Abstract
Plasma and B cells dyscrasias that overproduce monoclonal immunoglobulin free light chains (FLCs) affect the kidney frequently in various ways. The hematologic dyscrasia responsible for the production of FLCs may or may not meet the criteria for cancer, such as multiple myeloma (MM) or lymphoma, or may remain subclinical. If there is overt malignancy, the accompanying kidney disorder is called myeloma- or lymphoma-associated. If the dyscrasia is subclinical, the associated kidney disorders are grouped as monoclonal gammopathy of renal significance. Glomeruli and tubules may both be involved. The proximal tubule disorders comprise a spectrum of interesting syndromes, which range in severity. This review focuses on the recent insights gained into the patterns and the mechanisms of proximal tubule toxicity of FLCs, including subtle transport disorders, such as proximal tubule acidosis, partial or complete Fanconi syndrome, or severe acute or chronic renal failure. Histologically, there may be crystal deposition in the proximal tubule cells, acute tubule injury, interstitial inflammation, fibrosis, and tubule atrophy. Specific structural alterations in the V domain of FLCs caused by somatic hypermutations are responsible for crystal formation as well as partial or complete Fanconi syndrome. Besides crystal formation, tubulointerstitial inflammation and proximal tubulopathy can be mediated by direct activation of inflammatory pathways through cytokines and Toll-like receptors due to cell stress responses induced by excessive FLC endocytosis into the proximal tubule cells. Therapy directed against the clonal source of the toxic light chain can prevent progression to more severe lesions and may help preserve kidney function.
Keywords: chronic kidney disease, Fanconi syndrome, light chains, proximal tubule, proximal tubule crystallopathy, proximal tubulopathy
An abnormal monoclonal plasma cell proliferation is frequently observed in the elderly population.1 In most cases, this proliferation is asymptomatic, is only detected by the presence of a monoclonal immunoglobulin spike in serum protein electrophoresis, and is called monoclonal gammopathy of undetermined significance. However, monoclonal gammopathy of undetermined significance evolves at slow rate (1% per year) to MM,1 which is the malignant form of plasma cell proliferation with an incidence rate of approximately 1.1% among all cancers and constitutes 12% to 13% of hematologic malignancies in the United States.2 Despite the relatively low incidence rate among cancers, MM is considered the most common malignancy that leads to end-stage renal disease (ESRD). According to the US Renal Data System, approximately 1.2% of patients with ESRD have associated MM.3
Renal dysfunction, defined as serum creatinine elevation ≥1.3 mg/dl, occurs in 48% of patients with newly diagnosed MM.4 Approximately 19% of patient have an estimated glomerular filtration rate of <30 ml/min/1.73 m2 at the time of diagnosis,5 and 8% require renal replacement therapy.6 Importantly, one large study concluded that reversibility of renal dysfunction was a more important prognostic factor than the response to chemotherapy.7
Besides MM, small indolent B and plasma cell clones that do not meet the criteria of cancer can also associate with a large variety of monoclonal paraprotein-related kidney diseases, so this syndrome was recently regrouped under the term of monoclonal gammopathy of renal significance.8, 9, 10 Therefore, MM and other monoclonal lymphoproliferative disorders became diseases of rising interest to nephrologists and onconephrologists. This close association of plasma cell dyscrasias with kidney disease relates especially to the production of monoclonal immunoglobulin FLCs. Kidney lesions related to FLCs include a variety of glomerular diseases such as monoclonal light chain deposition disease and amyloid light-chain– type amyloidosis,8,11 myeloma cast nephropathy,12,13 and, particularly, proximal tubule injury, which is the subject of this review.
Unlike intact immunoglobulins, FLCs are low-molecular-weight proteins that are removed from the circulation through glomerular filtration.14 Filtered FLCs are reabsorbed into the proximal tubule by binding to a luminally expressed multiligand receptor composed of megalin and cubilin.15,16 After endocytosis, FLCs usually undergo degradation, permitting return of the amino acid components to the circulation.17, 18, 19 This system is highly efficient, with only approximately 1 to 10 mg of polyclonal FLCs escaping reabsorption by the proximal tubule and subsequently appearing in the urine each day.20 However, monoclonal B or plasma cell proliferation can lead to a significant increase of circulating FLCs, which can exceed 100,000 mg/l.21 As a result, monoclonal FLCs accumulate within the proximal tubular epithelium. Saturation of the megalin/cubilin receptor permits FLCs to escape absorption, appearing in the tubular fluid of distal nephron segments and finally in the urine as Bence Jones proteins.
An amazing feature of MM is that during the renal handling of FLCs, a broad spectrum of progressive kidney disorders can develop,22 and reducing the circulating levels is critical for renal function recovery.23 This update focuses on the pathogenesis of 2 major aspects of proximal tubule injury related to production and metabolism of these interesting biologically active proteins: proximal tubulopathy and proximal tubule crystallopathy. Myeloma cast nephropathy, which is the other major tubulointerstitial renal lesion associated with FLC, has been reviewed elsewhere.12
Proximal Tubulopathy
Proximal tubule cells (PTCs) are the most abundant cell type in the kidney, and they are always exposed to FLCs in high concentrations in patients with MM. It is therefore not surprising that proximal tubulopathy is common among myeloma-associated renal disorders. There is a large body of evidence that FLCs exert a variety of toxicity on PTCs. Although there is considerable variability in the potency of FLC toxicity—mostly linked to specific sequences in the variable region of the FLC molecule—some degree of overproduction appears as a prerequisite of toxicity in most cases.10,11,13,24,25
Proximal tubulopathy associated with FLCs comprises a broad spectrum of disorders. On one end may be subtle tubule function disorders, such as partial or complete Fanconi syndrome (FS), which is discussed in detail below. On the severe end of the spectrum is marked structural damage in the proximal tubules with varying levels of associated renal insufficiency.11,26 Although a significant portion of proximal tubulopathies results from crystalline or noncrystalline inclusions in PTC, recognition is increasing that there are a variety of inflammatory changes, as evidenced by occasional inflammatory cell infiltration, and more commonly, interstitial fibrosis and tubule atrophy.27 Proximal tubule changes also frequently accompany cast nephropathy, which is predominantly a distal tubule disorder associated with MM. Myeloma casts may further incite inflammatory responses in the kidney interstitium surrounding the proximal tubules.
Tubule disorders result from cytotoxic or inflammatory effects of FLCs, or a combination of both. Studies in vitro showed direct toxicity, which included cytoskeletal disruptions, generation of reactive oxygen species, apoptosis and necrosis, and direct interference with substrate transport functions, as well as activation of inflammatory pathways in kidney PTC.13,28,29 Early studies with isolated brush border membrane vesicles and in vivo microperfusion of the proximal tubule suggested direct interference with amino acid and glucose and phosphate transport, possibly through steric hindrance, independent of interaction with cytosolic elements.30, 31, 32
Other investigations with cell cultures in vitro and mice experiments in vivo demonstrated that FLCs activated nuclear transcription factor κ-B and mitogen-activated protein kinases, leading to transcription and release into the medium of inflammatory cytokines, including interleukins 6 and 8, monocyte chemoattractant protein 1, and transforming growth factor-β.13,29,33, 34, 35, 36 Activation of Toll-like receptors through the signal transducer and activators of transcription 1 (STAT1)-high-mobility group box-1 (HMGB1) axis led to oxidative stress and proinflammatory and profibrotic kidney injury.10,11,29,36
In vitro studies with FLC-exposed kidney PTC and animal studies in vivo both demonstrated FLCs can induce apoptosis, necrosis, and epithelial-mesenchymal transformation of proximal tubule epithelial cells.10,28,29 Many species of FLCs were able to generate these responses at FLC concentrations that may occur in the glomerular ultrafiltrate of a typical patient with MM, although there was some variability among them.
There is strong experimental evidence that the cytotoxic events are associated with FLC-induced redox stress in the PTCs.36,37 Detailed studies showed that the redox-sensitive mitogen-activated protein kinase kinase, known as apoptosis signal-regulating kinase 1 (ASK1), played a significant role in activating the intrinsic apoptotic pathway in FLC-exposed PTCs.37 These inflammatory effects require FLC endocytosis, because maneuvers that block endocytosis of light chains, such as cubilin-megalin knockdown, hypertonic media, and others, can abrogate the inflammatory phenomena.13,36 It is likely that endocytosis of increased quantities of FLCs produced by myeloma cells overload cell trafficking and induce additional endoplasmic reticulum stress responses that further promote inflammation.38,39
Kidney biopsy series by Ecotiere et al.40 and Herrera27 showed significant proximal tubule-associated lesions in MM patients. Interstitial fibrosis, tubule atrophy, and inflammatory cell infiltration are commonly present in the kidney of patients with myeloma, with and without casts. In the series of 70 patients with myeloma cast nephropathy, in addition to the extent of casts, the presence of tubule atrophy was associated with worse prognosis.40 In Herrera’s27 review of 5410 kidney biopsy specimens, paraproteinemia-associated lesions were present in 2.5% of the samples. Of these lesions, 46% were related to proximal tubules.27 Even if some proximal tubule lesions in MM could not be directly related to FLC, the combined clinical, laboratory, and pathology evidence showed that endocytosis of monoclonal FLCs into the proximal tubule epithelium can result in a spectrum of inflammatory and profibrotic phenomena that provide the mechanistic basis for the high frequency of proximal tubulopathy in patients with myeloma.
Proximal Tubule Crystallopathy
Proximal tubule crystallopathy remains a rare observation during plasma cell dyscrasias but represents quite a specific proximal tubulopathy associated with FS. The first observations of cytoplasmic crystalline inclusion bodies during myeloma, both in plasma cells and kidney, were done in the mid-20th century41, 42, 43 and were later associated with FLC-induced renal FS by Maldonado et al.44 The characteristic pathologic features of monoclonal FLC-induced FS typically comprise intracytoplasmic rhomboid or needle-shaped crystals located in endolysosomes of PTCs and a positive staining with anti-κ conjugates in immunofluorescence analysis.
Since the first descriptions of myeloma-associated FS, most proximal tubule crystallopathies have been shown to be associated with low-grade, slowly evolving, lymphoplasmacytic disorders (∼70%) and meet the criteria for monoclonal gammopathy of renal significance.26,45,46 Crystals are also usually observed in monoclonal plasma cells, and it was suggested that these contributed to the slow progression of the hematologic disorder.47 Several studies confirmed the association of proximal tubule crystallopathy with partial or full-blown FS,26,27 even if few cases of FLC-associated FS without crystals were also described.45,48,49
FS is characterized by a generalized dysfunction of the PTCs reabsorption processes, including all sodium-linked transports and megalin/cubilin-induced endocytosis of low-molecular-weight proteins. Consequently, clinical signs include hypophosphatemia, hypouricemia, normoglycemic glucosuria, proximal tubule acidosis, hypocalcemia, aminoaciduria, and low-molecular-weight proteinuria. Bone pain and progressive chronic kidney disease are the most frequent manifestations and usually precede the diagnosis of the underlying hematologic disorder. Interstitial inflammatory infiltration and interstitial fibrosis are common but not systematic.46
In contrast to FLC-induced tubulopathies (including cast nephropathy), the molecular and biochemical features of monoclonal FLC involved in FS present a striking homogeneity that has helped to a better understanding of the pathophysiology of the disease. With few exceptions,45,48, 49, 50 the involved monoclonal FLC is restricted to the κ isotype (>90%) with archetypical FLCs comprising variable (V) domains derived from the IGKV1-33 and IGKV1-39 germline genes.45,48,51 This restriction of V domains allowed structural and biochemical studies to decipher peculiarities leading to toxicity.
A first striking feature of these toxic FLCs is their resistance to proteolysis. Digestion of the FLCs with various proteases, including trypsin, pepsin, or the lysosomal cathepsin B, fails to fully degrade these proteins and release a 12-kDa fragment that corresponds to the V domain.48,52 A second common peculiarity of crystal-forming FLCs is the change of polar to hydrophobic residues in the complementarity determining regions (CDR) loops due to somatic hypermutations. Especially, all FS FLCs with a IGVK1-39 domain contained a hydrophobic amino acid residue in position 30 in CDR1.45,51,52 Directed mutagenesis experiments demonstrated the functional relevance of such mutations. In a mouse model of FLC-induced FS using pathogenic FLC-expressing tumor grafts, Decourt et al.53 demonstrated that the substitution of hydrophobic amino acid residues in CDR1 and CDR3 (residues 30 and 94) to the polar germline counterpart prevented the formation of intracellular crystals in PTCs.
Until recently, an elegant scenario associated these recurrent hydrophobic mutations to the resistance to proteolysis of FS FLCs, which in turn, could have explained their propensity to form crystals in lysosomes and induce PTC dysfunction. However, using in vitro experiments with primary PTCs, we recently confirmed that changing the hydrophobic to a polar residue change at position 30 completely abrogated FLC-induced toxicity but did not reverse the resistance to proteolysis.54 Although these studies were performed using a single FLC, it seems that the resistance to proteolysis cannot be associated with the toxicity of the FLC.
While the high excess of FLCs seen in MM can likely explain the cell trafficking overload leading to cellular stress and inflammatory signals, FS FLCs seem to be highly toxic to PTCs even at very low concentrations. This low threshold to exert toxicity is consistent with the high occurrence of FLC-induced FS in indolent lymphoplasmacytic proliferation with low FLC levels.26,45,46
Structural peculiarities in the V domain caused by somatic hypermutations clearly participate in the potency of FLC toxicity independently of crystal formation. In vitro, physiological concentrations of FS FLCs (25 μg/ml) induce significant morphologic and functional changes in primary PTCs, although no crystalline inclusions were present in the cells. In contrast, equivalent doses of FLCs from patients with cast nephropathy or amyloid light-chain–type amyloidosis, or mutated FS FLC (polar residue in position 30) had no detectable toxic effects.
Accordingly, in a transgenic mouse model of proximal tubule crystallopathy, producing only a slight excess of circulating monoclonal FLC resulted in pathologic lesions and PTC dysfunction that fully reproduced the patient’s disease.55 Interestingly, in this model, only the V domain sequence was from the patient, whereas the constant (C) domain was from the mouse. These findings definitively demonstrated the involvement of this region in the FLC toxicity. FS FLCs showed a highly specific toxicity in PTCs characterized by a loss of apical transporters together with megalin/cubilin and a decrease of lysosomal activity leading to the generalized proximal tubule dysfunction.
All of these effects seemed to result from a dedifferentiation/proliferation process directly induced by the toxic FLC and involving the transcription factor ZONAB.54 Similar molecular events were previously observed in other forms of renal FS56 but not in other FLC-induced proximal tubulopathies without FS.
Summary and Conclusions
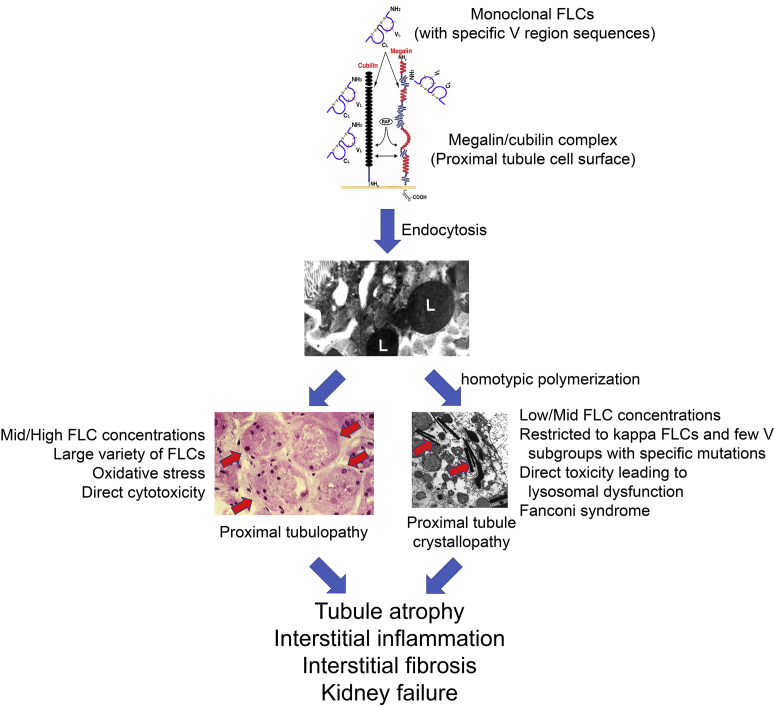
Progress with dissecting the complicated pathogenesis of tubular injury from FLC continues (Figure 1).29,51, 52, 53, 54, 55 Proximal tubulopathy and proximal tubule crystallopathy are related to the amino acid sequence and structure of the variable domain of the offending FLC.29,51, 52, 53, 54, 55 Proximal tubulopathy may be relatively nonspecific, because many FLCs can induce this disease. It is a frequent occurrence in MM and often requires significant concentrations of FLC to produce clinical manifestations. Reabsorption of the FLC leads to cellular stress and consequences of cytotoxicity as well as inflammation and interstitial fibrosis. Proximal tubule crystallopathy is a highly distinct toxicity associated with a few specific FLCs. These lesions may occur with low concentrations of the FLC and may be observed more frequently in monoclonal gammopathy of renal significance than in MM. Proximal tubule crystallopathy occurs with FLC-mediated defects in the reabsorption/degradation processes and associated cellular stress. The unique clinical manifestation is FS, but is often followed by a progressive evolution to include interstitial inflammation and fibrosis.
Figure 1.
Proximal tubule toxicity of immunoglobulin free light chains (FLC). Once certain monoclonal FLCs with specific V region sequences29,51, 52, 53, 54, 55 are endocytosed through the megalin/cubilin complex on the luminal surface of the proximal tubule, they are concentrated in the endolysosomal (L) system and promote cell injury. A large variety of FLCs produce proximal tubulopathy from the generation of oxidative stress and direct cytotoxicity (red arrows at left indicate proximal tubule segments with significant cellular injury). FLCs with κ restriction can specifically undergo homotypic polymerization to form crystals (red arrows at right), producing proximal tubule crystallopathy. Direct toxicity of these FLCs leads to lysosomal dysfunction and the clinical manifestations of Fanconi syndrome. The natural history of both conditions includes the clinical manifestations of progressive chronic kidney disease from tubule atrophy, interstitial inflammation, and interstitial fibrosis.
Disclosure
All the authors declared no competing interests.
Acknowledgements
CS is supported by grants from Fondation Française pour la Recherche contre le Myélome et les Gammapathies monoclonales (FFRMG) et Limousin committees of Ligue nationale contre le cancer. VB is supported by the Paul Teschan Research Fund. PWS is supported by a Merit Award (1 I01 CX001326) from the US Department of Veterans Affairs Clinical Sciences R&D (CSRD) Service and a National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases George M. O’Brien Kidney and Urological Research Centers Program grant (P30 DK079337).
References
- 1.Kyle R.A., Larson D.R., Therneau T.M. Long-term follow-up of monoclonal gammopathy of undetermined significance. N Engl J Med. 2018;378:241–249. doi: 10.1056/NEJMoa1709974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenlee R.T., Murray T., Bolden S., Wingo P.A. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 3.Reule S., Sexton D.J., Solid C.A. ESRD due to multiple myeloma in the United States, 2001-2010. J Am Soc Nephrol. 2016;27:1487–1494. doi: 10.1681/ASN.2014090876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyle R.A., Gertz M.A., Witzig T.E. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 5.Dimopoulos M.A., Delimpasi S., Katodritou E. Significant improvement in the survival of patients with multiple myeloma presenting with severe renal impairment after the introduction of novel agents. Ann Oncol. 2014;25:195–200. doi: 10.1093/annonc/mdt483. [DOI] [PubMed] [Google Scholar]
- 6.Blade J., Fernandez-Llama P., Bosch F. Renal failure in multiple myeloma: presenting features and predictors of outcome in 94 patients from a single institution. Arch Intern Med. 1998;158:1889–1893. doi: 10.1001/archinte.158.17.1889. [DOI] [PubMed] [Google Scholar]
- 7.Knudsen L.M., Hjorth M., Hippe E. Renal failure in multiple myeloma: reversibility and impact on the prognosis. Nordic Myeloma Study Group. Eur J Haematol. 2000;65:175–181. doi: 10.1034/j.1600-0609.2000.90221.x. [DOI] [PubMed] [Google Scholar]
- 8.Leung N., Bridoux F., Batuman V. The evaluation of monoclonal gammopathy of renal significance: a consensus report of the International Kidney and Monoclonal Gammopathy Research Group. Nat Rev Nephrol. 2019;15:45–59. doi: 10.1038/s41581-018-0077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung N., Bridoux F., Hutchison C.A. Monoclonal gammopathy of renal significance: when MGUS is no longer undetermined or insignificant. Blood. 2012;120:4292–4295. doi: 10.1182/blood-2012-07-445304. [DOI] [PubMed] [Google Scholar]
- 10.Sirac C., Herrera G.A., Sanders P.W. Animal models of monoclonal immunoglobulin-related renal diseases. Nat Rev Nephrol. 2018;14:246–264. doi: 10.1038/nrneph.2018.8. [DOI] [PubMed] [Google Scholar]
- 11.Doshi M., Lahoti A., Danesh F.R. Paraprotein-related kidney disease: kidney injury from paraproteins-what determines the site of injury? Clin J Am Soc Nephrol. 2016;11:2288–2294. doi: 10.2215/CJN.02560316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanders P.W. Mechanisms of light chain injury along the tubular nephron. J Am Soc Nephrol. 2012;23:1777–1781. doi: 10.1681/ASN.2012040388. [DOI] [PubMed] [Google Scholar]
- 13.Batuman V. The pathogenesis of acute kidney impairment in patients with multiple myeloma. Adv Chronic Kidney Dis. 2012;19:282–286. doi: 10.1053/j.ackd.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Wochner R.D., Strober W., Waldmann T.A. The role of the kidney in the catabolism of Bence Jones proteins and immunoglobulin fragments. J Exp Med. 1967;126:207–220. doi: 10.1084/jem.126.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Batuman V., Verroust P.J., Navar G.L. Myeloma light chains are ligands for cubilin (gp280) Am J Physiol. 1998;275:F246–F254. doi: 10.1152/ajprenal.1998.275.2.F246. [DOI] [PubMed] [Google Scholar]
- 16.Klassen R.B., Allen P.L., Batuman V. Light chains are a ligand for megalin. J Appl Physiol. 2005;98:257–263. doi: 10.1152/japplphysiol.01090.2003. [DOI] [PubMed] [Google Scholar]
- 17.Maack T., Johnson V., Kau S.T. Renal filtration, transport, and metabolism of low-molecular-weight proteins: a review. Kidney Int. 1979;16:251–270. doi: 10.1038/ki.1979.128. [DOI] [PubMed] [Google Scholar]
- 18.Christensen E.I., Devuyst O., Dom G. Loss of chloride channel ClC-5 impairs endocytosis by defective trafficking of megalin and cubilin in kidney proximal tubules. Proc Natl Acad Sci U S A. 2003;100:8472–8477. doi: 10.1073/pnas.1432873100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozyraki R., Fyfe J., Verroust P.J. Megalin-dependent cubilin-mediated endocytosis is a major pathway for the apical uptake of transferrin in polarized epithelia. Proc Natl Acad Sci U S A. 2001;98:12491–12496. doi: 10.1073/pnas.211291398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berggård I., Peterson P.A. Polymeric forms of free normal k and λ chains of human immunoglobulin. J Biol Chem. 1969;244:4299–4307. [PubMed] [Google Scholar]
- 21.Mead G.P., Carr-Smith H.D., Drayson M.T. Serum free light chains for monitoring multiple myeloma. Br J Haematol. 2004;126:348–354. doi: 10.1111/j.1365-2141.2004.05045.x. [DOI] [PubMed] [Google Scholar]
- 22.Sanders P.W., Herrera G.A., Kirk K.A. Spectrum of glomerular and tubulointerstitial renal lesions associated with monotypical immunoglobulin light chain deposition. Lab Invest. 1991;64:527–537. [PubMed] [Google Scholar]
- 23.Hutchison C.A., Cockwell P., Stringer S. Early reduction of serum-free light chains associates with renal recovery in myeloma kidney. J Am Soc Nephrol. 2011;22:1129–1136. doi: 10.1681/ASN.2010080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanders P.W. Light chain-mediated tubulopathies. Contrib Nephrol. 2011;169:262–269. doi: 10.1159/000313959. [DOI] [PubMed] [Google Scholar]
- 25.Hutchison C.A., Batuman V., Behrens J. The pathogenesis and diagnosis of acute kidney injury in multiple myeloma. Nat Rev Nephrol. 2011;8:43–51. doi: 10.1038/nrneph.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stokes M.B., Valeri A.M., Herlitz L. Light chain proximal tubulopathy: clinical and pathologic characteristics in the modern treatment era. J Am Soc Nephrol. 2016;27:1555–1565. doi: 10.1681/ASN.2015020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrera G.A. Proximal tubulopathies associated with monoclonal light chains: the spectrum of clinicopathologic manifestations and molecular pathogenesis. Arch Pathol Lab Med. 2014;138:1365–1380. doi: 10.5858/arpa.2013-0493-OA. [DOI] [PubMed] [Google Scholar]
- 28.Upadhyay R., Ying W.Z., Nasrin Z. Free light chains injure proximal tubule cells through the STAT1/HMGB1/TLR axis. JCI Insight. 2020;5 doi: 10.1172/jci.insight.137191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ying W.Z., Li X., Rangarajan S. Immunoglobulin light chains generate proinflammatory and profibrotic kidney injury. J Clin Invest. 2019;129:2792–2806. doi: 10.1172/JCI125517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Batuman V., Guan S., O’Donovan R., Puschett J.B. Effect of myeloma light chains on phosphate and glucose transport in renal proximal tubule cells. Ren Physiol Biochem. 1994;17:294–300. doi: 10.1159/000173861. [DOI] [PubMed] [Google Scholar]
- 31.Batuman V., Sastrasinh M., Sastrasinh S. Light chain effects on alanine and glucose uptake by renal brush border membranes. Kidney Int. 1986;30:662–665. doi: 10.1038/ki.1986.237. [DOI] [PubMed] [Google Scholar]
- 32.Sanders P.W., Herrera G.A., Galla J.H. Human Bence Jones protein toxicity in rat proximal tubule epithelium in vivo. Kidney Int. 1987;32:851–861. doi: 10.1038/ki.1987.286. [DOI] [PubMed] [Google Scholar]
- 33.Batuman V. Proximal tubular injury in myeloma. Contrib Nephrol. 2007;153:87–104. doi: 10.1159/000096762. [DOI] [PubMed] [Google Scholar]
- 34.Sengul S., Zwizinski C., Batuman V. Role of MAPK pathways in light chain-induced cytokine production in human proximal tubule cells. Am J Physiol Renal Physiol. 2003;284:F1245–F1254. doi: 10.1152/ajprenal.00350.2002. [DOI] [PubMed] [Google Scholar]
- 35.Sengul S., Zwizinski C., Simon E.E. Endocytosis of light chains induces cytokines through activation of NF-kappaB in human proximal tubule cells. Kidney Int. 2002;62:1977–1988. doi: 10.1046/j.1523-1755.2002.00660.x. [DOI] [PubMed] [Google Scholar]
- 36.Ying W.Z., Wang P.X., Aaron K.J. Immunoglobulin light chains activate NF-κB in renal epithelial cells through a Src-dependent mechanism. Blood. 2011;117:1301–1307. doi: 10.1182/blood-2010-08-302505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ying W.Z., Wang P.X., Sanders P.W. Pivotal role of apoptosis signal-regulating kinase 1 in monoclonal free light chain-mediated apoptosis. Am J Pathol. 2012;180:41–47. doi: 10.1016/j.ajpath.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sengul S., Erturk S., Khan A.M., Batuman V. Receptor-associated protein blocks internalization and cytotoxicity of myeloma light chain in cultured human proximal tubular cells. PLoS One. 2013;8 doi: 10.1371/journal.pone.0070276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li M., Balamuthusamy S., Simon E.E., Batuman V. Silencing megalin and cubilin genes inhibits myeloma light chain endocytosis and ameliorates toxicity in human renal proximal tubule epithelial cells. Am J Physiol Renal Physiol. 2008;295:F82–F90. doi: 10.1152/ajprenal.00091.2008. [DOI] [PubMed] [Google Scholar]
- 40.Ecotiere L., Thierry A., Debiais-Delpech C. Prognostic value of kidney biopsy in myeloma cast nephropathy: a retrospective study of 70 patients. Nephrol Dial Transplant. 2016;31:64–72. doi: 10.1093/ndt/gfv283. [DOI] [PubMed] [Google Scholar]
- 41.Neumann V. Multiple plasma-cell myeloma with crystalline deposits in the tumour cells and in the kidneys. J Pathol Bacteriol. 1949;61:165–169. doi: 10.1002/path.1700610203. pl. [DOI] [PubMed] [Google Scholar]
- 42.Maldonado J.E., Brown A.L., Jr., Bayrd E.D., Pease G.L. Cytoplasmic and intranuclear electron-dense bodies in the myeloma cell. Arch Pathol. 1966;81:484–500. [PubMed] [Google Scholar]
- 43.Ito S., Goshima K., Ninomi M. Electron microscopic studies of the crystalline inclusions in the myeloma cells and kidney of K-Bence Jones protein type myeloma. Nihon Ketsueki Gakkai Zasshi. 1970;33:598–617. [in Japanese] [PubMed] [Google Scholar]
- 44.Maldonado J.E., Velosa J.A., Kyle R.A. Fanconi syndrome in adults. A manifestation of a latent form of myeloma. Am J Med. 1975;58:354–364. doi: 10.1016/0002-9343(75)90601-4. [DOI] [PubMed] [Google Scholar]
- 45.Messiaen T., Deret S., Mougenot B. Adult Fanconi syndrome secondary to light chain gammopathy. Clinicopathologic heterogeneity and unusual features in 11 patients. Medicine (Baltimore) 2000;79:135–154. doi: 10.1097/00005792-200005000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Vignon M., Javaugue V., Alexander M.P. Current anti-myeloma therapies in renal manifestations of monoclonal light chain-associated Fanconi syndrome: a retrospective series of 49 patients. Leukemia. 2017;31:123–129. doi: 10.1038/leu.2016.195. [DOI] [PubMed] [Google Scholar]
- 47.Sirac C., Bridoux F., Essig M. Toward understanding renal Fanconi syndrome: step by step advances through experimental models. Contrib Nephrol. 2011;169:247–261. doi: 10.1159/000313962. [DOI] [PubMed] [Google Scholar]
- 48.Deret S., Denoroy L., Lamarine M. Kappa light chain-associated Fanconi’s syndrome: molecular analysis of monoclonal immunoglobulin light chains from patients with and without intracellular crystals. Protein Eng. 1999;12:363–369. doi: 10.1093/protein/12.4.363. [DOI] [PubMed] [Google Scholar]
- 49.Bridoux F., Sirac C., Hugue V. Fanconi’s syndrome induced by a monoclonal Vkappa3 light chain in Waldenstrom’s macroglobulinemia. Am J Kidney Dis. 2005;45:749–757. doi: 10.1053/j.ajkd.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 50.Isobe T., Kametani F., Shinoda T. V-domain deposition of lambda Bence Jones protein in the renal tubular epithelial cells in a patient with the adult Fanconi syndrome with myeloma. Amyloid. 1998;5:117–120. doi: 10.3109/13506129808995289. [DOI] [PubMed] [Google Scholar]
- 51.Rocca A., Khamlichi A.A., Touchard G. Sequences of V kappa L subgroup light chains in Fanconi’s syndrome. Light chain V region gene usage restriction and peculiarities in myeloma-associated Fanconi’s syndrome. J Immunol. 1995;155:3245–3252. [PubMed] [Google Scholar]
- 52.Leboulleux M., Lelongt B., Mougenot B. Protease resistance and binding of Ig light chains in myeloma-associated tubulopathies. Kidney Int. 1995;48:72–79. doi: 10.1038/ki.1995.269. [DOI] [PubMed] [Google Scholar]
- 53.Decourt C., Rocca A., Bridoux F. Mutational analysis in murine models for myeloma-associated Fanconi’s syndrome or cast myeloma nephropathy. Blood. 1999;94:3559–3566. [PubMed] [Google Scholar]
- 54.Luciani A., Sirac C., Terryn S. Impaired lysosomal function underlies monoclonal light chain-associated renal Fanconi syndrome. J Am Soc Nephrol. 2016;27:2049–2061. doi: 10.1681/ASN.2015050581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sirac C., Bridoux F., Carrion C. Role of the monoclonal kappa chain V domain and reversibility of renal damage in a transgenic model of acquired Fanconi syndrome. Blood. 2006;108:536–543. doi: 10.1182/blood-2005-11-4419. [DOI] [PubMed] [Google Scholar]
- 56.Raggi C., Luciani A., Nevo N. Dedifferentiation and aberrations of the endolysosomal compartment characterize the early stage of nephropathic cystinosis. Hum Mol Genet. 2014;23:2266–2278. doi: 10.1093/hmg/ddt617. [DOI] [PubMed] [Google Scholar]