Abstract
Clostridioides difficile infection (CDI) is the leading cause of nosocomial infectious diarrhea. Fecal microbiota transplantation (FMT) is a successful treatment for recurrent CDI (rCDI), and in some patients FMT has been associated with the resolution of recurrent urinary tract infections (rUTI). Recent evidence suggests that the origin of most bacterial infections in the urinary tract is the gut. Thus, the possibility of using FMT to displace pathogens commonly involved in rUTIs has major therapeutic implications. We report the case of a 93-year-old female patient with a rCDI and rUTI that underwent FMT and reported a complete clinical resolution of CDI; unexpectedly, no new symptomatic UTI episodes were diagnosed post-FMT. We characterized the gut microbiota of the stool donor and of the patient before and after the procedure. Our patient presented a dysbiosis with clear predominance of Enterobacteriaceae (74%) before FMT, which was significantly reduced to 0.07% after FMT. These findings were maintained for almost a year. We also observed an increase in microbial diversity indices compared with the pre-FMT sample reaching diversity values comparable to the donor stool samples. We reasoned that the disappearance of UTIs in our patient resulted from the reduction of Enterobacteriaceae in the gut microbiota. Our findings support previous evidence suggesting the potential of FMT for rUTI, particularly in cases due to multi-drug resistant pathogens where conventional antibiotic treatment is not an option.
Keywords: Clostridioides difficile infection, Fecal microbiota transplantation, Recurrent urinary tract infection
Key Summary Points
| Fecal microbiota transference (FMT) is a successful treatment for recurrent Clostridioides difficile infection (rCDI). In some patients FMT has been associated with the resolution of recurrent urinary tract infections (rUTI). |
| It has been demonstrated that a high predominance of Enterobacterales in the gut microbiota is associated with rUTIs. Using FMT to reduce this predominance could have a major therapeutic implication. |
| A 93-year-old female patient with rUTI and rCDI as a consequence of antibiotic treatment underwent FMT and reported a complete clinical resolution post-FMT. |
| The analysis of gut microbiota showed a marked dysbiosis with a clear predominance of Enterobacterales (74%) before FMT, which was significantly reduced to 0.07% 1 year after FMT. |
| The microbial diversity indices increased, reaching diversity values comparable to the donor stool samples. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.13118507.
Introduction
Clostridioides difficile infection (CDI) is a frequent cause of antibiotic-associated diarrhea. It is the leading cause of nosocomial infectious diarrhea with an incidence in Europe of 5 episodes for 10,000 days of hospitalization [1]. The number of community-acquired infections is also growing, representing up to 33% of all CDI cases [2]. The main risk factors of CDI are the use of antibiotics (especially fluoroquinolones, cephalosporin, ampicillin and amoxicillin) and age > 65 years [3], but also admission to health-care facilities, immunosuppression, chemotherapy, inflammatory bowel disease or proton pump inhibitors [1].
Fecal microbiota transplantation (FMT) restores the composition and functionality of the gut microbiota and is approved as a successful treatment for recurrent CDI [4]. Furthermore, it has been shown that FMT reduces the mortality rate in patients with severe CDI that fail to respond to conventional anti-clostridial antibiotic treatment [5, 6]. There are several mechanisms to explain the effectiveness of FMT, including direct competition of donor microbiota with C. difficile, restoration of secondary bile acid metabolism and repair of the gut barrier by stimulation of the mucosal immune system [5].
In some cases, FMT has been associated with the resolution of recurrent urinary tract infections (rUTI) in CDI patients who underwent FMT [7, 8]. rUTI in women is mostly associated with being a fecal carrier of specific serotypes of Escherichia coli expressing fimbriae that are highly adherent to the urothelium. This circumstance favors the colonization of the urethra and posterior invasion of the bladder, causing cystitis or even pyelonephritis [9]. Recent evidence suggests that the origin of the majority of bacterial infections in the urinary tract is the gut. Thus, the possibility to use FMT to displace pathogens commonly involved in rUTIs such as Proteobacteria (including E. coli) has major therapeutic implications [10].
Here we report the case of a patient who underwent FMT to treat a recurrent CDI (rCDI) and subsequently reported a complete clinical resolution of recurrent urinary infections. We studied the composition and diversity of the microbial communities of the stool donor and also the gut microbiota of the patient before and 1 year after the FMT.
Case
The patient was a 93-year-old female with a history of chronic infection by hepatitis C virus, end sigmoid colostomy for acute diverticulitis and rUTI (3 episodes) for 1 year preceding consultation caused by Escherichia coli and Pseudomonas aeruginosa; both were treated with ciprofloxacin at another institution. As a prophylaxis for rUTI, since the last episode the patient had received ciprofloxacin 250 mg daily for 3 months.
She was admitted to our hospital for severe diarrhea with positive toxin for C. difficile, which was treated successfully with oral (po) vancomycin 125 mg/6 h for 14 days and intravenous (iv) tigecycline 50 mg/12 h for 7 days. Four months later, the patient had a recurrent CDI with signs of severity (leukocytosis, high creatinine, hypotension, abdominal pain and metabolic acidosis), and she was treated with oral fidaxomicin 200 mg/12 h for 10 days and iv tigecycline 50 mg/12 h for 5 days. At the end of this regimen, fidaxomicin was maintained every 48 h, but diarrhea, fever and elevated white blood cell count recurred, and FMT was proposed and accepted by the patient and her relatives.
Potential stool donors were screened for potential infectious pathogens following the European recommendations [4]. Donor blood testing included complete blood count, blood chemistry tests, and serology for HIV, herpes simplex virus, Epstein-Barr virus, toxoplasma, HTLV, hepatitis B and C virus, and cytomegalovirus. Donor stools were examined to check for intestinal parasites, C. difficile toxin, bacterial culture and presence of multidrug-resistant microorganisms (including ESBLs and carbapenemases). A fresh donor stool was mixed with normal saline and homogenized using a Stomacher 400 Circulator for 1 min at 230 rpm. Then, the stool emulsion was filtered using a sterile strainer. The filtered stool emulsion was kept at 4 ºC until its use. Processed samples were directly infused into the gastrointestinal tract of the patient by colonoscopy.
For genomic analysis, fecal samples were collected from the two donors before FMT and from the patient at four time points: before FMT and 2, 16 and 299 days after the procedure. Samples were stored at – 80 ºC until processing.
DNA was extracted using the PureLinkTM Microbiome DNA Purification Kit (Invitrogen). The 16S rRNA gene V3-V4 region was amplified and sequenced on an Illumina MiSeq platform (2 × 300 bp) following the Illumina 16S Metagenomic Sequencing Library Preparation protocol. Diversity metrics, compositional and statistical analyses were performed using QIIME (QIIME 2 version 2020.2) and R version 3.4.4. Taxonomy was assigned using Silva version 132.
Samples with < 1000 sequence reads were removed. Singletons and features with a relative frequency < 0.01% were also removed. Finally, samples were rarefied to 4300 read sequencing depth for alpha diversity and compositional calculations.
From the genomic sequences, the microorganisms that shared > 97% sequence identity were grouped in operational taxonomic units (OTUs) obtaining the number of species in the sample or species richness [11]. For microbial diversity analysis, we used three different diversity indices. The Shannon index considers the number of species and their abundance in the sample and showed the heterogeneity of the microbial community [12]. Evenness was measured to observe differences in the relative abundance of the present species [11]. Finally, the Faith index or phylogenetic diversity evaluated the phylogenetic differences between the species in the sample and gave information on overall diversity [13].
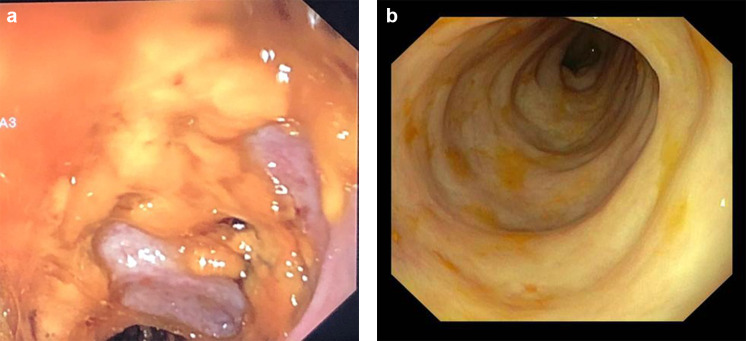
The patient received a FMT by colonoscopy through the colostomy using a solution of 350 ml corresponding to 35 g of homogenate feces as previously described. The donor for the FMT was a healthy relative. The mucosa presented diffuse typical pseudomembranes highly suggestive of CDI (Fig. 1a). No adverse events occurred during the procedure but, due to the absence of a sphincter at the end of the colostomy, most of the FMT solution was out of the gut within a few hours. Six days after FMT, because there was no resolution of diarrhea, she received a second FMT by gastroscopy from a different relative donor. The procedure was performed with 400 ml of solution corresponding to 50 g feces. Two weeks after the second FMT, a colonoscopy revealed resolution of pseudomembranes (Fig. 1b). No additional treatment for preventing CDI or UTI was added, and during the following year, the patient had no symptoms of CDI even though she received antibiotic treatment for acute respiratory tract infection once during this period. Unexpectedly, no new symptomatic UTI was diagnosed.
Fig. 1.
Images of gut mucosa from colonoscopy before FMT (a) and after FMT (b)
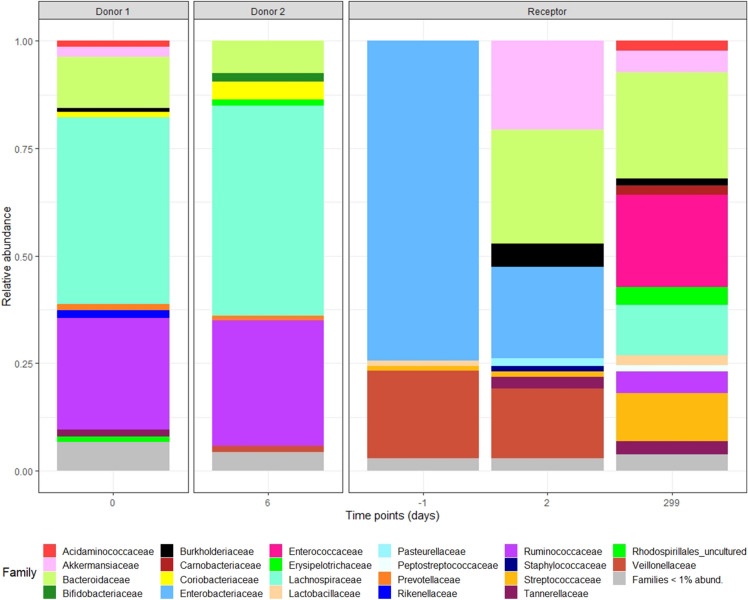
It has been previously reported that Bacteroidetes and Firmicutes are the predominant phyla in the the healthy intestinal microbiota [14]. In agreement with these data, the gut microbiota analyses from both donor samples presented very similar taxonomic compositions (Fig. 2) with a predominance of the Bacteroidaceae family (Bacteroidetes phylum) and Lachnospiraceae and Ruminoccoccaceae families (Firmicutes phylum). Before the FMT, the patient’s gut microbiota showed clear signs of dysbiosis with a marked predominance of Enterobacteriaceae (74.23% relative abundance) and more specifically of Klebsiella spp. The stool samples collected 2 and 299 days after the first FMT showed a major reduction of Enterobacteriaceae reaching a relative abundance of 0.07% (Fig. 2, Table 1) and, importantly, an increase in alpha diversity indices comparable to those of the donor stool samples (Table 1).
Fig. 2.
Relative abundances of bacterial taxonomical composition from donor samples and patient samples at different time points
Table 1.
Alpha diversity indices and Enterobacteriaceae relative abundance in the different samples
| Time (days) | Evenness | Faith index | Number of OTUs | Shannon diversity index | Enterobacteriaceae relative abundance (%) | |
|---|---|---|---|---|---|---|
| Donor1 | 0 | 0.933 | 15.20 | 279 | 7.58 | 0.49 |
| Donor2 | 6 | 0.931 | 11.31 | 148 | 6.71 | 0 |
| Receptor | −1 | 0.832 | 3.77 | 46 | 4.60 | 74.23 |
| 2 | 0.898 | 4.98 | 74 | 5.57 | 21.37 | |
| 299 | 0.893 | 8.26 | 146 | 6.42 | 0.07 |
OUT operational taxonomic unit [11]
Informed consent was obtained from the patient to be included in this case report.
Discussion
The efficacy of FMT for rCDI has been documented in clinical trials [15] and case series [16]. Our patient was elderly, fragile and exposed to antibiotic therapy because of rUTI and therefore at high risk of rCDI [3, 17] that would not respond to vancomycin nor fidaxomicin. Accordingly, she was a good candidate for FMT, and the response was in line with previous results. The reason for the good outcome could be related to the quality, in terms of diversity, of the transferred microbiota shown in Fig. 2 and Table 1 with a high number of OTUs, high values on the Faith and Shannon indices and low relative abundance of the Enterobacteriaceae family.
The main reason for rCDI in our patient was a history of antibiotic treatment due to rUTI, one of the most common causes of antibiotic consumption in the community [9]. Interestingly, our patient has had no more symptomatic UTIs since the FMT. The same experience was reported in two previous publications, including eight and one patients each [7, 8]. It has been documented in adult monkeys that the delivery of amoxicillin into the vagina significantly reduces the colonization resistance to E. coli by the intestinal microbiota, favoring its spread to the urethra and finally causing UTI [18]. This experimental study supported the concept that the source of uropathogenic bacteria is the gut microbiota, while predisposing factors include host characteristics (anatomical alterations, sexual behavior, disruption of the vaginal microbiota) and microbial pathogenicity (pathogenic islands) [19]. However, a recent article demonstrated that a 1% relative abundance of E. coli in the gut is an independent risk factor for UTI caused by this pathogen [10], suggesting that beyond the pathogenicity of a specific bacterial clone, the critical factor favoring rUTI is the relative abundance of this pathogen in the gut microbiota. Our patient had a gut predominance of Enterobacteriaceae (74%), which was significantly reduced to 0.07% after FMT, and this low proportion was maintained for almost a year. We consider it a reasonable interpretation that the disappearance of UTIs in our patient was the consequence of this relative reduction of Enterobacteriaceae in the gut microbiota. Our findings support the accumulating evidence for using FMT as a treatment for rUTI, particularly in cases due to multi-drug resistant pathogens where conventional antibiotic treatment is not an option.
The main limitation of our study is the low number of specimens collected after the FMT. Samples were collected 1 day before, 2 days and 299 days after the FMT. More frequent sampling would have been helpful to better understand the timing of the new microbiota acquisition and their fluctuation over time. On the other hand, the opportunity to evaluate long-term stability of the gut microbiota composition after FMT is of great interest.
Conclusions
FMT is one of the best alternatives to rCDI, and a few reports, with a low number of cases, suggest that it can be an alternative therapeutic option for rUTI, particularly when multidrug-resistant bacteria are responsible for recurrent infections. This case provides further evidence in favor of the potential benefit of FMT to treat rUTI. The potential benefit of FMT in rUTI should be further evaluated in well-designed randomized control trials.
Acknowledgements
We thank the participants of the study. We acknowledge the Infectious Disease, Microbiology and Gastroenterology Departments for their contributions.
Funding
This study has been funded by Instituto de Salud Carlos III through the project "PI/1601023" (Co-funded by European Regional Development Fund "Investing in your future"). No funding or sponsorship was received for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Alex Soriano has received grants from Pfizer and is part of the speaker's bureau of Pfizer, MSD, Angelini, Shionogy and Menarini. He has participated in advisory meetings for Pfizer, MSD, Menarini and Shionogy. Alex Soriano is also the Editor-in-Chief of this journal. Andrea Aira, Elisa Rubio, Andrea Vergara Gómez, Csaba Fehér, Climent Casals-Pascual, Begoña Gonzalez, Laura Morata and Verónica Rico have no disclosures.
Compliance with Ethics Guideline
Informed consent was obtained from the patient for being included in this case report.
References
- 1.Bouza E. Consequences of Clostridium difficile infection: Understanding the healthcare burden. Clin Microbiol Infect. 2012;18:5–12. doi: 10.1111/1469-0691.12064. [DOI] [PubMed] [Google Scholar]
- 2.Khanna S, Pardi DS, Aronson SL, Kammer PP, Orenstein R, St Sauver JL, et al. The epidemiology of community-acquired Clostridium difficile infection: a population-based study. Am J Gastroenterol. 2012;107:89–95. doi: 10.1038/ajg.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372:1539–1548. doi: 10.1056/NEJMra1403772. [DOI] [PubMed] [Google Scholar]
- 4.Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, Satokari R, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569–580. doi: 10.1136/gutjnl-2016-313017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khoruts A, Sadowsky MJ. Understanding the mechanisms of faecal microbiota transplantation. Nat Rev Gastroenterol Hepatol. 2016;13:508–516. doi: 10.1038/nrgastro.2016.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hocquart M, Lagier JC, Cassir N, Saidani N, Eldin C, Kerbaj J, et al. Early fecal microbiota transplantation improves survival in severe Clostridium difficile infections. Clin Infect Dis. 2018;66:645–650. doi: 10.1093/cid/cix762. [DOI] [PubMed] [Google Scholar]
- 7.Tariq R, Pardi DS, Tosh PK, Walker RC, Razonable RR, Khanna S. Fecal microbiota transplantation for recurrent Clostridium difficile infection reduces recurrent urinary tract infection frequency. Clin Infect Dis. 2017;65:1745–1747. doi: 10.1093/cid/cix618. [DOI] [PubMed] [Google Scholar]
- 8.Biehl LM, Cruz Aguilar R, Farowski F, Hahn W, Nowag A, Wisplinghoff H, et al. Fecal microbiota transplantation in a kidney transplant recipient with recurrent urinary tract infection. Infection. 2018;46:871–874. doi: 10.1007/s15010-018-1190-9. [DOI] [PubMed] [Google Scholar]
- 9.Glover M, Moreira CG, Sperandio V, Zimmern P. Recurrent urinary tract infections in healthy and nonpregnant women. Urol Sci. 2014;25:1–8. doi: 10.1016/j.urols.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magruder M, Sholi AN, Gong C, Zhang L, Edusei E, Huang J, et al. Gut uropathogen abundance is a risk factor for development of bacteriuria and urinary tract infection. Nat Commun. 2019;10:1–9. doi: 10.1038/s41467-019-13467-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young VB, Schmidt TM. GI microbiota and regulation of the immune system. New York: Springer; 2008. Overview of the gastrointestinal microbiota; pp. 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shannon CE. A Mathematical theory of communication. Bell Syst Tech J. 1948;27:379–423. doi: 10.1002/j.1538-7305.1948.tb01338.x. [DOI] [Google Scholar]
- 13.Faith DP. Conservation evaluation and phylogenetic diversity. Biol Conserv. 1992;61:1–10. doi: 10.1016/0006-3207(92)91201-3. [DOI] [Google Scholar]
- 14.Rodríguez JM, Murphy K, Stanton C, Ross RP, Kober OI, Juge N, et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol Heal Dis. 2015;26:1–17. doi: 10.3402/mehd.v26.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moayyedi P, Yuan Y, Baharith H, Ford AC. Faecal microbiota transplantation for Clostridium difficile associated diarrhoea: a systematic review of randomised controlled trials. Med J Aust. 2017;207:166–172. doi: 10.5694/mja17.00295. [DOI] [PubMed] [Google Scholar]
- 16.van Beurden YH, Nieuwdorp M, van de Berg PJEJ, Mulder CJJ, Goorhuis A. Current challenges in the treatment of severe Clostridium difficile infection: early treatment potential of fecal microbiota transplantation. Ther Adv Gastroenterol. 2017;10:373–381. doi: 10.1177/1756283X17690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson S. Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J Infect. 2009;58:403–410. doi: 10.1016/j.jinf.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Winberg J, Herthelius-Elman M, Möllby R, Nord CE. Pathogenesis of urinary tract infection -experimental studies of vaginal resistance to colonization. Pediatr Nephrol. 1993;7:509–514. doi: 10.1007/BF00852528. [DOI] [PubMed] [Google Scholar]
- 19.Marrs CF, Zhang L, Foxman B. Escherichia coli mediated urinary tract infections: are there distinct uropathogenic E. coli (UPEC) pathotypes? FEMS Microbiol Lett. 2005;252:183–190. doi: 10.1016/j.femsle.2005.08.028. [DOI] [PubMed] [Google Scholar]