Introduction
Active and chronic active antibody-mediated rejection (ABMR) are major causes of allograft loss after kidney transplantation.1 There is no standard treatment approach for ABMR, but intravenous immunoglobulin (IVIG), steroids, and targeted B-cell therapies have been found to have relative success in reducing allograft injury and preserving function.2, 3, 4,S1 In contrast, outcomes for patients with refractory or resistant, chronic active ABMR (cABMR) are generally poor with limited success reported with the addition of bortezomib, eculizumab, or IVIG and rituximab to the standard of care.S2–S4 Patients with refractory cABMR also require more intensive posttreatment monitoring. Renal biopsies remain the gold standard for diagnosis and therapeutic guidance, but longitudinal histologic data are limited in the studies of cABMR.5,6 Therefore, examining patients receiving prolonged treatment with comprehensive histologic assessment may yield valuable information for managing refractory rejection. In this study, we followed patients with refractory cABMR who required multiple follow-up biopsies and courses of treatment in the first year of diagnosis. We describe the clinical course of circulating donor-specific antibody (DSA), allograft pathology, and kidney function in patients with refractory cABMR.
Results
Patient Characteristics
Participants (n = 8) were 40 ± 14.5 years old with a mean transplant-to-index biopsy interval of 4.4 ± 3.7 years. A total of 3 participants (38%) had a history of rejection. Additional patient characteristics can be found in the Supplementary Materials (Tables S1 and S2). Kidney function was evaluated using serum creatinine, blood urea nitrogen, estimated glomerular filtration rate (eGFR), and urine protein creatinine ratio (Figure S1). There were no significant changes in kidney function during the 1-year study. The average change in eGFR in 1 year was –4.3 ± 12.8. The most recent values for serum creatinine and eGFR were also evaluated for patients with a viable graft. No significant differences were noted between serum creatinine and eGFR at index biopsy and the most recent values (interval from index biopsy 1.8–4.3 years). One patient lost their graft approximately 9 months after index biopsy. Another patient developed a polyomavirus BK virus infection, which resolved, and no other infectious complications were noted. Beyond the 1-year follow-up period, 1 additional patient lost their allograft owing to rejection approximately 17 months after index biopsy.
Treatment of Refractory cABMR
After confirmation of cABMR, patients were treated with IVIG, corticosteroids, and rituximab.S5 Persistent rejection after each biopsy was treated primarily with IVIG and corticosteroids (Table S3).
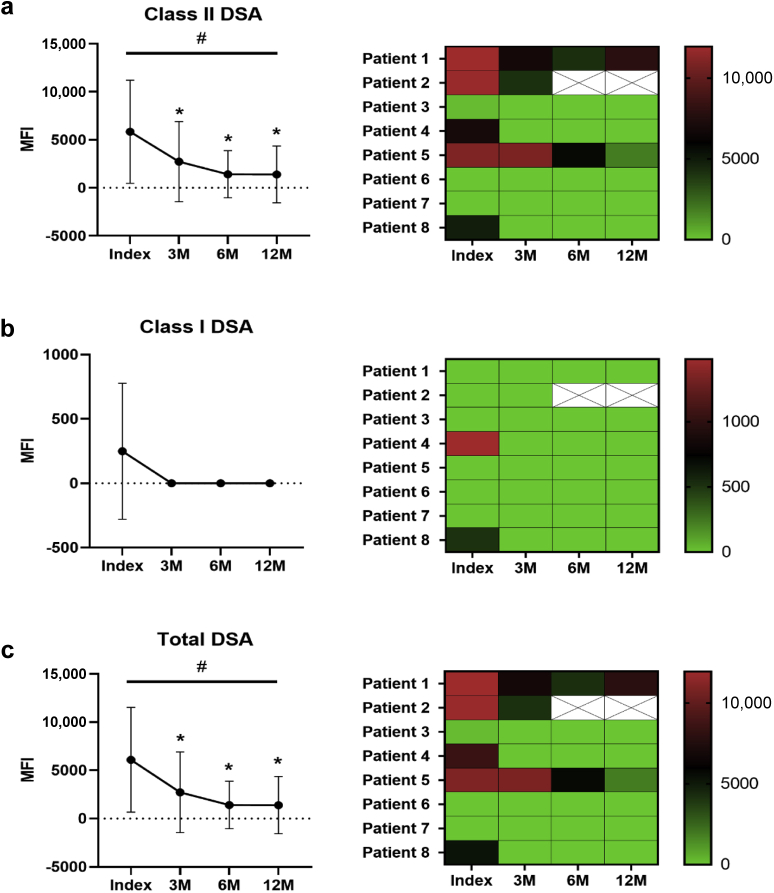
Early Reduction in Total and Class II DSA
There was a reduction in circulating class II DSA (P = 0.02) (Figure 1). Specifically, there was a decrease in class II DSA between index and follow-up biopsies at 3 (5845 ± 5366 vs. 2731 ± 4172, P = 0.03), 6 (5845 ± 5366 vs. 1415 ± 2457, P = 0.03), and 12 months (5845 ± 5366 vs. 1394 ± 2956, P = 0.04) (Figure 1a). In all but 3 patients, class II DSA resolved completely after the first round of treatment. This was reflected in a reduction in total circulating DSA (P = 0.02) (Figure 1c). Compared with index biopsy, total DSA was reduced at 3 (6093 ± 5424 vs. 2731 ± 4172, P = 0.04), 6 (6093 ± 5424 vs. 1415 ± 2457, P = 0.03), and 12 months (6093 ± 5424 vs. 1394 ± 2956, P = 0.04) (Figure 1c). Class I DSA resolved between index and the 3-month follow-up, but this was not statistically significant (Figure 1b).
Figure 1.
Changes in DSA across the first year after cABMR diagnosis. The MFI of (a) class II DSA, (b) class I DSA, and (c) total DSA was evaluated by Luminex at the time of index, 3-month, 6-month, and 12-month biopsies. There was a decrease in class II DSA (P = 0.02) and total DSA (P = 0.03) in the course of the follow-up period at 3-month, 6-month, and 12-month biopsies compared with the index biopsy. Class I DSA resolved by 3 months posttreatment, but it was not statistically significant owing to the small sample size. MFI values for patient 2 are missing at 6 and 12 months (designated by X) owing to the decline in graft function, but the patient remained positive for class II DSA until the graft was lost. Data were evaluated for normality using the D’Agostino and Pearson tests and then analyzed using the ANOVA mixed effects test with significance designated as #P ≤ 0.05. Follow-up with Student’s t test is found with significance designated as ∗P < 0.05 (compared with index biopsy). Data are expressed as the mean ± SD. ANOVA, analysis of variance; cABMR, chronic active antibody-mediated rejection; DSA, donor-specific antibody; MFI, mean fluorescence intensity; SD, standard deviation.
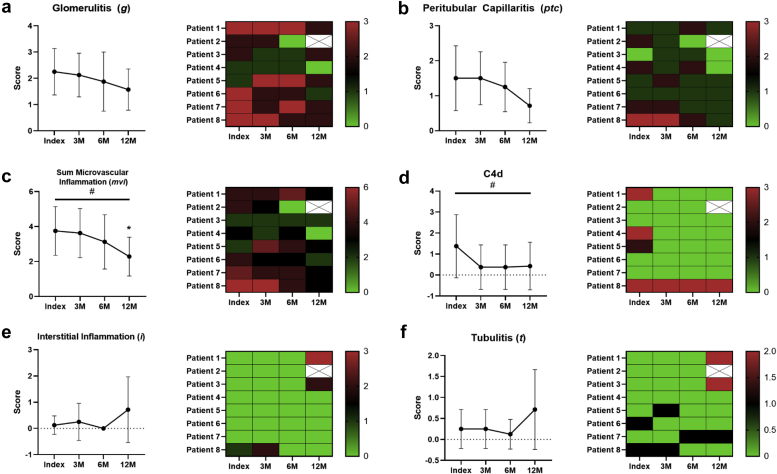
Delayed Improvement in Acute Histopathology
Acute allograft pathology (glomerulitis [g], peritubular capillaritis [ptc], sum microvascular inflammation [mvi], C4d, interstitial inflammation [i], tubulitis [t]) was evaluated at each biopsy (Figure 2). We observed significant reductions in mvi (P = 0.03) and C4d (P = 0.03) scores by analysis of variance. There were no statistically significant differences between the mvi score at index and at 3 (3.8 ± 1.4 vs. 3.6 ± 1.4, P = 0.8) or 6 months (3.8 ± 1.4 vs. 3.1 ± 1.6, P = 0.4); however, the improvement in mvi became significant at 12 months (3.8 ± 1.4 vs. 2.3 ± 1.1, P = 0.05) (Figure 2c). No other significant differences in acute pathology were observed. No significant correlations between changes in DSA, acute histopathology, and renal function were observed.
Figure 2.
Changes in acute histopathology within 1 year of diagnosis. Graft pathology was evaluated using the Banff 2017 criteria at index, 3-month, 6-month, and 12-month posttreatment biopsies. There was a significant change in the (c) mvi (P = 0.03) and (d) C4d (P = 0.03) scores over the follow-up period by ANOVA. Independent t test revealed a significant reduction in the mvi score (P = 0.05) at 12 months when compared with index biopsy. There were no significant differences in the mvi score at 3 months (P = 0.8) or 6 months (P = 0.4) after index biopsy. No differences were also detected between the (a) g (P = 0.2), (b) ptc (P = 0.07), (e) i (P = 0.4), or (f) t scores (P = 0.8). C4d scores between index biopsy and subsequent follow-up biopsies. Missing values for patient 2 are due to graft loss and are designated with an X. Data were evaluated for normality using the D’Agostino and Pearson tests and then analyzed using the ANOVA mixed effects test or Friedman test with significance designated as #P ≤ 0.05. Follow-up with Student’s t or Wilcoxon test is found with significance designated as ∗P ≤ 0.05. Data are expressed as the mean ± SD. ANOVA, analysis of variance; SD, standard deviation.
Chronic lesions (interstitial fibrosis [ci], tubular atrophy [ct], vascular fibrosis intimal thickening [cv], glomerular basement membrane double contours [cg], arteriolar hyalinosis [ah], and mesangial matrix expansion [mm]) were also evaluated. Chronic lesions neither progressed nor improved with treatment in the course of 12 months (Figure S2).
Discussion
In this case series of 8 patients undergoing treatment and sequential biopsies for refractory cABMR, we observed significant reductions in circulating DSA and C4d before improvements in microvascular inflammation. Chronicity scores and kidney function remained stable in patients with viable grafts. One patient lost their graft owing to ongoing rejection during the 1-year follow-up period. Our data suggest that modest improvements in histopathology may be delayed relative to declining DSA after prolonged treatment; however, this approach may not be sufficient to achieve resolution of refractory cABMR.
There are no approved therapeutics for refractory cABMR, and many studies exploring treatment modalities consist of a limited number of biopsies; so the histologic response is not well documented.S1–S4 In this cohort, we observed improvements in C4d staining at 3 months, which coincided with a reduction in circulating levels of class II and total DSA. The role of DSA in the management of ABMR is complex, but a reduction in circulating levels is generally indicative of a reduction in antibody-mediated injury.S6,S7 Studies with similar therapeutic approaches have also reported reductions in class II DSA paired with improvement in renal function or graft survival, but follow-up surveillance biopsies were not performed in these studies.3,7 We did not observe changes in overall kidney function in the first year of diagnosis or in the most recent laboratory values from this cohort; however, the patient with the lowest eGFR at index biopsy (26 ml/min per 1.73 m2) was also the only patient to lose their allograft during the 1-year follow-up period. Since then, 1 additional patient, who demonstrated the greatest decline in eGFR, has also required a retransplant. Although we were unable to establish a clear correlation between changes in eGFR, DSA, and histopathology, these observations potentially support the use of eGFR in future studies of refractory ABMR.
We observed improvements in microvascular inflammation 1 year after diagnosis. Microcirculation inflammation is an important predictor of outcomes in renal transplant recipients.8,9 Patients in this study demonstrated persistent allograft inflammation and required additional treatment but eventually had modest histologic improvement. The delay between improvements in DSA and C4d-staining and microcirculation inflammation suggests that high-intensity immune responses in refractory ABMR may require more time and therapy to resolve. We also evaluated chronic allograft injury and observed no significant changes in chronicity scores over the course of the study.S8 Mulley et al.S4 also reported that patients with cABMR and persistent peritubular capillaritis had improved allograft survival when given high-dose IVIG (1 g/kg per) and a single dose of rituximab (500 mg).S4 It is difficult to interpret the impact of our treatment approach on allograft survival given the short follow-up time. Finally, we observed only a single infectious complication in our study, which resolved. These preliminary observations suggest that the risk of infections in similar patients may be low as long as appropriate prophylaxis is provided. Though limited by its small, observational nature and short duration, this study provides the critical information that in patients with refractory cABMR, declining DSA precedes improvements in histopathologic evidence of disease activity; however, resolution of cABMR was not observed, suggesting that new methods for addressing refractory immune processes are needed.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work was partially supported by the UW Transplant Research Training Grant (T32 AI1256231). Additional grant support did not come from any specific funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
FA, NG, DAM, MM, SP, SRR, NAW, and WZ contributed to the acquisition of data, analysis and interpretation of data, and critical revision. PN contributed to the study conception and design, analysis and interpretation of data, and critical revision. SEP contributed to the analysis and interpretation of data and critical revision. KVH contributed to the acquisition of data and critical revision. KRD and AD contributed to the study conception and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and critical revision.
Footnotes
Supplementary Methods.
Table S1. Patient characteristics at index biopsy.
Table S2. Transplant and clinical characteristics.
Table S3. Treatment modalities after biopsy.
Figure S1. Kidney function in patients treated for refractory cABMR.
Figure S2. Chronic allograft pathology in the first year after diagnosis of cABMR.
Supplementary References.
Supplementary Material
Supplementary Methods
Table S1. Patient characteristics at index biopsy
Table S2. Transplant and clinical characteristics
Table S3. Treatment modalities after biopsy
Figure S1. Kidney function in patients treated for refractory cABMR
Figure S2. Chronic allograft pathology in the first year after diagnosis of cABMR
Supplementary References
References
- 1.Sellarés J., de Freitas D.G., Mengel M. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:388–399. doi: 10.1111/j.1600-6143.2011.03840.x. [DOI] [PubMed] [Google Scholar]
- 2.Parajuli S., Joachim E., Alagusundaramoorthy S. Subclinical antibody-mediated rejection after kidney transplantation. Transplantation. 2019;103:1722–1729. doi: 10.1097/TP.0000000000002566. [DOI] [PubMed] [Google Scholar]
- 3.Parajuli S., Mandelbrot D.A., Muth B. Rituximab and monitoring strategies for late antibody-mediated rejection after kidney transplantation. Transplant Direct. 2017;3:e227. doi: 10.1097/TXD.0000000000000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Djamali A., Kaufman D.B., Ellis T.M. Diagnosis and management of antibody-mediated rejection: current status and novel approaches. Am J Transplant. 2014;14:255–271. doi: 10.1111/ajt.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redfield R.R., McCune K.R., Rao A. Nature, timing, and severity of complications from ultrasound-guided percutaneous renal transplant biopsy. Transpl Int. 2016;29:167–172. doi: 10.1111/tri.12660. [DOI] [PubMed] [Google Scholar]
- 6.Parfrey P.S., Kuo Y.L., Hanley J.A. The diagnostic and prognostic value of renal allograft biopsy. Transplantation. 1984;38:586–590. doi: 10.1097/00007890-198412000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Kincaide E., Hitchman K., Hall R. Impact of active antibody-mediated rejection treatment on donor-specific antibodies in pediatric kidney transplant recipients. Pediatr Transplant. 2019;23 doi: 10.1111/petr.13590. [DOI] [PubMed] [Google Scholar]
- 8.de Kort H., Willicombe M., Brookes P. Microcirculation inflammation associates with outcome in renal transplant patients with de novo donor-specific antibodies. Am J Transplant. 2013;13:485–492. doi: 10.1111/j.1600-6143.2012.04325.x. [DOI] [PubMed] [Google Scholar]
- 9.Parajuli S., Redfield R.R., Garg N. Clinical significance of microvascular inflammation in the absence of anti-HLA DSA in kidney transplantation. Transplantation. 2019;103:1468–1476. doi: 10.1097/TP.0000000000002487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.