Abstract
Asthma as a chronic inflammatory disease can be expected to affect central nervous system structures but little is known about subcortical structures in asthma and their potential association with illness-specific outcomes and anxiety. A total of 40 young adults (20 with asthma and 20 gender- and age-matched controls) underwent high-resolution T1-weighted MRI scan, viewed short distressing film clips, and filled in questionnaires about anxious and depressed mood, as well as asthma history, control, and catastrophizing thoughts about asthma, for those with asthma. The structural scans were processed in FSL’s FIRST program to delineate subcortical structures of interest: amygdala, hippocampus, putamen, pallidum, caudate nucleus, nucleus accumbens, and thalamus. Findings showed no general reduction in subcortical gray matter volumes in asthma compared to controls. Asthma duration, asthma control, and catastrophizing of asthma and asthma attacks were negatively associated with volumes of putamen and pallidum, and to a weaker extent thalamus and amygdala, while controlling for gender, age, and corticosteroid inhaler use. In addition, stronger anxiety in response to distressing films was associated with lower volume of the pallidum, whereas general anxious and depressed mood was unrelated to subcortical structures. Thus, although there are no subcortical structural differences between young adults with asthma and healthy controls, longer asthma history, suboptimal management, and illness-related anxiety are reflected in lower gray matter volumes of subcortical structures, further emphasizing the importance of maintaining optimal asthma control.
Keywords: Structural magnetic resonance imaging, Gray matter volume, Asthma, Limbic system, Basal ganglia, Asthma management, Anxiety
Current knowledge about the brain in asthma is limited. Progress has been made in elucidating brain function related to respiratory sensations and stress in asthma (e.g., Davenport et al. 2000; Rosenkranz et al. 2016; von Leupoldt et al. 2009; Webster and Colrain 2002), however, the potential role of asthma in altering the shape of brain structure, in particular gray matter volume, remains largely unexplored. Given the central role of compromised lung function and airway inflammation in the asthmatic pathophysiology it is conceivable that longer lasting or uncontrolled disease could affect central nervous system structures and lead to accelerated degeneration. Two published studies have examined brain structures in asthma. Smaller hippocampal volumes have been found in a large sample of middle-aged asthma patients compared to matched controls (Carlson et al. 2017). In addition, one smaller study found that longer asthma duration was associated with larger periaqueductal gray volumes (von Leupoldt et al. 2011). Furthermore, reductions in gray matter volumes of a number of cortical and subcortical structures have been found in Chronic Obstructive Pulmonary Disease (COPD) in two other studies (Esser et al. 2016; Zhang et al. 2012). Both studies observed structural changes related to disease duration and one additionally found an association with patients’ fear of dyspnea and exercise (Esser et al. 2016).
High comorbidity of asthma with anxiety disorders, in particular panic disorder, has been documented frequently, both cross-sectionally and longitudinally (Favreau et al. 2014; Goodwin et al. 2012; Meuret et al. 2017; Scott et al. 2007). More specifically, panic and fear related to asthma have been shown to affect asthma management, including excessive self-medication with bronchodilators and more frequent emergency room visits as well as overuse or overprescription of medication (Janssens et al. 2009). Anxious thoughts about catastrophic outcomes of asthma attacks and asthma in general have been related to elevated symptom reports in respiratory challenges (De Peuter et al. 2007, 2008) and reduced asthma control (Janssens et al. 2012). Such catastrophic cognitions are also a characteristic feature of panic disorder and a growing literature suggests that panic, fear, and anxiety disorders are associated with reduced gray matter volume in subcortical structures. In particular, reduced volumes have been observed in limbic structures such as hippocampus and amygdala (Ahmed-Leitao et al. 2016; Fisler et al. 2013; Hayano et al. 2009; Morey et al. 2012; Yoon et al. 2016; although with less consistency for the amygdala, see Cacciaglia et al. 2017; Günther et al. 2018) and in basal ganglia (Hilbert et al. 2015; Uono et al. 2017; Yoo et al. 2005).
In this study, we sought to examine subcortical structures in asthma, for which prior research has previously shown associations with emotional processes. From an evolutionary and comparative perspective, subcortical regions are viewed as more central to the generation of affect, with cortical regions taking on a more modulatory role (e.g., Damasio and Carvalho 2013; Panksepp 2011). Amygdala and hippocampus are classically involved in negative emotion (LeDoux et al. 1988; Davis 1992) and are sensitive to effects of stress hormones (McEwen et al. 2015), but more recently have also been invoked in reward sensitivity and learning (Baxter and Murray 2002; Morrison and Salzman 2010). Similarly, basal ganglia have been implicated in both reward processing and positive emotion, but more recently also in negative states like anxiety and fear (Lago et al. 2017). In addition, the thalamus has been characterized in emotion theories as an important hub for proprioceptive and sensory afferents (Damasio and Carvalho 2013) as well as top-down modulation of affect by cortical input through the basal ganglia (Panksepp 2011).
The aims of the present study were, (1) to compare subcortical gray matter volumes between individuals with asthma and a group of age and gender-matched healthy controls, (2) to study associations of gray matter volumes in asthma with disease duration and control, and (3) to examine whether catastrophic thinking about asthma and anxious responding to distressing stimuli is related to gray matter volumes.
Materials and methods
Participants
Twenty volunteers with asthma and 20 healthy controls between the ages of 18–45 were recruited from a university campus and the community. Inclusion criteria for those with asthma were a physician-documented diagnosis of asthma. Exclusion criteria were treatment with oral corticosteroids in the previous two months, forced expiratory volume in the 1st second (FEV1) below 70% of predicted; pregnancy in women (confirmed via urine test); presence or history of medical or neurological disorder such as angina, myocardial infarction, congestive heart failure, transient ischemic attacks, cerebrovascular accidents, uncontrolled diabetes mellitus, emphysema, or chronic obstructive pulmonary disease, seizures or head trauma, endocrine disorders or renal disease. Major psychological disorders excluded were: presence or history of schizophrenia, bipolar disorder, or dementia; current presence of major depressive disorder or blood-injection-injury phobia; current or recent history (within 1 year) of substance-related disorders, current recreational drug use or consuming more than 20 alcoholic drinks per week; current smoking or recent history (within 6 months) of smoking (< 6 pack years of lifetime smoking history); use of medication with sympathetic and parasympathetic effects, anxiolytics or other psychoactive drugs, or previous electroconvulsive therapy. Presence of history of orthopaedic circumstances and metallic inserts was contraindicated for magnetic resonance scanning. Persons with a lack of proficiency in English were also excluded. Asthma severity was determined by relevant guidelines (National Heart, Lung, and Blood Institute/National Asthma Education and Prevention Program 2007) on the basis of % of predicted FEV1, symptoms, functional limitations, bronchodilator use, and exacerbations (in the past year) for those without maintenance medication use, or treatment step for those who initiated maintenance medication and whose asthma was well controlled.
All procedures were approved by institutional review boards of The University of Texas
Southwestern Medical Center (STU 082011–038) and Southern Methodist University (2015–007-RITT). All participants provided written informed consent. They received an honorarium of $50 for taking part in the imaging sessions or alternatively elected to receive course credit, in case they were undergraduate students.
Measures
FENO was measured with a handheld electrochemical analyzer (Niox mino, Aerocrine, Sweden; Alving et al. 2006) as an indicator of airway inflammation. FEV1 (expressed as % of predicted values) was measured with an electronic spirometer (Jaeger/Tönnies AM2, Höchberg, Germany) as a common indicator of mechanical lung function. Both measures were obtained at the beginning of the session. Participants completed an ad-hoc questionnaire on demographics, asthma history, manifestation and past treatment, the Catastrophizing of Asthma Scale (CAS; De Peuter et al. 2007) with subscales for Catastrophizing of Asthma (CAS-asthma) and Catastrophizing of Asthma Attacks (CAS-attacks) with 13 items each, and the Asthma Control Questionnaire (ACQ; Juniper et al. 1999), which measures asthma control impairment in the past week (including scoring % of predicted FEV1 as one of seven items), and the Hospital Anxiety and Depression Scale (HADS; Zigmond and Snaith 1983) with subscales for anxious and depressive mood. Participants completed ratings of their mood prior to entering the scanner, after entering it, and after each film stimulus block during the scan. The anxiety rating consisted of an 11-point rating scale ranging from 0 (“not at all”) to 10 (“extremely”).
Procedures
Following questionnaire measures, FENO, and spirometry at the beginning of the session, participants entered the scanner and structural images were acquired. Subsequently, participants were instructed to view two blocks of three negative films and one block of three neutral films. Each film lasted 44 s followed by a 120 s recovery period. Negative films contained scenes of surgery, injection and injury, mostly from medical education material, which had been effective in eliciting airway constriction and negative affect in prior studies (Janssens et al. 2017; Ritz et al. 2010, 2011). Neutral films were extracts from a geology lecture on sedimentary rock formation. Films were rear-projected onto a screen and viewed by participants via a mirror mounted on the head coil. After each run, participants provided self-report of anxiety. At debriefing, possible residual symptoms were explored by the experimenter and spirometry was repeated to ensure patient safety.
MRI acquisition and analysis
All images were collected using a high-field 3-Tesla (3 T) Philips Achieva scanner equipped with a 32-channel head coil. High-resolution anatomical (T1) images were acquired using magnetization-prepared rapid gradient-echo sequence: TR/TE/TI = 8.2/3.8/873 ms, voxel size = 1.0 × 1.0 × 1.0 mm3, flip angle = 12°; FOV = 256 × 256 mm2, 160 sagittal slices. The structural scans were then processed in FSL’s FIRST program (Patenaude et al. 2011) to delineate subcortical structures. In short, FSL-FIRST method spatially normalizes the T1 images into MNI space and then restricts the local registration to subcortical structures. The variation in shape and intensity is modeled by multivariate Gaussian distribution based on 336 T1-weighted MR images where the subcortical regions were delineated manually. In our analysis, we estimated the volumes of amygdala, hippocampus, putamen, pallidum, caudate nucleus, nucleus accumbens, and thalamus (Fig. 1). Next, we normalized the volume of each subcortical region by the intracranial volume to account for different brain sizes. Thus, the subcortical regions are reported in percent (%), which are in relation to intracranial volume.
Fig. 1.
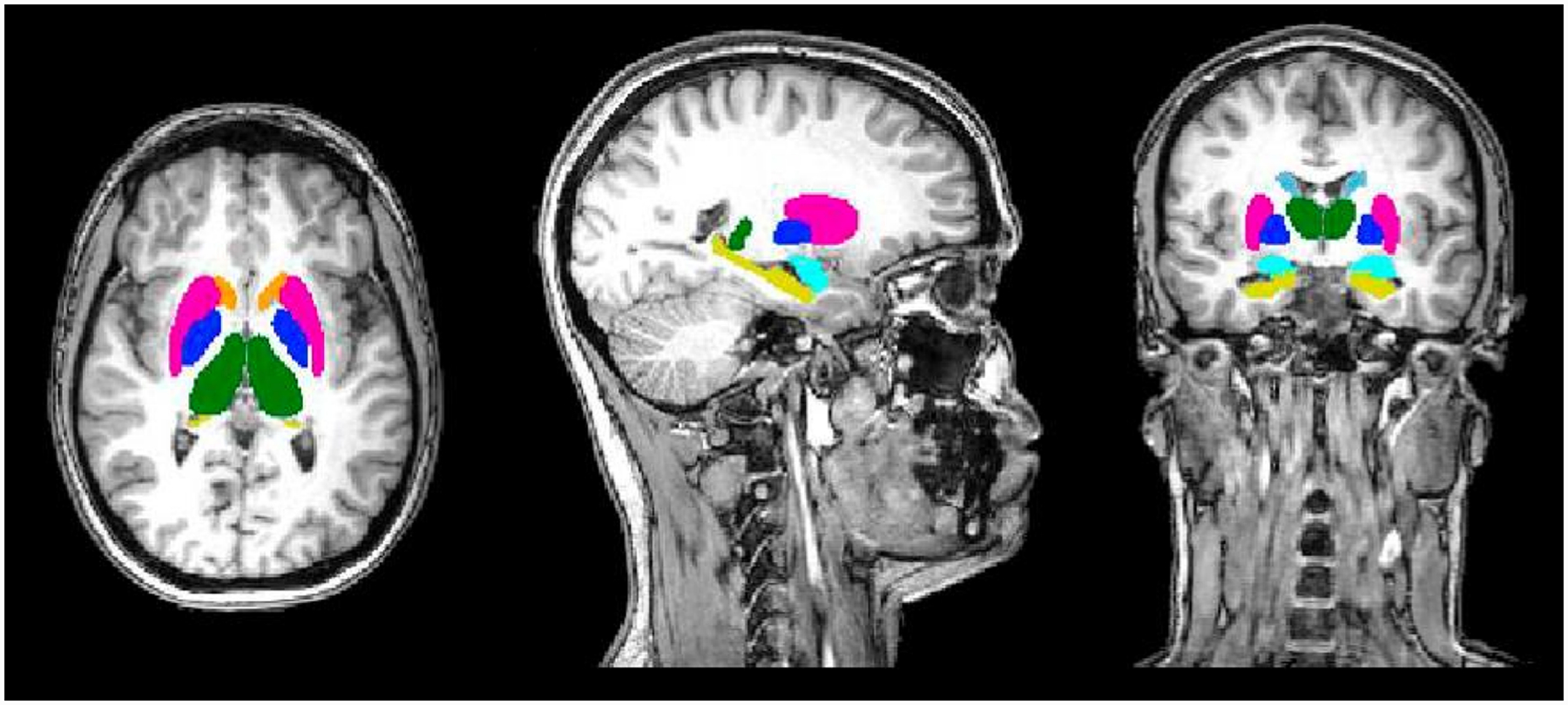
Subcortical structures analyzed for gray matter volume: pink – putamen; dark blue – pallidum; light blue – caudate nucleus; orange – nucleus accumbens; green – thalamus proper; yellow – hippocampus; teal – amygdala
Statistical analysis
Means or frequencies of demographic, anthropometric, and clinical variables were calculated for baseline characterization of groups. Group differences in gray matter volumes were examined by t-tests. Partial correlations controlling for age, gender, and inhaled corticosteroid intake were calculated for the association between gray matter volumes and asthma-related variables or anxiety response to negative film viewing (relative to baselines outside and inside the scanner). Log-transformation was applied for FENO and CAS-asthma because they deviated from normal distribution.
Results
Baseline sample characterization
Characteristics of the groups were largely similar in baseline characteristics, except for higher FENO in asthma (Table 1). Participants were young on average and asthma patients had predominantly mild severity and control was in an indeterminate range according to the ACQ26. Thirty-five percent were taking inhaled corticosteroids.
Table 1.
Demographics for participants with asthma (n = 20) and healthy controls (n = 20)
| Asthma | Control | p | |
|---|---|---|---|
| Age, M (SD) | 25.3 (8.8) | 25.1 (8.8) | .958 |
| Gender, female, n (%) | 10 (50.0) | 10 (50.0) | 1.00 |
| Race, Non-White, n (%) | 13 (59.1) | 9 (40.9) | .341 |
| Ethnicity, Hispanic n (%)a | 4 (20.0) | 4 (21.1) | 1.00 |
| BMI, M (SD) | 24.5 (4.9) | 26.4 (5.4) | .261 |
| FEV1, % of predicted, M (SD) | 95.0 (10.5) | 99.3 (14.5) | .280 |
| FENO, ppb, M (SD) | 51.4 (53.5) | 19.5 (13.4) | .028 |
| Asthma onset, M (SD) | 8.2 (6.8) | N/A | |
| Asthma duration, M (SD) | 17.1 (8.0) | N/A | |
| Asthma severityb | |||
| Intermittent, n (%) | 4 (22.2) | N/A | |
| Mild persistent, n (%) | 8 (44.4) | N/A | |
| Moderate Persistent, n (%) | 4 (22.2) | N/A | |
| Severe persistent, n (%) | 3 (11.1) | N/A | |
| Asthma Control Questionnaire, M (SD) | 0.99 (0.55) | N/A | |
| Inhaled corticosteroid use, n (%) | 7 (35.0) | N/A |
Abbreviations: BMI body mass index, FENO fractional exhaled nitric oxide, FEV1 forced expiratory volume in the 1st second
Missing ethnicity report for one control;
according to NHLBI/NAEEP (2007), rated for those without maintenance medication or well-controlled on maintenance medication
Gray matter volume differences between asthma and controls
Findings showed no general reduction in gray matter volumes in the asthma cohort compared to controls (Table 2).
Table 2.
Comparison of subcortical gray matter volumes in percent (relative to intracranial volume) between participants with asthma and healthy controls, means (SD)
| Asthma (n = 20) | Control (n = 20) | p | |
|---|---|---|---|
| Amygdala | 0.16 (0.05) | 0.16 (0.04) | .778 |
| Hippocampus | 0.49 (0.07) | 0.46 (0.06) | .169 |
| Caudate nucleus | 0.46 (0.06) | 0.46 (0.06) | .721 |
| Putamen | 0. 63 (0.05) | 0. 61 (0.07) | .252 |
| Pallidum | 0.21 (0.03) | 0.21 (0.02) | .731 |
| Nucleus accumbens | 0.06 (0.01) | 0.06 (0.01) | .691 |
| Thalamus | 1.00 (0.06) | 1.00 (0.08) | .842 |
Associations of gray matter volumes with asthma-relevant variables
Duration and asthma control were negatively associated with volumes of a number of subcortical areas, with significant or trend-level associations for amygdala, putamen, and pallidum while controlling for gender, age, and corticosteroid inhaler use (Table 3, Fig. 2). In addition, stronger catastrophizing of asthma attacks or asthma in general was consistently associated with lower volumes in the same structures and the thalamus. In significant associations with putamen and pallidum asthma-relevant variables accounted for 16.9–20.4% of variance beyond control variables. (See Supplemental Table 1 and 2 for associations between gray matter volumes with gender, age, and corticosteroid inhaler use).
Table 3.
Association of subcortical gray matter volumes with asthma-related variables and anxiety response to distressing films in participants with asthma
| Amygdala | Hippocampus | Caudate N. | Putamen | Pallidum | N. accumbens | Thalamus | |
|---|---|---|---|---|---|---|---|
| Asthma control (ACQ) | −.48† | −.15 | −.22 | −.53* | −.27 | −.22 | −.19 |
| Asthma duration (yrs) | −.46† | −.08 | .01 | −.08 | −.51* | .28 | −.34 |
| Catastrophizing of attacks (CAS-attacks) | −.48† | −.15 | −.05 | −.53* | −.48† | −.02 | −.45† |
| Catastrophizing of asthma (lnCAS-asthma) | −.41 | −.12 | −.19 | −.52* | −.52* | −.22 | −.52* |
| lnFENO (ln ppb) | −.24 | −.17 | −.27 | −.18 | −.48† | −.10 | −.06 |
| Δ anxiety, relative to baseline outside scanner | .03 | −.01 | −.08 | −.05 | −.44† | −.13 | .10 |
| Δ anxiety, relative to baseline inside scanner | −.10 | −.28 | −.25 | .04 | −.57* | −.25 | .02 |
Partial rs controlling for sex, age, and inhaled corticosteroid use; note that higher ACQ values indicate lower asthma control
p < .05,
p < .10
Fig. 2.
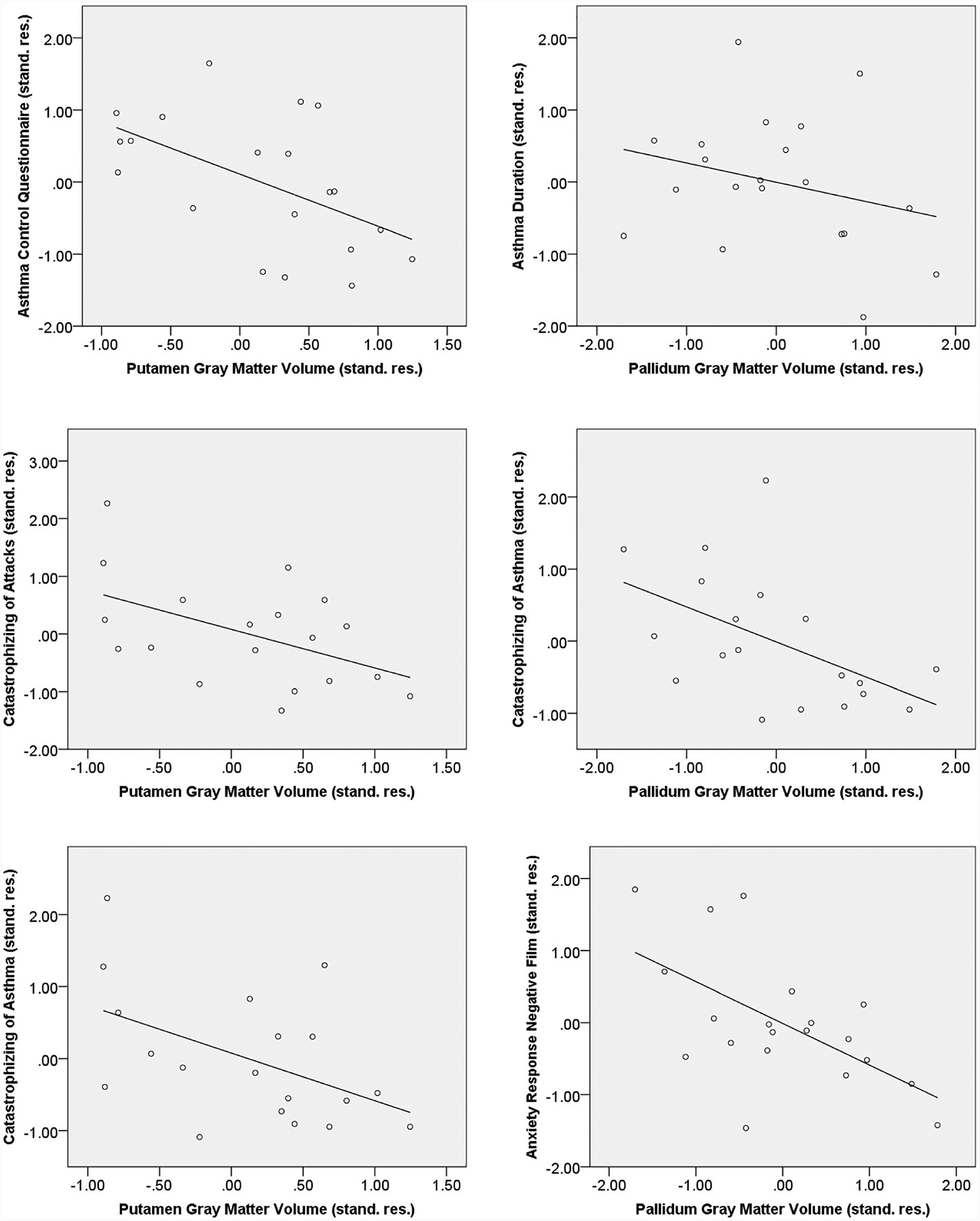
Scatter plots for selected partial correlations of putamen and pallidum gray matter volume for asthma
Associations of gray matter volumes with anxiety response to distressing films and mood
In asthma, lower volumes of the pallidum correlated with greater anxiety response to the presentation of the distressing films (Table 3, Fig. 2). The anxiety response after entering the scanner explained 26.3% of volume variance in the pallidum beyond control variables. In controls, anxiety response (film viewing minus baseline before entering the scanner), correlated negatively with volumes of amygdala (r[15] = −.55, p = .023) and hippocampus (r[15] = −.52, p = .027). No significant partial correlations were observed with the HADS anxious mood (rs = −.37 to .20) for those with asthma, whereas controls showed significant negative associations with amygdala volume (r[16] = −.52, p = .027) and a trend with caudate nucleus volume (r[16] = −.42, p = .086). No substantial associations were found for the HADS depressive mood subscale for both groups (rs = −.40 to .22).
Association of gray matter volumes with individual components of asthma control
Given the novelty of the uncovered associations for individuals with asthma we explored associations with individual items of the ACQ, which covered past-week night-time awaking by symptoms, severity of morning symptoms, shortness of breath, wheezing, functional limitations, β-adrenergic bronchodilator use, and current % of predicted FEV1. Controlling for age, gender, and ICS use, past-week wheezing and bronchodilator use showed significantly negative associations with putamen volume, r = .54 and .49, p = .025 and .045, respectively, while severity of morning symptoms after awaking were negatively associated with volume of the pallidum, r = .50, p = .039.
Discussion
Our study showed for the first time that asthma history and clinical outcomes are associated with differences in subcortical gray matter volume in young adult individuals with asthma. Although no substantial differences in volumes were found compared to healthy controls, aspects of the disease history, control, and experience are reflected in subcortical structures that are linked to processing of emotion, in particular anxiety and fear. Pallidum and putamen were the structures that were most likely to show these associations. Associations of gray matter volumes with distress over symptoms have been observed before in COPD (Esser et al. 2016) and anxiety disorders and fear have been linked to deficits in limbic and basal ganglia structures (Ahmed-Leitao et al. 2016; Fisler et al. 2013; Hayano et al. 2009; Hilbert et al. 2015; Uono et al. 2017; Yoo et al. 2005; Yoon et al. 2016), but the relationship of asthma-related variables with subcortical structure variations has not been reported before. This relationship is substantial, accounting for up to 20% of variation in gray matter volumes.
Asthma duration and control both showed relatively consistent associations with basal ganglia structures, in particular putamen and pallidum. Although we were not able to establish directions of effects with our cross-sectional study design, the association of lower volumes with longer disease duration makes it more likely that the asthmatic disease process negatively affects CNS structures, rather than structural variations determining the asthmatic disease process. Mechanisms through which asthma could affect the CNS have not been studied well, but speculations at this point could involve a variety of pathways linked to medication or stress hormone effects, brain oxygenation, inflammation, oxidative stress, or sleep disruption. Stress-related hypothalamus-pituitary adrenal axis activity and steroid medication are likely to unfold detrimental effects on CNS structures (Brown et al. 2004; McEwen and Gianaros 2011), and both are more likely seen in asthma patients with significant anxiety, who are known to report or receive more frequent oral steroid regimens (Hyland et al. 1993; Kinsman et al. 1977; Romero-Frais et al. 2005). However, effects likely differ by brain region and glucocorticoid and mineralcorticoid receptor density (Joëls 2018; Meijer et al. 2019) and for the hippocampus, where receptor density is greatest and glucocorticoid effects are well established (Lupien et al. 2018), we did not find associations of gray matter volume with disease outcomes and anxiety in those with asthma. Differential sensitivity of hippocampal substructures and types of neurons further complicate the interpretation of our findings as driven by corticosteroid effects (McEwen et al. 2016). The amydgala, another structure with rich glucocorticoid receptor density (Wang et al. 2014), which has shown volume reductions with corticosteroid treatment (Brown et al. 2008), only showed trend-level associations with disease outcome variables in asthma. Episodes of desaturation by exacerbations have been suspected as a major culprit (Albéri 2013), but problematic saturation levels in asthma appear to be more likely limited to severe exacerbations (Wagner et al. 1996), which may have been less typical for our sample. Associations between systemic inflammation markers (such as C-reactive protein and IL-6) and structural deterioration are well documented (Gu et al. 2017; Frodl and Amico 2014), although less is known about effects of Th2-driven inflammation more typical for young individuals with asthma. Recently, associations have been shown between eotaxin-1, a chemokine marker of eosinophilic inflammation that can cross the blood brain barrier, and brain neurodegeneration (Huber et al. 2018). Oxidative stress is elevated in asthma (Riedl and Nel 2008) and additionally in anxiety disorders (Hassan et al. 2014), making it a candidate mechanisms explaining both effects of asthma pathophysiology and psychopathology on gray matter volumes. Finally, nighttime symptoms in asthma are associated with sleep deficits and lack of sleep has been linked to brain structural deficits (Dai et al. 2018). Our exploratory analysis of ACQ items did not directly support this assumption, although patients’ emotional evaluation of morning symptom (“how bad” they felt) again was negatively associated with volume of the pallidum. However, given the lack of validation of the ACQ on the individual item level, the small number of participants, and the large number of computed correlations these findings should be treated with caution and may serve to generate hypothesis for future large-scale studies.
At this point it is difficult to determining a causal sequence of events related to anxiety. It is also possible that early insults through asthma and its treatment lead to brain atrophy with the subsequent development of anxiety. Gray matter reductions in cortical and subcortical (hippocampus) structures associated with early childhood maltreatment have been related to subsequent development of anxiety (Gorka et al. 2014). On the other hand, recent anxiety treatment studies have shown reductions rather than increases in amygdala and other subcortical gray matter volumes (Hölzel et al. 2010; Månsson et al. 2017; Schienle et al. 2014), complicating causal interpretations of the relation between asthma, brain structure, and anxiety at present.
The specificity of these findings for subcortical structures we studied is unknown. Although we found differences between the examined structures, with a majority of findings involving putamen and pallidum, associations were also found to a weaker extent with thalamus and amygdala. Whether our findings are evidence for a more general reduction in gray matter volumes across brain structures remains to be demonstrated with studies casting a wider net across brain regions. Our study was restricted to selected subcortical structures that are relevant for emotional processing and we cannot rule out that additional cortical structures, linked to emotion or wide ranges of function, might show similar associations. Prefrontal areas in particular play a role in brain networks processing and modulating affect (Berkowitz et al. 2007) and show neuronal remodeling in chronic stress (McEwen et al. 2016). We elected to restrict our focus to subcortical regions given the primary importance of these structures for the generation of affect (Panksepp 2011; Damasio and Carvalho 2013) and for practical reasons given the limited size of our study.
The specificity of our findings for asthma could also be questioned. Deficits in gray matter volumes of limbic and basal ganglia structure have been found in many other conditions, including depression (Grieve et al. 2013), schizophrenia (Kuo and Pogue-Geile 2019) and attention deficit/hyperactivity disorder (Frodl and Skokauskas 2012), the latter in particular for pallidum and putamen. It is also possible that the associations with catastrophizing of asthma and asthma attacks are reflections of the general association between subcortical gray matter volume, anxiety, and fear. However, it should be noted that in our study associations with general anxious mood were not significant for individuals with asthma. The fact that anxious responding to the distressing films was also selectively seen for the pallidum in asthma may also speak against a more generalized effect in asthma, while a more general association of limbic structure volumes (amygdala and hippocampus) with anxiety and anxious responding was seen in our control group. The observation of the specific role of the pallidum in asthma requires further examination.
Emerging research has pointed towards reductions in hippocampal gray matter volume in asthma, specifically in older patients and in men (Carlson et al. 2017). We could not confirm this finding in the present study, although our sample was much smaller and may not have been sufficiently sensitive to detect smaller differences. It is possible that more pronounced deficits could be shown in older patients and/or with greater severity through cumulative disease burden, as also suggested by the observed association with asthma duration. Our sample also had less severe asthma, with a predominance of mild persistent cases, which may have affected our ability to show volume differences.
In addition to the aforementioned limitations, our study was obviously limited by the small sample size and the focus on a younger, mostly well-controlled population of individuals with asthma. However, given the relative consistent effects observed with asthma-relevant variables and subcortical structures it is likely that findings with older and more severe patients could be even more pronounced. Since our findings are only cross-sectional, additional longitudinal brain imaging studies are needed to better determine cause and effect in the observed associations. Although our analysis prioritized subcortical areas, lack of predefined masks in a spatially normalized space (i.e. MNI/Talairach Atlas) and concerns over clear identification of anatomical boundaries of other emotion-relevant hindbrain and mid-brain areas, such as periaqueductal gray, bed nucleus of the stria terminalis, superior colliculus, nucleus tractus solitarius, or parabrachial nucleus (Damasio and Carvalho 2013; Davis 1992; Panksepp 2011), kept us from inspecting these areas, although they would certainly be important to explore in future studies. Similarly, because modulation of emotion by cortico-limbic and cortico-striatal networks is integral part of human emotion, future studies also need to expand scrutiny to cortical gray matter. Despite these limitations, our findings provide initial support for the suspicion that deficits in a range of important clinical outcomes of the asthmatic disease process are reflected in reduced gray matter volumes of subcortical brain structures, further emphasizing the importance of optimal control of the disease.
Supplementary Material
Acknowledgements
Partial funding for this study was provided by the National Institute on Aging (NIA, R24 AG048024), the University Research Council grant (URC 413876) at Southern Methodist University (SMU), and seed funds from Dedman College at SMU. We thank Binu Thomas, Lilly Yang, and Salvador Pena (University of Texas Southwestern Medical Center, Advanced Imaging Research Center) for their help with image acquisitions; Ashton Steele, Sheenal V. Patel, Justin R. Chen, Steve Dorman, Maryam Saifi, Sharon Deol, and Julie Kim for their help in data collection; and Alexandra Kulikova and Brittany Mason for their administrative support.
Funding
Partial funding for this study was provided by the National Institute on Aging (NIA, R24 AG048024), the University Research Council grant (URC 413876) at Southern Methodist University (SMU), and seed funds from Dedman College at SMU.
Abbreviations
- ACQ
Asthma Control Questionnaire
- CAS
Catastrophizing of Asthma Scale
- CNS
Central nervous system
- FENO
Fractional exhaled nitric oxide
- FEV1
Forced expiratory volume in the 1st second
- HADS
Hospital Anxiety and Depression Scale
- MRI
Magnetic resonance imaging
- NHLBI/NAEPP
National Heart, Lung, and Blood Institute/National Asthma Education and Prevention Program
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11682-019-00188-3) contains supplementary material, which is available to authorized users.
Conflict of interest TR, SA, JLK, TJ, AEP, and DAK, report no biomedical financial interests or potential conflicts of interest. ESB reports having received research funding from Otsuka and lecture fees from Genentech.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- Ahmed-Leitao F, Spies G, van den Heuvel L, & Seedat S (2016). Hippocampal and amygdala volumes in adults with posttraumatic stress disorder secondary to childhood abuse or maltreatment: A systematic review. Psychiatry Research: Neuroimaging, 256, 33–43. [DOI] [PubMed] [Google Scholar]
- Albéri L (2013). Asthma: a clinical condition for brain health. Experimental Neurology, 248, 338–342. [DOI] [PubMed] [Google Scholar]
- Alving K, Jansson C, & Nordvall L (2006). Performance of a new hand-held device for exhaled nitric oxide measurement in adults and children. Respiratory Research, 7, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, & Murray EA (2002). The amygdala and reward. Nature Reviews Neuroscience, 3, 563573. [DOI] [PubMed] [Google Scholar]
- Berkowitz RL, Coplan JD, Reddy DP, & Gorman JM (2007). The human dimension: how the prefrontal cortex modulates the subcortical fear response. Reviews in the Neurosciences, 18, 191–207. [DOI] [PubMed] [Google Scholar]
- Brown ES, Woolston D, Frol A, Bobadilla L, Khan DA, Hanczyc M, Rush AJ, Fleckenstein J, Babcock E, & Cullum CM (2004). Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid therapy. Biological Psychiatry, 55, 538–545. [DOI] [PubMed] [Google Scholar]
- Brown ES, Woolston DJ, & Frol AB (2008). Amygdala volume in patients receiving chronic corticosteroid therapy. Biological Psychiatry, 63, 705–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciaglia R, Nees F, Grimm O, Ridder S, Pohlack ST, Diener SJ, Liebscher C, & Flor H (2017). Trauma exposure relates to heightened stress, altered amygdala morphology and deficient extinction learning: implications for psychopathology. Psychoneuroendocrinology, 76, 19–28. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Kim J, Khan DA, King K, Lucarelli RT, McColl R, Peshock R, & Brown ES (2017). Hippocampal volume in patients with asthma: results from the Dallas Heart study. Journal of Asthma, 54, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai XJ, Jiang J, Zhang Z, Nie X, Liu BX, Pei L, Gong H, Hu J, Lu G, & Zhan Y (2018). Plasticity and susceptibility of brain morphometry alterations to insufficient sleep. Frontiers in Psychiatry, 9, 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio A, & Carvalho GB (2013). The nature of feelings: evolutionary and neurobiological origins. Nature Reviews Neuroscience, 14, 143–152. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Cruz M, Stecenko AA, & Kifle Y (2000). Respiratory-related evoked potentials in children with life-threatening asthma. American Journal of Respiratory and Critical Care Medicine, 161, 1830–1835. [DOI] [PubMed] [Google Scholar]
- Davis M (1992). The role of the amygdala in fear and anxiety. Annual Review of Neuroscience, 15, 353–375. [DOI] [PubMed] [Google Scholar]
- De Peuter S, Lemaigre V, Van Diest I, Verleden G, Demedts M, & Van den Bergh O (2007). Differentiation between the sensory and affective aspects of histamine-induced bronchoconstriction in asthma. Respiratory Medicine, 101, 925–932. [DOI] [PubMed] [Google Scholar]
- De Peuter S, Lemaigre V, Van Diest I, & Van den Bergh O (2008). Illness-specific catastrophic thinking and overperception in asthma. Health Psychology, 27, 93–99. [DOI] [PubMed] [Google Scholar]
- Esser RW, Stoeckel MC, Kirsten A, Watz H, Taube K, Lehmann K, Petersen S, Magnussen H, & von Leupoldt A (2016). Structural brain changes in patients with COPD. Chest, 149, 426–434. [DOI] [PubMed] [Google Scholar]
- Favreau H, Bacon SL, Labrecque M, & Lavoie KL (2014). Prospective impact of panic disorder and panic-anxiety on asthma control, health service use, and quality of life in adult patients with asthma over a 4-year follow-up. Psychosomatic Medicine, 76, 147–155. [DOI] [PubMed] [Google Scholar]
- Fisler MS, Federspiel A, Horn H, Dierks T, Schmitt W, Wiest R, de Quervain DJ, & Soravia LM (2013). Spider phobia is associated with decreased left amygdala volume: a cross-sectional study. BMC Psychiatry, 13, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, & Amico F (2014). Is there an association between peripheral immune markers and structural/functional neuroimaging findings? Progress in Neuro-Psychopharmacology & Biological Psychiatry, 48, 295–303. [DOI] [PubMed] [Google Scholar]
- Frodl T, & Skokauskas N (2012). Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatrica Scandinavica, 125, 114–126. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Bandiera FC, Steinberg D, Ortega AN, & Feldman JM (2012). Asthma and mental health among youth: etiology, current knowledge and future directions. Expert Reviews in Respiratory Medicine, 6, 397–406. [DOI] [PubMed] [Google Scholar]
- Gorka AX, Hanson JL, Radtke SR, & Hariri AR (2014). Reduced hippocampal and medial prefrontal gray matter mediate the association between reported childhood maltreatment and trait anxiety in adulthood and predict sensitivity to future life stress. Biology of Mood & Anxiety Disorders, 4, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve SM, Korgaonkar MS, Koslow SH, Gordon E, & Williams LM (2013). Widespread reductions in gray matter volume in depression. Neuroimage Clinical, 3, 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Vorburger R, Scarmeas N, Luchsinger JA, Manly JJ, Schupf N, Mayeux R, & Brickman AM (2017). Circulating inflammatory biomarkers in relation to brain structural measurements in a nondemented elderly population. Brain, Behavior, and Immunity, 65, 150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther V, Ihme K, Kersting A, Hoffmann KT, Lobsien D, & Suslow T (2018). Volumetric associations between amygdala, nucleus accumbens, and socially anxious tendencies in healthy women. Neuroscience, 374, 25–32. [DOI] [PubMed] [Google Scholar]
- Hassan W, Silva CE, Mohammadzai IU, da Rocha JB, & Landeira-Fernandez J (2014). Association of oxidative stress to the genesis of anxiety: implications for possible therapeutic interventions. Current Neuropharmacology, 12, 120–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayano F, Nakamura M, Asami T, Uehara K, Yoshida T, Roppongi T, Otsuka T, Inoue T, & Hirayasu Y (2009). Smaller amygdala is associated with anxiety in patients with panic disorder. Psychiatry and Clinical Neurosciences, 63, 266–276. [DOI] [PubMed] [Google Scholar]
- Hilbert K, Pine DS, Muehlhan M, Lueken U, Steudte-Schmiedgen S, & Beesdo-Baum K (2015). Gray and white matter volume abnormalities in generalized anxiety disorder by categorical and dimensional characterization. Psychiatry Research, 234, 314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel BK, Carmody J, Evans KC, Hoge EA, Dusek JA, Morgan L, Pitman RK, & Lazar SW (2010). Stress reduction correlates with structural changes in the amygdala. Social Cognitive and Affective Neuroscience, 5, 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber AK, Giles DA, Segal BM, & Irani DN (2018). An emerging role for eotaxins in neurodegenerative disease. Clinical Immunology, 189, 29–33. [DOI] [PubMed] [Google Scholar]
- Hyland ME, Kenyon CAP, Taylor M, & Morice AH (1993). Steroid prescribing for asthmatics: relationship with asthma symptom checklist and living with asthma questionnaire. British Journal of Clinical Psychology, 32, 505–511. [DOI] [PubMed] [Google Scholar]
- Janssens T, Verleden G, De Peuter S, Van Diest I, & Van den Bergh O (2009). Inaccurate perception of asthma symptoms: a cognitive-affective framework and implications for asthma treatment. Clinical Psychology Review, 29, 317–327. [DOI] [PubMed] [Google Scholar]
- Janssens T, Verleden G, & Van den Bergh O (2012). Symptoms, lung function, and perception of asthma control: an exploration into the heterogeneity of the asthma control construct. Journal of Asthma, 49, 63–69. [DOI] [PubMed] [Google Scholar]
- Janssens T, Steele AM, Rosenfield D, & Ritz T (2017). Airway reactivity in response to repeated emotional film clip presentation in asthma. Biological Psychology, 123, 1–7. [DOI] [PubMed] [Google Scholar]
- Joëls M (2018). Corticosteroids and the brain. Journal of Endocrinology, 238, R121–R130. [DOI] [PubMed] [Google Scholar]
- Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, & King DR (1999). Development and validation of a questionnaire to measure asthma control. European Respiratory Journal, 14, 902–907. [DOI] [PubMed] [Google Scholar]
- Kinsman RA, Dahlem NW, Spector S, & Staudenmayer H (1977). Observations on subjective symptomatology, coping behavior, and medical decisions in asthma. Psychosomatic Medicine, 36, 102–119. [DOI] [PubMed] [Google Scholar]
- Kuo SS, & Pogue-Geile MF (2019). Variation in fourteen brain structure volumes in schizophrenia: A comprehensive meta-analysis of 246 studies. Neuroscience and Biobehavioral Reviews, 98, 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lago T, Davis A, Grillon C, & Ernst M (2017). Striatum on the anxiety map: Small detours into adolescence. Brain Research, 1654, 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, & Reis DJ (1988). Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. Journal of Neuroscience, 8, 2517–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Juster RP, Raymond C, & Marin MF (2018). The effects of chronic stress on the human brain: From neurotoxicity, to vulnerability, to opportunity. Frontiers in Neuroendocrinology, 49, 91–105. [DOI] [PubMed] [Google Scholar]
- Månsson KNT, Salami A, Carlbring P, Boraxbekk CJ, Andersson G, & Furmark T (2017). Structural but not functional neuroplasticity one year after effective cognitive behaviour therapy for social anxiety disorder. Behavioral Brain Research, 318, 45–51. [DOI] [PubMed] [Google Scholar]
- McEwen BS, & Gianaros PJ (2011). Stress- and allostasis-induced brain plasticity. Annual Review of Medicine, 62, 431–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, & Nasca C (2015). Mechanisms of stress in the brain. Nature Neuroscience, 18, 1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Nasca C, & Gray JD (2016). Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology, 41, 3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer OC, Buurstede JC, & Schaaf MJM (2019). Corticosteroid receptors in the brain: Transcriptional mechanisms for specificity and context-dependent effects. Cellular and Molecular Neurobiology, 39, 539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Kroll J, & Ritz T (2017). Panic disorder comorbidity with medical conditions and treatment implications. Annual Review of Clinical Psychology, 13, 209–240. [DOI] [PubMed] [Google Scholar]
- Morey RA, Gold AL, LaBar KS, Beall SK, Brown VM, Haswell CC, Nasser JD, Wagner HR, McCarthy G & Mid-Atlantic MIRECC Workgroup. (2012). Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Archives of General Psychiatry, 69, 1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SE, & Salzman CD (2010). Re-valuing the amygdala. Current Opinion in Neurobiology, 20, 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute/National Asthma Education and Prevention Program (2007). Expert panel report: Guidelines for the diagnosis and management of asthma. Full report 2007. NIH Publication No. 07–4051 National Institutes of Health, Bethesda, MD, 2007. [Google Scholar]
- Panksepp J (2011). Cross-species affective neuroscience decoding of the primal affective experiences of humans and related animals. PLoS One, 6(9), e21236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy D, & Jenkinson M (2011). A Bayesian model of shape and appearance for subcortical brain. NeuroImage, 56, 907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl MA, & Nel AE (2008). Importance of oxidative stress in the pathogenesis and treatment of asthma. Current Opinion in Allergy and Clinical Immunology, 8, 49–56. [DOI] [PubMed] [Google Scholar]
- Ritz T, Kullowatz A, Goldman GD, Smith H-J, Kanniess F, Dahme B, & Magnussen H (2010). Airway response to emotional stimuli in asthma: the role of the cholinergic pathway. Journal of Applied Physiology, 108, 1542–1549. [DOI] [PubMed] [Google Scholar]
- Ritz T, Wilhelm FH, Meuret AE, Gerlach AL, & Roth WT (2011). Airway response to emotion- and disease-specific films in asthma, blood phobia, and health. Psychophysiology, 48, 121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Frais E, Vázquez MI, Sández E, Blanco-Aparicio M, Otero I, & Verea H (2005). Prescription of oral corticosteroids in near-fatal asthma patients: relationship with panic-fear, anxiety and depression. Scandinavian Journal of Psychology, 46, 459–465. [DOI] [PubMed] [Google Scholar]
- Rosenkranz MA, Esnault S, Christian BT, Crisafi G, Gresham LK, Higgins AT, Moore MN, Moore SM, Weng HY, Salk RH, Busse WW, & Davidson RJ (2016). Mind-body interactions in the regulation of airway inflammation in asthma: a PET study of acute and chronic stress. Brain, Behavior, and Immunity, 58, 18–30. 10.1016/j.bbi.2016.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schienle A, Wabnegger A, & Scharmüller W (2014). Effects of cognitive behavior therapy on regional brain volume in spider-phobic patients: preliminary results. Journal of Anxiety Disorders, 28, 276–279. [DOI] [PubMed] [Google Scholar]
- Scott KM, Von Korff M, Ormel J, Zhang MY, Bruffaerts R, Alonso J, Kessler RC, Tachimori H, Karam E, Levinson D, Bromet EJ, Posada-Villa J, Gasquet I, Angermeyer MC, Borges G, de Girolamo G, Herman A, & Haro JM (2007). Mental disorders among adults with asthma: results from the world mental health survey. General Hospital Psychiatry, 29, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uono S, Sato W, Kochiyama T, Kubota Y, Sawada R, Yoshimura S, & Toichi M (2017). Putamen volume is negatively correlated with the ability to recognize fearful facial expressions. Brain Topography, 30, 774–784. [DOI] [PubMed] [Google Scholar]
- von Leupoldt A, Sommer T, Kegat S, Eippert F, Baumann HJ, Klose H, Dahme B, & Büchel C (2009). Down-regulation of insular cortex responses to dyspnea and pain in asthma. American Journal of Respiratory and Critical Care Medicine, 180, 232–238. [DOI] [PubMed] [Google Scholar]
- von Leupoldt A, Brassen S, Baumann HJ, Klose H, & Büchel C (2011). Structural brain changes related to disease duration in patients with asthma. PLoS One, 6, e23739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner PD, Hedenstierna G, & Rodriguez-Roisin R (1996). Gas exchange, expiratory flow obstruction and the clinical spectrum of asthma. European Respiratory Journal, 9, 1278–1282. [DOI] [PubMed] [Google Scholar]
- Wang Q, Verweij EW, Krugers HJ, Joels M, Swaab DF, & Lucassen PJ (2014). Distribution of the glucocorticoid receptor in the human amygdala; changes in mood disorder patients. Brain Structure and Function, 219, 1615–1626. [DOI] [PubMed] [Google Scholar]
- Webster KE, & Colrain IM (2002). P3-specific amplitude reductions to respiratory and auditory stimuli in subjects with asthma. American Journal of Respiratory and Critical Care Medicine, 166, 47–52. [DOI] [PubMed] [Google Scholar]
- Yoo HK, Kim MJ, Kim SJ, Sung YH, Sim ME, Lee YS, Song SY, Kee BS, & Lyoo IK (2005). Putaminal gray matter volume decrease in panic disorder: an optimized voxel-based morphometry study. European Journal of Neuroscience, 22, 2089–2094. [DOI] [PubMed] [Google Scholar]
- Yoon S, Kim JE, Kim GH, Kang HJ, Kim BR, Jeon S, Im JJ, Hyun H, Moon S, Lim SM, & Lyoo IK (2016). Subregional shape alterations in the amygdala in patients with panic disorder. PLoS One, 11, e0157856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wang X, Lin J, Sun Y, Huang Y, Yang T, Zheng S, Fan M, & Zhang J (2012). Grey and white matter abnormalities in chronic obstructive pulmonary disease: a case control study. BMJ Open, 2, e000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond AS, & Snaith RP (1983). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67, 361–370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


