticulatus based on three specimens from Point Pinos, Monterey, California. MacFarland (1966) provided detailed anatomical descriptions of these specimens and concluded that in general they resembled P. californicus. However, MacFarland (1966) noted that the Point Pinos specimens differed from P. californicus in that the dorsum is distinctly tuberculate in life. MacFarland (1966) also pointed out that the gill rachis of P. californicus denticulatus has two rows of tubercles, whereas Dall (1900) described the gill of P. californicus as granular not tuberculate. Other differences between P. californicus denticulatus and P. californicus have been noted in the literature. These include the presence of a pedal gland in P. californicus, the size of the radular teeth, which are larger according to Bergh’s (1897) description, and the shape of the jaw elements, which are smooth in P. californicus but in MacFarland’s (1966) specimens have 1–4 denticles.
Lance (1966) examined specimens collected from Monterey Bay to San Diego, which he identified as B. californica, but did not comment on any consistent differences among the specimens examined. Roller (1970: 372–373) commented that “the naming of a new subspecies, Pleurobranchus californicus denticulatus by MacFarland presents a difficult nomenclatural problem.” According to Roller (1970), the problem stems from the contradiction between Dall’s and Bergh’s descriptions of the gill rachis as smooth or tuberculate, respectively, and the fact this is a generic difference, with Berthella being characterized by having smooth gill rachises and Pleurobranchus tuberculate. Roller (1970) proposed that since the gill rachis of P. californicus denticulatus is described as tuberculate, it must be placed in Pleurobranchus and cannot remain a subspecies. He also suggested that further study of live specimens from the type localities of both taxa will be required before this problem can be satisfactorily solved.
Bertsch et al. (1972: 307) reported P. californicus denticulatus from Pebble Beach, San Mateo County, California and commented “we have insufficient material to propose a solution to the taxonomic problems of this species, other than stating that we agree with Roller (1970; 372–373) that P. californicus denticulatus should be given specific status.” Lee & Foster (1985) reported animals from Point Craven, Alaska identified as Berthella denticulatus (MacFarland, 1966) [sic.], considerably expanding the known range of this nominal species from Pacific Grove, California to Alaska, but did not provide descriptions of the specimens. More recently, Gosliner & Bertsch (1988) included both B. chacei and P. californicus denticulatus in the synonymy of B. californica, arguing that the difference in jaw element morphology is not consistent even in the same individual and therefore not a reliable taxonomic trait. Moreover, Gosliner & Bertsch (1988) also discussed discrepancies in the reproductive morphology between their specimens and the illustrations of the reproductive system by MacFarland (1966: pl. 16, fig. 12). Specifically, MacFarland (1966) illustrated the reproductive anatomy of P. californicus denticulatus as lacking a seminal receptacle and with the bursa copulatrix connecting directly to the oviduct. However, Gosliner & Bertsch (1988) examined specimens from the same general area (Point Pinos, Monterey and Duxbury Reef, Marin County, California) and found that a seminal receptacle is present and that the oviduct enters the female gland mass near the albumen gland, rather than joining with the bursa copulatrix. Unfortunately, Bergh (1897) did not examine the reproductive anatomy of P. californicus, thus comparison between these two taxa has not been possible until now.
In this study we examined 11 specimens identified as the northern morphotype and sampled from Central California to South Korea. Sequences from these animals formed a well-supported clade, which was sister to the southern morphotype (Fig. 3), and the ABGD analysis of the 16S rRNA and COI DNA sequences recovered the northern morphotypes as a distinct species (Table 2). The shells of these specimens are semi-quadrangular, proportionally less elongate that those of the southern morphotype and the nucleus is conspicuously elevated (Fig. 4C, D); these details of the shell outline and nucleus agree with the original description by J. Q. Burch (1944) and the syntype of B. chacei (Fig. 7E, F). Thus, the name B. chacei is here resurrected for the northern morphotype. While we cannot confirm the identity of P. californicus denticulatus based on shell morphology, MacFarland (1966: 85) did describe its external morphology: “ground color of dorsum, frontal veil, and top of foot deep cream, the larger and smaller tubercles of mantle, frontal veil, and dorsal surface of foot dead white; the peripheral portion of mantle, top of foot, and frontal veil sprinkled with minute white flecks; the distal halves of the rhinophores thickly sprinkled with white.” This indicates that MacFarland’s subspecies is nearly identical to the specimens of B. chacei examined here, leaving no doubt that they belong to the same species. Therefore, we here consider P. californicus denticulatus as a junior synonym of B. chacei.
DISCUSSION
The main conclusion of this study is that the northern and southern morphotypes of the nominal species Berthella californica constitute distinct species. In addition to genetic differences, B. californica and B. chacei also exhibit consistent differences in size and in aspects of their external and internal morphology. Berthella chacei is typically smaller than B. californica. The largest specimen of B. californica examined anatomically in this study (34 mm long) was about 0.6 times as large as the largest specimen of B. chacei examined (20 mm long) and, as described in the Introduction, the two species are known to reach lengths of 127 mm and 70 mm, respectively. Berthella chacei has a white to creamy white mantle whereas B. californica has a creamy white to brown mantle. In B. chacei, the white dorsal spots on the mantle are on tubercles and the rest of the mantle is typically smooth, whereas in B. californica the white dorsal spots are not on the tubercles and the entire mantle is always smooth. The white dorsal spots on the mantle vary in size in B. chacei, but they are typically of uniform size in B. californica. White specks are present on the rhinophores, oral veil and the foot of B. chacei, whereas in B. californica typically no structure other than the mantle bears any white spots. Most specimens of B. californica possess a longitudinal white dorsal stripe on each rhinophore (Fig. 1A–C), a character not present in B. chacei (Fig. 1E–G). Finally, the egg mass of B. chacei (Fig. 1H) is white, wavy and relatively rigid, compared to the darker coloured, more delicate and less wavy egg mass of B. californica (Fig. 1C).
Due to these consistent external differences between B. californica and B. chacei, identification of published and online photographic records is possible and this allows a further refinement of our understanding of the distribution of the two species. Molecular systematic data have confirmed the range of B. chacei to extend from the Sea of Japan (East Sea) in the northwestern Pacific to Monterey County in central California, but based on online photographs, this species occurs as far south as San Diego, California. At the same time, molecular systematic data have confirmed that B. californica ranges from Ventura County to Panama, but published photographs extend this range from San Miguel Island, Santa Barbara County, California to the Galapagos Islands. This means that the range of the two species overlaps along the entire SBC. However, throughout its range, B. chacei is found both intertidally and subtidally, whereas B. californica is known only from subtidal habitats (it is most likely that Oldroyd’s original specimens of the latter were obtained by San Pedro fishermen through dredging or trawling).
In addition to the external differences between B. californica and B. chacei there are consistent differences in their radular teeth, jaw elements and reproductive anatomy. The eight to ten outer radular teeth of B. chacei (Fig. 8C) decrease in size progressively whereas the outer radular teeth of B. californica (Fig. 5C) do not. The jaw elements of both species may or may not have prominent denticles on each side of the central cusp; when present they number up to three in B. chacei (Fig. 8D), whereas only up to two (both very small) occur in B. californica (Fig. 5D). There are also differences in the reproductive morphology of these species. The ampulla is very narrow in B. californica (Fig. 6A) but much wider in B. chacei (Fig. 6B); the penis is hook-shaped in B. californica but not in B. chacei (Fig. 6B); the distal vaginal canal is convoluted in B. californica but not in B. chacei; finally, the bursa copulatrix is oval in B. californica but spherical in B. chacei. We found a direct connection between the ampulla and the seminal receptacle in our specimens of both B. californica and B. chacei. Gosliner & Bertsch (1988) also described the reproductive system of B. chacei (as Berthella californica) and stated that the seminal receptacle was adjacent to the ampulla but that there was no connection between the two structures. This connection was also not reported by MacFarland (1966) in the description of Pleurobranchus californicus denticulatus. However, MacFarland (1966) indicated that the course of the oviduct was not clear in his dissection, which suggests that he might have missed this connection. A direct connection between the ampulla and the seminal receptacle has not been reported in any other species of Berthella. This may provide additional support for the assignment of these two species to a putative new genus.
Allopatric speciation has been proposed as an important driver of cladogenesis in North Pacific marine organisms (Liu et al., 2011; Shen et al., 2011; Lindsay & Valdés 2016). In this scenario, genetic differences accumulate over time because of interruptions in gene flow between populations due to cycles of ice sheet formation and melting during glacial and interglacial periods, respectively (Liu et al., 2011; Shen et al., 2011; Lindsay & Valdés 2016). Evidence for this process can be seen in B. chacei where the populations in the northwestern Pacific are slightly different genetically compared to the populations in the eastern Pacific. Within the main B. chacei clade, specimens from Russia and South Korea formed a clade; although not well supported in the Bayesian analysis (PP = 0.59), support for this clade was strong in the ML analysis BS = 74). A future haplotype network analysis can help determine whether there is currently any gene flow between the northwestern and the eastern Pacific populations. More specimens must be obtained from the entire range of B. chacei to investigate the genetic structure of the different populations of this species.
There are other intriguing similarities between the B. californica species complex and organisms hypothesized to have diversified due to processes driven by glacial cycles. These similarities may shed light on the process of speciation between B. californica and B. chacei. For example, in the nudibranch Diaulula sandiegensis species complex examined by Lindsay et al. (2016), the two constituent species (D. sandiegensis and D. odonoghuei) are hypothesized to have evolved allopatrically in a process driven by glacial cycles. The hypothesis by Lindsay et al. (2016) requires that D. sandiegensis (the southern species) evolved in the eastern Pacific and D. odonoghuei (the northern species) in the western Pacific, and that the latter subsequently dispersed eastward. Lindsay et al. (2016) also indicated that D. sandiegensis is restricted mainly to subtidal habitats, where its range overlaps with that of D. odonoghuei, suggesting character displacement after secondary contact. The only differences between Diaulula and Berthella are as follows: (1) the range overlap between the two species of Diaulula is more extensive and further north, from Oregon to Washington; (2) the southern species of Berthella is restricted to subtidal habitats, whereas the southern species of Diaulula is intertidal; and (3) the northern species of Berthella is uncommon in the zone of overlap, whereas this is not the case for Diaulula. The overall similarities between these two unrelated taxa could be indicative of common evolutionary processes producing the observed distribution patterns. Finding additional examples of this general pattern will help shed light on the process of speciation of benthic organisms in the North Pacific.
At least two studies have used B. californica as a model organism in the past. LaForge & Page (2007) studied the larval development stages in B. californica to determine whether larval characters can be phylogenetically informative in heterobranch sea slugs, and Goddard (2007) used B. californica to investigate the diet of Berthella species from the eastern Pacific Ocean. However, from the current study it is clear that both Goddard (2007) and LaForge & Page (2007) have, in fact, studied B. chacei. This highlights the importance of documenting cryptic diversity in order to avoid potential erroneous conclusions when studying the biology and ecology of species complexes.
ACKNOWLEDGEMENTS
This study was supported by a grant from the National Institute of Biological Resources (grant no. NIBR-201801104), funded by the Ministry of Environment of the Republic of Korea. SEM work was conducted at the California State Polytechnic University SEM Laboratory and was supported by the National Science Foundation, USA (grant no. DMR-1429674). HG was supported by a NIH-funded MBRS RISE grant (no. 1R25GM11374801). Ellen Strong (USNM), Elizabeth Kools (CASIZ) and Lindsey Groves (LACM) assisted greatly in providing us with information on and/or photographs of type material and other museum specimens. Craig Hoover provided important specimens as well as ecological information.
Contributor Information
Hessam Ghanimi, Department of Biological Sciences, California State Polytechnic University, 3801 West Temple Avenue, Pomona, California 91768, USA.
Jeffrey H R Goddard, Marine Science Institute, University of California, Santa Barbara, California 93106-6150, USA.
Anton Chichvarkhin, National Scientific Center of Marine Biology, Russian Academy of Sciences, Palchevskogo 17, 690041 Vladivostok, Russia.
Terrence M Gosliner, Department of Invertebrate Zoology, California Academy of Sciences, 55 Music Concourse Drive, San Francisco, California 94118, USA.
Dae-Wui Jung, Department of Biological Sciences, California State Polytechnic University, 3801 West Temple Avenue, Pomona, California 91768, USA.
Ángel Valdés, Email: aavaldes@cpp.edu, Department of Biological Sciences, California State Polytechnic University, 3801 West Temple Avenue, Pomona, California 91768, USA.
References
- AKAIKE, H. 1974. A new look at the statistical model identifications. IEEE Transactions on Automatic Control, 19: 716–723. [Google Scholar]
- ALFARO, M.E., HOLDER, M.T.. 2006. The posterior and the prior in Bayesian phylogenetics. Annual Review of Ecology, Evolution, and Systematics, 37: 19–42. [Google Scholar]
- BEHRENS, D.W., HERMOSILLO, A.. 2005. Eastern Pacific nudibranchs. Sea Challengers, Monterey, CA. [Google Scholar]
- BERGH, R. 1897. Die Pleurobranchiden. In: Malacologische Untersuchungen, Reisen im Archipel der Philippinen Wissenschaftliche Resultate, Band 7, Thiel 5, Lieferung 1 (Semper C., ed.), pp. 1–115, pls 1–8. C. W. Kreidel’s Verlag, Wiesbaden, Germany. [Google Scholar]
- BERTSCH, H., GOSLINER, T.M., WHARTON, R., WILLIAMS, G.. 1972. Natural history and occurrence of opisthobranch gastropods from the open coast of San Mateo County, California. Veliger, 14: 302–314. [Google Scholar]
- BURCH, J.Q. 1944. (No title). Minutes of the Conchological Club of Southern California, 37: 17–18. [Google Scholar]
- BURN, R. 1962. On the new pleurobranch subfamily Berthellinae (Mollusca: Gastropoda); a revision and new classification of the species of New South Wales and Victoria. Memoirs. National Museum of Victoria, 25: 129–148pls 1, 2. [Google Scholar]
- CAMACHO-GARCÍA, Y., GOSLINER, T.M., VALDÉS, A.. 2005. Field guide to the sea slugs of the tropical Eastern Pacific. California Academy of Sciences, San Francisco. [Google Scholar]
- CASTRESANA, J. 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular Biology and Evolution, 17: 540–552. [DOI] [PubMed] [Google Scholar]
- CHICHVARKHIN, A. 2016. Shallow water sea slugs (Gastropoda: Heterobranchia) from the northwestern coast of the Sea of Japan, north of Peter the Great Bay, Russia. PeerJ, 4: e2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE, A., CRAME, J.A.. 2010. Evolutionary dynamics at high latitudes: speciation and extinction in polar marine faunas. Philosophical Transactions of the Royal Society of London B, 365: 3655–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLGAN, D.J., MCLAUCHLAN, A., WILSON, G.D.F., LIVINGSTON, S.P., EDGECOMBE, G.D., MACARANAS, J., CASSIS, G., GRAY, M.R.. 1998. Histone H3 and U2 snRNA DNA sequences and arthropod molecular evolution. Australian Journal of Zoology, 46: 419–437. [Google Scholar]
- COOKE, S., HANSON, D., HIRANO, Y., ORNELAS-GATDULA, E., GOSLINER, T.M., CHERNYSHEV, A.V., VALDÉS, A.. 2014. Cryptic diversity of Melanochlamys sea slugs (Gastropoda, Aglajidae) in the North Pacific. Zoologica Scripta, 43: 351–369. [Google Scholar]
- DALL, W.H. 1900. A new species of Pleurobranchus from California. Nautilus, 14: 92–93. [Google Scholar]
- DARRIBA, D., TABOADA, G.L., DOALLO, R., POSADA, D.. 2012. JModelTest 2: more models, new heuristics and parallel computing. Nature Methods, 9: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDGAR, R.C. 2004. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EKIMOVA, I., KORSHUNOVA, T., SCHEPETOV, D., NERETINA, T., SANAMYAN, N., MARTYNOV, A.. 2015. Integrative systematics of northern and Arctic nudibranchs of the genus Dendronotus (Mollusca, Gastropoda), with descriptions of three new species. Zoological Journal of the Linnean Society, 173: 841–886. [Google Scholar]
- EKIMOVA, I., VALDÉS, A., SCHEPETOV, D., CHICHVARKHIN, A.. 2016. Was Gordon Robilliard right? Integrative systematics suggests that Dendronotus diversicolor Robilliard, 1970 is a valid species. Canadian Journal of Zoology, 94: 793–799. [Google Scholar]
- FLAX, S. 2017. California sidegill Berthella californica. Available at:https://www.inaturalist.org/photos/11815469. Accessed 2 May 2019.
- FOLMER, O., BLACK, M., HOEH, W., LUTZ, R., VRIJENHOEK, R.. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology, 3: 294–299. [PubMed] [Google Scholar]
- GARCÍA-MÉNDEZ, K., CAMACHO-GARCÍA, Y.. 2016. New records of heterobranch sea slugs (Mollusca: Gastropoda) from Isla del Coco National Park, Costa Rica. Revista de Biología Tropical, 64: S205–S209. [Google Scholar]
- GARDNER, S. 1999. Berthella californica Dall, 1990. The Slug Site. Available at:http://slugsite.us/bow/nudiwk79.html. Accessed 2 May 2019.
- GAO, W. 2017. Point Loma – Dean’s Maze. Available at:http://www.weiweigao.com/point-loma-deans-maze-2. Accessed 2 May 2019.
- GIROUARD, R. 2005. California Berthella. Available at:http://week.divebums.com/Sep06-2005/index.html. Accessed 2 May 2019.
- GÖBBELER, K., KLUSSMANN-KOLB, A.. 2010. Out of Antarctica? New insights into the phylogeny and biogeography of the Pleurobranchomorpha (Mollusca, Gastropoda). Molecular Phylogenetics and Evolution, 55: 996–1007. [DOI] [PubMed] [Google Scholar]
- GODDARD, J.H.R. 1984. The opisthobranchs of Cape Arago, Oregon, with notes on their biology and a summary of benthic opisthobranchs known from Oregon. Veliger, 27: 143–163. [Google Scholar]
- GODDARD, J.H.R. 2007. Berthella (Opisthobranchia: Pleurobranchidae) from the northeast Pacific Ocean prey on plakinid sponges (Homoscleromorpha, Plakinidae). Veliger, 49: 97–100. [Google Scholar]
- GODDARD, J.H.R. 2010. Photo 30403473, iNaturalist. Available at:https://www.inaturalist.org/photos/30403473. Accessed 2 May 2019. [Google Scholar]
- GOODHEART, J., CAMACHO-GARCÍA, Y., PADULA, V., SCHRÖDL, M., CERVERA, J.L., GOSLINER, T.M., VALDÉS, A.. 2015. Systematics and biogeography of Pleurobranchus Cuvier, 1804, sea slugs (Heterobranchia: Nudipleura: Pleurobranchidae). Zoological Journal of the Linnean Society, 174: 322–362. [Google Scholar]
- GOSLINER, T.M. 1996. The Opisthobranchia. In: Taxonomic atlas of the Santa Maria Basin and western Santa Barbara Channel, Volume 9, The Mollusca, Part 2, The Gastropoda (Scott P.H., Blake J.A., Lissner A.L., eds), pp. 161–213. Santa Barbara Museum of Natural History, Santa Barbara, CA. [Google Scholar]
- GOSLINER, T.M., BERTSCH, H.. 1988. A review of the genus Berthella (Opisthobranchia: Notaspidea) from the Pacific coast of North America. Veliger, 31: 46–67. [Google Scholar]
- GRANT, W.S., BOWEN, B.W.. 2006. Living in a tilted world: climate change and geography limit speciation in Old World anchovies (Engraulis; Engraulidae). Biological Journal of the Linnean Society, 88: 673–689. [Google Scholar]
- HILDERING, J., MILLER, G.. 2007. Berthella californica – mating & egg-laying. Sea Slug Forum. Available at:http://www.seaslugforum.net/message/20109. Accessed 2 May 2019. [Google Scholar]
- HILLIS, D.M., BULL, J.J.. 1993. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology, 42: 182–192. [Google Scholar]
- INTERNATIONAL COMMISSION ON ZOOLOGICAL NOMENCLATURE (ICZN) 1999. International code of zoological nomenclature. Edn 4. International Trust for Zoological Nomenclature, London. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNG, D.-W. 2014. A systematic study of opisthobranchs from Korea. Masters thesis. Sangmyung University, Seoul, Republic of Korea. [Google Scholar]
- KEARSE, M., MOIR, R., WILSON, A., STONES-HAVAS, S., CHEUNG, M., STURROCK, S., BUXTON, S., COOPER, A., MARKOWITZ, S., DURAN, C., THIERER, T.. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28: 1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLY, R.P., PALUMBI, S.R.. 2010. Genetic structure among 50 species of the Northeastern Pacific intertidal community. PLoS One, 5: e8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUMAR, S., STECHER, G., TAMURA, K.. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFORGE, N.L., PAGE, L.R.. 2007. Development in Berthella californica (Gastropoda: Opisthobranchia) with comparative observations on phylogenetically relevant larval characters among nudipleuran opisthobranchs. Invertebrate Biology, 126: 318–334. [Google Scholar]
- LAMB, A., HANDBY, B.P.. 2005. Marine life of the Pacific Northwest. Harbour Publishing, Madiera Park, BC. [Google Scholar]
- LANCE, J.R. 1966. New distributional records of some northeastern Pacific Opisthobranchiata (Mollusca: Gastropoda) with descriptions of two new species. Veliger, 9: 69–81. [Google Scholar]
- LANCE, J.R. 1994. Berthella californica, 127 mm, 145’ depth SIO (La Jolla) Canyon, January 1994. 35 mm slide in the James R. Lance Collection. Department of Invertebrate Zoology, California Academy of Sciences, San Francisco, CA. [Google Scholar]
- LEE, K. (undated). Opisthobranch field guide: Eastern Pacific. Available at:https://www.diverkevin.com/NorthAmerica/Opisthobranch-Field-Guide. Accessed 2 May 2019.
- LEE, R.S., FOSTER, N.R.. 1985. A distributional list with range extensions of the opisthobranch gastropods of Alaska. Veliger, 27: 440–448. [Google Scholar]
- LINDSAY, T., VALDÉS, A.. 2016. The model organism Hermissenda crassicornis (Gastropoda: Heterobranchia) is a species complex. PLoS One, 11: E0154265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDSAY, T., KELLY, J., CHICHVARKHIN, A., CRAIG, S., KAJIHARA, H., MACKIE, J., VALDÉS, A.. 2016. Changing spots: pseudocryptic speciation in the North Pacific dorid nudibranch Diaulula sandiegensis (Cooper, 1863) (Gastropoda: Heterobranchia). Journal of Molluscan Studies, 82: 564–574. [Google Scholar]
- LIU, J.X., TATARENKOV, A., BEACHAM, T.D., GORBACHEV, V., WILDES, S., AVISE, J.C.. 2011. Effects of Pleistocene climatic fluctuations on the phylogeographic and demographic histories of Pacific Herring (Clupea pallasii). Molecular Ecology, 20: 3879–3893. [DOI] [PubMed] [Google Scholar]
- MacFARLAND, F.M. 1966. Studies of opisthobranchiate mollusks of the Pacific coast of North America. Memoirs of the California Academy of Sciences, 6: i–xvi1–84. [Google Scholar]
- MACNAE, W. 1962. Notaspidean opisthobranchiate molluscs from Southern Africa. Annals of the Natal Museum, 15: 167–181figs. 1–7. [Google Scholar]
- MANNING, C.M. 2016. Some off-shore marine species coming to light in Galapagos, Ecuador. Galapagos Research, 68: 28–33. [Google Scholar]
- MARKO, P.B., HOFFMAN, J.M., EMME, S.A., MCGOVERN, T.M., KEEVER, C.C., COX, L.N.. 2010. The ‘expansion-contraction’ model of Pleistocene biogeography: rocky shores suffer a sea change? Molecular Ecology, 19: 146–169. [DOI] [PubMed] [Google Scholar]
- NAKANO, R. 2018. Field guide to sea slugs and nudibranchs of Japan. Shosoichi Publishing, Tokyo. [Google Scholar]
- ODHNER, N.H.J. 1939. Opisthobranchiate Mollusca from the western and northern coasts of Norway. Det Kongelige Norske Videnskabers-Selskabs Skrifter, 1: 1–93. [Google Scholar]
- OLDROYD, I.S. 1927. The marine shells of the west coast of North America, Volume 2, Part 1. Stanford University Press, Stanford, CA. [Google Scholar]
- PUILLANDRE, N., LAMBERT, A., BROUILLET, S., ACHAZ, G.. 2012. ABGD, Automatic barcode gap discovery for primary species delimitation. Molecular Ecology, 21: 1864–1877. [DOI] [PubMed] [Google Scholar]
- REID, D.G. 1990. Trans-Arctic migration and speciation induced by climatic change: the biogeography of Littorina (Mollusca: Gastropoda). Bulletin of Marine Science, 47: 35–49. [Google Scholar]
- ROLLER, R.A. 1970. A list of recommended nomenclatural changes for MacFarland’s “Studies of opisthobranchiate mollusks of the Pacific Coast of North America.”. Veliger, 12: 371–374. [Google Scholar]
- RONQUIST, F., HUELSENBECK, J.P.. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- SHEN, K.N., JAMANDRE, B.W., HSU, C.C., TZENG, W.N., DURAND, J.D.. 2011. Plio-Pleistocene sea level and temperature fluctuations in the northwestern Pacific promoted speciation in the globally-distributed flathead mullet Mugil cephalus. BMC Evolutionary Biology, 11: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAMATAKIS, A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALDÉS, A. 2019. Northeast Pacific benthic shelled sea slugs. Zoosymposia, 13: 242–304. [Google Scholar]
- VONNEMANN, V., SCHRÖDL, M., KLUSSMANN-KOLB, A., WÄGELE, H.. 2005. Reconstruction of the phylogeny of the Opisthobranchia (Mollusca: Gastropoda) by means of 18S and 28S rRNA gene sequences. Journal of Molluscan Studies, 71: 113–125. [Google Scholar]
- WARES, J.P. 2001. Biogeography of Asterias: North Atlantic climate change and speciation. Biological Bulletin, 201: 95–103. [DOI] [PubMed] [Google Scholar]
- WIGHT, B. 2002. Berthella from San Miguel, California. Sea Slug Forum. Available from:http://www.seaslugforum.net/find/7710. Accessed 2 May 2019. [Google Scholar]
- WIGHT, B. 2004. Berthella californica from California. Sea Slug Forum. Available from:http://www.seaslugforum.net/message/12720. Accessed 2 May 2019. [Google Scholar]
- WILLAN, R. 1987. Phylogenetic systematics of the Notaspidea (Opisthobranchia) with reappraisal of families and genera. American Malacological Bulletin, 5: 215–241. [Google Scholar]
- WILSON, A.B. 2006. Genetic signature of recent glaciation on populations of a near-shore marine fish species (Syngnathus leptorhyncus). Molecular Ecology, 15: 1857–1871. [DOI] [PubMed] [Google Scholar]