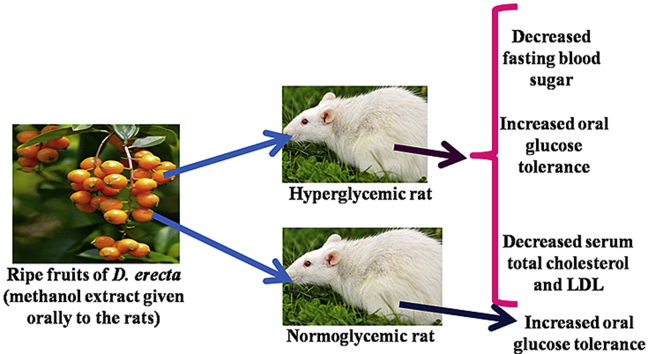
Graphical abstract
Keywords: Diabetes, South Eastern Nigeria, Igbo tribe, Traditional, Glucose tolerance
Highlights
-
•
MrFDE increased oral glucose tolerance in normoglycemic rats.
-
•
MrFDE caused 71.18% reduction in blood glucose, 6 h post-administration.
-
•
MrFDE caused progressive decline in FBS and increase in OGT in hyperglycaemic rats.
-
•
MrFDE showed antidyslipidemic effects in hyperglycaemic rats.
List of abbreviations
- MrFDE
methanol ripe fruit extract of Duranta erecta
- OGTT
oral glucose tolerance test
- OGT
oral glucose tolerance
- FBS
fasting blood sugar
- DM
diabetes mellitus
- WHO
World Health Organization
- HDL
high density lipoprotein
- LDL
low density lipoprotein
1. Introduction
Diabetes mellitus (DM) is a metabolic disease characterized by hyperglycemia and glucosuria due to total or relative lack of insulin or insulin resistance,1 the fasting plasma glucose cut off level being 126 mg/dl in man and 135 mg/dl in rats.2 It is a serious global health burden. According to W.H.O statistics, DM is the sixth leading cause of disease-related death in the world.3 The prevalence rate of DM in the developed countries is quite alarming and is expected to become one of the world’s leading debilitating and killer disease in a few years to come.4 Regions with greatest potential are Asia and Africa, where DM rates could rise to two-three folds.5 Dyslipidemia is one of the most important complications of DM, characterized by the elevation of the total cholesterol, triglycerides and LDL and decreased HDL.6 These anomalies are usually associated with atherosclerotic cardiovascular diseases encountered in DM patients. However, stringent glycemic control in clinical trials has failed to improve cardiovascular outcomes.7 Management of DM depends on both pharmacological means (oral hypoglycemic agents and insulin) and non-pharmacological means (diet, exercise and life style modification).8 The available oral hypoglycemic agents cause many adverse effects,9 while many only control blood sugar without affecting dyslipidemia, thus requiring concurrent administration of statins.7 Therefore, an ideal antidiabetic drug should not be toxic, should control the blood sugar as well as dyslipidemia. The limitations of these management methods have necessitated a search for better remedies.10 Herbal remedies have the potential of a viable alternative to allopathic medicines. Various plant species have been shown to have anti-diabetic activity. While few have been evaluated for authenticity of the claim, majority have not. Duranta erecta L (Verbenaceae) variously known as Golden dewdrop, sky flower, pigeon berry is also known as yellow bush in Nigeria, is an upright to drooping shrub that sometimes takes the form of a scrambling shrub or rarely a small tree.11,12 The unripe fruits are greenish in color, while the ripe ones are yellowish orange and hang in clusters.13 Various folkloric uses have been attributed to various parts of the plant. Fruit has been used as febrifuge, anti-malarial and anthelmintics.14 Fruits juice is used to manage urine related problems in the Philippines.15 The methanol leaf extract was shown to have antifungal activity and antiurolithiatic effects.16,17 The methanol ripe fruit extract did not show significant anthelmintic effects.14 Oral evidence from some traditional healers in the Igbo speaking part of Eastern Nigeria suggests the effectiveness of infusion of ripe fruits in the management of DM. Many phyto-chemicals with potential anti-hyperglycemic activity have been isolated from the fruits of the plant. These include saponins, alkaloids, glycosides and tannins.18,19 However, empirical data supporting the effectiveness of the ripe fruits as an antidiabetic agent are not available, hence this study. The main aim of this study was to validate or refute the effectiveness of the ripe fruits of D. erecta in the treatment of DM. To achieve this, we used a murine model of type 1 DM to evaluate the effectiveness of the methanol ripe fruit extract of D. erecta in the control of blood glucose and dyslipidemia associated with DM.
2. Materials and methods
2.1. Plant collection and extraction
Fresh ripe fruits of Duranta erecta were collected from the botanical garden of the University of Nigeria Nsukka. They were authenticated by Mr. A. Ozioko of the Biodiversity Conservation Program Nsukka (BDCP). Voucher specimen (UNVPP 2013/4506/21) was deposited in the herbarium of the Department of Botany University of Nigeria Nsukka. The fruits were dried under shed at room temperature (25 °C). The dried fruits were pulverized using hammer mill. The pulverized material was extracted in 70% methanol at room temperature with intermittent shaking for 72 h and the filtrate dried in vacuo in a rotary evaporator at 40 °C. The dried material was referred to as the methanol ripe fruit extract of D. erecta (MrFDE) and stored in the refrigerator at 4 °C until used. The yield of the extract was determined as described by Udobi et al., (2018)14
2.2. Experimental animals
Eighty seven 8 week old male Sprague Dawley rats weighing between 190 and 200 g were used in the study. They were housed in stainless steel rat cages. Food and water were provided ad libitum, except were fasting was necessary for experimental purposes. The rats were exposed to normal 12 h light and 12 h dark cycles and room temperature of 25 °C.20 They were acclimatized for 2 weeks before the experiments.
2.3. Ethical statement
The animal experimental protocol was in compliance with the Federation of European Laboratory Animal Science Association and the European Community Council Directive of November 24, 1986 (86/609/EEC). The animal experimental protocol was approved by the Experimental Animal Ethics Committee of the Faculty of Veterinary medicine, University of Nigeria, Nsukka.
2.4. Experimental
2.4.1. Acute toxicity test
Nine (9) rats were used for the first test. They were randomly assigned to 3 groups (n = 3) and treated with the extract orally by gastric gavage using curved rat feeding needle as follows: group A (10 mg/kg), group B (100 mg/kg), group C (1000 mg/kg). They were closely observed for 72 h for any sign of toxicity like death, excitement, changes in gait, weakness, diarrhea and sedation before the experiment was terminated. In the absence of any toxic signs 3 rats were administered the extract individually at the rate of 1600, 2900 and 5000 mg/kg respectively to determine the LD50.21
2.4.2. Dose response effects of MrFDE
2.4.2.1. Oral glucose tolerance test in normoglycemic rats
Twenty-five (25) rats were used for this experiment. They were randomly assigned to five (5) groups (n = 5). The animals were fasted overnight and fasting blood sugar (FBS) determined using Accu-chek® Active (Roche, Mannheim, Germany) glucose test kit. The rats were grouped and treated as follows: group A (distilled water 5 ml/kg; vehicle for the extract), group B (glibenclamide, 2 mg/kg), group C (MrFDE, 100 mg/kg), group D (MrFDE 200 mg/kg) and group E (MrFDE, 400 mg/kg). All treatments were by the oral route. One-hour post-treatment, 2000 mg/kg of glucose was administered orally to all the rats, and blood glucose was determined at 30, 60 and 120 min post-glucose administration.22
2.4.2.2. Induction of hyperglycemia
Animals were fasted overnight and their FBS was determined. Alloxan monohydrate was reconstituted using ice (cold) injection water and given at a dose of 160 mg/kg intraperitoneally once to all the experimental animals. Glucose was added to their drinking water overnight to prevent alloxan-induced hypoglycemic shock.23 The FBS level was checked every other day and animals with FBS >135 mg/dl were considered hyperglycemic.2
2.4.2.3. Effect of MrFDE on blood glucose level of hyperglycemic rats
Twenty-five (25) rats were randomly assigned to 5 groups (n = 5) and treated as follows: group A (distilled water 5 ml/kg), group B (2 mg/kg glibenclamide), group C (100 mg/kg MrFDE), group D (200 mg/kg MrFDE) and group E (400 mg/kg MrFDE). All treatments were given once orally. Overnight FBS of all the rats was determined before administration of glibenclamide or extract. Blood glucose of all the rats was determined at 1, 3 and 6 h post-administration of glibenclamide or MrFDE. Changes in blood glucose over time were recorded. Percentage reduction in blood glucose at 1, 3 and 6 h for each group was calculated as a percentage decrease in blood glucose from the FBS (0 h).24
2.4.3. Effect of sub-acute administration of MrFDE on FBS, oral glucose tolerance and lipid profile of hyperglycemic rats
Twenty-five (25) rats were used in this study. They were randomly assigned to 5 groups (n = 5). Induction of hyperglycemia was as already described in sub-section 2.4.2.2. They were treated as follows: group A (distilled water 5 ml/kg), group B (glibenclamide 0.6 mg/kg), group C (MrFDE, 25 mg/kg), group D (MrFDE, 50 mg/kg) and group E (MrFDE, 100 mg/kg). All treatments were through the oral route and were done once daily for 21 days. Overnight FBS was determined on days 0, 7, 14 and 21. Percentage reduction in blood glucose at day 7, 14 and 21 for each group was calculated as a percentage decrease in blood glucose from the day 0 FBS.
Oral glucose tolerance was done on days 7, 14 and 21. Mean OGT for days 7, 14 and 21 was calculated for each group as a percentage decrease in blood glucose between the 30 min blood glucose and 120 min blood glucose for each day. Serum samples were obtained from the rats on day 21 for analysis of the lipid profile (total cholesterol, triglyceride, HDL and LDL) using Randox® kits (Randox Laboratories Ltd, United Kingdom) according to the manufacturer’s instructions.
2.4.4. Phytochemical analyses
The methanol extract of the ripe fruits of Duranta erecta was subjected to phytochemical test.25 Presence of the following phytochemicals was evaluated: starch, carbohydrate, polyuronoids, terpens and sterols, flavonoids, tannins, alkaloids, saponins and glycosides.
2.5. Statistical analyses
Statistical analysis was conducted using SPSS version 15 for Windows. Data obtained were presented as arithmetic means with standard errors of mean in tables and figures. The data obtained from the lipid profile data in experiments in subsection 2.4.3 were analyzed using one-way analysis of variance. Repeated measures in general linear model was used to analyze the remaining data obtained in experiments in 2.4.2.1, 2.4.2.3, 2.4.3. The Greenhouse-Geisser correction was used where Mauchley’s test of sphericity was violated. Results are reported as main effects and simple main effects depending on the significance of any interactions. Where ANOVA showed significant difference, differences between means were confirmed using least significant difference (LSD) post hoc multiple comparison. Results were considered significant when p < 0.05.
3. Results
3.1. Extraction
The methanol extract of Duranta erecta ripe fruits gave a dark brown, jell-like and sticky substance with sweet aroma and readily soluble in water. The yield of MrFDE was 21.51% w/w.
3.2. Acute toxicity
The rats tolerated MrFDE up to 5000 mg/kg. Thus the LD50 was more than 5000 mg/kg. The animals showed no signs of toxicity (Table 1).
Table 1.
Acute toxicity effects of methanol ripe fruit extract of D. erecta.
| Signs of toxicity |
|||||||
|---|---|---|---|---|---|---|---|
| MrFDE (mg/kg) | N | death | excitement | Changes in gait | weakness | diarrhea | sedation |
| 10 | 3 | Nil | Nil | Nil | Nil | Nil | Nil |
| 100 | 3 | Nil | Nil | Nil | Nil | Nil | Nil |
| 1000 | 3 | Nil | Nil | Nil | Nil | Nil | Nil |
| 1600 | 1 | Nil | Nil | Nil | Nil | Nil | Nil |
| 2900 | 1 | Nil | Nil | Nil | Nil | Nil | Nil |
| 5000 | 1 | Nil | Nil | Nil | Nil | Nil | Nil |
N = no of animals.
3.3. Oral glucose tolerance test in normoglycemic rats
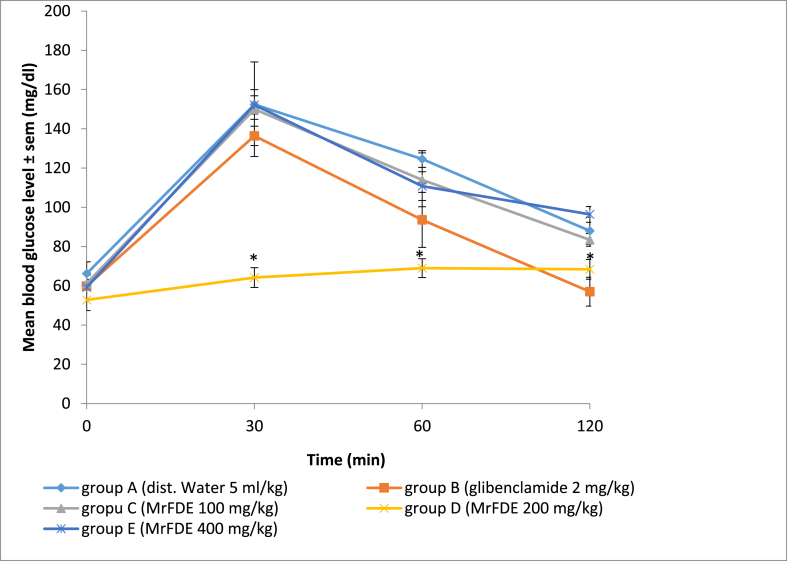
There was significant interaction between treatment doses of MrFDE and time in the OGTT (P = 0.004). There was significant main effect for treatment doses of MrFDE and time in OGT in treated rats (P = 0.000). Pairwise comparison for the treatment doses and duration of treatment showed that MrFDE at 200 mg/kg maintained significantly (p < 0.05) lower blood glucose level from 30 to 120 min post-glucose administration when compared with groups A, C and D and 30–60 min compared with group B. However, the 30 min blood glucose levels in all the treatment groups except group D increased significantly (P < 0.05) from the baseline levels (Fig. 1).
Fig. 1.
Mean blood glucose level of normoglycemic rats treated with methanol ripe fruit extract of Duranta erecta, 1 h before administration of 2000 mg/kg glucose in oral glucose tolerance test.
∗Significant (P < 0.05).
3.4. Effect of MrFDE on blood glucose level of hyperglycemic rats
ANOVA with Greenhouse-Geisser correction showed no significant interaction between treatment doses of MrFDE and time on the blood glucose levels of treated rats. However, there was significant main effect for the treatment doses (P = 0.00) and time (P = 0.02) on the blood glucose levels. Pairwise comparison of treatment doses and time showed that significant reduction in the blood glucose occurred in the glibenclamide-treated rats from 1 to 6 h post-treatment compared with groups A and D rats and 1–3 h post-treatment compared with groups C and E rats. Nevertheless, at 1 and 3 h post-treatment, the reduction in blood glucose levels in groups C and E rats were significant (P < 0.05) compared with group A. However, at 6 h post-treatment reduction in blood glucose level in all the MrFDE-treated rats were significant (P < 0.05) compared with group A. The blood glucose level of groups A and D rats did not change significantly from there pretreatment levels, while those of groups B and E rats showed significant (P < 0.05) decrease at all the time points post-treatment (Table 2).
Table 2.
Mean blood glucose level of hyperglycemic rats, treated with MrFDE once and monitored for 6 h.
| Mean blood glucose (mg/dl) |
||||
|---|---|---|---|---|
| 0 h | 1 h | 3 h | 6 h | |
| Dist. Water (5 ml/kg) | 399.50 ± 11.48 | 347.75 ± 3.55s | 363.75 ± 7.00a | 383.75 ± 7.26a |
| Glibenclamide (2 mg/kg) | 259.00 ± 21.07 | 113.25 ± 2.50b | 77.50 ± 2.00b | 77.50 ± 1.40b |
| Extract (100 mg/kg) | 289.00 ± 10.00 | 233.25 ± 3.80c | 155.25 ± 5.81c | 166.00 ± 12.80b |
| Extract (200 mg/kg) | 360.00 ± 7.20 | 316.67 ± 4.20a | 308.67 ± 1.82a | 228.00 ± 7.60c |
| Extract (400 mg/kg) | 293.25 ± 12.60 | 224.50 ± 12.0c | 162.25 ± 6.60c | 84.50 ± 3.22b |
Values down the column with different superscripts vary significantly (p < 0.05).
ANOVA with Greenhouse-Geisser correction showed no significant interaction between treatment doses of MrFDE and time in the percentage reduction in blood glucose levels. However, there was significant main effect for treatment doses (P = 0.05) and time (P = 0.01). Pairwise comparison for treatment doses showed that the percentage changes in the blood glucose levels in all the treatment doses did not vary significantly at 1 h post-treatment. But at 3 h, the changes in blood glucose level was significant (P < 0.05) in groups B, C and E compared to group A, while the changes at 6 h was significant (P < 0.05) in all the MrFDE and glibenclamide treated groups compared to group A. Pairwise comparison for time showed that the changes in blood glucose in groups A and B were not significant between the 1, 3 and 6 h time points (Table 3).
Table 3.
Percentage reduction in blood glucose of hyperglycemic rats, treated with MrFDE once and monitored for 6 h.
| Mean reduction in blood glucose (%) |
|||
|---|---|---|---|
| 1 h | 3 h | 6 h | |
| Dist. Water (5 ml/kg) | 12.95 ± 2.22 | 8.95 ± 0.58a | 3.94 ± 0.37a |
| Glibenclamide (2 mg/kg) | 49.39 ± 3.60 | 70.08 ± 3.67b | 70.08 ± 2.33b |
| Extract (100 mg/kg) | 19.29 ± 1.78 | 46.28 ± 1.96b | 42.56 ± 2.24b |
| Extract (200 mg/kg) | 12.22 ± 0.68 | 14.44 ± 1.67a | 36.67 ± 1.87b |
| Extract (400 mg/kg) | 23.44 ± 2.33 | 44.67 ± 2.50b | 71.18 ± 1.97b |
Values down the column with different superscripts vary significantly (p < 0.05.
3.5. Effect of sub-acute administration of MrFDE on the FBS, oral glucose tolerance and lipid profile of hyperglycemic rats
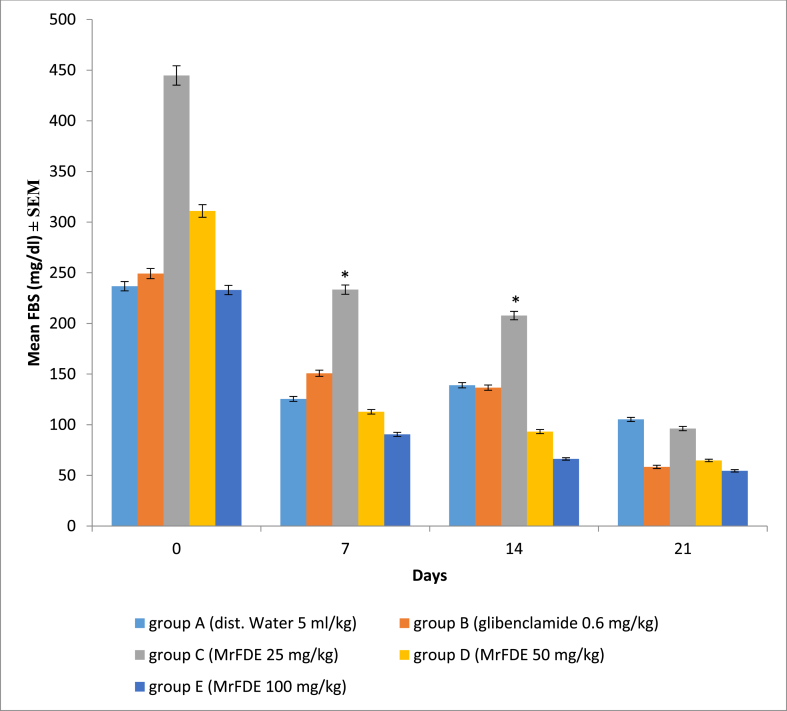
ANOVA with Greenhouse-Geisser correction showed no significant interaction between treatment doses of MrFDE and time in the FBS of treated hyperglycemic rats. There was also no significant main effect for treatment doses of MrFDE, but there was significant main effect for time (P = 0.000). Pairwise comparison for the treatment groups showed that on days 7 and 14, the FBS of group E rats were significantly (p < 0.05) lower than those of group C rats. Pairwise comparison for time showed that the decrease in the FBS of group C rats was significant between days 0, 7, 14 and 21 while those of group A were not throughout the same periods (Fig. 2).
Fig. 2.
Fasting blood sugar (FBS) of hyperglycemic rats, treated once daily for twenty-one days with methanol ripe fruit extract of D. erecta.
∗Significant (P < 0.05).
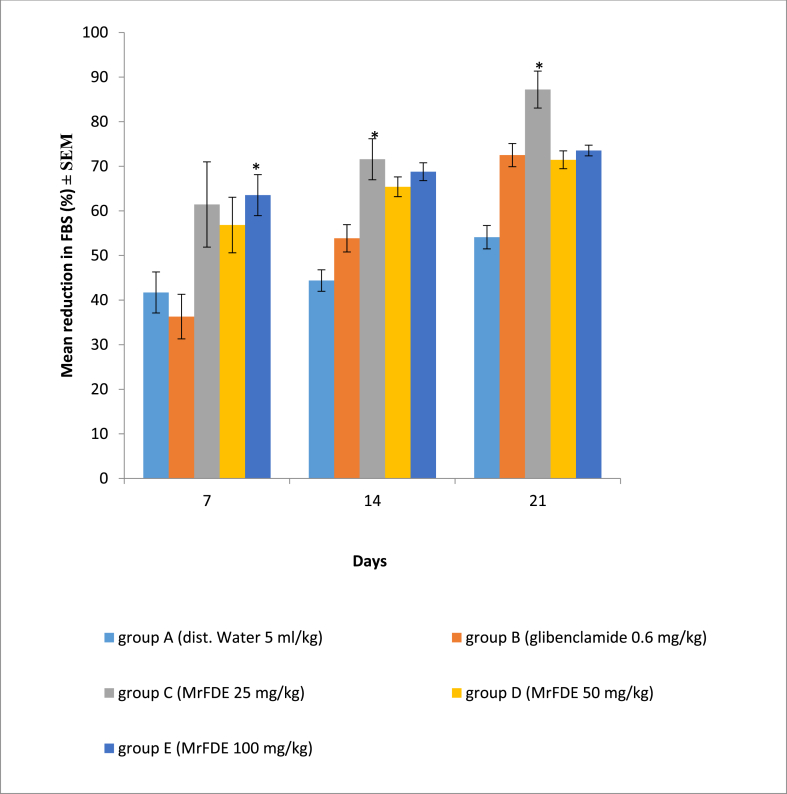
There was no significant interaction between treatment doses of MrFDE and time in the percentage reduction in FBS. There was also no significant main effect for treatment doses of MrFDE, however, there was significant main effect for time (P = 0.000). Pairwise comparison for treatment doses showed that the percentage reduction in FBS in group E was significantly (P = 0.043) higher than that of group B on day 7. The percentage reduction in the FBS in group C was significantly higher than that of group A on days 7 (P = 0.038) and 14 (P = 0.009). Pairwise comparison for time showed that the percentage reduction in FBS in group C on day 21 was significantly higher than that of day 7, while there was significant (P < 0.05) time dependent percentage reduction in the FBS in group B (Fig. 3)
Fig. 3.
Percentage reduction in the FBS of hyperglycemic rats, treated once daily for twenty-one days with methanol ripe fruit extract of D. erecta.
∗Significant (P < 0.05).
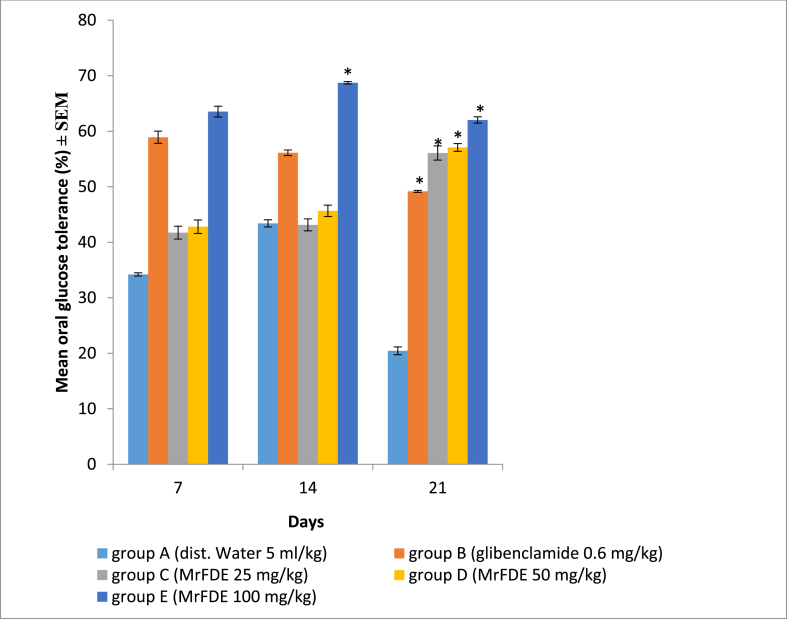
There was significant interaction between treatment doses of MrFDE and time (P = 0.03) in OGT in hyperglycemic rats. There was also significant main effect for treatment doses of MrFDE (P = 0.03) and time (P = 0.01). Pairwise comparison for treatment doses showed that there was no significant difference in OGT between the groups on day 7. On day 14, group E had significantly higher OGT than groups A and D. However, on day 21, all the MrFDE treated groups and the glibenclamide treated group had significantly (P < 0.05) higher OGT than the vehicle treated control (Fig. 4).
Fig. 4.
Oral glucose tolerance of hyperglycemic rats, treated once daily for twenty-one days with methanol extract of ripe fruit of D. erecta.
∗Significant (P < 0.05).
The lipid profile of the experimental animals showed that there was significantly (p < 0.05) lower total serum cholesterol concentration in group E rats compared to group A, B and D rats. There was no significant variation between the total serum cholesterol concentration of the rats in groups C and E. Triglyceride and high density lipoprotein (HDL) concentrations did not vary significantly among the experimental groups. The concentration of the low density lipoprotein (LDL) was significantly (p < 0.05) lower in group E rats compared to all the other experimental groups. However, the serum LDL concentration in group C rats was only significantly (p < 0.05) lower than those of groups A, B and D (Table 4).
Table 4.
Effect of methanol ripe fruit extract of D. erecta on the lipid profile of hyperglycemic rats treated once daily for twenty on days.
| Groups | Cholesterol (mg/dl) | Triglyceride (mg/dl) | HDL (mg/dl) | LDL (mg/dl) |
|---|---|---|---|---|
| A | 85.26 ± 0.42 | 11.89 ± 0.10 | 13.12 ± 0.38 | 69.76 ± 2.42 |
| B | 77.99 ± 0.01 | 23.03 ± 0.39 | 15.68 ± 0.14 | 57.70 ± 1.46 |
| C | 57.54 ± 1.49 | 14.61 ± 0.70 | 15.84 ± 0.16 | 38.78 ± 1.93∗ |
| D | 89.88 ± 1.31 | 20.56 ± 0.63 | 16.64 ± 0.45 | 69.13 ± 2.76 |
| E | 23.94 ± 3.03∗∗ | 24.77 ± 1.57 | 14.24 ± 0.48 | 4.75 ± 0.52∗ ∗ |
∗ ∗ significantly (p < 0.05) lower than A, B, C, D. ∗ significantly (p < 0.05) lower than A, B. D.
A: Distilled Water (5 ml/kg), B: Hyperglycemic and treated with glibenclamide (0.6 mg/kg), C: Hyperglycemic and treated with extract (25 mg/kg), D: Hyperglycemic and treated with extract (50 mg/kg), E: Hyperglycemic and treated with extract (100 mg/kg).
3.6. Phytochemical analysis
Phytochemical analyses of MrFDE showed the presence of flavonoids, tannins, terpenes, glycosides, polyuronides and saponins (Table 5).
Table 5.
Phytochemical constituents of methanol ripe fruit extract of D. erecta.
| Phytochemical test | Result |
|---|---|
| Starch | - |
| Carbohydrate | - |
| Polyuronides | + |
| Terpens | + |
| Sterols | - |
| Flavonoids | + |
| Tannins | + |
| Alkaloids | - |
| Saponins | + |
| Glycosides | - |
-: absent; +: present.
4. Discussion
The methanol ripe fruit extract of Duranta erecta increased significantly glucose tolerance in both normoglycemic and hyperglycemic rats. MrFDE also significantly decreased the FBS of hyperglycemic rats in a 6 h study and weekly FBS in a 21day study, with a significant reduction in serum total cholesterol and low density lipoprotein. This is a significant finding because there are few standard anti-diabetic drugs with both anti-hyperglycemic and anti-dyslipidemic properties. Acute toxicity study showed that MrFDE has a wide margin of safety, which makes it a promising candidate for further studies and possible clinical application.
Oral glucose tolerance test is standard diagnostic test for DM and measures glucose clearance rate which is a function of insulin production and sensitivity and glucose uptake by peripheral cells.26 Oral glucose tolerance study in normoglycemic rats showed that there was a significant interaction between treatment doses of MrFDE and time, hence, MrFDE at 200 mg/kg kept the blood glucose relatively constant with only a marginal rise after administration of 2000 mg/kg glucose from 30 to 120 min. This is an evidence of increased capacity for glucose tolerance in treated rats. It is possible that MrFDE at 200 mg/kg caused retardation of glucose absorption from the gastrointestinal tract, thereby leading to gradual release of glucose into the systemic circulation as evidenced by the gradual increase of blood sugar from 30 min to 120 min. The effect of MrFDE on glucose tolerance in normoglycemic rats peaked at 200 mg/kg, beyond which it depreciated. The reason for this effect is not clear. However, empirical evidence exists that shows that this is possible with medicinal plant product owing to the fact that they are made up of various phytochemicals which interactions can actually upregulate or downregulate a biological process depending on the dose. Suberu et al.,27 showed that some antiplasmodial constituents of Artemisia annua tea extract with minor structural difference from artemisinin (antiplasmodial principle of A. annua) antagonized or enhanced the antiplasmodial efficacy of artemisinin depending on the combination dose levels. It was therefore, suggested that these constituents could have acted as either pro-oxidants or antioxidant or compete for molecular target to increase or decrease activity depending on the dose level.28 These mechanisms could explain the behavior of MrFDE in this study. Hyperglycemia is induced by alloxan and the product of its reduction; dialuric acid, by establishing a redox cycle with the formation of superoxide radicals. These radicals undergo dismutation to hydrogen peroxide with massive increase in cytosolic calcium concentration, leading to rapid destruction of pancreatic beta-cells of Islets of Langerhans.29,30 In this study, the vehicle-treated rats remained hyperglycemic all through. The MrFDE-treated groups (100 mg/kg and 400 mg/kg) and glibenclamide (2 mg/kg) showed significant decrease in blood glucose level 1–6 h post treatment. MrFDE did not show a dose-dependent effect. Although, it seemed that doubling the initial dose depreciates the hypoglycemic effect of the extract, while quadrupling it produces greater hypoglycemic effect. Nevertheless, the overall effect of MrFDE on blood glucose in hyperglycemic rats at all doses used was a time-dependent decrease. Conversely the blood glucose of the vehicle-treated rats increased with time. Calculation of the percentage reduction in blood glucose showed that the effect of MrFDE at 400 mg/kg (71.18 ± 1.97%) at 6 h post-treatment was comparable to glibenclamide (70.08 ± 2.33%); a standard anti-diabetic drug.
Fasting blood sugar is a routine monitoring test for hyperglycemic patients.31 In the study of the effect of sub-acute administration of MrFDE in hyperglycemic rats, the FBS and percentage reduction in FBS was monitored weekly for 3 weeks. Here MrFDE showed a time-dependent decrease in the FBS and increase in percentage reduction in FBS, with the lowest dose (25 mg/kg) producing the highest effect after week 3. According to Malviva., et al.32 one of the antidiabetic mechanisms of medicinal plants is by restoration of pancreatic functions. The significant reduction of FBS by MrFDE could have been through the healing of the pancreas, thereby gradually restoring pancreatic functions previously distorted by alloxan. It is also possible that progressive administration of MrFDE lead to enhanced glucose utilization by cells. The weekly OGTT which was used as a stress test, to simulate post-prandial hyperglycemia, showed that MrFDE produced a time and dose-dependent increase in OGT throughout the duration of the study and the interaction between MrFDE doses and duration of treatment was statistically significant. When the OGT was calculated week by week, it was observed that the vehicle-treated group deteriorated with progressive decrease in OGT compared with all the MrFDE and glibenclamide treated groups which showed progressive increase in OGT. The highest dose of MrFDE used in this study (100 mg/kg) was most effective. This showed that in acute hyperglycemic attacks, higher doses of MrFDE is required to control blood glucose within a short period, while 25 mg/kg can be assumed to be a maintenance dose, since it was most effective in controlling FBS week on week.
One of the major complications of DM is dyslipidemia. Dyslipidemia has been implicated as the major cause of cardiovascular anomalies like atherosclerosis and cardiomyopathies seen in uncontrolled hyperglycemia.33,34 MrFDE showed potential anti-dyslipidemic effects in treated rats; lower total cholesterol and LDL at the doses of 25 and 100 mg/kg. However, the anti-dyslipidemic effect was more prominent at 100 mg/kg.
Anti-hyperglycemic activity of medicinal plants is mainly through restoration of the function of pancreatic cells or decreasing intestinal absorption of glucose.32 It could also be due to enhanced glucose uptake in cells.35 Glycosides, alkaloids, terpenoids, flavonoids, carotinoids from plants have been suggested to have anti-diabetic activity.32 The phytochemical analyses of the extract showed the presence of glycosides, flavonoids, terpenes, saponins, tannins and polyuronids. Various mechanisms of action have been postulated for these phyto-chemicals, which include inhibition of formation of advanced glycated end-products, increased glucose uptake by myocytes, increased insulin secretion by pancreas, increase in liver glycogen concentration and glucokinase activity and antioxidant effects 36, 37, 38, 39 The anti-hyperglycemic and anti-dyslipidemic effects of MrFDE could be as a result of combination of various mechanisms of anti-hyperglycemic and anti-dyslipidemic effects of the different phytochemical constituents. The availability of these phytochemicals with different mechanisms of anti-hyperglycemic effects could be responsible for the non-dose-dependent effect of the extract in some of the experiments, as combination of the different phytochemicals at certain doses of the extract are likely to interact in different ways to produce different results. Thus, at certain doses a synergistic interaction could occur to produce higher response, while increasing or decreasing the dose could produce antagonistic interaction, with lower response.
5. Conclusion
The methanol ripe fruit extract of Duranta erecta seemed to be safe in rats. It also caused significant increase in glucose tolerance in both normoglycemic and hyperglycemic rats. The extract also showed potential anti-dyslipidemic effect, inferring a potential cardiovascular protective effect. The lower dose (25 mg/kg) in the sub-acute study, suggests a maintenance daily dose for control of blood glucose, while the higher dose (100 mg/kg) may be particularly useful in acute cases of hyperglycemia often seen in diabetic patient where restoration of blood glucose to normalcy within a very short period of time is required. The anti-hyperglycemic and anti-dyslipidemic effects of MrFDE could be attributed to various phyto-chemicals which had been shown in other studies to poses potent anti-hyperglycemic and anti-dyslipidemic effects.
Declaration of competing interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.World Health Organization . vol. 99. WHO/NCD/NCS; Geneva: 1999. pp. 1–58. (Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications: Part1: Diagnosis and Classification of Diabetes). 2. [Google Scholar]
- 2.Wang Z., Yang Y., Xiang X., Zhu Y., Men J., He M. Estimation of the normal range of blood glucose in rats. Wei Sheng Yan Jiu. 2010;39:133–142. [PubMed] [Google Scholar]
- 3.Beverley B., Eschwège E. The diagnosis and classification of diabetes and impaired glucose tolerance. In: Pickup J.C., William G., editors. Textbook of Diabetes 1. 2003. pp. 2.1–2.11. [Google Scholar]
- 4.Ding C.H., Teng C.L., Koh C.N. Knowledge of diabetes mellitus among diabetic and Non-diabetic patients in klinikkesihatanseremban. Med J Malaysia. 2016;61:399–404. [PubMed] [Google Scholar]
- 5.Oputa R.N., Chinenye S. Dibetes mellitus: a global epidemic with potential solutions. Afri J Diabetes Med. 2012;20:33–35. [Google Scholar]
- 6.Goldberg I.J. Diabetic dyslipidemia: causes and consequences. J Clin Endocrinol Metab. 2001;86:965–971. doi: 10.1210/jcem.86.3.7304. [DOI] [PubMed] [Google Scholar]
- 7.Schofield J.D., Liu Y., Rao-Balakrishna P., Malik A.R., Soran H. Diabetes dyslipidemia. Diabetes Therapeut. 2016;7:203–219. doi: 10.1007/s13300-016-0167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gyawali B., Mishra S.R., Neupane D. Diabetes management training for female community health volunteers in Western Nepal: an implementation experience. BMC Publ Health. 2018;18:641. doi: 10.1186/s12889-018-5562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhury A., Duvoor C., Dendi V.S.R. Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Front Endocrinol. 2017;8:6. doi: 10.3389/fendo.2017.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osadebe P.O., Okide G.B., Akabogu I.C. Study on anti-diabetic activities of crude methanolic extracts of Loranthus micranthus (Linn.) sourced from five different Host trees. Ethnopharmacol. 2004;95:133–138. doi: 10.1016/j.jep.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 11.Little E.L., Jr., Woodbury R.O., Wadsworth F.H. vol. 2. U.S. Department of Agriculture; Washington, DC: 1974. (Trees of Puerto Rico and the Virgin Islands). Agriculture Handbook 449. 1,024. [Google Scholar]
- 12.Liogier H.A. vol. 4. Editorial de la Universidad de Puerto Rico; San Juan, PR: 1995. p. 617. (Descriptive Flora of Puerto Rico and Adjacent Islands). [Google Scholar]
- 13.Said R.M. Response of sky flower (Duranta erecta L. var. Variegata) transplants as pot plant to growing media and water amounts. Middle East J Agric Res. 2016;5:201–207. [Google Scholar]
- 14.Udobi M.I., Nzeakor T.A., Eke I.G. Evaluation of the anthelminthic potential of Duranta erecta L. (Verbenaceae) fruits used in Nigerian ethnomedicine as a vermifuge. J Ethnopharmacol. 2018;216:57–62. doi: 10.1016/j.jep.2018.01.030. [DOI] [PubMed] [Google Scholar]
- 15.Stuart G.U. Godofredo U. Stuart Jr., M.D/Stuart Xchange; 2015. Philippine Medicinal Plants.www.stuartxchange.org [Google Scholar]
- 16.Sharma P., Khandelwal S., Singh T., Vijayvergia R. Phytochemical analysis and antifungal potential of Duranta erecta against some phytopatogenic fungi. Int J Pharma Sci Res. 2012;3:2686–2689. [Google Scholar]
- 17.Agawane S.B., Gupta V.S., Kulkarni M.J., Bhattacharya A.K., Koratkar S.S., KoteswaraRao V. Patho-physiological evaluation of Duranta erecta for the treatment of urolithiasis. J Ayurveda Integr Med. 2019;10:4–11. doi: 10.1016/j.jaim.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao A.V., Gurfinkel D.M. The bioactivity of saponins: triterpenoid and steroidal glycosides. Drug Metabol Drug Interact. 2000;17:211–235. doi: 10.1515/dmdi.2000.17.1-4.211. [DOI] [PubMed] [Google Scholar]
- 19.Switi B., Gaikwad G., Krishna M., Sandhya Rani M. Phytochemicals for diabetes management. Bentham Pharmaceut Corps. 2014;5(Suppl 1: M2):11–28. [Google Scholar]
- 20.Gosh A., Banik S., Amin M.N., Ahmed J. Evaluation of antinociceptive, antihyperglycemic, and membrane stabilizing activities of Garcinia lancifolia Roxb. J Tradit Compl Med. 2018;8:303–307. doi: 10.1016/j.jtcme.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorke D. A new approach to acute toxicity testing. Arch Toxicol. 1983;53:275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 22.Chaimum-aom N., Chomko S., Talubmook C. Toxicology and oral glucose tolerance test (OGTT) of Thai medicinal plant used for diabetes control, Phyllanthus acidus L. (EUPHORBIACEAE) Pharmacogn J. 2017;9 58-6. [Google Scholar]
- 23.Ashok D.C., Shrimant N.P., Pradeep M.G., Akalpita U.A. Optimization of alloxan dose is essential to induce stable diabetes for prolonged period. Asian J Biochem. 2007;2:402–408. [Google Scholar]
- 24.Eke I.G., Omoja V.U., Echema C. Evaluation of methanol extract of Gongronema latifolium leaves singly and in combination with glibenclamide for antihyperglycemic effects in alloxan-induced hyperglycaemic rats. J Intercult Ethnopharmacol. 2014;3:119–122. doi: 10.5455/jice.20140610054950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trease G.E., Evans W.C. fifteenth ed. London W. B. Saunders; 2002. Pharmacognosy. [Google Scholar]
- 26.DeFronzo R.A., Abdul-Ghani M. Assessment and treatment of cardiovascular risk in prediabetes: impaired glucose tolerance and impaired fasting glucose. Am J Cardiol. 2011;108(3 Suppl):3B–24B. doi: 10.1016/j.amjcard.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Suberu J.O., Gorka A.P., Jacobs L. Anti-plasmodial polyvalent interactions in Artemisia annua L. aqueous extract--possible synergistic and resistance mechanisms. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0080790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caesar L.K., Cech N.B. Synergy and antagonism in natural product extracts: when 1 + 1 does not equal 2. Nat Prod Rep. 2019;36:869. doi: 10.1039/C9NP00011A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grover J.K., Vats V., Rathi S.S. Antihyperglycemic effects of Eugenia Jambulana and Tinospora Cordifolia in experimental diabetes and their effects on the key metabolic enzymes involved in carbohydrate metabolism. J Ethnopharmacol. 2000;73:461–470. doi: 10.1016/s0378-8741(00)00319-6. [DOI] [PubMed] [Google Scholar]
- 30.Szudelski T. The mechanism of Alloxan and Streptozotocin action in beta-cells of the rats’ pancreas. Physiol Res. 2001;50:536–546. [PubMed] [Google Scholar]
- 31.World Health Organization . 2006. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation. [Google Scholar]
- 32.Malviya N., Jain S., Malviya S. Antidiabetic potential of medicinal plants’. Acta Pol Pharm. 2010;67:113–118. [PubMed] [Google Scholar]
- 33.Abou-seif M.A., Youssef A.A. Evaluation of some biochemical changes in diabetic patients. Clin Chim Acta. 2004;346:161–170. doi: 10.1016/j.cccn.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 34.Elinasri H.A., Ahmed A.M. Patterns of lipid changes among type 2 diabetes patients in Sudan. East Mediterr Health J. 2008;14:314–324. [PubMed] [Google Scholar]
- 35.Kazumi Y. Anti-diabetic phytochemicals that promote GLUT4 translocation via AMPK signaling in muscle cells”. Nutr Aging. 2014;2:35–44. [Google Scholar]
- 36.Ramakrishnan M., Ramalingam S. Antidiabetic effect of d-limonene, a monoterpene in streptozotocin-induced diabetic rats. Biomed Prev Nutr. 2012;2:269–275. [Google Scholar]
- 37.Babby A., Elanchezhiyan C., Suhasini S., Chandirasegaran S.G. Antihyperglycemic effect of tannic acid in streptozotocin induced diabetic rats. Int J Cur Res. 2014;6:5396–5398. [Google Scholar]
- 38.Ayman M.M., Osama M.A., Mohamed B.A., Adel A.M. In vivo and in vitro antidiabetic effects of citrus flavonoids; a study on the mechanism of action. Int J Diabetes Dev Ctries. 2015;35:250–263. [Google Scholar]
- 39.Zang Y., Zhang L., Igarashi K., Yu C. The anti-obesity and anti-diabetic effects of Kaempferol glycosides from unripe soybean leaves in high fat diet mice. Food Funct. 2015;6:834–841. doi: 10.1039/c4fo00844h. [DOI] [PubMed] [Google Scholar]