Abstract
Cancer gene therapies are usually designed either to express wild-type copies of tumor suppressor genes or to exploit tumor-associated phenotypic changes to endow selective cytotoxicity. However, these approaches become less relevant to cancers that contain many independent mutations, and the situation is made more complex by our increased understanding of clonal evolution of tumors, meaning that different metastases and even regions of the same tumor mass have distinct mutational and phenotypic profiles. In contrast, the relatively genetically stable tumor microenvironment (TME) therefore provides an appealing therapeutic target, particularly since it plays an essential role in promoting cancer growth, immune tolerance, and acquired resistance to many therapies. Recently, a variety of different TME-targeted gene therapy and armed oncolytic strategies have been explored, with particular success observed in strategies targeting the cancer stroma, reducing tumor vasculature, and repolarizing the immunosuppressive microenvironment. Herein, we review the progress of these TME-targeting approaches and try to highlight those showing the greatest promise.
Keywords: gene therapy, oncolytic virus, tumor microenvironment, immunotherapy, combination therapy
Graphical abstract
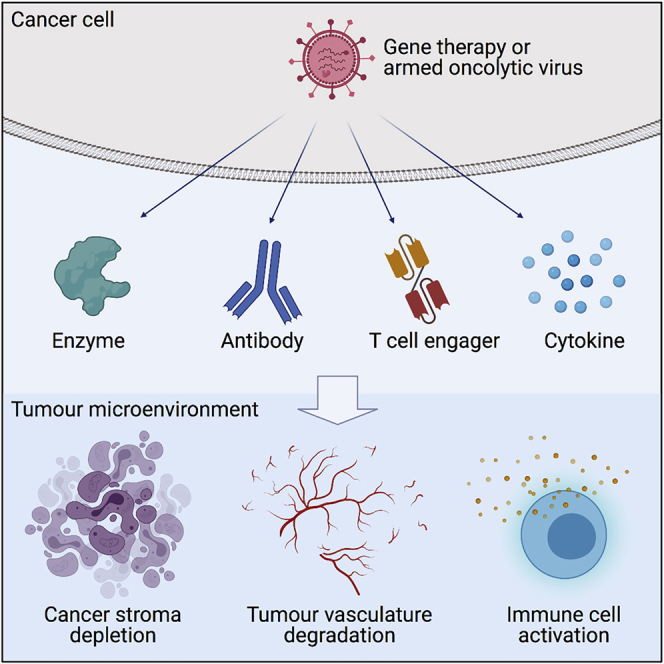
Recent advances in gene therapies and armed oncolytic viruses have been exploring therapeutic strategies that target not only cancer cells but also stromal populations. In this review, Wan et al. highlight different approaches to deplete cancer stroma, degrade tumor vasculature, and repolarize the immunosuppressive tumor microenvironment. The authors also discuss the opportunities this approach brings, together with challenges faced, and suggest directions the field may adopt.
Introduction
Importance of the tumor microenvironment (TME)
Components of the TME play essential roles in cancer development and growth. In most carcinomas, the malignant tumor cells are supported by a heterogeneous population of non-transformed cells in the TME, including fibroblasts, endothelial cells, immune cells, adipocytes, and pericytes.1 Developing tumors can recruit these stromal cells from adjacent non-tumor tissue and also from the bone marrow to support the emerging neoplastic phenotype.2 Bi-directional communication between the tumor and these microenvironmental cells appears to be critical for promoting tumor progression and metastasis, with tumors secreting cytokines and chemokines that alter surface markers on endothelial cells and induce tumor tolerance.3 Moreover, the components of the TME can change over time, providing supportive environments for different stages of tumor growth,4 and therefore the TME has been increasingly recognized as a valid target for therapeutic intervention.5
Gene therapy targeting the TME
Gene therapy has evolved rapidly in the last two decades. Early cancer gene therapy trials focused on the delivery of tumor suppressor genes, most notably wild-type p53,6 and later approaches often exploited the tumor phenotype to regulate a selective cytotoxic effect. However, with the availability of single-cell sequencing and recent insights into tumor heterogeneity and clonal evolution showing the multitude of tumor-associated mutations and phenotypic variation between different regions even of the same tumor,7 such “one size fits all” therapeutic strategies now appear less feasible. Accordingly, gene therapy approaches have recently been developed that aim to manipulate the relatively genetically stable TME, including modulation of the local immune system. In particular, progress has been made to deplete cancer stroma and to degrade the tumor vasculature,8,9 while cytokine-based gene therapies and vaccines have aimed to boost the antitumor response or suppress tumor tolerance.10 In addition, armed oncolytic viruses (OVs) are increasingly considered as appealing therapeutic agents because they mediate tumor-selective oncolytic cell-killing mechanisms,11 lysing tumor cells to expose proinflammatory signals (pathogen-associated and damage-associated molecular patterns [PAMPs and DAMPs]) and tumor antigens, combined with local expression of encoded immunomodulatory transgenes. This dual mechanism affords many innovations in therapeutic approach. In this review, we consider the most promising TME-targeting strategies using gene therapies and armed OVs, illustrated in Figure 1, and suggest which stromal cells and therapeutic modalities might provide the greatest therapeutic value.
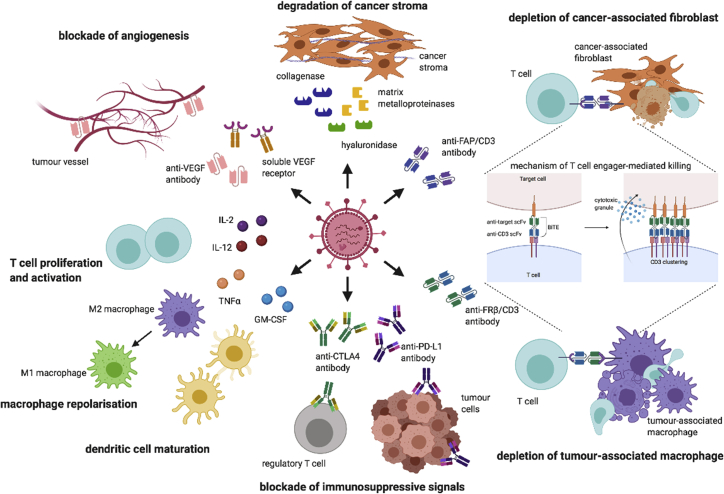
Figure 1.
Strategies of armed OVs for targeting the TME
OVs infect and lyse cancer cells, releasing the armed biologics to the TME. Matrix-degrading enzymes such as hyaluronidase, collagenase, and matrix metalloproteinase are used to degrade the dense and viscous cancer stroma. Bispecific antibodies such as bispecific T cell engager targeting FAP and FRβ are designed to selective deplete cancer-associated fibroblasts and immunosuppressive M2 macrophages, and activate endogenous T cells independent of the MHC class I molecule. Monovalent antibodies such as anti-CTLA-4 and anti-PD-L1 antibodies can also be encoded by OVs to block the inhibitory receptors on regulatory T cells and tumor cells. In addition, cytokines such as GM-CSF and TNF-α can be armed in OVs to promote dendritic cell maturation and macrophage repolarization. IL-2 and IL-12 have also been used to promote T cell proliferation and activation. Soluble VEGFR receptor composed of VEGFR fused to an Fc domain is encoded by OVs to block the VEGF/VEGFR pathway for suppressing angiogenesis. CTLA-4, cytotoxic T lymphocyte-associated protein 4; FAP, fibroblast activation protein α; FRβ, folate receptor β; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL-2, interleukin-2; IL-12, interleukin-12; PD-L1, programmed death-ligand 1; TNF-α, tumor necrosis factor α; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.
Choice of appropriate preclinical model systems is challenging for agents targeting the TME, particularly for immunomodulatory armed OVs, as these are often human selective and do not complete their full life cycle in most rodent cells. Some immune-competent animals, such as Syrian hamsters, are semi-permissive for some OVs,12 and this provides a handful of tumor models, although it is not clear whether the stromal composition of model tumors or the balance of direct oncolysis and transgene expression has clinical relevance.13,14 Similarly, xenografted tumors in transgenic mice provide improving model systems, although the stromal compartment is of murine origin and often poorly developed, and strict clinical relevance is still unclear. Patient-derived xenograft (PDX) models may provide better model systems going forward, since direct xenografts can retain some human stroma.15 Many of the pharmacodynamic studies discussed below make use of human ex vivo tumor culture systems, which usually contain all of the representative stromal cell compartments but suffer from limited viability and useful lifespan.16 All of these model systems bring their own technical challenges, and we have tried to interpret findings with this in mind throughout this review.
Pharmacokinetic aspects of tumor-targeted expression of biologics
One major advantage of encoding anti-cancer biologics within gene therapy vectors or OVs is the potential for selective expression within tumors. While this does not increase the amount of material being delivered to the tumor, local production in situ leads to high local concentrations of the secreted biologic within the TME and relatively low concentrations in the circulation, thereby maximizing the therapeutic effect and limiting systemic toxicities. This strategy is most effective when the expressed agents are relatively small macromolecules, improving their percolation through the TME and also minimizing their persistence in the bloodstream (by accelerated renal clearance) if they leach out of the tumor into the circulation. For example, small biologics such as nanobodies (∼15 kDa) and single-chain variable fragment (scFv) (∼28 kDa) have a relatively short half-life of <0.5 h.17,18 This is the converse of the desirable physical properties for systemic delivery, where a long half-life (for example using an intact antibody) is normally seen as preferred to maximize the opportunity to access disseminated targets. Accordingly, from a pharmacokinetic perspective, encoding antibody fragments or small biologics within gene therapy vectors or OVs may comprise a particularly powerful approach.
For gene therapies, transgene expression is normally regulated using tumor- or tissue-selective promoters.19 However, OVs provide the opportunity to use endogenous viral promoters to regulate expression, linking expression to the virus life cycle and thereby exploiting the cancer selectivity of the OV to ensure cancer-selective expression of the transgene.20 Alternatively, tumor- or tissue-specific promoters such as telomerase reverse transcriptase promoter21 and prostate-specific antigen promoter22 have been used to regulate both conditional replication and transgene expression in selected tissues. This also gives considerable flexibility over the duration of transgene expression. The use of AAV (adeno-associated virus) for gene therapy can give extended transgene expression; however, for most cancer therapies, where the expressed agents are likely to cause cytotoxicity, the intrinsically limited duration of expression afforded by most OVs may be preferable.
Cancer-associated fibroblasts as a therapeutic target
Cancer-associated fibroblasts (CAFs) orchestrate several aspects of cancer biology that contribute to poor prognosis, often producing cytokines, chemokines, and extracellular matrix (ECM). Notably, they secrete vascular endothelial growth factor (VEGF) to drive angiogenesis and transforming growth factor (TGF)-β to polarize immune cells to become immunosuppressive.23 In addition, CAFs can express high levels of programmed cell death ligand 2 (PD-L2) and FAS ligand (FASLG) to promote tumor immune evasion.24
Targeted degradation of protein components of the ECM
While supporting tumor progression in diverse ways, CAFs can also act to inhibit the infiltration and dissemination of gene therapy vectors and OVs.25 Most notably, CAFs secrete large quantities of extracellular proteins such as collagen and fibronectin, coupled with collagen cross-linking enzymes such as lysyl hydroxylases and prolyl hydrolases, which become embedded within a rigid framework of the densely packed ECM.26,27
Interstitial penetration and spreading of gene therapy vectors and OVs rely on fluid convection, and the densely packed ECM acts as a physical barrier that hinders their movement and thereby their dissemination throughout the tumor mass.28,29 There have been several attempts to address this challenge, for example Kim et al.30 and Choi et al.,31 respectively, engineered relaxin- and decorin-armed oncolytic adenoviruses (Ads) in order to degrade collagen components of the ECM. Relaxin and decorin are involved in the induction of matrix metalloproteinases (MMPs) and inhibition of collagen expression and assembly. Both armed Ads showed enhanced viral spread, replication, and transgene expression, remodeling the ECM composition by depleting collagen type I, type III, and elastin.31,32 Similarly, intratumoral expression of MMP-8 by oncolytic Ads effectively depleted collagen and broke down the fibrillar collagen matrix to enhance therapeutic efficacy.29 Improved viral distribution and therapeutic outcome were also observed using an oncolytic herpes simplex virus 1 (HSV-1) vector after co-injection with bacterial collagenase to degrade fibrillar collagen in a melanoma xenograft mouse model.25
Targeted degradation of glycan and nucleic acid components of the TME
While extracellular proteins provide the structural framework for ECM, more flexible hydrophilic components can associate with it and increase viscosity, further restricting virus spread by providing even greater resistance to fluid convection. These components include hyaluronic acid and extracellular DNA released during non-apoptotic tumor cell death. Building on observations that PEG-PH20 (hyaluronidase derivatized with poly(ethylene glycol) to improve its stability in the bloodstream) could be used to decrease the interstitial fluid pressure (IFP) and enhance the delivery of macromolecules and viruses into tumors,33 several groups have explored hyaluronidase-armed Ad or Ad co-injected with hyaluronidase to degrade the hyaluronan-rich matrix in an attempt to improve virus penetration. Guedan et al.34 showed that hyaluronidase (PH20)-armed oncolytic Ad improved viral spread and inhibited growth of human melanoma xenograft with high hyaluronan content in nude mice. Co-injection of hyaluronidase showed similar results.35 Importantly, despite the degradation of hyaluronic acid, the protein matrix remained intact and PH20 only allowed penetration of particles up to 200 nm in diameter, facilitating the efficient spreading of Ad but not metastasis of cancer cells.34,36 This hyaluronidase-expressing oncolytic Ad has now completed a phase I clinical trial and demonstrated modulation of the TME to a more pro-inflammatory state with a good safety profile.37
Extracellular genomic DNA within tumors, released from dying cells, may also act as a barrier to interstitial spread of viruses. To explore this a group B Ad11p/Ad3 chimeric oncolytic Ad enadenotucirev (EnAd) encoding actin-resistant DNAse I was developed and shown to improve viral infection and spread within the tumor, resulting in superior therapeutic activity.38 Using gene therapy vectors and OVs to express enzymes in this way overcomes the requirement for repeated injections of enzymes35 and highlights the importance of ECM remodeling to enhance efficacy of gene and oncolytic therapies.
CAFs can directly inhibit gene and oncolytic therapies
Stroma-tumor interactions have been reported to directly suppress the uptake of adenoviral vectors and the associated transgene expression.39 Intriguingly, the expression of essential Ad receptors (the coxsackievirus and Ad receptor, integrins αvβ3 and αvβ5, as well as clathrin) was found to be downregulated in pancreatic cancer cells exposed to media that had been conditioned with patient-derived fibroblasts in vitro.40 The study implicated hepatocyte growth factor (HGF) in the conditioned-causing phosphorylation of Met in the cancer cells, since inhibition of HGF/Met restored receptor expression. CAFs can also provide physical barriers to virus infection. For example, Vähä-Koskela et al.41 reported that initial oncolysis by an oncolytic Semliki Forest virus (SFV) strain A7 led to the formation of CAF-encapsulated tumor nodules containing both dying and regrowing cancer cells that were resistant to subsequent virus challenge. These studies illustrate the diverse ways that CAFs can directly impact successful gene and virotherapy by preventing infection of target cells.
Cytokines released by CAFs promote angiogenesis and immunosuppression
CAFs also secrete a wide range of signaling proteins, MMPs, and angiogenic factors, all of which can contribute to tumor growth and metastasis.42, 43, 44 They can also drive repolarization of tumor-associated macrophages (TAMs) towards more immunosuppressive phenotypes, induce differentiation of regulatory T cells (Tregs), and attenuate cytotoxic T cell responses.45 For all of these reasons, CAFs provide an important therapeutic target, and gene therapy strategies to achieve depletion of CAFs are considered in more detail below.
Strategies for targeting CAFs using gene therapies and armed OVs
Although the central paradigm for OV is to achieve selective replication and lysis of cancer cells, some studies have shown the possibility to include the stromal compartment in this activity. This is attractive given the frequent association of high stromal content and poor prognosis. For example, an oncolytic Ad with Rb-binding-deleted E1A under the transcriptional control of a truncated promoter from the stroma-associated gene SPARC (secreted protein, acidic, rich in cysteine) was reported to infect and lyse both tumor and stromal cells in human ovary cancer explants and human stroma-containing xenograft tumors in mice but showed no activity in non-malignant tissue explants.46 To enhance the replication capability of the Ad, motifs responsive to hypoxia-responsive element (HRE) and inflammation (nuclear factor κB) were incorporated into the SPARC promoter. This modified Ad demonstrated superior activity leading to complete regression of human melanoma in nude mice.47 In another approach, recombinant measles virus retargeting to urokinase receptor, which is abundantly expressed on tumor and stromal cells, was shown to delay growth of human breast cancer xenografts and also altered the pattern of gene expression associated with angiogenesis, cell survival, and inflammation.48
Targeting fibroblast activation protein-α (FAP)
Expression of FAP is a key characteristic of CAFs.49 FAP is upregulated in many cancer types and is frequently associated with poor patient prognosis.50,51 Although mechanisms underlying FAP-promoted tumorigenesis are not fully understood, a clear association of FAP with tumor progression, metastasis, and angiogenesis is observed.52 However, despite the considerable promise, phase II studies targeting FAP using the monoclonal antibody sibrotuzumab53 or blocking the enzymatic activity of FAP54 by talabostat showed no therapeutic efficacy in phase II trials in colorectal cancer, and later studies in animal raised the possibility of causing cachexia by depleting stromal fibroblasts from normal tissues.55
Bispecific antibodies are synthetic macromolecules that can be easily encoded into gene therapy vectors and OVs, and they provide great flexibility to orchestrate localized cellular functions within tumors. They represent a very popular emerging therapeutic strategy. Bispecific T cell engagers (BiTEs) are composed of two scFvs or nanobodies linked by a short serine-glycine linker with one arm binding to T cells, usually via T cell receptor (TCR) signaling complex CD3, and the other arm binding to the surface antigen on tumor cells or any target cells. The BiTE brings T cells and target cells into close proximity to form an immune synapse to initiate perforin/granzyme-killing mechanisms. An advantage of BiTE therapeutics is that the T cell-mediated cytotoxicity is independent of co-stimulatory signals or major histocompatibility complex class I (MHC class I), which is often downregulated in tumor cells, allowing them to evade immune surveillance.56
Armed oncolytic EnAd encoding anti-FAP/CD3 BiTE has been shown to target T cell cytotoxicity towards FAP-expressing cells in the TME.57,58 Using patient-derived immunosuppressive malignant ascites as an ex vivo model, the armed EnAd showed powerful cytotoxicity toward endogenous CAFs by activation of tumor-resident T cells. This is intriguing because it suggests that BiTEs may be able to overcome local immunosuppressive effects, a phenomenon that is documented increasingly widely.57 BiTE treatment also led to repolarization of M2 to M1 TAMs, perhaps due to the level of interferon γ (IFNγ) produced by activated T cells, or perhaps reflecting a broader reprogramming of the TME. Global upregulation of various immune stimulatory pathways was also observed.57 EnAd has been showing good blood stability, low immunogenicity, and an acceptable safety profile in preclinical trials,59 and the armed version expressing the FAP BiTE is now undergoing clinical assessment (ClinicalTrials.gov: NCT04053283).
“Double-deleted” Western Reserve vaccinia virus (vvDD, missing thymidine kinase and vaccinia growth factor) expressing anti-FAP/CD3 BiTE has also been shown to improve viral replication and promote intratumoral infiltration of activated T cells in a melanoma syngeneic model.60 Local and distant tumor regression were also observed in a subcutaneous melanoma model with local OV injection. In addition, anti-FAP/CD3 BiTE-expressing Ad ICOVIR15K showed superior antitumor responses and intratumoral T cell accumulation compared to the parental virus in xenograft mouse models of lung and pancreatic cancer with adoptive transfer of preactivated human T cells.58 Interestingly, FAP-negative expressing cells proximal to FAP-positive expressing cells were targeted by T cell-induced bystander cell killing, suggesting the potential of killing hard-to-target pro-tumorigenic cell populations (e.g. with lack of identified surface markers and/or with low abundance) in the TME. Apart from the BiTE approach, vaccinia virus (VV) encoding an anti-FAP scFv induced modest regressions in tumor growth compared with unarmed VV in lung and prostate cancer xenograft mouse models, although a significant reduction of CAFs was observed in both infected and uninfected areas.61 The modest antitumor activity may be due to the use of non-cytotoxic FAP-blocking antibody instead of the cytotoxic anti-FAP BiTE. The FAP-blocking antibody did not kill the CAFs, allowing them to regrow and restore the TME. These preclinical studies are summarized in Table 1.
Table 1.
Preclinical studies of antibody-armed oncolytic viruses targeting cancer stroma
| Target | Virus (species) | Antibody (promoter) | Effect ex vivo/in vivo | Delivery route (frequency) | Ref. |
|---|---|---|---|---|---|
| FAP | EnAd-SA-FAP-BiTE (Ad11p/Ad3; enadenotucirev) | anti-CD3/FAP BiTE (MLP) | malignant ascites: ↓ CAF (FC); ↑ T cell activation (FC, CD25, CD69); ↑ global immune response (DNA microarray); ↑ effector cytokines (IL-17F, IL-22, IFN-γ); TAM repolarization (FC, D206, CD163, CD64) prostate biopsy: ↓ CAF (CLSM, active caspase-3); ↑ TIL activation (IHC, CD25, 7 dpi) |
N/A (ex vivo) | 57 |
| FAP | ICO15K-FBiTE (Ad) | anti-CD3/FAP BiTE (MLP) | lung, pancreatic cancer/s.c. in NSG mice with pre-activated T cells: ↓ tumor mass; ↓ CAF (qPCR, IHC, FAP, 11 dpi); ↑ T cell infiltration (IVIS) |
i.t. (1) | 58 |
| FAP | mFAP-TEA-VV (VV) | anti-CD3/FAP BiTE (F17R late promoter) | melanoma/s.c. in C57 mice: ↓ tumor mass; ↑ TIL activation (FC, IL-2, IFN-γ, 8 dpi) |
i.t. (3; 0, 3, and 6 dpi) | 60 |
| FAP | GLV-1h282 (VV) | anti-FAP scFv (PSEL) | lung, prostate/ s.c. in nude mice: ↓ tumor mass; ↓ CAF (IHC, FAP, 36 dpi) |
r.o. (1) | 61 |
Ad, adenovirus; BiTE, bispecific T cell engager; CSLM, confocal laser scanning microscopy; dpi, days post-infection; ELISA, enzyme-linked immunosorbent assay; FAP, fibroblast activation protein-α; FC, flow cytometry; IFN-γ, interferon γ; IHC, immunohistochemistry; IL-2, interleukin 2; IL-17F, interleukin 17F; i.t., intratumoral; IVIS, in vivo imaging system; MLP, major late promoter; N/A, not applicable; PSEL, synthetic early/late promoter; Ref., reference; r.o., retro-orbital; s.c., subcutaneous; TAM, tumor-associated macrophage; TIL, tumor-infiltrating lymphocyte; VV, vaccinia virus.
Vaccines for depleting CAFs
Using a different approach, DNA vaccines recently showed preclinical success in depleting CAFs.62,63 This vaccine approach is designed to encourage antigen-presenting cells to process and present FAP and to activate both cell-mediated immune responses by cytotoxic T cells and humoral immune responses to produce antibodies. These studies showed the induction of FAP-specific CD8+ T cells and decreased production of stromal cytokines in syngeneic mice bearing colon62 and breast tumors.63 A modified DNA vaccine expressing the extracellular domain of human FAP with the tissue plasminogen activator signal sequence, to enhance antigen expression, also demonstrated a superior antitumor effect, particularly reducing the recruitment of myeloid-derived suppressor cells (MDSCs) and angiogenic cytokines to the TME.64
Tumor-associated endothelial cells (TECs) as a target for gene therapy
Tumor vasculature exhibits fundamentally different phenotypes from normal vasculature due to its disorganized structure and uneven vessel lumen diameter.65 Tumor blood vessels are often heterogeneous, dysfunctional, and leaky due to discontinuous basement membranes, incomplete endothelial linings, and inadequate pericyte coverage.66 The increased transcapillary flow and the abnormal or absence of lymphatic vessels in the tumor lead to an accumulation of interstitial fluid and subsequent elevated IFP. Furthermore, increased IFP, inconsistent lumen resistance, and abnormal vasculature result in blood stagnation and bi-directional blood flow that can significantly impair uniform vector and virus delivery.67 Although the leakiness of the vessels allows extravasation of the virus into the subendothelial regions, the high IFP and dense stromal matrix inhibit the convection of vectors and OVs from the periphery to the core tumor regions.
Strategies for targeting TECs using gene therapy and armed OVs
Targeting VEGF/VEGF receptor (VEGFR)
In cancer, targeting angiogenesis to block oxygen and nutrient supplies has attracted considerable attention, and several inhibitors, including bevacizumab and sunitinib, have been licensed for cancer treatment.68 VEGF acts as the key mediator of physiological angiogenesis to support the growth and remodeling processes of blood vessels by binding to and activating VEGFR-1 or VEGFR-2 expressed on vascular endothelial cells.69 Nonetheless, targeting the vasculature with highly selective VEGF signaling inhibitors (such as bevacizumab) used as monotherapy does not show significant anti-tumor activity in many clinical settings. Several unarmed OVs have been reported to exhibit direct anti-vascular properties,70,71 and a recent publication from Yousaf et al.72 suggests that in the case of Ad this may be due to virus-mediated suppression of HIF-1a, decreasing local VEGF levels. Nevertheless, armed OVs expressing antiangiogenic agents generally show more powerful anti-vascular effects.73
Replication-competent VV encoding anti-VEGF scFv has been shown to inhibit angiogenesis and formation of malignant effusion in xenograft mouse models of lung and prostate cancer.74,75 It also re-sensitized endothelial cells to ionizing irradiation.76 The combination of virus with irradiation facilitated viral replication and synergistically impaired the formation of tumor vasculature in a glioma xenograft mouse model. Arming vvDD with soluble VEGFR-1 fused with an Fc domain reduced the viral dose required to achieve a similar level of anti-tumor effect compared with unarmed vvDD in a xenograft mouse model of renal cancer, while lowering the potential toxicity and unwanted cytokine release.73
Non-replicating Ad-Flk1-Fc expressing VEGFR-2-Fc also inhibited microvessel formation.77 The antiangiogenic and antitumor activity of Ad-Flk1-Fc was further enhanced upon co-infection with replication-competent E1A-attenuated Ad strain dl922/924, which acts as helper virus to support replication and repackaging of Ad-Flk1-Fc by providing E1 functions in trans.78 In addition, Hou et al.79 reported that the timing of Ad-Flk1-Fc administration was also critical for the potency of the anti-tumor response in a xenograft mouse model of breast cancer. Intravenous injection of Ad-Flk1-Fc 7 days after the vvDD injection showed greater tumor regression than did co-injection, as Flk1-Fc blocked the revascularization after vvDD clearance.
Antivascular strategies targeting chemokine receptor 4 (CXCR4)
CXCL12 mediates an indirect angiogenesis program by recruiting CXCR4-expressing bone marrow-derived monocytes to the perivascular regions of the TME. The recruited monocytes then release angiogenic factors such as VEGF and angiopoietin, which subsequently recruit endothelial and pericyte progenitors for neovascularization.80. CXCL12-stimulated CXCR4 has also been shown to positively regulate VEGF promoter activity and VEGF secretion via AKT81 and also by inhibiting glycolytic enzyme phosphoglycerate kinase 1 (PGK1) expression and activating angiogenesis-associated genes.82
Systemic delivery of a VV expressing CXCR4 fused with murine Fc domain (CXCR4-mFc) was reported to disrupt tumor vasculature in both the tumor core and periphery and reduce the levels of CXCL12, VEGF, endothelial progenitor cells (EPCs), and MDSCs in tumor perfusion in an orthotopic breast cancer model.83 The suppression of lung metastasis in this tumor model further suggested that both VV-mediated direct cytotoxicity and the anti-tumor immunity (as detected by a sustained and elevated level of tumor-specific antibody in serum) contributed to the observed tumor regressions. It also generated immunological memory against tumor antigens as demonstrated by the resistance to tumor re-challenge. The post-operative setting, where the primary tumors are resected before oncolytic virotherapy, showed higher viral titers in the lung metastases than the pre-operative setting, further highlighting the importance of the timing of OV delivery.83 In additiona, CXCR4-mFc enhanced VV-disrupted immunosuppression by reducing the recruitment of Tregs and augmenting the ratio of IFNγ/interleukin (IL)-10-producing CD4+ and CD8+ lymphocytes in an ovarian cancer syngeneic model.84 These and other studies targeting the TECs are summarized in Table 2.
Table 2.
Preclinical studies of antibody-armed oncolytic viruses targeting tumor vasculature
| Target | Virus (species) | Antibody form (promoter) | Effect ex vivo/in vivo | Delivery route (frequency) | Ref. |
|---|---|---|---|---|---|
| VEGF/VEGFR | GLV-1h107; 1h108; 1h109 (VV) | anti-VEGF scFv (PSE, PSEL, PSL) | lung, prostate cancer/s.c. in nude mice: ↓ tumor mass; ↓ MVD (IHC, CD31, 21 dpi) | i.v. (1) | 74 |
| VEGF/VEGFR | GLV-1h109 (VV) | anti-VEGF scFv (PSL) | soft tissue sarcoma, prostate cancer/s.c. in nude mice: ↓ tumor mass; ↓ MVD (IHC, CD31, 7 dpi); ↑ intratumoral GR-1highCD11b+ granulocyte, GR-1intCD11b+ monocyte, and F4/80+CD45+ macrophage (FC, 7 dpi) | i.v. (1) | 85 |
| VEGF/VEGFR | GLV-108 (VV) | anti-VEGF scFv (PSEL) | lung cancer/s.c. in nude mice: ↓ tumor mass, malignant effusion |
i.v. (1) | 75 |
| VEGF/VEGFR | GLV-1h164 (VV) | anti-VEGF scFv (PSE) | lung, prostate cancer/s.c. in nude mice: ↓ tumor mass; ↓ MVD (IHC, CD31, 36 dpi) breast cancer/o.t. in nude mice: ↓ tumor mass; ↓ MVD (Doppler ultrasonography, 21 dpi) | r.o. (1) | 61,86 |
| VEGF/VEGFR | GLV-5b451 (VV) | anti-VEFG scFv (PSEL) | soft tissue sarcoma/s.c. in nude mice: ↓ tumor mass; ↓ MVD (IHC, CD31, 17 dpi) mammary cancer/s.c. in nude mice: ↓ tumor mass; ↓ MVD (IHC, CD31, 28 dpi) | i.v. (1) i.v. (1) |
87,88 |
| VEGF/VEGFR | AAVrh10.BevMab (AAV) | anti-VEGF IgG (bevacizumab) (CAG promoter) | ovarian cancer/i.p. in BALB/c nude mice: ↓ tumor mass; ↓ MVD (IHC, CD31, 24 dpi) | i.p. (1) | 89 |
| VEGF/VEGFR | Ad5/3-9HIF-Δ24-VEGFR-1-Ig (Ad) | soluble VEGFR1-Fc fusion protein (E3 promoter) | renal cancer/s.c. in nude mice: ↓ tumor mass; ↓ MVD (IHC, VWF, 16 dpi) | i.t. (1) | 90 |
| VEGF/VEGFR | vvDD-VEGFR-1-Ig (VV) | soluble VEGFR1-Fc fusion protein (PSEL) | renal cancer/s.c. in nude mice: ↓ tumor mass; ↓ MVD (IHC, VWF, 36 dpi) renal cancer/s.c. in nude mice: ↓ tumor mass; ↓ MVD (IHC, VWF, 24 dpi); ↑ effector cytokine (IFNγ, CCL5, MCP-1) renal cancer/s.c. in BALB/c mice: ↓ tumor mass; ↑ effector cytokine (IFNγ, CCL5, MCP-1) |
i.t. (1) i.v.(1) i.v. (1) |
73 |
| VEGF/VEGFR | Ad-Flk1-Fc (Ad) | soluble Flk1-Fc (CMV) | lung cancer, fibrosarcoma/s.c. in C57BL/6 mice: ↓ tumor mass; ↓ MVD (IHC, CD31; corneal micropocket assay) | i.v. (1) | 77 |
| VEGF/ VEGFR | Ad-Flk1-Fc (Ad) + dl922/947 (Ad) | soluble Flk1-Fc (CMV) | prostate, colorectal cancer/s.c. in CD1, SCID mice: ↓ tumor mass; ↓ MVD (IHC, CD31, 23 dpi) | Ad-Flk-Fc: i.t. (1) dl992/947: i.t. (3; 0, 2, 4 dpi) | 78 |
| VEGF/ VEGFR | vvDD (VV) + Ad-Flk1-Fc (Ad) |
anti-Flk1-Fc (CMV) | breast cancer/s.c. in nude mice: ↓ tumor mass |
i.v. (1) | 79 |
| CXCR4/CXCL12 | OVV-CXCR4-A-Fc (VV) | soluble CXCR4-A-Fc (PSEL) | ovarian cancer/o.t. in C57BL/6 mice: ↓ tumor mass, metastasis, cancer-initiating cells; ↑ survival; ↓ ascitic CXCL12, VEGF (ELISA, ~33 dpi); ↓ ascitic EPCs, MDSCs, pDCs, Tregs (FC, ~33 dpi); ↑ ascitic IFNγ/IL-10 CD4 CD8 T cell ratio (FC, ~33 dpi) | i.p. (3; 0, 7, 14 dpi) | 84 |
| CXCR4/CXCL12 | OVV-CXCR4-A-Fc (VV) | soluble CXCR4-A-Fc (PSEL) | breast cancer/o.t. in BALB/c mice: ↓ metastasis; ↓ MVD (IHC, CD31, Ki67, 14 dpi); ↓ intratumoral CXCL12, VEGF (ELISA, 14 dpi); ↓ intratumoral EPCs, MDSCs (FC, 14 dpi); ↑ serum antitumor antibody | i.p. (7; 0, 1, 2, 3, 4, 5, 6 dpi) | 83 |
| FAP | GLV-1h282 (VV) | anti-FAP scFv (PSEL) | lung, prostate/s.c. in nude mice: ↓ tumor mass; ↓MVD (IHC, CD31, 36 dpi) | r.o. (1) | 61 |
| VEGF/VEGFR + FAP | GLV1h446 (VV) | anti-FAP scFv (PSEL), anti-VEGF scFv (PSEL) | lung, prostate/s.c. in nude mice: ↓ tumor mass; ↓ MVD (IHC, CD31, 36 dpi) | r.o. (1) | 61 |
| VEGF/VEGFR | GLV-1h164 (VV) + radiation | anti-VEGF scFv (PSL) | glioma/s.c. in nude mice: ↓ tumor mass; ↓ MVD (IHC, CD31, 14 dpi); ↑ viral replication (CSLM, VV-GFP, 7, 14 dpi); ↑ radiosensitivity of TEC; ↓ intratumoral VEGF (ELISA, 3, 7, 14 dpi) | OV: r.o. (1) IR: (4; −1, 1, 6, 8 dpi) |
76 |
CAG, cytomegalovirus enhancer chicken β-actin promoter; CCL5, chemokine ligand 5; CSLM, confocal laser scanning microscopy; CXCL12, C-X-C motif chemokine ligand 12; EPC, endothelial progenitor cell; FC, flow cytometry; Flk-1, fetal liver kinase-1; HIF, hypoxia-inducible factor; HNSCC, head and neck squamous cell carcinoma; HSV, herpes simplex virus; IgG, immunoglobulin G; IHC, immunohistochemistry; i.p., intraperitoneal; i.t., intratumoral; i.v., intravenous; MCP-1, monocyte chemoattractant protein-1; MDSC, myeloid-derived suppressor cell; MVD, micro-vessel density; o.t., orthotopic; pDC, plasmacytoid dendritic cell; PSE, synthetic early promoter; PSL, synthetic late promoter; SCID, severe combined immunodeficiency; Treg, regulatory T cell; VWF, von Willebrand factor.
Vaccines for targeting TECs
A DNA vaccine expressing VEGFR2 has been reported to break peripheral T cell tolerance and exploit upregulation of VEGFR2 to elicit T cell-mediated killing of tumor vascular endothelial cells without impairing hematopoiesis and fertility in a syngeneic tumor mice model.91 In addition, non-human primates vaccinated with VEGF protein combined with a proteoliposome adjuvant can also break peripheral tolerance and elicit VEGF-neutralizing antibodies and a T cell response to VEGF-presenting cells.92 DNA vaccines targeting endoglin93 and delta-like ligand 4 (DLL4),94 expressed on endothelial tip cells during angiogenic sprouting, have also been reported to regress tumor formation and neoangiogenesis without affecting wound healing in murine models.
In contrast to the monovalent vaccine, placenta-derived endothelial cells primed with IFNγ, named ValloVax, is a whole cell-based polyvalent vaccine. The idea of using placenta as the source of antigens is based on the immunological similarities between pregnancy and cancer. Endothelial cells from placenta and tumors not only share many molecules for angiogenesis such as VEGF and angiopoietin receptors but also express similar tumor endothelial-like endosialin and roundabout guidance receptor 4 (ROBO4), suggesting that they may share a relatively comparable angiogenic mechanism and microenvironment.95 Immunization with ValloVax regressed tumor formation in syngeneic mice bearing melanoma, lung, and breast cancer96. Clinical trials showed that ValloVax immunization elicited antibody response against antigens associated with angiogenesis, including VEGFR1, VEGFR2, endoglin, and fibroblast growth factor receptor (FGFR).95,96
Gene therapies encoding antiangiogenic proteins
Several gene therapy strategies that express antiangiogenic proteins have shown promising results in preclinical and clinical trials. The recombinant pseudotyped AAV serotype 2/8 vector97 and human placenta-derived mesenchymal stem cells (MSCs) with fiber-modified Ad vector98 carrying the plasminogen kringle 1–5 (K1–5) gene, a 50-fold stronger inhibitor than angiostatin,99 reduced neovascularization and regressed primary and metastatic tumors in a syngeneic mouse model. In addition, MSCs expressing a bifunctional fusion protein composed of antiangiogenic tumstatin45–132 and tumor necrosis factor (TNF)-α promoted a death receptor-dependent apoptotic pathway and suppressed proliferation of both tumor and endothelial cells in a prostate cancer xenograft mouse model100. Similarly, electrotransfer of plasmids carrying recombination human disintegrin domain (RDD) showed an inhibitory effect on endothelial cell growth in a cancer-specific manner in a melanoma syngeneic mouse model.101 In a related strategy, NK4 is an angiogenesis inhibitor that acts as a competitive antagonist of HGF, which induces VEGF expression102. Gene transfer of NK4 by Ad vector,103 electrotransfer,104 or MSCs105 demonstrated significant suppression of angiogenesis with potent antitumor activity that could be usefully combined with cisplatin106 or gemcitabine.107
Apart from expressing antiangiogenic proteins or soluble receptors that bind to angiogenic molecules, another interesting approach has been the silencing of the angiogenic protein endoglin, a co-receptor of TGF-β, using small interference RNA (siRNA)108 and short hairpin RNA (shRNA)109 under the control of the endothelin-1 promoter. This new strategy was shown to suppress angiogenesis and tumor growth in mammary adenocarcinoma108 and melanoma109 syngeneic mouse models.
Tumor immune cells as a target for gene and virotherapy
During the early phase of tumor formation, innate and adaptive immune cells are thought to migrate to the tumor to destroy cancer cells and secrete cytokines to sustain a pro-inflammatory environment. However, these cells are later “educated,” directly or indirectly, by tumor-secreted factors, and acquire pro-tumorigenic phenotypes. For example, under the influence of tumor secreted CSF-1, type I cytokine-producing M1 macrophages repolarize into key immunosuppressive M2 TAMs.4 They secrete C-C motif chemokine ligand 22 (CCL22) to recruit Tregs to the TME.110 M2 TAMs and Tregs mediate tumor tolerance by secreting immunosuppressive cytokines such as TGF-β and IL-10 and by expressing ligands for programmed cell death 1 (PD-1) and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4).1,111 Moreover, not all activated T cells are similarly potent in killing tumor cells. Persistent activation drives T cells to an “exhausted” state, in which they have high levels of inhibitory receptors and a transcription profile distinctly different from their effector or memory counterparts, resulting in a loss of proliferative and cytotoxic potential.112 In addition, MDSCs promote immune suppression by impairing lymphocyte homing, upregulating immune regulatory molecules B7 and PD-L1, and suppressing the expression of TCR through free radical production and metabolic depletion.113 The respective functions of immune cells in the TME were summarized by Gajewski et al.114 Tumor-associated immune cells therefore provide compelling target populations for OVs, with key studies identified in Table 3.
Table 3.
Preclinical studies of antibody-armed oncolytic viruses targeting tumor immune cells
| Target | Virus (species) | Antibody form (promoter) | Effect ex vivo/in vivo | Delivery route (frequency) | Ref. |
|---|---|---|---|---|---|
| FRβ | EnAd-3FR (Ad11p/Ad3) | anti-FRβ/CD3 BiTE (MLP) | patient-derived malignant ascites: ↓ ascitic CD11b+CD64+ macrophages (FC); ↑ T cell activation (FC); ↑ TAM repolarization (FC, CD80, CD86); ↑ IFNγ (ELISA) | N/A (ex vivo) | 115 |
| PD-1/PD-L1 | WR-mAb1 (VV) | anti-PD-1 IgG | fibrosarcoma/s.c. in C57BL/6 mice: ↓ tumor mass; ↑ survival; ↑ intratumoral CD4+, IFNγ+TNF-α+CD8+ T cell, CD8+/Foxp3+ T cell ratio, ↓ Tregs (FC; 7 dpi) | i.t. (2; 0, dpi) | 116 |
| PD-1/PD-L1 | MV-aPD-L1 (MV) | anti-PD-L1 scFv-Fc | melanoma/s.c. in C57BL/6 mice: ↓ tumor mass; ↑ survival; ↑ IFNγ+CD8 T cells, CD8+/Treg ratio (FC; 5 dpi) primary melanoma biopsy: ↓ tumor mass | i.t. (5; 0, 1, 2, 3, 4 dpi) N/A (ex vivo) |
117 |
| PD-1/PD-L1 | OVH-aMPD-1 (HSV) | anti-PD-1 scFv (CMV promoter) | hepatoma/s.c. in C57BL/6 mice: ↓ local, distant tumor mass; ↑ survival; ↓ s.c. rechallenged tumor; ↑ local and distant intratumoral CD4+CD69, CD4+ICOS+, CD8+CD69, CD8+ICOS+, MDSCs (FC, 10 dpi) |
i.t. (2; 0, 3 dpi) | 118 |
| CTLA-4 | MV-aCTLA-4 (MV) | anti-CTLA-4-scFv-Fc | melanoma/s.c. in C57BL/6 mice: ↓ tumor mass; CD8+/Treg ratio (FC, 5 dpi) | i.t. (5; 0, 1, 2, 3, 4 dpi) | 117 |
| CTLA-4 | SKL002 (Ad) | anti-CTLA-4 IgG (E3 promoter) | melanoma/s.c. in C57BL/6 mice: ↓ tumor mass, metastasis |
i.v. (3; every 3 days) i.t. (4; every other day) |
119 |
| CTLA-4 | Ad5/3-Δ24aCTLA4 (Ad) | anti-CTLA-4 IgG (CMV promoter) | prostate and lung cancer/s.c. in nude mice: ↓ tumor mass; ↑ tumor apoptosis (IHC, active caspase-3, 5 dpi) PBMCs from patients with solid tumor refractory to chemotherapy: ↑ IL-2, IFNγ (FC) | i.t. (3; 0, 2, 4 dpi) N/A (ex vivo) |
120 |
| CTLA-4 | IAV-CTLA4 (orthomyxovirus) | anti-CTLA-4 scFv | Melanoma/i.d. in C57BL/6 mice: ↓ local, distant tumor mass; ↑ survival |
i.t. (4; 0, 2, 4, 6 dpi) | 121 |
| CTLA-4 | virus 27 (HSV) | anti-CTLA-4 Ab-like molecule | lymphoma/s.c. in BALB/c mice: ↓ local, distant tumor mass |
i.t. (3; every 2 days) | 122 |
| CTLA-4 | rNDV-CTLA-4 (NDV) + radiation | anti-CTLA-4 scFv | melanoma/i.d. in C57BL/6 mice: ↓ tumor mass; ↑ survival |
i.t. (5; 0, 2, 4, 6, 8 dpi) | 123 |
| TGF-β/ TGF-βR | rAd.sT (Ad) | soluble TGFβRII-Fc (TERT promoter) | breast, renal cancer/s.c. in BALB/c mice: ↓ tumor mass, metastasis; ↓ metastasis-related genes (PTHrP, CXCR4), angiogenesis-related genes (VEGFA, VEGFR) (qPCR, 5 dpi); ↑ intratumoral IFNγ, TNF-α, IL-2 (qPCR, 5 dpi); ↑ CD8+/CD4+ T cells ratio, CD44highCD62Lhigh memory T cells in PBMCs (FC, 5 dpi); ↓ MDSCs (FC, 5 dpi); ↓ splenic Tregs (FC, 24 dpi) | i.p. (2; 0, 3 dpi) | 124 |
| TGF-β/ TGF-βR | mAd.sTβRFc (Ad5/48) | soluble TGFβRII-Fc | prostate cancer injected into left heart ventricle of nude mice (bone metastasis model): ↓ tumor mass, bone metastasis; ↑ survival; ↓ tumor-induced osteolytic bone destructions (microCT, TRAP, 43 dpi) | i.v. (2; 0, 3 dpi) | 125,126 |
| TGF-β/ TGF-βR | Ad.sTβRFc (Ad dl01/07) mhTERTAd.sTβRFc (Ad dl01/07) | soluble TGFβRII-Fc | breast cancer injected into left heart ventricle of nude mice (bone metastasis model): ↓ tumor mass, bone metastasis; ↑ survival; ↓ tumor-induced osteolytic bone destructions (microCT, TRAP, 20 dpi) | i.v. (2; 0, 3 dpi) | 127 |
Ab, antibody; CTLA-4, cytotoxic T lymphocyte-associated protein 4; CMV, cytomegalovirus; CXCR4, C-X-C motif chemokine receptor 4; FC, flow cytometry; FRβ, folate receptor-β; i.d., intradermal; i.p., intraperitoneal; i.t., intratumoral; i.v., intravenous; IVA, influenza A virus; microCT, microcomputed tomography; MV, measles virus; NDV, Newcastle disease virus; o.t., orthotopic; PBMC, peripheral blood mononuclear cell; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; PTHrP, parathyroid hormone-related protein; TGF-β, transforming growth factor β; TGF-βRII, TGFβ receptor II; TNF-α, tumor necrosis factor α; TRAP, tartrate-resistant acid phosphatase; vvDD: vaccinia virus Western Reserve strain.
OVs creating a proinflammatory environment
OVs augment the process of antigen presentation and T cell priming by providing three crucial signals, i.e., release of cancer antigens following cell lysis, co-stimulation by release of PAMPs and DAMPs, and production of proinflammatory cytokines.128 Following the recognition of OVs as “foreign invaders” by the innate immune system, the release of type I IFNs often enhances the expression of MHC class I and II molecules and costimulatory signals on dendritic cells (DCs).129,130 Meanwhile, tumor antigens are released by cell lysis and may be cross-presented by DC-activated tumor-specific cytotoxic T cells. This process of uptake and cross-presentation of antigen by DCs is the central mechanism of epitope spreading, and it is essential for the recognition of tumor neoantigens.131,132 Epitope spreading facilitates the development of more diversified T cell clones against the tumors. Notably, an oncolytic Ad was reported to promote an oligoclonal T cell response against a panel of neoepitopes, while anti-PD-1 antibody was only able to elicit immune response against a single neoepitope in a murine lung adenocarcinoma model.133
Impairment of antigen presentation is one of the primary methods for tumor immune evasion. OVs have some potential to reverse this escape mechanism and thereby promote the APC-T cell interaction.128 For example, reovirus infection induced the expression of MHC class I, β2-microglobulin (β2M), transporter associated with antigen processing 1 (TAP-1), and TAP-2 on the tumor surface.134 Reovirus also restored the expression of MHC class II, CD40, CD80, and CD86 expression on DCs, which are initially downregulated by the tumors, and restored their overall antigen presentation ability to T cells. In addition, T cell priming was augmented by reovirus-induced proinflammatory cytokines such as TNF-α, granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL-6, which promoted DC maturation, antigen presentation, and trafficking of T cells and macrophages to the tumor region.135 This induction of type I IFN by OV can provide the third signal necessary for naive T cells to acquire effector phenotypes.136,137 OVs also promoted the tumor infiltration of DCs and promote their homing to the lymphoid organs for T cell priming.138
Nevertheless, one major challenge facing the field is how to direct the adaptive immune response towards recognition of tumor neoantigens rather than viral epitopes,139 particularly since the neoantigens are generally thought to comprise relatively weak immunogens. Considerable attention in the field is focused toward this goal, building on the other powerful attributes of the oncolytic approach.140
Immunosuppressive populations suppress efficacy of oncolytic virotherapy
Despite the pro-inflammation responses during oncolysis, anti-tumor activity is still partly impaired by peripheral tolerance. Sobol et al.139 reported that the ability of OV to induce CD8+ T cell responses against tumor-associated antigens (TAAs) was attenuated in a tolerized antigen model, potentially reflecting central tolerance or peripheral tolerance mediated by Tregs, TGF-β, and IL-10. In addition, TAMs can secrete macrophage-derived IFNβ and confer upon tumors a constitutive antiviral state with resistance to virotherapy by upregulating IFN-stimulated genes.141 In support of this, systemic depletion of CD11b+ myeloid cells has been shown to enhance oncolytic HSV anti-tumor responses, suggesting that targeted depletion of immunosuppressive macrophages may restore OV-mediated anti-tumor efficacy.142
Strategies for targeting immunosuppressive cells using OVs
Immunotherapy targeted to folate receptor β (FRβ)
FRβ was identified as a differential expressed marker on M2-polarized macrophages in vitro.143 Although a strong association of FRβ with anti-inflammation and tumor progression has been observed, only a few therapeutic approaches targeting FRβ have been reported. An earlier study reported the use of chimeric antigen receptor (CAR) T cells expressing anti-FRβ scFv to regress acute myeloid leukemia (AML) in a human xenograft mouse model, and the antitumor effect was augmented by FRβ-inducing agent all-trans retinoic acid (ATRA)144. Importantly, the CAR T cells were not toxic to normal healthy hematopoietic stem and progenitor cells (HSCs) or monocytes expressing low levels of FRβ. An anti-FRβ/CD3 BiTE was later reported and encoded in EnAd for targeting M2 macrophages. The armed OV triggered endogenous T cell activation and depleted the M2 macrophages within primary samples of malignant ascites from cancer patients, sparing the more pro-inflammatory M1 macrophages.115
Targeting PD-1/PD-L1
PD-1 is an inhibitory receptor expressed on activated T cells, B cells, natural killer (NK) cells, and some myeloid populations, yet its immunological functions are best characterized in conventional T cells. Persistent antigen stimulation (cancer or chronic infection) drives T cells to an exhausted state as indicated by PD-1 expression. Tumors express PD-L1 and PD-L2, which act as inhibitory signals to impair T cell effector functions and assist in immune evasion.145 Many OVs armed with anti-PD-1/PD-L1 antibodies have shown promising results in syngeneic melanoma mouse models. For example, measles virus (MV) encoding anti-PD-L1 scFv fused with Fc domain (MV-aPD-L1) demonstrated a greater cytotoxic potential than did the unarmed MV by increasing tumor-infiltrating IFNγ-producing CD8+ T cells and the ratio of CD8+ T cells to Tregs.117 This armed MV also killed primary melanoma ex vivo. In addition, Kleinpeter et al.116 reported that VV encoding anti-PD-1 immunoglobulin (Ig)G or scFv showed better fibrosarcoma regression and overall survival than did the unarmed virus in a syngeneic mouse model, although no significant difference was observed in CD4+, CD8+, and Treg populations. In addition, unarmed HSV was reported to induce a type I IFN response, which upregulated PD-L1 expression in the TME.118 An anti-PD-1 scFV-armed HSV enhanced phagocytosis of infected cancer cells and improved antigen presentation by DCs as compared to unarmed HSV. It regressed primary and distant tumor formation apparently by enhancing the activation of intratumoral CD4+ and CD8+ T cells in a syngeneic liver cancer model, although an infiltration of CD155+ MDSCs was observed. Long-term surviving mice were also protected in tumor rechallenge studies. Blockade of T cell immunoreceptor with Ig and ITIM domains (TIGIT), a receptor of CD155, further augmented the therapeutic efficacy. All three aforementioned studies, i.e., the armed MV, VV, HSV, showed similar therapeutic responses to the combined therapy of unarmed OVs with antibodies, confirming that the therapeutic effect is not virus type- or antibody form-dependent.
Targeting CTLA-4
CTLA-4 is an immune checkpoint that is closely associated with the modulation of T cell activity. CTLA-4 dampens TCR signaling by binding to B7-1 (CD80) and B7-2 (CD86) with higher affinity and avidity than CD28. Secondary lymphoid organs, primarily lymph nodes, are the sites of T cell priming, where CTLA-4 primarily regulates T cell activity.146 Anti-CTLA-4 monoclonal antibody (mAb)-armed Ads Ad5/3-Δ24aCTLA4120 and SKL002119 enhanced anti-tumor activity without affecting antiviral immunity or the viral titers in a xenograft mouse model of lung and prostate cancer. The combined therapy of SKL002 with GM-CSF-armed Ad further regressed the formation of lung tumor in the syngeneic mouse model. Nonetheless, Ad5 does not replicate productively in mice and may not fully represent the immune responses in humans. Recently, Hamilton et al.121 engineered the replication-competent influenza A virus (IAV) and inserted the anti-CTLA-4 scFv gene segment downstream of PB1 and PA viral polymerase, restricting the antibody expression during the viral life cycle. The armed IAV not only inhibited the growth of treated tumor but also demonstrated abscopal effects to regress distal tumor formation and prolong survival in a syngeneic melanoma mouse model. In addition, Engeland et al.117 reported that anti-CTLA-4 scFv-armed MV encoding (MV-aCTLA-4) increased the ratio of tumor-infiltrating CD8+ T cells to Tregs and the IFNγ response. Although the armed MV significantly regressed tumor formation in the syngeneic melanoma model, mice receiving armed MV injections showed inferior overall survival than did those receiving combination treatments of unarmed MV and systemic CTLA-4 antibody. Interestingly, Newcastle disease virus (NDV) expressing anti-CTLA-4 scFv induced potent anti-tumor responses comparable to systemic anti-CTLA-4 in combination with ionizing irradiation.123 Systemic anti-tumor responses were also observed in an armed HSV-1, which expressed anti-CTLA-4 antibody-like molecule, GM-CSF, and a fusogenic form of the envelope glycoprotein of gibbon ape leukemia virus (GALV-GP-R−).122
Future perspectives
Targeting the TME is still a relatively new concept in gene and virotherapy, and novel strategies continue to emerge. Of all of the challenges faced, those provided by CAFs and other immunosuppressive cells are perhaps the greatest since their many facets affect many different aspects of cancer biology as well as limiting the effectiveness of different therapeutic approaches.
Essentially, how to deplete CAFs and other immunosuppressive cells effectively and specifically comprises a major research direction, but one in which molecular approaches are uniquely able to address. Encouragingly, worldwide attention is increasingly being focused on these aspects of the TME, and particularly on reversing its local immunosuppressive effects. Depletion of CAFs is being explored in clinical trials using OVs expressing CAF-specific BiTEs for targeted T cell cytotoxicity, and it is encouraging that BiTEs recognizing FRβ115 have also been reported to selectively deplete M2 macrophages. There are also additional potential targets that have not been received much attention, for instance, fibroblast specific-protein-1 (FSP1) is a CAF marker that is present in multiple cancer stroma.147 Another CAF population expresses a different set of markers, including α-smooth muscle actin (α-SMA), neuron-glial antigen-2 chondroitin sulfate proteoglycan (NG2), and platelet-derived growth factor receptor-β (PDGRβ).148 Targeting multiple CAF markers using locally expressed BiTEs could allow simultaneously depletion of different CAF populations and may facilitate reversal of their deleterious effects.
Exploration of more sophisticated tri- or quadri-constructs, aiming at improving the efficacy of the T cell engager, is an important research area. One approach is to incorporate multiple domains targeting the antigens or CD3 to increase the avidity of the BiTE to the target cells or T cells and enhance the BiTE-mediated cytotoxicity. Another strategy is to incorporate an additional domain targeting the costimulatory signals such as CD28 and 4-1BB. Anti-CD28 bispecific antibody has been reported to improve the antitumor activity of anti-CD3 BiTE by enhancing the formation of “pseudo” immune synapse.149
Furthermore, exploring NK cells and macrophages as effector cells to which bispecific antibodies can crosslink the target cells may exploit new mechanisms of innate antitumor immunity. For example, NK cell-mediated cytotoxicity was enhanced by anti-CD16/CD33 bispecific killer cell engager (BiKE) against primary myelodysplastic syndromes (MDSs) and CD33+ MDSC populations.150 In 2019, a TriKE targeting CD16 and NKp46 on NK cell exhibited more potent immune responses with similar pharmacokinetics and improved unwanted effects compared with an IgG antibody targeting the same antigen.151 It follows that identifying gene and virotherapy strategies that can fully utilize the therapeutic power of NK cells and macrophages could unleash still more potent antitumor and anti-TME effects.
Acknowledgments
This work was supported by Cancer Research UK (grant no. C552/A29106).
Declaration of interests
L.W.S. owns equity or share options in PsiOxus Therapeutics, which is leading the clinical development of EnAd and its derivatives. The remaining authors declare no competing interests.
References
- 1.Balkwill F.R., Capasso M., Hagemann T. The tumor microenvironment at a glance. J. Cell Sci. 2012;125:5591–5596. doi: 10.1242/jcs.116392. [DOI] [PubMed] [Google Scholar]
- 2.Lamagna C., Bergers G. The bone marrow constitutes a reservoir of pericyte progenitors. J. Leukoc. Biol. 2006;80:677–681. doi: 10.1189/jlb.0506309. [DOI] [PubMed] [Google Scholar]
- 3.Motz G.T., Santoro S.P., Wang L.-P., Garrabrant T., Lastra R.R., Hagemann I.S., Lal P., Feldman M.D., Benencia F., Coukos G. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat. Med. 2014;20:607–615. doi: 10.1038/nm.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quail D.F., Joyce J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roma-Rodrigues C., Mendes R., Baptista P.V., Fernandes A.R. Targeting tumor microenvironment for cancer therapy. Int. J. Mol. Sci. 2019;20:840. doi: 10.3390/ijms20040840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang W.-W., Li L., Li D., Liu J., Li X., Li W., Xu X., Zhang M.J., Chandler L.A., Lin H. The first approved gene therapy product for cancer Ad-p53 (Gendicine): 12 years in the clinic. Hum. Gene Ther. 2018;29:160–179. doi: 10.1089/hum.2017.218. [DOI] [PubMed] [Google Scholar]
- 7.McGranahan N., Swanton C. Clonal heterogeneity and tumor evolution: Past, present, and the future. Cell. 2017;168:613–628. doi: 10.1016/j.cell.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Kakarla S., Song X.-T., Gottschalk S. Cancer-associated fibroblasts as targets for immunotherapy. Immunotherapy. 2012;4:1129–1138. doi: 10.2217/imt.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toro Bejarano M., Merchan J.R. Targeting tumor vasculature through oncolytic virotherapy: recent advances. Oncolytic Virother. 2015;4:169–181. doi: 10.2147/OV.S66045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchand J.-B., Semmrich M., Fend L., Rehn M., Silvestre N., Teige I., Foloppe F., Mårtensson L., Quéméneur E., Frendeus B. BT-001, an oncolytic vaccinia virus armed with a Treg-depletion-optimized recombinant human anti-CTLA4 antibody and GM-CSF to target the tumor microenvironment. Cancer Res. 2020;80(16 Suppl):5602. [Google Scholar]
- 11.Seymour L.W., Fisher K.D. Oncolytic viruses: Finally delivering. Br. J. Cancer. 2016;114:357–361. doi: 10.1038/bjc.2015.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhar D., Toth K., Wold W.S. Syrian hamster tumor model to study oncolytic Ad5-based vectors. Methods Mol. Biol. 2012;797:53–63. doi: 10.1007/978-1-61779-340-0_4. [DOI] [PubMed] [Google Scholar]
- 13.Guerin M.V., Finisguerra V., Van den Eynde B.J., Bercovici N., Trautmann A. Preclinical murine tumor models: A structural and functional perspective. eLife. 2020;9:e50740. doi: 10.7554/eLife.50740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamaguchi R., Perkins G. Animal models for studying tumor microenvironment (TME) and resistance to lymphocytic infiltration. Cancer Biol. Ther. 2018;19:745–754. doi: 10.1080/15384047.2018.1470722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida G.J. Applications of patient-derived tumor xenograft models and tumor organoids. J. Hematol. Oncol. 2020;13:4. doi: 10.1186/s13045-019-0829-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott E.M., Frost S., Khalique H., Freedman J.D., Seymour L.W., Lei-Rossmann J. Use of liquid patient ascites fluids as a preclinical model for oncolytic virus activity. Methods Mol. Biol. 2020;2058:261–270. doi: 10.1007/978-1-4939-9794-7_17. [DOI] [PubMed] [Google Scholar]
- 17.Hutt M., Färber-Schwarz A., Unverdorben F., Richter F., Kontermann R.E. Plasma half-life extension of small recombinant antibodies by fusion to immunoglobulin-binding domains. J. Biol. Chem. 2012;287:4462–4469. doi: 10.1074/jbc.M111.311522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamers-Casterman C., Atarhouch T., Muyldermans S., Robinson G., Hamers C., Songa E.B., Bendahman N., Hamers R. Naturally occurring antibodies devoid of light chains. Nature. 1993;363:446–448. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- 19.Hermiston T.W., Kuhn I. Armed therapeutic viruses: strategies and challenges to arming oncolytic viruses with therapeutic genes. Cancer Gene Ther. 2002;9:1022–1035. doi: 10.1038/sj.cgt.7700542. [DOI] [PubMed] [Google Scholar]
- 20.Robinson M., Ge Y., Ko D., Yendluri S., Laflamme G., Hawkins L., Jooss K. Comparison of the E3 and L3 regions for arming oncolytic adenoviruses to achieve a high level of tumor-specific transgene expression. Cancer Gene Ther. 2008;15:9–17. doi: 10.1038/sj.cgt.7701093. [DOI] [PubMed] [Google Scholar]
- 21.Wirth T., Zender L., Schulte B., Mundt B., Plentz R., Rudolph K.L., Manns M., Kubicka S., Kühnel F. A telomerase-dependent conditionally replicating adenovirus for selective treatment of cancer. Cancer Res. 2003;63:3181–3188. [PubMed] [Google Scholar]
- 22.DeWeese T.L., van der Poel H., Li S., Mikhak B., Drew R., Goemann M., Hamper U., DeJong R., Detorie N., Rodriguez R. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 2001;61:7464–7472. [PubMed] [Google Scholar]
- 23.Chen X., Song E. Turning foes to friends: Targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2019;18:99–115. doi: 10.1038/s41573-018-0004-1. [DOI] [PubMed] [Google Scholar]
- 24.Lakins M.A., Ghorani E., Munir H., Martins C.P., Shields J.D. Cancer-associated fibroblasts induce antigen-specific deletion of CD8 + T cells to protect tumour cells. Nat. Commun. 2018;9:948. doi: 10.1038/s41467-018-03347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKee T.D., Grandi P., Mok W., Alexandrakis G., Insin N., Zimmer J.P., Bawendi M.G., Boucher Y., Breakefield X.O., Jain R.K. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 2006;66:2509–2513. doi: 10.1158/0008-5472.CAN-05-2242. [DOI] [PubMed] [Google Scholar]
- 26.Erdogan B., Webb D.J. Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem. Soc. Trans. 2017;45:229–236. doi: 10.1042/BST20160387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henke E., Nandigama R., Ergün S. Extracellular matrix in the tumor microenvironment and its impact on cancer therapy. Front. Mol. Biosci. 2020;6:160. doi: 10.3389/fmolb.2019.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vähä-Koskela M., Hinkkanen A. Tumor restrictions to oncolytic virus. Biomedicines. 2014;2:163–194. doi: 10.3390/biomedicines2020163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng J., Sauthoff H., Huang Y., Kutler D.I., Bajwa S., Rom W.N., Hay J.G. Human matrix metalloproteinase-8 gene delivery increases the oncolytic activity of a replicating adenovirus. Mol. Ther. 2007;15:1982–1990. doi: 10.1038/sj.mt.6300264. [DOI] [PubMed] [Google Scholar]
- 30.Kim J.H., Lee Y.S., Kim H., Huang J.H., Yoon A.R., Yun C.O. Relaxin expression from tumor-targeting adenoviruses and its intratumoral spread, apoptosis induction, and efficacy. J. Natl. Cancer Inst. 2006;98:1482–1493. doi: 10.1093/jnci/djj397. [DOI] [PubMed] [Google Scholar]
- 31.Choi I.K., Lee Y.S., Yoo J.Y., Yoon A.R., Kim H., Kim D.S., Seidler D.G., Kim J.H., Yun C.O. Effect of decorin on overcoming the extracellular matrix barrier for oncolytic virotherapy. Gene Ther. 2010;17:190–201. doi: 10.1038/gt.2009.142. [DOI] [PubMed] [Google Scholar]
- 32.Jung K.H., Choi I.K., Lee H.S., Yan H.H., Son M.K., Ahn H.M., Hong J., Yun C.O., Hong S.S. Oncolytic adenovirus expressing relaxin (YDC002) enhances therapeutic efficacy of gemcitabine against pancreatic cancer. Cancer Lett. 2017;396:155–166. doi: 10.1016/j.canlet.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Thompson C.B., Shepard H.M., O’Connor P.M., Kadhim S., Jiang P., Osgood R.J., Bookbinder L.H., Li X., Sugarman B.J., Connor R.J. Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol. Cancer Ther. 2010;9:3052–3064. doi: 10.1158/1535-7163.MCT-10-0470. [DOI] [PubMed] [Google Scholar]
- 34.Guedan S., Rojas J.J., Gros A., Mercade E., Cascallo M., Alemany R. Hyaluronidase expression by an oncolytic adenovirus enhances its intratumoral spread and suppresses tumor growth. Mol. Ther. 2010;18:1275–1283. doi: 10.1038/mt.2010.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ganesh S., Gonzalez-Edick M., Gibbons D., Van Roey M., Jooss K. Intratumoral coadministration of hyaluronidase enzyme and oncolytic adenoviruses enhances virus potency in metastatic tumor models. Clin. Cancer Res. 2008;14:3933–3941. doi: 10.1158/1078-0432.CCR-07-4732. [DOI] [PubMed] [Google Scholar]
- 36.Bookbinder L.H., Hofer A., Haller M.F., Zepeda M.L., Keller G.A., Lim J.E., Edgington T.S., Shepard H.M., Patton J.S., Frost G.I. A recombinant human enzyme for enhanced interstitial transport of therapeutics. J. Control. Release. 2006;114:230–241. doi: 10.1016/j.jconrel.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Carbonero R., Gil Martín M., Alvarez Gallego R., Macarulla Mercade T., Riesco Martinez M.C., Guillen-Ponce C. Systemic administration of the hyaluronidase-expressing oncolytic adenovirus VCN-01 in patients with advanced or metastatic pancreatic cancer: First-in-human clinical trial. Ann. Oncol. 2019;30(Suppl 5):v271–v272. [Google Scholar]
- 38.Tedcastle A., Illingworth S., Brown A., Seymour L.W., Fisher K.D. Actin-resistant DNAse I expression from oncolytic adenovirus enadenotucirev enhances its intratumoral spread and reduces tumor growth. Mol. Ther. 2016;24:796–804. doi: 10.1038/mt.2015.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yasui T., Ohuchida K., Zhao M., Onimaru M., Egami T., Fujita H., Ohtsuka T., Mizumoto K., Tanaka M. Tumor-stroma interactions reduce the efficacy of adenoviral therapy through the HGF-MET pathway. Cancer Sci. 2011;102:484–491. doi: 10.1111/j.1349-7006.2010.01783.x. [DOI] [PubMed] [Google Scholar]
- 40.Nemerow G.R., Pache L., Reddy V., Stewart P.L. Insights into adenovirus host cell interactions from structural studies. Virology. 2009;384:380–388. doi: 10.1016/j.virol.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vähä-Koskela M.J., Kallio J.P., Jansson L.C., Heikkilä J.E., Zakhartchenko V.A., Kallajoki M.A., Kähäri V.M., Hinkkanen A.E. Oncolytic capacity of attenuated replicative Semliki Forest virus in human melanoma xenografts in severe combined immunodeficient mice. Cancer Res. 2006;66:7185–7194. doi: 10.1158/0008-5472.CAN-05-2214. [DOI] [PubMed] [Google Scholar]
- 42.Boire A., Covic L., Agarwal A., Jacques S., Sherifi S., Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120:303–313. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 43.Hanahan D., Coussens L.M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 44.Sottile J. Regulation of angiogenesis by extracellular matrix. Biochim. Biophys. Acta. 2004;1654:13–22. doi: 10.1016/j.bbcan.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Sahai E., Astsaturov I., Cukierman E., DeNardo D.G., Egeblad M., Evans R.M., Fearon D., Greten F.R., Hingorani S.R., Hunter T. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer. 2020;20:174–186. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lopez M.V., Rivera A.A., Viale D.L., Benedetti L., Cuneo N., Kimball K.J., Wang M., Douglas J.T., Zhu Z.B., Bravo A.I. A tumor-stroma targeted oncolytic adenovirus replicated in human ovary cancer samples and inhibited growth of disseminated solid tumors in mice. Mol. Ther. 2012;20:2222–2233. doi: 10.1038/mt.2012.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Viale D.L., Cafferata E.G., Gould D., Rotondaro C., Chernajovsky Y., Curiel D.T., Podhajcer O.L., Veronica Lopez M. Therapeutic improvement of a stroma-targeted CRAd by incorporating motives responsive to the melanoma microenvironment. J. Invest. Dermatol. 2013;133:2576–2584. doi: 10.1038/jid.2013.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jing Y., Chavez V., Ban Y., Acquavella N., El-Ashry D., Pronin A., Chen X., Merchan J.R. Molecular effects of stromal-selective targeting by uPAR-retargeted oncolytic virus in breast cancer. Mol. Cancer Res. 2017;15:1410–1420. doi: 10.1158/1541-7786.MCR-17-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garin-Chesa P., Old L.J., Rettig W.J. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc. Natl. Acad. Sci. USA. 1990;87:7235–7239. doi: 10.1073/pnas.87.18.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wikberg M.L., Edin S., Lundberg I.V., Van Guelpen B., Dahlin A.M., Rutegård J., Stenling R., Oberg A., Palmqvist R. High intratumoral expression of fibroblast activation protein (FAP) in colon cancer is associated with poorer patient prognosis. Tumour Biol. 2013;34:1013–1020. doi: 10.1007/s13277-012-0638-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sandberg T.P., Stuart M.P.M.E., Oosting J., Tollenaar R.A.E.M., Sier C.F.M., Mesker W.E. Increased expression of cancer-associated fibroblast markers at the invasive front and its association with tumor-stroma ratio in colorectal cancer. BMC Cancer. 2019;19:284. doi: 10.1186/s12885-019-5462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brennen W.N., Isaacs J.T., Denmeade S.R. Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol. Cancer Ther. 2012;11:257–266. doi: 10.1158/1535-7163.MCT-11-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hofheinz R.D., al-Batran S.E., Hartmann F., Hartung G., Jäger D., Renner C., Tanswell P., Kunz U., Amelsberg A., Kuthan H., Stehle G. Stromal antigen targeting by a humanised monoclonal antibody: An early phase II trial of sibrotuzumab in patients with metastatic colorectal cancer. Onkologie. 2003;26:44–48. doi: 10.1159/000069863. [DOI] [PubMed] [Google Scholar]
- 54.Narra K., Mullins S.R., Lee H.O., Strzemkowski-Brun B., Magalong K., Christiansen V.J., McKee P.A., Egleston B., Cohen S.J., Weiner L.M. Phase II trial of single agent Val-boroPro (talabostat) inhibiting fibroblast activation protein in patients with metastatic colorectal cancer. Cancer Biol. Ther. 2007;6:1691–1699. doi: 10.4161/cbt.6.11.4874. [DOI] [PubMed] [Google Scholar]
- 55.Roberts E.W., Deonarine A., Jones J.O., Denton A.E., Feig C., Lyons S.K., Espeli M., Kraman M., McKenna B., Wells R.J. Depletion of stromal cells expressing fibroblast activation protein-α from skeletal muscle and bone marrow results in cachexia and anemia. J. Exp. Med. 2013;210:1137–1151. doi: 10.1084/jem.20122344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Angell T.E., Lechner M.G., Jang J.K., LoPresti J.S., Epstein A.L. MHC class I loss is a frequent mechanism of immune escape in papillary thyroid cancer that is reversed by interferon and selumetinib treatment in vitro. Clin. Cancer Res. 2014;20:6034–6044. doi: 10.1158/1078-0432.CCR-14-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Freedman J.D., Duffy M.R., Lei-Rossmann J., Muntzer A., Scott E.M., Hagel J., Campo L., Bryant R.J., Verrill C., Lambert A. An oncolytic virus expressing a T-cell engager simultaneously targets cancer and immunosuppressive stromal cells. Cancer Res. 2018;78:6852–6865. doi: 10.1158/0008-5472.CAN-18-1750. [DOI] [PubMed] [Google Scholar]
- 58.de Sostoa J., Fajardo C.A., Moreno R., Ramos M.D., Farrera-Sal M., Alemany R. Targeting the tumor stroma with an oncolytic adenovirus secreting a fibroblast activation protein-targeted bispecific T-cell engager. J. Immunother. Cancer. 2019;7:19. doi: 10.1186/s40425-019-0505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Illingworth S., Di Y., Bauzon M., Lei J., Duffy M.R., Alvis S., Champion B., Lieber A., Hermiston T., Seymour L.W. Preclinical safety studies of enadenotucirev, a chimeric group B human-specific oncolytic adenovirus. Mol. Ther. Oncolytics. 2017;5:62–74. doi: 10.1016/j.omto.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu F., Hong B., Song X.-T. A T-cell engager-armed oncolytic vaccinia virus to target the tumor stroma. Cancer Transl. Med. 2017;3:122–132. [Google Scholar]
- 61.Huang T., Wang H., Chen N.G., Frentzen A., Minev B., Szalay A.A. Expression of anti-VEGF antibody together with anti-EGFR or anti-FAP enhances tumor regression as a result of vaccinia virotherapy. Mol. Ther. Oncolytics. 2015;2:15003. doi: 10.1038/mto.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wen Y., Wang C.-T., Ma T.-T., Li Z.-Y., Zhou L.-N., Mu B., Leng F., Shi H.S., Li Y.O., Wei Y.Q. Immunotherapy targeting fibroblast activation protein inhibits tumor growth and increases survival in a murine colon cancer model. Cancer Sci. 2010;101:2325–2332. doi: 10.1111/j.1349-7006.2010.01695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xia Q., Zhang F.-F., Geng F., Liu C.-L., Xu P., Lu Z.-Z., Yu B., Wu H., Wu J.X., Zhang H.H. Anti-tumor effects of DNA vaccine targeting human fibroblast activation protein α by producing specific immune responses and altering tumor microenvironment in the 4T1 murine breast cancer model. Cancer Immunol. Immunother. 2016;65:613–624. doi: 10.1007/s00262-016-1827-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Geng F., Guo J., Guo Q.-Q., Xie Y., Dong L., Zhou Y., Liu C.L., Yu B., Wu H., Wu J.X. A DNA vaccine expressing an optimized secreted FAPα induces enhanced anti-tumor activity by altering the tumor microenvironment in a murine model of breast cancer. Vaccine. 2019;37:4382–4391. doi: 10.1016/j.vaccine.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 65.Siemann D.W. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by tumor-vascular disrupting agents. Cancer Treat. Rev. 2011;37:63–74. doi: 10.1016/j.ctrv.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hashizume H., Baluk P., Morikawa S., McLean J.W., Thurston G., Roberge S., Jain R.K., McDonald D.M. Openings between defective endothelial cells explain tumor vessel leakiness. Am. J. Pathol. 2000;156:1363–1380. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carmeliet P., Jain R.K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 2011;10:417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 68.Vasudev N.S., Reynolds A.R. Anti-angiogenic therapy for cancer: Current progress, unresolved questions and future directions. Angiogenesis. 2014;17:471–494. doi: 10.1007/s10456-014-9420-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69(Suppl 3):4–10. doi: 10.1159/000088478. [DOI] [PubMed] [Google Scholar]
- 70.Ikeda Y., Kojima T., Kuroda S., Endo Y., Sakai R., Hioki M., Kishimoto H., Uno F., Kagawa S., Watanabe Y. A novel antiangiogenic effect for telomerase-specific virotherapy through host immune system. J. Immunol. 2009;182:1763–1769. doi: 10.4049/jimmunol.182.3.1763. [DOI] [PubMed] [Google Scholar]
- 71.Breitbach C.J., De Silva N.S., Falls T.J., Aladl U., Evgin L., Paterson J., Sun Y.Y., Roy D.G., Rintoul J.L., Daneshmand M. Targeting tumor vasculature with an oncolytic virus. Mol. Ther. 2011;19:886–894. doi: 10.1038/mt.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yousaf I., Kaeppler J., Frost S., Seymour L.W., Jacobus E.J. Attenuation of the hypoxia inducible factor pathway after oncolytic adenovirus infection coincides with decreased vessel perfusion. Cancers (Basel) 2020;12:851. doi: 10.3390/cancers12040851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guse K., Sloniecka M., Diaconu I., Ottolino-Perry K., Tang N., Ng C., Le Boeuf F., Bell J.C., McCart J.A., Ristimäki A. Antiangiogenic arming of an oncolytic vaccinia virus enhances antitumor efficacy in renal cell cancer models. J. Virol. 2010;84:856–866. doi: 10.1128/JVI.00692-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frentzen A., Yu Y.A., Chen N., Zhang Q., Weibel S., Raab V., Szalay A.A. Anti-VEGF single-chain antibody GLAF-1 encoded by oncolytic vaccinia virus significantly enhances antitumor therapy. Proc. Natl. Acad. Sci. USA. 2009;106:12915–12920. doi: 10.1073/pnas.0900660106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weibel S., Hofmann E., Basse-Luesebrink T.C., Donat U., Seubert C., Adelfinger M., Gnamlin P., Kober C., Frentzen A., Gentschev I. Treatment of malignant effusion by oncolytic virotherapy in an experimental subcutaneous xenograft model of lung cancer. J. Transl. Med. 2013;11:106. doi: 10.1186/1479-5876-11-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buckel L., Advani S.J., Frentzen A., Zhang Q., Yu Y.A., Chen N.G., Ehrig K., Stritzker J., Mundt A.J., Szalay A.A. Combination of fractionated irradiation with anti-VEGF expressing vaccinia virus therapy enhances tumor control by simultaneous radiosensitization of tumor associated endothelium. Int. J. Cancer. 2013;133:2989–2999. doi: 10.1002/ijc.28296. [DOI] [PubMed] [Google Scholar]
- 77.Kuo C.J., Farnebo F., Yu E.Y., Christofferson R., Swearingen R.A., Carter R., von Recum H.A., Yuan J., Kamihara J., Flynn E. Comparative evaluation of the antitumor activity of antiangiogenic proteins delivered by gene transfer. Proc. Natl. Acad. Sci. USA. 2001;98:4605–4610. doi: 10.1073/pnas.081615298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thorne S.H., Tam B.Y., Kirn D.H., Contag C.H., Kuo C.J. Selective intratumoral amplification of an antiangiogenic vector by an oncolytic virus produces enhanced antivascular and anti-tumor efficacy. Mol. Ther. 2006;13:938–946. doi: 10.1016/j.ymthe.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 79.Hou W., Chen H., Rojas J., Sampath P., Thorne S.H. Oncolytic vaccinia virus demonstrates antiangiogenic effects mediated by targeting of VEGF. Int. J. Cancer. 2014;135:1238–1246. doi: 10.1002/ijc.28747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Domanska U.M., Kruizinga R.C., Nagengast W.B., Timmer-Bosscha H., Huls G., de Vries E.G.E., Walenkamp A.M. A review on CXCR4/CXCL12 axis in oncology: No place to hide. Eur. J. Cancer. 2013;49:219–230. doi: 10.1016/j.ejca.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 81.Liang Z., Brooks J., Willard M., Liang K., Yoon Y., Kang S., Shim H. CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochem. Biophys. Res. Commun. 2007;359:716–722. doi: 10.1016/j.bbrc.2007.05.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guo F., Wang Y., Liu J., Mok S.C., Xue F., Zhang W. CXCL12/CXCR4: A symbiotic bridge linking cancer cells and their stromal neighbors in oncogenic communication networks. Oncogene. 2016;35:816–826. doi: 10.1038/onc.2015.139. [DOI] [PubMed] [Google Scholar]
- 83.Gil M., Seshadri M., Komorowski M.P., Abrams S.I., Kozbor D. Targeting CXCL12/CXCR4 signaling with oncolytic virotherapy disrupts tumor vasculature and inhibits breast cancer metastases. Proc. Natl. Acad. Sci. USA. 2013;110:E1291–E1300. doi: 10.1073/pnas.1220580110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gil M., Komorowski M.P., Seshadri M., Rokita H., McGray A.J.R., Opyrchal M., Odunsi K.O., Kozbor D. CXCL12/CXCR4 blockade by oncolytic virotherapy inhibits ovarian cancer growth by decreasing immunosuppression and targeting cancer-initiating cells. J. Immunol. 2014;193:5327–5337. doi: 10.4049/jimmunol.1400201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Patil S.S., Gentschev I., Adelfinger M., Donat U., Hess M., Weibel S., Nolte I., Frentzen A., Szalay A.A. Virotherapy of canine tumors with oncolytic vaccinia virus GLV-1h109 expressing an anti-VEGF single-chain antibody. PLoS ONE. 2012;7:e47472. doi: 10.1371/journal.pone.0047472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gholami S., Marano A., Chen N.G., Aguilar R.J., Frentzen A., Chen C.H., Lou E., Fujisawa S., Eveno C., Belin L. A novel vaccinia virus with dual oncolytic and anti-angiogenic therapeutic effects against triple-negative breast cancer. Breast Cancer Res. Treat. 2014;148:489–499. doi: 10.1007/s10549-014-3180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adelfinger M., Bessler S., Frentzen A., Cecil A., Langbein-Laugwitz J., Gentschev I., Szalay A.A. Preclinical testing oncolytic vaccinia virus strain GLV-5b451 expressing an anti-VEGF single-chain antibody for canine cancer therapy. Viruses. 2015;7:4075–4092. doi: 10.3390/v7072811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Adelfinger M., Gentschev I., Grimm de Guibert J., Weibel S., Langbein-Laugwitz J., Härtl B., Murua Escobar H., Nolte I., Chen N.G., Aguilar R.J. Evaluation of a new recombinant oncolytic vaccinia virus strain GLV-5b451 for feline mammary carcinoma therapy. PLoS ONE. 2014;9:e104337. doi: 10.1371/journal.pone.0104337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xie Y., Hicks M.J., Kaminsky S.M., Moore M.A., Crystal R.G., Rafii A. AAV-mediated persistent bevacizumab therapy suppresses tumor growth of ovarian cancer. Gynecol. Oncol. 2014;135:325–332. doi: 10.1016/j.ygyno.2014.07.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guse K., Diaconu I., Rajecki M., Sloniecka M., Hakkarainen T., Ristimäki A., Kanerva A., Pesonen S., Hemminki A. Ad5/3-9HIF-Delta24-VEGFR-1-Ig, an infectivity enhanced, dual-targeted and antiangiogenic oncolytic adenovirus for kidney cancer treatment. Gene Ther. 2009;16:1009–1020. doi: 10.1038/gt.2009.56. [DOI] [PubMed] [Google Scholar]
- 91.Niethammer A.G., Xiang R., Becker J.C., Wodrich H., Pertl U., Karsten G., Eliceiri B.P., Reisfeld R.A. A DNA vaccine against VEGF receptor 2 prevents effective angiogenesis and inhibits tumor growth. Nat. Med. 2002;8:1369–1375. doi: 10.1038/nm1202-794. [DOI] [PubMed] [Google Scholar]
- 92.Morera Y., Bequet-Romero M., Ayala M., Velazco J.C., Pérez P.P., Alba J.S., Ancizar J., Rodríguez M., Cosme K., Gavilondo J.V. Immunogenicity and some safety features of a VEGF-based cancer therapeutic vaccine in rats, rabbits and non-human primates. Vaccine. 2010;28:3453–3461. doi: 10.1016/j.vaccine.2010.02.069. [DOI] [PubMed] [Google Scholar]
- 93.Jarosz M., Jazowiecka-Rakus J., Cichoń T., Głowala-Kosińska M., Smolarczyk R., Smagur A., Malina S., Sochanik A., Szala S. Therapeutic antitumor potential of endoglin-based DNA vaccine combined with immunomodulatory agents. Gene Ther. 2013;20:262–273. doi: 10.1038/gt.2012.28. [DOI] [PubMed] [Google Scholar]
- 94.Haller B.K., Bråve A., Wallgard E., Roswall P., Sunkari V.G., Mattson U., Hallengärd D., Catrina S.B., Hellström M., Pietras K. Therapeutic efficacy of a DNA vaccine targeting the endothelial tip cell antigen delta-like ligand 4 in mammary carcinoma. Oncogene. 2010;29:4276–4286. doi: 10.1038/onc.2010.176. [DOI] [PubMed] [Google Scholar]
- 95.Wagner S.C., Riordan N.H., Ichim T.E., Szymanski J., Ma H., Perez J.A., Lopez J., Plata-Munoz J.J., Silva F., Patel A.N., Kesari S. Safety of targeting tumor endothelial cell antigens. J. Transl. Med. 2016;14:90. doi: 10.1186/s12967-016-0842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ichim T.E., Li S., Ma H., Yurova Y.V., Szymanski J.S., Patel A.N., Kesari S., Min W.P., Wagner S.C. Induction of tumor inhibitory anti-angiogenic response through immunization with interferon Gamma primed placental endothelial cells: ValloVax™. J. Transl. Med. 2015;13:90. doi: 10.1186/s12967-015-0441-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kuo C.-H., Chang B.-I., Lee F.-T., Chen P.-K., Lee J.-S., Shi G.-Y., Wu H.L. Development of recombinant adeno-associated virus serotype 2/8 carrying kringle domains of human plasminogen for sustained expression and cancer therapy. Hum. Gene Ther. 2015;26:603–613. doi: 10.1089/hum.2013.220. [DOI] [PubMed] [Google Scholar]
- 98.Chu Y., Liu H., Lou G., Zhang Q., Wu C. Human placenta mesenchymal stem cells expressing exogenous kringle1-5 protein by fiber-modified adenovirus suppress angiogenesis. Cancer Gene Ther. 2014;21:200–208. doi: 10.1038/cgt.2014.19. [DOI] [PubMed] [Google Scholar]
- 99.Cao R., Wu H.-L., Veitonmäki N., Linden P., Farnebo J., Shi G.-Y., Cao Y. Suppression of angiogenesis and tumor growth by the inhibitor K1–5 generated by plasmin-mediated proteolysis. Proc. Natl. Acad. Sci. USA. 1999;96:5728–5733. doi: 10.1073/pnas.96.10.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang X., Xu W., Qian H., Zhu W., Zhang R. Mesenchymal stem cells modified to express lentivirus TNF-α Tumstatin45–132 inhibit the growth of prostate cancer. J. Cell. Mol. Med. 2011;15:433–444. doi: 10.1111/j.1582-4934.2009.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Daugimont L., Vandermeulen G., Defresne F., Bouzin C., Mir L.M., Bouquet C., Feron O., Préat V. Antitumoral and antimetastatic effect of antiangiogenic plasmids in B16 melanoma: Higher efficiency of the recombinant disintegrin domain of ADAM 15. Eur. J. Pharm. Biopharm. 2011;78:314–319. doi: 10.1016/j.ejpb.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 102.Matsumoto K., Nakamura T. Mechanisms and significance of bifunctional NK4 in cancer treatment. Biochem. Biophys. Res. Commun. 2005;333:316–327. doi: 10.1016/j.bbrc.2005.05.131. [DOI] [PubMed] [Google Scholar]
- 103.Kishi Y., Kuba K., Nakamura T., Wen J., Suzuki Y., Mizuno S., Nukiwa T., Matsumoto K., Nakamura T. Systemic NK4 gene therapy inhibits tumor growth and metastasis of melanoma and lung carcinoma in syngeneic mouse tumor models. Cancer Sci. 2009;100:1351–1358. doi: 10.1111/j.1349-7006.2009.01184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tomioka D., Maehara N., Kuba K., Mizumoto K., Tanaka M., Matsumoto K., Nakamura T. Inhibition of growth, invasion, and metastasis of human pancreatic carcinoma cells by NK4 in an orthotopic mouse model. Cancer Res. 2001;61:7518–7524. [PubMed] [Google Scholar]
- 105.Zhu Y., Cheng M., Yang Z., Zeng C.Y., Chen J., Xie Y., Luo S.W., Zhang K.H., Zhou S.F., Lu N.H. Mesenchymal stem cell-based NK4 gene therapy in nude mice bearing gastric cancer xenografts. Drug Des. Devel. Ther. 2014;8:2449–2462. doi: 10.2147/DDDT.S71466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Matsumoto G., Omi Y., Lee U., Kubota E., Tabata Y. NK4 gene therapy combined with cisplatin inhibits tumour growth and metastasis of squamous cell carcinoma. Anticancer Res. 2011;31:105–111. [PubMed] [Google Scholar]
- 107.Ogura Y., Mizumoto K., Nagai E., Murakami M., Inadome N., Saimura M., Matsumoto K., Nakamura T., Maemondo M., Nukiwa T., Tanaka M. Peritumoral injection of adenovirus vector expressing NK4 combined with gemcitabine treatment suppresses growth and metastasis of human pancreatic cancer cells implanted orthotopically in nude mice and prolongs survival. Cancer Gene Ther. 2006;13:520–529. doi: 10.1038/sj.cgt.7700921. [DOI] [PubMed] [Google Scholar]
- 108.Dolinsek T., Markelc B., Sersa G., Coer A., Stimac M., Lavrencak J., Brozic A., Kranjc S., Cemazar M. Multiple delivery of siRNA against endoglin into murine mammary adenocarcinoma prevents angiogenesis and delays tumor growth. PLoS ONE. 2013;8:e58723. doi: 10.1371/journal.pone.0058723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tesic N., Kamensek U., Sersa G., Kranjc S., Stimac M., Lampreht U., Preat V., Vandermeulen G., Butinar M., Turk B., Cemazar M. Endoglin (CD105) silencing mediated by shRNA under the control of endothelin-1 promoter for targeted gene therapy of melanoma. Mol. Ther. Nucleic Acids. 2015;4:e239. doi: 10.1038/mtna.2015.12. [DOI] [PubMed] [Google Scholar]
- 110.Curiel T.J., Coukos G., Zou L., Alvarez X., Cheng P., Mottram P., Evdemon-Hogan M., Conejo-Garcia J.R., Zhang L., Burow M. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 111.Noy R., Pollard J.W. Tumor-associated macrophages: From mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wherry E.J., Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Groth C., Hu X., Weber R., Fleming V., Altevogt P., Utikal J., Umansky V. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br. J. Cancer. 2019;120:16–25. doi: 10.1038/s41416-018-0333-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gajewski T.F., Schreiber H., Fu Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Scott E.M., Jacobus E.J., Lyons B., Frost S., Freedman J.D., Dyer A., Khalique H., Taverner W.K., Carr A., Champion B.R. Bi- and tri-valent T cell engagers deplete tumour-associated macrophages in cancer patient samples. J. Immunother. Cancer. 2019;7:320. doi: 10.1186/s40425-019-0807-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kleinpeter P., Fend L., Thioudellet C., Geist M., Sfrontato N., Koerper V., Fahrner C., Schmitt D., Gantzer M., Remy-Ziller C. Vectorization in an oncolytic vaccinia virus of an antibody, a Fab and a scFv against programmed cell death -1 (PD-1) allows their intratumoral delivery and an improved tumor-growth inhibition. OncoImmunology. 2016;5:e1220467. doi: 10.1080/2162402X.2016.1220467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Engeland C.E., Grossardt C., Veinalde R., Bossow S., Lutz D., Kaufmann J.K., Shevchenko I., Umansky V., Nettelbeck D.M., Weichert W. CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Mol. Ther. 2014;22:1949–1959. doi: 10.1038/mt.2014.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin C., Ren W., Luo Y., Li S., Chang Y., Li L., Xiong D., Huang X., Xu Z., Yu Z. Intratumoral delivery of a PD-1-blocking scFv encoded in oncolytic HSV-1 promotes antitumor immunity and synergizes with TIGIT blockade. Cancer Immunol. Res. 2020;8:632–647. doi: 10.1158/2326-6066.CIR-19-0628. [DOI] [PubMed] [Google Scholar]
- 119.Du T., Shi G., Li Y.M., Zhang J.F., Tian H.W., Wei Y.Q., Deng H., Yu D.C. Tumor-specific oncolytic adenoviruses expressing granulocyte macrophage colony-stimulating factor or anti-CTLA4 antibody for the treatment of cancers. Cancer Gene Ther. 2014;21:340–348. doi: 10.1038/cgt.2014.34. [DOI] [PubMed] [Google Scholar]
- 120.Dias J.D., Hemminki O., Diaconu I., Hirvinen M., Bonetti A., Guse K., Escutenaire S., Kanerva A., Pesonen S., Löskog A. Targeted cancer immunotherapy with oncolytic adenovirus coding for a fully human monoclonal antibody specific for CTLA-4. Gene Ther. 2012;19:988–998. doi: 10.1038/gt.2011.176. [DOI] [PubMed] [Google Scholar]
- 121.Hamilton J.R., Vijayakumar G., Palese P. A recombinant antibody-expressing influenza virus delays tumor growth in a mouse model. Cell Rep. 2018;22:1–7. doi: 10.1016/j.celrep.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 122.Thomas S., Kuncheria L., Roulstone V., Kyula J.N., Mansfield D., Bommareddy P.K., Smith H., Kaufman H.L., Harrington K.J., Coffin R.S. Development of a new fusion-enhanced oncolytic immunotherapy platform based on herpes simplex virus type 1. J. Immunother. Cancer. 2019;7:214. doi: 10.1186/s40425-019-0682-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vijayakumar G., Palese P., Goff P.H. Oncolytic Newcastle disease virus expressing a checkpoint inhibitor as a radioenhancing agent for murine melanoma. EBioMedicine. 2019;49:96–105. doi: 10.1016/j.ebiom.2019.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang Y., Xu W., Peng D., Wang H., Zhang X., Wang H., Xiao F., Zhu Y., Ji Y., Gulukota K. An oncolytic adenovirus targeting transforming growth factor β inhibits protumorigenic signals and produces immune activation: A novel approach to enhance anti-PD-1 and anti-CTLA-4 therapy. Hum. Gene Ther. 2019;30:1117–1132. doi: 10.1089/hum.2019.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu W., Zhang Z., Yang Y., Hu Z., Wang C.H., Morgan M., Wu Y., Hutten R., Xiao X., Stock S. Ad5/48 hexon oncolytic virus expressing sTGFβRIIFc produces reduced hepatic and systemic toxicities and inhibits prostate cancer bone metastases. Mol. Ther. 2014;22:1504–1517. doi: 10.1038/mt.2014.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hu Z., Gupta J., Zhang Z., Gerseny H., Berg A., Chen Y.J., Zhang Z., Du H., Brendler C.B., Xiao X. Systemic delivery of oncolytic adenoviruses targeting transforming growth factor-β inhibits established bone metastasis in a prostate cancer mouse model. Hum. Gene Ther. 2012;23:871–882. doi: 10.1089/hum.2012.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hu Z., Gerseny H., Zhang Z., Chen Y.J., Berg A., Zhang Z., Stock S., Seth P. Oncolytic adenovirus expressing soluble TGFβ receptor II-Fc-mediated inhibition of established bone metastases: A safe and effective systemic therapeutic approach for breast cancer. Mol. Ther. 2011;19:1609–1618. doi: 10.1038/mt.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gujar S.A., Lee P.W.K. Oncolytic virus-mediated reversal of impaired tumor antigen presentation. Front. Oncol. 2014;4:77. doi: 10.3389/fonc.2014.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hervas-Stubbs S., Perez-Gracia J.L., Rouzaut A., Sanmamed M.F., Le Bon A., Melero I. Direct effects of type I interferons on cells of the immune system. Clin. Cancer Res. 2011;17:2619–2627. doi: 10.1158/1078-0432.CCR-10-1114. [DOI] [PubMed] [Google Scholar]
- 130.Kawai T., Akira S. Innate immune recognition of viral infection. Nat. Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 131.Nesslinger N.J., Ng A., Tsang K.-Y., Ferrara T., Schlom J., Gulley J.L., Nelson B.H. A viral vaccine encoding prostate-specific antigen induces antigen spreading to a common set of self-proteins in prostate cancer patients. Clin. Cancer Res. 2010;16:4046–4056. doi: 10.1158/1078-0432.CCR-10-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Brossart P. The role of antigen spreading in the efficacy of immunotherapies. Clin. Cancer. Res. 2020;26:4442–4447. doi: 10.1158/1078-0432.CCR-20-0305. [DOI] [PubMed] [Google Scholar]
- 133.Woller N., Gürlevik E., Fleischmann-Mundt B., Schumacher A., Knocke S., Kloos A.M., Saborowski M., Geffers R., Manns M.P., Wirth T.C. Viral infection of tumors overcomes resistance to PD-1-immunotherapy by broadening neoantigenome-directed T-cell responses. Mol. Ther. 2015;23:1630–1640. doi: 10.1038/mt.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gujar S., Dielschneider R., Clements D., Helson E., Shmulevitz M., Marcato P., Pan D., Pan L.Z., Ahn D.G., Alawadhi A., Lee P.W. Multifaceted therapeutic targeting of ovarian peritoneal carcinomatosis through virus-induced immunomodulation. Mol. Ther. 2013;21:338–347. doi: 10.1038/mt.2012.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gujar S.A., Marcato P., Pan D., Lee P.W.K. Reovirus virotherapy overrides tumor antigen presentation evasion and promotes protective antitumor immunity. Mol. Cancer Ther. 2010;9:2924–2933. doi: 10.1158/1535-7163.MCT-10-0590. [DOI] [PubMed] [Google Scholar]
- 136.Curtsinger J.M., Mescher M.F. Inflammatory cytokines as a third signal for T cell activation. Curr. Opin. Immunol. 2010;22:333–340. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hervas-Stubbs S., Riezu-Boj J.I., Gonzalez I., Mancheño U., Dubrot J., Azpilicueta A., Gabari I., Palazon A., Aranguren A., Ruiz J. Effects of IFN-α as a signal-3 cytokine on human naïve and antigen-experienced CD8(+) T cells. Eur. J. Immunol. 2010;40:3389–3402. doi: 10.1002/eji.201040664. [DOI] [PubMed] [Google Scholar]
- 138.Lapteva N., Aldrich M., Rollins L., Ren W., Goltsova T., Chen S.-Y., Huang X.F. Attraction and activation of dendritic cells at the site of tumor elicits potent antitumor immunity. Mol. Ther. 2009;17:1626–1636. doi: 10.1038/mt.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sobol P.T., Boudreau J.E., Stephenson K., Wan Y., Lichty B.D., Mossman K.L. Adaptive antiviral immunity is a determinant of the therapeutic success of oncolytic virotherapy. Mol. Ther. 2011;19:335–344. doi: 10.1038/mt.2010.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Twumasi-Boateng K., Pettigrew J.L., Kwok Y.Y.E., Bell J.C., Nelson B.H. Oncolytic viruses as engineering platforms for combination immunotherapy. Nat. Rev. Cancer. 2018;18:419–432. doi: 10.1038/s41568-018-0009-4. [DOI] [PubMed] [Google Scholar]
- 141.Liu Y.P., Suksanpaisan L., Steele M.B., Russell S.J., Peng K.W. Induction of antiviral genes by the tumor microenvironment confers resistance to virotherapy. Sci. Rep. 2013;3:2375. doi: 10.1038/srep02375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Currier M.A., Eshun F.K., Sholl A., Chernoguz A., Crawford K., Divanovic S., Boon L., Goins W.F., Frischer J.S., Collins M.H. VEGF blockade enables oncolytic cancer virotherapy in part by modulating intratumoral myeloid cells. Mol. Ther. 2013;21:1014–1023. doi: 10.1038/mt.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Puig-Kröger A., Sierra-Filardi E., Domínguez-Soto A., Samaniego R., Corcuera M.T., Gómez-Aguado F., Ratnam M., Sánchez-Mateos P., Corbí A.L. Folate receptor β is expressed by tumor-associated macrophages and constitutes a marker for M2 anti-inflammatory/regulatory macrophages. Cancer Res. 2009;69:9395–9403. doi: 10.1158/0008-5472.CAN-09-2050. [DOI] [PubMed] [Google Scholar]
- 144.Lynn R.C., Poussin M., Kalota A., Feng Y., Low P.S., Dimitrov D.S., Powell D.J., Jr. Targeting of folate receptor β on acute myeloid leukemia blasts with chimeric antigen receptor-expressing T cells. Blood. 2015;125:3466–3476. doi: 10.1182/blood-2014-11-612721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sharpe A.H., Pauken K.E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2018;18:153–167. doi: 10.1038/nri.2017.108. [DOI] [PubMed] [Google Scholar]
- 146.Wei S.C., Duffy C.R., Allison J.P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 147.Kahounová Z., Kurfürstová D., Bouchal J., Kharaishvili G., Navrátil J., Remšík J., Šimečková Š., Študent V., Kozubík A., Souček K. The fibroblast surface markers FAP, anti-fibroblast, and FSP are expressed by cells of epithelial origin and may be altered during epithelial-to-mesenchymal transition. Cytometry A. 2018;93:941–951. doi: 10.1002/cyto.a.23101. [DOI] [PubMed] [Google Scholar]
- 148.Sugimoto H., Mundel T.M., Kieran M.W., Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol. Ther. 2006;5:1640–1646. doi: 10.4161/cbt.5.12.3354. [DOI] [PubMed] [Google Scholar]
- 149.Skokos D., Waite J.C., Haber L., Crawford A., Hermann A., Ullman E., Slim R., Godin S., Ajithdoss D., Ye X. A class of costimulatory CD28-bispecific antibodies that enhance the antitumor activity of CD3-bispecific antibodies. Sci. Transl. Med. 2020;12:eaaw7888. doi: 10.1126/scitranslmed.aaw7888. [DOI] [PubMed] [Google Scholar]
- 150.Gleason M.K., Ross J.A., Warlick E.D., Lund T.C., Verneris M.R., Wiernik A., Spellman S., Haagenson M.D., Lenvik A.J., Litzow M.R. CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood. 2014;123:3016–3026. doi: 10.1182/blood-2013-10-533398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gauthier L., Morel A., Anceriz N., Rossi B., Blanchard-Alvarez A., Grondin G., Trichard S., Cesari C., Sapet M., Bosco F. Multifunctional natural killer cell engagers targeting NKp46 trigger protective tumor immunity. Cell. 2019;177:1701–1713.e16. doi: 10.1016/j.cell.2019.04.041. [DOI] [PubMed] [Google Scholar]