Abstract
The COVID-19 pandemic led to development of numerous serologic tests. To obviate the need for phlebotomy services, we validated dried blood spots (DBS) for anti-SARS-CoV-2 serologic testing. Immunoglobulins were extracted from 3 mm DBS punches and the extracts were analyzed using the Euroimmun anti-SARS-CoV-2 IgG ELISA. Various pre-analytical factors were studied. Results were favorable for DBS stored for at least 67 days at or below 37°C. Comparison between paired serum and DBS specimens tested by the Euroimmun ELISA showed 96.8% and 81.3% positive and negative agreement, respectively, indicating that confirmatory testing of positive Euroimmun results on DBS extracts is necessary to achieve clinical accuracy. Our findings suggest that any SARS-CoV-2 antibody assay that requires pre-dilution of serum is amenable to DBS as an alternate specimen type that is relatively low cost, self-collectable, stable, can be shipped by standard mail and could be used to establish the seroprevalence of large populations.
Keywords: SARS-CoV-2, COVID-19, antibodies, dried blood spot, home collection, seroprevalence
1. Introduction
The pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to put significant stress on communities worldwide (Chan et al., 2020, Christakis et al., 2020, Manabe et al., 2020). Testing for the presence of SARS-CoV-2 by molecular methods, e.g., real-time reverse transcription polymerase chain reaction (RT-PCR) or antigen detection are essential for diagnosis of active Coronavirus Disease 2019 (COVID-19) in both symptomatic and asymptomatic individuals with known exposure. In contrast, serologic testing for the presence of antibodies against SARS-CoV-2 is valuable for the assessment of prior COVID-19 symptomatic or asymptomatic infection, for qualification of convalescent plasma for therapeutic purposes, and if necessary, for assessment of vaccine response (CDC Interim guidelines for COVID-19 antibody testing. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html, last accessed: 1/31/2021) (Hanson et al., 2020, Theel et al., 2020b). Although the presence of antibodies to SARS-CoV-2 suggests immunity against re-infection, the duration and longevity of protective immunity against re-infection following natural or vaccine-induced seroconversion, remains unknown.
Early in the pandemic, when diagnostic tests were of limited availability, determination of seroprevalence was an alternative means to monitor spread of SARS-CoV-2 in asymptomatic or post-symptomatic individuals (Shook-Sa et al., 2020). However, the available serologic tests relied on venipuncture-collected serum or plasma specimens. This requires potential convalescents to visit a clinic or phlebotomy site for blood collection, which presents additional risk of (re)exposure for both the patients who may not have been infected or developed immunity, as well as for phlebotomists, other healthcare workers and patients who the individuals would encounter during their visits. In an effort to provide an alternative means for safe and efficient determination of prior SARS-CoV-2 infection, we modified the Euroimmun anti-SARS-CoV-2 IgG ELISA (Lübeck, Germany), which had received Emergency Use Authorization (EUA) from the Food and Drug Administration (FDA) for testing of serum and plasma specimens, to be performed on capillary whole blood collected on filter paper (i.e., dried blood spots, DBS).
2. Materials and methods
2.1. Samples
2.1.1. Contrived DBS
DBS were created by combining 0.5 mL washed red blood cells, collected from a SARS-CoV-2 seronegative donor, with equal volume of residual sera previously tested for IgG against the SARS-CoV-2 S1 protein using the Euroimmun anti-SARS-CoV-2 IgG ELISA; sixty total sera were selected, including 37 positive, 21 negative and 2 indeterminate specimens (Theel et al., 2020a). Following careful mixing by manual inversion, approximately 100 µL of the contrived blood was spotted onto Whatman 903 filter paper (GE Health Care, Cardiff, UK), allowed to dry at room temperature for at least 2 hours, and stored with desiccant at -20°C until analysis.
2.1.2. Patient DBS
DBS obtained by finger stick from 24 SARS-CoV-2 RT-PCR negative and 29 SARS-CoV-2 RT-PCR positive adult patients, collected at least 2 weeks post-diagnosis, were used to establish a preliminary index value threshold to distinguish positive from negative qualitative results for the Euroimmun anti-SARS-CoV-2 IgG ELISA.
Additionally, 65 paired serum and DBS samples were obtained from adult volunteers who were SARS-CoV-2 RT-PCR negative (N = 26 female, age range: 21−59 years; N = 4 male, age range: 26−48 years) or positive (N = 24 female, age range: 19-59 years; N = 11 male, age range: 26−59 years) at least 2 weeks prior to blood collection, except for a single RT-PCR negative specimen collected after 6 days (6−97 days). Serum samples were collected by phlebotomists via venipuncture and patients self-collected DBS under phlebotomist supervision. Volunteers were provided a survey regarding ease of DBS collection, which was completed by 61 of the 65 participants (Supplemental Table 1). All specimens were collected with informed consent and the study was approved by Mayo Clinic's Institutional Review Board.
2.2. Methods
Analysis of venipuncture-collected serum samples was performed without deviation from the manufacturer instructions using the Euroimmun anti-SARS-CoV-2 IgG ELISA assay.
Analysis of DBS was performed by first punching a single 3 mm DBS into a round U-bottom polypropylene 96-well plate (Greiner Bio-One, ChromTec, Apple Valley, MN) using a Perkin Elmer DBS Puncher (PerkinElmer Waltham, MA). To each well, 150 µL of extraction buffer (phosphate buffered saline [PBS]/2% bovine serum albumin/0.5% Tween-20; Sigma Aldrich, St. Louis, MO) was added using an Integra VIAFLO 96 channel semi-automated benchtop pipettor (Integra Biosciences Corp., Hudson, NH), fitted with a 1250 µL 96-channel head. An adhesive foil cover was placed on the plate, which was then rotated at 200 rpm for 2 hours at refrigerated (2°C−8°C) conditions. Using the semi-automated Integra ASSIST PLUS paired with a 300 µL 8-channel Voyager pipette, the DBS extract was transferred to a Sarstedt 12 × 75 mm polystyrene tube and subjected to a 2-fold dilution with PBS to yield the minimum volume required for automated handling (Supplemental Figure 1). The diluted DBS extracts were analyzed using the Euroimmun anti-SARS-CoV-2 IgG ELISA on the Dynex Agility (Dynex Technologies, Inc., Chantilly, VA) platform according to the manufacturer's instructions for serum testing with the following 2 deviations: (1) DBS extract, not serum or plasma was used, and (2) DBS extracts were diluted 2-fold with PBS, rather than a 100-fold dilution with kit buffer as required for serum. 100 μl of diluted DBS extract was transferred into the kit's SARS-CoV-2 S1 coated 96-well microplate, and incubated for 60-minutes at 37 ± 1°C. Wells were subsequently washed 3 times with 300 μl of the kit-provided wash buffer. Peroxidase-labelled anti-human IgG (100 µL) was added, incubated at 37 ± 1°C for 30 minutes, and wells washed 3 times with wash buffer. Chromogen/substrate solution (100 µL) was added, incubated for 30 minutes at room temperature, after which kit-provided stop solution (100 µL) was added. Optical density (OD) was measured at 450 nm with a 620-nm reference filter on an Agility analyzer.
The Euroimmun kit supplied negative human IgG calibrator was run in duplicate with each DBS plate batch. Per the manufacturer, serum or plasma results are expressed as an index value, calculated by dividing the patient sample OD value by the mean of the duplicate calibrator OD values, with index values of <0.8, ≥0.8 to <1.1, and ≥1.1 equivalent to negative, indeterminate and positive, respectively. This same approach was applied for determination of the results from DBS samples: the mean of the kit IgG calibrator was used to divide the DBS OD value to establish a DBS index value; qualitative DBS index value thresholds were determined using receiver-operator curve analysis.
3. Results
3.1. Filter paper study
Contrived whole blood samples using sera positive or negative for SARS-CoV-2 IgG by the Euroimmun IgG ELISA were created, and equal volumes spotted onto Whatman 903, Alhstrom 226, and Munktell filter paper, all of which have been found equivalent in past studies (Mei et al., 2010, Rottinghaus et al., 2013). All DBS were analyzed within the same batch. The mean index value percent difference for SARS-CoV-2 IgG measured from Alhstrom 226 and Munktell filter papers relative to the most commonly used Whatman 903 filter paper were 9.4% and -5.0%, respectively.
3.2. Lancet size study
DBS specimens were collected from 13 adults each of who used 3 FDA approved lancets of different sizes: 21 G needle with 2.2 mm depth, 21 G needle with 2.8 mm depth, and 18 G blade with 2.0 mm depth. Each style of lancet produced suitable DBS specimens, with most (6 of 13) participants noting the 21 G needle x 2.8 mm depth lancet as the easiest to use. Accordingly, the 21 G needle x 2.8 mm depth lancet was recommended for adult collections; otherwise guidelines established by the World Health Organization should be followed (WHO, 2010).
3.3. Desiccant study
Whole blood from a SARS-CoV-2 IgG borderline positive (Level 1) and contrived blood from an unequivocally positive donor (Level 2) were spotted on Whatman 903 filter paper and dried using one of the following conditions:
-
•
Appropriate air dry: DBS card spotted and maintained in the open at ambient temperature overnight.
-
•
Plastic bag with desiccant: DBS card spotted, immediately placed in a plastic bag containing a desiccant pack (10 g, Control Company, Webster, TX) and maintained at ambient temperature (20°C) overnight.
-
•
Plastic bag without desiccant: DBS card spotted, immediately placed in a plastic bag not containing desiccant, and maintained at ambient temperature overnight.
Replicates (N = 5) from both levels were analyzed in the same batch. While reactivity of the borderline positive (Level 1) DBS remained largely unchanged, an average decrease in reactivity of 17.2% and 23.1% was measured for the positive DBS (Level 2) maintained in plastic bags with and without desiccant, respectively (Table 1 ).
Table 1.
Desiccant study.
| Appropriate air dry Index mean (CV) | Bag with desiccant Index mean (CV) | Bag with no desiccant Index mean (CV) | |
|---|---|---|---|
| Level 1 | 1.05 (15%) | 1.09 (13%) | 1.02 (5%) |
| Level 2 | 8.09 (8%) | 6.69 (14%) | 6.17 (18%) |
Whole blood from a SARS-CoV-2 IgG borderline positive (Level 1) and an unequivocally positive donor (Level 2) were spotted onto filter paper and dried using different protocols listed (see text). All resulting DBS were analyzed (5 replicates each) within the same batch.
CV = coefficient of variation.
3.4. Blood drying time study
EDTA whole blood was added to Whatman 903 filter paper using a polyethylene transfer pipette to fill the indicated circle. Blood spots were generated with 0, 0.5, 1, 2, and 3 hours of drying time at ambient conditions. A single ply Kimtech Science Kimwipe was placed on top of the spots, with moderate, even pressure applied. The Kimwipe was then visually assessed for blood transfer. After one hour of drying at ambient conditions, the DBS were sufficiently dry, permitting transport without the risk of absorption losses to other wicking materials (Supplemental Figure 2).
3.5. DBS quality assessment
Contrived SARS-CoV-2 IgG negative and positive whole blood was used to create optimal, suboptimal and unacceptable DBS specimens (CLSI, 2013):
-
•
Optimal DBS: Applied whole blood visibly saturates through the filter paper.
-
•
Under-saturated DBS: Applied whole blood does not saturate through the filter paper.
-
•
Over-saturated DBS: Multiple applications of whole blood to a single location on the filter paper.
-
•
Smeared DBS: Whole blood is applied using an applicator (e.g., bulb from a disposable transfer pipette), which is wiped across the surface of the filter paper.
-
•
Smeared DBS with alcohol: Blood is applied using an applicator, damp with isopropyl alcohol, which is then wiped across the surface of the filter paper.
-
•
Shipped wet DBS: After application of whole blood, the DBS card is immediately placed in a plastic bag without a desiccator.
Despite the inappropriate specimen collections, analysis of these DBS yielded results similar to the optimally collected anti-SARS-CoV-2 IgG positive and negative DBS samples (Supplemental Figure 3).
3.6. Specimen stability
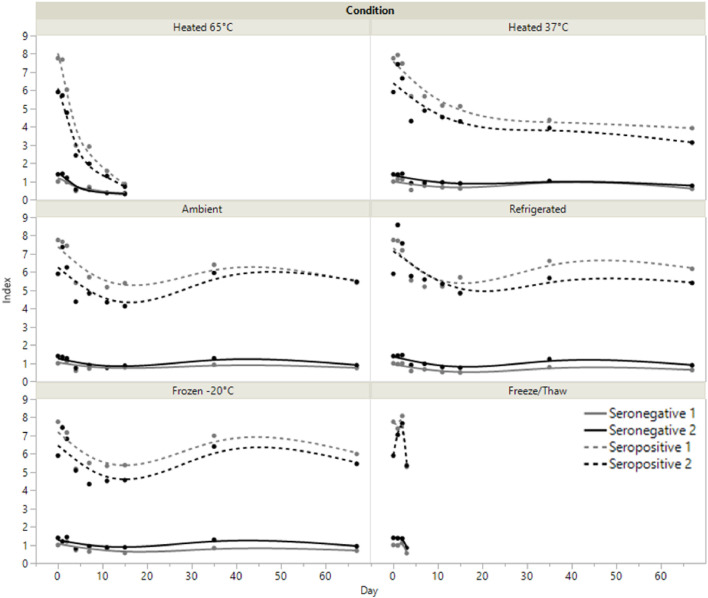
Contrived SARS-CoV-2 IgG positive (N = 2) and negative (N = 2) DBS specimens were tested following heated (65°C and 37°C), ambient (20°C), refrigerated (2°C −8°C), and frozen (-20°C) storage. The variation observed in specimens maintained at ambient, refrigerated, or frozen conditions was equivalent to kit lot-to-lot variability (Fig. 1 ) for at least 67 days. Specimen integrity rapidly deteriorated when stored at 65°C, with a more gradual decrease in SARS-CoV-2 IgG reactivity compared to samples maintained at 37°C. Up to 3 freeze/thaw cycles produce no meaningful change in the measured SARS-CoV-2 IgG index value.
Fig. 1.
Specimen stability. Two seropositive and 2 seronegative contrived DBS specimens were analyzed for up to 67 days while being stored at different temperatures or through 3 freeze/thaw cycles. Ambient corresponds to 20°C.
3.7. Shipping study
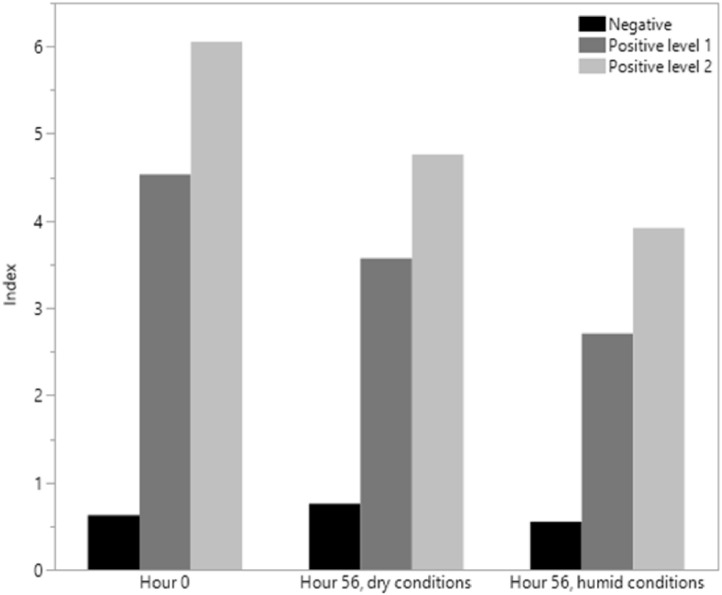
A set of contrived SARS-CoV-2 IgG DBS samples, including 20 negative, 20 replicates each of 2 different positive samples were prepared and analyzed on day 0 (immediately after being spotted and allowed to air dry) and through a 5-cycle temperature experiment using the FDA-recommended “Summer profile study” for home collection of nasal swab samples in media (Supplemental Table 2). This study was performed with a set of DBS cards placed in a sealed container with desiccant and another set placed in a sealed container with an open beaker of water, representing dry and humid shipping conditions respectively. Water did not come into direct contact with the cards. As shown in Fig. 2 , a reduction in the index value for positive specimens was observed after dry and humid temperature cycles. However, in each case sufficient anti-SARS-CoV-2 IgG antibody levels remained to yield a positive result for each replicate.
Fig. 2.
Temperature cycling study results (see text and Supplemental Table 2). Bars represent the mean of 20 measurements for each specimen.
3.8. Precision
Intra-assay precision (N = 10) was assessed using contrived negative (mean index value 0.9; 95% CI 0.8−1.0) and positive (mean index value 7.3; 95% CI 6.9−7.7) anti-SARS-CoV-2 DBS samples, which yielded coefficient of variation values of 8.9% and 7.3%, respectively. Inter-assay precision was assessed by analyzing contrived DBS anti-SARS-CoV-2 IgG negative (mean index value 0.7; 95% CI 0.5−0.8) and positive (mean index value 6.7; 95% CI 6.0−7.4) across 10 batches, which yielded coefficient of variations of 26.6% and 14.2%, respectively.
3.9. Determination of the DBS qualitative cut-off using the Euroimmun anti-SARS-CoV-2 IgG ELISA
DBS samples collected directly from 24 SARS-CoV-2 RT-PCR negative and 29 confirmed positive COVID-19 patients, tested at least 2 weeks after initial diagnosis, were used to establish the preliminary DBS index value threshold to distinguish positive from negative qualitative results. Receiver operating characteristic (ROC) curve analysis using this limited dataset identified cutoff index values of <0.71 for negative and ≥0.71 for positive results, yielding positive, negative and overall agreement values, relative to COVID-19 diagnosis of 96.2%, 84.0% and 86.8%, respectively.
3.10. Evaluation of contrived and authentic DBS samples using the Euroimmun anti-SARS-CoV-2 IgG ELISA
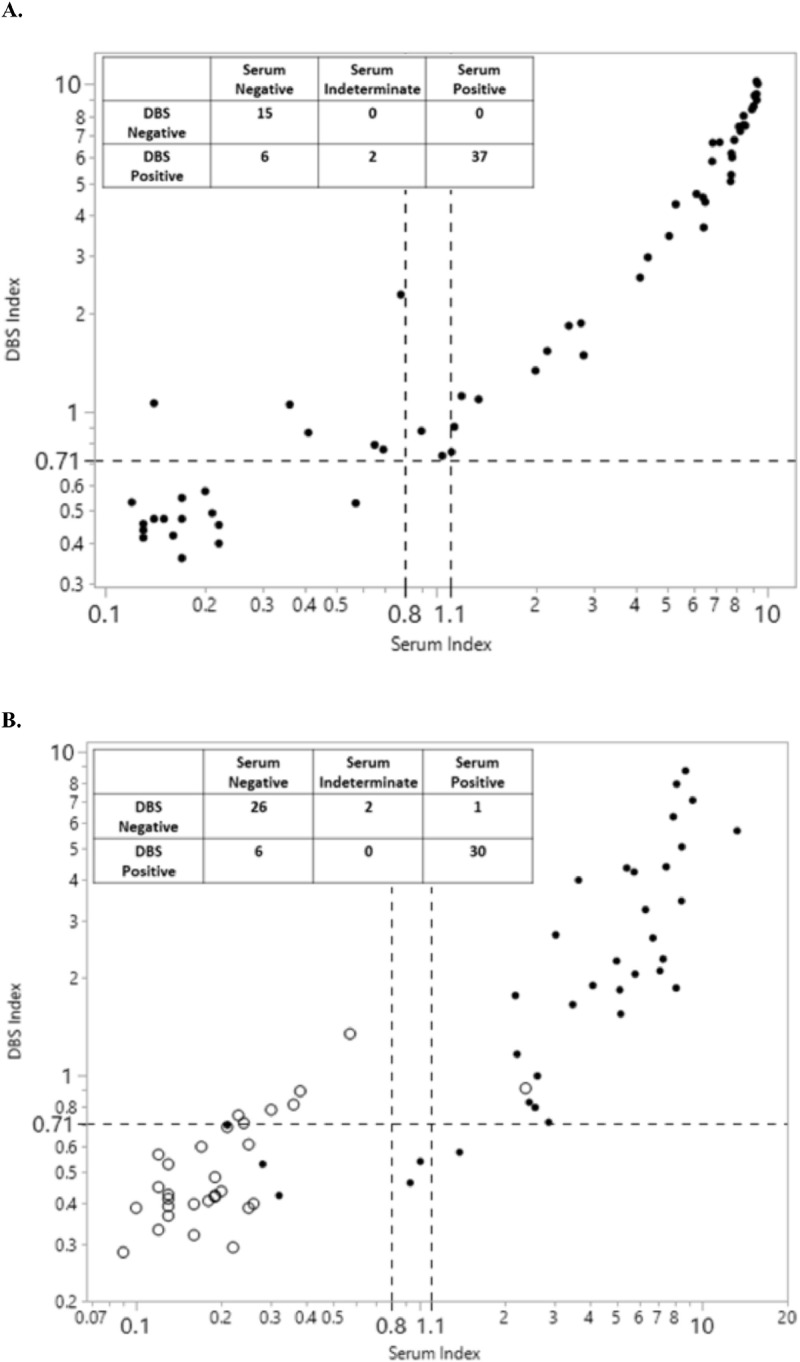
The first evaluation was performed with contrived DBS using sera from 21 negative, 37 positive and 2 indeterminate patients and the reactivity was compared to the results originally obtained in serum. Sera were analyzed and results interpreted according to manufacturer instructions, whereas DBS were analyzed as described above and results interpreted using the 0.71 index value threshold. Compared to serum, contrived DBS showed 100% (37/37) and 71% (15/21) positive and negative agreement, respectively. Bivariate analysis of this data set yielded a non-linear correlation (r2=0.97) applying a second order polynomial fit (Fig. 3 A).
Fig. 3.
Evaluation of DBS and serum tested by the Euroimmun anti-SARS-CoV-2 IgG ELISA. (A) Contrived DBS were manufactured using sera from SARS-CoV-2 IgG negative (N = 21), indeterminate (N = 2), and positive (N = 37) patients and results compared to those obtained in serum. (B) Comparison of paired serum and DBS collected at the same time from the same patients (N = 65) found to be SARS-CoV-2 positive (N = 35, black dots) or negative (N = 30, circles) by RT-PCR at least 6 days prior to blood collection. Table inserts summarize the result interpretations based on cutoffs represented by a dashed horizontal line (DBS positive index threshold: ≥0.71) and dashed vertical lines (serum positive index threshold: >1.1, incl. an intermediate range between 0.8-1.1).
The second comparison included data from 65 paired serum and DBS specimens, which were collected concurrently from RT-PCR confirmed SARS-CoV-2 negative (N = 30) and positive (N = 35) individuals. Relative to RT-PCR diagnosis, the positive and negative percent agreement for serum analysis was 86% (30/35) and 97% (29/30), respectively. For DBS analysis, positive agreement to RT-PCR diagnosis was 83% (29/35), whereas negative agreement was 77% (23/30). Compared to serology results in serum, DBS showed 96.8% (30/31) positive agreement and 81.3% (26/32) negative agreement (Fig. 3B). Bivariate analysis of serum vs authentic DBS sample index values showed a linear, but weaker, correlation (r2=0.72) relative to the comparison using contrived specimens (Fig. 3B). Correlation between the result interpretation of serum to DBS using the kit provided cutoffs for serum (≥1.1) and the preliminary cutoff for DBS (≥0.71) were improved for the paired, authentic specimens (negative percent agreement: 71% for contrived DBS vs. 81% for paired, authentic DBS specimens; Fig. 3).
4. Discussion
We developed and evaluated the performance of DBS specimens from capillary blood obtained by finger stick as an alternative to venipuncture-collected serum or plasma for detection of anti-SARS-CoV-2 IgG antibodies. Despite the limited utility of serologic testing for SARS-CoV-2 in the diagnosis of COVID-19, there remains public and private interest in antibody testing in order to establish and/or monitor seroprevalence rates, identify potential convalescent plasma donors, and potentially, to detect vaccine response. DBS specimens for these purposes, especially if self-collected, are advantageous as they would obviate the exposure risk to both the patient and healthcare provider, as well as eliminate the need for phlebotomist time and effort. Notably, DBS analyses have been conducted routinely for newborn screening for nearly 6 decades and for monitoring of patients with inborn errors of metabolism for at least 25 years (Guthrie and Susi, 1963, Moat et al., 2020, Randell and Lehotay, 1996). Other early applications of home-collected DBS for drug monitoring and infectious diseases, such as HIV, have also been successful (Rattenbury and Tsanakas, 1988, Spielberg et al., 2000). Not surprisingly, DBS applications for the COVID-19 pandemic are also starting to emerge (Karp et al., 2020, Valentine-Graves et al., 2020), with the FDA recently publishing a SARS-CoV-2 antibody DBS self-collection validation protocol.
Given the significant interest in serologic testing during the initial part of the pandemic, our approach for DBS testing was to first assess the feasibility of this alternative specimen type using an assay that had already received FDA EUA for serum, the Euroimmun anti-SARS-CoV-2 IgG ELISA. A DBS extraction protocol, based on current DBS assays used in our laboratories, was developed before a preliminary index value threshold for the Euroimmun IgG ELISA was established and analytical/clinical validation studies were conducted. The outcome of these preliminary studies supports the applicability of DBS as an alternative specimen type to serum for SARS-CoV-2 serologic testing. While DBS specimens showed high positive percent agreement (96.7%; 30/31) as compared to serum in paired samples using the Euroimmun anti-SARS-CoV-2 IgG ELISA, negative percent agreement was lower (81.5% 26/32). Collectively, this indicates that while negative results from DBS specimens using this method are largely reliable, positive results are not. Therefore, confirmatory or supplemental testing for DBS-positive results using the Euroimmun IgG ELISA would be recommended, ideally performed on a venipuncture-collected serum or plasma sample tested by an alternative assay with FDA EUA. Assay accuracy on DBS specimens is likely impacted by specimen type, but probably to a greater extent by the performance characteristics of the analytical assay system itself. Prior comparative studies for the Euroimmun IgG ELISA assay have found variable clinical performance characteristics versus COVID-19 diagnosis, including sensitivity and specificity values ranging from 78%-100% and 94.8%-100%, respectively (Nicol et al., 2020, Schnurra et al., 2020, Tang et al., 2020, Theel et al., 2020a). Notably, while Euroimmun has meanwhile also validated DBS extracts for their IgG ELISA and report 100% positive and negative percent agreement values (https://www.coronavirus-diagnostics.com/documents/Indications/Infections/Coronavirus/EI_2606_D_UK_A.pdf; accessed 2/1/2021), our data indicate significantly lower result concordance which should be recognized by laboratories considering implementation of this testing.
We performed a number of studies assessing pre-analytical factors that may affect accuracy of serologic testing on DBS, including the quality of the blood application to filter paper, DBS specimen stability under various conditions (e.g., temperature, humidity, etc.), and DBS drying time, among others, and found that the following conditions are optimal for DBS sampling and storage: drying time of at least 1 hour at ambient temperature in room air, and stability at room temperature, refrigerated or frozen conditions for at least 67 days. The quality of the blood application to the filter paper was not a major factor as long as there was sufficient blood to allow for at least one analysis. In contrast to recent publications that describe the use of larger amounts of DBS (Karp et al., 2020, Valentine-Graves et al., 2020), we found that a single 3 mm punch from the DBS sample was sufficient for reproducible results. Overall, our studies indicate that most DBS received in the laboratory would be of adequate quantity and quality for analysis.
We also assessed throughput of DBS analysis using the Euroimmun anti-SARS-CoV-2 IgG ELISA. DBS processing and testing was performed in a semi-automated fashion, which required 45 minutes of technologist effort and a total time of 5.25 hours from DBS punching to result availability for 96 samples (including 2 calibrators, 4 controls and 90 patient specimens). This allows for a throughput of 270 patient samples per 8-hour shift, by 2 laboratory technologists using one analyzer, where shifts are staggered by 2.5 hours.
These DBS validation studies were conducted between April and June 2020, before the FDA provided guidance on recommended studies for authorization of self-collected DBS specimens (https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/vitro-diagnostics-euas, last accessed: 1/31/2021). Our DBS validation studies involved identification of individuals who had tested positive by RT-PCR at least 2 weeks prior, and collection of paired serum and DBS. Patients were given the opportunity to self-collect the DBS specimen via finger stick after being provided the DBS collection kit (instructions, alcohol swab, sterile gauze, blood spot collection card with area to record patient information, lancet and Band-Aid) from the observing phlebotomist. In contrast to true home-collections, the DBS were not dried in the patient's home and were not sent through the mail. Instead, the phlebotomist placed the DBS in an open plastic bag for drying and within 1.5 hours submitted both specimens to the laboratory for subsequent testing. A survey was completed by 61 participating patients (46 females, 15 males; age range: 19−59 years) which indicated that the self-collection was overall acceptable (Supplemental Table 1). Consistent with prior self-collected DBS experiences, all but 2 of the 61 participants (97%) were satisfied with the DBS self-collection and would consider this type of home collection kit for future health care needs.
DBS specimens tested by the Euroimmun IgG ELISA were recently used for a study to determine the prevalence of SARS-CoV-2 antibodies among Mayo Clinic employees, with confirmatory testing of positive DBS results required (Carter et al., 2021). Nearly 30,000 employees volunteered to participate and were tested within 6 weeks. Each working day, an average of 1,215 (range: 2 to 2,549; median 1,019) DBS were received in the laboratory, extracted and tested using up to 8 FTE and 5 workstations. The mean daily time from sample receipt to routine reporting of results through the laboratory information system was 21 hours (range: 4−78 hours).
In summary, we provide further evidence that antibodies can be extracted efficiently from DBS and that this alternative specimen type may be used for detection of antibodies to SARS-CoV-2. Performance characteristics of DBS extracts require careful validation on anti-SARS-CoV-2 serologic assays in particular because this specimen type still awaits FDA approval and external quality assessment programs are not yet available. Notably, we report that while the positive percent agreement of DBS extract as compared to paired serum samples was high using the Euroimmun anti-SARS-CoV-2 IgG ELISA, the negative percent agreement was low, necessitating confirmatory testing. The need to confirm DBS results positive by the Euroimmun IgG ELISA is a significant limitation as was shown in Mayo Clinic's seroprevalence study where 14.5% of DBS samples that had above cutoff results required confirmation in a serum sample of which only 4% were found to be positive using the Roche Diagnostics Elecsys Anti-SARS-CoV-2 Total Antibody electrochemiluminescence immunoassay (ECLIA; Indianapolis, IN) (Carter et al., 2021). However, should better performing serologic assays applicable to DBS become available, self-collected DBS offer a means for efficient and effective population seroprevalence testing, without individuals having to risk exposure to potentially infectious individuals outside their home.
Author contributions
Coleman Turgeon: Methodology, Investigation, Validation, Data curation, Formal analysis, Visualization, Project administration, Writing-Original Draft; Karen Sanders: Methodology, Investigation, Validation, Data curation, Formal analysis, Visualization, Project administration, Writing - review & editing; Dane Granger: Investigation, Formal analysis, Data curation, Writing - review & editing; Stephanie Nett: Investigation, Writing - review & editing; Heather Hilgart: Project administration, Writing - review & editing; Dietrich Matern: Conceptualization, Methodology, Formal analysis, Resources, Project administration, Supervision, Writing-Review & Editing; Elitza S. Theel: Conceptualization, Methodology, Formal analysis, Resources, Project administration, Supervision, Writing-Review & Editing.
Acknowledgments
We are grateful to the patients who consented to participate in this study and the mobile phlebotomy unit at Mayo Clinic for their assistance in collecting sera and DBS from patients confirmed positive for COVID-19. We also thank Julie Faust, Julie Harring, and Brian Dukek for their invaluable assistance during this study.
Declaration of competing interest
The authors report no conflicts of interest relevant to this article.
Footnotes
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.diagmicrobio.2021.115425.
Appendix. Supplementary materials
Supplemental Figure 1. Effect of dilutions of DBS extract on assay results. For this study, one 3 mm punch was taken from each of 3 DBS (collected from patients proven to be seropositive by the Euroimmun IgG ELISA; serum index values were 9.26, 8.74, and 3.66 for samples 1, 2, and 3 respectively) and extracted with 150 µL of extraction buffer (phosphate buffered saline/2% bovine serum albumin/0.5% Tween-20). The extract (150 µL) was diluted 2, 5, 10, and 20-fold with 0.9% phosphate buffered saline. The index positivity cutoff for DBS (≥0.71) is represented by the dashed line.
Supplemental Figure 2. DBS drying time study. EDTA whole blood was added to Whatman 903 filter paper using a polyethylene transfer pipette. DBS were generated with 0, 0.5, 1, 2, and 3 hours of drying time at ambient (20°C) conditions. A single ply of Kimtech Science Kimwipes was then placed on top of the spots. After one hour of drying at ambient conditions the specimens were sufficiently dry, permitting transport without the risk of absorption losses to other wicking materials.
Supplemental Figure 3. DBS quality study. SARS-CoV-2 IgG negative and positive DBS were manufactured to represent what is generally considered optimal, suboptimal and unacceptable DBS quality (see text). Despite the inappropriate specimen collections, analysis of these DBS yielded the expected results. Each bar represents one analysis.
Supplemental Table 1. Survey questions, answer choices, responses and comments from 61 volunteers regarding the collection of capillary blood via fingerstick on filter paper.
Supplemental Table 2. “Summer profile study” performed under dry and humid conditions per FDA guidance. Two sets of SARS-CoV-2 IgG negative (N = 20), positive level 1 (N = 20) and positive level 2 (N = 20) DBS controls were analyzed 20 times each on day 0 (immediately after being spotted and allowed to air dry) and through a 5-cycle temperature experiment within a range from 22°C to 40°C. To model dry and humid shipping conditions, one set of samples was stored at the different temperatures with desiccant and the other with an open beaker of water. Results are shown in Figure 2.
References
- WHO Guidelines on Drawing Blood: Best Practices in Phlebotomy. WHO Guidelines Approved by the Guidelines Review Committee. Geneva; 2010.
- Carter RE, Theel ES, Breeher LE, Swift MD, Van Brunt NA, Smith WR. Prevalence of SARS-CoV-2 antibodies in a multistate academic medical center. Mayo Clin Proc. 2021;96:1165–1174. doi: 10.1016/j.mayocp.2021.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakis DA, Van Cleve W, Zimmerman FJ. Estimation of US children's educational attainment and years of life lost associated with primary school closures during the coronavirus disease 2019 pandemic. JAMA Netw Open. 2020;3(11) doi: 10.1001/jamanetworkopen.2020.28786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CLSI . Clinical and Laboratory Standards Institute; Wayne, PA: 2013. Blood Collection on Filter Paper for Newborn Screening Programs; Approved Standard-Sixth Edition. CLSI document01-A6. [Google Scholar]
- Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics. 1963;32:338–343. [PubMed] [Google Scholar]
- Hanson KE, Caliendo AM, Arias CA, Englund JA, Hayden MK, Lee MJ. Infectious Diseases Society of America guidelines on the diagnosis of COVID-19:Serologic Testing. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1343. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp DG, Danh K, Espinoza NF, Seftel D, Robinson PV, Tsai CT. A serological assay to detect SARS-CoV-2 antibodies in at-home collected finger-prick dried blood spots. Sci Rep. 2020;10(1):20188. doi: 10.1038/s41598-020-76913-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe YC, Sharfstein JS, Armstrong K. The need for more and better testing for COVID-19. JAMA. 2020;324:2153–2154. doi: 10.1001/jama.2020.21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei JV, Zobel SD, Hall EM, De Jesus VR, Adam BW, Hannon WH. Performance properties of filter paper devices for whole blood collection. Bioanalysis. 2010;2(8):1397–1403. doi: 10.4155/bio.10.73. [DOI] [PubMed] [Google Scholar]
- Moat SJ, George RS, Carling RS. Use of dried blood spot specimens to monitor patients with inherited metabolic disorders. Int J Neonatal Screen. 2020;6(2):26. doi: 10.3390/ijns6020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol T, Lefeuvre C, Serri O, Pivert A, Joubaud F, Dubee V. Assessment of SARS-CoV-2 serological tests for the diagnosis of COVID-19 through the evaluation of three immunoassays: two automated immunoassays (Euroimmun and Abbott) and one rapid lateral flow immunoassay (NG Biotech) J Clin Virol. 2020;129 doi: 10.1016/j.jcv.2020.104511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randell EW, Lehotay DC. An automated enzymatic method on the Roche COBAS MIRA S for monitoring phenylalanine in dried blood spots of patients with phenylketonuria. Clin Biochem. 1996;29(2):133–138. doi: 10.1016/0009-9120(95)02033-0. [DOI] [PubMed] [Google Scholar]
- Rattenbury JM, Tsanakas J. Acceptance of domiciliary theophylline monitoring using dried blood spots. Arch Dis Child. 1988;63(12):1449–1452. doi: 10.1136/adc.63.12.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottinghaus E, Bile E, Modukanele M, Maruping M, Mine M, Nkengasong J. Comparison of Ahlstrom grade 226, Munktell TFN, and Whatman 903 filter papers for dried blood spot specimen collection and subsequent HIV-1 load and drug resistance genotyping analysis. J Clin Microbiol. 2013;51(1):55–60. doi: 10.1128/JCM.02002-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnurra C, Reiners N, Biemann R, Kaiser T, Trawinski H, Jassoy C. Comparison of the diagnostic sensitivity of SARS-CoV-2 nucleoprotein and glycoprotein-based antibody tests. J Clin Virol. 2020;129 doi: 10.1016/j.jcv.2020.104544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook-Sa BE, Boyce RM, Aiello AE. Estimation without representation: early severe acute respiratory syndrome coronavirus 2 seroprevalence studies and the path forward. J Infect Dis. 2020;222(7):1086–1089. doi: 10.1093/infdis/jiaa429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg F, Critchlow C, Vittinghoff E, Coletti AS, Sheppard H, Mayer KH. Home collection for frequent HIV testing: acceptability of oral fluids, dried blood spots and telephone results. HIV early detection study group. AIDS. 2000;14(12):1819–1828. doi: 10.1097/00002030-200008180-00018. [DOI] [PubMed] [Google Scholar]
- Tang MS, Hock KG, Logsdon NM, Hayes JE, Gronowski AM, Anderson NW. Clinical performance of two SARS-CoV-2 serologic assays. Clin Chem. 2020;66(8):1055–1062. doi: 10.1093/clinchem/hvaa120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theel ES, Harring J, Hilgart H, Granger D. Performance characteristics of four high-throughput immunoassays for detection of IgG antibodies against SARS-CoV-2. J Clin Microbiol. 2020;58(8) doi: 10.1128/JCM.01243-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theel ES, Slev P, Wheeler S, Couturier MR, Wong SJ, Kadkhoda K. The role of antibody testing for SARS-CoV-2: is there one? J Clin Microbiol. 2020;58(8) doi: 10.1128/JCM.00797-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine-Graves M, Hall E, Guest JL, Adam E, Valencia R, Shinn K. At-home self-collection of saliva, oropharyngeal swabs and dried blood spots for SARS-CoV-2 diagnosis and serology: post-collection acceptability of specimen collection process and patient confidence in specimens. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0236775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Effect of dilutions of DBS extract on assay results. For this study, one 3 mm punch was taken from each of 3 DBS (collected from patients proven to be seropositive by the Euroimmun IgG ELISA; serum index values were 9.26, 8.74, and 3.66 for samples 1, 2, and 3 respectively) and extracted with 150 µL of extraction buffer (phosphate buffered saline/2% bovine serum albumin/0.5% Tween-20). The extract (150 µL) was diluted 2, 5, 10, and 20-fold with 0.9% phosphate buffered saline. The index positivity cutoff for DBS (≥0.71) is represented by the dashed line.
Supplemental Figure 2. DBS drying time study. EDTA whole blood was added to Whatman 903 filter paper using a polyethylene transfer pipette. DBS were generated with 0, 0.5, 1, 2, and 3 hours of drying time at ambient (20°C) conditions. A single ply of Kimtech Science Kimwipes was then placed on top of the spots. After one hour of drying at ambient conditions the specimens were sufficiently dry, permitting transport without the risk of absorption losses to other wicking materials.
Supplemental Figure 3. DBS quality study. SARS-CoV-2 IgG negative and positive DBS were manufactured to represent what is generally considered optimal, suboptimal and unacceptable DBS quality (see text). Despite the inappropriate specimen collections, analysis of these DBS yielded the expected results. Each bar represents one analysis.
Supplemental Table 1. Survey questions, answer choices, responses and comments from 61 volunteers regarding the collection of capillary blood via fingerstick on filter paper.
Supplemental Table 2. “Summer profile study” performed under dry and humid conditions per FDA guidance. Two sets of SARS-CoV-2 IgG negative (N = 20), positive level 1 (N = 20) and positive level 2 (N = 20) DBS controls were analyzed 20 times each on day 0 (immediately after being spotted and allowed to air dry) and through a 5-cycle temperature experiment within a range from 22°C to 40°C. To model dry and humid shipping conditions, one set of samples was stored at the different temperatures with desiccant and the other with an open beaker of water. Results are shown in Figure 2.