Abstract
Associations between sleep disorders and neurological autoimmunity have been notably expanding recently. Potential immune-mediated etiopathogenesis has been proposed for various sleep disorders including narcolepsy, Kleine-Levin syndrome, and Morvan syndrome. Sleep manifestations are also common in various autoimmune neurological syndromes, but may be underestimated as overriding presenting (and potentially dangerous) neurological symptoms often require more urgent attention. Even so, sleep dysfunction has been described with various neural-specific antibody biomarkers, including IgLON5; leucine-rich, glioma-inactivated protein 1 (LGI1); contactin-associated protein 2 (CASPR2); N-methyl-D-aspartate (NMDA)-receptor; Ma2; dipeptidyl-peptidase-like protein-6 (DPPX); alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA-R); anti-neuronal nuclear antibody type-1 (ANNA-1, i.e., Hu); anti-neuronal nuclear antibody type-2 (ANNA-2, i.e., Ri); gamma-aminobutyric acid (GABA)-B-receptor (GABA-B-R); metabotropic glutamate receptor 5 (mGluR5); and aquaporin-4 (AQP-4). Given potentially distinctive findings, it is possible that sleep testing could potentially provide objective biomarkers (polysomnography, quantitative muscle activity during REM sleep, cerebrospinal fluid hypocretin-1) to support an autoimmune diagnosis, monitor therapeutic response, or disease progression/relapse. However, more comprehensive characterization of sleep manifestations is needed to better understand the underlying sleep disruption with neurological autoimmunity.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-021-01020-x.
Keywords: Autoimmunity, Sleep disorders, Polysomnography, Neurological autoimmunity, Diagnosis, Therapy.
Introduction
Over the past decades, potential immune-mediated etiopathogenesis has been postulated for several sleep disorders, including narcolepsy, Kleine-Levin syndrome, and Morvan syndrome [1–5]. During this time, other autoimmune neurological syndromes (ANS) have also been discovered and characterized, including IgLON5-IgG, which has been associated with multiple, profound sleep disturbances [6]. Sleep manifestations in other ANS have also been described to varying degrees, but comprehensive characterization of associated sleep disorders and polysomnographic findings remain limited [7].
Although sleep symptoms can be found in 73% of autoimmune encephalitis patients [8], they are often overshadowed by severe and sometimes urgent or even life-threatening neurological symptoms (seizures, dysautonomia, neuropsychiatric agitation, etc.). There has been growing interest in characterization of sleep manifestations in autoimmune neurological conditions, however, given that these problems often substantially interfere with quality of life and functioning [8–12]. Sleep symptoms contribute to functional impairment in ANS patients and provide an additional opportunity for diagnostic insight and a symptomatic target to optimize recovery. Sleep manifestations may be a harbinger of a larger looming syndrome [13] or a lingering, residual deficit after other encephalitis symptoms have resolved [13].
Hypersomnias such as narcolepsy and Kleine-Levin syndrome are discussed in a separate article of this issue. This review focuses on a comprehensive discussion of sleep manifestations associated with various autoimmune neurological conditions and on autoimmune encephalitis in particular.
Epidemiology
Epidemiological studies have demonstrated that the incidence and prevalence of autoimmune encephalitis are comparable with infectious encephalitis [14]. A population-based study in Olmsted County, Minnesota, described the 2014 prevalence of autoimmune encephalitis as 13.7/100,000, comparable with the prevalence of confirmed infectious encephalitides at 11.6/100,000. In this study, the prevalence of specific autoantibodies was also described. The frequency ranged from 1.9/100,000 for myelin oligodendrocyte glycoprotein (MOG) to 0.6/100,000 for N-methyl-D-aspartate receptor (NMDA-R) [14].
A population-based study in northeastern Italy looked specifically at paraneoplastic neurological syndromes (PNS), a subset of autoimmune neurological syndromes. The prevalence of PNS was 4.37 per 100,000 [15]. Out of 89 definite PNS cases, well-characterized onconeural antibodies were identified in 23 patients (26%) including anti-neuronal nuclear antibody type-1 (ANNA-1, Hu, n = 6), Ma2 (n = 5), and anti-neuronal nuclear antibody type-2 (ANNA-2, Ri, n = 1). Another study based in Southwestern China included adult and pediatric autoantibody-positive cases. NMDA-R was the most commonly identified syndrome in this series, followed by gamma-aminobutyric acid (GABA)-B-receptor (GABA-B-R), leucine-rich, glioma-inactivated protein 1 (LGI1), and contactin-associated protein 2 (CASPR2) [16]. Interestingly, in all three studies, the incidence of ANS/PNS increased over time, which is likely related to increased recognition and testing for these antibodies [14–16].
Clinical Presentations
Neurological Autoimmune Presentations and Phenotypes
Neurological autoimmunity has varied presentations. Onset is typically subacute [17] with rapid progression. An infectious-like prodrome may occur (rhinorrhea, sore throat, low grade fevers), but is not typical [18]. Other prodromes can include gastrointestinal dysmotility (dipeptidyl-peptidase-like protein-6 [DPPX]-IgG-associated syndrome) or psychiatric dysfunction (NMDA-R encephalitis). Autoimmune neurological symptoms can vary according to the level of neuraxis involvement. Symptoms can range from peripheral neuropathy to cortical dysfunction [19]. These symptoms can manifest as independent, isolated symptoms, or occur concurrently with cerebellar degeneration, other movement disorders, myelopathy, peripheral nerve hyperexcitability, neuropathy, myasthenia gravis, or acute necrotizing myopathy.
Autoimmune encephalitis symptoms can include cognitive impairment, neuropsychiatric dysfunction, seizures, headache, visual disturbances, autonomic dysfunction, and/or movement disorders. Classic autoimmune encephalitis syndromes include limbic encephalitis, rhombencephalitis, opsoclonus-myoclonus, Morvan syndrome, and stiff-person spectrum disorders.
Some autoimmune neurological syndromes can arise from an immunogenic response to underlying malignancy. In 2004, experts in the field highlighted classic paraneoplastic neurological syndromes including encephalomyelitis, limbic encephalitis, cerebellar degeneration, opsoclonus-myoclonus, subacute sensory neuronopathy, chronic gastrointestinal pseudo-obstruction, Lambert-Eaton myasthenic syndrome, and dermatomyositis [20].
Sleep Manifestations in Neurological Autoimmunity
Sleep disturbances in autoimmune neurological syndromes include all domains of sleep disorders: insomnia, hypersomnia, parasomnia, movement disorders, circadian rhythm disorders, and sleep-disordered breathing. In a study that systematically screened for the presence of sleep symptoms in autoimmune encephalitis patients, new sleep complaints were reported in 73%: snoring/gasping (47%), insomnia (29%), hypersomnia (21%), dream enactment behaviors (32%), and other parasomnias (21%) [8]. The following discussion will focus on exemplary disorders (particularly autoimmune encephalitides) with prominent sleep manifestations (Table 1); however, this is not an exhaustive summary. Additionally, narcolepsy and Kleine-Levin syndrome will be discussed in a separate article in this issue of Neurotherapeutics.
Table 1.
Sleep manifestations in selected autoimmune neurological syndromes
| Syndrome | Insomnia | Narcolepsy or hypersomnia | RBD | Abnormal non-REM movement/behaviors | Sleep apnea | Stridor | Hypoventilation or respiratory failure | Sleep study abnormalities | Other |
|---|---|---|---|---|---|---|---|---|---|
| IgLON5 | + + + | + + + | + + | + + + | + + + (predominantly OSA, rarely CSA) | + + + | + + (41% respiratory failure, occasional central hypoventilation) |
+ + + reduced N1, reduced EEG sleep features (vertex waves, K-complexes, and spindles) |
Rapid periodic leg movements. Atypical rapid eye movement in non-REM |
| LGI1 | + + | + | + + + |
+ + low SE, reduced N3, reduced REM, low spindle density |
Morvan Syndrome in 8% | ||||
| CASPR2 | + + + | + |
+ + Agrypnia excitata |
Agrypnia excitata, reduced TST, reduced N3, reduced or abnormal N2 (reduced spindles, reduced K-complexes), reduced REM | Morvan syndrome in 23–29% | ||||
| NMDA-R | + + + (acute phase) | + + + (recovery phase) | + | + Predominantly central apneas | + + (hypoventilation) |
+ (excessive spindle activity) |
|||
| Ma2 | + + + |
+ reduced SE, sleep fragmentation, reduced sleep latency, reduced spindles, reduced N3, SOREMPs |
Reports of reduced CSF hypocretin-1 Occasional reports of cataplexy |
||||||
| DPPX | + | + | + |
+ Ambiguous sleep (simultaneous EEG features of REM and non-REM sleep |
+ PLMS | ||||
| AMPA-R | + | + | |||||||
| ANNA-1 | + | + Central apneas | + Central hypoventilation | ||||||
| ANNA-2 | + | + Central hypoventilation | Laryngospasm and jaw dystonia | ||||||
| GABA-B-R | + | + | + | + | |||||
| mGluR5 | + + | + + | + | altered sleep–wake cycle | |||||
| AQP-4 | + + | + central hypoventilation, depending on lesion burden | Shorted mean sleep latencies, SOREMP | Reports of mildly-markedly reduced CSF hypocretin-1 | |||||
| MS | + + | + | + (OSA more than CSA) |
+ + PLMS + + RLS |
|||||
| GBS | + | + | Reduced sleep latency, reduced TST, reduced SE, increased WASO, AHI, AI, PLMI, reduced N3, reduced spindles, SOREMP | + + Vivid dreams |
LGI1 = leucine-rich glioma inactivated-protein-1; CASPR2 contactin-associated protein 2; NMDA-R = N-methyl-D-aspartate: DPPX = dipeptidyl-peptidase-like protein-6; AMPA-R = alpha-amino-3-hydroxy-5-methyl-4isoxazolepropionic acid receptor; ANNA-1 = anti-neuronal nuclear antibody type 1; ANNA-2 = anti-neuronal nuclear antibody type 2; GABA-B-R = gamma-aminobutyric acid-B-receptor; mGluR5 = metabotropic glutamate receptor 5; AQP-4 = aquaporin-4; MS = multiple sclerosis; GBS = Guillain–Barré syndrome; REM = rapid eye movement sleep; RBD = REM behavior disorder; OSA = obstructive sleep apnea; CSA = central sleep apnea; N1 = N1 sleep; N2 = N2 sleep; N3 = N3 (slow wave) sleep; TST = total sleep time; SE = sleep efficiency; SOREMP = sleep onset REM period; EEG = electroencephalography; PLMS = periodic limb movements of sleep; PLMI = periodic limb movement index; WASO = wake time after sleep onset; AHI = apnea hypopnea index; AI = arousal index; RLS = restless leg syndrom
+ = a few associations; ++ = occasional associations; +++ = common associations
IgLON5 Syndrome
IgLON5-IgG is a serological biomarker that was discovered initially due to its distinctive, prominent sleep manifestations [6]. The neurological phenotype varies and can include bulbar dysfunction, parkinsonism, hyperkinetic movement disorders, and cognitive dysfunction [6, 21, 22]. The phenotype has subsequently proven to be considerably broader, with other neurological features reported: motor neuron disease [23], seizures, stiff person syndrome [21], fasciculations [24], epilepsy [21], respiratory failure, and/or dysautonomia [25].
The median patient age is 62 to 64 years old [21, 22]. IgLON5 syndrome is not considered paraneoplastic, although associated neoplasms have been reported in a subset of patients (hypernephroma, breast adenocarcinoma, remote prostate cancer, remote non-Hodgkin lymphoma) [21, 22].
Complex sleep symptoms are well documented with IgLON5-IgG and are seen in over 75% of cases [21, 22]. Sleep symptoms may be the primary reason for medical evaluation, so patients can present directly to sleep-medicine providers [6, 22]. The sleep manifestations associated with IgLON5-IgG can encompass multiple sleep domains simultaneously. Sleep-disordered breathing is frequent as obstructive sleep apnea (OSA, 55–95%) and/or sleep-related stridor (45–55%) [6, 21, 22]. However, IgLON5-IgG is rare in isolated sleep apnea presentations [26]. Rather, sleep-disordered breathing with IgLON5-IgG often accompanies insomnia and parasomnia. Abnormal sleep behaviors (parasomnias) occur frequently (15–86%), whether as atypical non-rapid eye movement (non-REM) parasomnias and/or REM sleep behavior disorder (RBD). Insomnia and hypersomnia are also frequently seen (73% and 59%, respectively) [22] and may be severe in some cases [22, 27]. Sleep attacks can also occur [28]. One study described respiratory failure in 41% of patients [22].
Polysomnographic observations of IglON5-IgG patients include abnormal sleep architecture (reduced N1, vertex waves, K-complexes, and sleep spindles) with prominent non-REM parasomnias, often during sleep initiation [6, 25, 29–31]. These non-REM parasomnias differ from classic arousal parasomnias (sleep walking, sleep talking) as they often do not arise out of N3 sleep. In some cases, this disorganized sleep architecture appears to normalize with uninterrupted sleep, corresponding with emergence of RBD and sleep-disordered breathing (OSA and stridor) [6, 29, 30]. In other cases or given night-to-night variation, there can be a complete lack of N3 or REM sleep [27, 30, 32]. RBD has been confirmed with polysomnography, often in the latter half of a study night [30, 31]. RBD with IgLON5-IgG has primarily been described as jerking body movements, typically of mild intensity. Vocalizations, violent movements, and quasi-purposeful movements are rare during REM sleep in IgLON5 syndrome [30]. Most respiratory events are obstructive hypopneas and apneas [6, 30]. Stridor was observed in 10 of 12 patients during polysomnography [22]. Vocal cord paralysis has also been reported [22, 33]. Central sleep apnea is infrequent [30, 33], but central hypoventilation can occur [22]. Additional polysomnographic features have also been described. Rapid, periodic leg movements can be seen, which differ from traditional periodic leg movements due to a shorter interval between movements (< 5 s versus 5–90 s). Eye movements also occur in non-REM with a shorter, smaller deflection and decreased frequency than the traditional REM-sleep eye movements.
Disrupted sleep architecture makes traditional sleep-stage scoring difficult and unreliable. Therefore, a proposed alternative scoring structure includes additional stages (undifferentiated non-REM and poorly structured N2 sleep) and additional features (rapid, periodic leg movements and atypical, isolated, rapid eye movements in non-REM). Audio-visual cues are particularly useful in applying this alterative scoring [30]. Actigraphy and multiple sleep latency test (MSLT) have shown increased nocturnal activity and abnormal sleep initiation during daytime naps [30].
In most cases, the sleep-disordered breathing associated with IgLON5-IgG can be successfully treated with CPAP or tracheostomy and mechanical ventilation [21, 25, 30]. However, abnormal sleep architecture can persist for years despite symptomatic or immune therapies [30]. There have been reports of subjective and/or objective (polysomnography, fiber optic swallow evaluation, etc.) response to immunotherapy, although sometimes these responses have been partial or transient [21, 25, 28, 30]. This is consistent with a recent systematic review in which IgLON5-IgG patients responded to immunotherapy 43% of the time. Unexpected or sudden death during sleep has been reported in several cases [6, 31]. However, a favorable response was seen more often with combination therapy and second-line therapies, suggesting a more refractory disease course [34]. Early initiation of immunotherapy may also have a favorable response [28].
The pathogenesis of IgLON5-IgG is not yet fully elucidated. The presence of IgLON5 autoantibodies, strong HLA associations, and occasional immuno-responsiveness suggest an autoimmune etiology. However, neuropathological associations (neuronal loss and tauopathy), chronic disease course in many cases, and lack of response to immunotherapy in many cases instead support the contention that it is a neurodegenerative disease [6, 35]. Taken together, it is possible that IgLON5 disease begins as an inflammatory, autoimmune course, and eventually develops irreversible damage corresponding to the pathological changes that blunt response to immunotherapy. IgLON5-IgG disruption of hippocampal cytoskeletal structures in animal models provides a potential link between the autoimmune and neurodegeneration pathogenesis [36].
Leucine-Rich, Glioma-Inactivated Protein 1 (LGI1), Contactin-Associated Protein 2 (CASPR2), and Voltage-Gated Potassium Channel Complex (VGKC) Syndromes
Both LGI1 and CASPR2 antibodies target proteins related to the voltage-gated potassium channel complex (VGKC). Historically, before the discovery and characterization of LGI1 and CASPR2 autoimmunity, the associated clinical syndromes (including Morvan syndrome) were attributed to VGKC antibodies [3, 4, 7, 37–40]. However, with evolving understanding and additional immunologic characterization, VGKC-IgG seropositivity without concurrent LGI1-IgG or CASPR2-IgG positivity (VGKC double negatives, VGKC-DN) has been found to lack a consistent or distinct clinical syndrome [41].
Morvan syndrome is a rare disorder now primarily associated with CASPR2-IgG and, less commonly, LGI1-IgG [40, 42]. Morvan syndrome is characterized by peripheral nerve hyperexcitability, autonomic dysfunction, and encephalopathy, which can manifest with severe insomnia and sleep disturbances [43]. Between 41 and 56% of Morvan syndrome cases are associated with neoplasms (primarily thymoma) [42, 44, 45]. Insomnia is frequent in Morvan syndrome, ~ 90% [42]. Oneiric stupor can be observed with excessive motor activity that can mimic complex daytime movements. Agrypnia excitata is also seen when these nocturnal movements are accompanied by autonomic agitation [4, 45, 46]. Despite profound insomnia and sleep-disrupting behaviors, daytime hypersomnia does not appear to be prominent in Morvan syndrome [45]. The sleep architecture in Morvan syndrome is significantly altered. Polysomnography has demonstrated reduced or absent total sleep, reduced or absent N3 sleep, and reduced or abnormal N2 sleep (paucity or absence of sleep spindles, K-complexes, etc.). There can also be invariant theta activity with or without intrusion of faster activity [4, 43, 45]. Abnormal REM sleep with reduced total amounts of REM, shortened REM periods, and lack of normal REM atonia have also been described [4, 45, 47]. The insomnia and sleep disturbances with Morvan syndrome can be severe and refractory to typical hypnotic treatment. However, most patients improve with immunotherapies [45, 48].
Morvan syndrome makes up only 23 to 29% of confirmed CASPR2-IgG positivity [49, 50]. CASPR2-IgG autoimmunity can also present without Morvan syndrome and is commonly associated with peripheral nerve hyperexcitability, limbic encephalitis, cerebellar dysfunction, and epilepsy [49, 50, 51]. About 20% of CASPR2-IgG autoimmunity is associated with thymoma, although other tumors were uncommon [49, 50]. Most, but not all, patients respond to immunomodulatory treatment [49].
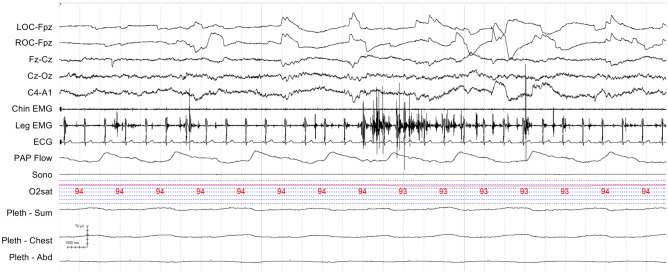
LGI1-IgG has commonly been associated with limbic encephalitis (43–90%), epilepsy (80–90%), and/or cognitive decline (70%). Although not found in every case, the presence of faciobrachial dystonic seizures or hyponatremia is suggestive of LGI1 autoimmunity. Additional presentations include cognitive impairment, peripheral hyperexcitability, and paroxysmal dizzy spells [51, 52]. Only 8% were associated with Morvan syndrome [52]. Insomnia was seen in 23 to 65% [51, 52], which may be associated with hypersomnolence [40, 53]. RBD has been associated with LGI1-IgG and with VGKC limbic encephalitis (now known to be LGI1 limbic encephalitis) [7, 10, 39, 51]. In one study, 57% of LGI1-IgG patients had dream-enactment behaviors [8]. In another study, 5 LGI1 limbic encephalitis patients had clinical RBD, 3 with documented increased REM sleep muscle activity (REM sleep without atonia) on polysomnography (Fig. 1) [7, 10].
Fig. 1.
Rapid eye movement (REM) sleep without atonia (RSWA). A 30 second epoch from the polysomnogram of a leucine-rich glioma inactivated-protein-1-IgG positive (LGI1+) patient demonstrating increased phasic muscle activity in the anterior tibialis (linked legs) during REM sleep. LOC, left outer canthus; ROC, right outer canthus; EMG, electromyogram; ECG, electrocardiogram; PAP Flow, positive airway pressure airflow; Sono, sonogram; O2Sat, oxyhemoglobin saturation; Pleth, impedance plethysmography; Abd, abdomen
Polysomnography with LGI1-IgG has been limited to case reports and small case series. In a case series including 4 LGI1-IgG-positive patients, polysomnogram (PSG) demonstrated reduced sleep efficiency (52–77%), low slow wave sleep (2–6%), and REM percentages (0–10%). They also found low spindle density in these LGI1-IgG patients. One patient had sleep apnea with an apnea-hypopnea index (AHI) of 20 per hour [54]. One of these cases was also written as a separate case report, which described both nighttime and daytime polysomnogram data. Nocturnal sleep appeared more disturbed with a sleep efficiency of 52% and REM sleep time of 8%. During daytime sleep, there was increased REM sleep (70%) and sleep efficiency (64%). They also described increased phasic muscle activity during REM sleep [53].
Most patients have favorable responses to immunomodulatory treatment overall, although detailed reports of the response of sleep disturbances is often lacking [51, 52]. In a Dutch study, 21% of patients with 2-year follow-up data continued to have insomnia without excessive daytime sleepiness (EDS) [52]. RBD can also improve with treatment. Three out of 5 cases of limbic encephalitis with RBD improved with immunotherapy; two had persistent RBD and limbic symptoms [7, 10].
N-Methyl-D-Aspartate-Receptor Encephalitis
N-methyl-D-aspartate-receptor (NMDA-R) encephalitis often starts with a viral-like prodrome followed by subacute progression of neuropsychiatric symptoms and seizures [13]. Days to weeks later, additional symptoms may variably ensue, involving decreased responsiveness, autonomic dysfunction, and characteristic movement disorders [55, 56]. Characteristic facial dyskinetic movement disorders may be present only during sleep [57]. Patients are disproportionately young (median age: 21–23 years old) with female predominance (80–91%) [56, 58, 59]. The syndrome is frequently paraneoplastic, with an underlying neoplasm discovered in 20–38%, usually an ovarian teratoma (94%) [55, 58]. Other tumors have been reported as well including extra-ovarian teratomas, Hodgkin’s lymphoma, small cell lung cancer, and neuroblastoma [60].
Several studies have suggested that sleep-related symptoms may be frequent in NMDA-R encephalitis [56, 61, 62], although details have been limited. For example, two recent systematic reviews of psychiatric symptoms in NDMA patients suggested that sleep disorders were commonly reported; however, insomnia and various sleep disturbances varied from 21% [62] to about 40% [62]. In a recent prospective observational trial, 89% of NMDA-R patients reported insomnia during the acute stage of their illness. In 72%, insomnia symptoms started potentially weeks before other encephalitis symptoms, frequently without excessive daytime sleepiness despite dramatically reduced sleep times, and did not improve with benzodiazepine treatment [13].
Only 11% reported hypersomnia during the acute phase. In the recovery phase, 78% had hypersomnia, which was generally mild and associated with hyperphagia, apathy, increased weight, and hypersexuality. Greater napping and 24-h sleep time than controls were also observed, although sedating medications may have been confounders [13].
Sleep studies have been limited in hospitalized NMDA-R encephalitis during acute illness given logistical challenges and prioritization of acute disorders including seizures, dysautonomia, and agitation. Video EEGs studies have described absent sleep patterns [63], and excessive spindle activity has been described in children with NMDA-R IgG [64]. One systematic study with polysomnography in NMDA-R encephalitis during recovery revealed comparable sleep time and efficiency to controls, with well-preserved sleep architecture. However, NMDA-R patients had longer, more frequent confusional arousals than controls. One patient had hypermotor seizures with normal REM atonia [13]. There have also been reports of central and obstructive sleep apnea [65].
Hypoventilation has been described in 23–66% of NMDA-R encephalitis patients, [56, 61] frequently necessitating mechanical ventilation [66] with an 8-week median duration [56]. In some cases, central hypoventilation may not be recognized until patients fail to wean from mechanical ventilation.
Fifty-three percent of NMDA patients improve with a first-line immunotherapy. Of those requiring a second-line immunotherapy, 57% show improvement. However, even with successful immunotherapy, recovery can take up to 18 months. There is a 12% risk of relapse within 2 years [58].
Ma2 ( i.e., anti-Ta) Antibody Encephalitis
Ma2-IgG (also known as anti-Ta-IgG) autoimmunity typically manifests as limbic, diencephalic, or brainstem encephalitis [67–71]. There have also been reports of cerebellar dysfunction [69], motor neuron disease-like presentations [72], atypical parkinsonism [70], and peripheral nervous system manifestations [73]. There are strong paraneoplastic associations with Ma2-IgG and dual positive Ma1/Ma2-IgGs; tumors are identified in 64–84%. Testicular tumors (commonly non-seminomatous) are the most commonly associated neoplasm with isolated Ma2-IgG positivity, while non-small cell lung cancer is more often associated with dual positive Ma1/Ma2-IgG. Other tumors have also been described [70]. The majority of cases present with a neurological syndrome before the tumor is identified [69, 70, 73]. Most patients (68–75%) with Ma2-IgG were men, with a median age of 60 years, although men may present at a younger median age of 34 years, while the median age for women may be older near 64 years [69, 70].
Several cases of Ma2-IgG-associated hypersomnia and/or secondary narcolepsy have been described [8, 70, 73–81], although autoantibodies against Ma1 or Ma2 are uncommon in idiopathic narcolepsy [78]. Hypersomnia was found in 32% of Ma2 encephalitis cases [70], and cataplexy has been described in at least 6 cases [70, 75, 77, 80, 81].
Polysomnography has demonstrated abnormal sleep architecture including reduced sleep efficiency, increased sleep fragmentation, reduced sleep latency, absent sleep spindles, and absent slow wave sleep [74, 75, 77, 80]. REM sleep without atonia (RSWA) has been confirmed in at least 4 cases [74, 77, 80, 81]. There is limited detailed discussion of RSWA. Multiple sleep-latency tests have demonstrated reduced mean sleep latency and multiple sleep-onset REM periods (SOREMP) consistent with symptomatic narcolepsy [8, 74, 75, 77, 80]. Available cerebrospinal fluid (CSF) hypocretin-1 levels have been low or absent in patients with excessive daytime sleepiness [70, 74–79] but did not have the HLA typing associated with idiopathic narcolepsy [74, 75]. Low CSF hypocretin-1 levels have been suggestive of inflammatory damage involving the hypothalamic hypocretinergic neurons [77, 78].
Many Ma2-IgG patients are refractory to treatment with immunotherapy. Studies have suggested that 33% improve, 21 to 40% stabilize, and 11 to 85% may continue to progress [69, 70, 73]. A favorable outcome has been associated with male sex, age < 45 years, absence of anti-Ma1 antibodies, and/or a testicular tumor with complete response to treatment [70].
Specific outcomes of sleep disturbances in anti-Ma/Ta syndrome have been less frequently reported. In one patient with low CSF hypocretin levels, excessive daytime sleepiness improved with tumor treatment and corticosteroids [70], suggesting that depleted hypocretin-1 levels may not indicate irreversible hypothalamic dysfunction. Another patient’s hypersomnia, ptosis, and ophthalmoplegia resolved with oral corticosteroids (despite untreated epidermoid carcinoma) [79]. In one case with detailed PSG and MSLT before and after therapy, no significant change occurred in sleep architecture, RSWA, or mean sleep latency [80]. One patient with narcolepsy with cataplexy was negative for HLA DR2 and DQW and refractory to high-dose modafinil and immunosuppression until a right orchiectomy was completed. Pathology confirmed testicular cancer. After orchiectomy, memory, thinking, and cataplexy improved [75].
Dipeptidyl-Peptidase-Like Protein-6 (DPPX/DPP6) Syndrome
DPPX autoimmunity typically has a subacutely progressive course (8-month median symptom onset to peak) involving prominent and significant weight loss in 77% of patients (20–34 kg median loss) and heterogeneous multifocal CNS dysfunction, which may include brainstem, cerebellar, spinal cord, or neuropsychiatric symptoms. There is often central hyperexcitability with prominent dysautonomia (especially with gastrointestinal involvement) or seizures. Progressive encephalomyelitis (PERM) presentations also occur infrequently [82, 83]. Demographically, the median age is 52 years old, and 69% are men. Rare paraneoplastic presentations in association with leukemia or lymphoma have been reported [82, 83].
Sleep disruption has been reported in 33 to 45% of DPPX patients including insomnia, periodic limb movements, sleep apnea, hypersomnia, parasomnia behaviors, and nocturnal jerking movements [82, 83]. Details concerning polysomnography in DPPX are limited, but ambiguous sleep was noted in a patient with simultaneous REM (sawtooth waves) and non-REM sleep (sleep spindles, K-complexes) architectural features [82]. Neuropathology in DPPX has shown inflammatory changes and/or neuronal loss in segments of the hippocampus, amygdala, cingulum, and cortex. Additionally, mild changes were seen in the pons, cerebellum, and medulla [83, 84].
Complete amelioration of sleep symptoms with immunotherapy and symptomatic treatment has been reported [85]. In a collective DPPX case analysis, 60% of treated patients improved, 23% had no improvement or progression, and 17% died [83]. Relapses were also common, but patients improved when re-challenged with immunosuppressive therapy [82, 83].
Alpha-Amino-3-Hydroxy-5-Methyl-4-Isoxazolepropionic Acid (AMPA) Receptor (AMPA-R) Encephalitis
AMPA-R-IgG encephalitis is rare [86] and will often present with limbic encephalitis but can manifest with extra-limbic symptoms including focal weakness, involuntary movements, autonomic dysfunction, incoordination, aphasia, and apraxia. Based on a systematic review of 53 patients with AMPA-R encephalitis with available clinical information, mean age was 53.1 years, and 65% were women. Tumors were identified in 62%, most frequently small-cell lung cancer and thymoma; 16% of patients died despite treatment with immunotherapy [87]. In another study, all 9 treated patients responded, although relapses were common [88].
Insomnia and hypersomnia can occur during initial presentation and later in the disease course [8, 88–90], but limited sleep details currently available. Co-existing neural specific antibodies with AMPA-R antibodies are associated with a poor outcome [90]. One case developed Cheyne-Stokes breathing and respiratory distress, which required tracheostomy and mechanical ventilation. The patient died with circulatory and respiratory failure [89].
Anti-neuronal Nuclear Antibody Type-1 (ANNA-1, i.e., Hu) Syndrome
Sensory neuronopathy is the most common phenotype with ANNA-1 syndrome, with ataxia, limbic encephalitis, brainstem encephalitis, and other manifestations. The median age is 63 years old, and 33 to 75% are men. There is a strong cancer association in 83–88%, especially small cell lung cancer in 52 to 81%. Most cases have a refractory course despite oncologic therapy and immunotherapy [91–93].
Sleep-symptom characterization and detailed polysomnographic reports are limited in ANNA-1 syndrome. There have been several reports of ANNA-1 presenting with central hypoventilation [94–99]; 1.5% of 202 ANNA-1 cases presented with Ondine’s curse with central sleep apnea leading to death despite immuno- or chemotherapy, but one patient stabilized with dependence on tracheostomy and nocturnal mechanical ventilation [94].
Another case report described an 85-year-old patient with hypersomnolence, sleep fragmentation, transient psychosis, and abnormal movements without an EEG correlate during wakefulness and sleep. A 24-h PSG/MSLT demonstrated nocturnal sleep-onset REM and reduced daytime mean sleep latency with 5 SOREMPs on MSLT. Several daytime cataplectic episodes were also recorded. DQB1*0602 HLA was negative and CSF hypocretin-1 was 147 pg/mL (borderline low). Additional workup revealed ANNA-1 seropositivity and an indeterminate lung mass. Symptomatic therapy was ineffective [100].
Another study described three ANNA-1 patients with sleep disturbance (2 with witnessed apneas, 1 with insomnia), 2 with small cell lung cancer. One patient with co-existing ANNA-1, NMDA-R, and CRMP5 antibodies had possible agrypnia excitata with abnormal sleep architecture [8].
Anti-neuronal Nuclear Antibody Type-2 (ANNA-2, i.e., Ri) Syndrome
ANNA-2-IgG is commonly associated with brainstem syndromes including opsoclonus-myoclonus and cerebellar ataxia. There is a higher frequency in women (64–78%) with a median age of 66 years. There is a strong tumor association (86–92%), particularly for lung and breast cancer [101, 102]. Symptoms can be severe, but there is potential for improvement with immunotherapy and oncologic treatments [102, 103].
Laryngospasm and jaw-opening dystonia have been described. In one study of 48 ANNA-2 patients, 19% had either laryngospasm or jaw-opening dystonia. These were associated with respiratory distress, with fatal asphyxiation in 1 patient and nocturnal stridor in another. Three patients needed respiratory support, and one underwent tracheostomy. However, laryngospasm and dystonia can improve with immunotherapy [102, 104]. Central hypoventilation (Ondine’s curse) has also been described occasionally [101, 105].
A case report of a patient with co-existing ANNA-2 and voltage-gated calcium-channel P/Q-type antibodies presented with a multifocal neurologic syndrome, insomnia, and semi-purposeful movements during sleep. Initial polysomnogram demonstrated reduced sleep efficiency (18%) with increased wake-after sleep-onset time, increased N1, decreased N2/REM, and absence of sleep spindles and N3. There were few respiratory events noted. A post-treatment polysomnogram after a 5-day course of intravenous immunoglobulin [IVIG] had a worsened sleep efficiency and sleep latency, one subclinical seizure but no parasomnia behaviors [106].
Gamma-aminobutyric Acid (GABA)-B-receptor (GABA-B-R) Syndrome
GABA-B-R-IgG is a rare antibody, often presenting with seizures, limbic encephalitis, gait disorders, and other symptoms. Average patient age is 60 years old, with 63% men. The syndrome can be severe, with about 15% becoming comatose or needing intensive care. About 50% of cases are associated with neoplasms, most being small-cell lung cancer; 86% improve with immunotherapy combined with oncologic therapy when appropriate [107].
Descriptions of sleep symptoms in GABA-B have been vague. Six of 14 GABA-B syndrome patients in one series had sleep changes, but details were not reported [108]. There was also another patient with hypersomnia, snoring, witnessed apnea [8], and occasional reports of “abnormal sleeping habits” [109] or “sleep disorders” [110] without further characterization. Several patients have been intubated and mechanically ventilated, most often for airway protection during seizures, but there are a few descriptions of poor respiratory effort and hypoventilation [109, 111].
However, one case report and subsequent experimental study described the detailed, longitudinal course of a 55-year-old GABA-B-R patient with serial polysomnograms. She initially presented with multiple cranial neuropathies. Two years later, she evolved stridor and respiratory failure requiring intubation and mechanical ventilation that improved with immunotherapy (corticosteroids and plasmapheresis). One year later, respiratory symptoms recurred. Polysomnogram showed reduced REM and non-REM sleep. Breathing again improved with immunotherapy (plasmapheresis). Three years later, she developed hypersomnia, sleep fragmentation, abnormal sleep movements, and recurrent nocturnal respiratory distress. Several polysomnograms during this acute exacerbation demonstrated a diffuse alpha rhythm with absence of typical non-REM or REM sleep EEG features. Episodes of complex motor behaviors with possible dream enactment were observed. Following additional immunotherapy with corticosteroids and plasmapheresis, she improved with re-emergence of EEG non-REM sleep features. Symptoms again recurred when maintenance azathioprine doses were decreased. Interestingly, passive transfer of this patient’s purified antibodies reproduced neurological and sleep disorders in mice [112, 113].
Metabotropic Glutamate Receptor 5 Syndrome
Metabotropic glutamate receptor 5 (mGluR5) autoimmunity has been associated with Ophelia syndrome which manifests with encephalopathy, mood disturbances, and seizures [114]. mGlur5 IgG is most commonly associated with Hodgkin’s lymphoma; small cell lung cancer has also been reported [115]. Sleep dysfunction has been described with mGluR5 autoimmunity. In one series, 7 of 11 (64%) mGluR5 patients had sleep dysfunction. This was further described as increased sleep duration (n = 3, 2 described as hypersomnia), decreased sleep duration (n = 4, 3 described as insomnia), and poor sleep quality. One patient was described as having an altered sleep-wake cycle and hypoventilation [115]. Detailed sleep descriptions and polysomnographic characteristics are lacking.
Aquaporin-4 Antibody-Associated Neuromyelitis Optica Spectrum Disorder
Neuromyelitis optica spectrum disorder (NMOSD) is characterized by relapsing inflammatory demyelination, most often in the optic nerves and spinal cord. Median age of NMO onset is 39 years old, disproportionally affecting women. More than 80% of NMO cases are positive for aquaporin-4 (AQP-4) IgG [116–118], but similar relapsing inflammatory CNS diseases may be AQP-4-IgG seronegative.
There have been several cases of hypersomnia and secondary narcolepsy associated with NMOSD and/or AQP-4-IgG [119–123]. These appear to be associated with hypothalamic lesions, which occur in 2.5% [124]. Most are in the Japanese population, which has a higher prevalence of NMO-SD [120–123]. Additional cases have been identified outside of Japan [119, 125, 126, 127]. Additional hypothalamic symptoms usually co-exist with hypersomnia, but cataplexy, hypnagogic hallucinations, and sleep paralysis have not been reported. Multiple cases have documented reduced CSF hypocretin-1 levels, which can increase or decrease corresponding with improvement or exacerbation of symptomatic hypersomnia. HLA DQB1*0602 testing has been negative [121, 122].
Objective sleep testing has been limited. MSLTs have described shortened mean sleep latencies and SOREMPs [121, 122, 128]. Polysomnography has described nocturnal SOREMP [121, 122]. Cervical spine lesions may also lead to central hypoventilation requiring mechanical ventilation [119]. Hypothalamic lesions and hypersomnia typically improve with immunotherapy, although residual EDS may remain [122].
Multiple Sclerosis
Multiple sclerosis (MS) can affect any level of the central nervous system causing multiple neurological symptoms including a variety of sleep disturbances that will be discussed only briefly here given other recent focused reviews on this subject [129–134].
Sleep symptoms are common in MS, impacting over 50%, with a higher frequency among women [130]. Sex and length of disease can impact sleep symptoms. A systematic review found several types of sleep disruption in MS including narcolepsy in 0–1.6%, periodic limb movements of sleep in 36%, restless leg syndrome in 14.4–57.5%, RBD in 2.2–3.2%, and sleep apnea in 7.1–58.1% (CSA in 4.17%) [132]. Insomnia and circadian rhythm disorders are also common, described in 40% and 30% respectively [129, 135].
Restless leg syndrome is more frequent and more severe in the multiple sclerosis population than in normal controls [136]. Although the etiology is not clearly understood, potential theories include neurotransmitter dysfunction, iron metabolism, and/or spinal cord lesions/atrophy [135]. Regardless of the pathophysiology, RLS in MS has been associated with worse quality of life, fatigue, and anxiety [137].
Multiple factors potentially contribute to sleep symptoms including critical lesions in sleep-related neurophysiologic structures [134], increased inflammatory cytokines, medication side effects [133], discomfort, urinary symptoms, and comorbid medical conditions (iron deficiency). Sleep disorders in MS have been associated with cognitive dysfunction [131], and treatment is thought to positively impact quality of life and functional status [129].
Fatigue is also common in MS (affecting up to 90% during their illness) and is associated with disability [129]. MS patients with fatigue should be screened for sleep disorders. Studies have suggested that fatigue in MS is associated with greater sleep fragmentation, PLMI, or RLS [138]. Some evidence also suggests that treatment of an underlying sleep disorder improves fatigue [129]. However, as fatigue is often multifactorial, additional factors should be considered.
Guillain–Barré Syndrome (GBS, Also Known as Acute Inflammatory Demyelinating Polyradiculoneuropathy, AIDP)
In Guillain–Barré syndrome (GBS), an acute inflammatory polyradiculoneuropathy, presenting sensorimotor symptoms predominate and can be severe, causing respiratory weakness that may progress rapidly to become life threatening. Restrictive lung disease is found in 79% of GBS patients in the subacute phase [139], and 20–30% of GBS patients require mechanical ventilation [140].
However, in addition to sensorimotor phenomenon, sleep disturbances are also frequent in GBS patients. In hospitalized GBS patients, sleep disturbances are found in at least 50%, likely multifactorial with contributions from pain, psychiatric sequela, immobility, medication effects, and treatments/procedures [141–143]. Detailed sleep descriptions are often lacking, but a few studies and case reports have discussed sleep disturbances in GBS in detail.
Vivid dreams have been described in 19% of GBS patients, accompanying other mental status changes. Hypnagogic hallucinations are also frequent [144]. Predominant insomnia has also been described in one case [145]. Low or undetectable CSF hypocretin-1 levels have been reported [144, 146], as have EDS and RBD [147, 148].
Objective sleep data in GBS have been limited. One study examined polysomnography and MSLT before and after immunotherapy. Pre-treatment assessments found reduced sleep latency on MSLT in GBS compared with controls (11.8 vs 16.6 min). Pre-treatment PSGs demonstrated decreased total sleep time (5.3 vs 7.3 h), decreased sleep efficiency (62.6 vs 86.4%), increased wake time after sleep-onset time, AHI, arousal index, and periodic limb movement index in GBS. On post-immunotherapy assessment, there were only minor sleep improvements, despite improved neuropathic symptoms, suggesting that GBS patients have longer lasting sleep disruption than sensorimotor manifestations [149].
In a prospective controlled study, there were multiple differences between GBS patients and ICU controls including more frequent hallucinations in GBS. Polysomnography was performed in 13 GBS patients and 6 controls. Sleep was poor (short and fragmented) in all patients. In some cases, there was absence of slow-wave sleep (n = 2) and sleep spindles (n = 1). The GBS hallucinators had more prominent sleep abnormalities when compared with non-hallucinators including shorter REM latency, multiple SOREMPs, RSWA, dream-enactment behavior, RBD, and abnormal eye movements during non-REM sleep. Collectively, these abnormalities suggest features of status dissociatus. Interestingly, these sleep abnormalities resolved when the mental status improved while the patients were still in the ICU [144].
Diagnosis of Sleep Disturbances in Autoimmune Encephalopathies
History
For autoimmune neurological syndromes and sleep manifestations, a careful history can guide diagnosis and identify treatment opportunities. While there are many clinical variations, some clues to suggest autoimmune encephalitis are a subacute onset, rapid progression, fluctuating clinical course, personal or family history of autoimmune conditions, recent history of cancer, and a robust objective response to immunotherapy [150](Table 2). As sleep symptoms in autoimmunity occur as part of a syndrome, predictive models for autoimmune encephalitis, such as the antibody-prevalence-in-epilepsy-and-encephalopathy (APE2) score, may be helpful [18]. Sleep specific features that could be suggestive of autoimmunity include late onset narcolepsy, agrypnia excitata, status dissociatus, rapid periodic leg movements, atypical rapid eye movements in non-REM sleep, etc. (Table 2). However, clinical judgment must be used to distinguish idiopathic sleep disorders and other non-autoimmune syndromes (for example, idiopathic parkinsonism).
Table 2.
Features that increase suspicion of an autoimmune etiology
| Clinical features | Polysomnographic features | Neurological workup |
|---|---|---|
| Subacute onset, rapid progression symptoms | Status dissociatus | CSF: inflammatory profile |
| History of other autoimmune conditions | Undifferentiated Non-REM sleep | MRI brain: limbic encephalitis, diencephalic encephalitis, rhombencephalitis |
| Recent history of cancer | Poorly structured N2 sleep | PET brain: occipital lobe hypometabolism |
| History of an infectious-like prodrome | Atypical rapid eye movements in Non-REM sleep | EEG: Delta Brush |
| New onset encephalopathy and/or seizuresa | Rapid periodic leg movements | EMG: peripheral nerve hyperexcitability |
| Other involuntary movements: chorea, oral dyskinesia | ||
| Autonomic dysfunction | ||
| Robust objective responsive to immunotherapy | ||
| Late onset narcolepsy | ||
| Agrypnia excitata |
REM = rapid eye movement sleep; N2 = N2 sleep; CSF = cerebrospinal fluid; MRI = magnetic resonance imaging; PET = positron emission tomography; EEG = electroencephalography; EMG = electromyography
aEspecially seizure semiologies such as faciobracial dystonic seizures and pilomotor seizures
Unless sleep symptoms are prominent, it is likely that they may be overlooked when more obvious, urgent, or life-threatening symptoms, such as seizures or autonomic dysfunction, are present. However, when the setting allows, screening for sleep symptoms may identify additional symptomatic clues or targets.
In a case of known or suspected ANS, sleep-history screening should include discussion of all sleep domains, as these patients may have multiple sleep issues [6] including sleep-disordered breathing, parasomnia, and insomnia. Obtaining collateral information from a bed partner can be helpful, as well as using systematic sleep questionnaires. Surveys such as the Epworth sleepiness scale (ESS), International Restless Legs Syndrome Rating Scale (IRLSRS), and Pittsburgh Sleep Quality Index can help quantify symptom severity at baseline and follow-up.
The temporal relationship between sleep-disturbance symptoms and other systemic and neurological symptoms can help determine whether sleep disturbances are related to the autoimmune condition. When possible, a well-delineated timeline should include symptom onset and progression, temporal relationship to other relevant events (other symptoms, medications, etc.), and any changes with immunotherapy. This can help differentiate new ANS-related symptoms from chronic/premorbid conditions or medication/treatment side effects. Symptoms may also change over the disease course (e.g., NMDA-R encephalitis will often manifest with insomnia in the acute phase, then hypersomnia in the recovery phase) [13]. In some cases, sleep symptoms may also linger longer than other presenting symptoms [149] or may evolve later, after resolution of other neurological or systemic symptoms. Medications such as corticosteroids or antiepileptics can have alerting or sedating side effects.
Sleep Work-up
Clinicians should pursue objective sleep testing as clinically indicated based on comprehensive sleep-symptom screening, remembering that some syndromes may manifest with multiple co-existing sleep symptoms. When feasible, usual diagnostic tests such as PSG, actigraphy, and MSLT can be employed. Additional features can be added to standard PSG for better characterization, such as arm EMG leads that can help characterize parasomnias and abnormal movements. The importance of audio-visual recordings has been recently highlighted in the alternative sleep stage scoring proposed for IgLON5-IgG disease [30]. An expanded EEG recording using the full 10–20 International Electrode Systems placements can help identify subtle sleep-related seizures.
When hypersomnia and narcolepsy symptoms are present, HLA testing and CSF hypocretin-1 testing can be considered. As discussed above, there have been several cases of low or absent CSF hypocretin-1 levels, some with fluctuation corresponding with symptom resolution or exacerbation. In most of the cases of low CSF hypocretin-1 level with neural-specific antibody positivity, the HLA haplotypes associated with idiopathic narcolepsy were absent [74, 75, 100, 121, 122].
While there are no pathognomonic polysomnography findings, certain uncommon features may suggest an autoimmune syndrome. Reduced or absent EEG markers of N2 or N3 sleep (vertex waves, K-complexes, sleep spindles, high voltage slow waves, etc.) can be seen with IgLON5-IgG [30] or Morvan syndrome [4, 43, 45]. Rapid, periodic leg movements can be seen in IgLON5-IgG, as well as grossly abnormal sleep architecture early in the night that normalizes later in the night [30]. RBD and RSWA have been associated with several ANS, such as IgLON5, VGKC/LGI1/CASPR2, and DPPX autoimmunity syndromes. Similar to other neurological conditions with stridor, laryngospasm, or vocal-cord paralysis, upper-airway evaluation (e.g., nasal endoscopy or laryngoscopy) may be considered in certain scenarios [22, 33].
Other Investigations for Autoimmune Encephalopathies (Imaging, CSF Studies, EEG)
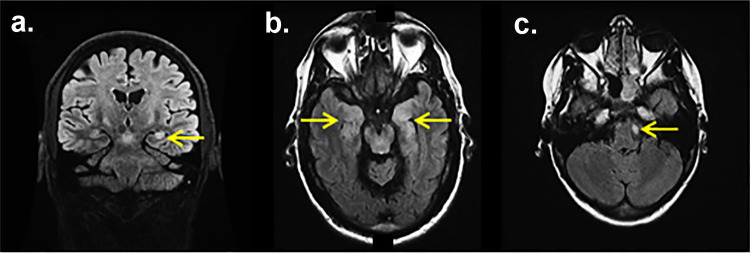
Magnetic resonance imaging (MRI) brain with gadolinium is an important evaluation for diagnosis of CNS autoimmune disorders. However, many autoimmune encephalitis patients, especially those with NMDA-R encephalitis, can have a normal MRI brain. When present, specific changes observed on an MRI brain can help diagnose an autoimmune neurological syndrome including mesial temporal FLAIR signal abnormalities and/or gadolinium enhancement suggestive of limbic encephalitis (Fig. 2a, b), multifocal T2/FLAIR hyperintensities suggestive of aquaporin-4 autoimmunity, or diencephalic signal abnormalities/enhancement suggestive of Ma2 autoimmunity [144]. [18F]fluorodeoxyglucose positron emission tomography (FDG-PET) brain can help and may show occipital hypometabolism in NMDA-R encephalitis [151] or altered basal ganglia metabolism in LGI1 encephalitis with faciobrachial dystonic seizures [152].
Fig. 2.
Magnetic resonance imaging (MRI) brain with leucine-rich glioma inactivated-protein-1 IgG. Coronal T2 fluid-attenuated inversion recovery (FLAIR) of an leucine-rich glioma inactivated-protein-1-IgG positive (LGI1+) limbic encephalitis patient with bilateral (left more than right) mesial temporal hyperintensities (a, arrow), axial T2 FLAIR of an LGI1+ limbic encephalitis patient with bilateral (mesial temporal hyperintensities consistent with limbic encephalitis (b, arrows), axial T2 of an LGI1+ patient with a non-enhancing caudal pontine lesion (c, arrow)
EEG is another useful modality commonly utilized in diagnosis of autoimmune encephalitis. Commonly observed EEG findings include diffuse non-specific delta or theta slowing, extreme delta brush activity at 20–30 Hz riding on each delta wave in NMDA-R encephalitis [153]. EEG monitoring in LGI1 encephalitis and faciobrachial dystonic seizures has demonstrated rhythmic slow-wave activity followed by attenuation [154].
CSF analysis can yield additional diagnostic insight. Since autoimmune encephalitides are thought to be inflammatory conditions, an inflammatory CSF profile (elevated CSF protein, elevated white blood cells, elevated IgG index, elevated synthesis rate, supernumerary oligoclonal bands, etc.) may be supportive. However, inflammatory CSF changes are neither sensitive for nor specific to ANS. CSF can be normal in many cases [150]. A recent systematic review discussed three CSF profiles in the most common neural specific antibodies. Inflammatory CSF was the most common profile found with NMDA, AMPA, DPPX, and GABA-B. CSF was usually normal with CASPR2, LGI1, GABA-A, and glycine. IgLON5 had elevated CSF protein without pleocytosis. GAD most often had supernumerary oligoclonal bands [155].
Autoantibody Testing and Interpretation
Testing for neural specific autoantibodies is critical in diagnosing an autoimmune neurological syndrome. When possible, it is beneficial to test both the serum and CSF, as certain antibodies are more readily detected in serum (LGI1 and CASPR2) or CSF (NMDA) [19, 156]. To optimize sensitivity, it is best to collect samples during an active disease episode and before initiation of immunotherapy [150]. Also, knowing whether the antibodies of interest are included in the antibody-panel evaluation ordered is important, since certain specific auto-antibodies such as IgLON5-IgG and Ma2-IgG may not be included in all commercially marketed panels in the USA. Phenotype-specific panels may aid in antibody selection.
A false-positive neural antibody result can lead to diagnostic error and unnecessary additional investigations or immunosuppressive therapy [157]. Neural antibody testing in patient populations with a low likelihood of neurological autoimmunity may generate false-positives. Since many different laboratories conduct neural-specific antibody testing, methodologies and reference values may differ.
A neural-specific antibody may not be identified despite a strong clinical suspicion of an autoimmune etiology. Since a seronegative autoimmune syndrome is possible, diagnostic criteria aid diagnosis [20, 158] by outlining suggestive features, classic phenotypes, and criteria for possible and definite diagnoses. Exclusion of alternative diagnoses is also important.
Tumor Screening
When associated with neoplasms, the neurological syndrome will often present before diagnosis of the neoplasm [159]. When a paraneoplastic etiology is suspected based on clinical symptoms or the presence of a paraneoplastic-associated neural-specific antibody, neoplastic screening should be completed. Depending on the level of suspicion and most likely neoplasm, this might include computed tomography (CT), FDG-PET, colonoscopy, scrotal or transvaginal ultrasound, mammogram, MRI, or thorough skin exam [160, 161, 162]. If screening is negative, a repeat screen should be pursued every 3 to 6 months for 2 to 5 years [162].
Differential Diagnosis
The sleep manifestations of autoimmune neurological syndromes can mimic several other primary sleep disorders and other diseases. Sleep disturbance symptoms can be mistaken for sleep disorders secondary to other medical conditions such as insomnia secondary to pain or anxiety, hypersomnolence related to metabolic derangements, or RBD related to medullary strokes [163]. Prion diseases such as fatal familial insomnia in particular can have significant overlap with certain autoimmune syndromes [45], while VGKC-IgG, NMDA, and glycine receptor-associated autoimmunity can closely resemble Creutzfeldt-Jakob disease (CJD) [164]. CJD can also be associated with complete lack of sleep, poorly structured N2 sleep, sleep disordered breathing, and RBD. This highlights the fact that while these features may be suggestive of an underlying neurological etiology, they are usually not specific to autoimmune syndromes [165].
An autoimmune etiology should be considered when there are autoimmune risk factors or atypical, concurrent sleep manifestations, such as narcolepsy symptoms at an advanced age [100]. Sleep disorders are common in neurodegenerative conditions such as Alzheimer’s disease (AD), dementia with Lewy bodies (DLB), Parkinson’s disease, multiple system atrophy (MSA), and amyotrophic lateral sclerosis (ALS). Given the pleiotropic neurological manifestations of autoimmune encephalopathies, which may involve cognitive, autonomic, extrapyramidal motor, and peripheral nerve/muscle manifestations, neurodegenerative disorder with prominent sleep manifestations are one of the chief differential diagnostic categories to consider. CNS infections can have similar manifestations as autoimmune encephalitis, especially when flu-like prodromal symptoms are present. Neuroinflammatory conditions, such as neurosarcoidosis, can also cause hypothalamic lesions and symptoms consistent with secondary narcolepsy [166].
Treatment
Symptomatic Sleep Therapies
Symptomatic treatment of autoimmune-related sleep symptoms should be implemented similarly to uses in other sleep disorders; for detailed discussion, please see the other articles included in this sleep specific issue of Neurotherapeutics. Ideally, symptom management should be coordinated with immunotherapy or oncologic therapy. If there is a favorable response to immunotherapy or oncologic therapy, symptom management can be reevaluated and adjusted. If symptoms of sleep disturbances are only mild, symptomatic therapy can be held during an initial acute immunotherapy trial to limit variables influencing response.
Objective Markers
Objective baseline and comparative outcome measures should be utilized to prevent over reliance on isolated subjective improvement. These objective measures should be individualized to the patient and care environment; these may include neurological examination, sleep questionnaires, PSG, MSLT, hypocretin-1 level, MRI brain with gadolinium, PET brain, neuropsychological testing, and/or electrodiagnostic studies. In our experience, objective comparison has traditionally been neurologic testing (MRI brain, EEG, neuropsychiatric cognitive testing, etc.) given symptom prominence, accessibility, and/or cost. However, sleep specific measures could also be used when available and feasible. Choice of questionnaire can be based on symptoms and provider preference (Epworth sleepiness scale, international restless legs syndrome rating scale, Pittsburgh sleep quality index, insomnia severity index, etc.); however, continuity of the same standardized questionnaire(s) is important for longitudinal comparison. Repeat polysomnography has documented improvement in select cases [7, 30]. If a hypocretin-1 deficiency is identified, then a repeat CSF hypocretin-1 testing could be considered as normalization has been documented [121]. Given limited data on repeat testing and documented improvement, it is difficult to generalize the utility of repeat sleep specific testing; rather decisions for repeat sleep studies or hypocretin-1 testing should be determined on a case-by-case basis considering symptoms, syndrome, and accessibility of neurologic/sleep testing. Additionally, not all symptoms or objective measures improve, so careful consideration of the overall clinical scenario is crucial when making treatment and monitoring decisions. One should also be mindful of other medications or factors being adjusted during the immunotherapy trial, as these could impact patient outcome and interpretation of improvement or deterioration following immunotherapy.
Oncologic Therapy
If a malignancy is identified, appropriate oncologic treatment should be initiated as soon as feasible. Complete oncologic response often has a favorable influence on the neurologic syndrome [167]. In some cases, oncologic treatment alone can adequately treat the paraneoplastic disease; however, a combination of oncologic and immunotherapy is usually necessary for stabilization or improvement [58, 150].
Immunotherapy
Initiation of immunotherapy should be considered as soon as an autoimmune etiology is suspected. However, evidence-guided therapy is limited, so these practices are primarily based on retrospective studies, with only one small randomized placebo-controlled trial demonstrating efficacy of immunotherapy for management of autoimmune encephalitis [168]. In many cases, utilization of clinically based diagnostic criteria for co-existing neurological syndromes may enable immunotherapy even before autoantibody-testing results are available or when no antibody is detected [158, 159].
Acute therapies commonly utilized in both inpatient and outpatient settings include intravenous methyl prednisone (IVMP), IVIG, and plasma exchange (PLEX) (Table 3). For most autoimmune CNS conditions, high-dose IVMP is the first-line acute immunotherapy, especially for those presumed to be mediated by autoantigen-specific cytotoxic T cells. In antibody-mediated conditions, IVIG or plasmapheresis are also potential options, especially when patients have contraindications to high-dose corticosteroids. The risks of allergic reaction, thromboembolic events, and bleeding are also possible when considering IVIG or plasmapheresis. At times, in cases with a rapid decline or severe presentation, a combination of therapies is utilized, IVMP and plasmapheresis or IVIG and IVMP, rather than staggering therapies and evaluating response between use of different agents [17].
Table 3.
Summary of immunomodulatory agents.
Adapted from Husari and Dubey Autoimmune Epilepsy. Neurotherapeutics. 2019 [174]
| Immunomodulatory agent | Route of administration | Dosing/regimen | Mechanism of action | Adverse effects | Monitoring and prophylaxis |
|---|---|---|---|---|---|
| Corticosteroids | IV or PO |
Initial dose: IV methylprednisolone (1 g per day for 3–5 days) 6-week trial: 1 g per day for 3 days followed by once weekly for 5 weeks 12 week trial: 1 g per day for 3 days followed by once weekly for 5 weeks, followed by once every 2 weeks for 6 weeks Oral maintenance: 60–80 mg prednisone daily, duration of taper variable |
-Acts on nuclear glucocorticoids receptors to reduce cytokine and chemokine production -Reduces migration of leukocytes to the target tissue |
Insomnia, psychiatric dysfunction, hyperglycemia, electrolyte imbalances, fluid retention, hypertension, peptic ulcer, Cushing syndrome, cataracts, infections, osteoporosis and avascular necrosis Addisonian crisis on rapid withdrawal of corticosteroids |
-Blood pressure, serum electrolytes and glucose monitoring - PJP ppx: TMP/SMX - osteoporosis ppx: Vitamin D + calcium - GI ppx: PPI or H2 blocker |
| Plasmapheresis | IV | 1 exchange every other day for 10–14 days | -Extracorporeal blood filtration designed to remove large molecular weight molecules, including immunoglobulins, immune complex, and complements | Hypotension, electrolyte imbalance, perioral paresthesia (hypocalcemia), coagulopathy, central line infection, hemorrhage, thrombosis and pneumothorax | PT, INR, PTT, Fibrinogen |
| IVIG | IV |
Initial dose 2 g/kg daily divided over 4–5 days maintenance: 1–2 g/kg every 3–4 weeks |
Remains unclear Interaction with antigen-binding fragment on the antibodies and/or crystalizable fragment on the antibodies or the antigen presenting cells |
Headache, aseptic meningitis, acute renal failure, thrombotic/thromboembolic events anaphylaxis due to IgA deficiency | Electrolytes, renal function |
| Rituximab | IV | 1000 mg followed by 2nd dose in 2 weeks, or 375 mg/m2 weekly for 4 weeks | B-cell depletion by antibody dependent cellular cytotoxicity, complement dependent cytotoxicity and apoptosis | Allergic reaction, opportunistic infection, reactivation of tuberculosis infection or hepatitis B infection, PML | Hepatitis B antibodies, Quatiferon test (for latent tuberculosis), pregnancy test, Liver function test |
| Cyclophosphamide | IV or PO |
IV: 500-1000 mg/m2/month PO: 1–2 mg/kg/day (renal adjustment necessary) |
Alkylating agent with interferes with DNA synthesis | Gastrointestinal (nausea, vomiting), hair loss, mucositis, hemorrhagic cystitis, infertility and myelosuppression |
CBC, liver function test, creatinine CBC at 8–14 days post infusion, increased hydration recommended |
| Mycophenolate | PO | Initially 500 mg twice daily, target 1000 mg twice daily | Inhibition of inosine monophosphate dehydrogenase mediated guanosine nucleotide synthesis | Gastrointestinal (nausea, vomiting, diarrhea), hypertension, peripheral edema, infections, myelosuppression, lymphoma |
CBC, creatinine, urine pregnancy test CBC once per week for 1 month, then once every 2 weeks for 2 months then once every 1–3 months for the duration of therapy |
| Azathioprine | PO | Initially 1.5 mg/kg/day, target 2–3 mg/kg/day (guided by 5-point MCV increase from baseline) | Converted to cytotoxic 6-thioguanine nucleotides which leads to incorporation as a false base into DNA inducing lymphocyte apoptosis | Gastrointestinal symptoms (nausea, vomiting, diarrhea), hypersensitivity reactions, hair loss, cytopenia, hepatotoxicity, lymphoma and infections |
CBC, liver function test, creatinine, TPMT and urine pregnancy CBC should be checked once per week for 1 month, then once every 2 weeks for 2 months, then once every 1–3 months for the duration of therapy |
IV = intravenous, PO = per oral; PLEX = plasmapheresis; IVIG = intravenous immunoglobulin; PT = prothrombin time; INR = international normalized ratio; PTT = partial thromboplastin time; CBC = complete blood count; TPMT = thiopurine S-methyltransferase; TMP = trimethoprim; SMX = sulfamethoxazole; PPI = proton pump inhibitor; H2 = histamine 2; PML = progressive multifocal leukoencephalopathy; ppx = prophylaxis
Common initial inpatient doses for these therapies are IVMP, 1 g daily for 3 to 5 days; IVIG, 2 g/kg divided over 4 to 5 days; and plasmapheresis, 5 to 7 sessions over 10 to 14 days. In the outpatient setting, some centers utilize a 6- or 12-week immunotherapy regimen (IVMP 1 g/week or IVIG 0.4 mg/kg/week) with gradual reduction in immunotherapy dose [169]. Patients are evaluated at the end of the recommended regimen (ideally within 1 week of the last infusion) to ascertain the treatment response. These evaluations should include objective markers such as neurological examination, sleep questionnaires, PSG, MSLT, hypocretin-1 level, MRI brain with gadolinium, PET brain, neuropsychological testing, and/or electrodiagnostic studies.
Although often used for maintenance therapy, second-line agents such as rituximab and cyclophosphamide can also be utilized early in the disease course, especially among ANS with high risk of relapse or persistent neuroinflammatory disease activity. Potential dosing for rituximab includes 375 mg/m2/week for 4 weeks or 1 g/week for 2 weeks. Cyclophosphamide can be given as 1–2 mg/kg/day orally or 500–1000 mg/m2/month IV for 6 months [150].
Among patients with onco-neural antibodies targeting an intracellular epitope (such as ANNA-1or ANNA-2), preferred options for chronic immunosuppression are azathioprine (oral; initial dose of 1.5 mg/kg/day; goal dose of 2–3 mg/kg/day), mycophenolate (oral; initial dose of 500 mg twice per day; goal dose of 1000 mg twice per day), and cyclophosphamide (oral or IV, doses as discussed above), which target both T- and B-lymphocytes because pathogenesis of these PNS are presumed to involve cellular immune response. Whereas ANS mediated by direct effect of neural-specific antibodies (such as NMDA-R or DPPX), initiation of rituximab (IV, dosing as discussed above), IVIG and/or low-dose oral corticosteroids (prednisone 10 mg/day to 20 mg/day or alternate day) can also be used as maintenance therapy [150].
Prophylactic measures are recommended with long-term corticosteroid therapy. For bone health, recommendations include supplemental calcium and vitamin D along with bone-density screening and bisphosphonate treatment if indicated. The gastrointestinal lining should be protected, commonly with a proton pump inhibitor or histamine-2 blocker [17]. Pneumocystis jiroveci pneumonia prophylaxis commonly includes double-strength trimethoprim/sulfamethoxazole tablets administered three times per week. Alternatives include daily oral dapsone or monthly inhaled pentamidine [150].
Treatment of multiple sclerosis, NMO, and GBS, has been discussed extensively in the October 2017 and the January 2016 issues of Neurotherapeutics [170–172]. Further specific details on the treatment of autoimmune encephalopathies were also discussed in the January 2016 issue [173].
Reassessment and Relapses
When severe neurological deficits are already present, improvement may be limited. In these cases, halting progression (disease stability, without improvement of existing symptoms) may be considered successful treatment [150]. If disease stability has been achieved, a trial of weaning acute or maintenance immunotherapy can be attempted. If symptoms exacerbate during the trial of weaning, then the taper should be reversed. Again, utilizing objective testing and/or questionnaires may help judge patient response and guide therapy adjustment over time. While a clinician can use their questionnaire of choice, continuity of the same standardized questionnaire is important for longitudinal comparison.
Disease relapse may occur despite stable therapy, in which case sleep, neurologic, and tumor workup/screening may need to be repeated. Immunotherapy escalation and additional oncologic treatment should be implemented as appropriate. However, it is important to consider other reasons for residual symptoms before escalating immunotherapy. Some syndromes may improve slowly over months to years before reestablishing a new baseline [58], which may not be equivalent to pre-morbid baseline. Exacerbation of prior deficits may also occur with metabolic or infectious derangements. Medication adjustments and comorbid medical conditions (depression, sleep-disordered breathing, etc.) can contribute to symptomatology (fatigue, insomnia, or drowsiness).
Conclusions
Sleep manifestations are common in autoimmune neurological syndromes but may be overlooked due to other predominant, more urgent neurological symptoms. As some syndromes involve multiple sleep symptoms, screening all sleep domains allows for complete characterization. Certain phenotypes appear to be more commonly associated with specific autoimmune neurological phenotypes. Some phenotypes are similar to those seen in other conditions (e.g., idiopathic narcolepsy or fatal familial insomnia), and some present with a defining constellation of sleep symptoms and polysomnographic findings (e.g., IgLON5 IgG-associated, undifferentiated non-REM sleep, rapid periodic limb movements, and normalization of sleep architecture in the second half of the night). With certain neural-specific antibody phenotypes, sleep testing may provide objective biomarkers (polysomnography, CSF hypocretin-1, etc.) to support an autoimmune diagnosis, monitor therapeutic response, or disease progression/relapse. However, more comprehensive characterization of sleep manifestations and sleep testing is needed to better understand underlying sleep disruption in autoimmune conditions.
Supplementary Information
Below is the link to the electronic supplementary material.
(DOCX 430 KB)
(DOCX 432 KB)
(PDF 1.19 MB)
(PDF 1.19 MB)
Acknowledgements
We gratefully acknowledge the contributions of Lea Dacy, Mayo Clinic Department of Neurology, for secretarial support with manuscript formatting and submission.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Funding
This publication was supported by NIH/NCRR/NCATS CCaTS Grant Number UL1 TR002377 and by NIH/NIA R34AG056639 (NAPS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/3/2021
A Correction to this paper has been published: 10.1007/s13311-021-01058-x
References
- 1.Juji T, Satake M, Honda Y, Doi Y. HLA antigens in Japanese patients with narcolepsy. All the patients were DR2 positive. Tissue Antigens. 1984;24(5):316–9. [DOI] [PubMed]
- 2.Langdon N, Welsh KI, van Dam M, Vaughan RW, Parkes D. Genetic markers in narcolepsy. Lancet. 1984;2(8413):1178–1180. doi: 10.1016/s0140-6736(84)92742-9. [DOI] [PubMed] [Google Scholar]
- 3.Barber PA, Anderson NE, Vincent A. Morvan's syndrome associated with voltage-gated K+ channel antibodies. Neurology. 2000;54(3):771–772. doi: 10.1212/wnl.54.3.771. [DOI] [PubMed] [Google Scholar]
- 4.Liguori R, Vincent A, Clover L, Avoni P, Plazzi G, Cortelli P, et al. Morvan's syndrome: peripheral and central nervous system and cardiac involvement with antibodies to voltage-gated potassium channels. Brain. 2001;124(Pt 12):2417–2426. doi: 10.1093/brain/124.12.2417. [DOI] [PubMed] [Google Scholar]
- 5.Dauvilliers Y, Mayer G, Lecendreux M, Neidhart E, Peraita-Adrados R, Sonka K, et al. Kleine-Levin syndrome: an autoimmune hypothesis based on clinical and genetic analyses. Neurology. 2002;59(11):1739–1745. doi: 10.1212/01.wnl.0000036605.89977.d0. [DOI] [PubMed] [Google Scholar]
- 6.Sabater L, Gaig C, Gelpi E, Bataller L, Lewerenz J, Torres-Vega E, et al. A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: a case series, characterisation of the antigen, and post-mortem study. Lancet Neurol. 2014;13(6):575–586. doi: 10.1016/S1474-4422(14)70051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iranzo A, Graus F, Clover L, Morera J, Bruna J, Vilar C, et al. Rapid eye movement sleep behavior disorder and potassium channel antibody-associated limbic encephalitis. Ann Neurol. 2006;59(1):178–181. doi: 10.1002/ana.20693. [DOI] [PubMed] [Google Scholar]
- 8.Blattner MS, de Bruin GS, Bucelli RC, Day GS. Sleep disturbances are common in patients with autoimmune encephalitis. J Neurol. 2019;266(4):1007–1015. doi: 10.1007/s00415-019-09230-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silber MH. Autoimmune sleep disorders. Handb Clin Neurol. 2016;133:317–326. doi: 10.1016/B978-0-444-63432-0.00018-9. [DOI] [PubMed] [Google Scholar]
- 10.Iranzo A. Sleep and neurological autoimmune diseases. Neuropsychopharmacology. 2020;45(1):129–140. doi: 10.1038/s41386-019-0463-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blattner MS, Day GS. Sleep disturbances in patients with autoimmune encephalitis. Curr Neurol Neurosci Rep. 2020;20(7):28. doi: 10.1007/s11910-020-01048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muñoz-Lopetegi A, Graus F, Dalmau J, Santamaria J. Sleep disorders in autoimmune encephalitis. Lancet Neurol. 2020;19(12):1010–1022. doi: 10.1016/S1474-4422(20)30341-0. [DOI] [PubMed] [Google Scholar]
- 13.Ariño H, Muñoz-Lopetegi A, Martinez-Hernandez E et al. Sleep disorders in anti-NMDAR encephalitis. Neurology. 2020. [DOI] [PubMed]
- 14.Dubey D, Pittock SJ, Kelly CR, et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol. 2018;83(1):166–177. doi: 10.1002/ana.25131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogrig A, Gigli GL, Segatti S, Corazza E, Marini A, Bernardini A, et al. Epidemiology of paraneoplastic neurological syndromes: a population-based study. J Neurol. 2020;267(1):26–35. doi: 10.1007/s00415-019-09544-1. [DOI] [PubMed] [Google Scholar]
- 16.Gu Y, Zhong M, He L, et al. Epidemiology of antibody-positive autoimmune encephalitis in Southwest China: a multicenter study. Front Immunol. 2019;10:2611. doi: 10.3389/fimmu.2019.02611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKeon A. Autoimmune encephalopathies and dementias. CONTINUUM: Lifelong Learning in Neurology. 2016;22(2):538–58. [DOI] [PubMed]
- 18.Dubey D, Kothapalli N, McKeon A, Flanagan EP, Lennon VA, Klein CJ, et al. Predictors of neural-specific autoantibodies and immunotherapy response in patients with cognitive dysfunction. J Neuroimmunol. 2018;323:62–72. doi: 10.1016/j.jneuroim.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Gadoth A, Pittock SJ, Dubey D, McKeon A, Britton JW, Schmeling JE, et al. Expanded phenotypes and outcomes among 256 LGI 1/CASPR 2-I g G–positive patients. Annals of neurology. 2017;82(1):79–92. doi: 10.1002/ana.24979. [DOI] [PubMed] [Google Scholar]
- 20.Graus F, Delattre JY, Antoine JC, Dalmau J, Giometto B, Grisold W, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004;75(8):1135–1140. doi: 10.1136/jnnp.2003.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honorat JA, Komorowski L, Josephs KA, Fechner K, St Louis EK, Hinson SR, et al. IgLON5 antibody: neurological accompaniments and outcomes in 20 patients. Neurol Neuroimmunol Neuroinflamm. 2017;4(5):e385. doi: 10.1212/NXI.0000000000000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaig C, Graus F, Compta Y, Högl B, Bataller L, Brüggemann N, et al. Clinical manifestations of the anti-IgLON5 disease. Neurology. 2017;88(18):1736–1743. doi: 10.1212/WNL.0000000000003887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tao QQ, Wei Q, Song SJ, Yin XZ. Motor neuron disease-like phenotype associated with anti-IgLON5 disease. CNS Neurosci Ther. 2018;24(12):1305–1308. doi: 10.1111/cns.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wenninger S. Expanding the clinical spectrum of IgLON5-syndrome. J Neuromuscul Dis. 2017;4(4):337–339. doi: 10.3233/JND-170259. [DOI] [PubMed] [Google Scholar]
- 25.Brunetti V, Della Marca G, Spagni G, Iorio R. Immunotherapy improves sleep and cognitive impairment in anti-IgLON5 encephalopathy. Neurol Neuroimmunol Neuroinflamm. 2019;6(4):e577. doi: 10.1212/NXI.0000000000000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasselbacher K, Steffen A, Wandinger KP, Brüggemann N. IgLON5 antibodies are infrequent in patients with isolated sleep apnea. Eur J Neurol. 2018;25(4):e46–e47. doi: 10.1111/ene.13566. [DOI] [PubMed] [Google Scholar]
- 27.Nissen MS, Blaabjerg M. Anti-IgLON5 disease: a case with 11-year clinical course and review of the literature. Front Neurol. 2019;10:1056. doi: 10.3389/fneur.2019.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grüter T, Behrendt V, Bien CI, Gold R, Ayzenberg I. Early immunotherapy is highly effective in IgG1/IgG4 positive IgLON5 disease. J Neurol. 2020;267(7):2151–2153. doi: 10.1007/s00415-020-09924-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaig C, Iranzo A, Santamaria J, Graus F. The sleep disorder in anti-lgLON5 disease. Curr Neurol Neurosci Rep. 2018;18(7):41. doi: 10.1007/s11910-018-0848-0. [DOI] [PubMed] [Google Scholar]
- 30.Gaig C, Iranzo A, Cajochen C, Vilaseca I, Embid C, Dalmau J, et al. Characterization of the sleep disorder of anti-IgLON5 disease. Sleep. 2019;42(9). [DOI] [PubMed]
- 31.Erro ME, Sabater L, Martínez L et al. Anti-IGLON5 disease: A new case without neuropathologic evidence of brainstem tauopathy. Neurol Neuroimmunol Neuroinflamm. 2020;7(2). [DOI] [PMC free article] [PubMed]
- 32.Moreno-Estébanez A, Garcia-Ormaechea M, Tijero B, Fernández-Valle T, Gómez-Esteban JC, Berganzo K. Anti-IgLON5 disease responsive to immunotherapy: a case report with an abnormal MRI. Mov Disord Clin Pract. 2018;5(6):653–656. doi: 10.1002/mdc3.12679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J, Vasani S. A Novel Presentation of the Acute Airway: Anti-IgLON5 Disease. Laryngoscope. 2020. [DOI] [PubMed]
- 34.Cabezudo-García P, Mena-Vázquez N, Estivill Torrús G, Serrano-Castro P. Response to immunotherapy in anti-IgLON5 disease: a systematic review. Acta Neurol Scand. 2020;141(4):263–270. doi: 10.1111/ane.13207. [DOI] [PubMed] [Google Scholar]
- 35.Gaig C, Ercilla G, Daura X, Ezquerra M, Fernández-Santiago R, Palou E, et al. HLA and microtubule-associated protein tau H1 haplotype associations in anti-IgLON5 disease. Neurol Neuroimmunol Neuroinflamm. 2019;6(6). [DOI] [PMC free article] [PubMed]
- 36.Landa J, Gaig C, Planagumà J, Saiz A, Antonell A, Sanchez-Valle R, et al. Effects of IgLON5 antibodies on neuronal cytoskeleton: a link between autoimmunity and neurodegeneration. Ann Neurol. 2020. [DOI] [PubMed]
- 37.Vincent A, Jacobson L, Plested P, Polizzi A, Tang T, Riemersma S, et al. Antibodies affecting ion channel function in acquired neuromyotonia, in seropositive and seronegative myasthenia gravis, and in antibody-mediated arthrogryposis multiplex congenita. Ann N Y Acad Sci. 1998;841:482–496. doi: 10.1111/j.1749-6632.1998.tb10968.x. [DOI] [PubMed] [Google Scholar]
- 38.Kleopa KA, Elman LB, Lang B, Vincent A, Scherer SS. Neuromyotonia and limbic encephalitis sera target mature Shaker-type K+ channels: subunit specificity correlates with clinical manifestations. Brain. 2006;129(Pt 6):1570–1584. doi: 10.1093/brain/awl084. [DOI] [PubMed] [Google Scholar]
- 39.Lai M, Huijbers MG, Lancaster E, Graus F, Bataller L, Balice-Gordon R, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. 2010;9(8):776–785. doi: 10.1016/S1474-4422(10)70137-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Irani SR, Alexander S, Waters P, Kleopa KA, Pettingill P, Zuliani L, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis. Morvan's syndrome and acquired neuromyotonia. Brain. 2010;133(9):2734–2748. doi: 10.1093/brain/awq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michael S, Waters P, Irani SR. Stop testing for autoantibodies to the VGKC-complex: only request LGI1 and CASPR2. Pract Neurol. 2020. [DOI] [PubMed]
- 42.Irani SR, Pettingill P, Kleopa KA, Schiza N, Waters P, Mazia C, et al. Morvan syndrome: clinical and serological observations in 29 cases. Ann Neurol. 2012;72(2):241–255. doi: 10.1002/ana.23577. [DOI] [PubMed] [Google Scholar]
- 43.Josephs KA, Silber MH, Fealey RD, Nippoldt TB, Auger RG, Vernino S. Neurophysiologic studies in Morvan syndrome. J Clin Neurophysiol. 2004;21(6):440–445. doi: 10.1097/00004691-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Lee W, Day TJ, Williams DR. Clinical, laboratory and electrophysiological features of Morvan's fibrillary chorea. J Clin Neurosci. 2013;20(9):1246–1249. doi: 10.1016/j.jocn.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 45.Cornelius JR, Pittock SJ, McKeon A, Lennon VA, Aston PA, Josephs KA, et al. Sleep manifestations of voltage-gated potassium channel complex autoimmunity. Arch Neurol. 2011;68(6):733–738. doi: 10.1001/archneurol.2011.106. [DOI] [PubMed] [Google Scholar]
- 46.Lugaresi E, Provini F, Cortelli P. Agrypnia excitata. Sleep Med. 2011;12(Suppl 2):S3–10. doi: 10.1016/j.sleep.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 47.Devine M, Feemster J, Lieske E, McCarter S, Sandness D, Steele T, et al. REM Sleep Without Atonia and Sleep Disturbances in Patients with LGI1 and CASPR2 Autoimmunity. Neurology; 2020.
- 48.Lee EK, Maselli RA, Ellis WG, Agius MA. Morvan's fibrillary chorea: a paraneoplastic manifestation of thymoma. J Neurol Neurosurg Psychiatry. 1998;65(6):857–862. doi: 10.1136/jnnp.65.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Sonderen A, Ariño H, Petit-Pedrol M, Leypoldt F, Körtvélyessy P, Wandinger KP, et al. The clinical spectrum of Caspr2 antibody-associated disease. Neurology. 2016;87(5):521–528. doi: 10.1212/WNL.0000000000002917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boyko M, Au KLK, Casault C, de Robles P, Pfeffer G. Systematic review of the clinical spectrum of CASPR2 antibody syndrome. J Neurol. 2020;267(4):1137–1146. doi: 10.1007/s00415-019-09686-2. [DOI] [PubMed] [Google Scholar]
- 51.Gadoth A, Pittock SJ, Dubey D, McKeon A, Britton JW, Schmeling JE, et al. Expanded phenotypes and outcomes among 256 LGI1/CASPR2-IgG-positive patients. Ann Neurol. 2017;82(1):79–92. doi: 10.1002/ana.24979. [DOI] [PubMed] [Google Scholar]
- 52.van Sonderen A, Thijs RD, Coenders EC, Jiskoot LC, Sanchez E, de Bruijn MA, et al. Anti-LGI1 encephalitis: clinical syndrome and long-term follow-up. Neurology. 2016;87(14):1449–1456. doi: 10.1212/WNL.0000000000003173. [DOI] [PubMed] [Google Scholar]
- 53.Tezer FI, Erdener E, Sel CG, Mehdikanova L, Saygi S, Topcuoglu M. Daytime polysomnography recording in LIG1 --related limbic encephalitis. Arch Neurol. 2012;69(1):145–6; author reply 6. [DOI] [PubMed]
- 54.Serdaroğlu E, Tezer F, Saygi S. Autoimmune epilepsy and/or limbic encephalitis can lead to changes in sleep spindles. Noro Psikiyatr Ars. 2018;55(4):320–324. doi: 10.5152/npa.2017.19442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Irani SR, Bera K, Waters P, Zuliani L, Maxwell S, Zandi MS, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain. 2010;133(Pt 6):1655–1667. doi: 10.1093/brain/awq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morales-Briceño H, Fung VSC. Isolated nocturnal occurrence of orofacial dyskinesias in N-methyl-D-aspartate receptor encephalitis-a new diagnostic clue. Mov Disord Clin Pract. 2017;4(6):884–886. doi: 10.1002/mdc3.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Titulaer MJ, McCracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157–165. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10(1):63–74. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Irani SR, Vincent A. NMDA receptor antibody encephalitis. Curr Neurol Neurosci Rep. 2011;11(3):298–304. doi: 10.1007/s11910-011-0186-y. [DOI] [PubMed] [Google Scholar]
- 61.Florance NR, Davis RL, Lam C, Szperka C, Zhou L, Ahmad S, et al. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol. 2009;66(1):11–18. doi: 10.1002/ana.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gurrera RJ. Recognizing psychiatric presentations of anti-NMDA receptor encephalitis in children and adolescents: a synthesis of published reports. Psychiatry Clin Neurosci. 2019;73(5):262–268. doi: 10.1111/pcn.12821. [DOI] [PubMed] [Google Scholar]
- 63.Marques IB, Teotónio R, Cunha C, Bento C, Sales F. Anti-NMDA receptor encephalitis presenting with total insomnia–a case report. J Neurol Sci. 2014;336(1–2):276–280. doi: 10.1016/j.jns.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 64.Mohammad SS, Soe SM, Pillai SC, Nosadini M, Barnes EH, Gill D, et al. Etiological associations and outcome predictors of acute electroencephalography in childhood encephalitis. Clin Neurophysiol. 2016;127(10):3217–3224. doi: 10.1016/j.clinph.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 65.Anderson KN, Kelly TP, Griffiths TD. Primary sleep disorders can cause long-term sleep disturbance in patients with autoimmune mediated limbic encephalitis. Clin Neurol Neurosurg. 2013;115(7):1079–1082. doi: 10.1016/j.clineuro.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 66.Dalmau J, Tüzün E, Wu HY, Masjuan J, Rossi JE, Voloschin A, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Voltz R, Gultekin SH, Rosenfeld MR, Gerstner E, Eichen J, Posner JB, et al. A serologic marker of paraneoplastic limbic and brain-stem encephalitis in patients with testicular cancer. N Eng J Med. 1999;340(23):1788–1795. doi: 10.1056/NEJM199906103402303. [DOI] [PubMed] [Google Scholar]
- 68.Rosenfeld MR, Dalmau JO. Paraneoplastic disorders of the CNS and autoimmune synaptic encephalitis. Continuum (Minneap Minn). 2012;18(2):366–383. doi: 10.1212/01.CON.0000413664.42798.aa. [DOI] [PubMed] [Google Scholar]
- 69.Ortega Suero G, Sola-Valls N, Escudero D, Saiz A, Graus F. Anti-Ma and anti-Ma2-associated paraneoplastic neurological syndromes. Neurologia. 2018;33(1):18–27. doi: 10.1016/j.nrl.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 70.Dalmau J, Graus F, Villarejo A, Posner JB, Blumenthal D, Thiessen B, et al. Clinical analysis of anti-Ma2-associated encephalitis. Brain. 2004;127(Pt 8):1831–1844. doi: 10.1093/brain/awh203. [DOI] [PubMed] [Google Scholar]
- 71.Dubey D, Wilson MR, Clarkson B, Giannini C, Gandhi M, Cheville J, et al. Expanded Clinical Phenotype, Oncological Associations, and Immunopathologic Insights of Paraneoplastic Kelch-like Protein-11 Encephalitis. JAMA Neurol. 2020. [DOI] [PMC free article] [PubMed]
- 72.Vogrig A, Fouret M, Joubert B et al. Increased frequency of anti-Ma2 encephalitis associated with immune checkpoint inhibitors. Neurol Neuroimmunol Neuroinflamm. 2019;6(6). [DOI] [PMC free article] [PubMed]
- 73.Hoffmann LA, Jarius S, Pellkofer HL, Schueller M, Krumbholz M, Koenig F, et al. Anti-Ma and anti-Ta associated paraneoplastic neurological syndromes: 22 newly diagnosed patients and review of previous cases. J Neurol Neurosurg Psychiatry. 2008;79(7):767–773. doi: 10.1136/jnnp.2007.118588. [DOI] [PubMed] [Google Scholar]
- 74.Compta Y, Iranzo A, Santamaría J, Casamitjana R, Graus F. REM sleep behavior disorder and narcoleptic features in anti-Ma2-associated encephalitis. Sleep. 2007;30(6):767–769. doi: 10.1093/sleep/30.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Landolfi JC, Nadkarni M. Paraneoplastic limbic encephalitis and possible narcolepsy in a patient with testicular cancer: case study. Neuro Oncol. 2003;5(3):214–216. doi: 10.1215/S1152-8517-02-00046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blumenthal DT, Salzman KL, Digre KB, Jensen RL, Dunson WA, Dalmau J. Early pathologic findings and long-term improvement in anti-Ma2-associated encephalitis. Neurology. 2006;67(1):146–149. doi: 10.1212/01.wnl.0000223647.83708.20. [DOI] [PubMed] [Google Scholar]
- 77.Dauvilliers Y, Bauer J, Rigau V, Lalloyer N, Labauge P, Carlander B, et al. Hypothalamic immunopathology in anti-Ma-associated diencephalitis with narcolepsy-cataplexy. JAMA Neurol. 2013;70(10):1305–1310. doi: 10.1001/jamaneurol.2013.2831. [DOI] [PubMed] [Google Scholar]
- 78.Overeem S, Dalmau J, Bataller L, Nishino S, Mignot E, Verschuuren J, et al. Hypocretin-1 CSF levels in anti-Ma2 associated encephalitis. Neurology. 2004;62(1):138–140. doi: 10.1212/01.wnl.0000101718.92619.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rojas-Marcos I, Graus F, Sanz G, Robledo A, Diaz-Espejo C. Hypersomnia as presenting symptom of anti-Ma2-associated encephalitis: case study. Neuro Oncol. 2007;9(1):75–77. doi: 10.1215/15228517-2006-013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Adams C, McKeon A, Silber MH, Kumar R. Narcolepsy, REM sleep behavior disorder, and supranuclear gaze palsy associated with Ma1 and Ma2 antibodies and tonsillar carcinoma. Arch Neurol. 2011;68(4):521–524. doi: 10.1001/archneurol.2011.56. [DOI] [PubMed] [Google Scholar]
- 81.Kritikou I, Vgontzas AN, Rapp MA, Bixler EO. Anti-Ma1- and anti-Ma2-associated encephalitis manifesting with rapid eye movement sleep disorder and narcolepsy with cataplexy: a case report. Biol Psychiatry. 2018;83(5):e39–e40. doi: 10.1016/j.biopsych.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 82.Tobin WO, Lennon VA, Komorowski L, Probst C, Clardy SL, Aksamit AJ, et al. DPPX potassium channel antibody: frequency, clinical accompaniments, and outcomes in 20 patients. Neurology. 2014;83(20):1797–1803. doi: 10.1212/WNL.0000000000000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hara M, Ariño H, Petit-Pedrol M, Sabater L, Titulaer MJ, Martinez-Hernandez E, et al. DPPX antibody-associated encephalitis: main syndrome and antibody effects. Neurology. 2017;88(14):1340–1348. doi: 10.1212/WNL.0000000000003796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stokin GB, Popović M, Gelpi E, Kogoj A, Dalmau J, Graus F. Neuropathologic features of anti-dipeptidyl-peptidase-like protein-6 antibody encephalitis. Neurology. 2015;84(4):430–432. doi: 10.1212/WNL.0000000000001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou Q, Zhu X, Meng H, Zhang M, Chen S. Anti-dipeptidyl-peptidase-like protein 6 encephalitis, a rare cause of reversible rapid progressive dementia and insomnia. J Neuroimmunol. 2020;339:577114. doi: 10.1016/j.jneuroim.2019.577114. [DOI] [PubMed] [Google Scholar]
- 86.Byun JI, Lee ST, Jung KH, Sunwoo JS, Moon J, Kim TJ, et al. Prevalence of antineuronal antibodies in patients with encephalopathy of unknown etiology: data from a nationwide registry in Korea. J Neuroimmunol. 2016;293:34–38. doi: 10.1016/j.jneuroim.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 87.Laurido-Soto O, Brier MR, Simon LE, McCullough A, Bucelli RC, Day GS. Patient characteristics and outcome associations in AMPA receptor encephalitis. J Neurol. 2019;266(2):450–460. doi: 10.1007/s00415-018-9153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lai M, Hughes EG, Peng X, Zhou L, Gleichman AJ, Shu H, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol. 2009;65(4):424–434. doi: 10.1002/ana.21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jia Y, Wang J, Xue L, Hou Y. Limbic encephalitis associated with AMPA receptor and CRMP5 antibodies: a case report and literature review. Brain Behav. 2020;10(3):e01528. doi: 10.1002/brb3.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Höftberger R, van Sonderen A, Leypoldt F, Houghton D, Geschwind M, Gelfand J, et al. Encephalitis and AMPA receptor antibodies: novel findings in a case series of 22 patients. Neurology. 2015;84(24):2403–2412. doi: 10.1212/WNL.0000000000001682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Graus F, Keime-Guibert F, Reñe R, Benyahia B, Ribalta T, Ascaso C, et al. Anti-Hu-associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain. 2001;124(Pt 6):1138–1148. doi: 10.1093/brain/124.6.1138. [DOI] [PubMed] [Google Scholar]
- 92.Sillevis Smitt P, Grefkens J, de Leeuw B, van den Bent M, van Putten W, Hooijkaas H, et al. Survival and outcome in 73 anti-Hu positive patients with paraneoplastic encephalomyelitis/sensory neuronopathy. J Neurol. 2002;249(6):745–753. doi: 10.1007/s00415-002-0706-4. [DOI] [PubMed] [Google Scholar]
- 93.Lucchinetti CF, Kimmel DW, Lennon VA. Paraneoplastic and oncologic profiles of patients seropositive for type 1 antineuronal nuclear autoantibodies. Neurology. 1998;50(3):652–657. doi: 10.1212/wnl.50.3.652. [DOI] [PubMed] [Google Scholar]
- 94.Gómez-Choco MJ, Zarranz JJ, Saiz A, Forcadas MI, Graus F. Central hypoventilation as the presenting symptom in Hu associated paraneoplastic encephalomyelitis. J Neurol Neurosurg Psychiatry. 2007;78(10):1143–1145. doi: 10.1136/jnnp.2007.117994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee KS, Higgins MJ, Patel BM, Larson JS, Rady MY. Paraneoplastic coma and acquired central alveolar hypoventilation as a manifestation of brainstem encephalitis in a patient with ANNA-1 antibody and small-cell lung cancer. Neurocrit Care. 2006;4(2):137–139. doi: 10.1385/NCC:4:2:137. [DOI] [PubMed] [Google Scholar]
- 96.Ball JA, Warner T, Reid P, Howard RS, Gregson NA, Rossor MN. Central alveolar hypoventilation associated with paraneoplastic brain-stem encephalitis and anti-Hu antibodies. J Neurol. 1994;241(9):561–566. doi: 10.1007/BF00873520. [DOI] [PubMed] [Google Scholar]
- 97.Brookes C, McIntosh R, Twigg S. Ondine's curse: a case of anti-Hu paraneoplastic syndrome. J Intensive Care Soc. 2014;15(4):355–357. [Google Scholar]
- 98.Kay L, Bauer S, Koczulla AR, Librizzi D, Vadasz D, Knake S, et al. Ondine's curse and temporal lobe seizures: anti-Hu- and Zic4-associated paraneoplastic brainstem and limbic encephalitis. Eur J Neurol. 2018;25(5):e59–e60. doi: 10.1111/ene.13629. [DOI] [PubMed] [Google Scholar]
- 99.Najjar M, Taylor A, Agrawal S, Fojo T, Merkler AE, Rosenblum MK, et al. Anti-Hu paraneoplastic brainstem encephalitis caused by a pancreatic neuroendocrine tumor presenting with central hypoventilation. J Clin Neurosci. 2017;40:72–73. doi: 10.1016/j.jocn.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 100.Vitiello M, Antelmi E, Pizza F, Postiglione E, Poggi R, Liguori R, et al. Type 1 narcolepsy in anti-Hu antibodies mediated encephalitis: a case report. Sleep Med. 2018;52:23–25. doi: 10.1016/j.sleep.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 101.Simard C, Vogrig A, Joubert B, Muniz-Castrillo S, Picard G, Rogemond V, et al. Clinical spectrum and diagnostic pitfalls of neurologic syndromes with Ri antibodies. Neurol Neuroimmunol Neuroinflamm. 2020;7(3). [DOI] [PMC free article] [PubMed]
- 102.Pittock SJ, Lucchinetti CF, Lennon VA. Anti-neuronal nuclear autoantibody type 2: paraneoplastic accompaniments. Ann Neurol. 2003;53(5):580–587. doi: 10.1002/ana.10518. [DOI] [PubMed] [Google Scholar]
- 103.Dropcho EJ, Kline LB, Riser J. Antineuronal (anti-Ri) antibodies in a patient with steroid-responsive opsoclonus-myoclonus. Neurology. 1993;43(1):207–211. doi: 10.1212/wnl.43.1_part_1.207. [DOI] [PubMed] [Google Scholar]
- 104.Pittock SJ, Parisi JE, McKeon A, Roemer SF, Lucchinetti CF, Tan KM, et al. Paraneoplastic jaw dystonia and laryngospasm with antineuronal nuclear autoantibody type 2 (anti-Ri) Arch Neurol. 2010;67(9):1109–1115. doi: 10.1001/archneurol.2010.209. [DOI] [PubMed] [Google Scholar]
- 105.Kim KJ, Yun JY, Lee JY, Kim YE, Jeon BS. Ondine's curse in anti-Ri antibody associated paraneoplastic brainstem syndrome. Sleep Med. 2013;14(4):382. doi: 10.1016/j.sleep.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 106.Hoque R, DelRosso L. Paraneoplastic overlap syndrome in non-small squamous cell lung carcinoma. BMJ Case Rep. 2014;2014. [DOI] [PMC free article] [PubMed]
- 107.McKay JH, Dimberg EL, Lopez Chiriboga AS. A systematic review of gamma-aminobutyric acid receptor type B autoimmunity. Neurol Neurochir Pol. 2019;53(1):1–7. doi: 10.5603/PJNNS.a2018.0005. [DOI] [PubMed] [Google Scholar]
- 108.Zhu F, Shan W, Lv R, Li Z, Wang Q. Clinical characteristics of anti-GABA-B receptor encephalitis. Front Neurol. 2020;11:403. doi: 10.3389/fneur.2020.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lancaster E, Lai M, Peng X, Hughes E, Constantinescu R, Raizer J, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2010;9(1):67–76. doi: 10.1016/S1474-4422(09)70324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guan HZ, Ren HT, Yang XZ, Lu Q, Peng B, Zhu YC, et al. Limbic encephalitis associated with anti-γ-aminobutyric acid B receptor antibodies: a case series from China. Chin Med J (Engl). 2015;128(22):3023–3028. doi: 10.4103/0366-6999.168989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Höftberger R, Titulaer MJ, Sabater L, Dome B, Rózsás A, Hegedus B, et al. Encephalitis and GABAB receptor antibodies: novel findings in a new case series of 20 patients. Neurology. 2013;81(17):1500–1506. doi: 10.1212/WNL.0b013e3182a9585f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Batocchi AP, Della Marca G, Mirabella M, Caggiula M, Frisullo G, Mennuni GF, et al. Relapsing-remitting autoimmune agrypnia. Ann Neurol. 2001;50(5):668–671. doi: 10.1002/ana.1261. [DOI] [PubMed] [Google Scholar]
- 113.Frisullo G, Della Marca G, Mirabella M, Caggiula M, Broccolini A, Rubino M, et al. A human anti-neuronal autoantibody against GABA B receptor induces experimental autoimmune agrypnia. Exp Neurol. 2007;204(2):808–818. doi: 10.1016/j.expneurol.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 114.Lancaster E, Martinez-Hernandez E, Titulaer MJ, Boulos M, Weaver S, Antoine JC, et al. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology. 2011;77(18):1698–1701. doi: 10.1212/WNL.0b013e3182364a44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Spatola M, Sabater L, Planagumà J, Martínez-Hernandez E, Armangué T, Prüss H, et al. Encephalitis with mGluR5 antibodies: symptoms and antibody effects. Neurology. 2018;90(22):e1964–e1972. doi: 10.1212/WNL.0000000000005614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Iorio R, Pittock SJ. Neuromyelitis optica and the evolving spectrum of autoimmune aquaporin-4 channelopathies. Clin Exp Neuroimmunol. 2014;5(2):175–187. [Google Scholar]
- 117.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6(9):805–815. doi: 10.1016/S1474-4422(07)70216-8. [DOI] [PubMed] [Google Scholar]
- 118.Jiao Y, Fryer JP, Lennon VA, Jenkins SM, Quek AM, Smith CY, et al. Updated estimate of AQP4-IgG serostatus and disability outcome in neuromyelitis optica. Neurology. 2013;81(14):1197–1204. doi: 10.1212/WNL.0b013e3182a6cb5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Carlander B, T V, A LF, N P, W C, Y D. Hypocretinergic dysfunction in neuromyelitis optica with coma-like episodes. BMJ case reports. 2009;2009. [DOI] [PMC free article] [PubMed]
- 120.Kanbayashi T, T S, I N, H Y, I Y, M N, et al. Symptomatic narcolepsy in patients with neuromyelitis optica and multiple sclerosis: new neurochemical and immunological implications. Arch Neurol. 2009;66(12). [DOI] [PubMed]
- 121.Baba T, Nakashima I, Kanbayashi T, Konno M, Takahashi T, Fujihara K, et al. Narcolepsy as an initial manifestation of neuromyelitis optica with anti-aquaporin-4 antibody. J Neurol. 2009;256(2):287–288. doi: 10.1007/s00415-009-0139-4. [DOI] [PubMed] [Google Scholar]
- 122.Suzuki K, Nakamura T, Hashimoto K, Miyamoto M, Komagamine T, Nagashima T, et al. Hypothermia, hypotension, hypersomnia, and obesity associated with hypothalamic lesions in a patient positive for the anti-aquaporin 4 antibody: a case report and literature review. Arch Neurol. 2012;69(10):1355–1359. doi: 10.1001/archneurol.2012.300. [DOI] [PubMed] [Google Scholar]
- 123.Deguchi K, Kono S, Deguchi S, Morimoto N, Ikeda M, Kurata T, et al. A patient with anti-aquaporin 4 antibody presenting hypersomnolence as the initial symptom and symmetrical hypothalamic lesions. J Neurol Sci. 2012;312(1–2):18–20. doi: 10.1016/j.jns.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 124.Pittock SJ, Weinshenker BG, Lucchinetti CF, Wingerchuk DM, Corboy JR, Lennon VA. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol. 2006;63(7):964–968. doi: 10.1001/archneur.63.7.964. [DOI] [PubMed] [Google Scholar]
- 125.El Otmani H, Z A, B EM, MA R. Hypersomnia as an isolated symptom of neuromyelitis optica. Intern Med J. 2018;48(12). [DOI] [PubMed]
- 126.Kallollimath P, Gujjar A, Patil M. Symptomatic Narcolepsy as a Presenting Feature of Neuromyelitis Optica. Ann Indian Acad Neurol. 2018;21(2). [DOI] [PMC free article] [PubMed]
- 127.Viegas S, Weir A, Esiri M, Kuker W, Waters P, Leite MI, et al. Symptomatic, radiological and pathological involvement of the hypothalamus in neuromyelitis optica. J Neurol Neurosurg Psychiatry. 2009;80(6):679–682. doi: 10.1136/jnnp.2008.157693. [DOI] [PubMed] [Google Scholar]
- 128.Sekiguchi T, Ishibashi S, Kubodera T, Fukabori J, Uezato A, Kanbayashi T, et al. Anhidrosis associated with hypothalamic lesions related to anti-aquaporin 4 autoantibody. J Neurol. 2011;258(12):2293–2295. doi: 10.1007/s00415-011-6112-z. [DOI] [PubMed] [Google Scholar]
- 129.Braley TJ, Chervin RD. A practical approach to the diagnosis and management of sleep disorders in patients with multiple sclerosis. Ther Adv Neurol Disord. 2015;8(6):294–310. doi: 10.1177/1756285615605698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bamer AM, Johnson KL, Amtmann D, Kraft GH. Prevalence of sleep problems in individuals with multiple sclerosis. Mult Scler. 2008;14(8):1127–1130. doi: 10.1177/1352458508092807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hughes AJ, Dunn KM, Chaffee T. Sleep disturbance and cognitive dysfunction in multiple sclerosis: a systematic review. Curr Neurol Neurosci Rep. 2018;18(1):2. doi: 10.1007/s11910-018-0809-7. [DOI] [PubMed] [Google Scholar]
- 132.Marrie RA, Reider N, Cohen J, Trojano M, Sorensen PS, Cutter G, et al. A systematic review of the incidence and prevalence of sleep disorders and seizure disorders in multiple sclerosis. Mult Scler. 2015;21(3):342–349. doi: 10.1177/1352458514564486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lanza G, Ferri R, Bella R, Ferini-Strambi L. The impact of drugs for multiple sclerosis on sleep. Mult Scler. 2017;23(1):5–13. doi: 10.1177/1352458516664034. [DOI] [PubMed] [Google Scholar]
- 134.Foschi M, Rizzo G, Liguori R, Avoni P, Mancinelli L, Lugaresi A, et al. Sleep-related disorders and their relationship with MRI findings in multiple sclerosis. Sleep Med. 2019;56:90–97. doi: 10.1016/j.sleep.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 135.Sakkas GK, Giannaki CD, Karatzaferi C, Manconi M. Sleep abnormalities in multiple sclerosis. Curr Treat Options Neurol. 2019;21(1):4. doi: 10.1007/s11940-019-0544-7. [DOI] [PubMed] [Google Scholar]
- 136.Manconi M, Ferini-Strambi L, Filippi M, Bonanni E, Iudice A, Murri L, et al. Multicenter case-control study on restless legs syndrome in multiple sclerosis: the REMS study. Sleep. 2008;31(7):944–952. [PMC free article] [PubMed] [Google Scholar]
- 137.Cederberg KLJ, Jeng B, Sasaki JE, Braley TJ, Walters AS, Motl RW. Restless legs syndrome and health-related quality of life in adults with multiple sclerosis. J Sleep Res. 2020;29(3):e12880. doi: 10.1111/jsr.12880. [DOI] [PubMed] [Google Scholar]
- 138.Strober LB. Fatigue in multiple sclerosis: a look at the role of poor sleep. Front Neurol. 2015;6:21. doi: 10.3389/fneur.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Khanna M, Rawat N, Gupta A, Nagappa M, Taly AB, Rukmani MR, et al. Pulmonary involvement in patients with Guillain-Barré syndrome in subacute phase. J Neurosci Rural Pract. 2017;8(3):412–416. doi: 10.4103/jnrp.jnrp_11_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Orlikowski D, Prigent H, Sharshar T, Lofaso F, Raphael JC. Respiratory dysfunction in Guillain-Barré syndrome. Neurocrit Care. 2004;1(4):415–422. doi: 10.1385/NCC:1:4:415. [DOI] [PubMed] [Google Scholar]
- 141.Karkare K, Sinha S, Taly AB, Rao S. Prevalence and profile of sleep disturbances in Guillain-Barre syndrome: a prospective questionnaire-based study during 10 days of hospitalization. Acta Neurol Scand. 2013;127(2):116–123. doi: 10.1111/j.1600-0404.2012.01688.x. [DOI] [PubMed] [Google Scholar]
- 142.Gao J, Li Y, Sun Y, Hu W, Liu Y, An D, et al. The study of sleep disorder factors in patients with Guillain-Barré syndrome. Int J Neurosci. 2016;126(10):893–898. doi: 10.3109/00207454.2015.1080699. [DOI] [PubMed] [Google Scholar]
- 143.Ranjani P, Khanna M, Gupta A, Nagappa M, Taly AB, Haldar P. Prevalence of fatigue in Guillain-Barre syndrome in neurological rehabilitation setting. Ann Indian Acad Neurol. 2014;17(3):331–335. doi: 10.4103/0972-2327.138521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cochen V, Arnulf I, Demeret S, Neulat ML, Gourlet V, Drouot X, et al. Vivid dreams, hallucinations, psychosis and REM sleep in Guillain-Barré syndrome. Brain. 2005;128(Pt 11):2535–2545. doi: 10.1093/brain/awh585. [DOI] [PubMed] [Google Scholar]
- 145.Tagami S, Susuki K, Takeda M, Koga M. Fulminant case of Guillain-Barré syndrome with poor recovery and depression following Haemophilus influenzae infection. Psychiatry Clin Neurosci. 2008;62(4):486. doi: 10.1111/j.1440-1819.2008.01834.x. [DOI] [PubMed] [Google Scholar]
- 146.Nishino S, Kanbayashi T, Fujiki N, Uchino M, Ripley B, Watanabe M, et al. CSF hypocretin levels in Guillain-Barré syndrome and other inflammatory neuropathies. Neurology. 2003;61(6):823–825. doi: 10.1212/01.wnl.0000081049.14098.50. [DOI] [PubMed] [Google Scholar]
- 147.Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9(2):293–308. doi: 10.1093/sleep/9.2.293. [DOI] [PubMed] [Google Scholar]
- 148.Guilleminault C, Mondini S. Mononucleosis and chronic daytime sleepiness. A long-term follow-up study. Arch Intern Med. 1986;146(7):1333–5. [PubMed]
- 149.Bahnasy WS, El-Heneedy YAE, El-Shamy AM, Badr MY, Amer RA, Ibrahim ISE. Sleep and psychiatric abnormalities in Gullian Barré syndrome. Egypt J Neurol Psychiatr Neurosurg. 2018;54(1):5. doi: 10.1186/s41983-018-0007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.McKeon A. Autoimmune Encephalopathies and Dementias. Continuum (Minneap Minn). 2016;22(2 Dementia):538–58. [DOI] [PubMed]
- 151.Solnes LB, Jones KM, Rowe SP, Pattanayak P, Nalluri A, Venkatesan A, et al. Diagnostic value of. J Nucl Med. 2017;58(8):1307–1313. doi: 10.2967/jnumed.116.184333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu X, Shan W, Zhao X, Ren J, Ren G, Chen C, et al. The Clinical Value of. Front Neurol. 2020;11:418. doi: 10.3389/fneur.2020.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schmitt SE, Pargeon K, Frechette ES, Hirsch LJ, Dalmau J, Friedman D. Extreme delta brush: a unique EEG pattern in adults with anti-NMDA receptor encephalitis. Neurology. 2012;79(11):1094–1100. doi: 10.1212/WNL.0b013e3182698cd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Navarro V, Kas A, Apartis E, Chami L, Rogemond V, Levy P, et al. Motor cortex and hippocampus are the two main cortical targets in LGI1-antibody encephalitis. Brain. 2016;139(Pt 4):1079–1093. doi: 10.1093/brain/aww012. [DOI] [PubMed] [Google Scholar]
- 155.Blinder T, Lewerenz J. Cerebrospinal fluid findings in patients with autoimmune encephalitis-a systematic analysis. Front Neurol. 2019;10:804. doi: 10.3389/fneur.2019.00804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gresa-Arribas N, Titulaer MJ, Torrents A, Aguilar E, McCracken L, Leypoldt F, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. 2014;13(2):167–177. doi: 10.1016/S1474-4422(13)70282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Budhram A, Mills JR, Shouman K, Dyck PJB, Hassan A, Zalewski NL. False-positive anti-neuronal nuclear antibody type 1 in a patient with RFC1 repeat expansion: Preventing "phenotype creep" in autoimmune neurology. J Neurol Sci. 2020;416:117018. doi: 10.1016/j.jns.2020.117018. [DOI] [PubMed] [Google Scholar]
- 158.Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391–404. doi: 10.1016/S1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Graus F, Delattre J, Antoine J, Dalmau J, Giometto B, Grisold W, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004;75(8):1135–1140. doi: 10.1136/jnnp.2003.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vedeler C, Antoine J, Giometto B, Graus F, Grisold W, Hart I, et al. Management of paraneoplastic neurological syndromes: report of an EFNS Task Force. Euro J Neurol. 2006;13(7):682–690. doi: 10.1111/j.1468-1331.2006.01266.x. [DOI] [PubMed] [Google Scholar]
- 161.Pittock SJ, Palace J. Paraneoplastic and idiopathic autoimmune neurologic disorders: approach to diagnosis and treatment. Handbook of clinical neurology. 133: Elsevier; 2016. p. 165–83. [DOI] [PubMed]
- 162.Titulaer MJ, Soffietti R, Dalmau J, Gilhus NE, Giometto B, Graus F, et al. Screening for tumours in paraneoplastic syndromes: report of an EFNS task force. Eur J Neurol. 2011;18(1):19–e3. doi: 10.1111/j.1468-1331.2010.03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tang WK, Hermann DM, Chen YK, Liang HJ, Liu XX, Chu WC, et al. Brainstem infarcts predict REM sleep behavior disorder in acute ischemic stroke. BMC Neurol. 2014;14:88. doi: 10.1186/1471-2377-14-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Geschwind MD, Tan KM, Lennon VA, Barajas RF, Haman A, Klein CJ, et al. Voltage-gated potassium channel autoimmunity mimicking Creutzfeldt-Jakob disease. Arch Neurol. 2008;65(10):1341–6. doi: 10.1001/archneur.65.10.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kang P, de Bruin GS, Wang LH, Ward BA, Ances BM, Lim MM, et al. Sleep Pathology in Creutzfeldt-Jakob Disease. J Clin Sleep Med. 2016;12(7):1033–9. doi: 10.5664/jcsm.5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rubinstein I, Gray TA, Moldofsky H, Hoffstein V. Neurosarcoidosis associated with hypersomnolence treated with corticosteroids and brain irradiation. Chest. 1988;94(1):205–6. doi: 10.1378/chest.94.1.205. [DOI] [PubMed] [Google Scholar]
- 167.Keime-Guibert F, Graus F, Broët P, Reñé R, Molinuevo JL, Ascaso C, et al. Clinical outcome of patients with anti-Hu-associated encephalomyelitis after treatment of the tumor. Neurology. 1999;53(8):1719–23. doi: 10.1212/wnl.53.8.1719. [DOI] [PubMed] [Google Scholar]
- 168.Dubey D, Britton J, McKeon A, Gadoth A, Zekeridou A, Lopez Chiriboga SA, et al. Randomized placebo-controlled trial of intravenous immunoglobulin in autoimmune LGI1/CASPR2 epilepsy. Ann Neurol. 2020;87(2):313–23. doi: 10.1002/ana.25655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Toledano M, Britton JW, McKeon A, Shin C, Lennon VA, Quek AM, et al. Utility of an immunotherapy trial in evaluating patients with presumed autoimmune epilepsy. Neurology. 2014;82(18):1578–86. doi: 10.1212/WNL.0000000000000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shirani A, Okuda DT, Stüve O. Therapeutic advances and future prospects in progressive forms of multiple sclerosis. Neurotherapeutics. 2016;13(1):58–69. doi: 10.1007/s13311-015-0409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kleiter I, Gold R. Present and future therapies in neuromyelitis optica spectrum disorders. Neurotherapeutics. 2016;13(1):70–83. doi: 10.1007/s13311-015-0400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Léger JM, Guimarães-Costa R, Muntean C. Immunotherapy in peripheral neuropathies. Neurotherapeutics. 2016;13(1):96–107. doi: 10.1007/s13311-015-0401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gastaldi M, Thouin A, Vincent A. Antibody-mediated autoimmune encephalopathies and immunotherapies. Neurotherapeutics. 2016;13(1):147–62. doi: 10.1007/s13311-015-0410-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Husari KS, Dubey D. Autoimmune epilepsy. Neurotherapeutics. 2019;16(3):685–702. doi: 10.1007/s13311-019-00750-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 430 KB)
(DOCX 432 KB)
(PDF 1.19 MB)
(PDF 1.19 MB)