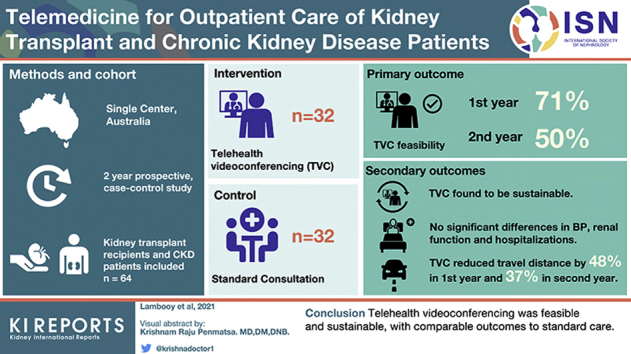
Abstract
Introduction
Telehealth videoconferencing (TVC) may improve access in rural areas, but reported uptake and outcomes among kidney transplant recipients (KTRs) and chronic kidney disease (CKD) patients are limited. This study aimed to assess the feasibility, sustainability, and clinical outcomes of TVC for this patient population.
Methods
A total of 64 participants were recruited in this single-center, prospective, 2-year longitudinal, case-control study. Inclusion criteria for the telemedicine group included travel of ≥15 km to the hospital, and the control group was matched for transplant or CKD status, age, and sex. The primary outcome was feasibility (≥50% of consultations for each individual patient in the telemedicine group being conducted by TVC in year 1). Secondary outcomes were sustainability of telemedicine, change in blood pressure and creatinine, hospitalization, and travel distance.
Results
There were 32 participants in both the telemedicine and control arms, with no baseline differences. The majority were male (65.6%) and the mean age was 63.9 years (SD = 12.3 years). TVC uptake in year 1 in the telemedicine arm was 71% (interquartile range [IQR] = 50.0−100.0) but reduced significantly in year 2 (50.0% [IQR = 33.3−71.4], P < 0.01). No significant differences in creatinine or blood pressure were observed between groups, including in the KTRs and CKD subgroup analysis. Patient satisfaction remained high for both groups. Compared with travel distance required if TVC was unavailable, travel distance in the TVC group decreased by 48% (16,644 km) in year 1 and by 37.0% (8177 km) in year 2.
Conclusion
TVC was feasible and sustainable, with outcomes comparable to those of standard care. Larger studies, especially among KTRs, are needed to confirm these findings.
Keywords: chronic kidney disease, kidney, telehealth, telemedicine, transplant
Graphical abstract
Kidney disease is associated with high morbidity and mortality. Poorer outcomes from kidney disease have been shown among Indigenous and non-Indigenous people living in rural areas or more distant from nephrology services.1, 2, 3, 4, 5 Residents of rural areas are often of low socioeconomic status, which is also associated with poorer outcomes.6, 7, 8
Nephrology care is critical to improve outcomes; however, disadvantaged groups may not access care or may experience poorer quality of care. Canadian data have shown that people with chronic kidney disease (CKD) in rural areas are less likely to see a nephrologist, and that those with diabetes are less likely to have an HbA1c or urine albumin measured or to receive an angiotensin-converting enzyme inhibitor or a receptor blocker.5 Hemodialysis patients more distant from a nephrologist are less likely to have seen a nephrologist within 90 days and have poorer Kt/V and suboptimal phosphate control.9 Aboriginal Australians are less likely to be waitlisted or to undergo kidney transplantation once undergoing dialysis10 and have poorer transplantation outcomes, especially in rural areas.3
Telemedicine (or telehealth), including the modalities of Web-based applications, videoconferencing, and remote monitoring devices, has been proposed to improve healthcare access and outcomes for rural populations with kidney disease.11 In Australia, telehealth videoconferencing (TVC) between a medical practitioner and patient has become a standard of care, supported by Medicare reimbursement for the provider.12 However, the uptake of TVC for management of kidney transplant recipients (KTRs) has been lagging,13 and reports of outcomes of care are limited.14,15 Telehealth videoconferencing has been more widely reported among the CKD population, including in observational studies16, 17, 18 and a randomized controlled trial,19 all suggesting improved patient access and clinical outcomes comparable to those of standard care. A systematic review of telemedicine for blood pressure control in nondialysis CKD found only 3 studies with no difference compared to standard care.20
Studies of telemedicine typically report positive or neutral findings, are of relatively short duration,21 or report patient satisfaction.22 We aimed to examine the feasibility, sustainability, and clinical outcomes of TVC for chronic care of KTRs and CKD patients in a case-matched observational cohort study.
Materals and Methods
We performed a case-controlled longitudinal observational cohort study, with each participant of a matched pair having nephrology care by telemedicine or standard care with 2 years’ follow-up. Case matching (1:1) was for transplant or CKD status, age, and sex. Inclusion criteria for the telemedicine arm were ≥18 years of age and living at least 15 km from the specialized clinic or in an aged care facility (to comply with Medicare telehealth payment requirements). Exclusion criteria included requiring dialysis, poor compliance (i.e., a history of regular nonattendance at outpatient appointments), cognitive impairment (documented in the medical record), life expectancy <1 year, requirement of an interpreter, nephrologist discretion (i.e., where the nephrologist was of the opinion that face-to-face appointments were essential for patient care), inability to access or use a computer, and inability to measure blood pressure or weight or to obtain pathology results prior to the appointment. The control arm had the same inclusion and exclusion criteria except for the requirement to live >15 km from the specialized clinic.
Participants were recruited opportunistically from a single tertiary hospital outpatient clinic that serviced an area of 10,000 km2 (3900 square miles). The recruitment target for this pilot study was 30 in each arm, divided between KTRs and nondialysis CKD patients. Recruitment commenced on 15 May 2015 and was completed on 17 May 2016, with the last follow-up on 7 June 2018 All participants were followed for 2 years unless they withdrew from the study, died, started hemodialysis, or were lost to follow-up.
The TVC was delivered with the nephrologist at the tertiary hospital clinic and the patient either in their own home or at the health facility nearest to their residence. The hospital telehealth service assisted staff and patients to establish telehealth capability. Staff used a desktop computer with specific telehealth software and linked to the patient in the virtual waiting room using a dial code. The patient could choose where to receive TVC. If it was conducted to the patient’s home, the telehealth service assisted the patient with initial software set-up on their desktop computer, tablet, or smartphone, and a dial-up code was provided prior to each appointment. If the patient preferred, they could attend a telehealth clinic at their nearest healthcare facility, where a nurse measured blood pressure, noted other observations, and facilitated the TVC. The telemedicine group aimed to receive up to 75% of consultations by TVC, with the remainder delivered by standard face-to-face care, whereas the control group received only face-to-face consultations.
The primary outcome of the study was feasibility of telemedicine, defined as at least 50% of consultations for each individual patient in the telemedicine group being conducted by TVC in the first year. This measure was chosen pragmatically, prior to study commencement, as a target that would justify establishing TVC capabilities at patients’ homes or local health care facilities. Secondary outcomes were sustainability of telemedicine (defined as percentage consultations for each individual patient in the telemedicine arm being conducted by TVC in year 2); change in blood pressure, serum creatinine, and estimated glomerular filtration rate (eGFR) at 1 and 2 years; hospitalizations; and travel distance.
The study was approved by The Prince Charles Hospital Human Research Ethics Committee (HREC/14/QPCH/250) and local governance. It was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12614001237673). All participants provided written informed consent.
Data Collection
Baseline data for participants in both the telemedicine and control groups were collected at the enrollment visit. This included both demographic (age, sex, race, marital status, first language, education level, family income, occupation, home Internet access, computer at home, home address) as well as health-related data (comorbidities, smoking status, medications, serum creatinine, total cholesterol, blood pressure, height, and weight). Participants were asked “Out of 10, how would you rate your entire experience with all staff and services at the Sunshine Coast Hospital and Health Service Renal Unit?” and scored from 0 to 10 on a visual analogue scale at baseline, month 12, and month 24.
Follow-up data were collected by telephone or in person every 6 months for a total of 2 years from enrollment. Pathology results were either those taken at the face-to-face appointment or those closest to the time of the telemedicine appointment and the 6-monthly dataset. Pathology was from either the hospital laboratory or a private laboratory as part of the patients’ routine medical assessment. Blood pressure for the TVC group was as provided by the participant or as recorded during a face-to-face visit at the relevant time point.
Travel Distance
Travel distance used the patients’ home address as collected with baseline data. Travel distance (in kilometers) to each appointment was calculated using Google Maps. For those patients having standard care (or a face-to-face appointment when in the telemedicine group), travel was from home to the tertiary hospital clinic. For those having TVC, travel distance was either 0 km, if staying at home, or was calculated to the nearest health facility that they attended with TVC facilities. Travel distance to the tertiary hospital clinic was used as the comparator for the telemedicine group.
Hospitalization
Overnight hospitalizations were recorded throughout the study for each subject. Hospitalizations were identified by hospital record review and by asking the participants at each 6-month study visit. The hospitalization rate was calculated by dividing the number of days in the hospital by the number of days that each subject was in the study and multiplied by 100 to give a rate per 100 at-risk days.
Data Analysis
Data analyses were performed using STATA SE 16.1 (Statcorp LLC, College Station, TX), and figures were produced with GraphPad Prism version 8.4.2 for Windows, GraphPad Software, La Jolla, CA. Normality plots and histograms were used for evaluating normality of data. For baseline data with non-parametric distribution, Mann−Whitney U test was used to compare telemedicine and standard care groups. For baseline data with normal distribution, t tests were used. Complete data was available for the primary outcome which was assessed using Wilcoxon signed rank test. Other secondary outcomes were analyzed using the Mann−Whitney U test or Wilcoxon signed rank test for unpaired and paired data, respectively. Subgroup analysis for CKD and transplant groups were conducted for feasibility, blood pressure, creatinine, and glomerular filtration rate. A 2-tailed P value of <0.05 was considered significant. Data are expressed as mean ± standard deviation or as median (interquartile range) unless otherwise stated.
Results
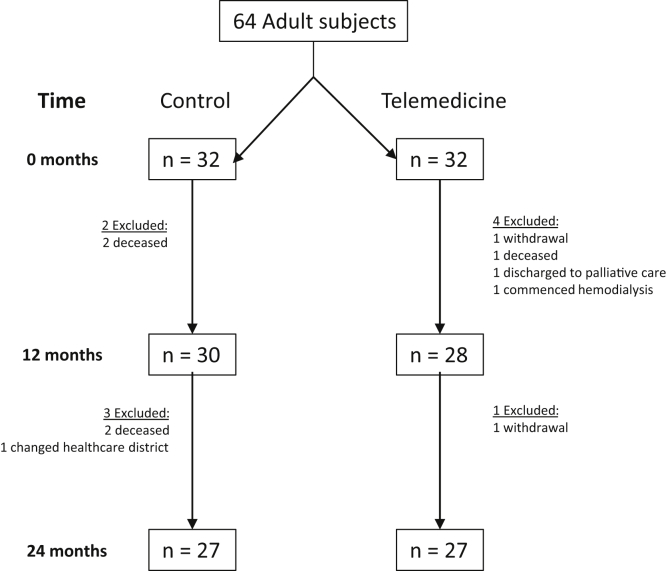
A total of 64 subjects were included, 32 each in both the telemedicine and control arms, evenly divided in each group between KTRs and CKD patients. After 1 year, 28 patients were available for analysis in the telemedicine arm and 30 in the standard care arm (Figure 1). After 2 years, there were 27 participants in each group. Throughout the study, there were 4 deaths and 1 patient lost to follow-up in the control group, and 2 withdrawals of consent and 1 each of death, transfer to palliative care, and commencement of hemodialysis in the telemedicine group.
Figure 1.
Study flow chart describing subjects excluded from study at 12 and 24 months.
At baseline, the mean age was 64.4 ± 12.0 years and 63.4 ± 12.7 years (P = 0.74) for the control and telemedicine subjects, respectively (Table 1). The majority of subjects were male (65.6%), and there were no significant differences between groups for primary renal disease, comorbidity status, smoking status, medication use, or income. There were also no significant differences between groups in serum creatinine, estimated glomerular filtration rate (eGFR), blood pressure, cholesterol, or satisfaction with care.
Table 1.
Baseline characteristics of study population
| Control (n = 32) | Telemedicine (n = 32) | P value | |||
|---|---|---|---|---|---|
| Age, yr mean (SD) | 64.41 | 12 | 63.37 | 12.74 | 0.74 |
| Female sex, (%) | 11 | 34.40% | 11 | 34.40% | 1.00 |
| Transplant, (%) | 16 | 50.00% | 16 | 50.00% | 1.00 |
| Caucasian race, (%) | 32 | 100% | 32 | 100% | 1.00 |
| Primary renal disease | 0.07 | ||||
| Diabetes | 9 | 28.1% | 2 | 6.3% | |
| Hypertension | 2 | 6.3% | 4 | 12.5% | |
| Vascular | 2 | 6.3% | 5 | 15.6% | |
| Glomerulonephritis | 6 | 18.8% | 13 | 40.6% | |
| Cystic disease | 2 | 6.3% | 1 | 3.1% | |
| Other | 11 | 34.4% | 7 | 21.9% | |
| Time since transplantation, yr [IQR] | 4.74 | [2.39– 9.47] | 0.95 | [0.57– 5.54] | 0.13 |
| Smoking statusa | 0.59 | ||||
| Current/former | 17 | 53.1% | 18 | 56.3% | |
| Never | 15 | 46.9% | 12 | 37.5% | |
| Comorbidities | |||||
| Diabetes | 13 | 40.6% | 10 | 31.3% | 0.43 |
| Peripheral vascular disease | 3 | 9.4% | 1 | 3.1% | 0.30 |
| Ischemic heart disease | 6 | 18.8% | 4 | 12.5% | 0.49 |
| Medication use | |||||
| ACEi or ARB | 22 | 68.8% | 22 | 68.8% | 1.00 |
| Loop diuretic | 7 | 21.9% | 7 | 21.9% | 1.00 |
| β-Blocker or CCB | 16 | 50.0% | 12 | 37.5% | 0.31 |
| Corticosteroid | 15 | 46.9% | 18 | 56.3% | 0.45 |
| Azathioprine | 1 | 3.1% | 1 | 3.1% | 1.00 |
| Mycophenolate | 13 | 40.6% | 16 | 50.0% | 0.45 |
| Tacrolimus or cyclosporin | 13 | 40.6% | 13 | 40.6% | 1.00 |
| Sirolimus or everolimus | 2 | 6.3% | 2 | 6.3% | 1.00 |
| Household characteristics | |||||
| Home computer (%)b | 27 | 84.4% | 26 | 81.3% | 0.53 |
| Home Internet (%)b | 28 | 87.5% | 25 | 78.1% | 0.19 |
| Income (AUD) | 0.69 | ||||
| < $30k | 17 | 53.1% | 12 | 37.5% | |
| $30k to $60k | 8 | 25.0% | 9 | 28.1% | |
| $60k to $100k | 3 | 9.4% | 3 | 9.4% | |
| > $100k | 1 | 3.1% | 2 | 6.3% | |
| Declined to answer | 3 | 9.4% | 6 | 18.8% | |
| Employment status | |||||
| Retired | 23 | 71.9% | 19 | 59.4% | 0.26 |
| Occupation unknown | 2 | 6.3% | 2 | 6.3% | |
| Metabolic parameters | |||||
| Creatinine, μmol/l [IQR] | 155.5 | [108.5–215] | 129 | [94–185] | 0.49 |
| eGFR, ml/min per 1.73 m2 [IQR] | 37 | [23–58] | 50 | [26–60.50] | 0.46 |
| Systolic BP, mm Hg (SD) | 132.4 | 16.3 | 134.9 | 15.2 | 0.53 |
| Diastolic BP, mm Hg (SD) | 75.1 | 11.3 | 77.8 | 8.5 | 0.29 |
| Cholesterol, mmol/l [IQR] | 4.05 | [3.5–4.85] | 4.75 | [3.85–5.5] | 0.19 |
| BMI, kg/m2 [IQR] | 28.93 | [24.5–35.3] | 28.11 | [25.3–30.8] | 0.65 |
| HbA1c, %c [IQR] | 7.1 | [5.9–8.2] | 6.55 | [6.1–7.8] | 0.78 |
| Satisfaction, Likert scale score 0−10 [IQR] | 10 | [10–10] | 10 | [10–10] | 0.15 |
Data are presented as n (%), mean (standard deviation), or median [interquartile range].
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; AUD, Australian dollars; BMI, body mass index; BP, blood pressure; CCB, calcium channel blocker; eGFR, estimated glomerular filtration rate.
Two absent in telemedicine group.
One absent in control group.
Includes only diabetic patients (control, n = 13; telemedicine, n = 10),
Baseline characteristics of KTRs and CKD patients allocated to the telemedicine and control arms were also not statistically different (Supplementary Table S1).
Feasibility
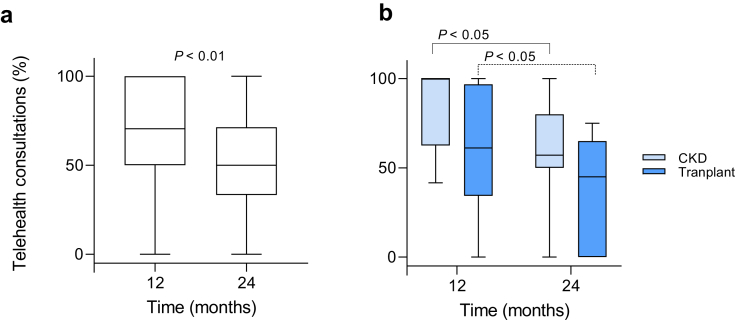
Uptake of TVC for consultations by each participant in the first year among the telemedicine group was 71% (IQR = 50.0−100.0) (Figure 2a), meeting the prespecified definition of feasibility. Telemedicine was sustainable, although patient uptake was lower in year 2 compared with year 1 (50% [IQR = 33.3−71.4] vs. 71% [IQR 50.0−100]; P < 0.01), respectively. Both CKD and KTR subgroups had significant reductions in telemedicine uptake in year 2 compared with year 1 (Figure 2b), 57% (IQR = 50.0−80.0) vs. 100% (IQR = 62.5−100.0), P < 0.05 and 45.0% (IQR = 0.0−63.0) vs. 61.1% (IQR = 35.4−93.8), P < 0.05, respectively. The broad interquartile ranges show significant variability in uptake of TVC at the patient level. Over the 2 years of the study, 177 TVC consultations were conducted, comprising 48.6% of total consultations in the telemedicine arm.
Figure 2.
Individual uptake of telemedicine per year. (a) Telemedicine consultations are shown as a percentage of total consultations for each patient at 12 months and 24 months in the telemedicine arm. (b) Subgroup analysis of chronic kidney disease (CKD) patients and kidney transplant recipients. Data expressed as median and interquartile range.
Secondary Outcomes
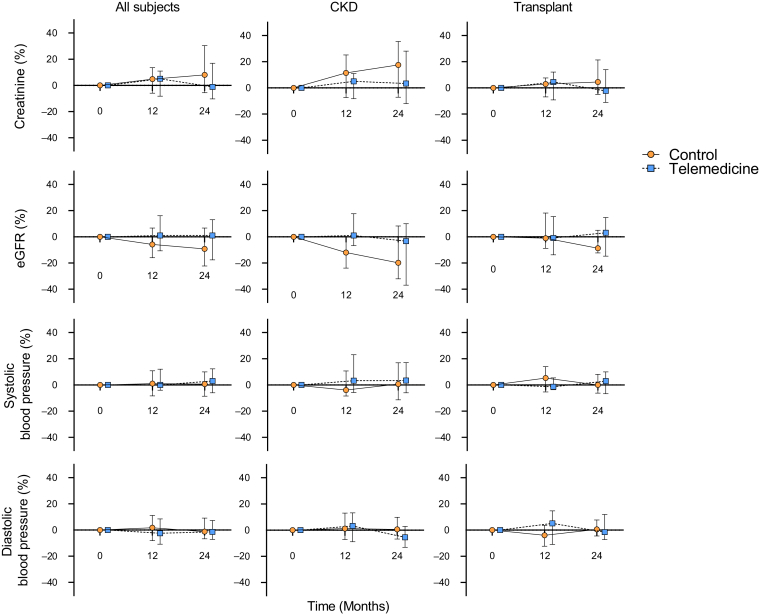
Change in creatinine, eGFR, and systolic/diastolic blood pressure for each group was expressed as percentage change from baseline at 12 and 24 months (Figure 3). No significant changes were measured at 1 and 2 years compared to baseline for the above parameters in either group, nor were there any differences between the control and telemedicine groups at 1 or 2 years (Supplementary Table S2). There were no graft failures in the transplant recipients in either group. Patient satisfaction with the care provided during the study was high throughout, measured at 10 (IQR = 9−10) and 10 (IQR = 10−10) at baseline for the standard care and telemedicine group, respectively, and 10 (IQR = 10−10) and 10 (10−10) at 2 years. There was no significant change in satisfaction over time in the KTR and CKD subgroup analysis (Supplementary Table S2).
Figure 3.
Change (%) in creatinine, estimated glomerular filtration rate (eGFR), and blood pressure over time. Percentage change in secondary outcomes at 12 and 24 months normalized to baseline. Data are expressed as median and interquartile range. CKD, chronic kidney disease.
At 24 months, the number of overnight days admitted to hospital per 100 at-risk days remained low in both the control and telemedicine groups 0 (IQR = 0−0.55) vs. 0 (IQR = 0−0.48) (P > 0.05), respectively.
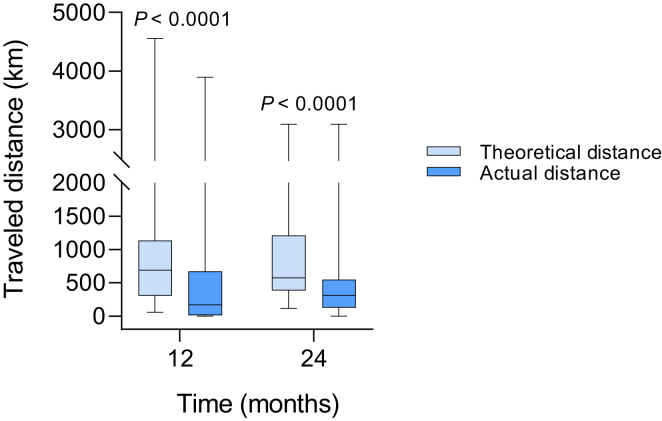
Travel distance to the tertiary hospital outpatient clinic in the standard care group was significantly less than the telemedicine group (21.0 km [IQR = 12.6−32.9] vs. 65.4 km [IQR = 31.8−106.7], P < 0.0001). To investigate whether TVC had a significant reduction in distance traveled, the theoretical distance (the distance to travel to the outpatient clinic for a face-to-face appointment if telemedicine was unavailable) was calculated and compared to actual distance traveled. Travel distance in the TVC group reduced by 47.9% (16,644 km) in year 1 and 37.0% (8177 km) in year 2 (Figure 4).
Figure 4.
Travel distance in the telemedicine group. Theoretical (if telemedicine was not available) and actual distance traveled annually at 12 and 24 months in the telemedicine group. Data are expressed as median and interquartile range.
Discussion
We conducted a case-matched longitudinal observational cohort study of telemedicine compared with standard care including KTRs and CKD patients, and found that the intervention was feasible at 1 year. Furthermore, uptake of TVC in the telemedicine group remained at 50% of consultations in the second year, although it was lower than in the initial 12 months. Travel distance reduced significantly in the TVC group, and there were no between-group differences during follow-up for 2 years in kidney function, blood pressure, mortality, or hospitalization.
Studies of TVC are often brief and examine feasibility and satisfaction, without continuing follow-up for long enough to assess whether the initial enthusiasm for telemedicine wanes or whether relevant clinical outcomes are comparable. We have shown that feasibility of TVC persists beyond 1 year, albeit with lower uptake. It is possible this decrease in TVC may be due to both patient and clinician factors, including comfort with standard care, concerns with technology, failure to consider TVC as an option, and additional reasons to travel from home to the tertiary hospital area such as shopping, other appointments, or visiting family members. Nevertheless, 50% of consultations were with TVC in the second year of the study and reduced travel distance by 8177 km (37%).
There are limited studies of TVC for KTRs. In the United States, the Department of Veterans Affairs has reported that TVC resulted in reduced travel time for patients and reduced travel costs for both patients and healthcare providers.14 An Australian group has reported on 263 clinical consultations delivered by TVC, saving significant travel distance for patients with resultant reductions in carbon dioxide emissions.15 A small randomized controlled trial from Germany comparing standard care with standard care plus case management and telemedicine found lower hospitalizations and less medication nonadherence.23 Our study showing that TVC has clinical outcomes equivalent to those of standard care has expanded on the reported literature for management of KTRs by TVC; however, the results of larger studies24,25 are needed to confirm our findings.
Our findings among the CKD population are similar to those of previous studies.16,17 Interestingly, Ladino et al. found improved outcomes for blood pressure, although this was among an underserviced population compared with our study population, who were already accessing care.18
The experience of patients is important to consider. Overall patient satisfaction with the care provided was very high in both groups. The TVC was comparable to standard care, which may be due to an established relationship with the staff and the opportunity to have face-to-face consultations if desired. The extremely high satisfaction with care in both groups suggests that the question asked did not adequately explore the impact of TVC on patients. Future work should examine the ease of using the technology, adequacy of video and audio quality, perceived quality of care, and preference for TVC versus travel for face-to-face appointments.
We did not explore why patients may not access TVC when available. A study of transplant recipients from Belgium found that there was limited smartphone ownership but that 72% of patients owned a computer with Internet access. Several patient variables affected the willingness to use interactive health technology, including marital status and previous use of information and communications technology.26 Patients may also identify the risks and barriers of TVC, such as cost of telehealth equipment, poor Internet access, loss of personal interaction with the multidisciplinary team, or concerns with data breach as reasons not to pursue TVC.27
The COVID-19 pandemic highlights another role for telemedicine, whereby patients can receive routine clinical care without attending a hospital clinic with the associated risks of infection.28 Telemedicine was used in New York to deliver care to KTRs in response to COVID-19.29 In Australia, COVID-19 prompted an expansion of the criteria for reimbursement for TVC. As a result, routine outpatient appointments were able to be undertaken by TVC as previously, but also by standard telephone call without any restrictions on distance to the treating practitioner.30 It is likely that the ability to use a telephone will benefit patients with poor or no Internet access and those who are not technology literate, especially elderly and socioeconomically disadvantaged individuals, allowing them to access healthcare safely during a pandemic.
Reimbursement and regulation related to telehealth is central to its uptake and acceptance. In Australia, the Medicare Benefits Schedule details criteria that allow medical practitioners to claim reimbursement for TVC.30 In the United States, there is a need to show cost-effectiveness or superior outcomes to allow reimbursement.11 Furthermore, a number of specifications and legislative requirements are listed relevant to dialysis patients, including that only 2 of 3 monthly visits may be conducted via telehealth, and the provider must be registered in the state the patient resides.27
This study has several limitations. It was performed at a single Australian center in a Caucasian population, which limits the generalizability of the results. Pathology was not analyzed at a central laboratory, and blood pressure in the TVC group was measured either at the hospital clinic or was measured and reported by the participant at home, who may not have followed the standard protocol. The study is small, and larger studies are needed to confirm these findings, especially in the transplant population, in which important end points must include patient and graft survival, whereas among the CKD population, progression to kidney failure and mortality must be examined. Nevertheless, the study has strengths, including the 2-year follow-up and high retention rate.
In conclusion, in this study, telemedicine delivered as TVC was shown to be feasible and had outcomes similar to those of standard care for both KTRs and CKD patients. The slow uptake of telemedicine among the nephrology community, especially for KTRs, should be an area of attention so as to improve access to specialist care for patients who have difficulty attending clinics.
Disclosure
All the authors declare no competing interests.
Acknowledgments
This work was funded by the Sunshine Coast Hospital and Health Service Private Practice Trust Fund.
Footnotes
Table S1. Subgroup (CKD and KTRs) baseline characteristics
Table S2. Percentage change in secondary outcomes compared with baseline
Supplementary Material
Table S1. Subgroup (CKD and KTRs) baseline characteristics
Table S2. Percentage change in secondary outcomes compared with baseline
References
- 1.Gray N.A., Dent H., McDonald S.P. Renal replacement therapy in rural and urban Australia. Nephrol Dial Transplant. 2012;27:2069–2076. doi: 10.1093/ndt/gfr584. [DOI] [PubMed] [Google Scholar]
- 2.Tonelli M., Hemmelgarn B., Culleton B. Mortality of Canadians treated by peritoneal dialysis in remote locations. Kidney Int. 2007;72:1023–1028. doi: 10.1038/sj.ki.5002443. [DOI] [PubMed] [Google Scholar]
- 3.Barraclough K.A., Grace B.S., Lawton P., McDonald S.P. Residential location and kidney transplant outcomes in indigenous compared with nonindigenous Australians. Transplantation. 2016;100:2168–2176. doi: 10.1097/TP.0000000000001007. [DOI] [PubMed] [Google Scholar]
- 4.Harasemiw O., Milks S., Oakley L. Remote dwelling location is a risk factor for CKD among indigenous Canadians. Kidney Int Rep. 2018;3:825–832. doi: 10.1016/j.ekir.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rucker D., Hemmelgarn B.R., Lin M. Quality of care and mortality are worse in chronic kidney disease patients living in remote areas. Kidney Int. 2011;79:210–217. doi: 10.1038/ki.2010.376. [DOI] [PubMed] [Google Scholar]
- 6.Krishnasamy R., Gray N.A. Low socio-economic status adversely effects dialysis survival in Australia. Nephrology. 2018;23:453–460. doi: 10.1111/nep.13053. [DOI] [PubMed] [Google Scholar]
- 7.Kimmel P.L., Fwu C.-W., Eggers P.W. Segregation, income disparities, and survival in hemodialysis patients. J Am Soc Nephrol JASN. 2013;24:293–301. doi: 10.1681/ASN.2012070659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naylor K.L., Knoll G.A., Shariff S.Z. Socioeconomic status and kidney transplant outcomes in a universal healthcare system: a population-based cohort study. Transplantation. 2019;103:1024–1035. doi: 10.1097/TP.0000000000002383. [DOI] [PubMed] [Google Scholar]
- 9.Thompson S., Bello A., Wiebe N. Quality-of-care indicators among remote-dwelling hemodialysis patients: a cohort study. Am J Kidney Dis. 2013;62:295–303. doi: 10.1053/j.ajkd.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Khanal N., Lawton P.D., Cass A., McDonald S.P. Disparity of access to kidney transplantation by Indigenous and non-Indigenous Australians. Med J Aust. 2018;209:261–266. doi: 10.5694/mja18.00304. [DOI] [PubMed] [Google Scholar]
- 11.Rohatgi R., Ross M.J., Majoni S.W. Telenephrology: current perspectives and future directions. Kidney Int. 2017;92:1328–1333. doi: 10.1016/j.kint.2017.06.032. [DOI] [PubMed] [Google Scholar]
- 12.MBS and telehealth-services Australia. https://www.servicesaustralia.gov.au/organisations/health-professionals/services/medicare/mbs-and-telehealth Available at:
- 13.Concepcion B.P., Forbes R.C. The role of telemedicine in kidney transplantation: opportunities and challenges. Kidney360. 2020;1:420–423. doi: 10.34067/KID.0000332020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalil R., Thomas C., Winetroub C., Abel S. US Department of Veterans Affairs; Iowa City, IA: 2013. Improving access to specialized care: the telehealth kidney transplant clinic at the Iowa City VAMC. [Google Scholar]
- 15.Andrew N., Barraclough K.A., Long K. Telehealth model of care for routine follow up of renal transplant recipients in a tertiary centre: a case study. J Telemed Telecare. 2020;26:232–238. doi: 10.1177/1357633X18807834. [DOI] [PubMed] [Google Scholar]
- 16.Venuthurupalli S.K., Rolfe A., Fanning J. Chronic Kidney Disease, Queensland (CKD.QLD) Registry: management of CKD with telenephrology. Kidney Int Rep. 2018;3:1336–1343. doi: 10.1016/j.ekir.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narva A.S., Romancito G., Faber T. Managing CKD by telemedicine: the Zuni Telenephrology Clinic. Adv Chronic Kidney Dis. 2017;24:6–11. doi: 10.1053/j.ackd.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ladino M.A., Wiley J., Schulman I.H. Tele-nephrology: a feasible way to improve access to care for patients with kidney disease who reside in underserved areas. Telemed E-Health. 2016;22:650–654. doi: 10.1089/tmj.2015.0197. [DOI] [PubMed] [Google Scholar]
- 19.Ishani A., Christopher J., Palmer D. Telehealth by an interprofessional team in patients with CKD: a randomized controlled trial. Am J Kidney Dis. 2016;68:41–49. doi: 10.1053/j.ajkd.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 20.Luo L., Ye M., Tan J. Telehealth for the management of blood pressure in patients with chronic kidney disease: a systematic review. J Telemed Telecare. 2019;25:80–92. doi: 10.1177/1357633X17743276. [DOI] [PubMed] [Google Scholar]
- 21.Wootton R. Twenty years of telemedicine in chronic disease management—an evidence synthesis. J Telemed Telecare. 2012;18:211–220. doi: 10.1258/jtt.2012.120219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell M., Akbari A., Amos S., Keyes C. Feasibility of providing nephrology services to remote communities with videoconferencing. J Telemed Telecare. 2012;18:13–16. doi: 10.1258/jtt.2011.110321. [DOI] [PubMed] [Google Scholar]
- 23.Schmid A., Hils S., Kramer-Zucker A. Telemedically supported case management of living-donor renal transplant recipients to optimize routine evidence-based aftercare: a single-center randomized controlled trial. Am J Transplant. 2017;17:1594–1605. doi: 10.1111/ajt.14138. [DOI] [PubMed] [Google Scholar]
- 24.Pape L., de Zwaan M., Tegtbur U. The KTx360°-study: a multicenter, multisectoral, multimodal, telemedicine-based follow-up care model to improve care and reduce health-care costs after kidney transplantation in children and adults. BMC Health Serv Res. 2017;17:587. doi: 10.1186/s12913-017-2545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foucher Y., Meurette A., Daguin P. A personalized follow-up of kidney transplant recipients using video conferencing based on a 1-year scoring system predictive of long term graft failure (TELEGRAFT study): protocol for a randomized controlled trial. BMC Nephrol. 2015;16:6. doi: 10.1186/1471-2369-16-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanhoof J.M.M., Vandenberghe B., Geerts D. Technology experience of solid organ transplant patients and their overall willingness to use interactive health technology. J Nurs Scholarsh. 2018;50:151–162. doi: 10.1111/jnu.12362. [DOI] [PubMed] [Google Scholar]
- 27.Lew S.Q., Sikka N. Operationalizing telehealth for home dialysis patients in the United States. Am J Kidney Dis Off. 2019;74:95–100. doi: 10.1053/j.ajkd.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 28.Hollander J.E., Carr B.G. Virtually perfect? Telemedicine for COVID-19. N Engl J Med. 2020;382:1679–1681. doi: 10.1056/NEJMp2003539. [DOI] [PubMed] [Google Scholar]
- 29.Chang J.-H., Diop M., Burgos Y.L. Telehealth in outpatient management of kidney transplant recipients during COVID-19 pandemic in New York. Clin Transplant. 2020;34 doi: 10.1111/ctr.14097. [DOI] [PubMed] [Google Scholar]
- 30.Medicare Benefits Schedule COVID-19 bulk-billed MBS telehealth services—specialists 220520. 2020. http://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/0C514FB8C9FBBEC7CA25852E00223AFE/$File/COVID-19%20Bulk-billed%20MBS%20telehealth%20Services%20-%20Specialists%20220520.pdf Available at:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.