Abstract
OBJECTIVE.
Imaging plays a critical role in the assessment of patients with femoroacetabular impingement (FAI). With better understanding of the underlying pathomechanics and advances in joint-preserving surgery, there is an increasing need to define the most appropriate imaging workup. The purpose of this article is to provide guidance on best practices for imaging of patients with FAI in light of recent advances in corrective FAI surgery.
CONCLUSION.
Pelvic radiography with dedicated hip projections is the basis of the diagnostic workup of patients with suspected FAI to assess arthritic changes and acetabular coverage and to screen for cam deformities. Chondrolabral lesions should be evaluated with unenhanced MRI or MR arthrography. The protocol should include a large-FOV fluid-sensitive sequence to exclude conditions that can mimic or coexist with FAI, radial imaging to accurately determine the presence of a cam deformity, and imaging of the distal femoral condyles for measurement of femoral torsion. CT remains a valuable tool for planning of complex surgical corrections. Advanced imaging, such as 3D simulation, biochemical MRI, and MR arthrography with application of leg traction, has great potential to improve surgical decision-making. Further research is needed to assess the added clinical value of these techniques.
Keywords: femoroacetabular impingement, imaging workup, MR arthrography, MRI, radiography
Clinical Vignettes and Images
Imaging plays a critical role in the assessment of patients with suspected femoroacetabular impingement (FAI). It provides objective information to the orthopedic surgeon and can help to differentiate FAI from confounding intraarticular or extraarticular abnormalities. As a result, during initial workup and preoperative planning, it is critical to choose the correct imaging studies to determine extent of disease and to identify candidates for joint-preserving surgery. This article summarizes best practices of imaging for FAI. The clinical vignettes presented in Figures 1–6 serve as examples of imaging workups and pitfalls in the care of patients with FAI.
Fig. 1—
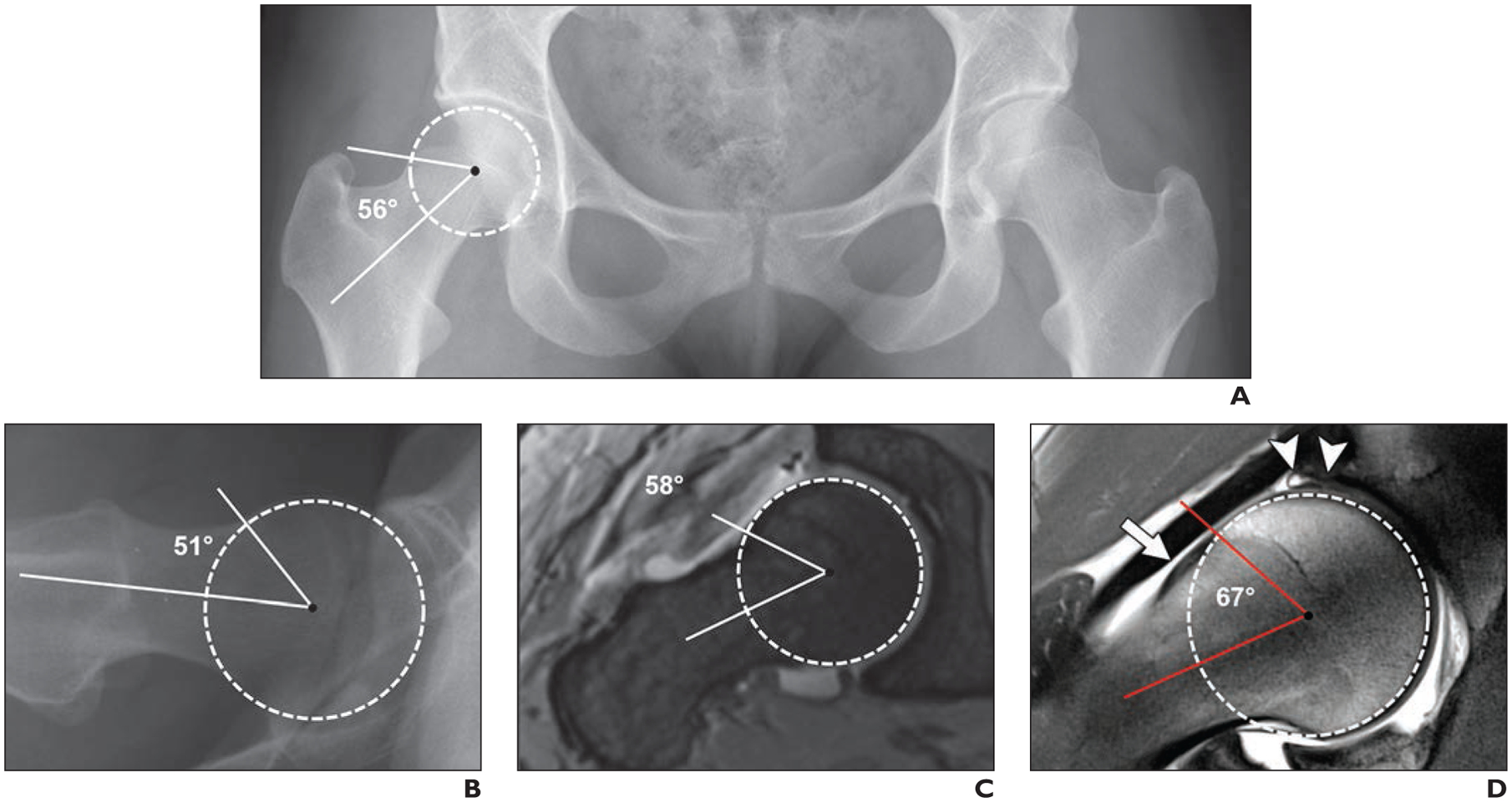
28-year-old woman with 2-year history of right-sided groin pain and positive anterior impingement test (pain with hip flexion, adduction, and internal rotation). Case emphasizes importance of appropriate imaging workup of patients with suspected femoroacetabular impingement to arrive at correct diagnosis. Alpha angles are measured between femoral neck axis (bottom line) and line that connects proximal part of asphericity (top line), determined by best-fitting circle (circle), with femoral rotation center.
A, Anteroposterior radiograph of pelvis shows normal pelvic morphology, preserved hip joint space, and no arthritic change.
B, Cross-table lateral radiograph of symptomatic right hip shows no obvious deformity. Patient was referred for MR arthrography to evaluate morphology of femoral head-neck junction.
C, Oblique axial MR arthrogram shows normal femoral head-neck junction.
D, Radial proton density–weighted MR image shows mild cam deformity (arrow) with alpha angle of 67°. Intrasubstance tear of superior labrum with adjacent cartilage damage (arrowheads) is evident. Patient was referred for hip arthroscopy for cam resection and labral repair. Cam deformities are frequently located in anterolateral aspect of femoral head-neck junction and therefore are not visible on standard anteroposterior and cross-table lateral radiographs.
Fig. 6—
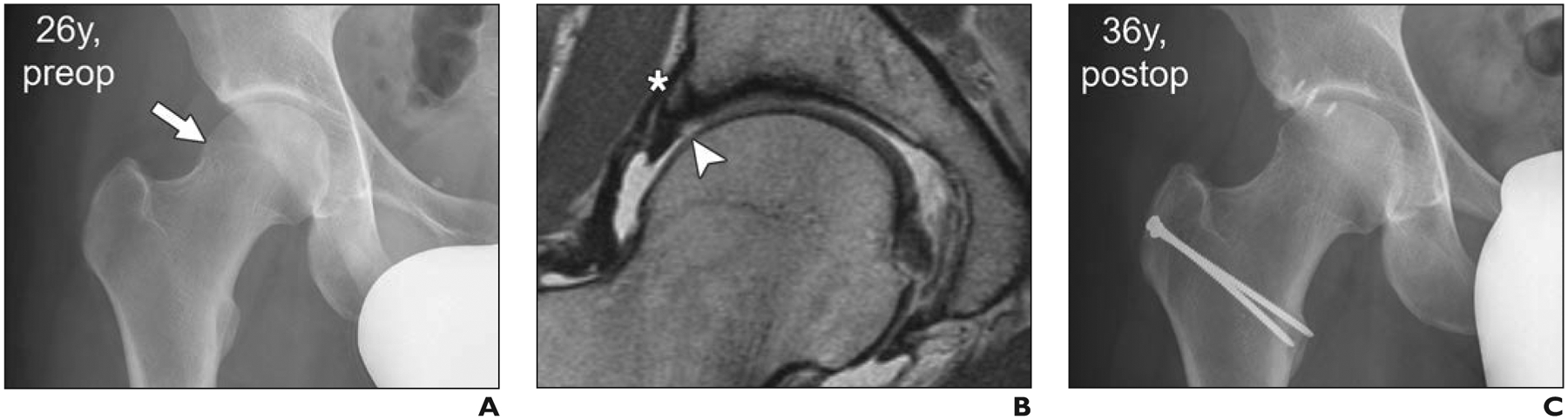
26-year-old man with acute onset of groin pain. Case supports role of femoroacetabular impingement surgery in appropriate clinical setting and absence of joint degeneration and cartilage loss.
A, Anteroposterior radiograph of pelvis shows preserved joint space and cam deformity (arrow).
B, Midcoronal proton density–weighted MR image shows focal superior cartilage defect (arrowhead) and perilabral ossification (asterisk). Overall, no extensive joint degeneration was detected.
C, Ten years after surgical hip dislocation, cam resection, and acetabular rim trimming with labral refixation, patient presented with tenderness over greater trochanter but no clinical signs of impingement. Radiograph shows no evidence of progression of osteoarthritis.
The Imaging Question
What is the appropriate imaging workup for patients who present with hip or groin pain and suspected FAI?
Background and Importance
According to the Warwick agreement, an international expert consensus initiative, FAI syndrome (FAIS) is defined as a triad of symptoms, clinical signs of impingement, and corresponding imaging findings [1]. Since the first description of FAI more than 15 years ago [2], an increasing number of studies have shown that open and arthroscopic FAI surgery has decreased pain, restored hip function, and prevented progression of osteoarthritis in as many as 80% (95% CI, 72–88%) of patients [3] in 5–10 years of follow-up [3–6]. Imaging has played a vital part in understanding and establishing FAIS as a clinical entity [7]. Despite that, physicians and radiologists face numerous challenges when it comes to improving surgical decision-making based on the preoperative diagnostic workup [8]. To date, the natural course of FAI remains the subject of ongoing research. It is commonly accepted that not all patients with FAI deformities will experience end-stage osteoarthritis. However, in a systematic review of longitudinal and cross-sectional cohort studies that included over 6000 patients participating in 5–20 years of follow-up [9], cam FAI was consistently associated with the risk of development of osteoarthritis. However, multiple studies [10–12] have shown imaging findings of FAI in individuals without symptoms; therefore, clinical decision-making should be based on the patient history and physical examination findings in conjunction with imaging findings.
Originally, two pathomorphologies, cam impingement, which refers to a decreased femoral head-neck offset, and pincer impingement, which refers to acetabular overcoverage or retroversion, were linked to FAIS, early chondrolabral damage, and osteoarthritis of the hip [2, 13, 14]. However, mounting evidence suggests that abnormal femoral torsion considerably affects impingement-free range of motion and can compensate for or aggravate the impingement conflict [15–18]. In brief, increased femoral torsion increases the passive range of internal rotation and decreases the passive range of external rotation, and vice versa for decreased femoral torsion [17–20]. Therefore, femoral torsion is currently considered the third pillar of FAIS. Given the increasing evidence that FAIS results from the complex interplay between alignment and morphology of the proximal femur and the acetabulum, including abnormalities in coverage and version, there is a need for thorough assessment of individual impingement morphologies [21].
In light of the growing number of hip arthroscopies performed for FAI correction worldwide, there is an increasing need to better define which patients can benefit from a joint-preserving procedure. Radiography and MRI play key roles in the initial assessment of patients with FAI or suspected FAI and in identifying patients who are candidates for joint-preserving surgery. MRI is the cornerstone in the diagnostic workup of FAIS because it can be used to assess intraarticular damage, including damage to the labrum and cartilage, the main predictors of outcome [7, 8].
The aim of this article is to review the literature on current best practices in diagnostic imaging for FAI and to provide an outlook on emerging imaging techniques for the assessment of FAI and degenerative hip disease.
Synopsis and Synthesis of Evidence
We performed a systematic literature review using PubMed for English-language articles. No beginning date limit was used, and the search was last updated in December 2019. The search terms were “femoroacetabular impingement” AND “imaging.” A total of 1215 records, including 31 clinical trials and 187 review articles, were identified. From this literature search, 64 original articles that focused on imaging of FAI and 30 articles that focused on surgical techniques for and outcome from FAI were selected for detailed review and inclusion in this article. Among the studies on diagnostic imaging of FAI, whenever possible we selected those in which subsequent surgical procedures and intraoperative evaluation were documented or in which a clinical endpoint was used as the outcome parameter. Among the clinical studies, we selected the ones with the longest clinical follow-up. We summarized the relevant literature on the role of imaging in the workup and surveillance of patients with FAI to determine best practices.
Evidence-Based Guidelines
Radiography of the Acetabulum
Evidence—
Conventional radiographs of the hip acquired in two planes form the basis of the diagnostic workup of patients with FAI or suspected FAI (Fig. 1). They provide an excellent overview of the pelvic anatomy and enable assessment of arthritic changes in the hip [22]. This is crucial because patients with mild or moderate joint space narrowing benefit less from corrective FAI surgery. Performing joint-preserving surgery is not a reasonable option at most institutions if the patient has moderate joint space narrowing consistent with Tönnis grade 2 [3–5]. Acetabular coverage and version are assessed on anteroposterior pelvic radiographs with established reference values [22, 23]. A standardized acquisition technique is of utmost importance because the anatomy of the acetabulum is strongly affected by many factors, including patient positioning and centering and positioning of the central beam [22]. For assessment of FAI, anteroposterior pelvic radiographs are typically acquired with the patient supine with a film-focus distance of 1.2 m and the central beam directed at the midpoint between a line that connects the anterior-superior iliac spine with the symphysis pubis [22]. Radiologists must be aware of the underlying acetabular morphology to identify FAI-related abnormalities on cross-sectional imaging.
In young patients with hip pain, evaluation of acetabular coverage should be performed with the lateral center-edge angle and the acetabular index [23]. Developmental dysplasia of the hip (DDH) is the most important intraarticular differential diagnosis of FAI and may be present in as many as 17% (78/463) of patients presenting with hip pain [16]. Correctly identifying DDH is critical. Periacetabular osteotomy to correct deficient acetabular coverage is the surgical treatment of choice for DDH because it prevents osteoarthritis and total hip arthroplasty and restores hip function in 61% (95% CI, 49–72%) of patients after 20 years and in 29% (95% CI, 17–42%) of patients after 30 years [24]. In mildly dysplastic hips (lateral center-edge angle, 20–25°), arthroscopic labral and capsular repair with correction of an associated cam deformity is controversially discussed as a less invasive alternative to periacetabular osteotomy [4, 25]. However, it is commonly accepted that trimming of the acetabulum should not be performed for DDH because it further decreases hip stability with detrimental effects on the joint [4, 25] (Fig. 2).
Fig. 2—
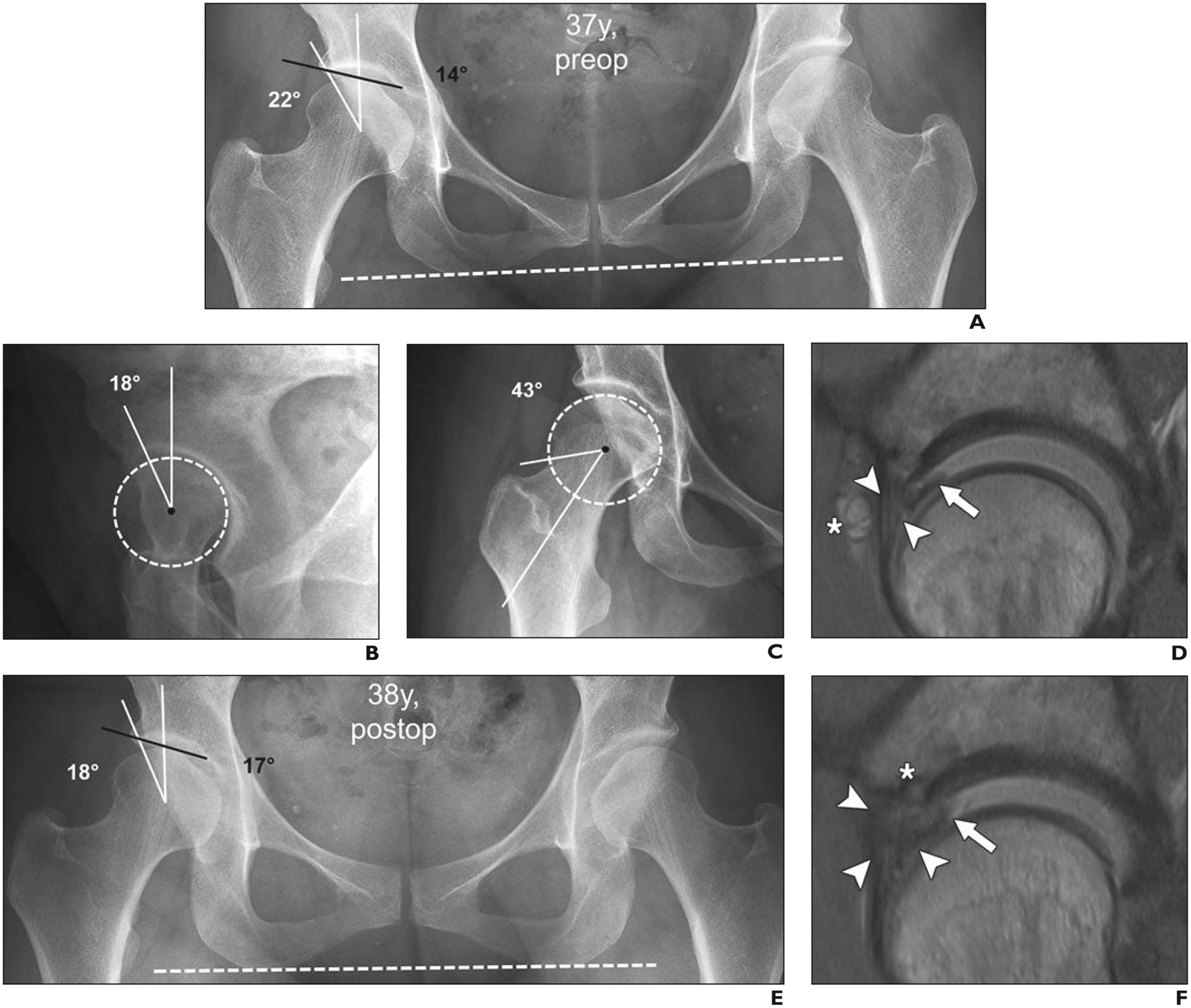
37-year-old woman with right-sided groin pain with prolonged standing and provocative pain with flexion and internal rotation during clinical examination (positive anterior impingement test). This case of incorrect management of developmental dysplasia of hip (DDH) as femoroacetabular impingement (FAI) underlines importance of correct identification of DDH before FAI surgery.
A, Anteroposterior radiograph of pelvis shows preserved joint space and DDH on right (lateral center-edge angle, 22° [white lines]; acetabular index, 14° [black line]). Dashed line indicates anatomic horizontal reference axis.
B, False-profile-view radiograph shows deficient anterior coverage with anterior center-edge angle of 18°. Dashed circle indicates best-fitting circle of femoral head to define femoral rotation center.
C, Forty-five-degree Dunn-view radiograph shows no cam deformity (alpha angle, 43°) at anterosuperior femoral head-neck junction. Dashed circle indicates best-fitting circle of femoral head to define femoral rotation center.
D, Sagittal proton density–weighted image from direct MR arthrogram obtained with application of leg traction shows hypertrophic anterosuperior labrum (arrowheads) with mucoid degeneration and associated paralabral cyst (asterisk) and flap tear (arrow) at chondrolabral junction. Overall, these findings are indicative of hip instability rather than impingement conflict. Patient underwent arthroscopic labral repair with trimming of acetabular rim and reattachment of labrum.
E, Anteroposterior radiograph of pelvis 1 year after arthroscopic labral repair with trimming of acetabular rim and reattachment of labrum shows iatrogenic progression of dysplasia of right hip after acetabuloplasty (lateral center-edge angle, 18° [white lines]; acetabular index, 17° [black line]). Dashed line indicates anatomic horizontal reference axis.
F, Sagittal proton density–weighted MR arthrogram obtained with application of leg traction shows massive hypertrophy of labrum with diffuse contrast interposition, consistent with retear (arrowheads) and unstable chondrolabral flap (arrow). Anchor from labrum refixation (asterisk) is evident.
Although DDH most commonly affects the anterolateral aspect of the acetabulum, it can predominantly affect the anterior or posterior wall [26]. Thus, anterior and posterior coverage should be assessed separately in cases of suspected DDH (Fig. 2). At many institutions, extension of the anterior sclerotic rim relative to a vertical reference line is routinely measured on a false-profile-view radiograph to screen for anterior wall deficiency with the anterior center-edge angle [27]. Angles less than 20° are used to define DDH, and those less than 25° define borderline DDH [27]. The anterior and posterior wall indexes have been introduced to assess anterior and posterior coverage on an anteroposterior pelvic radiograph [26].
Guidance—
Anteroposterior radiographs of the pelvis should be used to assess the presence of pincer deformities, such as acetabular overcoverage, defined as a lateral center-edge angle larger than 39°, or protrusio acetabuli (i.e., femoral head contour touches or overlaps with the ilioischial line), which reflects the rarest (reported prevalence, 3% [32/1206]) and most severe form of acetabular overcoverage [22, 23, 28]. This is important because hips with excessive acetabular coverage reportedly have worse outcome (10-year survivorship, 51% [95% CI, 34–67%]) after FAI surgery compared with hips with normal coverage (10-year survivorship, 83% [95% CI, 75–91%]) [28]. By contrast, the presence of a coxa profunda sign (floor of the acetabular fossa touches or overlaps the ilioischial line) reportedly [29] does not reflect acetabular overcoverage because it was present in only 22% (19/86) of hips with a lateral center-edge angle greater than 40° and in 41% (24/58) of hips with hip dysplasia. Thus, hips with a coxa profunda sign should not be referred to as having pincer deformity [29].
Diagnosis of acetabular retroversion is based on the presence of radiographic retroversion signs. A normal acetabular opening plane is directed anteriorly. This is referred to as acetabular anteversion and corresponds to a more lateral projection of the posterior acetabular wall relative to the anterior wall. In acetabular retroversion, the anterior wall projects laterally to the posterior wall cranially and crosses the posterior wall as it approaches the inferior acetabulum. This characteristic appearance has been described as the crossover sign and is present mostly at the superior aspect of the acetabulum, where it should be sought [30].
With increasing craniocaudal extension of the crossover sign, the posterior wall sign (medial projection of the posterior wall relative to femoral head rotation center) and the ischial spine sign (ischial spine projecting into the pelvic inlet) indicate severe acetabular retroversion and can be found in 14% (65/463) of patients with hip pain [31]. In contrast to the lateral center-edge angle and the acetabular index, these signs are subject to pelvic malpositioning and pelvic tilt [31]. Recognition of these signs is important because increasing evidence suggests that severe acetabular retroversion reflects malrotation of the hemipelvis rather than actual increased coverage with an increased lunate surface [32, 33]. This provides the rationale for acetabular reorientation instead of acetabular rim trimming [32, 33]. Anteverting periacetabular osteotomy can be performed to correct acetabular retroversion with durable mid- to long-term results [34, 35] that are reportedly [36] superior to those of acetabular rim trimming (10-year survivorship, 79% [95% CI, 68–90%] vs 23% [95% CI, 6–40%]).
Radiography of the Proximal Femur
Evidence—
Diagnosis of femoral abnormalities requires biplanar radiographs to screen for the presence of a cam deformity. Although anteroposterior pelvic views best depict superiorly located cam deformities (i.e., pistol-grip deformities), lateral views such as the cross-table lateral view, frogleg lateral view, and the 45°/90° Dunn view are used to visualize the anterior to anterosuperior extension of the cam lesion. The cross-table lateral view (3-o’clock position) and frogleg lateral view (2-o’clock position) show the more anterior aspect of the femoral neck [37, 38]. Because cam deformity is typically most prominent anterosuperiorly, it is profiled most accurately in the 45° Dunn view, which is acquired in 45° of hip flexion, 20° abduction, and neutral rotation.
On the basis of reported sensitivity of 71% (21/30) to 96% (27/28) and specificity of 36% (4/11) to 90% (9/10), the 45° Dunn view is widely considered the most appropriate view for primary screening for cam deformity [37–41] (Fig. 2C). However, even a combination of different radiographic views cannot definitely exclude the presence of cam deformity [37–41], because as many as 35% (19/55) of cam deformities can reportedly be missed with radiographic assessment alone [42]. Thus, most authors recommend acquisition of radial images, either MRI or CT, for all patients eligible for joint-preserving hip surgery [38–42].
Guidance—
The workup of patients with suspected FAI should start with an anteroposterior radiograph of the pelvis and a dedicated lateral radiograph of the hip. Radiographs should be assessed for the presence of osteoarthritis, acetabular coverage, acetabular version, and cam deformity.
MRI and CT to Assess Abnormal Hip Morphology
Evidence—
The aspherical portion of the proximal femur is typically located anterosuperiorly, which is why assessment on biplanar radiographs alone cannot be used to rule out the presence of a cam deformity [38, 42]. Although oblique axial MR images were initially used to describe the alpha angle, the extent of cam deformity is underestimated on these images, which are aligned with the femoral neck, because they show only the anterior position of the proximal femur [38, 43, 44] (Fig. 1C). Assessment of the femoral head-neck junction on oblique axial images reportedly results in 17° underestimation of maximal alpha angles, and as many as 54% (22/41) of cam deformities are missed compared with the rate of detection on radial images [44]. By contrast, radial images oriented perpendicular to the femoral neck axis can be used for circumferential orthogonal assessment of the femoral head-neck junction, and this imaging plane is considered the most accurate for assessment of the extent of a cam deformity [44–46] (Fig. 1D).
Anatomic landmarks, such as the most prominent appearance of the greater trochanter (femoral 12-o’clock position) and the acetabular teardrop (acetabular 12-o’clock position) can be used for accurate topographic allocation of the cam deformity on radial images. The number of slices used determines the clockface interval between two consecutive radial images in 30-minute intervals when 12 radial slices are used [45]. This is important for surgical planning because some lesions, such as posterosuperior cam deformities, which are close to the retinacular vessels that maintain the blood supply to the femoral head, are difficult to correct arthroscopically [47].
Radial images can be reformatted with an isovoxel 3D sequence or can be acquired directly as 2D images. Most commonly, the alpha angle is used to quantify the extent of the cam deformity. A threshold of 60° has been introduced for an imaging diagnosis because it is associated with progression of osteoarthritis within 2–5 years in patients with early symptoms [46, 48]. However, because the alpha angle reflects only the proximal extension of the osseous deformity, it does not necessarily reflect the configuration of the proximal femur, as in hips with an overall thick or short femoral neck [22, 49]. More distally located cam deformities have been described more recently as a secondary sign of extraarticular subspinal impingement in 80% (16/20) of patients without classic signs of subspinal impingement, which includes a too prominent or downsloping anterior-inferior iliac spine [50, 51]. Such deformities would go unnoticed if one were to rely solely on measurement of the alpha angle. For clinical routine, morphologic and topographic assessment of osseous deformities of the femoral head and neck should be performed. Alpha angles are useful for identifying where the deformity is most pronounced [21].
Because of its wide availability, low cost, and high level of detail in assessing cortical and cancellous bone, CT remains an important modality in the diagnostic workup of FAI. CT is still considered the imaging reference standard for measurement of femoral torsion. Furthermore, CT-based 3D models of the pelvis are important for preoperative planning of complex osteotomies and can be used for surgical navigation [52]. Although MRI is the modality of choice for assessment of chondrolabral lesions, CT arthrography is a viable alternative in patients with claustrophobia or surgical implants or at centers with limited MRI capacity [53]. Because patients eligible for FAI surgery are typically young, CT protocols should be set at 100 kV and 100 mAs to reduce the radiation dose to approximately 1 mSv [54]. In FAI, there is typically an overlap in deformities involving the proximal femur and acetabulum [16]. Furthermore, clinical presentation is often nonspecific, and it may be difficult to differentiate between intraarticular and extraarticular hip impingement [51].
Because standard imaging techniques are static [55], there has been interest in CT-based virtual impingement simulation by use of validated collision detection software with algorithms to adjust the femoral head rotation center and detect the acetabular rim for surgical planning in patients with complex FAI deformities [19, 20, 56, 57] (Fig. 4D). To date, the application of virtual impingement simulation software to MRI has been limited because 3D volume rendering of the pelvis is usually performed with CT.
Fig. 4—
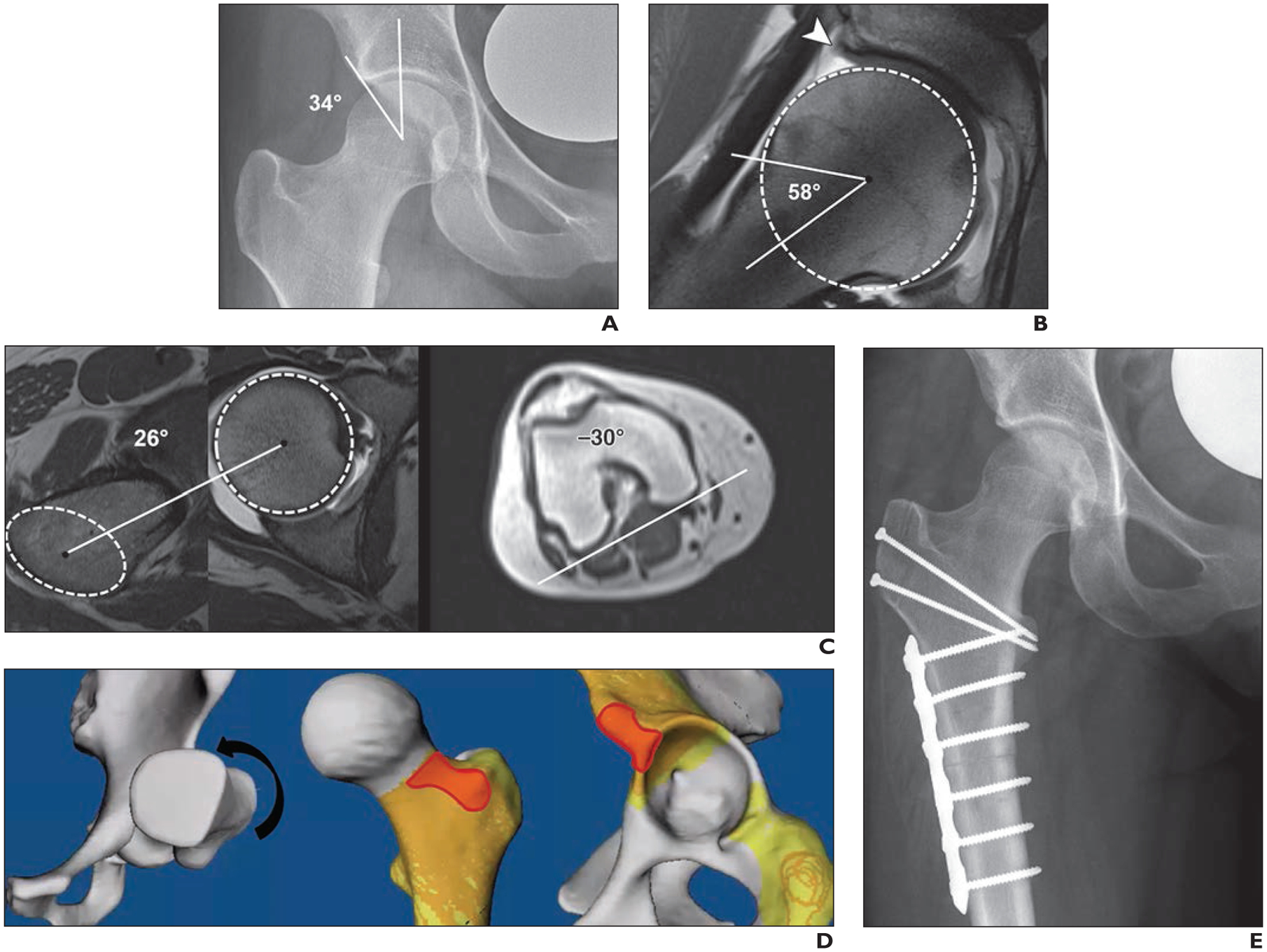
23-year-old male semiprofessional ice hockey player with right-sided hip pain and restricted range of motion. Case illustrates abnormal femoral torsion as cause of hip pain and emphasizes assessment of femoral torsion in workup of patients with femoroacetabular impingement.
A, Anteroposterior radiograph of hip shows normal acetabular coverage (lateral center-edge angle of 34°) and normal femoral head-neck junction.
B, Radial proton density–weighted MR arthrogram shows normal femoral head-neck junction (alpha angle of 58°) but degenerative fraying of labrum (arrowhead) and thinning at chondrolabral junction. Dashed circle indicates best-fitting circle of femoral head to define femoral rotation center.
C, Measurement of femoral torsion according to Tomczak et al. [91] was performed. On axial MR images of hip (left, center), line connecting femoral head center, as determined with best-fitting circle (circle), with center of greater trochanter at base of femoral neck (ellipse) serves as proximal reference axis. On axial MR image of knee (right), line connecting posterior contours of distal femoral condyles serves as distal reference axis. Resulting calculation shows femoral retrotorsion of −4° (26° – 30° = −4°).
D, Three-dimensional reformation from CT of osseous pelvis with virtual impingement simulation (flexion, 90°; adduction, 20°; internal rotation, 30° [arrow]) shows intraarticular and extraarticular anterior hip impingement (red).
E, Patient underwent open surgical dislocation without trochanteric advancement; intraarticular abutment was confirmed intraoperatively, and 20° subtrochanteric derotational osteotomy was performed to increase femoral torsion and to restore normal range of motion. Anteroposterior radiograph of hip shows postsurgical change from subtrochanteric derotational osteotomy.
Preliminary reports, however, have shown that 3D segmentation of the hip can be performed with high accuracy with fast MRI sequences of the hip and pelvis [58].
Guidance—
MRI assessment of hip morphology should include small-FOV imaging of the hip that includes oblique axial and radial imaging for assessment of cam deformities. CT remains a valuable tool for planning of complex surgical corrections.
MRI to Assess Intraarticular Hip Pathology
Evidence—
For imaging of intraarticular lesions of the hip, a tailored multiplanar protocol with a small FOV (< 20 cm) and large matrix (minimum, 256 × 256) is required to ensure adequate in-plane resolution [59]. Because of the obliquity of the hip, a combination of coronal, sagittal, and oblique axial images is needed to achieve a comprehensive overview [60]. Whereas the anterior and posterior aspects of the joint are best visualized on sagittal and oblique axial images (Fig. 3B), midcoronal images best depict the superior to posterosuperior acetabular rim and the acetabular fossa, including the fovea capitis, where tears of the ligamentum teres and damage to the adjacent femoral cartilage typically occur [21]. Although radial images with sufficient resolution to depict cam deformities and chondrolabral lesions are frequently obtained, their diagnostic benefit compared with a multiplanar standard protocol has not been shown to date [61–63]. Owing to the anatomic characteristics of the hip, fast isotropic 3D sequences have been widely included because they minimize partial volume effects and enable further image reformatting with diagnostic performance comparable to that of standard 2D imaging [64–66]. However, current T1-weighted and proton density–weighted 2D turbo and fast spin-echo sequences remain the workhorse for evaluation of intraarticular lesions.
Fig. 3—
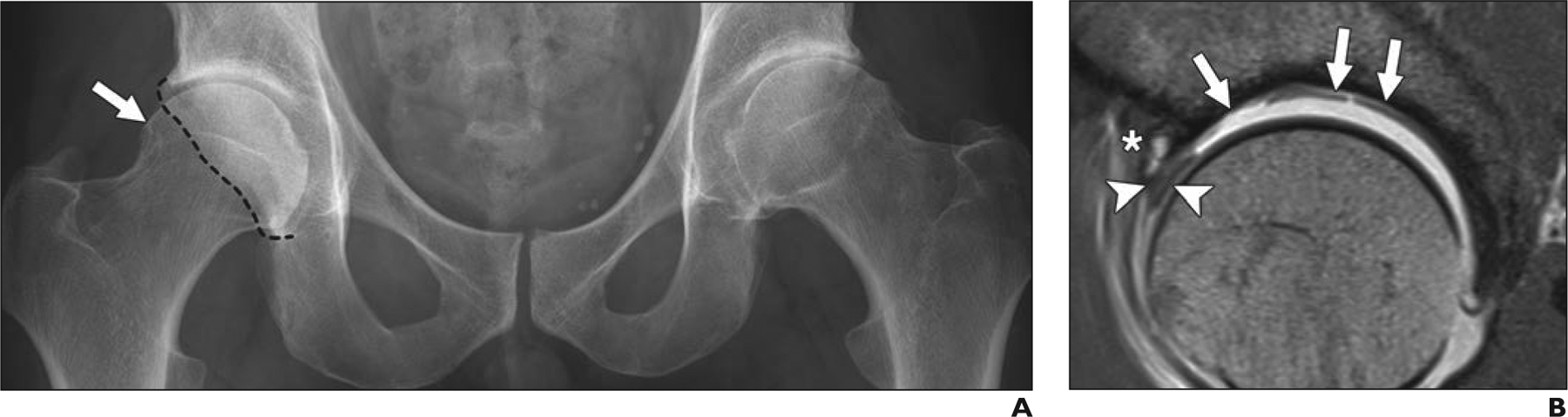
36-year-old man with gradually increasing bilateral groin pain over 2 years. Case shows value of MRI in surgical decision-making for patients with early signs of osteoarthritis on radiographs.
A, Anteroposterior radiograph of pelvis shows advanced arthritis with moderate joint space narrowing on left. On right side, beginning joint space narrowing and mixed-type femoroacetabular impingement (FAI) with cam deformity (arrow) and prominent posterior wall (dashed line) indicating focal posterior overcoverage are evident.
B, Sagittal proton density–weighted image from direct MR arthrogram obtained with application of leg traction of right hip to assess extent of intraarticular damage shows intrasubstance tearing of anterosuperior labrum (arrowheads). Excessive cartilage delamination and full-thickness cartilage loss (arrows) and subchondral cyst (asterisk) at acetabular rim are evident. These MRI findings are strong predictors of poor outcome after FAI surgery. Consequently, joint-preserving procedure on hip was not considered reasonable. Patient was scheduled for total hip arthroplasty of left hip and symptomatic treatment, including activity modification and physical therapy for right hip.
Direct MR arthrography (MRA) at 3 T is commonly considered the diagnostic reference standard for detection of chondrolabral lesions [67, 68], lesions of the ligamentum teres [69], and intraarticular loose bodies [70]. Encouraging results have been reported for unenhanced 3-T MRI in the detection of labral and cartilage damage [62, 63, 65]. Higher diagnostic performance has been found for direct MRA than for unenhanced MRI of the hip at 1.5 T and 3 T [71–73].
Sutter et al. [72] reported that direct MRA in the detection of labral lesions had sensitivity of 85% (22/26); specificity, 100% (2/2); PPV, 100% (22/22); and NPV, 33% (2/6). Direct MRA for cartilage lesions had sensitivity of 71% (17/24); specificity, 100% (4/4); PPV, 100% (17/17); and NPV, 36% (4/11). The corresponding values for unenhanced 1.5-T MRI for labral lesions were sensitivity, 77% (20/26); specificity, 50% (1/2); PPV, 95% (20/21); and NPV, 14% (1/7). For cartilage lesions, the values were sensitivity, 58% (14/24); specificity, 100% (4/4); PPV, 100% (14/14); and NPV, 29% (4/14). Results of more recent studies [74, 75], however, suggest that the accuracy of unenhanced MRI at 3 T is comparable to that of MRA at 1.5 T for detection of chondrolabral lesions.
Crespo-Rodriguez and colleagues [75] reported that direct MRA for detection of labral lesions at 1.5 T had sensitivity of 86% (31/36); specificity, 50% (7/14); PPV, 82% (31/38); and NPV, 58% (7/12). The corresponding values for detection of lesions at the chondrolabral transition zone were sensitivity, 100% (43/43); specificity, 86% (6/7); PPV, 98% (43/44); and NPV, 100% (6/6). Unenhanced 3-T MRI in the detection of labral lesions had sensitivity of 89% (32/36); specificity, 79% (11/14); PPV, 91% (32/35); and NPV, 73% (11/15). The corresponding values for detection of lesions at the chondrolabral transition zone were sensitivity, 98% (42/43); specificity, 100% (7/7); PPV, 100% (42/42); and NPV, 88% (7/8).
There is sparse evidence about the role of MRI in improving patient selection for FAI surgery and planning of surgical approaches and procedures [76, 77]. However, extensive cartilage damage (> 2-hour interval on the clockface) (hazard ratio, 4.6 [95% CI, 3.6–5.6]) and the presence of a subchondral cyst at the acetabular rim (hazard ratio, 4.1 [95% CI, 3.1–5.2]) are reportedly [76] associated with increased risk of failure of FAI surgery 10 years postoperatively. Accordingly, the 10-year survivorship of FAI surgery decreases from 73% to 40% in hips with extensive cartilage [76]. The study by Hanke et al. [76] showed the great potential of MRA to help surgeons identify which patients can receive long-term benefit from joint-preserving surgery on the basis of the presence or absence of extensive joint damage (Figs. 3 and 6).
According to results of a 2017 meta-analysis [67], the diagnostic accuracy of conventional MRI in detecting cartilage lesions is lower than the diagnostic accuracy of unenhanced MRI (pooled sensitivity, 86% vs 76%) and direct MRA of the hip (pooled sensitivity, 91% vs 75%). Despite the importance of cartilage integrity, the evaluation of early cartilage damage, such as cartilage delamination, often remains elusive with conventional MRI owing to the tight anatomy of the hip cartilage surfaces [78, 79]. More specifically, Pfirrmann and colleagues [78] reported that direct MRA cannot be used to reliably rule out cartilage delamination, which makes planning of cartilage repair difficult. According to their study, the sensitivity of direct MRA for detecting cartilage delamination ranged from 22% (5/23) to 74% (17/23) and the NPV from 53% (20/38) to 76% (19/25).
To overcome this limitation, joint distention by arthrography can be further combined with leg traction with MRI-compatible devices. Traction MRA by a standardized approach with weight-adapted traction force (15–23 kg) improves differentiation of cartilage layers from 19% (14/75) to 96% (72/75) of cases [80, 81]. With this technique, detection of overall acetabular cartilage damage, more specifically cartilage delamination with and without intact surface, was possible with sensitivity of 88% (46/52) and 91% (32/35) according to a promising early report [81].
A further promising application of MRA was presented in a recent study [82] that showed the potential of anesthetic MRA for improving differentiation between intraarticular and extraarticular hip abnormalities (sensitivity, 80% [41/51]; specificity, 83% [20/24]; PPV, 91% [41/45]; NPV, 67% [20/30]) based on pain response at clinical examination. In contrast to morphologic MRI techniques, quantitative techniques for cartilage mapping, such as delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) and T2-/T2*-weighted and T1rho mapping, enable objective quantitative estimation of cartilage properties based on different substrates and therefore have great potential for improving detection of early cartilage damage [83]. In the hip, dGEMRIC has had clinical value in predicting failure of periacetabular osteotomies for DDH at 1.5 T with high accuracy (AUC, 0.977 for T1 ≤ 370 ms) [84], has had moderate to high correlation with histologic Mankin scores of cartilage degeneration (r = −0.515 to −0.716) [85, 86], and has been used to monitor response of cartilage composition to surgery [87].
Guidance—
For assessment of intraarticular abnormalities, small-FOV MRI or MRA is recommended. Given the critical role of cartilage integrity for the success of hip-preserving surgery, MRA with or without traction is recommended to increase the sensitivity of cartilage lesions and cartilage delamination.
MRI to Assess Femoral Torsion
Evidence—
Although abnormal femoral torsion, which describes the orientation of the proximal femur in the sagittal plane, was linked to hip pain and development of early osteoarthritis more than 30 years ago, the underlying pathomechanism has remained unclear [88]. Improved understanding of the pathomechanics of the hip joint has led to integration of femoral torsion into the concept of FAIS and hip instability. The prevalence of torsional deformities, that is, femoral retrotorsion and excessively high femoral torsion, may be as high as 17% and is associated with cam and pincer deformities in young patients with hip pain [16].
Excessively high femoral torsion can compensate an anterior intraarticular impingement conflict due to cam and pincer deformities. However, it can predispose to a posterior extraarticular impingement (ischiofemoral) conflict during external rotation and hip extension [19]. Conversely, low femoral torsion can lead to anterior, intraarticular, and extraarticular impingement against the acetabulum and the anterior-inferior iliac spine, even in the absence of cam deformity or a prominent anterior-inferior iliac spine [20]. These deformities can be treated with additional derotational femoral osteotomies; however, indications are evolving [89]. Femoral torsion should be routinely assessed in patients eligible for joint-preserving hip surgery [15, 20] (Fig. 4).
Femoral torsion can be measured on MR and CT images. Regardless of the modality, image acquisition must be planned with extended scout images including the knees and the hip and without moving the patient. The coils integrated in the MRI table should be used to obtain the knee images to facilitate and accelerate the workflow [15]. There is controversy regarding the accuracy of MRI for measurement of femoral torsion. Some authors [90, 91] have found considerable mean differences (up to 8.9°) between MRI and CT, which they attributed to involuntary patient movement and nonstandardized patient positioning. By contrast, others [92] reported negligible (1.9°) mean differences between MRI and CT with use of a standardized protocol for patient positioning and fast image acquisition.
Overall, more studies are needed to definitively confirm that MRI is as accurate as CT. Research findings [93] suggest that the level of the anatomic landmark selected to define the proximal femoral reference has a strong impact on the resulting femoral torsion angles. Mean differences as high as 17° have been reported when comparing the most proximal method of Lee et al. [94] (level of the greater trochanter) with the most distal measurement method of Murphy et al. [95] (level of the lesser trochanter). Consequently, normal values also vary among the different measurement methods. Although the method described by Murphy et al. [95] most closely reflects true anatomic femoral torsion found in cadaveric studies, more research is needed to better understand how different measurement methods affect femoral torsion angles [95]. We routinely apply the method of Murphy et al. [95] using 10–25° as normal values, angles less than 0° to define retrotorsion, and angles greater than 35° to define excessively high femoral torsion [16]. This measurement method is based on two superimposed images on which the femoral head center is connected with the center of the base of the femoral neck directly superior to the lesser trochanter [95].
Guidance—
Large-FOV axial images of the proximal femur and the distal femoral condyles should be obtained to assess femoral torsion. Various methods of measurement of femoral torsion have been described, and they yield different femoral torsion angles. In clinical routine, a consistent measurement method should be applied and the results reported accordingly.
MRI to Screen for Extraarticular Pathology
Evidence—
The clinical presentation and history of FAIS can be nonspecific and insidious in onset. Because of the deep location of the hip joint and its proximity to anatomic structures that can be frequent sources of hip pain in young and active patients, making the correct diagnosis can be challenging [96]. Consequently, a wide range of extraarticular differential diagnoses or associated conditions can cause or contribute to hip pain and require different treatment strategies. This includes muscular and osseous overuse injuries, subgluteal syndrome, ischiofemoral impingement, and neoplastic or inflammatory disease [21, 97].
Acquisition of axial or coronal fluid-sensitive fat-suppressed MR images that cover the pelvis, including the pubic symphysis, sacroiliac joints, and the lesser trochanter, is generally recommended in the workup of FAI to screen for bone or soft-tissue abnormalities [21] (Fig. 5). However, adding such a sequence cannot replace a tailored imaging protocol for the pubic symphysis, lower spine, or sacroiliac joint performed in cases of specific referrals.
Fig. 5—
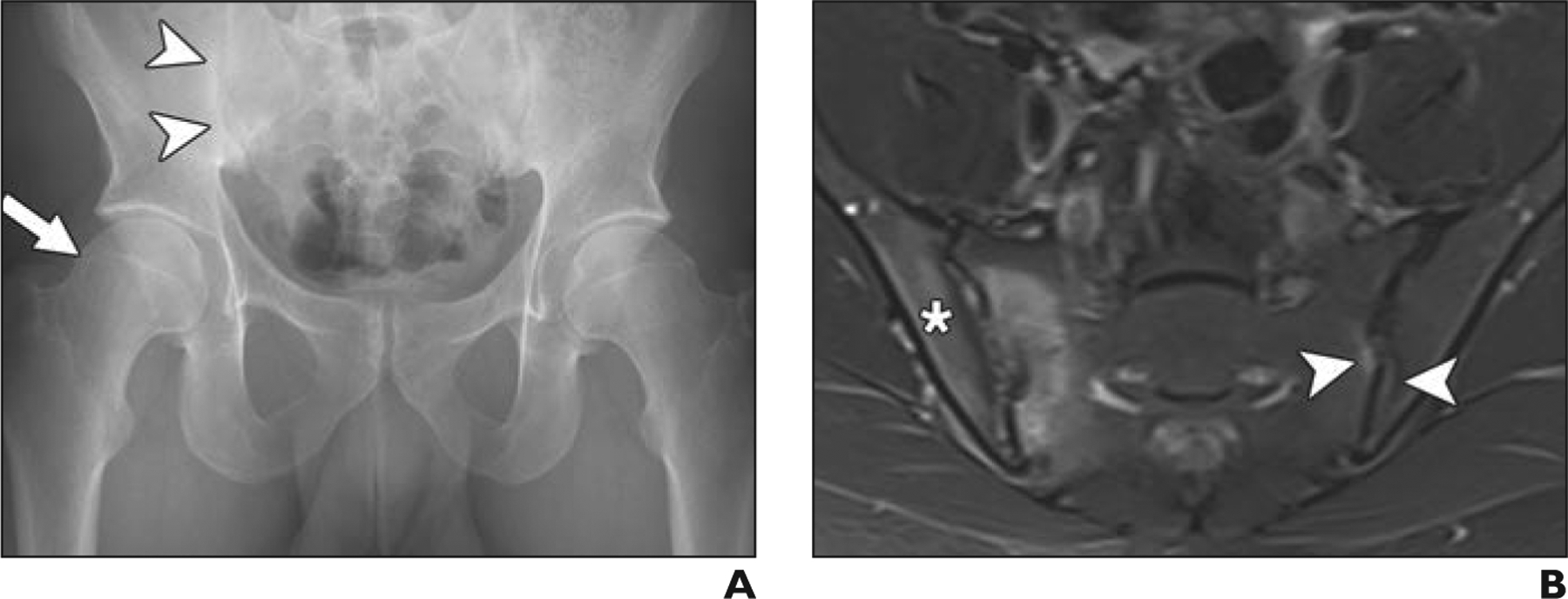
24-year-old man with groin and buttock pain for 1 year. Case emphasizes importance of screening for extraarticular hip abnormalities in young patients with suspected femoroacetabular impingement.
A, Anteroposterior radiograph of pelvis shows cam deformity (arrow) and normal acetabular coverage. Subtle subchondral sclerosis (arrowheads) of right sacroiliac joint is evident. MR arthrography revealed intact labrum and cartilage (not shown).
B, Axial STIR MR image of pelvis shows evidence of periarticular osteitis (asterisk), joint effusion, subchondral erosions of right sacroiliac joint, and subtle edema and sclerosis on left side (arrowheads). Patient was referred to rheumatologist, who confirmed diagnosis of seronegative spondyloarthritis.
Guidance—
A large-FOV axial or coronal fluid-sensitive imaging sequence is recommended to assess for pathologic entities that can mimic or coexist with FAI.
Outstanding Issues That Warrant Research
Much of the controversy regarding the surgical treatment of FAI centers on the difficulty of identifying the predominant osseous deformity to be addressed surgically. Although CT-based impingement simulation has shown promise in this regard, it is still unclear how such tools affect surgical planning and outcome in clinical routine [19, 20, 56]. The use of MRI has great potential to further improve the accuracy of virtual impingement simulation by implementing the labrum, cartilage, joint capsule, and periarticular muscles [58]. Given the advent of deep learning technologies and their ability to fully automate tasks like segmentation, technologies such as virtual impingement simulation will become more widely used [98]. Furthermore, accurate MRI-based 3D rendering of bone and soft tissue would pave the way to more widespread use of 3D printing for surgical planning [98].
The second major challenge in treating patients with FAI is the inaccuracy of current standard imaging techniques in estimating the extent of intraarticular damage [76]. Few studies have been conducted to assess the predictive value of MRI findings in clinical outcome of FAI surgery [76]. In recent years, advanced morphologic imaging with traction MRA has been introduced, and the first clinical studies of the use of cartilage mapping techniques to predict outcome have been conducted [81, 84]. To date, however, the clinical value of these techniques, specifically the role of quantitative imaging techniques such as dGEMRIC, has yet to be reproduced at 3 T. In this regard, the introduction of fully automatic cartilage segmentation tools may represent an important step for their integration into clinical practice [99]. Furthermore, the increasing availability of ultrahigh-field-strength 7-T MRI systems will enable studies of the use of more specific, unenhanced quantitative imaging techniques, such as 23Na-MRI and chemical exchange saturation transfer imaging of glycosaminoglycans (gagCEST) MRI for FAI.
Summary
Recommendations for Best Practices
An overview of the recommended algorithm for the workup of patients with FAI is shown in Figure 7. Figure 8 shows an overview of imaging findings and reference values of the abnormalities in FAI. Radiography of the pelvis and hip should be performed first to assess for osteoarthritis and morphologic abnormalities of the acetabulum and proximal femur. Large-FOV MRI of the pelvis and knees should be performed to determine femoral torsion and to exclude abnormalities that can clinically mimic FAI, such as sacroiliitis and stress fractures. Small-FOV MRI, including radial imaging without or with intraarticular administration of contrast material, is used to detect and quantify cam deformities and to determine the extent of intraarticular abnormalities, such as labral and cartilage damage.
Fig. 7—
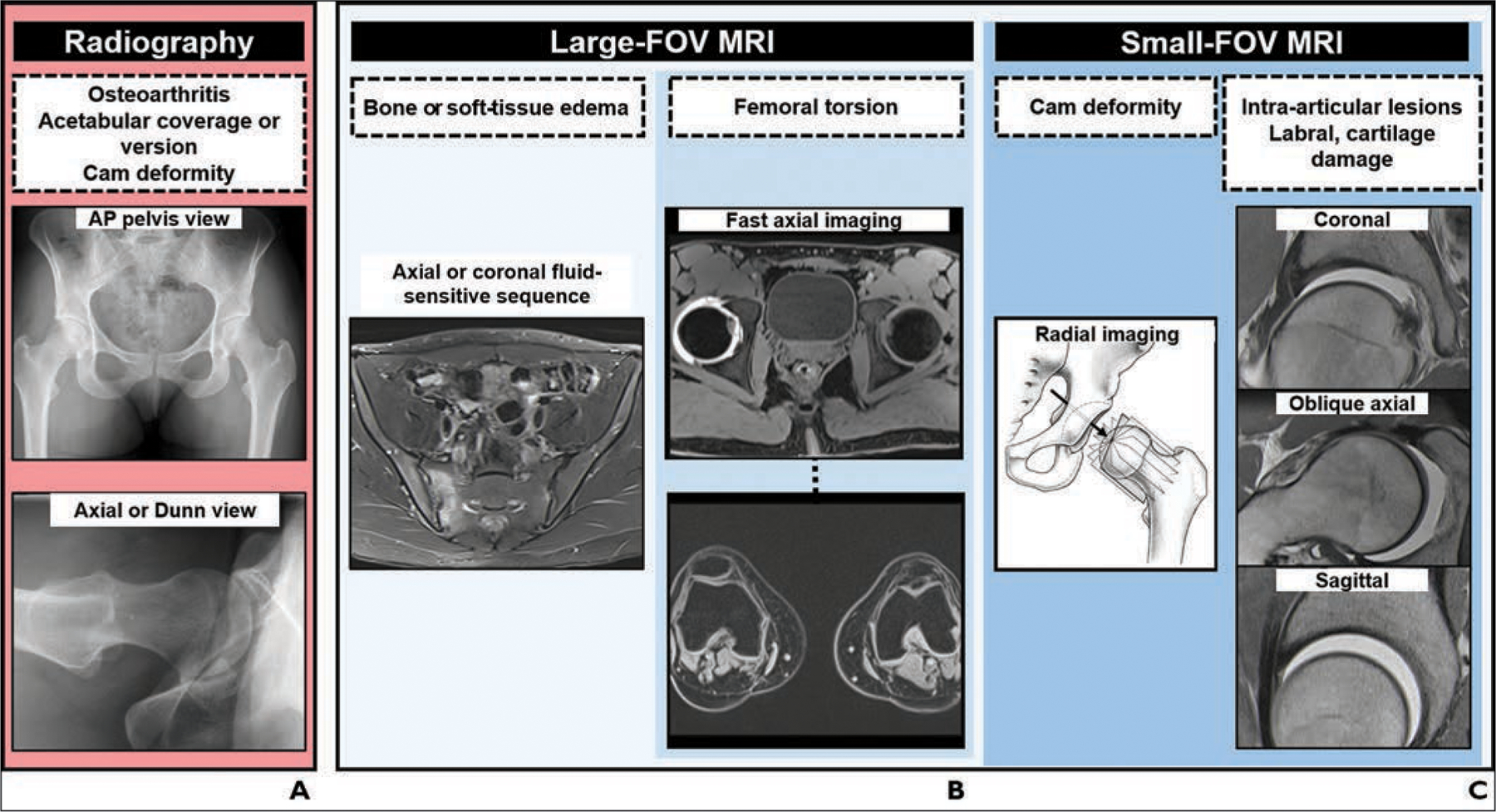
Charts show algorithm for imaging workup of patients with femoroacetabular impingement.
A, Radiography. AP = anteroposterior.
B, Large-FOV MRI.
C, Small-FOV MRI. (Drawing adapted with permission of Wolters Kluwer Health, Inc. from Steppacher SD, Tannast M, Werlen S, Siebenrock KA, Femoral morphology differs between deficient and excessive acetabular coverage, Clinical Orthopaedics and Related Research, 466, 4, 782–790, journals.lww.com/clinorthop/, a publication of The Association of Bone and Joint Surgeons)
Fig. 8—
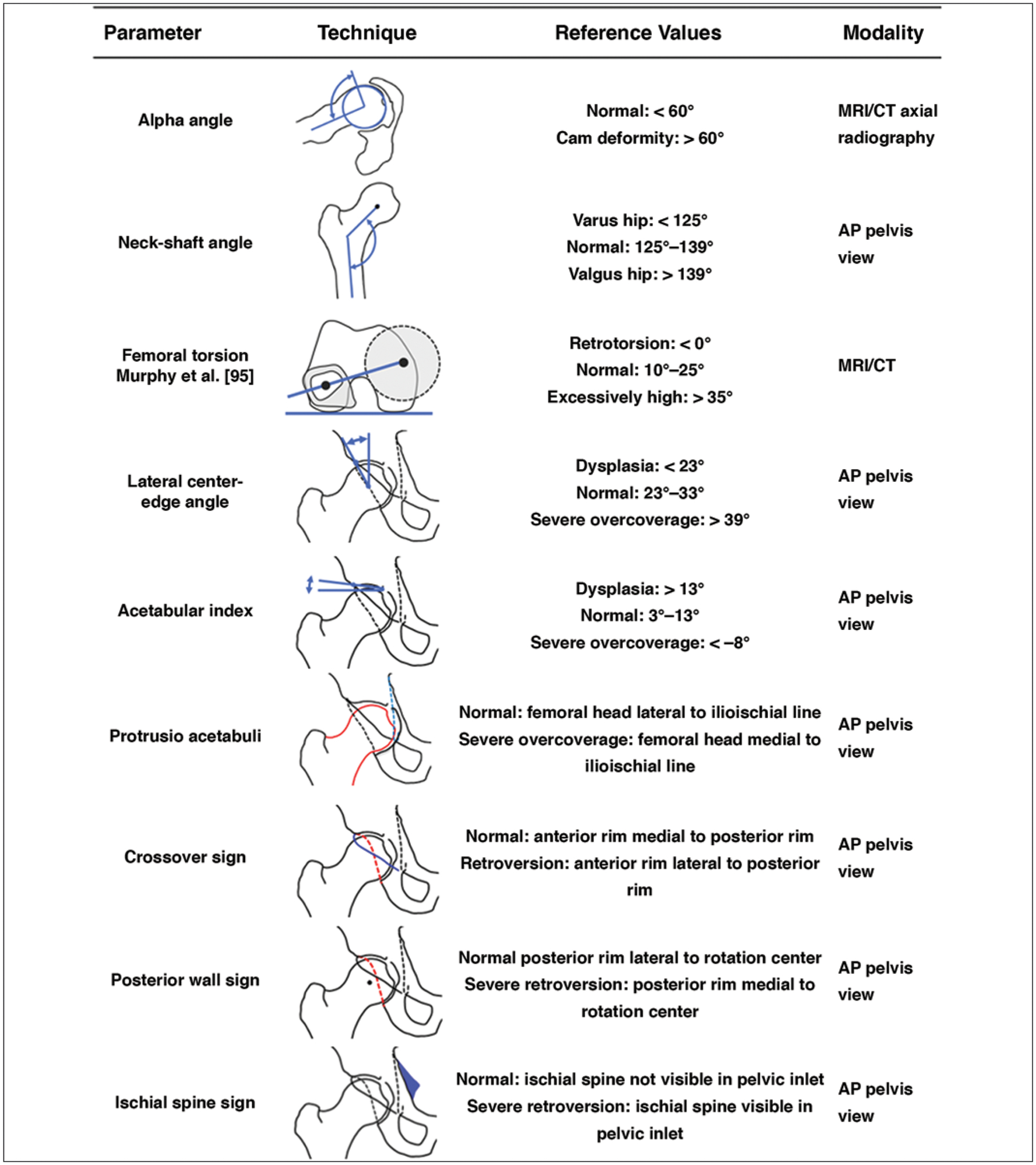
Chart shows imaging findings and reference values in femoroacetabular impingement. Alpha angle is measured between femoral neck axis (bottom line) and line that connects proximal part of asphericity (top line), determined by best-fitting circle, with femoral rotation center. Neck-shaft angle is measured between femoral neck axis (top line) and femoral shaft axis (bottom line). Femoral torsion is measured between top line connecting femoral head center, determined by perfectly fitting circle, to center of femoral neck base directly superior to lesser trochanter and bottom line connecting posterior contours of proximal femoral condyles. Lateral center-edge angle is measured between left line connecting femoral rotation center to lateral extension of sclerotic rim and vertical reference line. Acetabular wall lines shown are anterior rim (solid black lines) and posterior rim (dashed lines). Angle shown for acetabular index is formed between top line connecting medial and lateral sclerotic margins and horizontal reference line. For protrusio acetabuli, femoral head contour (red line) overlaps with ilioischial line (blue line). In crossover sign, anterior rim (blue line) projects laterally to posterior rim (red line). In posterior wall sign, posterior rim (red line) projects medially to rotation center. In ischial spine sign, ischial spine (blue shading) is visible in pelvic inlet. References values are recommendations and may differ by institutional approach [22, 23, 58]. AP = anteroposterior. (Translated by permission from Springer Nature Customer Service Centre GmbH: Springer Nature Radiologe [Impingement of the hip], Schmaranzer F, Hanke M, Lerch T, Steppacher S, Siebenrock K, Tannast M, Copyright 2016)
Further Research
Emerging techniques include 3D CT or MRI with virtual impingement simulation, MRA with application of leg traction, and quantitative techniques for cartilage mapping.
Acknowledgments
Supported in part by NIH grant K24 DK-109940 (M. A. Bredella).
Footnotes
The authors declare that they have no disclosures relevant to the subject matter of this article.
References
- 1.Griffin DR, Dickenson EJ, O’Donnell J, et al. The Warwick agreement on femoroacetabular impingement syndrome (FAI syndrome): an international consensus statement. Br J Sports Med 2016; 50:1169–1176 [DOI] [PubMed] [Google Scholar]
- 2.Ganz R, Parvizi J, Beck M, Leunig M, Nötzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res 2003; 417:112–120 [DOI] [PubMed] [Google Scholar]
- 3.Steppacher SD, Anwander H, Zurmühle CA, Tannast M, Siebenrock KA. Eighty percent of patients with surgical hip dislocation for femoroacetabular impingement have a good clinical result without osteoarthritis progression at 10 years. Clin Orthop Relat Res 2015; 473:1333–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menge TJ, Briggs KK, Dornan GJ, McNamara SC, Philippon MJ. Survivorship and outcomes 10 years following hip arthroscopy for femoroacetabular impingement: labral debridement compared with labral repair. J Bone Joint Surg Am 2017; 99:997–1004 [DOI] [PubMed] [Google Scholar]
- 5.Kaldau NC, Brorson S, Hölmich P, Lund B. Good midterm results of hip arthroscopy for femoroacetabular impingement. Dan Med J 2018; 65:A5483. [PubMed] [Google Scholar]
- 6.Perets I, Chaharbakhshi EO, Shapira J, Ashberg L, Mu BH, Domb BG. Hip arthroscopy for femoroacetabular impingement and labral tears in patients younger than 50 years: minimum five-year outcomes, survivorship, and risk factors for reoperations. J Am Acad Orthop Surg 2019; 27:e173–e183 [DOI] [PubMed] [Google Scholar]
- 7.Sutter R, Pfirrmann CWA. Update on femoroacetabular impingement: what is new, and how should we assess it? Semin Musculoskelet Radiol 2017; 21:518–528 [DOI] [PubMed] [Google Scholar]
- 8.Mascarenhas VV, Ayeni OR, Egund N, et al. Imaging methodology for hip preservation: techniques, parameters, and thresholds. Semin Musculoskelet Radiol 2019; 23:197–226 [DOI] [PubMed] [Google Scholar]
- 9.Wylie JD, Kim YJ. The natural history of femoroacetabular impingement. J Pediatr Orthop 2019; 39(1 suppl 1):S28–S32 [DOI] [PubMed] [Google Scholar]
- 10.Mascarenhas VV, Rego P, Dantas P, et al. Hip shape is symmetric, non-dependent on limb dominance and gender-specific: implications for femoroacetabular impingement:—a 3D CT analysis in asymptomatic subjects. Eur Radiol 2018; 28:1609–1624 [DOI] [PubMed] [Google Scholar]
- 11.Mascarenhas VV, Rego P, Dantas P, et al. Imaging prevalence of femoroacetabular impingement in symptomatic patients, athletes, and asymptomatic individuals: a systematic review. Eur J Radiol 2016; 85:73–95 [DOI] [PubMed] [Google Scholar]
- 12.Frank JM, Harris JD, Erickson BJ, et al. Prevalence of femoroacetabular impingement imaging findings in asymptomatic volunteers: a systematic review. Arthroscopy 2015; 31:1199–1204 [DOI] [PubMed] [Google Scholar]
- 13.Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br 2005; 87:1012–1018 [DOI] [PubMed] [Google Scholar]
- 14.Tannast M, Goricki D, Beck M, Murphy SB, Siebenrock KA. Hip damage occurs at the zone of femoroacetabular impingement. Clin Orthop Relat Res 2008; 466:273–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutter R, Dietrich TJ, Zingg PO, Pfirrmann CWA. Femoral antetorsion: comparing asymptomatic volunteers and patients with femoroacetabular impingement. Radiology 2012; 263:475–483 [DOI] [PubMed] [Google Scholar]
- 16.Lerch TD, Todorski IAS, Steppacher SD, et al. Prevalence of femoral and acetabular version abnormalities in patients with symptomatic hip disease: a controlled study of 538 hips. Am J Sports Med 2018; 46:122–134 [DOI] [PubMed] [Google Scholar]
- 17.Kraeutler MJ, Chadayammuri V, Garabekyan T, Mei-Dan O. Femoral version abnormalities significantly outweigh effect of cam impingement on hip internal rotation. J Bone Joint Surg Am 2018; 100:205–210 [DOI] [PubMed] [Google Scholar]
- 18.Chadayammuri V, Garabekyan T, Bedi A, et al. Passive hip range of motion predicts femoral torsion and acetabular version. J Bone Joint Surg Am 2016; 98:127–134 [DOI] [PubMed] [Google Scholar]
- 19.Siebenrock KA, Steppacher SD, Haefeli PC, Schwab JM, Tannast M. Valgus hip with high antetorsion causes pain through posterior extraarticular FAI. Clin Orthop Relat Res 2013; 471:3774–3780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lerch TD, Boschung A, Todorski IAS, et al. Femoroacetabular impingement patients with decreased femoral version have different impingement locations and intra- and extraarticular anterior subspine FAI on 3D-CT-based impingement simulation: implications for hip arthroscopy. Am J Sports Med 2019; 47:3120–3132 [DOI] [PubMed] [Google Scholar]
- 21.Schmaranzer F, Cerezal L, Llopis E. Conventional and arthrographic magnetic resonance techniques for hip evaluation: what the radiologist should know. Semin Musculoskelet Radiol 2019; 23:227–251 [DOI] [PubMed] [Google Scholar]
- 22.Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis—what the radiologist should know. AJR 2007; 188:1540–1552 [DOI] [PubMed] [Google Scholar]
- 23.Tannast M, Hanke MS, Zheng G, Steppacher SD, Siebenrock KA. What are the radiographic reference values for acetabular under- and overcoverage? Clin Orthop Relat Res 2015; 473:1234–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lerch TD, Steppacher SD, Liechti EF, Tannast M, Siebenrock KA. One-third of hips after periacetabular osteotomy survive 30 years with good clinical results, no progression of arthritis, or conversion to THA. Clin Orthop Relat Res 2017; 475:1154–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larson CM, Ross JR, Stone RM, et al. Arthroscopic management of dysplastic hip deformities: predictors of success and failures with comparison to an arthroscopic FAI cohort. Am J Sports Med 2016; 44:447–453 [DOI] [PubMed] [Google Scholar]
- 26.Siebenrock KA, Kistler L, Schwab JM, Büchler L, Tannast M. The acetabular wall index for assessing anteroposterior femoral head coverage in symptomatic patients. Clin Orthop Relat Res 2012; 470:3355–3360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beltran LS, Rosenberg ZS, Mayo JD, et al. Imaging evaluation of developmental hip dysplasia in the young adult. AJR 2013; 200:1077–1088 [DOI] [PubMed] [Google Scholar]
- 28.Hanke MS, Steppacher SD, Zurmühle CA, Siebenrock KA, Tannast M. Hips with protrusio acetabuli are at increased risk for failure after femoroacetabular impingement surgery: a 10-year followup. Clin Orthop Relat Res 2016; 474:2168–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nepple JJ, Lehmann CL, Ross JR, Schoenecker PL, Clohisy JC. Coxa profunda is not a useful radiographic parameter for diagnosing pincer-type femoroacetabular impingement. J Bone Joint Surg Am 2013; 95:417–423 [DOI] [PubMed] [Google Scholar]
- 30.Jamali AA, Mladenov K, Meyer DC, et al. Anteroposterior pelvic radiographs to assess acetabular retroversion: high validity of the “cross-over-sign.” J Orthop Res 2007; 25:758–765 [DOI] [PubMed] [Google Scholar]
- 31.Tannast M, Fritsch S, Zheng G, Siebenrock KA, Steppacher SD. Which radiographic hip parameters do not have to be corrected for pelvic rotation and tilt? Clin Orthop Relat Res 2015; 473:1255–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steppacher SD, Lerch TD, Gharanizadeh K, et al. Size and shape of the lunate surface in different types of pincer impingement: theoretical implications for surgical therapy. Osteoarthritis Cartilage 2014; 22:951–958 [DOI] [PubMed] [Google Scholar]
- 33.Pun SY, Hingsammer A, Millis MB, Kim YJ. Is increased acetabular cartilage or fossa size associated with pincer femoroacetabular impingement? Clin Orthop Relat Res 2017; 475:1013–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siebenrock KA, Anwander H, Zurmühle CA, Tannast M, Slongo T, Steppacher SD. Head reduction osteotomy with additional containment surgery improves sphericity and containment and reduces pain in Legg-Calvé-Perthes disease. Clin Orthop Relat Res 2015; 473:1274–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parry JA, Swann RP, Erickson JA, Peters CL, Trousdale RT, Sierra RJ. Midterm outcomes of reverse (anteverting) periacetabular osteotomy in patients with hip impingement secondary to acetabular retroversion. Am J Sports Med 2016; 44:672–676 [DOI] [PubMed] [Google Scholar]
- 36.Zurmühle CA, Anwander H, Albers CE, et al. Periacetabular osteotomy provides higher survivorship than rim trimming for acetabular retroversion. Clin Orthop Relat Res 2017; 475:1138–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nepple JJ, Martel JM, Kim YJ, Zaltz I, Clohisy JC; ANCHOR Study Group. Do plain radiographs correlate with CT for imaging of cam-type femoroacetabular impingement? Clin Orthop Relat Res 2012; 470:3313–3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Domayer SE, Ziebarth K, Chan J, Bixby S, Mamisch TC, Kim YJ. Femoroacetabular cam-type impingement: diagnostic sensitivity and specificity of radiographic views compared to radial MRI. Eur J Radiol 2011; 80:805–810 [DOI] [PubMed] [Google Scholar]
- 39.Saito M, Tsukada S, Yoshida K, Okada Y, Tasaki A. Correlation of alpha angle between various radiographic projections and radial magnetic resonance imaging for cam deformity in femoral head-neck junction. Knee Surg Sports Traumatol Arthrosc 2017; 25:77–83 [DOI] [PubMed] [Google Scholar]
- 40.Barton C, Salineros MJ, Rakhra KS, Beaulé PE. Validity of the alpha angle measurement on plain radiographs in the evaluation of cam-type femoroacetabular impingement. Clin Orthop Relat Res 2011; 469:464–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hipfl C, Titz M, Chiari C, et al. Detecting cam-type deformities on plain radiographs: what is the optimal lateral view? Arch Orthop Trauma Surg 2017; 137:1699–1705 [DOI] [PubMed] [Google Scholar]
- 42.Dudda M, Albers C, Mamisch TC, Werlen S, Beck M. Do normal radiographs exclude asphericity of the femoral head-neck junction? Clin Orthop Relat Res 2009; 467:651–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nötzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br 2002; 84:556–560 [DOI] [PubMed] [Google Scholar]
- 44.Rakhra KS, Sheikh AM, Allen D, Beaulé PE. Comparison of MRI alpha angle measurement planes in femoroacetabular impingement. Clin Orthop Relat Res 2009; 467:660–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klenke FM, Hoffmann DB, Cross BJ, Siebenrock KA. Validation of a standardized mapping system of the hip joint for radial MRA sequencing. Skeletal Radiol 2015; 44:339–343 [DOI] [PubMed] [Google Scholar]
- 46.Sutter R, Dietrich TJ, Zingg PO, Pfirrmann CWA. How useful is the alpha angle for discriminating between symptomatic patients with cam-type femoroacetabular impingement and asymptomatic volunteers? Radiology 2012; 264:514–521 [DOI] [PubMed] [Google Scholar]
- 47.Büchler L, Neumann M, Schwab JM, Iselin L, Tannast M, Beck M. Arthroscopic versus open cam resection in the treatment of femoroacetabular impingement. Arthroscopy 2013; 29:653–660 [DOI] [PubMed] [Google Scholar]
- 48.Agricola R, Heijboer MP, Bierma-Zeinstra SMA, Verhaar JAN, Weinans H, Waarsing JH. Cam impingement causes osteoarthritis of the hip: a nationwide prospective cohort study (CHECK). Ann Rheum Dis 2013; 72:918–923 [DOI] [PubMed] [Google Scholar]
- 49.Ehrmann C, Rosskopf AB, Pfirrmann CWA, Sutter R. Beyond the alpha angle: alternative measurements for quantifying cam-type deformities in femoroacetabular impingement. J Magn Reson Imaging 2015; 42:1024–1031 [DOI] [PubMed] [Google Scholar]
- 50.Samim M, Walter W, Gyftopoulos S, Poultsides L, Youm T. MRI assessment of subspine impingement: features beyond the anterior inferior iliac spine morphology. Radiology 2019; 293:412–421 [DOI] [PubMed] [Google Scholar]
- 51.Wong TT, Igbinoba Z, Bloom MC, Kazam JK, Ahmed FS, Rasiej MJ. Anterior inferior iliac spine morphology: comparison of symptomatic hips with femoroacetabular impingement and asymptomatic hips. AJR 2019; 212:166–172 [DOI] [PubMed] [Google Scholar]
- 52.Ecker TM, Puls M, Steppacher SD, et al. Computer-assisted femoral head-neck osteochondroplasty using a surgical milling device: an in vitro accuracy study. J Arthroplasty 2012; 27:310–316 [DOI] [PubMed] [Google Scholar]
- 53.Tobalem F, Dugert E, Verdun FR, et al. MDCT arthrography of the hip: value of the adaptive statistical iterative reconstruction technique and potential for radiation dose reduction. AJR 2014; 203:[web]W665–W673 [DOI] [PubMed] [Google Scholar]
- 54.Su AW, Hillen TJ, Eutsler EP, et al. Low-dose computed tomography reduces radiation exposure by 90% compared with traditional computed tomography among patients undergoing hip-preservation surgery. Arthroscopy 2019; 35:1385–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sutter R, Pfirrmann CWA. Atypical hip impingement. AJR 2013; 201:[web] W437–W442 [DOI] [PubMed] [Google Scholar]
- 56.Lerch TD, Siegfried M, Schmaranzer F, et al. Location of intra- and extra-articular hip impingement is different in patients with pincer-type and mixed-type femoroacetabular impingement due to acetabular retroversion or protrusio acetabuli on 3D CT–based impingement simulation. Am J Sports Med 2020; 48:661–672 [DOI] [PubMed] [Google Scholar]
- 57.Hetsroni I, Poultsides L, Bedi A, Larson CM, Kelly BT. Anterior inferior iliac spine morphology correlates with hip range of motion: a classification system and dynamic model. Clin Orthop Relat Res 2013; 471:2497–2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lerch TD, Degonda C, Schmaranzer F, et al. Patient-specific 3-D magnetic resonance imaging-based dynamic simulation of hip impingement and range of motion can replace 3-D computed tomography-based simulation for patients with femoroacetabular impingement: implications for planning open hip preservation surgery and hip arthroscopy. Am J Sports Med 2019; 47:2966–2977 [DOI] [PubMed] [Google Scholar]
- 59.Toomayan GA, Holman WR, Major NM, Kozlowicz SM, Vail TP. Sensitivity of MR arthrography in the evaluation of acetabular labral tears. AJR 2006; 186:449–453 [DOI] [PubMed] [Google Scholar]
- 60.Ziegert AJ, Blankenbaker DG, De Smet AA, Keene JS, Shinki K, Fine JP. Comparison of standard hip MR arthrographic imaging planes and sequences for detection of arthroscopically proven labral tear. AJR 2009; 192:1397–1400 [DOI] [PubMed] [Google Scholar]
- 61.Yoon LS, Palmer WE, Kassarjian A. Evaluation of radial-sequence imaging in detecting acetabular labral tears at hip MR arthrography. Skeletal Radiol 2007; 36:1029–1033 [DOI] [PubMed] [Google Scholar]
- 62.Petchprapa CN, Rybak LD, Dunham KS, Lattanzi R, Recht MP. Labral and cartilage abnormalities in young patients with hip pain: accuracy of 3-Tesla indirect MR arthrography. Skeletal Radiol 2015; 44:97–105 [DOI] [PubMed] [Google Scholar]
- 63.Linda DD, Naraghi A, Murnaghan L, Whelan D, White LM. Accuracy of non-arthrographic 3T MR imaging in evaluation of intra-articular pathology of the hip in femoroacetabular impingement. Skeletal Radiol 2017; 46:299–308 [DOI] [PubMed] [Google Scholar]
- 64.Blankenbaker DG, Ullrick SR, Kijowski R, et al. MR arthrography of the hip: comparison of IDEAL-SPGR volume sequence to standard MR sequences in the detection and grading of cartilage lesions. Radiology 2011; 261:863–871 [DOI] [PubMed] [Google Scholar]
- 65.Schleich C, Hesper T, Hosalkar HS, et al. 3D double-echo steady-state sequence assessment of hip joint cartilage and labrum at 3 Tesla: comparative analysis of magnetic resonance imaging and intraoperative data. Eur Radiol 2017; 27:4360–4371 [DOI] [PubMed] [Google Scholar]
- 66.Kraus MS, Notohamiprodjo M, Partovi S, et al. MR arthrography of the hip: diagnostic performance and image quality of 3D-steady state free precession versus 2D turbo spin echo sequences. Skeletal Radiol 2018; 47:811–819 [DOI] [PubMed] [Google Scholar]
- 67.Saied AM, Redant C, El-Batouty M, et al. Accuracy of magnetic resonance studies in the detection of chondral and labral lesions in femoroacetabular impingement: systematic review and meta-analysis. BMC Musculoskelet Disord 2017; 18:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith TO, Hilton G, Toms AP, Donell ST, Hing CB. The diagnostic accuracy of acetabular labral tears using magnetic resonance imaging and magnetic resonance arthrography: a meta-analysis. Eur Radiol 2011; 21:863–874 [DOI] [PubMed] [Google Scholar]
- 69.Shakoor D, Farahani SJ, Hafezi-Nejad N, et al. Lesions of ligamentum teres: diagnostic performance of MRI and MR arthrography-a systematic review and meta-analysis. AJR 2018; 211:[web]W52–W63 [DOI] [PubMed] [Google Scholar]
- 70.Schmaranzer F, Lerch TD, Strasser U, Vavron P, Schmaranzer E, Tannast M. Usefulness of MR arthrography of the hip with and without leg traction in detection of intra-articular bodies. Acad Radiol 2019; 26:e252–e259 [DOI] [PubMed] [Google Scholar]
- 71.Tian CY, Wang JQ, Zheng ZZ, Ren AH. 3.0 T conventional hip MR and hip MR arthrography for the acetabular labral tears confirmed by arthroscopy. Eur J Radiol 2014; 83:1822–1827 [DOI] [PubMed] [Google Scholar]
- 72.Sutter R, Zubler V, Hoffmann A, et al. Hip MRI: how useful is intraarticular contrast material for evaluating surgically proven lesions of the labrum and articular cartilage? AJR 2014; 202:160–169 [DOI] [PubMed] [Google Scholar]
- 73.Magee T Comparison of 3.0-T MR vs 3.0-T MR arthrography of the hip for detection of acetabular labral tears and chondral defects in the same patient population. Br J Radiol 2015; 88:20140817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chopra A, Grainger AJ, Dube B, et al. Comparative reliability and diagnostic performance of conventional 3T magnetic resonance imaging and 1.5T magnetic resonance arthrography for the evaluation of internal derangement of the hip. Eur Radiol 2018; 28:963–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Crespo-Rodríguez AM, De Lucas-Villarrubia JC, Pastrana-Ledesma M, Hualde-Juvera A, Méndez-Alonso S, Padron M. The diagnostic performance of non-contrast 3-Tesla magnetic resonance imaging (3-T MRI) versus 1.5-Tesla magnetic resonance arthrography (1.5-T MRA) in femoroacetabular impingement. Eur J Radiol 2017; 88:109–116 [DOI] [PubMed] [Google Scholar]
- 76.Hanke MS, Steppacher SD, Anwander H, Werlen S, Siebenrock KA, Tannast M. What MRI findings predict failure 10 years after surgery for femoroacetabular impingement? Clin Orthop Relat Res 2017; 475:1192–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hartigan DE, Perets I, Yuen LC, Domb BG. Results of hip arthroscopy in patients with MRI diagnosis of subchondral cysts: a case series. J Hip Preserv Surg 2017; 4:324–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pfirrmann CWA, Duc SR, Zanetti M, Dora C, Hodler J. MR arthrography of acetabular cartilage delamination in femoroacetabular cam impingement. Radiology 2008; 249:236–241 [DOI] [PubMed] [Google Scholar]
- 79.Konstantinidis G, Mitchell M, Boyd G, Coady C, Ghosh S, Wong I. Poor sensitivity of magnetic resonance arthrography to detect hip chondral delamination: a retrospective follow-up of 227 FAI-operated patients. Cartilage 2019. January 23 [published online] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schmaranzer F, Klauser A, Kogler M, et al. Improving visualization of the central compartment of the hip with direct MR arthrography under axial leg traction: a feasibility study. Acad Radiol 2014; 21:1240–1247 [DOI] [PubMed] [Google Scholar]
- 81.Schmaranzer F, Klauser A, Kogler M, et al. Diagnostic performance of direct traction MR arthrography of the hip: detection of chondral and labral lesions with arthroscopic comparison. Eur Radiol 2015; 25:1721–1730 [DOI] [PubMed] [Google Scholar]
- 82.Kheterpal AB, Bunnell KM, Husseini JS, et al. Value of response to anesthetic injection during hip MR arthrography to differentiate between intra- and extra-articular pathology. Skeletal Radiol 2020; 49:555–561 [DOI] [PubMed] [Google Scholar]
- 83.Sutter R, Stoel BC, Buck FM, et al. Internal derangements of joints-past, present, and future. Invest Radiol 2015; 50:601–614 [DOI] [PubMed] [Google Scholar]
- 84.Kim SD, Jessel R, Zurakowski D, Millis MB, Kim YJ. Anterior delayed gadolinium-enhanced MRI of cartilage values predict joint failure after periacetabular osteotomy. Clin Orthop Relat Res 2012; 470:3332–3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schmaranzer F, Arendt L, Liechti EF, et al. Do dGEMRIC and T2 imaging correlate with histologic cartilage degeneration in an experimental ovine FAI model? Clin Orthop Relat Res 2019; 477:990–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zilkens C, Miese F, Herten M, et al. Validity of gradient-echo three-dimensional delayed gadolinium-enhanced magnetic resonance imaging of hip joint cartilage: a histologically controlled study. Eur J Radiol 2013; 82:e81–e86 [DOI] [PubMed] [Google Scholar]
- 87.Schmaranzer F, Haefeli PC, Hanke MS, et al. How does the dGEMRIC index change after surgical treatment for FAI? A prospective controlled study: preliminary results. Clin Orthop Relat Res 2017; 475:1080–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tönnis D, Heinecke A. Acetabular and femoral anteversion: relationship with osteoarthritis of the hip. J Bone Joint Surg Am 1999; 81:1747–1770 [DOI] [PubMed] [Google Scholar]
- 89.Buly RL, Sosa BR, Poultsides LA, Caldwell E, Rozbruch SR. Femoral derotation osteotomy in adults for version abnormalities. J Am Acad Orthop Surg 2018; 26:e416–e425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Botser IB, Ozoude GC, Martin DE, Siddiqi AJ, Kuppuswami S, Domb BG. Femoral anteversion in the hip: comparison of measurement by computed tomography, magnetic resonance imaging, and physical examination. Arthroscopy 2012; 28:619–627 [DOI] [PubMed] [Google Scholar]
- 91.Tomczak RJ, Guenther KP, Rieber A, Mergo P, Ros PR, Brambs HJ. MR imaging measurement of the femoral antetorsional angle as a new technique: comparison with CT in children and adults. AJR 1997; 168:791–794 [DOI] [PubMed] [Google Scholar]
- 92.Hesham K, Carry PM, Freese K, et al. Measurement of femoral version by MRI is as reliable and reproducible as CT in children and adolescents with hip disorders. J Pediatr Orthop 2017; 37:557–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schmaranzer F, Lerch TD, Siebenrock KA, Tannast M, Steppacher SD. Differences in femoral torsion among various measurement methods increase in hips with excessive femoral torsion. Clin Orthop Relat Res 2019; 477:1073–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee YS, Oh SH, Seon JK, Song EK, Yoon TR. 3D femoral neck anteversion measurements based on the posterior femoral plane in ORTHODOC system. Med Biol Eng Comput 2006; 44:895–906 [DOI] [PubMed] [Google Scholar]
- 95.Murphy SB, Simon SR, Kijewski PK, Wilkinson RH, Griscom NT. Femoral anteversion. J Bone Joint Surg Am 1987; 69:1169–1176 [PubMed] [Google Scholar]
- 96.Philippon MJ, Maxwell RB, Johnston TL, Schenker M, Briggs KK. Clinical presentation of femoroacetabular impingement. Knee Surg Sports Traumatol Arthrosc 2007; 15:1041–1047 [DOI] [PubMed] [Google Scholar]
- 97.Torriani M, Souto SCL, Thomas BJ, Ouellette H, Bredella MA. Ischiofemoral impingement syndrome: an entity with hip pain and abnormalities of the quadratus femoris muscle. AJR 2009; 193:186–190 [DOI] [PubMed] [Google Scholar]
- 98.Gyftopoulos S, Lin D, Knoll F, Doshi AM, Rodrigues TC, Recht MP. Artificial intelligence in musculoskeletal imaging: current status and future directions. AJR 2019; 213:506–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schmaranzer F, Helfensteinw R, Zeng G, et al. Automatic MRI-based three-dimensional models of hip cartilage provide improved morphologic and biochemical analysis. Clin Orthop Relat Res 2019; 477:1036–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]


