Abstract
Great strides have been made in understanding and treating hepatitis C virus (HCV) thanks to the development of various experimental systems including cell-culture-proficient HCV, the HCV pseudoparticle system and soluble envelope glycoproteins. The HCV pseudoparticle (HCVpp) system is a platform used extensively in studies of cell entry, screening of novel entry inhibitors, assessing the phenotypes of clinically observed E1 and E2 glycoproteins and, most pertinently, in characterizing neutralizing antibody breadth induced upon vaccination and natural infection in patients. Nonetheless, some patient-derived clones produce pseudoparticles that are either non-infectious or exhibit infectivity too low for meaningful phenotyping. The mechanisms governing whether any particular clone produces infectious pseudoparticles are poorly understood. Here we show that endogenous expression of CD81, an HCV receptor and a cognate-binding partner of E2, in producer HEK 293T cells is detrimental to the infectivity of recovered HCVpp for most strains. Many HCVpp clones exhibited increased infectivity or had their infectivity rescued when they were produced in 293T cells CRISPR/Cas9 engineered to ablate CD81 expression (293TCD81KO). Clones made in 293TCD81KO cells were antigenically very similar to their matched counterparts made parental cells and appear to honour the accepted HCV entry pathway. Deletion of CD81 did not appreciably increase the recovered titres of soluble E2 (sE2). However, we did, unexpectedly, find that monomeric sE2 made in 293T cells and Freestyle 293-F (293-F) cells exhibit important differences. We found that 293-F-produced sE2 harbours mostly complex-type glycans whilst 293T-produced sE2 displays a heterogeneous mixture of both complex-type glycans and high-mannose or hybrid-type glycans. Moreover, sE2 produced in 293T cells is antigenically superior; exhibiting increased binding to conformational antibodies and the large extracellular loop of CD81. In summary, this work describes an optimal cell line for the production of HCVpp and reveals that sE2 made in 293T and 293-F cells are not antigenic equals. Our findings have implications for functional studies of E1E2 and the production of candidate immunogens.
Keywords: human hepacivirus, pseudoparticle, receptor, viral glycoprotein, human betacoronavirus
Introduction
HCV is a significant human pathogen infecting more than 70 million people worldwide, of whom the majority are chronically infected. Current WHO estimates suggest around half a million patients succumb to the disease annually, mostly due to complications arising from cirrhosis or hepatocellular carcinoma. Transmission still continues unabated with incidence rates rising in North America as the majority of infected individuals are unaware of their status [1]. The recent development of curative direct-acting antivirals has revolutionized HCV therapy as well as raised the possibility of eliminating HCV. Nonetheless, the high cost of treatment, the risk of reinfection following successful treatment and poor awareness of HCV status in high-risk groups necessitate the development of a prophylactic vaccine [2].
As viruses are genetically diverse it is imperative that experimental systems have the capacity to include many isolates allowing for comprehensive assessment of the effectiveness of therapeutic and prophylactic interventions [3–5]. There are two major systems to assess HCV infection in vitro: HCV pseudoparticles (HCVpp) and cell-culture-derived HCV (HCVcc). HCVpp are based on a disabled retroviral construct, which encodes a reporter gene and incorporates the HCV glycoproteins E1 and E2 in their lipid envelope [6, 7]. HCVcc are full-length replicative viruses generated by the introduction of in vitro transcribed RNA genome into permissive cells; HCVcc are typically based on the JFH-1 clone or chimaeras consisting of the JFH-1 replicase genes NS3-NS5B and Core-NS2 regions of alternative HCV genomes [8–10].
The HCVcc system represents a more physiological model of infection and allows for a broader study of the HCV life cycle [11]. However, this system still largely depends on JFH-1 chimaeras, which often rely on culture adaptation for optimal infectivity, and has challenging production and handling protocols given its biosafety level III designation in most regions [12]. In contrast, producing HCVpp is a relatively simple task and the ease of handling allows for the simultaneous generation of a multitude of clones if one requires [13]. The major disadvantage of HCVpp is that their application is only limited to studying viral entry. Regardless, due to its flexibility, the HCVpp system is preferred for characterizing neutralizing antibody breadth, an essential component for screening HCV vaccine candidates. To this end, Wasilewski and colleagues recently observed a very strong positive correlation between the relative neutralization resistance of E1E2-matched HCVcc and HCVpp variants indicating that either system can be used to phenotype neutralizing antibodies [14]. Furthermore, diverse HCVpp panels were key in identifying the relationship between the development of broadly neutralizing antibodies (bNAbs) and spontaneous clearance of HCV infection [15–17].
Another important tool for studying HCV entry and vaccine development is a soluble form of its glycoprotein E2 (sE2), which is devoid of its transmembrane region and is expressed in the absence of E1. Screenings based on sE2 binding to human hepatoma cell lines identified the first two HCV entry receptors as being the tetraspanin CD81 and scavenger receptor class type B 1 (SR-B1) [18, 19]. sE2 retains proper folding as evidenced by its ability to recapitulate HCV binding to CD81 and SR-B1, block HCVcc infection and bind various antibodies [20], therefore allowing for functional, structural and biophysical characterization of E2. The partial structures of sE2 revealed a globular shape with IgG-like folds and flexible regions [21, 22]. Interestingly, computational models and antibody competition studies suggest there may be an interdependence in E2-SR-B1/CD81 interactions, whereby SR-B1 binding enhances E2-CD81 engagement [23, 24]. The SR-B1 and CD81 binding epitopes on E2 are predicted to be in close proximity thus steric clashes may prevent simultaneous engagement of the two receptors. However, several studies have recently suggested this region may be highly flexible thereby potentially introducing a greater spatial separation between the epitopes [25–27]. Investigating these hypotheses will further clarify our understanding of HCV entry as well as aid vaccine development and sE2 will doubtless continue to be a key tool to decipher these mechanisms.
HEK 293-derived cell lines are extensively used to produce many recombinant proteins and pseudoviruses, including sE2 and HCVpp. HEK 293 cells express CD81 [28, 29], despite this, it remains unknown whether the presence of this cognate E2 receptor during production affects the functionality and antigenicity of pseudovirus and/or sE2. Here, we describe the generation of a 293T cell line knocked out for CD81 expression (293TCD81KO). Producing HCVpp in this cell line significantly improved or rescued the infectivity of the majority of clinical isolates screened [30] without altering particle antigenicity. Finally, we show that while sE2 molecules produced in 293TCD81KO and 293T cells are very similar, they are antigenically superior to that produced in Freestyle 293-F cells (293-F); another 293-derived cell line preferred for the large-scale production of recombinant viral glycoproteins [31–33]. We propose this is likely due to observed differences in glycosylation status between the two.
Results
HCVpp supernatant produced in CD81 knock-down 293T cells exhibits enhanced infectivity
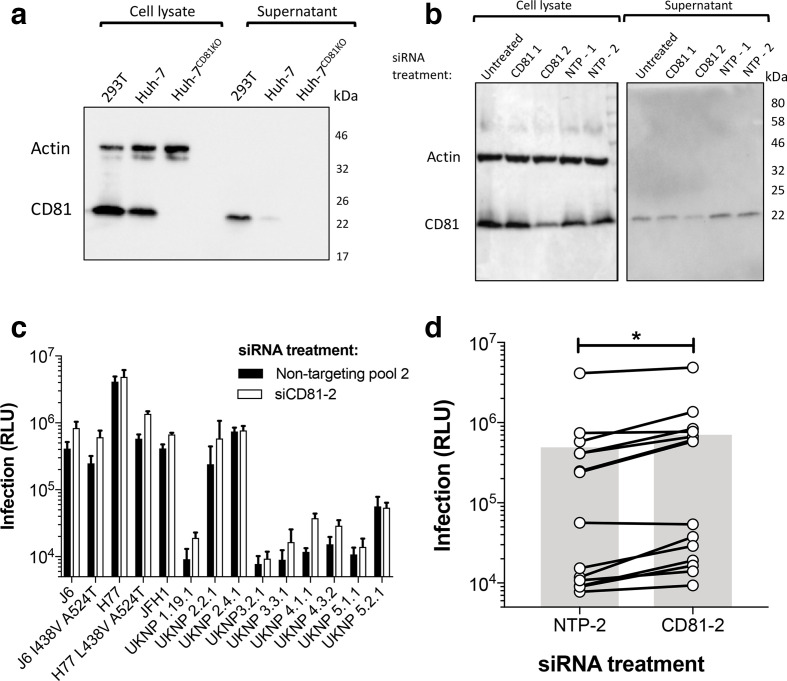
Our study was initiated following an observation we made during routine experiments: conditioned media taken from 293T cells contains a high level of CD81. Fig. 1a displays extracellular CD81 in filtered conditioned cell-culture supernatant from two cell lines extensively used in HCV research (the Huh-7 and 293T cell lines). We detected CD81 in both 293T and Huh-7 conditioned supernatant, although the former contained considerably more (Fig. 1a). The extracellular source of CD81 is likely to be exosomes: small extracellular vesicles formed by the inward budding of the late endosomal membrane. They measure 30–120 nm in size and are composed of proteins, lipids, nucleic acids and other metabolites. Notably, their surfaces are highly enriched in tetraspanins such as CD81, which consequently serves as an exosome biomarker.
Fig. 1.
Knock-down of CD81 in producer 293T cells increases HCVpp infectivity. (a) Cell lysates and unconcentrated conditioned supernatant of cell lines involved in HCV research were analysed by SDS-PAGE and Western blot with a specific anti-CD81 antibody (note: a CD81 knock-out Huh-7 line is included as a negative control). (b) Cell lysates and unconcentrated conditioned supernatant of 293T cells transfected with CD81 targeting siRNA were analysed by SDS-PAGE and Western blot with a specific anti-CD81 antibody. Molecular mass markers are indicated on the right (kDa). (c) Huh-7 cells were infected with an HCVpp panel consisting of 14 E1E2 clones produced in 293T cells exhibiting appreciable CD81 knock-down (white bars) or 293T cells treated with a non-targeting siRNA pool (NTP-2) (black bars). Data are represented as raw luciferase units (RLU) and are from a single experiment performed in triplicate. Error bars indicate the standard deviations between three replicate wells. (d) A compiled summary of the data shown in (c), connected points indicate a single E1E2 clone and the grey bars represent the mean RLU of all clones. Paired t-test, (*P <0.05).
We theorized that exosomes and HCV particles may complex extracellularly through interactions between surface CD81 and E2, and that this may be detrimental for HCVpp infectivity. To test this, we screened the infectivity of a panel of HCVpp produced in 293T cells treated with siRNA targeting CD81 (Fig. 1b). We consistently observed greater infection of Huh-7 cells for HCVpp produced in CD81 knock-down 293T cells (CD81-2) compared to their E1E2 matched equivalents produced in non-targeted pool siRNA (NTP-2) treated 293T cells (Fig. 1c). Collectively, CD81 knock-down led to a twofold increase in infection for viruses in the panel (Fig. 1d) indicating that the expression of CD81 in 293T cells affects HCVpp production. However, we were unable to demonstrate cell-free interactions between HCVpp and CD81 positive exosomes (data not shown). Therefore, this phenotype may not be due to extracellular CD81 though it is also possible that CD81 affects HCVpp production intracellularly or during viral egress (see discussion).
Deletion of CD81 in 293T cells enhances or rescues infectivity of patient-derived HCVpp
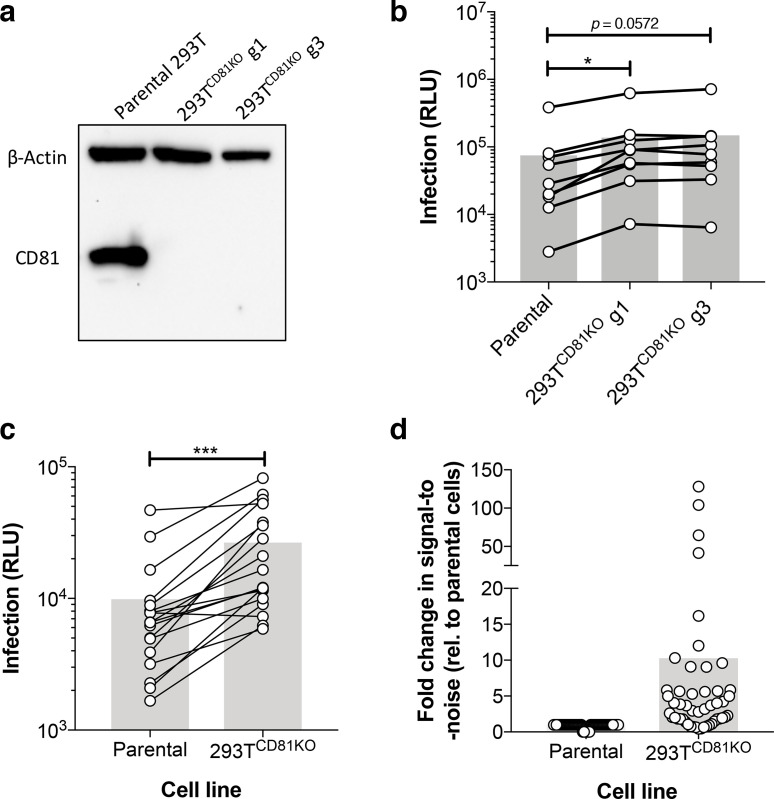
Following the above observations (Fig. 1d) we decided to generate stable cell lines ablated of CD81 expression as they could be a useful resource for the production of HCVpp [30, 34]. To delete CD81, we engineered 293T cells with the CRISPR-Cas9 gene-editing system. As CD81 is a cell-surface protein we were able to sort edited cells by flow cytometry to obtain pure CD81 negative populations (coloured boxes in Fig. S1a, available in the online version of this article). Sorted cells were then diluted to obtain single cell clones and expanded (Fig. S1b) until we obtained two stable cell lines: 293TCD81KO guide 1 and guide 3 (g1 and g3) (Fig. 2a).
Fig. 2.
HCVpp made in CD81 knock-out 293T cells exhibit enhanced infectivity. Huh-7 cells were infected with HCVpp produced in parental or 293TCD81KO cell lines. (a) CD81 ablation was confirmed by SDS-PAGE and Western blot analysis. (b) A compiled summary of the infection levels for an HCVpp panel consisting of both prototypical and clinical isolates. Paired t-test with virus produced in parental 293Ts (*P <0.05). (c) A compiled summary of the infection levels of a panel of clinical HCVpp isolates. Paired t-test, (***P <0.001). Data in (b) and (c) are from lone experiments performed in triplicate and data are represented as RLU. (d) A compiled summary of the fold signal-to-noise ratio (S/N) for all screened clones, fold values are relative to virus produced in parental 293T cells. Connected points indicate a single E1E2 clone and the grey bars represent the mean value.
To comprehensively determine whether CD81 deletion enhanced HCVpp infection, three independent labs screened the infectivities of HCVpp produced in parental 293Ts and CD81 knock-out cell lines. Firstly, a panel consisting of both prototypical strains (e.g. J6 and H77) and clinical isolates was made in the aforementioned cell lines. We consistently observed higher infection across the panel for HCVpp harvested from both 293TCD81KO lines (g1 and g3) compared to matched viruses made in parental cells (Figs 2b and S2a, b). As the infectivities of HCVpp recovered from the two 293TCD81KO cell lines were very similar, we chose the g3 CD81 knock-out lineage for subsequent experiments, these cells are, henceforth, referred to as 293TCD81KO. The next screen was of a panel of HCVpp carrying patient-derived E1E2 observed in patient cohorts from hospitals in Amsterdam (32 and unpublished) or Nottingham [35]. Here, the improvement in infection for HCVpp produced in 293TCD81KO cells compared to their equivalents made in parental cells was markedly more apparent (Figs 2c and S2c, d). Indeed, we observed a fivefold increase in infectivity for four of the tested clones and this effect reached tenfold for the UKNP 5.2.1 clone (Fig. S2c).
Lastly, we made a panel of HCVpp expressing E1E2 observed in HCV+ individuals from the Australian Hepatitis C Incidence and Transmission Studies in prisons (HITS-p) cohort in the 293TCD81KO cells [36]. Following infection read-out, we used the signal observed in cells infected with a non-enveloped control to calculate the signal-to-noise ratio (S/N) for each clone in the panel and compared it to the S/N when the clone was produced in parental 293Ts (Table S1). We saw an increase in the S/N for the majority of screened clones when made in 293TCD81KO cells. Strikingly, 5 of the 22 screened clones previously found to be non-infectious [30] were now infectious (S/N ≥5) and a further 8 of the 22 screened clones exhibited at least a fivefold increase in S/N (Table S1, Fig. S2e). Finally, a comparison of calculated S/N for all screened clones from all three panels generally demonstrated an upward trend in infectivity for HCVpp following production in 293TCD81KO cells (Figs 2d and S2f). Combined, the above data suggest the 293TCD81KO cells we have produced could be a useful resource for HCV research.
Next, we sought to ensure deletion of CD81 in producer 293T cells did not affect the entry pathway of HCVpp. The E1 and E2 glycoproteins mediate the virus’ intricate entry pathway. The E1 and E2 genes display the greatest diversity in the HCV genome, yet receptor interactions remain highly conserved across distinct genotypes and entry is widely understood to involve the essential receptors CD81, SR-B1, Claudin and Occludin [37, 38]. We infected Huh-7 cells ablated for these proteins [39] with HCVpp produced in parental or 293TCD81KO cells. Whilst ablation of CD81, Claudin-1 and Occludin abolished infection for all four tested strains, a proportion of all strains was still able to infect SR-B1 knock-out cells (Fig. S3). These data are consistent with previous findings for both HCVcc and HCVpp particles [23, 39, 40] and indicate pseudoparticles made in 293TCD81KO cells follow the correct HCV entry pathway.
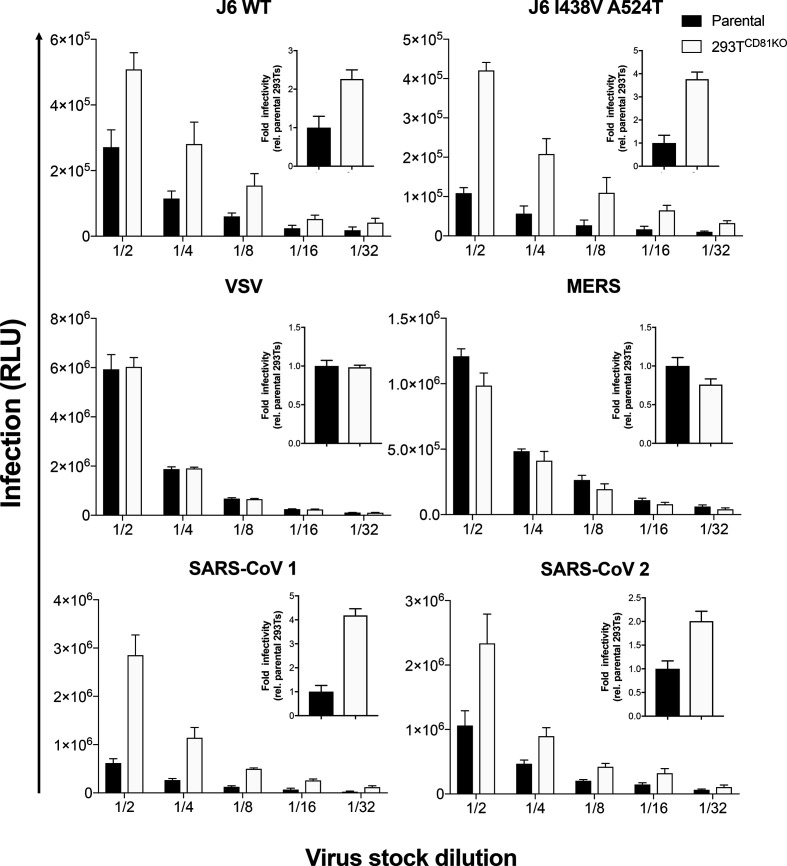
To determine whether CD81 deletion in producer 293T cells specifically affects the infectivity of HCV, we produced lentiviral packaged pseudoparticles bearing the glycoproteins of Indiana vesiculovirus (VSV), Middle East respiratory syndrome-related coronavirus (MERS) and severe acute respiratory syndrome coronaviruses 1 and 2 (SARS-CoV 1 and SARS-CoV 2) glycoproteins in parental 293Ts or 293TCD81KO cells. Following infection of the appropriate cell line, we observed no difference in the infectivity of VSV or MERS pseudoparticles when produced in 293TCD81KO cells whereas the two HCV J6 strain viruses tested exhibited an improvement in infectivity, as expected (Fig. 3). Remarkably, we also observed an increase in infectivity for both SARS-CoV 1 and 2 (Fig. 3, bottom panels). These data indicate that whilst CD81 ablation in producer 293Ts does not improve the infectivity of all pseudoparticles, enhanced infection is not limited to HCVpp. However, it is difficult to speculate on the mechanism(s) of enhancement without further investigation.
Fig. 3.
Effect of CD81 knock-out on lentiviral-based pseudovirus infectivity. Huh-7 (HCV J6 and VSV), Caco-2 (MERS) and HeLa-Ace2 (SARS-CoVs) cell lines were infected with a twofold serial dilution of viral pseudoparticles produced in parental or 293TCD81KO cell lines. Data are represented as RLU and the error bars indicate the standard deviations between five replicate wells. Insert graphs show the overall fold infectivity of pseudoparticles across all five concentrations used, data are presented relative to virus produced in parental 293Ts. Data are representative of two independent experiments.
Production in CD81 knock-out 293T cells does not alter HCVpp antibody sensitivity
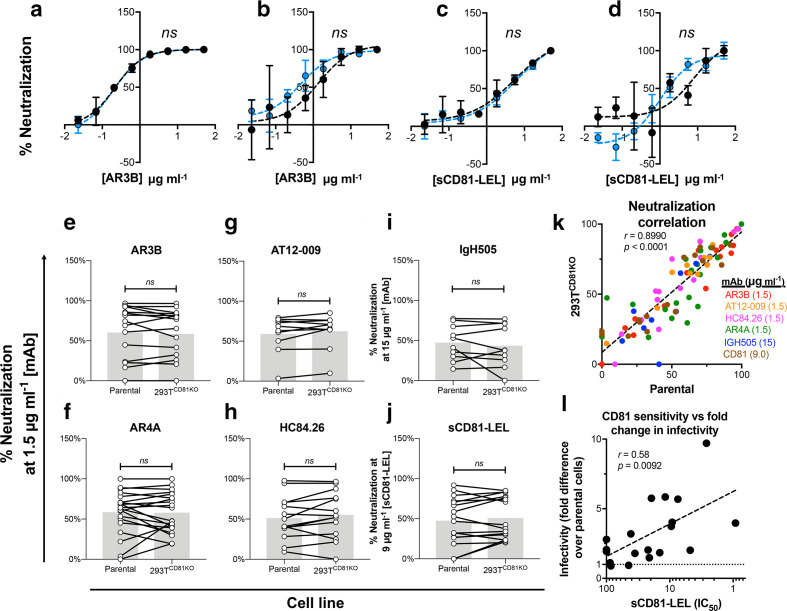
HCVpp are widely used to examine the neutralizing breadth and potency of monoclonal antibodies (mAbs) and polyclonal sera from humans and immunized animals [17, 30]. Therefore, it was imperative to ensure that HCVpp produced in 293TCD81KO cells are antigenically similar to those produced in parental cells. First, we challenged H77 and UKNP 5.2.1 pseudoparticles with a serial dilution of AR3B, a bNAb that targets the CD81 binding site (CD81-bs) [41]. We observed similar neutralization for each clone irrespective of the cell line of production; indeed, the hyperbola curves generated for the H77 pair overlapped and, accordingly, their calculated IC50 was identical (Fig. 4a, b). We also challenged H77 and UKNP 5.2.1 pseudoparticles with a serial dilution of a soluble form of the large extracellular loop of CD81 (sCD81-LEL). As with AR3B, the IC50 of sCD81-LEL toward H77 was indistinguishable regardless of the cellular source of the HCVpp (Fig. 4c). The IC50 of sCD81-LEL toward the matched UKNP 5.2.1 HCVpp pair was also similar (Fig. 4d).
Fig. 4.
Absence of CD81 in producer 293T cells does not affect antibody and CD81 sensitivity of HCVpp. The prototypical lab strain (a) H77 and the clinical isolate (b) UKNP 5.2.1 were incubated with a serial dilution of the mAb AR3B prior to infection. The prototypical lab strain (c) H77 and the clinical isolate (d) UKNP 5.2.1 were incubated with a serial dilution of the sCD81-LEL prior to infection. Data in (a–d) are expressed as percent neutralization relative to wells not preincubated with antibody or receptor molecules and each point is the mean of three replicate values. Error bars indicate the standard deviations between replicate wells. Data were fitted using the log (inhibitor) vs. response (four parameters) function on GraphPad Prism. ns indicates best fit curves do not differ significantly, F-test. Compiled summaries of the neutralization of HCVpp panels when challenged with a single concentration of the indicated mAb or soluble protein prior to infection (e–j). Connected points indicate a single E1E2 clone and the grey bars represent the mean value. Paired t-test, no significance (ns). (k) A scatter plot of the percentage neutralization for a panel of HCVpp clones produced in parental 293T cells (x-axis) vs. when produced in 293TCD81KO cells (y-axis) after challenge with a single concentration of indicated mAb or sCD81-LEL. (l) A scatter plot of HCVpp CD81 sensitivity (sCD81-LEL IC50) (x-axis) and fold change in infectivity following production in 293TCD81KO cells (y-axis). Each point in (k) and (l) represents a lone HCV isolate. Spearman correlation (r) and P values were computed on GraphPad Prism. All data are from lone experiments performed in triplicate.
Next, we compared the neutralization of an HCVpp panel following incubation with a fixed concentration of AR3B. We found the infectivities of most clones was similar irrespective of the cell line of production and saw no significant difference for the panel as a whole (Fig. 4e). This experiment was conducted for an additional four mAbs: (i) AR4A, which targets an epitope composed of both E1 and E2 residues, (ii) AT-12009 (CD81-bs), (iii) HC84.26 (domain D) and (iv) IgH505, which targets a discontinuous epitope in E1 (residues 313–327) [41–44]. We saw no significant difference in the neutralization levels of the panel against all four mAbs irrespective of the cell line of production (Fig. 4f–i). Similar observations were also made when pseudoparticles were pre-incubated with sCD81 (Fig. 4j). Finally, a scatter plot of the data shown in figures Fig. 4e–j revealed a very strong correlation for neutralization sensitivity between HCVpp produced in 293TCD81KO cells and HCVpp made in parental 293T cells (Fig. 4k). These data indicate ablation of CD81 in producer 293T cells does not change the sensitivity of pseudoparticles to neutralization by sCD81 or mAbs. However, we did find a significant positive correlation (r=0.58, P=0.0092) between the sensitivity of HCVpp to sCD81 neutralization and their fold increase in infectivity following production in 293TCD81KO cells (Fig. 4l). This suggests that HCVpp that are sensitive to CD81 neutralization benefit more from production in 293TCD81KO cells. In summary, the data demonstrate that producing HCVpp in 293TCD81KO cells does not alter their antigenicity or sensitivity to mAbs. These findings considered along with their ability to improve virus infectivity suggest that the 293TCD81KO cell line is a superior system for assessing the functionality and neutralization of diverse HCVpp.
sE2 produced in 293TCD81KO or parental 293Ts displays a mixture of high-mannose and complex-type glycans
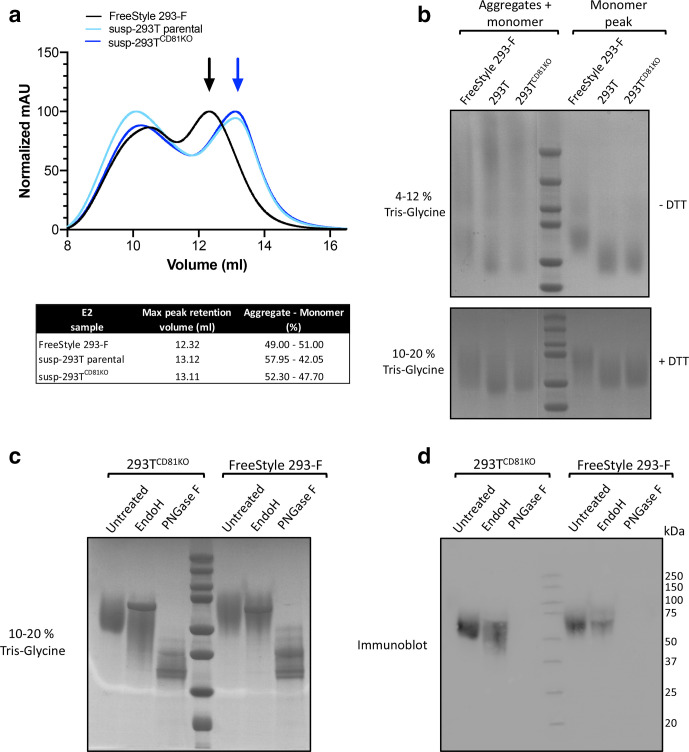
HEK 293T cells are commonly used for the production of sE2. Consequently, we examined whether 293TCD81KO cells improve sE2 production, as they did HCVpp production, without altering the properties of the resultant protein. To do this, we first adapted the 293TCD81KO and parental 293T cell lines to grow in suspension, which makes the cells more suitable for large-scale protein production. We refer to these cell lines as susp-293T and susp-293TCD81KO cells. We used conventional transient transfection to produce sE2 in these two cell lines. In parallel, we also compared these to sE2 generated in the 293-F cells. Although this cell line is also derived from HEK 293 cells, and grows in suspension, it is preferred for the production of recombinant viral glycoproteins, including those of HIV-1, influenza and HCV, because of the high production yields [31, 32, 45]. We isolated sE2 monomers from harvested supernatants using affinity purification followed by size exclusion chromatography (SEC). Very similar yields of sE2 were obtained following purification of harvested susp-293TCD81KO, susp-293T (both ~14 mg l−1) and 293-F (12 mg l−1) supernatant, although relatively more aggregates were observed in sE2 from the susp-293T lineages. Furthermore, the retention volumes for monomeric sE2 made in susp-293TCD81KO and susp-293T cells were practically identical (~13.1 ml) yet monomeric sE2 produced in 293-F cells eluted at a lower volume (~12.3 ml) suggesting it is larger than that produced in the susp-293T cell lines (Fig. 5a). Indeed, SDS-PAGE analysis of unfractionated sE2 and their monomeric peak fractions, under both reducing and non-reducing conditions, revealed sE2 made in 293-F cells migrated more slowly than sE2 from the two susp-293T lines (Fig. 5b). These data suggest that sE2 undergoes distinct post-translational modifications in 293-F and 293T cells.
Fig. 5.
Soluble E2 is differentially glycosylated in susp-293T and 293-F cells. (a) Superdex 200 size-exclusion chromatography profile of susp-293T- (turquoise), susp-293TCD81KO- (blue) and 293-F- (black) derived sE2 following Strep-II-tag affinity chromatography. Blue arrow corresponds to the peak fraction of monomeric sE2 from susp-293T and susp-293TCD81KO cells and the black arrow corresponds to monomeric sE2 from 293-F cells. mAU, milliabsorbance units. (b) Nonreducing BN-PAGE gel (top panel) and reducing SDS-PAGE gel (bottom panel) of the purified sE2 derived from the aforementioned cell lines (Coomassie Brilliant Blue G-250 staining). (c) Reducing SDS-PAGE gel of monomeric susp-293TCD81KO- and 293-F- derived sE2 after treatment with PNGase F or EndoH (Coomassie Brilliant Blue G-250 staining). (d) Western blot of SDS-PAGE gel shown in (c). Please note, we failed to detect PNGase F treated E2 with AP33 on the Western blot on multiple attempts. Molecular mass markers are indicated on the right (kDa). Data is from a single experiment.
Up to 11 Asn-linked glycosylation sites can be detected in most E2 sequences and fully processed E2, in both its soluble form or in the context of viral particles, is heavily glycosylated and displays a mixture of high-mannose and complex-type glycans [45, 46]. As a result, the molecular mass of E2 is significantly influenced by glycans. To test whether differences in glycosylation account for the disparate molecular weight between sE2 from 293T and 293-F cells, we treated purified monomeric sE2 with PNGase F or EndoH deglycosylation enzymes. PNGase F cleaves both high-mannose and complex-type glycans yielding a mostly deglycosylated protein whereas Endo H specifically cleaves high-mannose and some hybrid glycans.
We compared sE2 produced in 293-F cells to susp-293TCD81KO-derived sE2 (glycoprotein produced in either the edited or parental 293-T cells migrated identically suggesting their glycosylation status is similar, Fig. 5b). As expected, PNGase F treatment confirmed sE2 made in both susp-293TCD81KO cells and 293 F cells to be heavily glycosylated. Undigested sE2 migrated to around ~65 kDa and its deglycosylated form migrated to ~35 kDa irrespective of the cell line of production (Fig. 5c). Strikingly, the sensitivity of sE2 from 293 F and susp-293TCD81KO cells to EndoH treatment was different. sE2 made in 293 F cells was mostly unaffected by EndoH treatment as evidenced by the similar migration observed for the untreated and EndoH-digested samples (Fig. 5c). This suggests that 293-F-produced sE2 is composed of mostly complex-type glycans, a hallmark of maturation through the Golgi apparatus. On the other hand, sE2 from susp-293TCD81KO was sensitive to EndoH as evidenced by the faster migrating smear on SDS-PAGE after treatment. Furthermore, the smear length indicates glycans on susp-293TCD81KO-produced sE2 ranged from fully EndoH resistant to almost completely EndoH sensitive. This suggests that 293TCD81KO cells produce a heterogeneous population of sE2 molecules in terms of glycosylation and that some sE2 molecules are only partially matured as some remain high-mannose type, while others have been heavily modified by Golgi enzymes (Fig. 5c, d). As an aside, we noted an unexpected loss of AP33 binding upon PNGase F treatment in the Western blot (Fig. 5d). AP33 binding is not dependent on glycosylation, however, its contact residues are proximal to Asn-417 and enzymatic removal of the glycan at this position may have affected the AP33 epitope [47]. Notably, heterogeneity in the glycosylation status of E2 has also been observed in HCVcc-associated E2 (46). This implies that the processing of sE2 through the Golgi apparatus of susp-293TCD81KO cells more closely resembles that of E2 destined to be incorporated in authentic HCV virions than sE2 from 293-F cells. In summary, these data demonstrate compositional differences in the glycans of sE2 produced in susp-293TCD81KO and 293-F, based on previous findings it is likely that 293T produced sE2 is more representative of E2 on HCVcc particles.
CD81 binding and antigenicity of 293T lineage produced sE2 is superior to that of 293-F-produced sE2
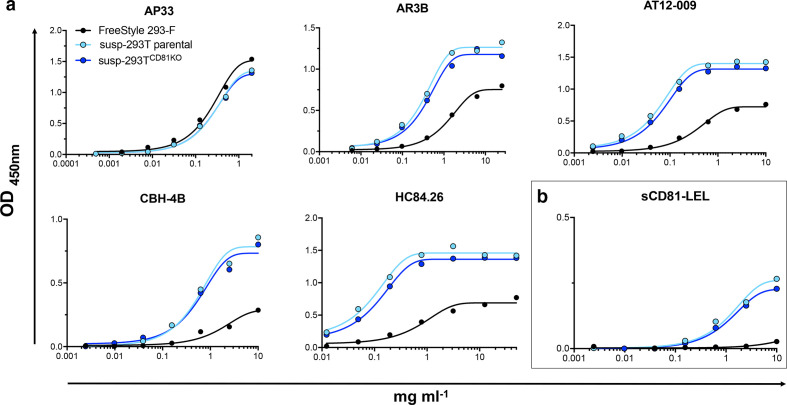
To investigate whether producing sE2 in cells lacking CD81 altered its antigenicity, we first tracked the binding of immobilized sE2 monomers from susp-293T, susp-293TCD81KO and 293-F cells to serially diluted mAbs and human Fc-tagged soluble CD81 (sCD81-LEL-hFc) by ELISA. The mAb panel included the AS412 targeting AP33, AR3B and AT12-009, which target the front layer and binding loop of the CD81 binding site, CBH-4B (domain A) and HC84.26 (domain E) [41–43, 48]. All tested antibodies and sCD81-LEL-hFc bound equally well to sE2 produced in susp-293TCD81KO or parental susp-293T cells, suggesting that the expression of CD81 in 293T cells does not affect the antigenicity of sE2 (Fig. 6a). sE2 produced in 293F cells exhibited reduced binding to conformational antibodies, with equivalent binding observed only for AP33, which targets a continuous epitope (AS412). These results suggest there is a difference in epitope accessibility between the two forms of sE2. This difference was even more apparent when comparing the binding of sCD81-LEL-hFc to the different sE2 types (Fig. 6b).
Fig. 6.
ELISA analysis of antibody and CD81 binding to sE2 form susp-293T, susp-293TCD81KO and 293-F cells. sE2 was immobilized to Strep-TactinXT-coated microplates and incubated with a serial dilution of the indicated mAb (a) or CD81-LEL-hFc (b). Each point represents a single value from a lone experiment. Data were fitted using a sigmoidal curve in GraphPad Prism.
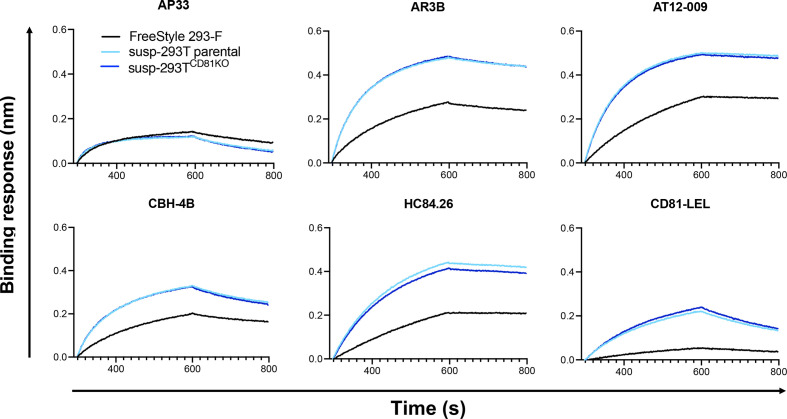
The binding of the three types of sE2 to the mAb panel and sCD81-LEL-hFc was further characterized by biolayer interferometry (BLI) (Fig. 7). Antibodies and CD81-LEL-hFc were immobilized on a protein A biosensor and incubated with the same concentration of sE2. Consistent with the ELISA data, near-identical binding profiles were observed for sE2 made in susp-293TCD81KO and parental susp-293T cells; again, besides AP33, sE2 made in 293-F exhibited lower binding to all mAbs and sCD81-LEL. Together, the BLI and ELISA data reveal a previously unappreciated difference between the antigenicity of sE2 produced in 293-F and 293T cells. This may have implications for functional/structural studies of sE2 and/or future immunogen production.
Fig. 7.
BLI analysis of antibody and CD81 binding to sE2 form susp-293T, susp-293TCD81KO and 293 F cells. CD81-LEL-hFc and the indicated mAbs were immobilized onto protein A biosensors and incubated with a single concentration of sE2. Sensorgrams were obtained from an Octet K2 instrument. Data is from a single experiment.
Discussion
The HCV pseudoparticle system is a flexible platform for evaluating the phenotypes of clinical isolates and has proven to be a crucial tool for defining the sequence of virion and receptor interactions occurring during HCV entry; it is likely to be an important component for the assessment of future HCV vaccine candidates [13, 28, 29]. Nonetheless, producing infectious viruses from polymorphic HCV populations circulating in patients has repeatedly proved challenging [35, 49, 50]. This is problematic for vaccine screens and antibody-neutralizing breadth studies, as only strains that recover workable infectivity are included in such analyses and they may not necessarily be representative of the diversity circulating in the population. Moreover, some infectious strains give low yields, which fail to reach a signal-to-noise threshold sufficient for neutralization analysis, a signal at least tenfold above background is favoured but can be lowered to a minimum of 5 for clinical isolates [30, 51]. Perhaps owing to its flexibility, several studies have demonstrated the HCVpp system is amenable to optimization. Urbanowicz, Tarr and colleagues have shown infectivity for some strains in the HCVpp system partly depends on the choice of retroviral background and the stoichiometry of transfected plasmids [34]. More recently, it has been shown that the cellular co-expression of the HCV p7NS2 open reading frame in producer cells enhances pseudoparticle infectivity for the H77 strain [52]. Others have also proposed that the inclusion of an intact HCV Core at the N-terminal of the glycoprotein plasmid is required for efficient pseudoparticle morphogenesis for some strains [53]. Nonetheless, it is now generally recognized that the inclusion of the last 21 residues of the Core/E1 peptide is sufficient for the vast majority of clones. Here, we disclose that a 293T cell lineage genetically edited to not express CD81 improves or rescues the infectivity of a wide range of HCVpp strains.
Producing HCVpp in 293TCD81KO cells is a facile method for improving HCVpp infectivity as it does not require empirical optimization of the plasmid ratios or co-expression of additional HCV genome elements. Unlike Soares and colleagues, we demonstrate an increase for the infectivities of a broad range of strains representing multiple genotypes [52]. Furthermore, producing HCVpp in 293TCD81KO cells had a more dramatic effect on viral infectivity compared to plasmid ratio optimization [34]. Moreover, none of the 45 clones became non-infectious after production in 293TCD81KO cells (see Table S1). Therefore, we would recommend the routine adoption of this cell system for the generation of HCVpp and are happy to provide this cell line to any interested investigators.
We are yet to uncover the mechanism by which the presence of CD81 in the 293-T cells influences the infectivity of HCV. However, the correlation between HCVpp sCD81 neutralization sensitivity and the fold increase in infectivity following production 293TCD81KO cells (Fig. 4k) suggests that there is a direct interaction between CD81 produced by regular 293T cells and HCVpp. There are several points during HCVpp production where E2 could encounter its cognate binding partner. The E1E2 glycoprotein and CD81 undergo glycosylation and palmitoylation in the Golgi compartment, respectively. When co-transfected, CD81 associates with E1E2 in the endoplasmic reticulum (ER) and influences the glycoprotein’s maturation through the Golgi membranes [54]. This association redirects the glycoprotein toward the endocytic pathway culminating in its incorporation into exosomes possibly reducing the amount of E1E2 available at the cell surface, where retroviral budding occurs. CD81 is also incorporated into retroviral pseudoparticles and its deletion possibly reduces protein density in lipid rafts at the cell membrane thereby permitting for increased E1E2 incorporation into budding HCVpp. However, glycoprotein incorporation is a poor predictor of HCVpp infectivity as others have shown that the majority of E2 in the supernatant of cells producing pseudoparticles does not sediment with infectious particles [55]. Additionally, as released virions in suspension will behave as colloidal matter [56] it is highly probable that many particles contact the cell surface and are retained or fated to non-productive uptake since 293T cells do not express the additional HCV entry factor claudin [28, 29].
Our findings somewhat contrast a recent report by Soares and colleagues who concluded that CD81 was required to generate HCVpp [57]. This is likely because Soares et al. did not perform infections with the HCVpp they generated from cells silenced for CD81 expression and instead relied on the quantities of E2 in viral harvests as their gauge. Furthermore, as mentioned above, E2 incorporation is a poor predictor of HCVpp infectivity [55] aand the observed decrease in E2 in their HCVpp harvests following CD81 silencing is more likely a reflection of reduced E2 incorporation into exosomes since CD81 chaperons E2 into the endocytic pathway, from where exosomes are biosynthesized [54].
The infectivities of VSV and MERS pseudoparticles were unaltered when made in 293TCD81KO cells, ruling out a general enhancement of pseudoparticle production; however, SARS-CoV-1/2pp infection was increased by ablation of CD81. A role for CD81 in the life cycles of the SARS-CoVs is yet to be identified, nonetheless, the related tetraspanin CD9 influences MERS entry by scaffolding dipeptidyl peptidase 4 (DPP4) and type II transmembrane serine protease (TTSP), the cell receptor and CoV activating protease used by MERS, respectively [58, 59]. CD81 may play an analogous role during SARS-CoV 1 and 2 entry by scaffolding their receptor angiotensin-converting enzyme 2 (ACE2) [60, 61]. Thus, CD81 deletion may reduce 293T-surface ACE2 or host CoV protease which, in turn, may reduce both pseudoparticle retention and non-productive uptake, as discussed for HCVpp above. Work to ensure SARS-CoV-1/2pp produced in 293TCD81KO cells are similar to their counterparts made in parental 293Ts and the authentic full-length virus is ongoing.
Considering CD81 ablation evidently improves the production of HCVpp, it is surprising we did not observe an increase in the amount sE2 recovered from susp-293TCD81KO. This may be due to the absence of the signal peptide at the intersection of core and E1, which retains E1E2 in the ER lumen, where virion morphogenesis would normally commence in full-length virus [62]. Therefore, it is plausible that, unlike the E1E2 heterodimer, sE2 has reduced opportunity for interaction with CD81 as it matures through the Golgi membranes.
The observation that sE2 made in susp-293T and 293-F cells is differentially glycosylated was unexpected and requires further scrutiny. EndoH digestion indicated that some E2 glycans remain shielded from Golgi glycosidases and glycosyltransferases in 293Ts, whereas a much higher proportion of total sE2 efficiently matured through the Golgi apparatus when produced in 293-Fs. Differential glycosylation patterns for the same viral glycoprotein have been demonstrated in cell lines from different species but not in cell lines sharing an immediate predecessor, as 293Ts and 293-Fs do [33, 45, 63]. There are likely many differences between the two lines; however, the most obvious are that 293-Fs are usually grown in suspension and do not express Simian virus large T 40 antigen. We adapted the 293TCD81KO to suspension thus this cannot explain the difference in glycosylation. The SV40 origin of replication is present on the H77 sE2 expression vector (pPPI4) we used [64]; however, it is highly improbable that an improvement in plasmid stability would affect the distal process of sE2 glycosylation. A recent omics study of the HEK 293 line and its progeny not only revealed transcriptome profile differences between adherent 293T cells and 293-F cells but also between 293H cells adapted to suspension and 293-F cells [65]. This indicates genes other than those involved in the adherent to suspension transition are also differentially expressed in 293 progeny cell lines. Hence, we suspect that some genetic difference(s) between 293-F and 293T cells ultimately underlies our observation.
The observed disparity in the antigenicity between 293T- and 293-F-produced sE2 is probably a direct result of the difference in glycosylation pattern between the two. Glycans, directly and indirectly, influence E1E2 folding through their interactions with ER chaperones such as calnexin [66, 67]. Several E2 glycans have been shown to play an important role in folding and E1 and E2 heterodimerization [68]. Furthermore, it is well documented that glycans protect conserved viral glycoprotein epitopes, including those on E1E2, from recognition by neutralizing antibodies through a phenomenon termed glycan shielding [67, 69, 70]. Our deglycosylation experiments indicate that 293-F-produced sE2 molecules harbour mostly complex glycans, while susp-293TCD81KO-produced sE2 also contain high-mannose or hybrid glycans. Complex glycans are usually larger and this could explain why some epitopes on 293-F-produced E2 seem to be less accessible for most mAbs and CD81 as measured by ELISA and BLI (Figs. 6 and 7). Our results suggest that apparently highly similar 293-derived cell lines can produce glycoproteins with different glycan species. Site-specific glycan analysis [45, 71] is needed to more thoroughly compare the difference in glycosylation between two lines and its effect on antigenicity.
Deleting one of the main receptors of HCV from producer cells significantly increased HCVpp infectivity. It is plausible that similar receptor-deleting strategies could be applied to increase the recovered infectious titres of other pseudotyped viruses. However, one should always carefully consider the biological role of these receptors. For example, integrins and lipoprotein receptors are targeted by a range of viruses, but integrins also play a significant role in maintaining cell integrity and cell-cycle progression, while many lipoprotein receptors are essential for proper cholesterol homeostasis [72, 73]. Disruption of either cellular processes would likely reduce infectious titres. Therefore, yielded viruses must be phenotyped for cell-entry pathway and antibody sensitivity to ensure there are no disparities with pseudoparticles obtained from the parental lineage, as we have done here.
In summary, here we have demonstrated that the presence of the HCV receptor CD81 in producer 293T cells used for HCVpp production has an effect on recoverable titres. We proceeded to generate a new 293T cell line lacking CD81 that substantially improved infectivity for most clinical HCVpp isolates. Crucially, these HCVpp are functionally similar to those made in conventional 293Ts. This cell line can easily be generated through CRISPR-Cas9 gene-editing but is also readily available from us. Furthermore, we disclose differences in the glycosylation patterns and antigenicity of sE2 produced in 293-F and 293T cells; this could have important implications for functional studies of E2 and for immunogen production.
Methods
Cell lines and culture
The receptor knock-out Huh-7 cell lines were a kind gift from Professor Yoshiharu Matsuura (Osaka University). Huh-7 cells were acquired from Apath LLC. All cells were grown at 37 °C in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco) supplemented with 10 % foetal calf serum (FCS), 1 % non-essential amino acids and 1 % penicillin/ streptomycin (P/S) (DMEM/FCS). For soluble E2 production, 293T (ATCC) and 293TCD81KO cells were initially maintained in DMEM/FCS. For large-scale purifications, these cells were adapted to suspension culture by growing the cells in a mix of FreeStyle 293 Expression medium (Gibco) and DMEM/FCS and decreasing the concentration of DMEM/FCS at each passage. Finally, both cell lines were grown in pure FreeStyle medium. 293-F cells (Invitrogen) were maintained in FreeStyle 293 Expression. All suspension cell lines were maintained in a shaking incubator (125 r.p.m.) at 37 °C and 8 % CO2.
Antibody source table
|
Antibody |
Source |
DOI |
|---|---|---|
|
Goat anti-human IgG (HRP) |
Jackson Immunoresearch |
n/a |
|
Goat anti-mouse IgG (HRP) |
CST |
n/a |
|
2.131 (anti-CD81) |
Grove et al. 2017 [74] |
10.12688/wellcomeopenres.12058.1 |
|
AP33 |
Clayton R et al. 2002 [75] |
10.1128/JVI.76.15.7672–7682.2002 |
|
AR3B |
Law et al. 2009 [76] |
10.1038/nm1698 |
|
AR4A |
Giang et al. 2012 [41] |
10.1073/pnas.1114927109 |
|
AT12-009 |
Merat et al. 2016 [42] |
10.1371/journal.pone.0165047 |
|
CBH-4B |
Keck et al. 2004 [77] |
10.1128/JVI.78.17.9224–9232.2004 |
|
H77.16 |
Sabo et al. 2011 [78] |
10.1128/JVI.00586–11 |
|
HC84.26 |
Keck et al. 2012 [43] |
10.1371/journal.ppat.1002653 |
|
IGH505 |
Meunier et al. 2008 [44] |
10.1128/JVI.01872–07 |
Western blot
Two days prior to study, HEK 293T and Huh-7 cells+/-CD81 were seeded into a standard six-well plates at 2.5×105 cells ml−1. Cells were then lysed using a buffer containing 20 mM Tris-HCl, 135 mM NaCl, 1 % Triton-X 100 and 10 % glycerol. The samples were then run on a TruPage 4–12% gel under non-reducing conditions and transferred on to nitrocellulose membrane. The blots were blocked in PBS+2 % milk solution +0.1 % Tween-20 and then probed by serial incubation with anti-receptor antibodies and goat anti-mouse secondary conjugated to horseradish peroxidase (HRP). Chemiluminescence signal was then measured in a Chemidoc MP (Bio Rad).
Cell lines and culture
The receptor knock-out Huh-7 cell lines were a kind gift from Professor Yoshiharu Matsuura (Osaka University). Huh-7 cells were acquired from Apath LLC and Caco-2 from ATCC. All cells were grown at 37 °C in DMEM (Gibco) supplemented with 10 % FCS, 1 % non-essential amino acids and 1 % penicillin/streptomycin (P/S) (DMEM/FCS). For soluble E2 production, 293T (ATCC) and 293T 293TCD81KO cells were initially maintained in DMEM/FCS. For large-scale purifications, these cells were adapted to suspension culture by growing the cells in a mix of FreeStyle 293 Expression medium (Gibco) and DMEM/FCS and decreasing the concentration of DMEM/FCS at each passage. Finally, both cell lines were grown in pure FreeStyle medium. 293-F cells (Invitrogen) were maintained in FreeStyle 293 Expression. All suspension cell lines were maintained in a shaking incubator (125 r.p.m.) at 37 °C and 8 % CO2.
Generation of CD81 knock-out cell lines
CD81KO 293T cell lines were established by following the Invitrogen TrueGuide sgRNA CRISPRMAX transfection protocol. Briefly, 293T cells were first transfected with one of the following CD81 sgRNAs: CRISPR661076_CR, CRISPR661085_CR and CRISPR661093_CR (Thermo Fisher Scientific). Then, 72 h later, the cells were then sorted on a FACSAria II instrument (BD Biosciences) to obtain CD81-/- populations. Cells were then expanded for 48 h before dilution for single-cell cloning and expansion in a 96-well plate. Knock-out of CD81 was confirmed by flow cytometry and Western blot.
Generation of HCV pseudoparticles
To generate pseudoparticles, 293T cells were co-transfected with three plasmids: an HIV (pCMV-dR8.91) or MLV (phCMV-5349) packaging construct, a luciferase reporter plasmid and an expression vector encoding the appropriate viral glycoprotein. For all experiments, a non-enveloped control (empty plasmid) was employed as a negative control. Supernatants containing HCVpp were collected at 48 and 72 h.
Infection and neutralization assays
Huh-7 cells were seeded at 1.5×104 cells per well of a 96-well plate 24 h prior to the experiment; to infect cells were incubated with HCVpp supernatant. For neutralization studies, a volume of virus stock was incubated in triplicates with a dilution series of anti-E2 antibody or sCD81-LEL prior to infection. Virus-antibody or virus-sCD81-LEL mixtures were incubated for 1 h at 37 ˚C and were added to Huh-7 cells and infection was allowed to proceed for 72 h. For readout, the samples were lysed and assayed using the SteadyGlo reagent kit and a GloMax luminometer (Promega, USA). A no-envelope pseudovirus was used to calculate the signal-to-noise ratio. Data was analysed in GraphPad Prism 7.0 c.
Soluble E2 protein expression and purification
Strep-II-tagged soluble E2 (sE2) was expressed using the same transient transfection protocol in all three HEK293-based cell lines. The supernatant containing sE2 was harvested after 6 days and clarified using vacuum-filtration (0.2 µm) and sE2 was purified using Strep-TactinXT columns (IBA Life Sciences) by gravity flow (~0.5–1.0 ml supernatant/min). The eluted proteins were then concentrated, and buffer exchanged into Tris-buffered saline (TBS, pH 7.5) using Vivaspin 6, 10.000 MWCO PES (Sartorius). Finally, purified sE2 was fractionated using size-exclusion chromatography (SEC) (Superdex 200 Increase 10/300 GL, GE) and the fractions corresponding to E2 monomers were pooled and concentrated. The concentration of the monomer fraction was then determined by Nanodrop (Thermo Fisher Scientific) using theoretical molecular weight and extinction coefficient.
Blue native (BN-)PAGE and SDS-PAGE
BN-PAGE and SDS-PAGE analyses were performed as described elsewhere [79] with some modifications. Briefly, for BN-PAGE, 5 µg sE2 was mixed with PBS and 4× loading dye [a mix of 500 µl 20× MOPS Buffer (1M MOPS+1M Tris, pH 7.7), 1000 µl 100 % glycerol, 50 µl 5 % Coomassie Brilliant Blue G-250, 600 µl milli-Q] and directly loaded onto a 4–12 % Bis-Tris NuPAGE gel (Thermo Fisher Scientific). The gels were run for 1 h at 200 V at 4 °C using NativePAGE Running Buffer (Invitrogen). BN-PAGE gels were stained using the Colloidal Blue Staining Kit according to the manufacturer’s instructions (Life Technologies). For SDS PAGE, 5 µg of sE2 was mixed with loading dye (25 mM Tris, 192 mM Glycine, 20 % v/v glycerol, 4 % m/v SDS, 0.1 % v/v bromophenol blue in milli-Q water) and incubated at 95 °C for 10 min prior to loading on a 4–12 % Tris-Glycine gel (Invitrogen). For reducing SDS-PAGE, dithiothreitol (DTT; 100 mM) was included in the loading dye and loaded on a Novex 10–20 % Tris-Glycine gel (Thermo Fisher Scientific). Gels were run in a buffer containing 25 mM Tris, 192 mM glycine and 0.5 % SDS for 1 h at 200 V at 4 °C. Coomassie blue staining of SDS-PAGE gels was performed using the PageBlue Protein Staining Solution (Thermo Fisher Scientific).
Deglycosylation of purified E2 monomers
Purified sE2 monomers were treated with PNGase F or EndoH (New England BioLabs) following the manufacturer’s protocol. The samples were then run on a reducing Novex 10–20% Tris-Glycine gel (Thermo Fisher Scientific) and stained by Coomassie blue as described above. Hereafter, the samples from this same gel were transferred onto a nitrocellulose membrane. The blots were blocked in PBS+5% milk solution+0.1% Tween-20 and then probed by serial incubation with an anti-E2 antibody followed by a goat anti-human secondary conjugated to HRP. Chemiluminescence signal was then determined via the use of the Western Lightning Plus-ECL system (PerkinElmer).
ELISA
Purified Strep-II-tagged sE2 monomer (1.0 µg ml−1 in TBS) was coated for 2 h at room temperature on 96-well Strep-TactinXT coated microplates (IBA LifeSciences). Plates were washed with TBS twice before incubating with serially diluted mAbs and CD81-LEL-hFc (R and D systems) in casein blocking buffer (Thermo Fisher Scientific) for 90 min. After three washes with TBS, a 1 : 3000 dilution of HRP-labelled goat anti-human IgG (Jackson Immunoresearch) in casein blocking buffer was added for 45 min. After washing the plates five times with TBS +0.05 % Tween-20, plates were developed by adding develop solution [1 % 3,3′,5,5′-tetraethylbenzidine (Sigma-Aldrich), 0.01 % H2O2, 100 mM sodium acetate, 100 mM citric acid] and the reaction was stopped after 3 min by adding 0.8 M H2SO4. Absorbance was measured at 450 nm and data was visualized and analysed on GraphPad Prism.
Biolayer interferometry (BLI)
BLI assays were performed using the Octet K2 instrument (FortéBio). All assays were performed at 30 °C and with agitation set at 1000 r.p.m. Antibodies, CD81-LEL-hFc and sE2 samples were dissolved in running buffer [PBS pH 7.5 with 0.1 % bovine serum albumin (BSA) and 0.02 % Tween-20 in a volume of 250 µl/well. Antibodies (3.0 µg ml−1) and CD81-LEL-hFc (5.0 µg ml−1] were immobilized onto protein A biosensors (FortéBio, cat no. 18–5010) until a loading threshold of 1.0 nm was reached followed by a 30 s baseline measurement in the running buffer. Purified sE2 monomers were diluted to 250 nM and association and dissociation were measured for 300 s and 200 s, respectively. A well without E2 was used for background correction. Data was analysed and visualized in GraphPad Prism.
Statistical analysis
All statistical analysis and data fitting were performed on GraphPad Prism 8.3. Data were analysed using paired t-tests, Spearman’s correlation or curve fit F-test, and data were fitted using by linear regression, hyperbola of log (inhibitor) vs. response (four parameters) function or the sigmoidal curves where X is log(concentration) function. Please see figure legends for accompanying analyses.
Supplementary Data
Funding information
This work was funded by a Sir Henry Dale Fellowship to J.G. from the Wellcome Trust (wellcome.ac.uk) and the Royal Society (royalsociety.org) (grant number 107653/Z/15/Z). M.D.K. is funded by an MRC PhD studentship (mrc.ukri.org) and A.C. is funded by an AMC PhD Scholarship (amc.nl). This work was also supported by a Vici grant from the Netherlands Organization for Scientific Research (NOW). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Acknowledgements
We are grateful to Alexander Tarr and Jonathan Ball for providing E1E2 expression plasmids for HCVpp with the prefix UKNP. We also thank the Fondation Dormeur, Vaduz and Marit J van Gils for funding and acquiring the Octet K2 instrument.
Author contributions
Conceptualization: M.D.K., K.S., J.G. Formal analysis: M.D.K., J.C.P., A.C., A.U. Funding acquisition: R.A.B., J.S., J.G. Investigation: M.D.K., J.C.P., A.C., A.U. Methodology: R.A.B., K.S., J.G. Project administration: M.D.K., R.A.B., K.S., J.G. Resources: R.A.B., J.S., J.G. Supervision: R.A.B., K.S., J.G. Validation: R.A.B., K.S., J.G. Visualization: M.D.K., J.C.P., A.C., A.U. Writing – original draft: M.D.K. Writing – reviewing and editing: All authors
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: EndoH, endoglycosidase H; HCVpp, HCV pseudoparticles; HEK, human embryonic kidney cells; IC50, 50% inhibitory concentration; PNGase F, peptide: N-glycosidase F; sCD81-LEL, soluble CD81 large extracellular loop; S/N, signal-to-noise ratio; 293TCD81KO, CD81 knock-out 293T cells; UKNP, United Kingdom Nottingham Panel.
Three supplementary figures and one supplementary table are available with the online version of this article.
References
- 1.Suryaprasad AG, White JZ, Xu F, Eichler BA, Hamilton J, et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006-2012. Clin Infect Dis. 2014;59:1411–1419. doi: 10.1093/cid/ciu643. [DOI] [PubMed] [Google Scholar]
- 2.Ding Q, von Schaewen M, Ploss A. The impact of hepatitis C virus entry on viral tropism. Cell Host Microbe. 2014;16:562–568. doi: 10.1016/j.chom.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burton DR, Poignard P, Stanfield RL, Wilson IA. Broadly neutralizing antibodies present new prospects to counter highly antigenically diverse viruses. Science. 2012;337:183–186. doi: 10.1126/science.1225416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.deCamp A, Hraber P, Bailer RT, Seaman MS, Ochsenbauer C, et al. Global panel of HIV-1 env reference strains for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2014;88:2489–2507. doi: 10.1128/JVI.02853-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pietschmann T, Kaul A, Koutsoudakis G, Shavinskaya A, Kallis S, et al. Construction and characterization of infectious intragenotypic and intergenotypic hepatitis C virus chimeras. Proc Natl Acad Sci U S A. 2006;103:7408–7413. doi: 10.1073/pnas.0504877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartosch B, Dubuisson J, Cosset F-L. Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J Exp Med. 2003;197:633–642. doi: 10.1084/jem.20021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu M, Zhang J, Flint M, Logvinoff C, Cheng-Mayer C, et al. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc Natl Acad Sci U S A. 2003;100:7271–7276. doi: 10.1073/pnas.0832180100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong J, Gastaminza P, Cheng G, Kapadia S, Kato T, et al. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci USA. 2005;102:9294–9299. doi: 10.1073/pnas.0503596102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindenbach BD, Evans MJ, Syder AJ, Wölk B, Tellinghuisen TL, et al. Complete replication of hepatitis C virus in cell culture. Science. 2005;309:623–626. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- 10.Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riva L, Dubuisson J. Similarities and differences between HCV Pseudoparticle (HCVpp) and cell culture HCV (HCVcc) in the study of HCV. Methods in Molecular Biology. 2019:33–45. doi: 10.1007/978-1-4939-8976-8_2. [DOI] [PubMed] [Google Scholar]
- 12.Catanese MT, Dorner M. Advances in experimental systems to study hepatitis C virus in vitro and in vivo . Virology. 2015;479-480:221–233. doi: 10.1016/j.virol.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Bartosch B, Cosset F-L. Studying HCV cell entry with HCV pseudoparticles (HCVpp) Methods Mol Biol. 2009;510:279–293. doi: 10.1007/978-1-59745-394-3_21. [DOI] [PubMed] [Google Scholar]
- 14.Wasilewski LN, Ray SC, Bailey JR. Hepatitis C virus resistance to broadly neutralizing antibodies measured using replication-competent virus and pseudoparticles. J Gen Virol. 2016;97:2883–2893. doi: 10.1099/jgv.0.000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osburn WO, Snider AE, Wells BL, Latanich R, Bailey JR, et al. Clearance of hepatitis C infection is associated with the early appearance of broad neutralizing antibody responses. Hepatology. 2014;59:2140–2151. doi: 10.1002/hep.27013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merat SJ, Bru C, van de Berg D, Molenkamp R, Tarr AW, et al. Cross-genotype AR3-specific neutralizing antibodies confer long-term protection in injecting drug users after HCV clearance. J Hepatol. 2019;71:14–24. doi: 10.1016/j.jhep.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Kinchen VJ, Zahid MN, Flyak AI, Soliman MG, Learn GH, et al. Broadly neutralizing antibody mediated clearance of human hepatitis C virus infection. Cell Host Microbe. 2018;24:717–730.:e5. doi: 10.1016/j.chom.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, et al. Binding of hepatitis C virus to CD81. Science. 1998;282:938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- 19.Scarselli E, Ansuini H, Cerino R, Roccasecca RM, Acali S, et al. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. Embo J. 2002;21:5017–5025. doi: 10.1093/emboj/cdf529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whidby J, Mateu G, Scarborough H, Demeler B, Grakoui A, et al. Blocking hepatitis C virus infection with recombinant form of envelope protein 2 ectodomain. J Virol. 2009;83:11078–11089. doi: 10.1128/JVI.00800-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan AG, Whidby J, Miller MT, Scarborough H, Zatorski AV, et al. Structure of the core ectodomain of the hepatitis C virus envelope glycoprotein 2. Nature. 2014;509:381–384. doi: 10.1038/nature13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kong L, Giang E, Nieusma T, Kadam RU, Cogburn KE, et al. Hepatitis C virus E2 envelope glycoprotein core structure. Science. 2013;342:1090–1094. doi: 10.1126/science.1243876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalemera M, Mincheva D, Grove J, Illingworth CJR. Building a mechanistic mathematical model of hepatitis C virus entry. PLoS Comput Biol. 2019;15:e1006905. doi: 10.1371/journal.pcbi.1006905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mankowski MC, Kinchen VJ, Wasilewski LN, Flyak AI, Ray SC, et al. Synergistic anti-HCV broadly neutralizing human monoclonal antibodies with independent mechanisms. Proc Natl Acad Sci U S A. 2018;115:E82–E91. doi: 10.1073/pnas.1718441115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kong L, Lee DE, Kadam RU, Liu T, Giang E, et al. Structural flexibility at a major conserved antibody target on hepatitis C virus E2 antigen. Proc Natl Acad Sci U S A. 2016;113:12768–12773. doi: 10.1073/pnas.1609780113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vasiliauskaite I, Owsianka A, England P, Khan AG, Cole S, et al. Conformational flexibility in the immunoglobulin-like domain of the hepatitis C virus glycoprotein E2. mBio. 2017;8:e00382–17. doi: 10.1128/mBio.00382-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stejskal L, Lees WD, Moss DS, Palor M, Bingham RJ, et al. Flexibility and intrinsic disorder are conserved features of hepatitis C virus E2 glycoprotein. PLoS Comput Biol. 2020;16:e1007710. doi: 10.1371/journal.pcbi.1007710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans MJ, von Hahn T, Tscherne DM, Syder AJ, Panis M, et al. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446:801–805. doi: 10.1038/nature05654. [DOI] [PubMed] [Google Scholar]
- 29.Ploss A, Evans MJ, Gaysinskaya VA, Panis M, You H, et al. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature. 2009;457:882–886. doi: 10.1038/nature07684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker MR, Leung P, Eltahla AA, Underwood A, Abayasingam A, et al. Clearance of hepatitis C virus is associated with early and potent but narrowly-directed, envelope-specific antibodies. Sci Rep. 2019;9:13300. doi: 10.1038/s41598-019-49454-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sliepen K, Han BW, Bontjer I, Mooij P, Garces F, et al. Structure and immunogenicity of a stabilized HIV-1 envelope trimer based on a group-M consensus sequence. Nat Commun. 2019;10:2355. doi: 10.1038/s41467-019-10262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marín MQ, Sliepen K, García-Arriaza J, Koekkoek SM, Pérez P, et al. Optimized hepatitis C virus (HCV) E2 glycoproteins and their immunogenicity in combination with MVA-HCV. Vaccines. 2020;8:440.:E440. doi: 10.3390/vaccines8030440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Struwe WB, Chertova E, Allen JD, Seabright GE, Watanabe Y, et al. Site-Specific glycosylation of Virion-Derived HIV-1 env is mimicked by a soluble trimeric immunogen. Cell Rep. 2018;24:1958–1966. doi: 10.1016/j.celrep.2018.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urbanowicz RA, McClure CP, King B, Mason CP, Ball JK, et al. Novel functional hepatitis C virus glycoprotein isolates identified using an optimized viral pseudotype entry assay. J Gen Virol. 2016;97:2265–2279. doi: 10.1099/jgv.0.000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Urbanowicz RA, McClure CP, Brown RJP, Tsoleridis T, Persson MAA, et al. A diverse panel of hepatitis C virus glycoproteins for use in vaccine research reveals extremes of monoclonal antibody neutralization resistance. J Virol. 2015;90:3288–3301. doi: 10.1128/JVI.02700-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cunningham EB, Hajarizadeh B, Bretana NA, Amin J, Betz-Stablein B, et al. Ongoing incident hepatitis C virus infection among people with a history of injecting drug use in an Australian prison setting, 2005-2014: the HITS-p study. J Viral Hepat. 2017;24:733–741. doi: 10.1111/jvh.12701. [DOI] [PubMed] [Google Scholar]
- 37.Baktash Y, Madhav A, Coller KE, Randall G. Single particle imaging of polarized hepatoma organoids upon hepatitis C virus infection reveals an ordered and sequential entry process. Cell Host Microbe. 2018;23:382–394.:e5. doi: 10.1016/j.chom.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindenbach BD, Rice CM. The Ins and outs of hepatitis C virus entry and assembly. Nat Rev Microbiol. 2013;11:688–700. doi: 10.1038/nrmicro3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto S, Fukuhara T, Ono C, Uemura K, Kawachi Y, et al. Lipoprotein receptors redundantly participate in entry of hepatitis C virus. PLoS Pathog. 2016;12:e1005610. doi: 10.1371/journal.ppat.1005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lavillette D, Tarr AW, Voisset C, Donot P, Bartosch B, et al. Characterization of host-range and cell entry properties of the major genotypes and subtypes of hepatitis C virus. Hepatology. 2005;41:265–274. doi: 10.1002/hep.20542. [DOI] [PubMed] [Google Scholar]
- 41.Giang E, Dorner M, Prentoe JC, Dreux M, Evans MJ, et al. Human broadly neutralizing antibodies to the envelope glycoprotein complex of hepatitis C virus. [published correction appears in Proc Natl Acad Sci U S A. 2017 Dec 11] Proc Natl Acad Sci U S A. 2012;109:6205–6210. doi: 10.1073/pnas.1114927109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merat SJ, Molenkamp R, Wagner K, Koekkoek SM, van de Berg D, et al. Hepatitis C virus broadly neutralizing monoclonal antibodies isolated 25 years after spontaneous clearance. PLoS One. 2016;11:e0165047. doi: 10.1371/journal.pone.0165047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keck Z-yong, Xia J, Wang Y, Wang W, Krey T, et al. Human monoclonal antibodies to a novel cluster of conformational epitopes on HCV E2 with resistance to neutralization escape in a genotype 2A isolate. PLoS Pathog. 2012;8:e1002653. doi: 10.1371/journal.ppat.1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meunier J-C, Russell RS, Goossens V, Priem S, Walter H, et al. Isolation and characterization of broadly neutralizing human monoclonal antibodies to the E1 glycoprotein of hepatitis C virus. J Virol. 2008;82:966–973. doi: 10.1128/JVI.01872-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urbanowicz RA, Wang R, Schiel JE, Keck Z-Y, Kerzic MC, et al. Antigenicity and immunogenicity of differentially glycosylated hepatitis C virus E2 envelope proteins expressed in mammalian and insect cells. J Virol. 2019;93:e01403–01418. doi: 10.1128/JVI.01403-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vieyres G, Thomas X, Descamps V, Duverlie G, Patel AH, et al. Characterization of the envelope glycoproteins associated with infectious hepatitis C virus. J Virol. 2010;84:10159–10168. doi: 10.1128/JVI.01180-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pantua H, Diao J, Ultsch M, Hazen M, Mathieu M, et al. Glycan shifting on hepatitis C virus (HCV) E2 glycoprotein is a mechanism for escape from broadly neutralizing antibodies. J Mol Biol. 2013;425:1899–1914. doi: 10.1016/j.jmb.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 48.Hadlock KG, Lanford RE, Perkins S, Rowe J, Yang Q, et al. Human monoclonal antibodies that inhibit binding of hepatitis C virus E2 protein to CD81 and recognize conserved conformational epitopes. J Virol. 2000;74:10407–10416. doi: 10.1128/JVI.74.22.10407-10416.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McKeating JA, Zhang LQ, Logvinoff C, Flint M, Zhang J, et al. Diverse hepatitis C virus glycoproteins mediate viral infection in a CD81-dependent manner. J Virol. 2004;78:8496–8505. doi: 10.1128/JVI.78.16.8496-8505.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lavillette D, Pécheur E-I, Donot P, Fresquet J, Molle J, et al. Characterization of fusion determinants points to the involvement of three discrete regions of both E1 and E2 glycoproteins in the membrane fusion process of hepatitis C virus. J Virol. 2007;81:8752–8765. doi: 10.1128/JVI.02642-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bailey JR, Urbanowicz RA, Ball JK, Law M, Foung SKH. Standardized method for the study of antibody neutralization of HCV pseudoparticles (HCVpp) Methods Mol Biol. 2019;1911:441–450. doi: 10.1007/978-1-4939-8976-8_30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soares HR, Ferreira-Fernandes M, Almeida AI, Marchel M, Alves PM, et al. Enhancing hepatitis C virus pseudoparticles infectivity through p7NS2 cellular expression. J Virol Methods. 2019;274:113714. doi: 10.1016/j.jviromet.2019.113714. [DOI] [PubMed] [Google Scholar]
- 53.Shukla P, Faulk KN, Emerson SU. The entire core protein of HCV JFH1 is required for efficient formation of infectious JFH1 pseudoparticles. J Med Virol. 2010;82:783–790. doi: 10.1002/jmv.21660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Masciopinto F, Giovani C, Campagnoli S, Galli-Stampino L, Colombatto P, et al. Association of hepatitis C virus envelope proteins with exosomes. Eur J Immunol. 2004;34:2834–2842. doi: 10.1002/eji.200424887. [DOI] [PubMed] [Google Scholar]
- 55.Flint M, Logvinoff C, Rice CM, McKeating JA. Characterization of infectious retroviral pseudotype particles bearing hepatitis C virus glycoproteins. J Virol. 2004;78:6875–6882. doi: 10.1128/JVI.78.13.6875-6882.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andreadis S, Lavery T, Davis HE, Le Doux JM, Yarmush ML, et al. Toward a more accurate quantitation of the activity of recombinant retroviruses: alternatives to titer and multiplicity of infection. J Virol. 2000;74:1258–1266. doi: 10.1128/JVI.74.3.1258-1266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soares HR, Castro R, Tomás HA, Carrondo MJT, Alves PM, et al. Pseudotyping retrovirus like particles vaccine candidates with hepatitis C virus envelope protein E2 requires the cellular expression of CD81. AMB Express. 2019;9:22. doi: 10.1186/s13568-019-0741-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Earnest JT, Hantak MP, Li K, McCray PB, Perlman S, et al. The tetraspanin CD9 facilitates MERS-coronavirus entry by scaffolding host cell receptors and proteases. PLoS Pathog. 2017;13:e1006546. doi: 10.1371/journal.ppat.1006546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Earnest JT, Hantak MP, Park JE, Gallagher T. Coronavirus and influenza virus proteolytic priming takes place in tetraspanin-enriched membrane microdomains. J Virol. 2015;89:6093–6104. doi: 10.1128/JVI.00543-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coller KE, Heaton NS, Berger KL, Cooper JD, Saunders JL, et al. Molecular determinants and dynamics of hepatitis C virus secretion. PLoS Pathog. 2012;8:e1002466. doi: 10.1371/journal.ppat.1002466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raska M, Takahashi K, Czernekova L, Zachova K, Hall S, et al. Glycosylation patterns of HIV-1 gp120 depend on the type of expressing cells and affect antibody recognition. J Biol Chem. 2010;285:20860–20869. doi: 10.1074/jbc.M109.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Binley JM, Sanders RW, Clas B, Schuelke N, Master A, et al. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J Virol. 2000;74:627–643. doi: 10.1128/JVI.74.2.627-643.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malm M, Saghaleyni R, Lundqvist M, Giudici M, Chotteau V, et al. Evolution from adherent to suspension – systems biology of HEK293 cell line development. [DOI] [PMC free article] [PubMed]
- 66.Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291:2364–2369. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- 67.Lavie M, Hanoulle X, Dubuisson J. Glycan shielding and modulation of hepatitis C virus neutralizing antibodies. Front Immunol. 2018;9:910. doi: 10.3389/fimmu.2018.00910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goffard A, Callens N, Bartosch B, Wychowski C, Cosset F-L, et al. Role of N-linked glycans in the functions of hepatitis C virus envelope glycoproteins. J Virol. 2005;79:8400–8409. doi: 10.1128/JVI.79.13.8400-8409.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crispin M, Ward AB, Wilson IA. Structure and immune recognition of the HIV glycan shield. Annu Rev Biophys. 2018;47:499–523. doi: 10.1146/annurev-biophys-060414-034156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kobayashi Y, Suzuki Y. Evidence for N-glycan shielding of antigenic sites during evolution of human influenza A virus hemagglutinin. J Virol. 2012;86:3446–3451. doi: 10.1128/JVI.06147-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Behrens A-J, Vasiljevic S, Pritchard LK, Harvey DJ, Andev RS, et al. Composition and antigenic effects of individual glycan sites of a trimeric HIV-1 envelope glycoprotein. Cell Rep. 2016;14:2695–2706. doi: 10.1016/j.celrep.2016.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mahley RW, Innerarity TL. Lipoprotein receptors and cholesterol homeostasis. Biochim Biophys Acta. 1983;737:197–222. doi: 10.1016/0304-4157(83)90001-1. [DOI] [PubMed] [Google Scholar]
- 73.Streuli CH. Integrins and cell-fate determination. J Cell Sci. 2009;122:171–177. doi: 10.1242/jcs.018945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grove J, Hu K, Farquhar MJ, Goodall M, Walker L, et al. A new panel of epitope mapped monoclonal antibodies recognising the prototypical tetraspanin CD81. Wellcome Open Res. 2017;2:82. doi: 10.12688/wellcomeopenres.12058.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clayton RF, Owsianka A, Aitken J, Graham S, Bhella D, et al. Analysis of antigenicity and topology of E2 glycoprotein present on recombinant hepatitis C virus-like particles. J Virol. 2002;76:7672–7682. doi: 10.1128/JVI.76.15.7672-7682.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Law M, Maruyama T, Lewis J, Giang E, Tarr AW, et al. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat Med. 2008;14:25–27. doi: 10.1038/nm1698. [DOI] [PubMed] [Google Scholar]
- 77.Keck Z-Y, Op De Beeck A, Hadlock KG, Xia J, Li TK, et al. Hepatitis C virus E2 has three immunogenic domains containing conformational epitopes with distinct properties and biological functions. J Virol. 2004;78:9224–9232. doi: 10.1128/JVI.78.17.9224-9232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sabo MC, Luca VC, Prentoe J, Hopcraft SE, Blight KJ, et al. Neutralizing monoclonal antibodies against hepatitis C virus E2 protein bind discontinuous epitopes and inhibit infection at a postattachment step. J Virol. 2011;85:7005–7019. doi: 10.1128/JVI.00586-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Taeye SW, Ozorowski G, Torrents de la Peña A, Guttman M, Julien J-P, et al. Immunogenicity of stabilized HIV-1 envelope trimers with reduced exposure of non-neutralizing epitopes. Cell. 2015;163:1702–1715. doi: 10.1016/j.cell.2015.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.