Abstract
Objective:
To characterize endothelial function, inflammation and immunosuppression in surgical patients with distinct clinical trajectories of acute kidney injury (AKI) and to determine the impact of persistent kidney injury and renal non-recovery on clinical outcomes, resource utilization, and long-term disability and survival.
Summary Background Data:
AKI is associated with increased healthcare costs and mortality. Trajectories that account for duration and recovery of AKI have not been described for sepsis patients, who are uniquely vulnerable to renal dysfunction.
Methods:
This prospective observational study included 239 sepsis patients admitted and enrolled between January 2015 and July 2017. KDIGO and ADQI criteria were used to classify subjects as having no AKI, rapidly reversed AKI, persistent AKI with renal recovery, or persistent AKI without renal recovery. Serial biomarker profiles, clinical outcomes, resource utilization, and long-term physical performance status and survival were compared among AKI trajectories.
Results:
Sixty-two percent of the study population developed AKI. Only one third of AKI episodes rapidly reversed within 48 hours; the remaining had persistent AKI, among which 57% did not have renal recovery by discharge. One-year survival and proportion of subjects fully active one year after sepsis was lowest among patients with persistent AKI compared with other groups. Long-term mortality hazard rates were five-fold higher for persistent AKI without renal recovery compared with no AKI.
Conclusions:
Among critically ill surgical sepsis patients, persistent AKI and the absence of renal recovery are associated with distinct early and sustained immunologic and endothelial biomarker signatures and decreased long-term physical function and survival.
Keywords: Acute kidney injury, renal recovery, persistent acute kidney injury, acute kidney disease, sepsis, chronic critical illness, recovery, persistent inflammation, immunosuppression, mortality, long-term survival, physical function
MINI-ABSTRACT
In a prospective observational study of 239 critically ill surgical sepsis patients, we have shown that persistent AKI and the absence of renal recovery are associated with distinct early and sustained increase in inflammation, immunosuppression and endothelial dysfunction and independently increase the risk for long term disability and mortality.
INTRODUCTION
The delayed or incomplete recovery of acute organ dysfunction during sepsis leads to chronic critical illness and is an important determinant of survival and functional recovery.1 The kidney is highly susceptible to acute dysfunction during sepsis. Acute kidney injury (AKI) affects more than half of septic patients.2,3 Despite evolving understanding of mechanisms by which inflammation and endothelial dysfunction lead to increased metabolic and oxidative stress in complex septic AKI pathophysiology, targeted therapies remain elusive.4–6
The clinical presentation and trajectories of AKI in sepsis are similarly complex and insufficiently described.7 Severity and duration of AKI, as measured by magnitude and persistence of decline in glomerular filtration rate (GFR), are obvious determinants of adverse outcomes. A growing body of evidence suggests that short- and long-term outcomes are influenced by recovery of renal function, measured by change in serum creatinine relative to baseline levels and residual renal function at discharge. 8–12 The 16th Acute Disease Quality Initiative proposed distinct clinical trajectories of rapidly reversed and persistent AKI, with or without renal recovery, assuming that timelines of renal recovery differentiate underlying pathophysiology and associated risk of complications and progression to chronic kidney disease.13 Although potential benefits of classifying sepsis patients into unique clinical phenotypes has been recently emphasized,14 the prospective characterization and validation of proposed clinical AKI trajectories is lacking.15
We performed a prospective longitudinal study of surgical sepsis patients to characterize endothelial function, inflammation and immunosuppression in patients with distinct clinical trajectories of AKI and to determine the impact of persistent kidney injury and renal non-recovery on clinical outcomes, resource utilization, and long-term survival and functional recovery.
METHODS
Study design
This single-center prospective one-year longitudinal cohort study (NCT02276417) included 239 septic patients hospitalized in a 48-bed surgical intensive care unit (ICU) at a quaternary care academic hospital between January 2015 and July 2017 (Supplemental Digital Content (SDC) Figure 1). Ethics approval was obtained from the University of Florida Institutional Review Board (201400611). Informed consent was obtained from each subject or their surrogate decision-maker.
Inclusion criteria were admission to the ICU, age ≥18 years, and a clinical diagnosis of sepsis with subsequent initiation of a clinical decision support-directed sepsis management protocol.16,17 Patients with pre-admission end-stage renal disease, advanced liver or heart disease, systemic immunological disorders, and those who were transplant recipients, immunosuppressed, or under cancer chemotherapy were excluded.16 During weekly meetings study investigators clinically adjudicated sepsis according to study protocols.16,18
Blood and urine samples were collected to characterize selected biological correlates of endothelial function, inflammation and immunosuppression, starting within twelve hours of enrollment and at predefined time points up to fourteen days (SDC Table 1). Clinical assessments of functional and renal status were performed at three, six, and twelve months after enrollment (a detailed description is available in Supplemental Methods).1,19
Assessment of kidney function
Three independent nephrologists (AB, MS, RM) clinically adjudicated all AKI episodes using all available clinical information, according to Kidney Disease: Improving Global Outcomes criteria (0.3 mg/dl increase in serum creatinine within 48 hours or 50% increase from baseline within seven days or decrease in urine output to less than 0.5 ml/kg/hr for six hours).20 The maximum AKI stage was determined based on the highest ratio between serum creatinine and reference serum creatinine and need for renal replacement therapy (RRT). Duration of AKI and evidence of renal recovery13 defined clinical trajectories of rapidly reversed and persistent AKI with and without renal recovery at discharge. Persistent AKI was defined as an episode of AKI by any serum or urine output criteria lasting for at least 48 hours. Any episode of shorter duration was characterized as rapidly reversed AKI. Renal recovery was adjudicated for each episode based on normalization of AKI criteria at the time of hospital discharge. Reference creatinine was determined using available preadmission measurements (n=175)21–23 or the estimated creatinine using the Modification of Diet in Renal Disease Study equation assuming that baseline estimated glomerular filtration rate (eGFR) is 75 ml/min per 1.73 m2 (n=64).20,24,25 Reference creatinine was used to estimate preadmission reference glomerular filtration rate using Chronic Kidney Disease Epidemiology Collaboration equation. 26 For each patient, we calculated daily kinetic GFR using the estimate of creatinine production rate and percent change in creatinine over time.27 Cumulative net fluid balance was calculated daily as the ratio between total net fluid balance from hospital admission and admission weight.28
Biological correlates, functional assessment and clinical outcomes
Severity of illness was characterized using Acute Physiology, Age and Chronic Health Evaluation (APACHE) II and Sequential Organ Failure Assessment Score (SOFA) scores.16 Longitudinal changes in ten serum biomarkers reflecting the host response to sepsis were categorized under endothelial function, inflammation and immunosuppression (Supplemental Methods and SDC Table 1). The five-point Zubrod Scale was used to measure physical performance and functional recovery at follow-up assessments, with higher scores indicating worse condition.1,19
Primary clinical outcomes were thirty-day mortality and one-year survival. Primary renal outcomes were major adverse kidney events at 30 and 365 days after enrollment, defined as the composite of death, dependency on RRT, or decrease in eGFR < 60 ml/min/1.72m2. Other exploratory outcomes included hospital-free, ICU-free, and mechanical ventilation-free days within 28 days of enrollment and functional recovery at one-year (Supplemental Method).
Statistical analysis
The sample size offered 80% power to detect an 8% difference in thirty-day mortality and two-fold increase in the hazard ratio for one-year mortality between patients with and without AKI, assuming AKI prevalence of 62% and thirty-day and one-year mortality in the no AKI group of 1% and 10%, respectively (Type 1 error < 0.05). Overall survival of each trajectory group was evaluated using log-rank and Kaplan–Meier methods. Propensity score based inverse weighting29 was used to plot adjusted Kaplan Meier curves where propensity of being in a trajectory group was calculated using multinomial logistic model that included patient demographics (age, gender, African-American ethnicity), Charlson comorbidity score, and septic shock and non-renal SOFA score on sepsis onset. Cox proportional-hazards regression was used to assess associations between AKI trajectories and one-year mortality while controlling for total ICU length of stay in addition to the same variables included in the propensity score models. Model discrimination was assessed using Harrell’s concordance index (c-index). Cumulative net fluid balance and kinetic GFR values over time were shown by locally estimated scatterplot smoothers calculated using local regression in R function “loess”.30 All p-values were adjusted for multiple comparisons using Dwass-Steel-Critchlow-Fligner and Bonferroni methods.31 Statistical analyses were performed with SAS (v.9.4, Cary, NC), R 3.5.3, and Python 3.6.6 software.
RESULTS
Clinical characteristics of patients with surgical sepsis
During enrollment period, among 1,643 patients initiated on sepsis protocol 245 consented for the study and 239 was eligible for this analysis (SDC Figure 1). Sepsis was diagnosed within 48 hours of hospital admission for 66% (n=158) of the cohort and 23% (n=56) presented with septic shock (Table 1 and SDC Table 2–3). Illness severity was high with average enrollment APACHE II and SOFA scores of 18 and 7, respectively. One-fourth of the cohort had non-renal organ dysfunction affecting three or more organ systems, the most common being respiratory (71%, n=170) and cardiovascular (29%, n=69). Most patients underwent major non-cardiac surgery prior to sepsis onset. Abdominal (46%, n=110), pulmonary (18%, n=44), and soft tissue infections (18%, n=43) were the most common sources of sepsis.
Table 1.
Patient characteristics at sepsis onset stratified by acute kidney injury trajectories.
| Variable | All cohort (N=239) | Persistent AKI without renal recovery (N=58, 24%) | Persistent AKI with renal recovery (N=43, 18%) | Rapidly reversed AKI (N=47, 20%) | No AKI (N=91, 38%) |
|---|---|---|---|---|---|
| Demographics | |||||
| Male gender, n (%) | 130 (54) | 38 (66) | 26 (61) | 25 (53) | 41 (45) |
| Age in years | 59 (15) | 61 (17) | 63 (14)a | 61 (14) | 55 (15) |
| Body mass index (kg/m2) | 31.2 (9.5) | 30.7 (9.7) | 33.1 (11.1) | 32.9 (10.5) | 29.7 (7.6) |
| Comorbidities | |||||
| Charlson comorbidity index, median (25th,75th) | 4 (2, 5) | 5 (3, 7)a | 5 (2, 7)a | 4 (3, 6)a | 3 (1, 5) |
| Chronic kidney disease, n (%) | 36 (15) | 12 (21) | 10 (23) | 8 (17) | 6 (7) |
| Moderate/Severe (≥ Stage 3), n (%) | 24 (10) | 8 (14) | 7 (16) | 6 (13) | 3 (3) |
| Preadmission estimated glomerular filtration rate (mL/min per 1.73 m2), median (25th, 75th) | 84 (73, 105) | 76 (70, 99)a | 78 (69, 95)a | 78 (73, 91)a | 98 (82, 108) |
| Admission characteristics, n (%) | |||||
| Transfer from another hospital | 112 (47) | 33 (57) | 27 (63) | 16 (34) | 36 (40) |
| Surgery type | |||||
| Vascular surgery | 40 (17) | 21 (36)a, b | 12 (28)a | 5 (11) | 2 (2) |
| Trauma and Acute Care surgery | 86 (36) | 13 (22)a | 8 (19)a | 20 (43) | 45 (50) |
| General surgery | 50 (21) | 12 (21) | 11 (26) | 8 (17) | 19 (21) |
| Other surgical subspecialtyd | 63 (26) | 12 (21) | 12 (28) | 14 (30) | 25 (28) |
| Characteristics of initial sepsis episode | |||||
| Late sepsis (after 48 hours of admission) | 81 (34) | 19 (33) | 18 (42) | 16 (34) | 28 (31) |
| Sepsis severity at onset, n (%) | |||||
| Sepsis 2 criteria (all patients) | 239 (100) | 58 (100) | 43 (100) | 47 (100) | 91 (100) |
| Sepsis/Severe Sepsis | 183 (77) | 31 (53) a, b | 29 (67) a | 40 (85) | 83 (91) |
| Septic Shock | 56 (23) | 27 (47) a, b | 14 (33) a | 7 (15) | 8 (9) |
| Sepsis 3 criteria (all patients) | 230 (96) | 57 (98) | 42 (98) | 47 (100) | 84 (92) |
| Sepsis | 177 (74) | 32 (55) a, b | 26 (60) a, b | 40 (85) | 79 (87) |
| Septic Shock | 53 (22) | 25 (43) a, b | 16 (37) a | 7 (15) | 5 (5) |
| Acuity scores within 24 hours of sepsis onset | |||||
| APACHE II score | 18 (8) | 23 (9)a, b | 22 (8)a, b | 16 (6) | 15 (7) |
| Total SOFA score | 6.9 (3.7) | 9.3 (4.1)a, b | 7.8 (3.6)a | 6.3 (2.9) | 5.3 (3.0) |
| Total SOFA score, non-renal | 5.8 (3.3) | 7.6 (3.8)a, b | 6.5 (3.1)a | 5.0 (2.8) | 4.7 (2.8) |
| ≥3 non-renal SOFA organ dysfunctione, n (%) | 60 (25) | 24 (41)a | 16 (37)a | 8 (17) | 12 (13) |
Results show mean (standard deviation) unless indicated otherwise.
All p-values were adjusted for multiple comparisons using Dwass-Steel-Critchlow-Fligner multiple comparison procedure for nonparametric comparison of continuous variables and Bonferroni method for others.
Abbreviations. AKI, acute kidney injury; APACHE II, Acute Physiology and Chronic Health Evaluation II score; SOFA, Sequential Organ Failure Assessment.
Significantly different from No AKI group (adjusted p<0.05)
Significantly different from rapidly reversed AKI group (adjusted p<0.05)
More than two drinks per day averaged over two weeks prior to admission, one drink consists of 2 ounces or liquor, 6 ounces of wine, or 12 ounces of beer
Specialty surgery included urology, obstetrics and gynecology, orthopedics, transplant surgery, otolaryngology and oral surgery.
Organ dysfunction is defined as a SOFA score of 2 or more. This criteria is satisfied by having a Glasgow coma scale <13 for central nervous system, being on any vasopressors (including epinephrine, norepinephrine, dopamine, dobutamine, phenylephrine, and vasopressin) for cardiovascular, being on mechanical ventilation or having the ratio of partial pressure arterial oxygen and fraction of inspired oxygen < 300 mmHg for respiratory, having serum creatinine ≥ 2 mg/dl, having platelets <100,000/¼l for coagulation, and having bilirubin ≥ 2 mg/dl for liver organ dysfunction.
Clinical trajectories of patients with acute kidney injury during hospitalization
Overall, 62% (n=148) of the cohort developed AKI; 52% (n=124) had AKI within 48 hours of sepsis onset while 10% (n=24) developed AKI later (Figure 1A). Only 32% (47/148) of AKI episodes rapidly reversed within 48 hours with sustained renal recovery until discharge, while the remaining 68% (101/148) had persistent AKI. By the time of discharge or death, 57% (58/101) of all subjects with persistent AKI did not recover renal function, and 21% (21/101) were dependent on RRT.
Figure 1.
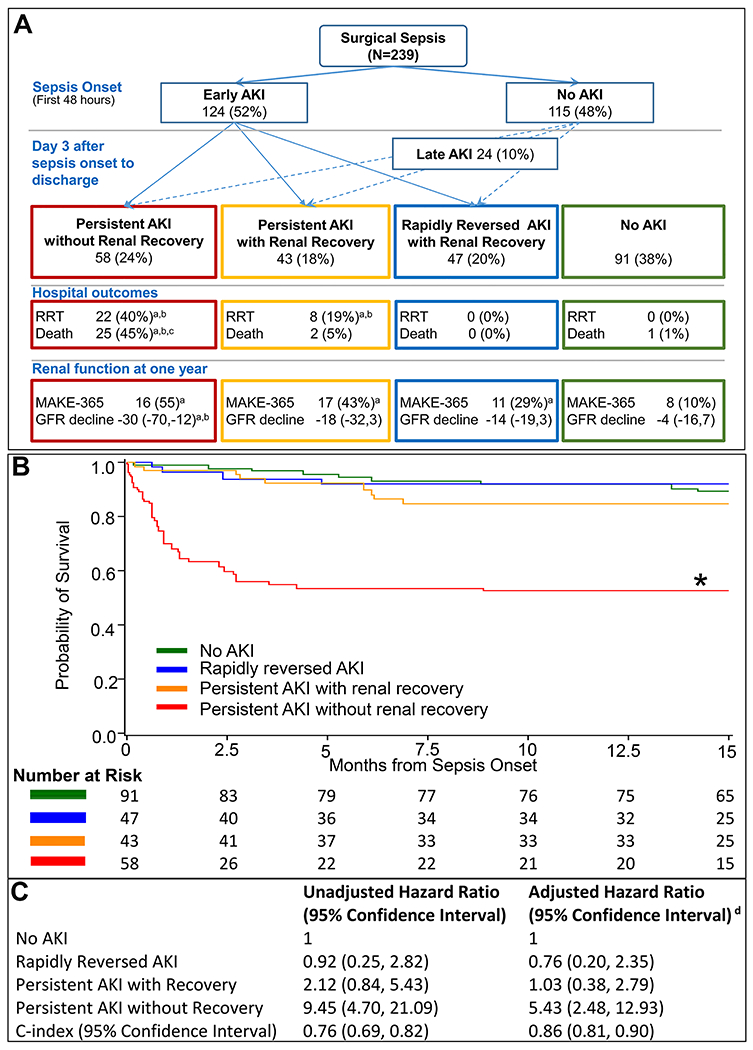
A. Trajectories of acute kidney injury in sepsis
a Significantly different from No AKI group (Bonferroni-adjusted p<0.05)
b Significantly different from rapidly reversed AKI group (Bonferroni-adjusted p<0.05)
c Significantly different from persistent AKI with recovery (Bonferroni-adjusted p<0.05)
B. Adjusted Kaplan-Meier survival curves and number at risk by AKI trajectories Propensity score based inverse weighting was used to plot adjusted Kaplan Meier curves where propensity of being in a trajectory group was calculated using multinomial logistic model that included patient demographics (age, gender, ethnicity), Charlson comorbidity score, septic shock and non-renal SOFA score on sepsis onset.
C. Hazard ratios for all-cause mortality by AKI trajectories
* p<0.05 for Bonferroni-adjusted log-rank test when compared to all other groups
d Adjusted for age, gender, ethnicity, Charlson comorbidity score, sepsis shock status and non-renal SOFA score on sepsis onset, and total ICU length of stay.
Abbreviations. RRT, renal replacement therapy; GFR, glomerular filtration rate ; MAKE-365, major adverse kidney events composite outcome of death, renal replacement therapy dependence, or having an estimated GFR less than 60 ml/min/1.72m2 at one year of sepsis onset. Hospital outcomes were reported among patients who were not withdrawn from the study at the time of hospital discharge and one year outcomes were reported among hospital survivors were not withdrawn from the study and had renal data available at one year.
Regardless of trajectory, AKI patients had greater comorbidity burden and lower reference eGFR compared to patients without AKI (Table 1 and SDC Table 2–3). Patients with persistent AKI had the worst early physiologic derangement, with more severe cardiovascular (more profound hypotension despite receiving significantly more vasopressors and intravenous crystalloid fluids and worse lactic acidosis) and hematologic organ dysfunctions (worse thrombocytopenia, coagulopathy and anemia) compared to others (Table 2 and SDC Table 4–5).
Table 2.
Early changes in renal function, blood product administration, and resuscitation volumes within 48 hours of sepsis onset, stratified by acute kidney injury trajectories.
| Variable | All cohort (N=239) | Persistent AKI without renal recovery (N=58, 24%) | Persistent AKI with renal recovery (N=43, 18%) | Rapidly reversed AKI (N=47, 20%) | No AKI (N=91, 38%) |
|---|---|---|---|---|---|
| Kidney function within 48 hours of the sepsis onset | |||||
| AKI, n (%) | 117 (49) | 41 (71)b | 34 (79) | 42 (89) | NA |
| Stage 1 | 58 (24) | 15 (26) b | 12 (28) b | 31 (66) | NA |
| Stage 2 | 32 (13) | 10 (17) | 15 (35) | 7 (15) | NA |
| Stage 3 | 27 (11) | 16 (27) b | 7 (17) | 4 (9) | NA |
| Renal replacement therapy, n (%) | 15 (6) | 10 (17) b | 5 (12) | 0 (0) | NA |
| Highest blood urea nitrogen (mg/dl) | 29 (21) | 42 (29)a, b | 37 (22)a | 29 (15)a | 18 (10) |
| Highest serum creatinine (mg/dl), median (25th, 75th) | 1.10 (0.80, 1.70) | 1.63 (1.01, 2.56)a | 1.71 (1.12, 2.57)a | 1.24 (1.04, 1.72)a | 0.80 (0.66, 1.07) |
| Highest cystatin C (mg/dl), median (25th, 75th) | 0.9 (0.7, 1.5) | 1.5 (0.9, 1.8)a, b | 1.5 (0.9, 2.2)a, b | 1.0 (0.8, 1.2)a | 0.7 (0.6, 0.9) |
| Urine microalbumin/creatinine ratio ≥30 mcg/mg, n (%) | 149 (62) | 36 (62) | 30 (70) | 30 (64) | 53 (58) |
| Urine output (L/day), median (25th, 75th) | 1.7 (1.1, 2.6) | 1.3 (0.6, 2.0)a, b | 1.3 (0.1, 2.5)a, b | 2.1 (1.4, 2.9) | 2.1 (1.4, 2.7) |
| Resuscitation volume within 48 hours of the sepsis onset, median (25th, 75th) | |||||
| Blood products, n (%)d | 33 (14) | 12 (21) | 11 (26)a | 4 (9) | 6 (7) |
| Saline (L), median (25th, 75th) | 2.7 (1.1, 5.2) | 3.8 (1.8, 6.2)a | 3.6 (1.5, 5.5) | 2.5 (1, 4.8) | 2 (1, 4.7) |
| Balanced crystalloids (L), median (25th, 75th) | 4.6 (2.4, 7) | 6.3 (2.6, 9.6) | 3.6 (1.8, 8.7) | 4.6 (2.5, 6.8) | 4 (2.3, 6.1) |
| Cumulative net fluid balance (L), median (25th, 75th) | 2.5 (−0.4, 5.1) | 4.1 (0.8, 6.3)a | 3.4 (−0.4, 6.2) | 1.3 (−1.2, 4.7) | 1.7 (−0.1, 4.1) |
| Cumulative net fluid balance (% admission weight) | 3.4 (5.2) | 5.4 (6.8)a, b | 3.7 (5.3) | 2.3 (4.4) | 2.6 (4) |
Data are represented as mean (standard deviation) unless indicated otherwise.
All p-values were adjusted for multiple comparisons using Dwass-Steel-Critchlow-Fligner multiple comparison procedure for nonparametric comparison of continuous variables and Bonferroni method for others.
Abbreviations. PO2, partial pressure of oxygen; FiO2: fraction of inspired oxygen.
Significantly different from No AKI group (adjusted p<0.05)
Significantly different from rapidly reversed AKI (adjusted p<0.05)
Significantly different from persistent AKI with renal recovery group (adjusted p<0.05)
Blood products considered were red blood cells, plasma, platelets, and cryoprecipitate.
Within 48 hours of sepsis onset, median values for serum creatinine (1.66 mg/dl, 25th-75th 1.03 - 2.56 mg/dl) and urine output (1.3 L, 25th-75th 0.8-2.1 L) were significantly higher among patients with persistent AKI compared to others, yet remained below the thresholds defining renal dysfunction by SOFA score (SDC Table 5). Subsequently 61% (90/148) of AKI patients were not identified as having renal dysfunction using SOFA (Table 1 and SDC Table 2–3). Compared to patients with rapidly reversed AKI, those with persistent AKI were more likely to present with more severe stage (23%, n=23 vs 9%, n=4, p<0.05) and had higher requirement for RRT in the first 48 of sepsis (15%, n=15 vs 0%, n=0, p<0.01) (SDC Table 5). Two thirds of all sepsis patients had early microalbuminuria, with similar prevalence among AKI subgroups (Table 2 and SDC Table 5).
Early and sustained decline in kinetic GFR below 60 ml/min/1.72m2 throughout hospitalization was demonstrated among patients with persistent AKI and even those who recovered renal function at discharge had significantly lower kinetic GFR than patients without AKI (86 versus 113 ml/min/1.72m2, respectively, p ≤ 0.01). Patients with persistent AKI exhibited significant fluid retention with an average fluid overload of approximately 20% of admission weight at discharge or death (Figure 2 A–B, SDC Figure 2 A–B).
Figure 2.
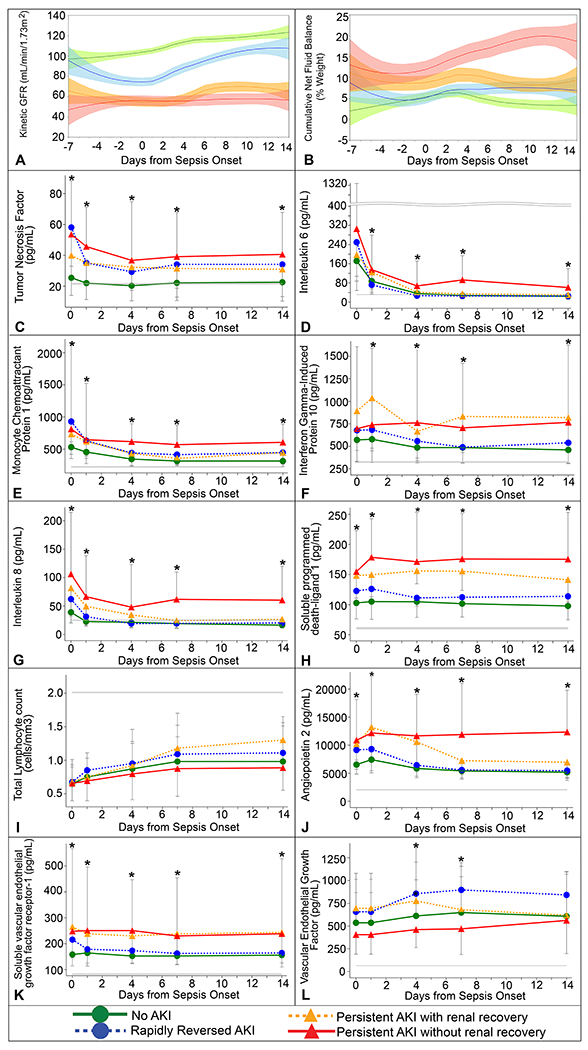
Kinetic glomerular filtration rate, cumulative net fluid balance, and immune and endothelial biomarker trajectories by AKI trajectory groups
A. Kinetic glomerular filtration rate B. Cumulative net fluid balance as a percentage of admission weight C. Tumor necrosis factor (TNF-alpha) D. Interleukin 6 (IL-6) E. Monocyte chemoattractant protein 1 (MCP1) F. Interferon gamma-induced protein 10 (IP-10) G. Interleukin 8 (IL-8) H. Soluble programmed death-ligand 1 (sPDL-1) I. Total lymphocyte count J. Angiopoietin 2 (ANG-2) K. Soluble vascular endothelial growth factor receptor-1 (sFLT-1) L. Vascular Endothelial Growth Factor (VEGF)
Gray lines represent median values for healthy controls provided by manufacturer of the ELISA and Luminex multiplex kits used as detailed in Supplemental Methods.
Persistent kidney dysfunction is characterized by sustained immune dysregulation and endothelial dysfunction
We observed a significant difference in systemic levels of biomarkers of inflammation, immunosuppression and endothelial dysfunction among different AKI trajectories (SDC Table 4–5, Figure 2 C–L, and SDC Figure 2 C–L in the Supplement). Compared to others, patients with persistent AKI had a significant increase in biomarkers of inflammation and immunosuppression. The most pronounced elevation was observed in levels of interleukin 8 and soluble programmed death-ligand-1 starting within 12 hours of sepsis and continuing for 14 days (p<0.001). Similarly, they had a significant increase in biomarkers of endothelial dysfunction, angiopoietin-2 and soluble vascular endothelial growth factor receptor-1, sustained for 14 days after sepsis (p<0.01).
Persistence of kidney dysfunction and absence of recovery affect short and long-term outcomes
Median duration of AKI was eight days (25th-75th 5-16 days) among patients with persistent AKI and ten days (25th-75th 5-18 days) among those who failed to recover at hospital discharge (Table 3 and SDC Table 6). Patients with persistent AKI required significantly more hospital resources within 28 days of sepsis onset (Table 4 and SDC Table 6). Compared to patients without AKI, they had more days requiring mechanical ventilation (7 days), ICU (16 days) and hospital stay (14 days) (p<0.001). Thirty-day mortality of 24% (n=24) for patients with persistent AKI was significantly higher compared to patients rapidly reversed AKI (5%, n=2) or those without AKI (1%, n=1) (p<0.01). Among them, those who failed to recover renal function by the time of discharge had the worse thirty-day mortality of 40% (n=22), significantly higher than any other group (Figure 1A). The rate of major adverse kidney events at 30 days was significantly higher among patients with persistent AKI (64%, n=63) compared with patients without AKI (5%, n=4) and those with rapidly reversed AKI (14%, n=6) (p<0.001).
Table 3.
Kidney function during hospitalization stratified by acute kidney injury trajectories.
| Kidney function during hospitalization | All cohort (N=239) | Persistent AKI without renal recovery (N=58, 24%) | Persistent AKI with renal recovery (N=43, 18%) | Rapidly reversed AKI (N=47, 20%) | No AKI (N=91, 38%) |
|---|---|---|---|---|---|
| AKI timing, n (%) | |||||
| Early (within 48 hours of sepsis onset) | 124 (52) | 42 (72)b | 37 (86) | 45 (96) | NA |
| Late (>48 hours after sepsis onset) | 24 (10) | 16 (28)b | 6 (14) | 2 (4) | NA |
| Maximum AKI stage, n (%) | 148 (62) | ||||
| Stage 1 | 59 (25) | 12 (21)b | 14 (33)b | 33 (70) | NA |
| Stage 2 | 45 (19) | 16 (28) | 19 (44) | 10 (21) | NA |
| Stage 3 | 44 (18) | 30 (52)b,c | 10 (24) | 4 (9) | NA |
| Renal replacement therapy, n (%) | 30 (13) | 22 (38)b | 8 (19)b | 0 (0) | NA |
| AKI duration, days | 5 (2, 11) | 10 (5, 18)b | 6 (4, 11)b | 2 (1, 2) | NA |
| Recurrent AKI, n (%) | 41 (17) | 19 (33)b | 19 (44) b | 3 (6) | NA |
| No renal recovery at discharge, n (%) | 58 (24) | 58 (100) b, c | 0 (0) | 0 (0) | NA |
All p-values were adjusted for multiple comparisons using Bonferroni method.
Abbreviations. AKI, acute kidney injury.
Significantly different from No AKI group (adjusted p<0.05)
Significantly different from rapidly reversed AKI group (adjusted p<0.05)
Significantly different from persistent AKI with renal recovery group (adjusted p<0.05)
Table 4.
Clinical outcomes stratified by acute kidney injury trajectories.
| Clinical Outcomes | All cohort (N=239) | Persistent AKI without renal recovery (N=58, 24%) | Persistent AKI with renal recovery (N=43, 18%) | Rapidly reversed AKI (N=47, 20%) | No AKI (N=91, 38%) |
|---|---|---|---|---|---|
| Thirty-day mortality, n (%)d | 27 (12) | 22 (40) a,b,c | 2 (5) | 2 (5) | 1 (1) |
| Major adverse kidney events -30, n (%)d, e | 73 (32) | 44 (80) a,b,c | 19 (44) a,b | 6 (14) | 4 (5) |
| Resource Utilization in the first 28 days d,f | |||||
| Mechanical ventilation-free days, median (25th, 75th) | 22 (13, 25) | 4 (0, 23)a,b,c | 20 (14, 24)a,b | 25 (24, 26) | 24 (20, 26) |
| Intensive care unit-free days, median (25th, 75th) | 20 (8, 25) | 1 (0, 14)a,b,c | 13 (2, 20)a,bb | 23 (20, 25) | 24 (18, 26) |
| Hospital-free days, median (25th, 75th) | 7 (0, 18) | 0 (0, 6)a,b | 0 (0, 10)a,b | 13 (5, 20) | 14 (3, 21) |
| One-year outcomes | |||||
| One-year survival, % (95% confidence interval), median (25th, 75th) h | 77 (71, 82) | 40 (28, 53) a,b,c | 79 (63, 88) | 91 (77, 96) | 92 (83, 96) |
| Major adverse kidney events - 365, n (%)e, h | 52 (28) | 16 (55) a | 17 (43) a | 11 (29) | 8 (10) |
| Functional status, n (%)h, i | |||||
| Asymptomatic and fully active | 44 (23) | 3 (10) | 5 (13) | 11 (29) | 25 (31) |
| Mild to moderate functional limitation | 83 (44) | 13 (45) | 19 (48) | 17 (45) | 34 (42) |
| Severe functional limitation | 36 (19) | 5 (17) | 9 (23) | 6 (16) | 16 (20) |
| Dead | 8 (28) | 8 (28) | 7 (18) | 4 (11) | 6 (7) |
All p-values were adjusted for multiple comparisons using Dwass-Steel-Critchlow-Fligner multiple comparison procedure for nonparametric comparison of continuous variables and Bonferroni method for others. Abbreviations. AKI, acute kidney injury.
Significantly different from No AKI group (adjusted p<0.05)
Significantly different from rapidly reversed AKI group (adjusted p<0.05)
Significantly different from persistent AKI with renal recovery group (adjusted p<0.05)
Among patients with available discharge data (10 patients withdrawn before discharge day), which includes 55 patients in persistent AKI with no recovery, 43 in persistent AKI with recovery, 43 in rapidly reversed AKI, and 88 in No AKI groups.
Major adverse kidney event is a composite outcome of death, renal replacement therapy dependency or having an estimated glomerular filtration rate (eGFR) less than 60 ml/min/1.72m2.
Complication free days are calculated as days free of complication within 28 days of hospital admission. For patients discharged before day 28, days after discharge are considered as complication free days. For patients who died in hospital, complication free days are considered as 0.
One-year survival probability along with 95% confidence interval using life-table estimates.
Percentages are shown among 188 hospital survivors who have not withdrawn from the study. Percentage of missingness in data is 21% (n=6) in persistent AKI with no recovery, 30% (n=12) in persistent AKI with recovery, 24% (n=9) in rapidly reversed AKI, and 26% (n=21) in No AKI groups.
Mild to moderate functional limitation group includes patients with Zubrod score of 1 (Symptomatic but completely ambulatory) and 2 (Symptomatic but completely ambulatory). Severe functional limitation group includes patients with Zubrod score of 3 (Symptomatic, >50% in bed, but not bedbound) and 4 (Bedbound/Completely disabled). For patients with missing Zubrod score at 12 months, last available Zubrod score was used.
One-year survival following persistent AKI was 57%, significantly lower than for rapidly reversed AKI and no AKI (91% and 92%, respectively, p <0.001) (SDC Table 6 and SDC Figure 3). Patients with persistent AKI who failed to recover renal function by the time of discharge had the worst one-year survival rate of 40% (p <0.001). In two separate multivariate models adjusted for comorbidities, demographics, sepsis severity and ICU length of stay, persistent AKI and persistent AKI without renal recovery were independently associated with all-cause mortality with adjusted hazard ratios of 2.78 (95% CI 1.30-6.48) and 5.43 (95% CI 2.48-12.93), respectively (Figure 1C and SDC Figure 3). C-index values of 0.86 (0.81, 0.90) and 0.81 (0.76, 0.86) were achieved in these models, respectively, indicating good model performance. At one-year follow-up, among sepsis survivors who had persistent AKI, the proportion of patients who could carry on all pre-disease activities without restrictions was only 12%, compared with 31% in the rapidly reversed AKI group and 29% in the no AKI group (p<0.05).
The rate of major adverse kidney events at one-year follow-up among sepsis survivors was 43% (n=17) and 55% (n=16) for patients with persistent AKI with and without renal recovery, respectively, and was significantly worse compared to the rate of 10% among patients without AKI (p<0.001) (Table 4 and SDC Table 6). Among 140 patients with available renal functional tests at one-year follow-up, those who had had persistent AKI exhibited significantly greater decline in eGFR (23 ml/min/1.72m2) compared to patients with rapidly reversed AKI (14 ml/min/1.72m2, p<0.05) and no AKI (4 ml/min/1.72m2, p<0.001).
DISCUSSION
In a single-center prospective longitudinal cohort of patients with sepsis, we characterized distinct clinical trajectories of persistent and rapidly reversed AKI and demonstrated that subtype of persistent AKI was not only common but also carried the highest burden of the adverse outcomes. More than half of the patients with sepsis developed AKI and for two-thirds of them renal injury will persisted after three days. One out of two patients with persistent AKI did not recover kidney function by the time of discharge or death. Patients with persistent AKI had three-fold increase in thirty-days and one-year mortality. At one-year follow up, only 57% of patients with persistent AKI were alive and among survivors, only 12% could carry on pre-disease activities without restrictions. Patients with persistent AKI had a 50% rate of major adverse kidney events at one year, a five-fold increase compared to patients without AKI and a two-fold increase compared to patients with rapidly reversed AKI. They exhibited more profound early physiologic derangement and persistent immune dysregulation and endothelial dysfunction that distinctly differentiated them from patients with rapidly reversed AKI or no AKI.
To our knowledge, clinical trajectories of sepsis patients with AKI have not been sufficiently described. Single-center retrospective analysis of ICU patients identified several AKI recovery phenotypes, based on AKI reversal within seven days of onset and renal recovery at discharge.11 Similar to our findings, septic patients were more likely to have AKI relapse without recovery suptype, and non-recovery was associated with increased mortality at one year. A study of AKI in patients with sepsis and pneumonia reported higher three-year mortality in patients without renal recovery.12 A study of patients with septic shock and AKI reported decreased hospital mortality for AKI episodes reversed within 24 hours of onset but did not evaluate long-term outcomes.32
The magnitude of change in serum creatinine within 48 hours of sepsis onset could not differentiate subsequent AKI trajectories. More than half of AKI patients had creatinine values below the threshold used for renal SOFA calculation, confirming its limited utility in early identification of high-risk AKI patients.2,33–35 Interestingly, lower urine output and higher cystatin C in the first 48 hours differentiated patient who developed persistent AKI from those with rapid recovery but other urinary indices, such as albuminuria, did not. Early and prolonged hypotension despite administration of larger volume of crystalloid fluids characterized all AKI patients but did not discriminate between AKI trajectories. These observations are consistent with recent literature supporting the role of higher blood pressure targets36,37 and alternative vasopressors such as angiotensin II to prevent progression of renal injury by abrogating vasoplegia.38 Significant volume overload and the use of high saline volumes among patients with persistent AKI started in the first 48 hours and persisted until discharge, which could have contributed to adverse outcomes.39,40 However, AKI in sepsis may occur in the presence of normal or increased renal blood flow, an observation which partially explains why normalization of hemodynamics alone is often insufficient to prevent or reverse AKI, and underscores the importance of endothelial dysfunction and inflammation in the pathophysiology of AKI.4,35 Patients with severe trajectory of persistent AKI exhibited biomarker evidence of sustained inflammation, immunosuppression, and endothelial dysfunction starting within 24 hours of sepsis onset. If inflammation and changes in renal perfusion persist, they can lead to irreparable damage to the epithelium of the glomerulus and tubules through apoptosis and necrosis.2–4 Our findings suggest that AKI trajectories could be differentiated by early assessment of comprehensive clinical, immune and endothelial biomarker profile, highlighting the need for structured, patient-centered early assessment of sepsis patients to identify those with high risk for progressing to the subtype of persistent AKI which is associated with worse outcomes.
Several mechanisms, including inflammation, endothelial dysfunction with blood flow redistribution, oxidative stress and hypoxia, contribute to the pathophysiology of AKI in sepsis.2–4,41 Inflammatory cytokine biomarkers are effective measures of both local and systemic inflammation associated with AKI and CKD.42,43 A prospective multicenter study of sepsis survivors identified a subpopulation of patients with a pro-inflammatory, immunosuppressive phenotype with elevated C-reactive protein and soluble programmed death-ligand levels and poor long-term outcomes, consistent with our findings.44 In a prospective study of cardiac surgery patients, high vascular endothelial growth factor and low soluble vascular endothelial growth factor receptor-1 levels within 6 hours of surgery were associated with lower incidence of AKI and one-year mortality.45
This study was constrained by a single-institution design with a limited sample size restricting the number of confounder variables that could be adjusted for. Findings may not generalize to all sepsis patients due to the predominance of surgical sepsis in this cohort. Although the generalizability of the findings is limited due to the single-institution design, the study site is a large quaternary care center receiving referrals from a large geographic area, and therefore has a diverse patient population. The observational study design identified associations without establishing causality. Despite these limitations, the availability of serial biomarker measurements and longitudinal clinical assessments allowed for a comprehensive description of AKI trajectories and their impact on short and long-term outcomes. Future investigations should seek development and validation of models to identify patients at risk of developing persistent AKI, and assess the efficacy of targeted preventative and therapeutic measures2,35 for these patients.
CONCLUSIONS
Among critically ill surgical sepsis patients, persistent AKI and the absence of renal recovery were associated with distinct early endothelial dysfunction, inflammation and immunosuppression, as well as decreased long-term physical function and survival. Identifying patients at increased risk for developing persistent AKI, establishing preventive strategies, and promoting renal recovery by discharge has the potential to improve survival and quality of life among sepsis patients.
Supplementary Material
SDC Figure 1. Patient enrollment and inclusion flow chart
SDC Table 1. Summary of measured biomarkers
SDC Figure 2. Kinetic glomerular filtration rate, cumulative net fluid balance, immune and endothelial biomarker trajectories by persistence of acute kidney injury
A. Kinetic glomerular filtration rate B. Cumulative net fluid balance as a percentage of admission weight C. Tumor necrosis factor (TNF-alpha) D. Interleukin 6 (IL-6) E. Monocyte chemoattractant protein 1 (MCP1) F. Interferon gamma-induced protein 10 (IP-10) G. Interleukin 8 (IL-8) H. Soluble programmed death-ligand 1 (sPDL-1) I. Total lymphocyte count J. Angiopoietin 2 (ANG-2) K. Soluble vascular endothelial growth factor receptor-1 (sFLT-1) L. Vascular Endothelial Growth Factor (VEGF) Gray lines represent median values for healthy controls provided by manufacturer of the ELISA and Luminex multiplex kits used as detailed in SDC Methods.
SDC Figure 3. Survival by AKI persistence
A. Adjusted Kaplan-Meier survival curves and number at risk by persistence of AKI
Propensity score based inverse weighting was used to plot adjusted Kaplan Meier curves where propensity of being in a trajectory group was calculated using multinomial logistic model that included patient demographics (age, gender, ethnicity), Charlson comorbidity score, septic shock and non-renal SOFA score on sepsis onset.
B. Hazard ratios for all-cause mortality by persistence of AKI
* p<0.05 for Bonferroni-adjusted log-rank test when compared to all other groups
a Adjusted for age, gender, ethnicity, Charlson comorbidity score, sepsis shock status and non-renal SOFA score on sepsis onset, and total ICU length of stay.
SDC Table 4. Early changes in renal, physiologic, immune and endothelial biomarkers stratified by acute kidney injury trajectories
SDC Table 5. Early changes in renal, physiologic, immune and endothelial biomarkers stratified by persistence of acute kidney injury
SDC Methods. Supplemental Methods
SDC Table 2. Patient characteristics at sepsis onset stratified by acute kidney injury trajectories
SDC Table 3. Patient characteristics at sepsis onset stratified by persistence of acute kidney injury
SDC Table 6. Resource utilization and clinical outcomes stratified by persistence of acute kidney injury
ACKNOWLEDGMENT
Conflicts of Interest Disclosures and Source of Funding: A.B. and T.O.B. were supported by R01 GM110240 from the National Institute of General Medical Sciences. A.B., T.O.B., Q.W., S.B., B.B., P.E., S.A., F.M., L.L., and M.S. were supported by Sepsis and Critical Illness Research Center Award P50 GM-111152 from the National Institute of General Medical Sciences. T.O.B. has received grant that was supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001427 and received grant from Gatorade Trust (127900), University of Florida. T.J.L. was supported by a post-graduate training grant (T32 GM-008721) in burns, trauma, and perioperative injury from the National Institute of General Medical Sciences. S.A. was supported by the NIH funded Claude D. Pepper Older Americans Independence Center (P30AG028740). This work was supported in part by the NIH/NCATS Clinical and Translational Sciences Award to the University of Florida UL1 TR000064. Partial results from this research were presented as an abstract presentation at the Society of Critical Care Medicine and American Society of Nephrology conferences and at the University of Florida Research Day.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health and other funding sources.
Footnotes
Publisher's Disclaimer: Disclaimer: The contents of this article do not represent the views of the US Department of Veterans Affairs or the US government.
Conflict of Interest Disclosures: None reported.
REFERENCES
- 1.Gardner AK, Ghita GL, Wang Z, et al. The Development of Chronic Critical Illness Determines Physical Function, Quality of Life, and Long-Term Survival Among Early Survivors of Sepsis in Surgical ICUs. Crit Care Med. 2019;47(4):566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poston JT, Koyner JL. Sepsis associated acute kidney injury. BMJ. 2019;364:k4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellomo R, Kellum JA, Ronco C, et al. Acute kidney injury in sepsis. Intensive Care Med. 2017;43(6):816–828. [DOI] [PubMed] [Google Scholar]
- 4.Gomez H, Ince C, De Backer D, et al. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poyan Mehr A, Tran MT, Ralto KM, et al. De novo NAD(+) biosynthetic impairment in acute kidney injury in humans. Nat Med. 2018;24(9):1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun J, Zhang J, Tian J, et al. Mitochondria in Sepsis-Induced AKI. J Am Soc Nephrol. 2019;30(7):1151–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kellum JA, Chawla LS, Keener C, et al. The Effects of Alternative Resuscitation Strategies on Acute Kidney Injury in Patients with Septic Shock. Am J Respir Crit Care Med. 2016;193(3):281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bihorac A, Yavas S, Subbiah S, et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249(5):851–858. [DOI] [PubMed] [Google Scholar]
- 9.Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119(18):2444–2453. [DOI] [PubMed] [Google Scholar]
- 10.Korenkevych D, Ozrazgat-Baslanti T, Thottakkara P, et al. The Pattern of Longitudinal Change in Serum Creatinine and 90-Day Mortality After Major Surgery. Ann Surg. 2016;263(6):1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kellum JA, Sileanu FE, Bihorac A, et al. Recovery after Acute Kidney Injury. Am J Respir Crit Care Med. 2017;195(6):784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiorentino M, Tohme FA, Wang S, et al. Long-term survival in patients with septic acute kidney injury is strongly influenced by renal recovery. PLoS One. 2018;13(6):e0198269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chawla LS, Bellomo R, Bihorac A, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13(4):241–257. [DOI] [PubMed] [Google Scholar]
- 14.Seymour CW, Kennedy JN, Wang S, et al. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA. 2019;321(20):2003–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrell ED, Kellum JA, Pastor-Soler NM, et al. Septic acute kidney injury: molecular mechanisms and the importance of stratification and targeting therapy. Crit Care. 2014;18(5):501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loftus TJ, Mira JC, Ozrazgat-Baslanti T, et al. Sepsis and Critical Illness Research Center investigators: protocols and standard operating procedures for a prospective cohort study of sepsis in critically ill surgical patients. BMJ Open. 2017;7(7):e015136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Croft CA, Moore FA, Efron PA, et al. Computer versus paper system for recognition and management of sepsis in surgical intensive care. J Trauma Acute Care Surg. 2014;76(2):311–319. [DOI] [PubMed] [Google Scholar]
- 18.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256. [DOI] [PubMed] [Google Scholar]
- 19.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. [PubMed] [Google Scholar]
- 20.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. Clinical Practice Guideline for Acute Kidney Injury. Kidney inter, Suppl. 2012;2(1):1–138. [Google Scholar]
- 21.Ozrazgat-Baslanti T, Thottakkara P, Huber M, et al. Acute and Chronic Kidney Disease and Cardiovascular Mortality After Major Surgery. Ann Surg. 2016;264(6):987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selby NM, Hill R, Fluck RJ, et al. Standardizing the Early Identification of Acute Kidney Injury: The NHS England National Patient Safety Alert. Nephron. 2015;131(2):113–117. [DOI] [PubMed] [Google Scholar]
- 23.Ozrazgat-Baslanti T, Hobson C, Motaei A, et al. Development and validation of computable phenotype to identify and characterize kidney health in adult hospitalized patients. arXiv. 2019. https://arxiv.org/abs/1903.03149. Accessed 04/01/2019.
- 24.Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zavada J, Hoste E, Cartin-Ceba R, et al. A comparison of three methods to estimate baseline creatinine for RIFLE classification. Nephrol Dial Transplant. 2010;25(12):3911–3918. [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen S Retooling the creatinine clearance equation to estimate kinetic GFR when the plasma creatinine is changing acutely. J Am Soc Nephrol. 2013;24(6):877–888. [DOI] [PubMed] [Google Scholar]
- 28.Balakumar V, Murugan R, Sileanu FE, et al. Both Positive and Negative Fluid Balance May Be Associated With Reduced Long-Term Survival in the Critically Ill. Crit Care Med. 2017;45(8):e749–e757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie J, Liu C. Adjusted Kaplan-Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat Med. 2005;24(20):3089–3110. [DOI] [PubMed] [Google Scholar]
- 30.Cleveland WS, Grosse E, Shyu WM. Local Regression Models. 1992. [Google Scholar]
- 31.Dolgun A, Demirhan H. Performance of nonparametric multiple comparison tests under heteroscedasticity, dependency, and skewed error distribution. Communications in Statistics - Simulation and Computation. 2017;46(7):5166–5183. [Google Scholar]
- 32.Sood MM, Shafer LA, Ho J, et al. Early reversible acute kidney injury is associated with improved survival in septic shock. J Crit Care. 2014;29(5):711–717. [DOI] [PubMed] [Google Scholar]
- 33.Bihorac A, Brennan M, Ozrazgat-Baslanti T, et al. National surgical quality improvement program underestimates the risk associated with mild and moderate postoperative acute kidney injury. Crit Care Med. 2013;41(11):2570–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hobson CE, Ozrazgat-Baslanti T, Kuxhausen A, et al. Cost and Mortality Associated With Postoperative Acute Kidney Injury. Ann Surg. 2015;261(6):1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Honore PM, Jacobs R, Joannes-Boyau O, et al. Septic AKI in ICU patients. diagnosis, pathophysiology, and treatment type, dosing, and timing: a comprehensive review of recent and future developments. Ann Intensive Care. 2011;1(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Futier E, Lefrant JY, Guinot PG, et al. Effect of Individualized vs Standard Blood Pressure Management Strategies on Postoperative Organ Dysfunction Among High-Risk Patients Undergoing Major Surgery: A Randomized Clinical Trial. JAMA. 2017;318(14):1346–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asfar P, Meziani F, Hamel JF, et al. High versus low blood-pressure target in patients with septic shock. N Engl J Med. 2014;370(17):1583–1593. [DOI] [PubMed] [Google Scholar]
- 38.Tumlin JA, Murugan R, Deane AM, et al. Outcomes in Patients with Vasodilatory Shock and Renal Replacement Therapy Treated with Intravenous Angiotensin II. Crit Care Med. 2018;46(6):949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Payen D, de Pont AC, Sakr Y, et al. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12(3):R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prowle JR, Echeverri JE, Ligabo EV, et al. Fluid balance and acute kidney injury. Nat Rev Nephrol. 2010;6(2):107–115. [DOI] [PubMed] [Google Scholar]
- 41.Schouten M, Wiersinga WJ, Levi M, et al. Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol. 2008;83(3):536–545. [DOI] [PubMed] [Google Scholar]
- 42.Bihorac A, Baslanti TO, Cuenca AG, et al. Acute kidney injury is associated with early cytokine changes after trauma. J Trauma Acute Care Surg. 2013;74(4):1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang WR, Parikh CR. Biomarkers of Acute and Chronic Kidney Disease. Annu Rev Physiol. 2019;81:309–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yende S, Kellum JA, Talisa VB, et al. Long-term Host Immune Response Trajectories Among Hospitalized Patients With Sepsis. JAMA Netw Open. 2019;2(8):e198686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mansour SG, Zhang WR, Moledina DG, et al. The Association of Angiogenesis Markers With Acute Kidney Injury and Mortality After Cardiac Surgery. Am J Kidney Dis. 2019;74(1):36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SDC Figure 1. Patient enrollment and inclusion flow chart
SDC Table 1. Summary of measured biomarkers
SDC Figure 2. Kinetic glomerular filtration rate, cumulative net fluid balance, immune and endothelial biomarker trajectories by persistence of acute kidney injury
A. Kinetic glomerular filtration rate B. Cumulative net fluid balance as a percentage of admission weight C. Tumor necrosis factor (TNF-alpha) D. Interleukin 6 (IL-6) E. Monocyte chemoattractant protein 1 (MCP1) F. Interferon gamma-induced protein 10 (IP-10) G. Interleukin 8 (IL-8) H. Soluble programmed death-ligand 1 (sPDL-1) I. Total lymphocyte count J. Angiopoietin 2 (ANG-2) K. Soluble vascular endothelial growth factor receptor-1 (sFLT-1) L. Vascular Endothelial Growth Factor (VEGF) Gray lines represent median values for healthy controls provided by manufacturer of the ELISA and Luminex multiplex kits used as detailed in SDC Methods.
SDC Figure 3. Survival by AKI persistence
A. Adjusted Kaplan-Meier survival curves and number at risk by persistence of AKI
Propensity score based inverse weighting was used to plot adjusted Kaplan Meier curves where propensity of being in a trajectory group was calculated using multinomial logistic model that included patient demographics (age, gender, ethnicity), Charlson comorbidity score, septic shock and non-renal SOFA score on sepsis onset.
B. Hazard ratios for all-cause mortality by persistence of AKI
* p<0.05 for Bonferroni-adjusted log-rank test when compared to all other groups
a Adjusted for age, gender, ethnicity, Charlson comorbidity score, sepsis shock status and non-renal SOFA score on sepsis onset, and total ICU length of stay.
SDC Table 4. Early changes in renal, physiologic, immune and endothelial biomarkers stratified by acute kidney injury trajectories
SDC Table 5. Early changes in renal, physiologic, immune and endothelial biomarkers stratified by persistence of acute kidney injury
SDC Methods. Supplemental Methods
SDC Table 2. Patient characteristics at sepsis onset stratified by acute kidney injury trajectories
SDC Table 3. Patient characteristics at sepsis onset stratified by persistence of acute kidney injury
SDC Table 6. Resource utilization and clinical outcomes stratified by persistence of acute kidney injury


