Abstract
Introduction
Impaired physical fitness is prevalent in people with chronic kidney disease (CKD), associating with an increased risk of mortality, falls, and hospitalization. A plethora of physical fitness outcomes have been reported in randomized trials. This study aimed to assess the scope and consistency of physical fitness outcomes and outcome measures reported in trials in CKD.
Methods
A systematic review of randomized trials reporting physical fitness outcomes in adults with CKD (not requiring kidney replacement therapy) receiving hemodialysis (HD) or peritoneal dialysis and kidney transplant recipients was conducted. Studies were identified from MEDLINE, Embase, and the Cochrane Library from 2000 to 2019. The scope, frequency, and characteristics of outcome measures were categorized and analyzed.
Results
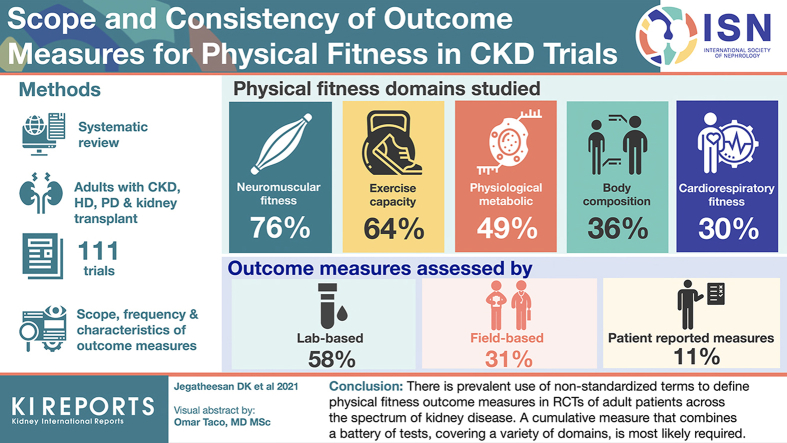
From 111 trials, 87 tests/measurements were used to evaluate 30 outcomes measures that reported on 23 outcomes, categorized into five domains of physical fitness: neuromuscular fitness (reported in 76% of trials), exercise capacity (64%), physiological-metabolic (49%), body composition (36%), and cardiorespiratory fitness (30%). Neuromuscular fitness was examined by 37 tests/measurements including the physical function component of questionnaires (27%), one-repetition maximum (9%), and hand-grip strength (9%). Outcome measures were assessed by lab-based (58% of all trials), field-based (31%), and patient-reported measures (11%), and commonly evaluated at 12 (30%), 26 (23%) and 52 weeks (10%), respectively.
Conclusion
There is large heterogeneity in the reporting of physical fitness outcomes, with inconsistencies particularly in the definitions of outcome measures. Standardization in the assessment of physical fitness will likely improve the comparability of trial outcomes and enhance clinical recommendations.
Keywords: exercise, kidney disease, outcomes, physical fitness, randomized controlled trials
Graphical abstract
Impaired physical fitness is prevalent in patients with CKD and is associated with an increased risk of mortality, progression of kidney disease requiring kidney replacement therapy, falls, and hospitalization.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 Patients with kidney disease experience greater rates of functional decline and accelerated ageing compared to the general population.12, 13, 14, 15, 16, 17, 18 The reasons are multifactorial including uremic muscle dysfunction, shared cardio-metabolic risk factors, and complications of the disease process itself.16,18
Physical fitness broadly implies that an individual has acquired the attributes needed to perform a given physical task, at an acceptable level, within a certain physical, social, and psychological environment.19 The reporting of physical fitness in the nephrology literature has lacked clarity due to the lack of standard definitions, the absence of an agreed classification system of the subcomponents of physical fitness, and poor identification of the appropriate tools to measure outcomes. For example, there appears to be haphazard use of terms such as functional capacity, aerobic capacity, and exercise performance, and it is important to determine what aspect of fitness (e.g., cardiorespiratory fitness or exercise capacity) is being reported. Outcomes relating to physical fitness, such as mobility, were identified as important by patients, caregivers, and health professionals through the Standardized Outcomes in Nephrology initiative.20, 21, 22 How to best assess such metrics in a standardized way is as yet unclear and critical to allow reporting of patient important outcomes.
Although interventions to improve physical fitness have been assessed in randomized controlled trials (RCTs),23, 24, 25, 26, 27 outcomes must be consistent, standardized, and patient-important to allow meaningful comparisons across studies and to inform clinical decision-making. The aim of this study is to assess the scope and consistency of outcomes and outcome measures used to assess physical fitness in RCTs in patients with CKD.
Methods
Selection Criteria
An electronic search using MEDLINE, Embase, and the Cochrane Library databases without language restriction was conducted using search strategies provided in the Supplemental Material. Randomized trials (including post hoc analyses of RCTs) that reported at least one physical fitness outcome in adult patients aged 18 years or older at any stage of kidney disease (CKD not requiring kidney replacement therapy, HD, peritoneal dialysis, kidney transplantation) published from March 2000 to March 2019 were eligible. This time frame was chosen to capture the outcome measures used in contemporary trials as they were more likely to reflect current clinical practice. Relevant systematic reviews were screened to identify additional RCTs published within the specified time frame. Review articles, observational studies, conference abstracts, study protocols, and non–English language publications were excluded. The full list of articles is provided in Supplementary Table S1.
Data Extraction
Trial characteristics were extracted by two reviewers (DKJ and RM), including first author, year of publication, participating countries, sample size, stage of kidney disease, baseline fitness levels, study duration, type of intervention, timing of intervention (intradialytic vs. nondialytic for HD trials), and all outcomes. Each physical fitness outcome measure had accompanying data extracted including assessment type (e.g., field test, lab-based test, or patient-reported measure), specific measure (e.g., distance walked in meters), method of aggregation (e.g., mean or median), and time point of measurement.
Classification of Outcome Measures and Tests
Individual outcomes assessing similar aspects of physical fitness were grouped together by one reviewer (DKJ). The grouping was reviewed by three reviewers (DKJ, RM, and JSC), and discrepancies were discussed to reach agreement. Outcome measures were identified and categorized into five domains according to the framework for health-related physical fitness endorsed by Exercise & Sports Science Australia: cardiorespiratory fitness (e.g., objectively measured or estimated peak oxygen uptake, anaerobic threshold), exercise capacity (e.g., peak power output, time on test, distance travelled, and number of steps), neuromuscular fitness (e.g., strength, power, strength endurance, balance, and submaximal speed), body composition (e.g., fat mass, lean muscle mass, and bone mineral density), and physiological-metabolic (e.g., glycemic parameters, lipid metabolism, and cardiovascular and respiratory function) domains.28 Outcome measures that were defined by terms such as physical functioning, functional capacity, physical performance, physical capacity, physical fitness, exercise tolerance, and work performance were confusing to interpret. To minimize overlaps and to further categorize these terms, outcome measures were reclassified according to the tests/measurements they were assessed by and their relevant physical fitness domains as outlined in Table 1. The list of outcomes was reviewed and agreed upon by five additional reviewers (RK, AT, AV, DWJ, and NI). Categories and classifications were finally agreed on by all reviewers. Tests/measurements were classified into field tests, lab-based tests, and patient-reported outcome measures by reviewers DKJ and JSC. Tests that required specialized equipment such as a dynamometer (e.g., hand grip strength [HGS]) or weights were also classified as lab-based tests.
Table 1.
Tests/Measurements Used Across the Trials, Reclassified Into Physical Fitness Domains and Subdomains (%)
| Neuromuscular Fitness | Physiological-Metabolic | Exercise Capacity | Cardiorespiratory Fitness | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Strength | Power | Vascular structure/function | Submaximal exercise capacity | Aerobic power | |||||
| Maximal torque | TUG | 8 | Pulse wave velocity | 18 | Distance travelled | VO2 max/peak | |||
| Quad strength | 12 | Power rig | <1 | Arterial stiffness index | 3 | 6MWT | 57 | Maximal or submaximal test | 76 |
| HGS | 10 | ||||||||
| Maximal weight lifted | Balance | Arterial FMD | 3 | NSRI walk | 4 | Exercise threshold | |||
| 1-RM | 5 | Tinetti balance instrument | 1 | Baroreflex sensitivity | 3 | Number of steps | Anaerobic, ventilatory or lactate threshold | ||
| Repetition maximum | 1-leg balance test | 1 | Artery/vein diameter | 3 | Stair climbing test | 4 | Maximal or submaximal test | 17 | |
| 5-RM | 1 | Berg balance scale | 1 | Toe-brachial index | 3 | 2-minute step test | 4 | HR response | |
| Squat test <1 | Four square step test | <1 | CIMT | 1 | 2-minute stair climb | 1 | HR max | ||
| Submaximal speed | Balance confidence scale | <1 | Doppler flow | 1 | 4-minute step test | 1 | Maximal exercise test | 7 | |
| Gait speed | 3 | Mini-BEST | <1 | Vein distensibility | 1 | Incremental step test | 1 | Body composition | |
| Function | Static GSI | <1 | Respiratory parameters | Time | Fat | ||||
| Physical function component of a | 27 | Dynamic GSI | <1 | Maximal inspiratory and expiratory pressure | 11 | Normal walking test | 1 | BMI | 26 |
| Barthel ADL index | <1 | Strength endurance | Spirometry | 10 | Fast walking test | 1 | DEXA | 15 | |
| FIM | <1 | 30-s STS | 8 | Lipid metabolism | Maximal exercise capacity | BIA | 5 | ||
| Flexibility | 60-s STS | 4 | Serum lipids | 7 | Time on test | Waist to hip ratio | 5 | ||
| Sit and reach test | 3 | 10 times STS | 5 | Glycemic parameters | Graded exercise treadmill or ergometer | 11 | Air displacement plethysmography | 3 | |
| Shoulder flexibility | <1 | 5 times STS | 4 | Fasting glucose | 10 | ISWT | 7 | Skinfold thickness | 3 |
| Hamstring flexibility | <1 | Arm curl test | 2 | HbA1c | 4 | METs | CT abdomen | 1 | |
| Heel rises | 1 | OGTT | 3 | Peak METs | 6 | Muscle | |||
| Chair stands | 1 | Fasting insulin | 3 | Maximal speed | DEXA | 16 | |||
| Toe lifts | <1 | Muscle characteristics | Graded exercise test | 1 | CSA/volume — US, CT or MRI | 14 | |||
| Forearm endurance | <1 | Histology | 7 | BIA | 6 | ||||
| Leg extensor endurance | <1 | Resting muscle VO2 | 1 | Muscle circumference | 5 | ||||
| Cardiac function | Bone density | ||||||||
| TTE | 4 | BMD | 1 | ||||||
| HR variability | 4 | ||||||||
6MWT, 6-minute walk test; ADL, activities of daily living; BEST, balance evaluation systems test; BIA, bio-electrical impedance analysis; BMD, bone mineral density; BMI, body mass index; CIMT, carotid intima-media thickness; CSA, cross-sectional area; CT, computed tomography; DEXA, dual-energy x-ray absorptiometry; FIM, functional independence measure; FMD, flow-mediated dilation; GSI, global stability index; HbA1c, glycosylated hemoglobin; HGS, hand grip strength; HR, heart rate; ISWT, ; MET, metabolic equivalent; MRI, magnetic resonance imaging; NSRI, North Staffordshire Royal Infirmary; OGTT, oral glucose tolerance test; RM, repetition maximum; STS, sit to stand; TUG, timed up and go; TTE, transthoracic echocardiography; US, ultrasound; VO2, peak oxygen uptake;
Kidney disease quality of life, short form 36, short-form 8, Spitzer index, sickness impact profile.
Results
Trial Characteristics
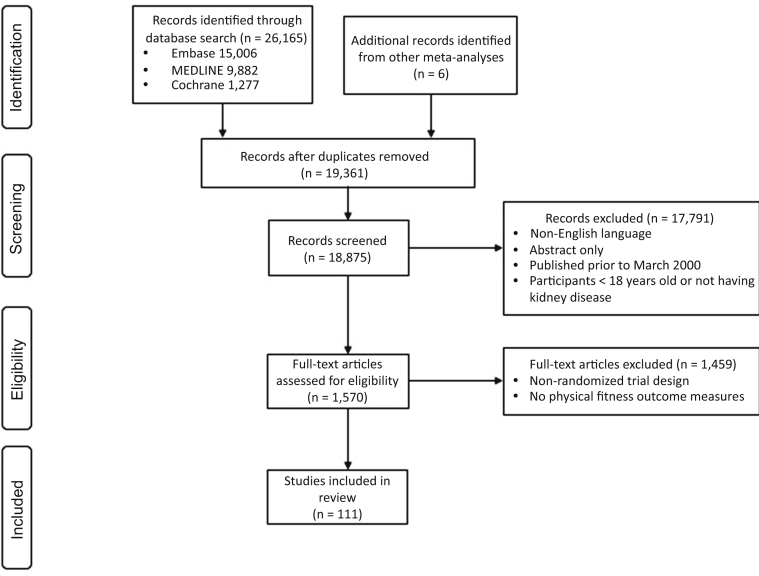
We included 111 relevant RCTs involving 6049 participants (1644 CKD; 3716 HD; 121 peritoneal dialysis; and 568 kidney transplantations) (Figure 1). Trial characteristics are provided in Table 2. Trials were conducted in 25 countries, including Brazil (23 [21%] trials), United States (22 [20%]), Greece (10 [9%]), United Kingdom (8 [7%]), and Canada (8 [7%]). The median duration of trials was 4 (interquartile range: 3–6) months, and the median sample size was 39 (interquartile range: 26–60) patients. Studies included interventions that mostly involved aerobic training (39%), and were supervised (78%) and delivered during HD sessions (90% of trials that included patients receiving HD; 58% of all trials). Of the 73 trials that studied exercise in people receiving HD, 77% had outcomes measured on dialysis days (91% intradialytic), 16% on nondialysis days, and 7% on either day or not specified.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the systematic review.
Table 2.
Characteristics of Included Trials
| Trial Characteristic | n (%) |
|---|---|
| Year of Publication | |
| 2000–2004a | 14 (13) |
| 2005–2009 | 9 (8) |
| 2010–2014 | 31 (28) |
| 2015–2019a | 57 (51) |
| Country | |
| Brazil | 23 (21) |
| United States | 22 (20) |
| Greece | 10 (9) |
| United Kingdom | 8 (7) |
| Canada | 8 (7) |
| Australia | 7 (6) |
| Otherb | 33 (30) |
| Kidney disease cohort | |
| HD | 69 (62) |
| CKD | 27 (24) |
| KT | 9 (8) |
| HD and PD | 3 (3) |
| PD | 1 (1) |
| CKD and HD | 1 (1) |
| CKD and KT | 1 (1) |
| Sample size | |
| 1–50 | 76 (68) |
| 51–100 | 21 (20) |
| 101–150 | 7 (6) |
| ≥151 | 7 (6) |
| Study duration, months | |
| ≤1 | 6 (5) |
| >1 to ≤3 | 38 (35) |
| >3 to ≤6 | 44 (39) |
| >6 to <12 | 7 (6) |
| ≥12 | 16 (14) |
| Intervention typec | |
| Exercise | |
| Aerobic | 44 (39) |
| Resistance | 34 (30) |
| Combined | 30 (27) |
| Otherd | 40 (36) |
| Number of outcome measures reported in each trial | |
| 1–3 | 58 (53) |
| 4–6 | 37 (33) |
| 7–11 | 16 (14) |
HD, hemodialysis; PD, peritoneal dialysis; KT, kidney transplantation; CKD, chronic kidney disease.
March 2000 to March 2019.
Other countries included Italy (4 trials), Japan (4), Tunisia (3), Taiwan (2), Iran (2), China (2), Spain (2), France (2), Denmark (2), Mexico (2), Turkey (1), Belgium (1), Czech Republic (1), Hong Kong (1), Netherlands (1), South Korea (1), Sweden (1), Switzerland (1), and Thailand (1).
Sum is greater than 100% as some trials compared different interventions (e.g., aerobic versus resistance training was regarded as two separate interventions).
Other interventions included electromyostimulation (5 trials), supplements (5), flexibility (4), respiratory muscle training (4), balance training (3), lifestyle counseling (3), stretching (2), whole body vibration (1), antidepressant (1), steroid withdrawal (1), L-carnitine infusion (1), blood flow restriction (1), Quran recitation (1), dopamine agonist (1), and progressive muscle relaxation (1).
Outcomes, Measures, Tests, and Domains
Nonspecific outcome measures were frequently reported, including functional capacity (17 trials), physical function (reported in 15 trials), physical performance (12 trials), physical capacity (seven trials), physical fitness (three trials), exercise tolerance (three trials), and work performance (one trial). Heterogeneity in outcome measure assessment was also noted. The 6-minute walk test (6MWT), as a measure of distance walked, was reported to be an assessment of functional capacity (24% of trials), walking capacity (16%), exercise capacity/tolerance (16%), physical functioning (14%), physical performance (10%), physical capacity (8%), aerobic capacity (6%), exercise performance (2%), function (2%), and muscular endurance (2%). Similarly, HGS was reported as an assessment of muscular strength (63%), physical functioning (21%), physical performance (5%), exercise capacity/tolerance (5%), and physical fitness (5%) (Supplemental Table S1).
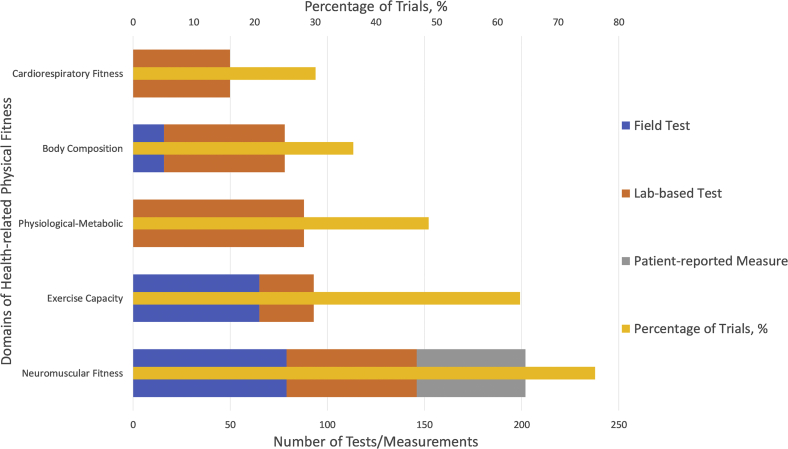
After reclassification, we identified that 87 different tests/measurements were used to evaluate 30 outcome measures that reported on 23 physical fitness outcomes over 17 separate time points across the trials. The breakdown of outcomes, tests/measurements, assessment types, and time points within each physical fitness domain are summarized in Figures 2 and 3, respectively. Lab-based tests were the most common assessment type (58% of all trials), followed by field tests (31%), and patient-reported outcome measures (11%). Outcome measures were most frequently assessed at 12 weeks (30%), 26 weeks (23%), and 52 weeks (10%). Tests/measurements that were used to assess the different fitness domains (neuromuscular fitness, exercise capacity, cardiorespiratory fitness, body composition, and physiological-metabolic) and their respective subdomains are presented in Table 2.
Figure 2.
The reported frequency of each domain (as percentage of all trials) and the number of tests/measurements reported within each domain (categorized according to assessment type — field test, lab-based test, or patient-reported measure).
Figure 3.
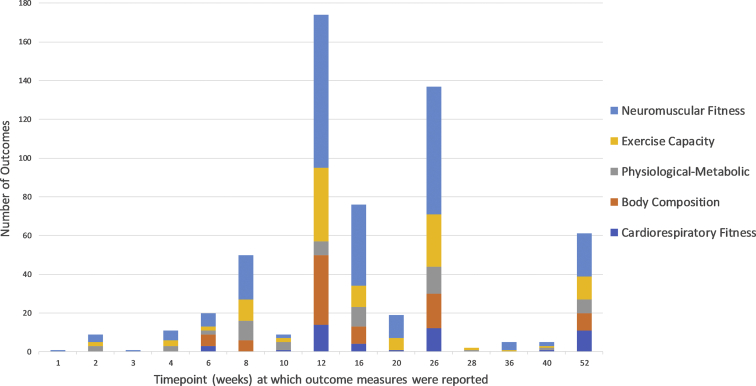
The number of outcomes measured at different time points, after baseline testing, separated by physical fitness domain. Additionally, three outcome measures were recorded at 208 weeks (not shown).
Neuromuscular Fitness
Neuromuscular fitness was reported as nine different outcome measures and at 14 separate time points. The examined subdomains were strength (28%, reported as maximal torque [22%], maximum weight lifted [5%], and repetition maximum [1%]), function (28%), strength endurance (25%), power (9%), balance (5%), flexibility (3%), and submaximal speed (2%). Neuromuscular fitness was assessed using 37 different tests/measurements including the physical function component of patient questionnaires (27%), quadriceps strength (12%), HGS (10%), and timed up-and-go tests (8%).
Exercise Capacity
Exercise capacity was reported as six different outcome measures and at 13 separate time points. Distance travelled was the most common outcome measure (60%), followed by time on test (19%), number of steps (11%), metabolic equivalents from time on test or peak power (6%), walking time (2%), and speed (1%). Exercise capacity was assessed using 13 different tests/measurements including 6MWT (57%), graded exercise treadmill or ergometer (19%), and incremental shuttle walk test (7%).
Cardiorespiratory Fitness
Cardiorespiratory fitness was reported as three different outcome measures, peak oxygen uptake (VO2 max/peak, [76%]), anaerobic/ventilatory/lactate threshold (17%), and heart rate maximum (7%). These outcomes were measured at nine separate time points and were all assessed through maximal or submaximal exercise tests.
Body Composition
Body composition was reported as five different outcome measures and at six separate time points. Muscle mass (measured in millimeters or percent of body weight) was the most commonly reported outcome measure (41%), followed by fat mass (26%, measured in millimeters or percent of body weight), weight for height (26%), waist-to-hip ratio (5%), and bone mineral density (1%). The most frequently reported tests/measurements were dual-energy x-ray absorptiometry (DEXA) (33%), body mass index (26%), and muscle cross-sectional area (14%, measured by ultrasound, computed tomography, or magnetic resonance imaging).
Physiological-Metabolic
Components of the physiological-metabolic domain were reported as seven different outcome measures and at 11 separate time points. These included vascular structure/function (36%), glycemic parameters (19%), respiratory muscle strength (11%), and respiratory function (10%). The most commonly used tests/measurements were pulse wave velocity (18%), spirometry (11%), and respiratory manometry (11%).
Patient-Reported Outcomes
Patient-reported outcomes were evaluated by 11% of all tests/measurements and reported exclusively in trials that assessed neuromuscular fitness. The physical function component of the kidney disease quality of life, short-form 36 (SF-36), short-form 8 (SF-8), Spitzer index, and the sickness impact profile accounted for 96% of patient-reported outcomes, with one trial using “Barthel activities of daily living” and another trial reporting on the “functional independence measure.”
Tests/Measurements Across Stages of Kidney Disease
The frequency and distribution of tests/measurement were similar between the stages of kidney disease (Supplementary Table S2): HD/ peritoneal dialysis (6MWT [11%], SF-36 [10%], VO2 peak/max [6%], quadriceps strength [5%], HGS [5%], and DEXA [5%]); CKD (VO2 peak/max [13%], 6MWT [9%], body mass index [7%], SF-36 [5%], and DEXA [5%]); kidney transplantation (VO2 peak/max [14%], body mass index [9%], DEXA [9%], SF-36 [7%], pulse wave velocity [7%],dual and quadriceps strength [7%]).
Discussion
This review highlights the prevalent use of nonstandardized terms to define physical fitness outcome measures in RCTs of adult patients across the spectrum of kidney disease. Overall, 87 tests/measurements were used to assess 30 different outcome measures across 112 RCTs. Physical fitness outcome measures have primarily assessed the domains of neuromuscular fitness, followed by exercise capacity, physiological-metabolic, body composition, and cardiorespiratory fitness. These outcomes have generally been assessed using lab-based and field tests, with few patient-reported outcome measures included. The findings of this systematic review emphasize the inconsistency and diversity in the measurement and reporting of outcome measures in trials of physical fitness interventions in people with kidney disease. Furthermore, the metric, method of aggregation, and time point of when outcomes were measured varied considerably. Finally, the trials were small (87% had 100 or fewer participants) and short (79% running for 6 months or less). Overall, these inconsistencies and limitations restrict the ability to accurately assess the efficacy of an intervention and compare different interventions, ultimately impairing the quality of clinical recommendations.
Categorization of outcome measures and tests/measurements within fitness domains was a critical first step to analyzing the data in this review. Cardiorespiratory fitness is a very specific component of physical fitness, defined as the integrated ability to transport oxygen from the atmosphere to the mitochondria to perform physical work.29 It is either measured directly with a gas analyzer or estimated from the heart rate response of a submaximal test. Exercise capacity is another component of physical fitness that refers to the ability to complete a physical task (e.g., peak work rate achieved on a graded cycle ergometer test, “time on test” during a standard incremental treadmill protocol, or from a field test such as the 6MWT). Although tests of exercise capacity can be used to estimate VO2 max/peak using formulae, they were often inappropriately reported as measures of cardiorespiratory fitness. For classifying tests into neuromuscular fitness subdomains, consideration was given to the contribution of the different energy systems based on the usual time taken to complete the test. For example, the five times sit to stand test would take approximately 10 to 15 seconds in older individuals; therefore, it relies more on the anaerobic glycolysis system leading to its classification as strength endurance, whereas the time up-and-go test is classified as a measure of power as it would usually be completed in less than 10 seconds.
In addition, outcome measures that were defined by terms such as physical functioning, functional capacity, physical performance, physical capacity, physical fitness, exercise tolerance, and work performance were frequently reported but did not reflect a standardized outcome measure or domain of physical fitness. For example, the term “physical functioning” was used to report outcome measures of (i) neuromuscular fitness; (ii) composite of neuromuscular fitness and exercise capacity; and (iii) composite of neuromuscular fitness, exercise capacity, and cardiorespiratory fitness. Similarly, “functional capacity” was reported as an outcome measure of (i) exercise capacity; (ii) composite of exercise capacity and neuromuscular fitness; and (iii) neuromuscular fitness. Furthermore, the tests/measurements used to assess these outcome measures were also heterogenous. To rationalize these inconsistencies, we propose that physical fitness outcome measures should be defined by what the test or measurement was designed to measure, and not the other way around (e.g., the 6MWT to be reported as a measure of “distance travelled” or “submaximal exercise capacity” [Table 1], rather than “physical performance” or “exercise performance”). Categorization into physical fitness domains provides further granularity to the outcome measures. The use of nonspecific outcomes such as physical performance, physical capacity, or exercise performance must be more clearly defined and ideally avoided. The proposed model of using tests/measurements and domains to classify outcome measures offers a structured and comprehensive approach for future physical fitness research (Table 1).
The timing of outcome measurement was also inconsistent across trials, particularly relevant in people receiving HD. The cardiovascular physiology of an individual treated with HD varies considerably, depending on the time of day (especially if pre-, intra- or immediately post-HD) and day of the week (particularly in relation to when they last dialyzed).30 For instance, in a cross-sectional study of 156 people receiving HD, there was a significant reduction in HGS over the duration of a single HD session.31 The timing of outcome measurement, regardless of physical fitness domain, is thus likely to influence the interpretation of results. This is therefore an important consideration when comparing study outcomes, particularly in trials involving participants treated with HD.
This review also highlights the apparent disconnect between trial design and patient preferences. For instance, a survey of 423 dialysis patients in Canada found that 70% of HD patients preferred to exercise at home.32 In contrast, fewer than 10% of HD trials in this review evaluated home-based interventions. Similarly, although combined aerobic and resistance training was the most preferred type of exercise (selected by 40% of surveyed HD patients), this was the least trialled intervention (28%) across all the included studies.32 Overall, it remains unclear the extent to which patients were involved in the selection of outcomes in these trials. It is plausible that increased consumer engagement at the trial design stage may improve the relevance of the research, its external validity, and dissemination of findings as well as the uptake of effective interventions.33 Critically important outcomes identified from the Standardized Outcomes in Nephrology initiative such as mortality, fatigue, hospitalization, life participation, ability to work, sleep quality, and ability to travel were mostly not reported in any of the trials, and how to best incorporate these patient important outcomes warrants further study.20, 21, 22 It would be pertinent to evaluate these outcomes in conjunction with physical fitness outcomes in future trials.
Study Limitations
There are some potential limitations. We limited the search to trials published with a 20-year time frame from March 2000 because we focused on contemporary trials. However, the inclusion of nonrandomized and older trials would likely further increase heterogeneity and inconsistency, and thus be unlikely to change the conclusion of this study.
Conclusion
Compromised physical fitness in patients with CKD needs ongoing high-quality research to ensure that effective interventions and improved patient outcomes can be translated to routine clinical practice. It is apparent that there is considerable heterogeneity in the outcome measures used to report on physical fitness in kidney disease trials. A multitude of outcome measures have been presented, often to assess different outcomes, with disparity in how these measures report on the key domains of physical fitness. Given the multidimensional nature of physical fitness, a cumulative measure that combines a battery of tests, covering a variety of domains, is most likely required. Furthermore, there are limited patient-reported outcome measures for assessing physical fitness, and it is not clear if these are directly measuring factors that are important to patients.
Future research should firstly aim to identify the physical fitness outcome measures that are valid, reliable, and patient-important. Classifying outcomes within an accepted framework for physical fitness offers the first step to better defining and informing the selection of outcome measures in this process. Further investigation into the validity, reliability, and practicality of outcome measures is necessary. Defining consistent, relevant, and patient-important outcome measures will potentially allow for more meaningful comparisons across studies to inform clinical decisions. These advances will lay the foundation for more effective clinical trials, ultimately aspiring towards improving the physical fitness of people with kidney disease.
Disclosures
DKJ has received travel sponsorship from Roche. DWJ has received consultancy fees, research grants, speaker’s honoraria and travel sponsorships from Baxter Healthcare and Fresenius Medical Care, consultancy fees from Astra Zeneca and AWAK, speaker’s honoraria and travel sponsorships from ONO, and travel sponsorships from Amgen. He is a current recipient of an Australian National Health and Medical Research Council (NHMRC) Practitioner Fellowship. NI has received consultancy fees, speaker’s honoraria and travel sponsorship from Alexion Pharmaceuticals. RK has received consultancy fees, speaker’s honoraria and travel sponsorship from Baxter Healthcare and travel sponsorship from Amgen. The remaining authors declare that they have no relevant financial interests.
Acknowledgments
We acknowledge the support provided by Christine Dalais for the literature review search strategy. Aspects of this work have been presented in abstract form at the European Renal Association-European Dialysis and Transplant Association Congress 2020 and the Australian and New Zealand Society of Nephrology Annual Scientific Meeting 2020.
Footnotes
Table S1. Summary of intervention type, fitness outcome and assessment method across the studies.
Table S2. Distribution of tests/measurements across the stages of kidney disease.
PRISMA Checklist
Supplementary Material
Table S1. Summary of intervention type, fitness outcome and assessment method across the studies.
Table S2. Distribution of tests/measurements across the stages of kidney disease.
PRISMA Checklist
References
- 1.Beddhu S., Baird B.C., Zitterkoph J., Neilson J., Greene T. Physical activity and mortality in chronic kidney disease (NHANES III) Clin J Am Soc Nephrol. 2009;4:1901–1906. doi: 10.2215/CJN.01970309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avesani C.M., Trolonge S., Deleaval P. Physical activity and energy expenditure in haemodialysis patients: an international survey. Nephrol Dial Transplant. 2012;27:2430–2434. doi: 10.1093/ndt/gfr692. [DOI] [PubMed] [Google Scholar]
- 3.Gordon E.J., Prohaska T.R., Gallant M.P. Longitudinal analysis of physical activity, fluid intake, and graft function among kidney transplant recipients. Transpl Int. 2009;22:990–998. doi: 10.1111/j.1432-2277.2009.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cobo G., Gallar P., Gama-Axelsson T. Clinical determinants of reduced physical activity in hemodialysis and peritoneal dialysis patients. J Nephrol. 2015;28:503–510. doi: 10.1007/s40620-014-0164-y. [DOI] [PubMed] [Google Scholar]
- 5.MacKinnon H.J., Wilkinson T.J., Clarke A.L. The association of physical function and physical activity with all-cause mortality and adverse clinical outcomes in nondialysis chronic kidney disease: a systematic review. Ther Adv Chronic Dis. 2018;9:209–226. doi: 10.1177/2040622318785575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stack A.G., Molony D.A., Rives T., Tyson J., Murthy B.V. Association of physical activity with mortality in the US dialysis population. Am J Kidney Dis. 2005;45:690–701. doi: 10.1053/j.ajkd.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 7.White S.L., Dunstan D.W., Polkinghorne K.R., Atkins R.C., Cass A., Chadban S.J. Physical inactivity and chronic kidney disease in Australian adults: the AusDiab study. Nutr Metab Cardiovasc Dis. 2011;21:104–112. doi: 10.1016/j.numecd.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein J., Joshi A., Hise M.K. Association of physical activity and renal function in subjects with and without metabolic syndrome: a review of the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2006;48:372–382. doi: 10.1053/j.ajkd.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Robinson-Cohen C., Katz R., Mozaffarian D. Physical activity and rapid decline in kidney function among older adults. Arch Intern Med. 2009;169:2116–2123. doi: 10.1001/archinternmed.2009.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desmet C., Beguin C., Swine C., Jadoul M., Universite Catholique de Louvain Collaborative Group Falls in hemodialysis patients: prospective study of incidence, risk factors, and complications. Am J Kidney Dis. 2005;45:148–153. doi: 10.1053/j.ajkd.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 11.Kutner N.G., Zhang R., Huang Y., Painter P. Gait speed and mortality, hospitalization, and functional status change among hemodialysis patients: a US Renal Data System special study. Am J Kidney Dis. 2015;66:297–304. doi: 10.1053/j.ajkd.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng L., Yap K.B., Yeoh L.Y., Ng T.P. Kidney function and cognitive and functional decline in elderly adults: findings from the Singapore longitudinal aging study. J Am Geriatr Soc. 2012;60:1208–1214. doi: 10.1111/j.1532-5415.2012.04043.x. [DOI] [PubMed] [Google Scholar]
- 13.Odden M.C., Whooley M.A., Shlipak M.G. Association of chronic kidney disease and anemia with physical capacity: the heart and soul study. J Am Soc Nephrol. 2004;15:2908–2915. doi: 10.1097/01.ASN.0000143743.78092.E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Painter P. Physical functioning in end-stage renal disease patients: update 2005. Hemodial Int. 2005;9:218–235. doi: 10.1111/j.1492-7535.2005.01136.x. [DOI] [PubMed] [Google Scholar]
- 15.Painter P., Marcus R. Physical function and gait speed in patients with chronic kidney disease. Nephrol Nurs J. 2013;40:529–538. quiz 539. [PubMed] [Google Scholar]
- 16.Roshanravan B., Gamboa J., Wilund K. Exercise and CKD: skeletal muscle dysfunction and practical application of exercise to prevent and treat physical impairments in CKD. Am J Kidney Dis. 2017;69:837–852. doi: 10.1053/j.ajkd.2017.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newman A.B., Cauley J.A. Springer; New York, New York: 2012. The Epidemiology of Aging. [Google Scholar]
- 18.Kooman J.P., Kotanko P., Schols A.M.W.J., Shiels P.G., Stenvinkel P. Chronic kidney disease and premature ageing. Nat Rev Nephrol. 2014;10:732–742. doi: 10.1038/nrneph.2014.185. [DOI] [PubMed] [Google Scholar]
- 19.Bouchard C.B., Haskell WL S.N. Second Edition. Human Kinetic; Champaign, Illinois: 2012. Physical Activity and Health. [Google Scholar]
- 20.Standardised Outcomes in Nephrology (SONG) HD Core Outcomes. http://songinitiative.org/hd-core-outcomes/ Available at:
- 21.Standardised Outcomes in Nephrology (SONG) SONG-Tx Core Outcomes. http://songinitiative.org/projects/song-tx/ Available at:
- 22.Standardised Outcomes in Nephrology (SONG) PD Core Outcomes. 2019. https://songinitiative.org/projects/song-pd/ Available at:
- 23.Heiwe S., Jacobson S.H. Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis. 2014;64:383–393. doi: 10.1053/j.ajkd.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Barcellos F.C., Santos I.S., Umpierre D., Bohlke M., Hallal P.C. Effects of exercise in the whole spectrum of chronic kidney disease: a systematic review. Clin Kidney J. 2015;8:753–765. doi: 10.1093/ckj/sfv099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Q.G., Zhang H.R., Wen X. Exercise interventions on patients with end-stage renal disease: a systematic review. Clin Rehabil. 2019;33:147–156. doi: 10.1177/0269215518817083. [DOI] [PubMed] [Google Scholar]
- 26.Clarkson M.J., Bennett P.N., Fraser S.F., Warmington S.A. Exercise interventions for improving objective physical function in patients with end-stage kidney disease on dialysis: a systematic review and meta-analysis. Am J Physiol Renal Physiol. 2019;316:F856–F872. doi: 10.1152/ajprenal.00317.2018. [DOI] [PubMed] [Google Scholar]
- 27.Gomes Neto M., de Lacerda F.F.R., Lopes A.A., Martinez B.P., Saquetto M.B. Intradialytic exercise training modalities on physical functioning and health-related quality of life in patients undergoing maintenance hemodialysis: systematic review and meta-analysis. Clin Rehabil. 2018;32:1189–1202. doi: 10.1177/0269215518760380. [DOI] [PubMed] [Google Scholar]
- 28.Coombes JS T. Elsevier Australia; New South Wales, Australia: 2018. ESSA’s Student Manual for Health, Exercise & Sport Assessment. [Google Scholar]
- 29.Ross R., Blair S.N., Arena R. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134:e653–e699. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 30.Debowska M., Poleszczuk J., Dabrowski W., Wojcik-Zaluska A., Zaluska W., Waniewski J. Impact of hemodialysis on cardiovascular system assessed by pulse wave analysis. PLoS One. 2018;13 doi: 10.1371/journal.pone.0206446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinto A.P., Ramos C.I., Meireles M.S., Kamimura M.A., Cuppari L. Impact of hemodialysis session on handgrip strength. J Bras Nefrol. 2015;37:451–457. doi: 10.5935/0101-2800.20150072. [DOI] [PubMed] [Google Scholar]
- 32.Moorman D., Suri R., Hiremath S. Benefits and barriers to and desired outcomes with exercise in patients with ESKD. Clin J Am Soc Nephrol. 2019;14:268–276. doi: 10.2215/CJN.09700818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gutman T., Tong A., Howell M. Principles and strategies for involving patients in research in chronic kidney disease: report from national workshops. Nephrol Dial Transplant. 2020;35:1585–1594. doi: 10.1093/ndt/gfz076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.