Abstract
Objective
To estimate the real world effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S vaccines against confirmed covid-19 symptoms (including the UK variant of concern B.1.1.7), admissions to hospital, and deaths.
Design
Test negative case-control study.
Setting
Community testing for covid-19 in England.
Participants
156 930 adults aged 70 years and older who reported symptoms of covid-19 between 8 December 2020 and 19 February 2021 and were successfully linked to vaccination data in the National Immunisation Management System.
Interventions
Vaccination with BNT162b2 or ChAdOx1-S.
Main outcome measures
Primary outcomes were polymerase chain reaction confirmed symptomatic SARS-CoV-2 infections, admissions to hospital for covid-19, and deaths with covid-19.
Results
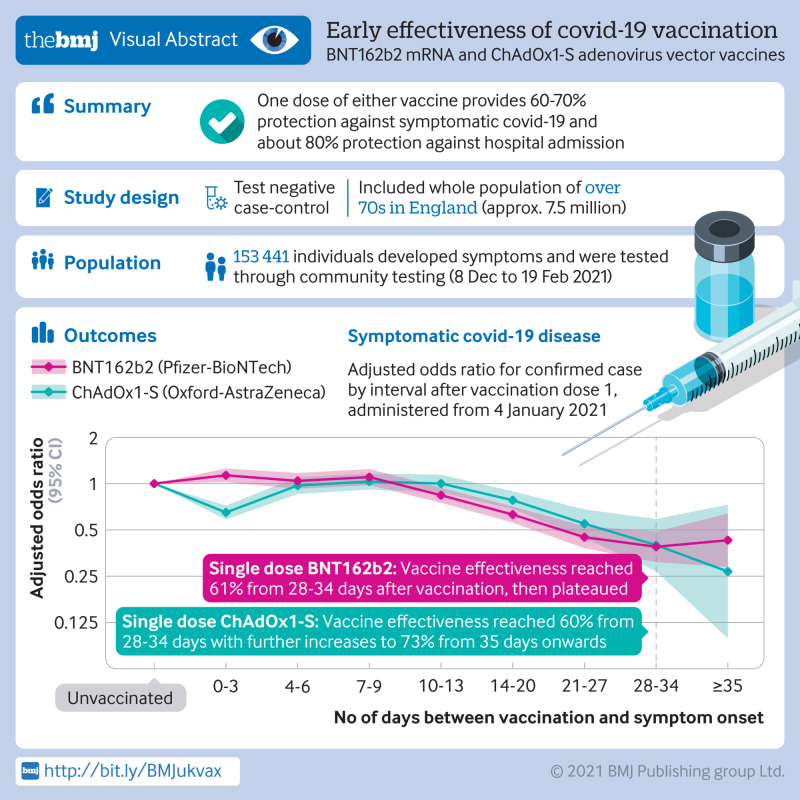
Participants aged 80 years and older vaccinated with BNT162b2 before 4 January 2021 had a higher odds of testing positive for covid-19 in the first nine days after vaccination (odds ratio up to 1.48, 95% confidence interval 1.23 to 1.77), indicating that those initially targeted had a higher underlying risk of infection. Vaccine effectiveness was therefore compared with the baseline post-vaccination period. Vaccine effects were noted 10 to 13 days after vaccination, reaching a vaccine effectiveness of 70% (95% confidence interval 59% to 78%), then plateauing. From 14 days after the second dose a vaccination effectiveness of 89% (85% to 93%) was found compared with the increased baseline risk. Participants aged 70 years and older vaccinated from 4 January (when ChAdOx1-S delivery commenced) had a similar underlying risk of covid-19 to unvaccinated individuals. With BNT162b2, vaccine effectiveness reached 61% (51% to 69%) from 28 to 34 days after vaccination, then plateaued. With ChAdOx1-S, effects were seen from 14 to 20 days after vaccination, reaching an effectiveness of 60% (41% to 73%) from 28 to 34 days, increasing to 73% (27% to 90%) from day 35 onwards. On top of the protection against symptomatic disease, a further 43% (33% to 52%) reduced risk of emergency hospital admission and 51% (37% to 62%) reduced risk of death was observed in those who had received one dose of BNT162b2. Participants who had received one dose of ChAdOx1-S had a further 37% (3% to 59%) reduced risk of emergency hospital admission. Follow-up was insufficient to assess the effect of ChAdOx1-S on mortality. Combined with the effect against symptomatic disease, a single dose of either vaccine was about 80% effective at preventing admission to hospital with covid-19 and a single dose of BNT162b2 was 85% effective at preventing death with covid-19.
Conclusion
Vaccination with either one dose of BNT162b2 or ChAdOx1-S was associated with a significant reduction in symptomatic covid-19 in older adults, and with further protection against severe disease. Both vaccines showed similar effects. Protection was maintained for the duration of follow-up (>6 weeks). A second dose of BNT162b2 was associated with further protection against symptomatic disease. A clear effect of the vaccines against the B.1.1.7 variant was found.
Introduction
On 8 December 2020 the UK became the first country to implement a covid-19 vaccination programme after the approval of the Pfizer-BioNTech messenger RNA (mRNA) vaccine, BNT162b2, for emergency use.1 The programme has since expanded to include the Oxford-AstraZeneca adenovirus vector vaccine, ChAdOx1-S, and more than 28 million people have now been vaccinated. The burden of covid-19 in the UK remains high, and early evidence on the effectiveness of vaccines is essential for informing policy decisions on the ongoing delivery of the programme and the use of other non-drug interventions.2
During the first few weeks of the programme, the priority groups for vaccination included older residents of care homes and their carers, those aged 80 years and older, and frontline health and social care workers.3 From 18 January, vaccine delivery was extended to those aged 70 years and older and those in clinically extremely vulnerable groups. Delivery was initially through hospital trusts and care homes, when possible, then subsequently also through primary care providers and mass vaccination centres. Interim results from phase III clinical trials have found the BNT162b2 and ChAdOx1-S vaccines to be highly effective when using a two dose schedule with a target interval of three and four weeks, respectively, between doses.4 5 Data from the ChAdOx1-S trial suggests that protection might be greater with a longer dosing interval.5 A reanalysis of the BNT162b2 trial data suggests that a single dose of this vaccine has an efficacy of 92.6% in the early post-vaccination period.6 Furthermore, with other vaccines an extended interval between the prime and booster doses typically provides a better immune response to the booster dose.7 8 Based on this evidence, the increasing incidence of covid-19 in the UK and the need to rapidly vaccinate as many vulnerable people as possible, on 20 December 2020 the Joint Committee on Vaccination and Immunisation advised that the dose interval for both vaccines could be extended to up to 12 weeks. A policy decision was subsequently made to prioritise vaccinating as many people as possible with the first dose.
Also in December 2020, a new covid-19 variant of concern (B.1.1.7) was found to be associated with increasing case numbers in Kent in south east England.9 Recent analyses suggest that this variant has increased transmissibility, and it has since become the dominant strain in large parts of the UK.10 11 The variant is characterised by 23 mutations, including mutations to genes encoding the spike protein, the target of the two vaccines currently in use, as well as the majority of vaccine candidates.9 Concerns have been raised about the possible impact of the new variant on vaccine effectiveness.12
Public Health England has undertaken its first analysis of the early effect of covid-19 vaccination using routine testing and vaccination data. In this analysis we estimated the effect of vaccination with the BNT162b2 and ChAdOx1-S vaccines on confirmed symptomatic covid-19 in adults aged 70 years and older with one and two doses; estimated vaccine effectiveness against the B.1.1.7 variant; and estimated covid-19 hospital admissions and case fatality rates among vaccinated and unvaccinated people.
Methods
A test negative case-control design was used to estimate odds ratios for testing positive to SARS-CoV-2 in all vaccinated compared with unvaccinated people with compatible symptoms who were tested using polymerase chain reaction (PCR). Test negative case-control designs are considered powerful enough to estimate vaccine effectiveness and are used extensively for estimating effectiveness of influenza vaccines and vaccines against other respiratory viruses.13 14 15 They have been found to have high concordance with findings in randomised controlled trials.16 17 Vaccination status is compared in people who test positive for the target organism compared with those who test negative. Comparing to others who present for testing but test negative helps to control for factors that are typically difficult to estimate in observational studies, including differences in health seeking behaviours, access to testing, and case ascertainment.
Data sources
Outcome assessment
All adults aged 70 years or older in England (>7.5 million people) were eligible for inclusion. Testing for covid-19 in the UK is done through hospital and public health laboratories for those with a clinical need as well as some healthcare workers (pillar 1 testing), and through community testing (pillar 2 testing).18 Anybody can access a pillar 2 test if they have symptoms of covid-19 (high temperature, new continuous cough, loss or change in sense of smell or taste) or if they are part of a local or national mass testing programme. For this analysis, we extracted PCR testing data from pillar 2 in those who reported having symptoms for all tests between 26 October 2020 and 21 February 2021.
Mutations to the spike gene in the B.1.1.7 variant cause a reproducible spike gene target failure in laboratories using a three target PCR assay (TaqPath; Thermo Fisher).9 Between the week commencing 7 December 2020 and week commencing 25 January 2021, the B.1.1.7 variant accounted for between 98% and 100% of spike gene target failures in England.19 Spike gene target failure therefore provides a good proxy for identification of the B.1.1.7 variant without relying on sequencing. We undertook an analysis of vaccine effects against covid-19 detections with spike gene target failures restricted to data from laboratories using the TaqPath assay.
Exposure assessment
Testing data were linked to individual vaccination histories in the national vaccination register (the National Immunisation Management System, NIMS) using National Health Service number, date of birth, surname, first name, and postcode. All covid-19 vaccines administered in England are recorded in NIMS by clinicians through point of care applications. NIMS data were extracted on 22 February 2021 with immunisations to 21 February 2021. To allow for delayed entry of data into NIMS, we only included samples in analyses that were taken from 19 February 2021.
Secondary outcomes
We also linked the data to hospital admission data from the Emergency Care Dataset, which includes hospital admissions through emergency departments but not elective admissions, and to mortality data from NHS records.20
Covariates
A range of factors might be associated with both the likelihood of being offered or accepting a vaccine and the risk of exposure to SARS-CoV-2 or propensity to be tested. These include personal factors, such as age, sex, index of multiple deprivation, and ethnicity; geography and period (incidence of covid-19 varied by region and by week over the study period, as did vaccine delivery); and care home status, because care homes have been high exposure settings during the pandemic.
We extracted age, sex, date of birth, ethnicity, and residential address from the testing data and NIMS. Addresses were used to determine index of multiple deprivation fifth and were also linked to Care Quality Commission registered care homes using the unique property reference number.21 Data were restricted to those older than 70 (defined as those aged 70 and older on 31 March 2021).
Statistical analysis
Logistic regression was used to estimate the odds of vaccination in PCR confirmed cases compared with those who tested negative for SARS-CoV-2. Only those swabbed within 0-10 days of symptom onset were included in the analysis because sensitivity of PCR testing decreases beyond 10 days after symptom onset.22 Individuals only contribute their first positive test result from 8 December (as this was the date that the vaccination programme was introduced) and if they did not test positive in the previous six weeks (which could have indicated a single prolonged illness episode). Participants contributed a maximum of three randomly chosen negative test results in the follow-up period after excluding any tests taken within three weeks before a positive result, or after a positive result, which are more likely to be false negatives, or taken within seven days of a previous negative sample; again, because these could represent a single illness episode. In addition, we excluded any negative test result associated with a symptom date within 10 days after a previous symptom date for the same reason.
To estimate vaccine effectiveness in fully susceptible people, we excluded from the primary analysis those with a previous positive PCR or antibody test result at any time before 8 December. Sensitivity analyses included those with a history of a positive PCR test result.
Week of symptom onset was included in a crude model because the variation in both disease incidence and vaccine delivery in England over the study period meant that an analysis without including time would not be meaningful. Several possible confounders were included in the fully adjusted logistic regression model: age (in five year age groups, at 31 March 2021), sex, ethnicity, geography (NHS region), index of multiple deprivation, care home residence, and week of symptom onset.
To understand how quickly a vaccine effect becomes apparent and when a full effect is first reached, as well as to better understand potential biases in the analysis, we chose narrow follow-up windows (two periods each week up to 14 days and weekly thereafter). Vaccination status was categorised as unvaccinated and the following intervals (in days) between vaccination and symptom onset were selected: post-dose 1: 0-3, 4-6, 7-9, 10-13, 14-20, 21-27, 28-34, 35-41, and ≥42; and post-dose 2: 0-3, 4-6, 7-13, and ≥14. We estimated odd ratios for each period. For ChAdOx1-S the final interval was ≥35 days because of the shorter follow-up time for this vaccine. For the analysis of either vaccine, we excluded those participants who had already been vaccinated with the other vaccine.
Analyses were also stratified by vaccination period—before 4 January (age ≥80 years only) and from 4 January (when ChAdOx1-S was introduced), spike gene target failure (for vaccinations given in the period before 4 January, age ≥80 years, BNT162b2 only). The comparator group at baseline comprised unvaccinated participants; however, for the earlier vaccination period and overall period we also performed a post hoc analysis comparing with days 4-9 after vaccination to help account for the likely higher underlying risk of covid-19 among those groups targeted for vaccination first.
We estimated the number of people with covid-19 admitted to hospital within 14 days of a positive test result, and the number of deaths within 21 days of a positive test result, by vaccination status at the date of test (unvaccinated, vaccinated within 0-13 days, vaccinated at least 14 days before). Proportional hazards survival analyses were also conducted for these outcomes, adjusting for age, care home status, sex, and period. This analysis was restricted to those older than 80 years as this age group was targeted first and follow-up in the 70-79 years age group is still too short to monitor these endpoints. To allow for delays in reporting of hospital admissions and deaths, we censored these data at 16 February and 9 February for survival, respectively, and 14 and 21 days earlier than this for hospital admissions of people with covid-19 and case fatality rates, respectively. We repeated the analysis in people with a negative test result (controls) to assess healthy vaccine bias.
Patient and public involvement
Members of the public were not directly involved in this study as this was an unfunded study using routine surveillance data sources. The study was, however, conducted in consultation with advice from the UK Joint Committee on Vaccination and Immunisation, which includes lay membership to represent the perspective of patients or users of NHS services.
Results
Overall, 174 731 pillar 2 PCR tested samples were available for people who reported symptoms within 10 days of the sample date; 156 930 of these (89.8%) were successfully linked to vaccination data in NIMS—44 590 (28.4%) tested positive for covid-19 and 112 340 (71.6%) tested negative. The negative control samples were from a total of 108 851 people of whom 105 302 contributed one negative sample, 2977 two samples, and 256 three samples. Three hundred and sixteen individuals contributed a negative sample and then a positive sample at least three weeks later. Supplementary table 1 shows the differences in characteristics of the participants with linked and unlinked test data. Characteristics were generally similar, although a higher proportion of people of non-white ethnicity and aged 85 years and older were among those with no linked test data.
Table 1 shows vaccine coverage by vaccine brand at 21 February 2021 according to positive and negative test results. The results relate to vaccines given both before and after the onset date. Person time is greater with BNT162b2 because of the earlier rollout of this vaccine.
Table 1.
Vaccination coverage of Oxford-AstraZeneca (ChAdOx1-S) and Pfizer-BioNTech (BNT162b2) vaccines according to covid-19 test result at end of study period (21 February), by age group. Values are numbers (percentages) unless stated otherwise
| ChAdOx1-S | BNT162b2 | Any vaccine | Unvaccinated | Total No | |
|---|---|---|---|---|---|
| Positive test result | |||||
| Age group (years): | |||||
| 70-74 | 10 073 (50) | 4932 (24) | 15 005 (74) | 5214 (25.8) | 20 219 |
| 75-79 | 5227 (47) | 3196 (28) | 8423 (75) | 2816 (25.1) | 11 239 |
| 80-84 | 2320 (36) | 2706 (42) | 5026 (77) | 1487 (22.8) | 6513 |
| 85-89 | 1355 (35) | 1356 (35) | 2711 (70) | 1180 (30.3) | 3891 |
| ≥90 | 985 (36) | 682 (25) | 1667 (61) | 1061 (38.9) | 2728 |
| Total | 19 960 (45) | 12 872 (29) | 32 832 (74) | 11 758 (26.4) | 44 590 |
| Negative test result | |||||
| Age group (years): | |||||
| 70-74 | 33 756 (59) | 20 251 (35) | 54 007 (95) | 3137 | 57 144 |
| 75-79 | 14 605 (50) | 13 375 (45) | 27 980 (95) | 1439 | 29 419 |
| 80-84 | 3955 (28) | 9366 (67) | 13 321 (95) | 721 | 14 042 |
| 85-89 | 2243 (30) | 4559 (62) | 6802 (92) | 555 | 7357 |
| ≥90 | 1866 (43) | 2061 (47) | 3927 (90) | 451 | 4378 |
| Total | 56 425 (50) | 49 612 (44) | 106 037 (94) | 6303 | 112 340 |
Figure 1 shows the number of cases and controls by intervals around the first and second vaccination doses. The number of people who were tested beyond 42 days after vaccination with BNT162b2 is relatively small, as is the number of people who were tested after two doses. The maximum duration of follow-up after one dose was 56 days. The number of people who were tested beyond 28 days after vaccination with ChAdOx1-S was small, with a maximum follow-up of 41 days. In the seven days before vaccination the number of tests decreased, and the results were mainly negative. A notable increase was observed in tests immediately after vaccination with ChAdOx1-S. Supplementary figure 1 shows the number of cases and controls by week and vaccination status.
Fig 1.
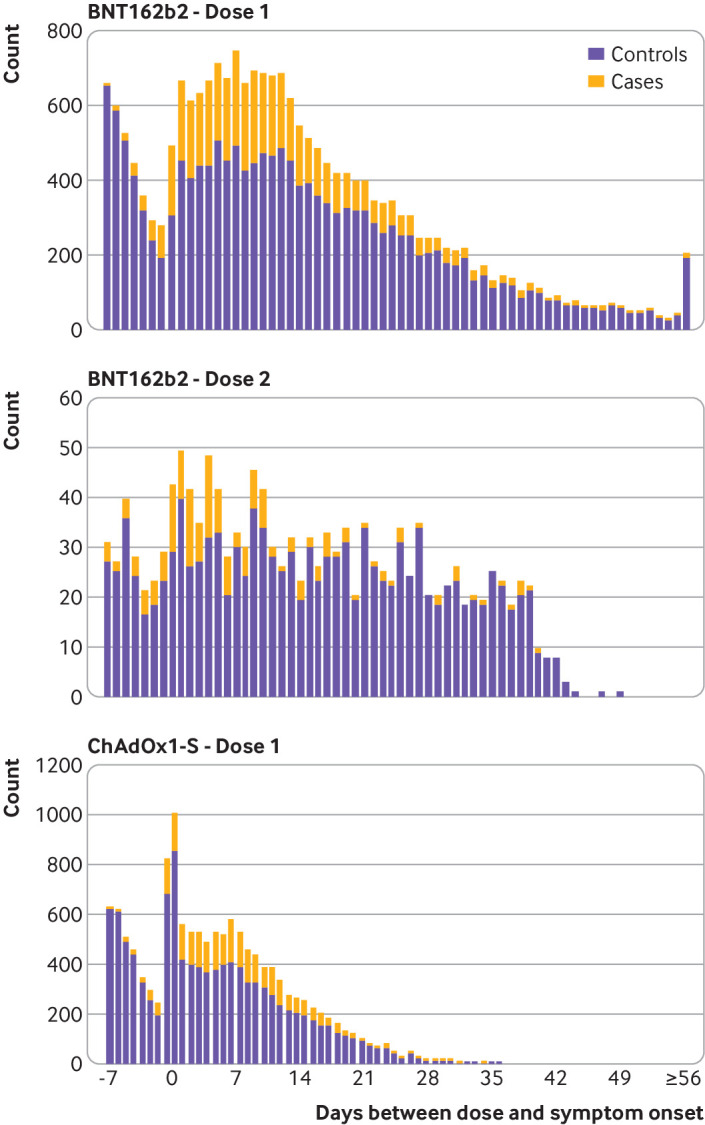
Number of cases and controls by interval from vaccination with Pfizer-Biontech BNT162b2 and Oxford Astra-Zeneca ChAdOx1-S vaccines
The odds of testing positive by interval after vaccination with BNT162b2 compared with being unvaccinated was initially analysed for the full period from the roll-out of the BNT162b2 vaccination programme on 8 December 2020 (supplementary table 2 and supplementary fig 2). During the first few days after vaccination (before an immune response would be anticipated), the odds of vaccinated people testing positive was higher, suggesting that vaccination was being targeted at those at higher risk of infection. The odds ratios then began to decrease from 14 days after vaccination, reaching 0.50 (95% confidence interval 0.42 to 0.59) during days 28 to 34, and remained stable thereafter. When those who had previously tested positive were included, results were almost identical (supplementary table 3). Stratifying by period indicated that vaccination before 4 January was targeted at those at higher baseline risk of covid-19, whereas from 4 January (when ChAdOx1-S was introduced), delivery was more accessible for those with a similar baseline risk to the unvaccinated group. A stratified approach was therefore considered more appropriate for the primary analysis.
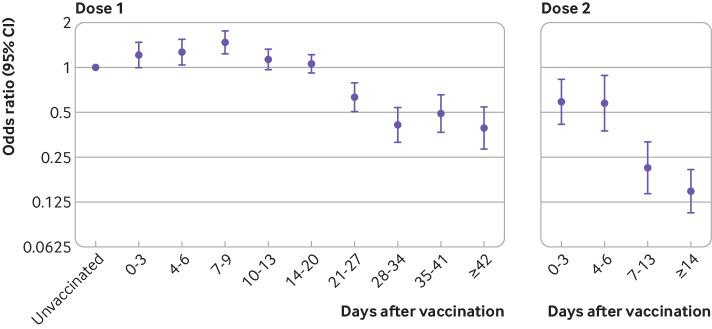
Table 2 and figure 2 show the results for vaccinations with BNT162b2 administered before 4 January—this analysis was restricted to those aged 80 years and older as younger age groups were not eligible for vaccination before 4 January. The odds of testing positive among vaccinated people increased during the early period, up to days 7 to 9, reaching 1.48 (95% confidence interval 1.23 to 1.77). The odds ratios then began to decrease from 10 to 13 days after vaccination, reaching 0.41 (0.32 to 0.54) on days 28 to 34, and remained at a similar level from 35 days onwards. Compared with an unvaccinated baseline group, vaccine effectiveness was equivalent to 59%. Relative to the higher baseline risk seen during days 4 to 9, the odds ratio reached 0.30 (0.22 to 0.41), equivalent to a vaccine effectiveness of 70%. From seven days after a second dose of BNT162b2, the odds ratio was 0.21 (0.14 to 0.32) and then 0.15 (0.11 to 0.21) from 14 days after the second dose, indicating a vaccine effectiveness of 85%. Relative to the higher baseline risk seen during days 4 to 9, the odds ratio reached 0.11 (0.07 to 0.15), equivalent to a vaccine effectiveness of 89%.
Table 2.
Adjusted odds ratios for confirmed cases of covid-19 by interval after vaccination with the Pfizer-BioNTech BNT162b2 vaccine before 4 January 2021 in those aged 80 years and older
| No of controls | No of cases | Odds ratio* (95% CI) | Adjusted odds ratio† (95% CI) | Odds ratio v post-dose days 4-9† (95% CI) | |
|---|---|---|---|---|---|
| Unvaccinated | 15 718 | 8988 | Base | Base | |
| First dose | |||||
| Interval after dose (days): | |||||
| 0-3 | 277 | 167 | 1.17 (0.96 to 1.42) | 1.22 (1.00 to 1.48) | |
| 4-6 | 241 | 179 | 1.26 (1.03 to 1.54) | 1.28 (1.05 to 1.56) | |
| 7-9 | 252 | 257 | 1.47 (1.23 to 1.76) | 1.48 (1.23 to 1.77) | |
| 10-13 | 361 | 284 | 1.12 (0.95 to 1.31) | 1.13 (0.96 to 1.33) | 0.82 (0.67 to 1.01) |
| 14-20 | 462 | 336 | 1.03 (0.89 to 1.19) | 1.06 (0.92 to 1.23) | 0.77 (0.63 to 0.94) |
| 21-27 | 288 | 118 | 0.60 (0.48 to 0.75) | 0.64 (0.51 to 0.79) | 0.46 (0.35 to 0.60) |
| 28-34 | 290 | 72 | 0.40 (0.30 to 0.52) | 0.41 (0.32 to 0.54) | 0.30 (0.22 to 0.41) |
| 35-41 | 274 | 65 | 0.45 (0.34 to 0.60) | 0.49 (0.37 to 0.66) | 0.36 (0.26 to 0.49) |
| ≥42 | 396 | 59 | 0.34 (0.25 to 0.47) | 0.39 (0.29 to 0.55) | 0.28 (0.20 to 0.40) |
| Second dose | |||||
| Interval after dose (days): | |||||
| 0-3 | 116 | 45 | 0.55 (0.39 to 0.77) | 0.59 (0.41 to 0.83) | 0.42 (0.29 to 0.62) |
| 4-6 | 80 | 30 | 0.52 (0.34 to 0.80) | 0.57 (0.37 to 0.88) | 0.41 (0.26 to 0.65) |
| 7-13 | 201 | 28 | 0.20 (0.13 to 0.29) | 0.21 (0.14 to 0.32) | 0.15 (0.10 to 0.23) |
| ≥14 | 634 | 41 | 0.13 (0.09 to 0.18) | 0.15 (0.11 to 0.21) | 0.11 (0.07 to 0.15) |
Odds ratio period adjusted by week of onset.
Adjusted for age, period, sex, region, ethnicity, care home, and index of multiple deprivation fifth.
Fig 2.
Adjusted odds ratios for confirmed cases of covid-19 by interval after vaccination with Pfizer-BioNTech BNT162b2 before 4 January 2021 in those aged 80 years and older
Table 3 and figure 3 show the results for BNT162b2 and ChAdOx1-S administered from 4 January. In this analysis, no significantly increased risk was observed during the early post-vaccination period for either vaccine. For ChAdOx1-S, the odds ratio decreased on days 0 to 3 after vaccination, which is associated with increased testing immediately after vaccination. For BNT162b2, the odds ratio started to decline 10 to 13 days after vaccination, reaching 0.39 (95% confidence interval 0.31 to 0.49) from 28 days after vaccination, equivalent to a vaccine effectiveness of 61%, then remained at a similar level. For ChAdOx1-S, the decline began on days 14 to 20 after vaccination, reaching an odds ratio of 0.40 (0.27 to 0.59) from 28 days after vaccination, equivalent to a vaccine effectiveness of 60%, and then reached 0.27 (0.10 to 0.73) from 35 days after vaccination, equivalent to a vaccine effectiveness of 73% and with wide confidence intervals. Confidence intervals for the two vaccines overlapped, and further follow-up was needed to understand whether the effects had plateaued for ChAdOx1-S. Notable differences were seen between the adjusted and unadjusted odds ratios in this analysis, but this was not seen in the analysis before 4 January. This was due to confounding by age and care home status, probably because few care home residents were vaccinated in the early period and this period was restricted to a smaller age group (≥80 years). Supplementary table 4 shows similar effects in the analysis of ChAdOx1-S including previous individuals with positive test results.
Table 3.
Adjusted odds ratios for confirmed cases of covid-19 by interval after vaccination with the Pfizer-BioNTech BNT162b2 and Oxford-AstaZeneca ChAdOx1-S vaccines from 4 January 2021in those aged 70 years and older
| BNT162b2 | ChAdOx1-S | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No of controls | No of cases | Odds ratio* (95% CI) | Adjusted odds ratio† (95% CI) | No of controls | No of cases | Odds ratio* (95% CI) | Adjusted odds ratio† (95% CI) | ||
| Unvaccinated | 36 668 | 15 287 | Base | Base | 36 668 | 15 287 | Base | Base | |
| Interval after first dose (days): | |||||||||
| 0-3 | 1311 | 622 | 1.29 (1.17 to 1.42) | 1.13 (1.02 to 1.25) | 2360 | 568 | 0.80 (0.72 to 0.88) | 0.65 (0.59 to 0.72) | |
| 4-6 | 1130 | 474 | 1.21 (1.08 to 1.35) | 1.04 (0.93 to 1.17) | 1141 | 405 | 1.25 (1.10 to 1.41) | 0.97 (0.85 to 1.10) | |
| 7-9 | 1091 | 463 | 1.30 (1.16 to 1.46) | 1.10 (0.98 to 1.24) | 1193 | 437 | 1.42 (1.26 to 1.61) | 1.03 (0.90 to 1.16) | |
| 10-13 | 1499 | 489 | 1.07 (0.96 to 1.19) | 0.84 (0.75 to 0.94) | 1235 | 441 | 1.44 (1.28 to 1.63) | 1.00 (0.88 to 1.14) | |
| 14-20 | 1956 | 448 | 0.83 (0.74 to 0.93) | 0.63 (0.56 to 0.71) | 1342 | 396 | 1.29 (1.13 to 1.47) | 0.78 (0.68 to 0.89) | |
| 21-27 | 1345 | 224 | 0.65 (0.56 to 0.76) | 0.45 (0.39 to 0.53) | 628 | 147 | 1.16 (0.95 to 1.41) | 0.55 (0.45 to 0.68) | |
| 28-34 | 717 | 99 | 0.60 (0.48 to 0.76) | 0.39 (0.31 to 0.49) | 176 | 39 | 1.18 (0.82 to 1.70) | 0.40 (0.27 to 0.59) | |
| ≥35 | 222 | 32 | 0.73 (0.49 to 1.08) | 0.43 (0.29 to 0.64) | 31 | 5 | 0.96 (0.37 to 2.50) | 0.27 (0.10 to 0.73) | |
Odds ratio period adjusted by week of onset.
Adjusted for age, period, sex, region, ethnicity, care home, and index of multiple deprivation fifth.
Fig 3.
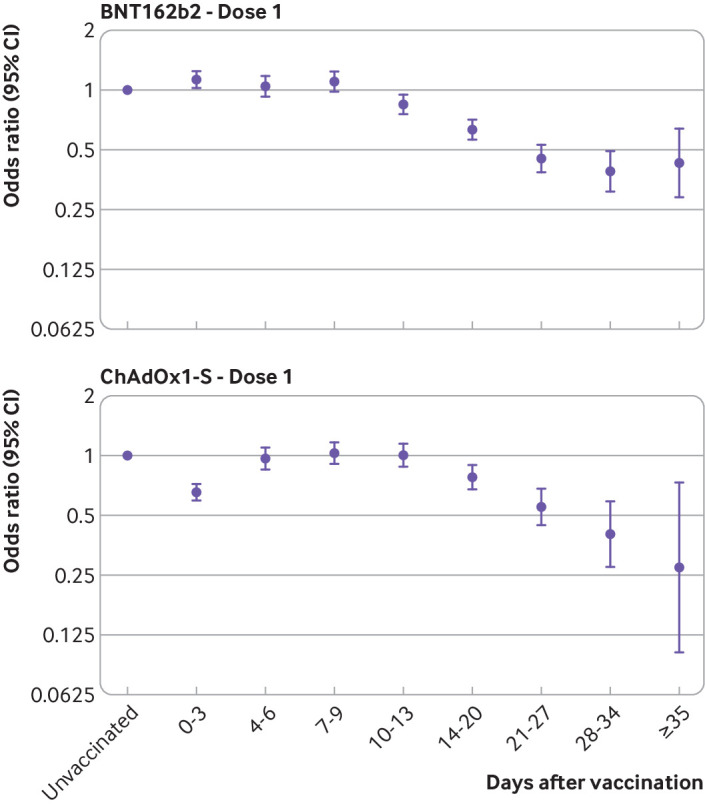
Adjusted odds ratios for confirmed cases of covid-19 by interval after vaccination with Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S from 4 January 2021
Supplementary table 2A shows further analysis by spike gene target failure status to indicate those with and without the B.1.1.7 variant. When comparing with the period days 4-9 after vaccination to account for differences in baseline risk in those vaccinated, the results were similar with and without spike gene target failure. The point estimate for vaccine effectiveness without spike gene target failure 28 to 41 days after vaccination was slightly bigger, but the effects were almost the same as those 42 days or more after vaccination and confidence intervals overlap throughout. Numbers without spike gene target failure were small, particularly during the later follow-up periods, because the B.1.1.7 variant is now dominant in England.
Table 4 shows hospital admissions for covid-19 cases within 14 days of a positive test result and deaths within 21 days of a positive test result by vaccination status among those aged 80 years and older. Hazard ratios from the survival analyses are also shown (see supplementary figure 3 for Kaplan-Meier curves). Hazard ratios for both vaccines were similar: 0.57 (95% confidence interval 0.48 to 0.67) for BNT162b2 and 0.63 (0.41 to 0.97) for ChAdOx1-S, among those vaccinated at least 14 days before the test date, indicating that vaccinated individuals who do start to show symptoms have an additional 43% and 37% protection against hospital admission within 14 days of a positive covid-19 test result. Mortality rates by vaccination status did not differ in the control analysis among those with a negative test result, indicating no evidence of a healthy vaccinee effect (see supplementary figure 4).
Table 4.
Risk of admission to hospital within 14 days of a positive covid-19 test result in vaccinated and unvaccinated people aged 80 years and older
| BNT162b2 | ChAdOx1-S | ||||||
|---|---|---|---|---|---|---|---|
| Total No of cases | No (%) admitted to hospital | Hazard ratio (95% CI) | Total No of cases | No (%) admitted to hospital | Hazard ratio (95% CI) | ||
| Unvaccinated | 8892 | 1365 (15.35) | 1.00 | 8892 | 1365 (15.35) | 1.00 | |
| Test date after first dose: | |||||||
| <14 days | 2084 | 293 (14.06) | 0.98 (0.86 to 1.11) | 562 | 64 (11.39) | 0.98 (0.78 to 1.24) | |
| ≥14 days | 1400 | 128 (9.14) | 0.57 (0.48 to 0.67) | 126 | 9 (7.14) | 0.63 (0.41 to 0.97) | |
| Total | 123 76 | 1786 (14.43) | 9580 | 1438 (15.01) | |||
Table 5 shows deaths within 21 days of a positive covid-19 test result by vaccination status among those aged 80 years and older who were vaccinated with BNT162b2 or unvaccinated. The hazard ratio for death compared with being unvaccinated was 0.49 (0.38 to 0.63) for those vaccinated at least 14 days before the test date (see supplementary figure 5 for Kaplan-Meier curve). This indicates that vaccinated individuals who go on to have symptoms have an additional 51% protection against death within 21 days of a positive covid-19 test result. Mortality rates by vaccination status did not differ in the control analysis among those with a negative test result, indicating no significant evidence of a healthy vaccinee effect (see supplementary figure 6).
Table 5.
Risk of death within 21 days of a positive covid-19 test result in those aged 80 years and older who were vaccinated with Pfizer-BioNTech BNT162b2 or unvaccinated
| Total No of cases | No (%) of deaths | Hazard ratio (95% CI) | |
|---|---|---|---|
| Unvaccinated | 8096 | 1063 (13.13) | 1.00 |
| Test date after first dose: | |||
| <14 days | 1096 | 114 (10.40) | 0.74 (0.62 to 0.90) |
| ≥14 days | 750 | 51 (6.80) | 0.49 (0.38 to 0.63) |
| Total | 9942 | 1228 (12.35) |
Discussion
This study provides early real world evidence for the effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-Asta-Zeneca ChAdOx1-S vaccines against symptomatic covid-19, hospital admissions, and death in older people in England. We found that a single dose of the BNT162b2 vaccine is about 60-70% effective at preventing symptomatic disease in adults aged 70 years and older in England and that two doses are about 85-90% effective. Those who were vaccinated and went on to have symptoms had a 44% lower risk of being admitted hospital and a 51% lower risk of death compared with people who were unvaccinated. We also found that a single dose of the ChAdOx1-S vaccine was about 60-75% effective against symptomatic disease and provided an additional protective effect against hospital admission—it is too early to assess the effect on mortality. The B.1.1.7 variant now dominates in the UK and these results will largely reflect vaccine effectiveness against this variant.
Interpretation
These data are observational and a range of factors will influence the odds of a positive covid-19 test result, which might also be associated with vaccination, thereby acting as confounders when examining vaccine effectiveness through routine testing, in particular in the early stages of the vaccination programme. A key factor that is likely to increase the odds of vaccinees testing positive (therefore underestimating vaccine effectiveness) is that individuals initially targeted for vaccination might be at increased risk of SARS-CoV-2 infection. For example, those accessing hospital may have been offered vaccination early in hospital hubs but might also be at higher risk of covid-19. This could explain the higher odds of a positive test result in vaccinees in the first few days after vaccination with BNT162b2 (before they would have been expected to develop an immune response to the vaccine) among those vaccinated during the first month of the roll-out.4 23 This effect appears to lessen as the roll-out of the vaccination programme progresses, suggesting that access to vaccines initially focused on those at higher risk, although this bias might still affect the longer follow-up periods (to which those vaccinated earliest will contribute) more than the earlier follow-up periods. This could also mean that lower odds ratios might be expected in later periods (ie, estimates of vaccine effectiveness could increase further). In the opposite direction, vaccinees might have a lower odds of a positive covid-19 test result in the first few days after vaccination because individuals are asked to defer vaccination if they are acutely unwell, have been exposed to someone who tested positive for covid-19, or had a recent coronavirus test.24 This explains the lower odds of a positive test result in the week before vaccination and may also persist for some time after vaccination if the recording of the date of symptom onset is inaccurate. Vaccination can also cause systemic reactions, including fever and fatigue.23 24 This might prompt more testing for covid-19 in the first few days after vaccination, which, if due to a vaccine reaction, will produce a negative result. This is likely to explain the increased testing immediately after vaccination with ChAdOx1-S and leads to an artificially low vaccine effectiveness in that period.25 26
An alternative explanation that vaccination caused an increased risk of covid-19 among those vaccinated before 4 January through some immunological mechanism is not plausible as this would also have been seen among those vaccinated from 4 January, as well as in clinical trials and other real world studies. Another explanation that some aspect of the vaccination event increases the risk of infection is possible, for example, through exposure to others during the vaccination event or while travelling to or from a vaccination site. However, the increase occurs within three days, before the typical incubation period of covid-19. Furthermore, if this were the cause, we would also expect this increase to occur beyond 4 January.
We also provide evidence that a single vaccine dose provides additional protection against covid-19 related admissions to hospital and deaths, with vaccinated individuals showing around half the risk of these severe outcomes compared with unvaccinated individuals. Combining this finding with our minimum vaccine effectiveness against symptomatic disease estimate would suggest that a single dose of BNT162b2 is around 80% effective at preventing hospital admission for covid-19 and around 85% effective at preventing death with covid-19.
We found that ChAdOx1-S reaches 75% effectiveness from 35 days after the first dose in those aged 70 years and older. As this had not yet plateaued during our study period, we are not able to estimate the level of effectiveness that this vaccine will reach or the duration of this effect. As with BNT162b2, additional protection against hospital admissions was shown, suggesting vaccine effectiveness against admission of at least 80% after a single dose of ChAdOx1-S. The initial phase III trial found a two dose efficacy against symptomatic disease of 70.4% (all ages).5 Efficacy results for older adults have not been reported, but immune response was similar.26 An analysis by duration of interval between doses suggested that a longer duration provided increased protection.27 The efficacy of a single dose was estimated at 76% with follow-up for up to 90 days in all age groups, which is in line with our findings.27
Comparison with other studies
Our results and those seen in the phase III clinical trials show similarities.4 5 27 As in the trial of BNT162b2, we saw a decline in the odds of a positive test result among vaccinees from 10 to 13 days after the first dose. The trial found an overall efficacy of 94.7% after the second dose in those aged 65 years and older. We estimated a vaccine effectiveness of 90% in those aged 80 years and older. In the trial the reported vaccine effectiveness in the interval between the first and second doses was 52.4% (95% confidence interval 29.5% to 68.4%). However, this included cases from the first two weeks after vaccination when we would not expect any effect. When the trial data were reanalysed using only cases observed between days 15 and 21 after the first dose, efficacy against symptomatic covid-19 was estimated at 92.6% (95% confidence interval 69.0% to 98.3%).6 28 Our analysis using observational data suggests that vaccine effectiveness against symptomatic disease in those aged 70 years and older reaches about 70% from 28 days after the first dose of vaccine.
Real world evidence on the early effectiveness of a single dose of BNT162b2 has also started to emerge from Israel: Chodick et al estimated a vaccine effectiveness of 52% during the first 24 days after vaccination, although a reanalysis of the same data by Hunter et al estimated that vaccine effectiveness had reached 90% by day 24.29 30 Dagan et al found a vaccine effectiveness against symptomatic disease of 57% after 14 to 20 days and 66% after 21 to 27 days, which are similar to our estimates. Amit et al estimated a vaccine effectiveness of 85% on days 15 to 28 after the first dose, although they also estimated a 45% reduction from days 1 to 14, which might indicate that those who were vaccinated had a lower baseline risk.31 It is not clear whether this analysis is based on the date of symptom onset or date of the test. The differences between some of the Israeli results and those seen in England might be explained by different testing strategies, populations analysed, case definitions, or analytical approaches. For example, greater vaccine effectiveness might be expected against symptomatic disease than against asymptomatic disease, therefore results might differ in settings with differing routine asymptomatic testing; similarly if testing is only offered to people with more severe disease (eg, after hospital admission), then the result for vaccine effectiveness might differ yet again. Where vaccines are offered to younger age groups first or to specific clinical risk groups (eg, those with immunosuppression) this may also influence vaccine effectiveness. The test negative case-control design used in our study may also not be suitable in settings where testing is not offered on the basis of symptoms. Another possible explanation is differential effectiveness against different variants. In England, the B.1.1.7 variant was the dominant virus throughout the study period. However, our analysis by variant based on spike gene target failure suggests that there is little difference in effects by variant. This is supported by recent evidence showing that sera from vaccinated individuals elicits equivalent neutralising titres to the B.1.1.7 variant and similar variants to that seen with previous strains.32 33
Strengths and limitations of this study
This study has several strengths: the large sample size, including all community covid-19 testing in England since the start of the vaccination programme, data on symptoms and date of onset, detailed vaccine history, and data on all previous testing. We provide evidence of vaccine effectiveness without restricting to the defined populations and storage, maintenance, and cold chains that can be well controlled in trial conditions but may be more challenging in the real world. The large sample size allowed us to look at fine intervals after vaccination, which helps to understand possible biases that need to be accounted for in this early phase of the vaccination programme. The large sample size also allowed us to estimate effects on severe outcomes, which may not be possible from the trials. Using a test negative case-control design helped to control for confounders that are difficult to measure as a result of differences in health seeking behaviour between vaccinated and unvaccinated individuals because outcomes are estimated within a group that we know has presented for testing. In cohort and other observational analyses that compare laboratory confirmed cases with other population based controls, it may be difficult to differentiate between effects being related to vaccinated individuals being less likely to develop disease or less likely to present for a test.
Limitations related to the observational nature of this analysis mean that the results should be interpreted with caution. Factors that could increase the risk of covid-19 in vaccinees (and therefore result in underestimation of vaccine effects) are that individuals may have more risky behaviours after vaccination if they believe they are protected; also, presenting for vaccination may be a risk factor in itself (eg, travelling to a vaccination centre with a friend or relative). Conversely, individuals who have been self-isolating may defer vaccination and may also be at lower risk of infection, this could underestimate vaccine effectiveness in the short period after vaccination. Misclassification is also likely to be a factor in this study. Symptoms are self-reported and may not be specific to covid-19 without clinician diagnosis. Furthermore, individuals may falsely report symptoms to have a test, which will include asymptomatic individuals in the symptomatic analysis and means that symptom onset dates are incorrect. Low sensitivity or specificity of PCR testing may also mean that cases and controls are misclassified. Failure to exclude those with past infection because of low testing rates in wave 1 is another possibility. Lags in vaccination data could also lead to misclassification; however, we excluded the most recent two days from the analysis, and a review of NIMS data showed that it is more than 90% complete beyond two days after vaccination (see supplementary figure 7). Furthermore, these lags would only affect the very early post-vaccination period, which is not of primary interest in this analysis. Any misclassification would attenuate vaccine effects. Also, at this stage in the vaccination programme, the length of follow-up in this analysis is limited. Further estimates in the coming weeks will include larger sample sizes and longer follow-up. We found a higher proportion of people from non-white ethnic groups and those aged 85 years and older among those who we were unable to link to vaccination histories. Although this could affect generalisability of the results, given the high linkage rates overall and the fact that the study covers the whole population of England, this is unlikely. Importantly, we restricted our analysis to those who report symptoms. The effect of the vaccines against asymptomatic disease may differ. This would require analysis of repeat asymptomatic PCR screening or serology.
Conclusions
This study provides early evidence that vaccination against covid-19 is having an important effect in England. We found a clear effect from the first dose of the BNT162b2 and ChAdOx1-S vaccines, and, in particular, a large effect of a single dose against severe outcomes of covid-19 related hospital admissions and mortality, supporting the decision to maximise the number of individuals vaccinated with a single dose—although we have limited evidence on the duration of this effect. An important number of vaccinated people still go on to develop covid-19 and our study indicates that vaccinated individuals must maintain other precautions, particularly in the first two to three weeks after vaccination. We also provide evidence that BNT162b2 is effective at preventing severe disease. Further evidence is needed on the duration of any effect and the effect against asymptomatic infection and transmission, and the four UK nations will work closely to develop and share evidence on this as it becomes available. Nevertheless, the fact that the vaccine appears to be preventing symptomatic disease, including with the B.1.1.7 variant, is encouraging, and this is likely to have an important impact on the detection of people with covid-19 and severe outcomes at a population level.
What is already known on this topic
Clinical trials and emerging real world data have shown that the Pfizer-BioNTech BNT162b2 vaccine is effective at preventing symptomatic disease using a schedule of two doses with an interval of three weeks between doses
Clinical trials have shown that the Oxford-AstraZeneca ChAdOx1-S vaccine is effective at preventing symptomatic disease in adults, although evidence in adults aged 70 years and older is limited
What this study adds
A single dose of either BNT162b2 or ChAdOx1-S provides significant protection against covid-19 and further protection against severe disease lasting at least six weeks, including against the UK variant of concern (B.1.1.7)
BNT162b2 and ChAdOx1-S offer similar levels of protection in adults aged 70 and older
Acknowledgments
We thank the Public Health England covid-19 Data Science Team, NHS England, NHS Digital, and NHS Test and Trace for their roles in developing and managing the covid-19 testing and vaccination systems and datasets as well as reporting NHS vaccinators, NHS laboratories, PHE laboratories, and lighthouse laboratories; and we thank the Joint Committee on Vaccination and Immunisation and the UK covid-19 Vaccine Effectiveness Working Group for advice and feedback in developing this study.
Web extra.
Extra material supplied by authors
Supplementary information: additional tables 1-5 and figures 1-6
Contributors: JLB, NA, CG, and MR designed the study and developed the protocol and analysis plan. NA, CG, and JS cleaned and analysed the data. JLB drafted the manuscript. All authors contributed to the study design and revised the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. JLB and MR are the guarantors.
Funding: There was no external funding for this study.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: funding from Public Health England for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years, no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Surveillance of covid-19 testing and vaccination is undertaken under Regulation 3 of The Health Service (Control of Patient Information) Regulations 2002 to collect confidential patient information (www.legislation.gov.uk/uksi/2002/1438/regulation/3/made) under Sections 3(i) (a) to (c), 3(i)(d) (i) and (ii) and 3(3). The study protocol was subject to an internal review by the Public Health England Research Ethics and Governance Group and was found to be fully compliant with all regulatory requirements. As no regulatory issues were identified, and ethical review is not a requirement for this type of work, it was decided that a full ethical review would not be necessary.
Data sharing: No additional data available.
The lead author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained
Dissemination to participants and related patient and public communities: The findings of this study will be disseminated through presentations to the Joint Committee on Vaccination and Immunisation and other National Immunisation Technical Advisory Groups as well as the World Health Organization. The findings will also be included in clinical guidance such as the Green Book. Blogs and press releases will also be prepared for public dissemination by UK public health agencies.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Medicines and Healthcare products Regulatory Agency . Vaccine BNT162b2: conditions of authorisation under Regulation 174. 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/948935/Conditions_of_authorisation_for_Pfizer_BioNTech_311220.pdf. [Google Scholar]
- 2.Public Health England. National flu and COVID-19 surveillance report: 21 January 2021 (week 3). 2020. www.gov.uk/government/statistics/national-flu-and-covid-19-surveillance-reports
- 3.Department of Health and Social Care. Joint Committee on Vaccination and Immunisation: advice on priority groups for COVID-19 vaccination, 30 December 2020. www.gov.uk/government/publications/priority-groups-for-coronavirus-covid-19-vaccination-advice-from-the-jcvi-30-december-2020/joint-committee-on-vaccination-and-immunisation-advice-on-priority-groups-for-covid-19-vaccination-30-december-2020#contents
- 4. Polack FP, Thomas SJ, Kitchin N, et al. C4591001 Clinical Trial Group . Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 2020;383:2603-15. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Voysey M, Clemens SAC, Madhi SA, et al. Oxford COVID Vaccine Trial Group . Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Skowronski DM, De Serres G. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 2021;384:1576-7. 10.1056/NEJMc2036242 [DOI] [PubMed] [Google Scholar]
- 7. Ledgerwood JE, Zephir K, Hu Z, et al. VRC 310 Study Team . Prime-boost interval matters: a randomized phase 1 study to identify the minimum interval necessary to observe the H5 DNA influenza vaccine priming effect. J Infect Dis 2013;208:418-22. 10.1093/infdis/jit180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shukarev G, Callendret B, Luhn K, Douoguih M, EBOVAC1 consortium . A two-dose heterologous prime-boost vaccine regimen eliciting sustained immune responses to Ebola Zaire could support a preventive strategy for future outbreaks. Hum Vaccin Immunother 2017;13:266-70. 10.1080/21645515.2017.1264755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Public Health England. Investigation of novel SARS-COV-2 variant - Variant of Concern 202012/01 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/947048/Technical_Briefing_VOC_SH_NJL2_SH2.pdf
- 10. Davies NG, Barnard RC, Jarvis CI, et al. Estimated transmissibility and severity of novel SARS-CoV-2 Variant of Concern 202012/01 in England. medRxiv. 2020:2020.12.24.20248822.
- 11.Public Health England. Investigation of novel SARS-CoV-2 variant - Variant of Concern 202012/01 - Technical briefing 4. 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/952490/Variant_of_Concern_VOC_202012_01_Technical_Briefing_4_England.pdf
- 12.European Centre for Disease Prevention and Control. Threat Assessment Brief: Rapid increase of a SARS-CoV-2 variant with multiple spike protein mutations observed in the United Kingdom. 20 December 2020. www.ecdc.europa.eu/sites/default/files/documents/SARS-CoV-2-variant-multiple-spike-protein-mutations-United-Kingdom.pdf
- 13.World Health Organization. Design of vaccine efficacy trials to be used during public health emergencies—points of considerations and key principles. 2020.
- 14. Jackson ML, Chung JR, Jackson LA, et al. Influenza Vaccine Effectiveness in the United States during the 2015-2016 Season. N Engl J Med 2017;377:534-43. 10.1056/NEJMoa1700153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pebody R, Djennad A, Ellis J, et al. End of season influenza vaccine effectiveness in adults and children in the United Kingdom in 2017/18. Euro Surveill 2019;24:1800488. 10.2807/1560-7917.ES.2019.24.31.1800488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Serres G, Skowronski DM, Wu XW, Ambrose CS. The test-negative design: validity, accuracy and precision of vaccine efficacy estimates compared to the gold standard of randomised placebo-controlled clinical trials. Euro Surveill 2013;18:20585. 10.2807/1560-7917.ES2013.18.37.20585 [DOI] [PubMed] [Google Scholar]
- 17. Schwartz LM, Halloran ME, Rowhani-Rahbar A, Neuzil KM, Victor JC. Rotavirus vaccine effectiveness in low-income settings: An evaluation of the test-negative design. Vaccine 2017;35:184-90. 10.1016/j.vaccine.2016.10.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Department of Health and Social Care. COVID-19 testing data: methodology note. 2020. www.gov.uk/government/publications/coronavirus-covid-19-testing-data-methodology/covid-19-testing-data-methodology-note
- 19.Public Health England. Investigation of novel SARS-CoV-2 variants of concern in England - Technical briefing 6. 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/961299/Variants_of_Concern_VOC_Technical_Briefing_6_England-1.pdf
- 20.Public Health England. Technical summary: Public Health England data series on deaths in people with COVID-19. 2020. www.gov.uk/government/publications/phe-data-series-on-deaths-in-people-with-covid-19-technical-summary
- 21.Care Quality Commission. CQC care directory. 2021. www.cqc.org.uk/files/cqc-care-directory-zip
- 22. Singanayagam A, Patel M, Charlett A, et al. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill 2020;25:2001483. 10.2807/1560-7917.ES.2020.25.32.2001483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Walsh EE, Frenck RW, Jr, Falsey AR, et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med 2020;383:2439-50. 10.1056/NEJMoa2027906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramsay M. COVID-19: the green book, chapter 14a. Immunisation against infectious diseases: Public Health England. 2020.
- 25. Folegatti PM, Ewer KJ, Aley PK, et al. Oxford COVID Vaccine Trial Group . Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020;396:467-78. 10.1016/S0140-6736(20)31604-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ramasamy MN, Minassian AM, Ewer KJ, et al. Oxford COVID Vaccine Trial Group . Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021;396:1979-93. 10.1016/S0140-6736(20)32466-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Voysey M, Costa Clemens SA, Madhi SA, et al. Single Dose Administration, And The Influence Of The Timing Of The Booster Dose On Immunogenicity and Efficacy Of ChAdOx1 nCoV-19 (AZD1222) Vaccine. SSRN 2021. 10.2139/ssrn.3777268. [DOI] [PMC free article] [PubMed]
- 28.Joint Committee on Vaccination and Immunisation. Optimising the COVID-19 vaccination programme for maximum short-term impact 2021. www.gov.uk/government/publications/prioritising-the-first-covid-19-vaccine-dose-jcvi-statement/optimising-the-covid-19-vaccination-programme-for-maximum-short-term-impact
- 29. Chodick G, Tene L, Patalon T, et al. The effectiveness of the first dose of BNT162b2 vaccine in reducing SARS-CoV-2 infection 13-24 days after immunization: real-world evidence. medRxiv 2021:2021.01.27.21250612.
- 30. Hunter PR, Brainard J. Estimating the effectiveness of the Pfizer COVID-19 BNT162b2 vaccine after a single dose. A reanalysis of a study of ‘real-world’ vaccination outcomes from Israel. medRxiv 2021:2021.02.01.21250957.
- 31. Amit S, Regev-Yochay G, Afek A, Kreiss Y, Leshem E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet 2021;397:875-7. 10.1016/S0140-6736(21)00448-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xie X, Zou J, Fontes-Garfias CR, et al. Neutralization of N501Y mutant SARS-CoV-2 by BNT162b2 vaccine-elicited sera. bioRxiv 2021:2021.01.07.425740.
- 33. Muik A, Wallisch A-K, Sänger B, et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera. bioRxiv 2021:2021.01.18.426984. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: additional tables 1-5 and figures 1-6