Abstract
Introduction
We aimed to assess episodic memory in genetic frontotemporal dementia (FTD) with the Free and Cued Selective Reminding Test (FCSRT).
Methods
The FCSRT was administered in 417 presymptomatic and symptomatic mutation carriers (181 chromosome 9 open reading frame 72 [C9orf72], 163 progranulin [GRN], and 73 microtubule‐associated protein tau [MAPT]) and 290 controls. Group differences and correlations with other neuropsychological tests were examined. We performed voxel‐based morphometry to investigate the underlying neural substrates of the FCSRT.
Results
All symptomatic mutation carrier groups and presymptomatic MAPT mutation carriers performed significantly worse on all FCSRT scores compared to controls. In the presymptomatic C9orf72 group, deficits were found on all scores except for the delayed total recall task, while no deficits were found in presymptomatic GRN mutation carriers. Performance on the FCSRT correlated with executive function, particularly in C9orf72 mutation carriers, but also with memory and naming tasks in the MAPT group. FCSRT performance also correlated with gray matter volumes of frontal, temporal, and subcortical regions in C9orf72 and GRN, but mainly temporal areas in MAPT mutation carriers.
Discussion
The FCSRT detects presymptomatic deficits in C9orf72‐ and MAPT‐associated FTD and provides important insight into the underlying cause of memory impairment in different forms of FTD.
Keywords: cognition, episodic memory, executive function, frontal lobe, frontotemporal dementia, genetic disorders, neuropsychology, temporal lobe, voxel‐based morphometry
1. BACKGROUND
Memory deficits are often considered indicative of the onset of Alzheimer's disease (AD), but an increasing number of studies have reported episodic memory impairment in the frontotemporal dementia (FTD) 1 , 2 spectrum as well, even at initial presentation. 3 There is ongoing discussion on what underlies episodic memory impairment in FTD, with some studies suggesting that it may be a consequence of poor organization and a lack of efficient learning and retrieval strategies (i.e., due to a dysexecutive syndrome caused by [pre]frontal cortical damage) and others suggesting that it is due to “true” consolidation problems, as is the case in AD, as a result of damage to mesiotemporal, including hippocampal, structures of the brain. 4 , 5 , 6 , 7
Delineating the contribution of executive/frontal and memory/hippocampal functioning to memory impairment can be performed using memory tests that separate learning, storage, and retrieval processes. The Free and Cued Selective Reminding Test (FCSRT) was designed specifically for this purpose. 8 The FCSRT uses semantic cues to, first, test if words were effectively encoded, and, second, facilitate subsequent cued recall of words that were not spontaneously retrieved during free recall. Specifically, the performance on cued recall is assumed to provide a measure of “true” memory consolidation, while performance on free recall also relies on executive functioning as it requires people to apply an effective learning and retrieval strategy. 5 Some studies have shown that this paradigm is effective in differentiating behavioral variant FTD (bvFTD) from AD, 6 , 7 , 9 , 10 , 11 , 12 while others have failed to show this distinction, or showed that the FTD sample could be split, with approximately half of the patients performing as poorly as patients with AD and the other half performing similarly to healthy controls. 6 , 11 , 13 Indeed, several neuroimaging studies have shown differences in temporal lobe involvement between amnesic and non‐amnesic patients with FTD, 4 , 11 , 14 , 15 underlining the pathological and clinical heterogeneity of this disease spectrum.
In approximately 30% of cases, FTD is caused by genetic mutations in progranulin (GRN), microtubule‐associated protein tau (MAPT), and chromosome 9 open reading frame 72 (C9orf72). 16 GRN mutations often lead to an asymmetrical pattern of atrophy in the frontal, temporal, and parietal lobes, whereas MAPT mutations show localized temporal lobe involvement. 17 The atrophy associated with the C9orf72 repeat expansion is rather diffuse with degeneration of the frontal and temporal cortices but also involvement of the subcortical and cerebellar regions. 18 Memory impairment has been described in GRN 19 , 20 and C9orf72 18 mutation carriers as a prominent symptom of later disease stages, whereas in MAPT mutation carriers memory decline has been previously described in the presymptomatic stage. 21 A recent study has shown that patients with a GRN mutation or C9orf72 repeat expansion were impaired on immediate recall, whereas MAPT mutation carriers were impaired on both immediate and delayed recall. According to the classic view, this suggests a “pure” memory impairment due to temporal involvement in MAPT, whereas the immediate recall impairment in C9orf72 and GRN mutation carriers are potentially a consequence of prefrontal and thus executive dysfunction, with relatively spared delayed recall performance. 22 However, systematic investigations of episodic memory performance using paradigms that can differentiate between primary executive versus true amnestic mechanisms have not been performed in detail in genetic FTD, and in particular, not in the presymptomatic stage. Clinical trials targeting specific pathologies are currently being developed and implemented for both early symptomatic and presymptomatic mutation carriers and it is important to identify gene‐specific sensitive outcome measures for signaling disease onset, tracking disease progression, and measuring potential treatment effects at an early disease stage.
The aim of this study is therefore to assess memory performance in a large cohort of genetic FTD families by means of the FCSRT and correlate performance with gray matter volume using voxel‐based morphometry. We compared both presymptomatic individuals and those with symptomatic FTD with pathogenic mutations in MAPT, GRN, or C9orf72 to a control group of mutation‐negative individuals from the same families. Data was collected within the Genetic Frontotemporal Dementia Initiative (GENFI), an international genetic FTD cohort study aimed at developing novel markers of disease onset and progression. 23
2. METHODS
2.1. Participants
Baseline data was included from the fifth GENFI data freeze in which participants from confirmed genetic FTD families were recruited between January 30, 2012 and May 31, 2019 in 24 centers. The FCSRT was administered in a total of 417 mutation carriers (181 C9orf72, 163 GRN, and 73 MAPT) and 290 mutation negative controls. Of the mutation carrier group 96 participants were symptomatic, fulfilling diagnostic criteria for bvFTD 1 (44 C9orf72, 19 GRN, 17 MAPT), non‐fluent variant primary progressive aphasia (nfvPPA; 2 1 C9orf72, 8 GRN), or FTD with amyotrophic lateral sclerosis (FTD‐ALS; 24 4 C9orf72). The presymptomatic mutation carrier group did not fulfill these diagnostic criteria, had a Clinical Dementia Rating scale plus behavioral and language domains from the National Alzheimer's Coordinating Center (NACC) FTLD module (CDR plus NACC FTLD) ≤0.5 25 and consisted of 129 C9orf72 repeat expansion, 136 GRN, and 56 MAPT mutation carriers. There were 352 mutation carriers with an FCSRT at baseline that also had a structural (T1‐weighted) magnetic resonance imaging (MRI) brain scan (148 C9orf72, 139 GRN, and 65 MAPT mutation carriers). All GENFI sites had local ethical approval for the study and all participants gave written informed consent. The study was in accordance with the Declaration of Helsinki.
2.2. Procedure
We administered the Mini‐Mental State Examination (MMSE) 26 to measure global cognitive functioning and determined clinical status by means of a structured clinical interview, including the CDR plus NACC FTLD, 25 with the participant and a knowledgable informant. The FCSRT was administered as part of the GENFI neuropsychological test battery. 23 From this test battery we also collected data on visual episodic memory (Benson figure recall), language (30‐item Boston Naming Test [BNT] 27 and category fluency 27 ), and executive function tests (Trail Making Test part B [TMT‐B 28 ] and the Delis–Kaplan Executive Function System Color‐Word Interference Test [D‐KEFS Color‐Word] card III 29 ) to correlate with FCSRT performance. The test battery was administered in the same order to all participants and no semantic tests were administered during the delay phase of the FCSRT.
HIGHLIGHTS
The Free and Cued Selective Reminding Test (FCSRT) is able to detect presymptomatic episodic memory impairment in both chromosome 9 open reading frame 72 (C9orf72)‐ and microtubule‐associated protein tau [MAPT]‐associated frontotemporal dementia (FTD).
Deficits in presymptomatic MAPT mutation carriers are likely to be due to “true” episodic memory deficits.
Impaired memory performance in progranulin (GRN)‐ and C9orf72‐associated FTD is likely to be mainly related to executive dysfunction.
FCSRT performance is associated with temporal lobe regions in MAPT‐associated FTD, with additional frontal lobe involvement in GRN‐ and C9orf72‐associated FTD.
The FCSRT provides insight into the underlying cause of memory impairment in FTD.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using PubMed. While episodic memory functioning has not been investigated systematically in (presymptomatic) genetic frontotemporal dementia (FTD), there have been several publications describing neuropsychological test results, including memory, in genetic FTD. Relevant citations are cited.
Interpretation: Our findings demonstrate that memory deficits are an integral part of the clinical spectrum in microtubule‐associated protein tau (MAPT) mutation carriers, whereas lower memory test scores in chromosome 9 open reading frame 72 (C9orf72) repeat expansion and progranulin (GRN) mutation carriers are more likely to be the consequence of executive dysfunction. These results are consistent with previous studies showing degradation of memory‐related temporal areas in MAPT‐associated FTD, and more executive function‐related frontal areas being implicated in GRN‐ and C9orf72‐associated FTD.
Future directions: Results from this study provide new insights and guidance for additional studies, such as investigating longitudinal trajectories of the FCSRT in genetic FTD as well as investigating the sensitivity of the FCSRT as a potential outcome measure for upcoming clinical trials.
2.3. Free and Cued Selective Reminding Test (FCSRT)
The FCSRT consists of 16 words to be learned, presented four at a time on successive cards. Each word belongs to a different semantic category (e.g., herring in the semantic category “fish”). The first presentation is aimed at inducing semantic encoding, for which subjects are asked to read aloud the word corresponding to a specific semantic category (e.g., “What is the name of the fish?”). After all four items are named, the card is removed and the test administrator asks for immediate recall of the four words in response to the semantic cue. This procedure of encoding is repeated a maximum of three times, until the participant is able to recall all four words or has completed the third round, after which the following card is administered and this encoding process is then repeated for the second, third, and fourth cards. Subsequently, three successive trials of free recall are administered, where participants are asked to remember as many of the 16 words as possible within two minutes. Each free recall trial is followed by a selective semantic cuing of the words that are not spontaneously recalled. After 20 to 30 minutes, a delayed free recall and then cued recall of words not spontaneously recalled is administered. This results in four scores to be analyzed: immediate free recall (max. score = 48), immediate total recall (free+cued; max. score = 48), delayed free recall (max. score = 16), delayed total recall (delayed free+cued; max. score = 16). The test was administered across the GENFI centers in eight languages: English, Dutch, Swedish, Spanish, Italian, Portuguese, German, and French.
2.4. Structural brain imaging and voxel‐based morphometry
Participants underwent volumetric T1‐weighted MRI according to the GENFI imaging protocol on a 3T scanner. Different scanners were used across GENFI sites: Siemens Trio 3T (n = 105), Siemens Skyra 3T (n = 55), Siemens Prisma 3T (n = 57), and Philips Achieva 3T (n = 101). All scans underwent extensive visual quality checke and those with artifacts or incidental brain abnormalities unrelated to FTD were excluded from analysis. Voxel‐based morphometry (VBM) was performed using Statistical Parametric Mapping (SPM) 12 software, version 6225 (www.fil.ion.ucl.ac.uk/spm) running under Matlab R2018a (Mathworks). T1‐weighted images were normalized and segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) probability maps, using standard procedures and the fast diffeomorphic image registration algorithm (DARTEL). 30 GM segmentations were affine transformed into the Montreal Neurological Institute (MNI) space, modulated and smoothed using a Gaussian kernel with 6 mm full width at half maximum. Finally, a mask was applied as reported in Ridgway et al. 31 All segmentations were visually checked at each stage. Total intracranial volume (TIV; i.e., GM+WM+CSF) was calculated using SPM 12. 32
2.5. Statistical analysis
Statistical analyses were performed using Stata version 14 (StataCorp). The significance level was set at P < 0.05 (two‐tailed) across all comparisons. We compared demographic data between groups with linear regression models except for sex, which was compared using a chi‐square test.
Performance in controls was assessed by calculating the cumulative frequency of test scores (and therefore percentile scores) as well as investigating the effect of age (Spearman rank correlation), years of education (Spearman rank correlation), sex (Mann–Whitney U test), and the language in which the test was administered (Kruskal–Wallis H test).
Mean differences on each FCSRT score between groups were analyzed with mixed models correcting for age, years of education, sex, language in which the test was administered, and family clustering with 95% bias‐corrected bootstrapped confidence intervals with 1000s repetitions (due to non‐normality).
Spearman rank correlations were used to investigate the association of each FCSRT test score with the Benson figure recall, BNT, category fluency, TMT‐B, and D‐KEFS Color‐Word tasks.
The relationship of performance on each FCSRT test score with GM density was explored in each mutation carrier (presymptomatic and symptomatic combined) group within the VBM analysis using multiple regression models. Age, sex, scanner, and TIV were included as covariates. All comparisons were corrected for a family‐wise error (FWE) rate of 0.05.
3. RESULTS
3.1. Demographic data
Demographic data are shown in Table 1. There was a significant difference in sex between the groups, X2(6, N = 707) = 16.8, P = 0.010, with more females in the presymptomatic and control group and more males in the symptomatic groups. Symptomatic groups were significantly older than controls and presymptomatic groups (all P < 0.001). In addition, presymptomatic MAPT mutation carriers were significantly younger than controls (P < 0.001), presymptomatic C9orf72 (P = 0.009), and GRN mutation carriers (P = 0.001). Symptomatic C9orf72 and GRN mutation carriers had significantly lower years of education than controls and presymptomatic C9orf72, GRN, and MAPT mutation carriers (all P < 0.013). All symptomatic mutation carriers performed significantly lower on the MMSE and had higher CDR plus NACC FTLD global scores than controls and presymptomatic C9orf72, GRN, and MAPT mutation carriers (all P < 0.005). In addition, symptomatic GRN mutation carriers had lower MMSE scores than symptomatic C9orf72 and MAPT mutation carriers (both P < 0.003) and symptomatic C9orf72 mutation carriers had higher CDR plus NACC FTLD global scores than symptomatic MAPT mutation carriers (P = 0.028).
TABLE 1.
Demographic information and FCSRT scores
| C9orf72 | GRN | MAPT | Controls | ||||
|---|---|---|---|---|---|---|---|
| PS | S | PS | S | PS | S | – | |
| n | 129 | 52 | 136 | 27 | 56 | 17 | 290 |
|
Age, y [range] |
44.6 ± 11.1 [20.1–69.3] |
62.0 ± 7.6 [39.4–74.5] |
46.1 ± 12.4 [20.2–75.5] |
60.8 ± 7.9 [49.2–78.5] |
39.8 ± 10.5 [20.6–74.1] |
58.6 ± 6.8 [44.0–78.9] |
45.9 ± 12.6 [19.5–82.3] |
| Sex ratio f:m | 77:52 | 19:33 | 84:52 | 10:17 | 34:22 | 7:10 | 167:123 |
| Education, y | 14.4 ± 3.0 | 12.8 ± 3.3 | 14.7 ± 3.5 | 12.0 ± 3.5 | 14.5 ± 3.0 | 14.5 ± 3.9 | 14.6 ± 3.4 |
| MMSE | 29.0 ± 2.1 | 25.3 ± 3.9 | 28.7 ± 4.6 | 22.9 ± 6.8 | 29.5 ± 0.9 | 26.2 ± 3.1 | 29.3 ± 2.1 |
| CDR plus NACC FTLD global | 0.1 ± 0.3 | 1.9 ± 1.0 | 0.1 ± 0.3 | 1.8 ± 0.9 | 0.1 ± 0.3 | 1.6 ± 0.9 | 0.1 ± 0.2 |
| FCSRT immediate free recall | 28.8 ± 7.1 | 13.9 ± 8.4 | 31.2 ± 6.2 | 13.8 ± 12.5 | 31.6 ± 7.0 | 12.8 ± 10.2 | 31.5 ± 6.8 |
| FCSRT immediate total recall | 44.4 ± 5.4 | 34.2 ± 13.1 | 45.8 ± 2.5 | 26.4 ± 17.5 | 45.3 ± 4.6 | 29.7 ± 13.1 | 45.7 ± 3.5 |
| FCSRT delayed free recall | 11.0 ± 2.9 | 4.7 ± 3.5 | 11.9 ± 2.8 | 5.2 ± 4.7 | 12.0 ± 3.1 | 4.5 ± 4.7 | 12.0 ± 3.1 |
| FCSRT delayed total recall | 15.3 ± 1.4 | 11.5 ± 4.7 | 15.5 ± 0.9 | 10.0 ± 6.3 | 15.3 ± 1.8 | 10.3 ± 4.9 | 15.5 ± 1.2 |
Note: All data are shown as mean ± standard deviation.
Abbreviations: C9orf72, chromosome 9 open reading frame 72; CDR plus NACC FTLD global, Clinical Dementia Rating scale plus National Alzheimer's Coordinating Center Frontotemporal Lobar Degeneration global score; FCSRT, Free and Cued Selective Reminding Test; GRN, progranulin; MAPT, microtubule‐associated protein tau; MMSE, Mini‐Mental State Examination; PS, presymptomatic; S, symptomatic.
3.2. Normative data in the control population
Cumulative frequencies (Table A.1), percentile scores (Table A.2), and mean score stratified by age group and sex (Table A.3) for mutation negative controls can be found in the supporting information. Fifthpercentile cut‐off scores were 19 (immediate free), 40 (immediate total), 7 (delayed free), and 13 (delayed total) for each of the FCSRT scores (Table A.2). There was a weak negative correlation with age (r between –0.14 and –0.36) and a weak positive correlation with years of education (r between 0.16 and 0.22) for each FCSRT score. Females performed better than males on all parts of the FCSRT: immediate free recall (z = 3.6, P < 0.001), immediate total recall (z = 2.6, P = 0.010), delayed free recall (z = 4.4, P < 0.001), and delayed total recall (z = 3.1, P = 0.002). There was also a significant effect of language on FCSRT immediate free recall (H[7] = 24.3, P = 0.001), immediate total recall (H[7] = 26.6, P < 0.001), and delayed free recall (H[7] = 25.9, P < 0.001) but not delayed total recall (H[7] = 11.3, P = 0.127).
3.3. Group comparisons
All three symptomatic mutation carrier groups performed significantly worse than controls on FCSRT immediate free recall, immediate total recall, delayed free recall, and delayed total recall (all P ≤ 0.001; Tables 1 and 2). In addition, symptomatic GRN mutation carriers performed significantly worse on the FCSRT immediate total score than symptomatic C9orf72 repeat expansion carriers (P = 0.047). All symptomatic mutation carriers performed significantly worse than presymptomatic mutation carriers (all P ≤ 0.004).
TABLE 2.
The adjusted mean differences between groups and 95% confidence intervals for all four FCSRT measures
| FCSRT immediate free recall | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C9orf72 | GRN | MAPT | |||||||||||
| PS | S | PS | S | PS | S | ||||||||
| Controls | –2.9 | –12.5 | 0.4 | –11.7 | –2.4 | –15.7 | |||||||
| –4.1 | –1.7 | –14.8 | –10.2 | –0.8 | 1.6 | –16.3 | –7.1 | –4.0 | –0.7 | –20.8 | –10.6 | ||
| C9orf72 | PS | ‐9.6 | 3.3 | –8.8 | 0.5 | –12.8 | |||||||
| –12.0 | –7.2 | 1.8 | 4.8 | –13.6 | –4.0 | –1.3 | 2.3 | –18.0 | –7.6 | ||||
| S | 12.9 | 0.8 | 10.1 | ‐3.2 | |||||||||
| 10.5 | 15.3 | –4.2 | 5.7 | 7.3 | 12.9 | –8.76 | 2.3 | ||||||
| GRN | PS | –12.1 | –2.8 | –16.1 | |||||||||
| –16.6 | –7.6 | –4.6 | –0.9 | –21.3 | –10.9 | ||||||||
| S | 9.3 | –4.0 | |||||||||||
| 4.7 | 14.0 | –10.4 | 2.5 | ||||||||||
| MAPT | PS | –13.3 | |||||||||||
| –18.4 | –.8.3 | ||||||||||||
| FCSRT immediate total recall | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C9orf72 | GRN | MAPT | |||||||||||
| PS | S | PS | S | PS | S | ||||||||
| Controls | –1.3 | –8.7 | 0.7 | –16.3 | –2.1 | 14.4 | |||||||
| –2.3 | –0.3 | –12.0 | –5.3 | 0.1 | 1.2 | –22.8 | –9.8 | –3.3 | –0.8 | –21.2 | –7.5 | ||
| C9orf72 | PS | –7.4 | 1.9 | –15.0 | –0.8 | –13.1 | |||||||
| –10.8 | –4.0 | 0.8 | 3.1 | –21.7 | –8.4 | –2.2 | 0.7 | –20.0 | –6. 1 | ||||
| S | 9.3 | –7.6 | 6.6 | –5.7 | |||||||||
| 5.9 | 12.7 | –15.2 | –0.1 | 3.0 | 10.2 | –13.6 | 2.2 | ||||||
| GRN | PS | –17.0 | –2.7 | –15.0 | |||||||||
| –23.5 | –10.4 | –4.1 | –1.3 | –21.9 | –8.1 | ||||||||
| S | 14.2 | 1.9 | |||||||||||
| 7.7 | 20.8 | –7.1 | 11.0 | ||||||||||
| MAPT | PS | –12.3 | |||||||||||
| –19.2 | –5.4 | ||||||||||||
| FCSRT delayed free recall | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C9orf72 | GRN | MAPT | |||||||||||
| PS | S | PS | S | PS | S | ||||||||
| Controls | –1.0 | –5.3 | 0.1 | –4.5 | –0.9 | –6.4 | |||||||
| –1.6 | –0.5 | –6.3 | –4.3 | –0.3 | 0.6 | –6.2 | –2.8 | –1.7 | –0.1 | –8.7 | –4.0 | ||
| C9orf72 | PS | –4.3 | 1.1 | –3.5 | 0.1 | –5.4 | |||||||
| –5.4 | –3.2 | 0.5 | 1.8 | –5.3 | –1.7 | –0.8 | 1.0 | –7.8 | –3.0 | ||||
| S | 5.5 | 0.8 | 4.4 | –1.0 | |||||||||
| 4.4 | 6.5 | –1.1 | 2.7 | 3.2 | 5.7 | –3.5 | 1.5 | ||||||
| GRN | PS | –4.6 | –1.0 | –6.5 | |||||||||
| –6.3 | –3.0 | –1.9 | –0.2 | –8.9 | –4.1 | ||||||||
| S | 3.6 | –1.9 | |||||||||||
| 1.8 | 5.4 | –4.7 | 1.0 | ||||||||||
| MAPT | PS | –5.5 | |||||||||||
| –7.8 | –3.2 | ||||||||||||
| FCSRT delayed total recall | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C9orf72 | GRN | MAPT | |||||||||||
| PS | S | PS | S | PS | S | ||||||||
| Controls | –0.3 | –3.1 | 0.1 | –4.3 | –0.7 | –4.5 | |||||||
| –0.6 | 0.0 | –4.4 | –1.9 | –0.1 | 0.4 | –6.7 | –1.9 | –1.3 | –0.2 | –7.1 | –1.9 | ||
| C9orf72 | PS | –2.8 | 0.4 | –4.0 | –0.4 | –4.2 | |||||||
| –4.1 | –1.5 | 0.1 | 0.8 | –6.4 | –1.6 | –1.1 | 0.2 | –6.8 | –1.6 | ||||
| S | –3.3 | –1.2 | 2.4 | –1.4 | |||||||||
| 2.0 | 4.5 | –3.9 | 1.6 | 1.1 | 3.8 | –4.2 | 1.5 | ||||||
| GRN | PS | –4.5 | –0.9 | –4.6 | |||||||||
| –6.8 | –2.1 | –1.5 | –0.3 | –7.2 | –2.1 | ||||||||
| S | 3.6 | –0.2 | |||||||||||
| 1.1 | 6.1 | –3.6 | 3.3 | ||||||||||
| MAPT | PS | –3.8 | |||||||||||
| –6.3 | –1.2 | ||||||||||||
Notes: Values in bold are significant at P < 0.05. Values are adjusted for age, years of education, sex, and language in which the test was administered.
Abbreviations: C9orf72, chromosome 9 open reading frame 72; FCSRT, Free and Cued Selective Reminding Test; GRN, progranulin; MAPT, microtubule‐associated protein tau; PS, presymptomatic; S, symptomatic.
Presymptomatic C9orf72 repeat expansion carriers performed significantly worse on FCSRT immediate free recall (P < 0.001), immediate total recall (P = 0.010), and delayed free recall (P < 0.001) than controls, but not delayed total recall (p = 0.066) (Tables 1 and 2). Presymptomatic MAPT mutation carriers had significantly lower FCSRT immediate free recall (P = 0.005), immediate total recall (P = 0.002), delayed free recall (P = 0.024), and delayed total recall (P = 0.011) scores than controls. In addition, presymptomatic C9orf72 and MAPT mutation carriers performed significantly worse than presymptomatic GRN mutation carriers on all four FCSRT test scores (all P < 0.017).
3.4. Association with other neuropsychological tests
Correlation coefficients for each FCSRT score with other neuropsychological tests by genetic group can be seen in Table 3. In the C9orf72 mutation carriers, the strongest correlations were with the D‐KEFS Color‐Word task, particularly for the free recall scores, as well as category fluency, with additional significant correlations with the BNT and Benson figure recall, particularly in the symptomatic group. In the GRN mutation carriers, the strongest correlations were with TMT‐B as well as with the Benson figure recall and BNT for the majority of scores, particularly for the symptomatic group. In the MAPT mutation carriers the strongest correlations were with the Benson figure recall (all significant except delayed free recall in the symptomatic group), followed by the BNT (for all scores), with no significant correlations with any of the executive function tasks or category fluency in the symptomatic group.
TABLE 3.
Correlation coefficients between FCSRT scores and other neuropsychological tests in each genetic group
| Benson figure recall | BNT | Category fluency | TMT‐B | D‐KEFS Color‐Word | ||||
|---|---|---|---|---|---|---|---|---|
| C9orf72 | PS | Immediate | Free | 0.14 | 0.12 | 0.28** | –0.22* | –0.36*** |
| Total | 0.21* | 0.27** | 0.30*** | –0.22** | –0.30*** | |||
| Delayed | Free | 0.20* | 0.22** | 0.28** | –0.26** | –0.41*** | ||
| Total | 0.23** | 0.23** | 0.26** | –0.29*** | –0.27** | |||
| S | Immediate | Free | 0.28 | 0.49*** | 0.46** | –0.21 | –0.42** | |
| Total | 0.29 | 0.55*** | 0.44** | –0.24 | –0.28 | |||
| Delayed | Free | 0.46** | 0.47** | 0.49*** | –0.25 | –0.54*** | ||
| Total | 0.36* | 0.56*** | 0.54*** | –0.29 | –0.44** | |||
| GRN | PS | Immediate | Free | 0.27** | 0.21* | 0.36*** | –0.31*** | –0.29*** |
| Total | 0.33*** | 0.26** | 0.22** | –0.24** | –0.39*** | |||
| Delayed | Free | 0.30*** | 0.26** | 0.31*** | –0.42*** | –0.40*** | ||
| Total | 0.34*** | 0.21* | 0.24** | –0.21* | –0.19* | |||
| S | Immediate | Free | 0.52* | 0.41 | 0.43 | –0.50* | 0.27 | |
| Total | 0.62** | 0.53** | 0.57* | –0.55* | 0.25 | |||
| Delayed | Free | 0.70** | 0.59** | 0.39 | –0.58** | 0.05 | ||
| Total | 0.45 | 0.57* | 0.56* | –0.51* | –0.03 | |||
| MAPT | PS | Immediate | Free | 0.40** | 0.38** | 0.38** | –0.49*** | –0.52*** |
| Total | 0.45*** | 0.37** | 0.36** | –0.41** | –0.50*** | |||
| Delayed | Free | 0.44*** | 0.38** | 0.45*** | –0.51*** | –0.46*** | ||
| Total | 0.45*** | 0.37** | 0.25 | –0.47*** | –0.32* | |||
| S | Immediate | Free | 0.74*** | 0.59** | 0.39 | –0.30 | –0.17 | |
| Total | 0.70** | 0.62** | 0.42 | –0.31 | –0.22 | |||
| Delayed | Free | 0.48 | 0.60** | 0.35 | –0.34 | –0.13 | ||
| Total | 0.76*** | 0.53* | 0.20 | –0.31 | 0.07 |
P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Abbreviations: BNT, Boston Naming Test; C9orf72, chromosome 9 open reading frame 72; D‐KEFS Color‐Word, Delis–Kaplan Executive Function System Color‐Word Interference Test; FCSRT, Free and Cued Selective Reminding Test; GRN, progranulin; MAPT, microtubule‐associated protein tau; PS, presymptomatic; S, symptomatic; TMT‐B, Trail Making Test Part B.
3.5. Neuroanatomical correlates of performance on the FCSRT
The VBM analyses revealed particular involvement of frontal (orbitofrontal and dorsolateral prefrontal cortices), insula, temporal (particularly medial cortical areas), and parietal (angular gyrus and precuneus) regions as well as the hippocampus in immediate free recall score in GRN and C9orf72 mutation carriers, with additional involvement of the thalamus and amygdala in the latter (Figure 1, Table A.4 in supporting information). For the immediate total recall score, a similar network was found in GRN mutation carriers as well as the thalamus, but in C9orf72 mutation carriers exclusively areas in the medial temporal lobe including the hippocampus were found (Figure 2, Table A.4). In MAPT mutation carriers, both immediate free and total recall were correlated with atrophy of the medial temporal lobes bilaterally (Figures 1 and 2, Table A.4). The overlap and differences in statistical parametric maps between immediate free and total recall can be seen in Figure A.1 in supporting information. For C9orf72 mutation carriers, similar findings were seen for delayed total recall (Table A.4), although only frontal areas were associated with delayed total recall for GRN mutation carriers. There were no associations in GRN and MAPT mutation carriers for delayed free recall or in MAPT mutation carriers for delayed total recall after FWE correction (Table A.4). All significant correlations were positive (i.e., lower gray matter volume associated with worse performance).
FIGURE 1.
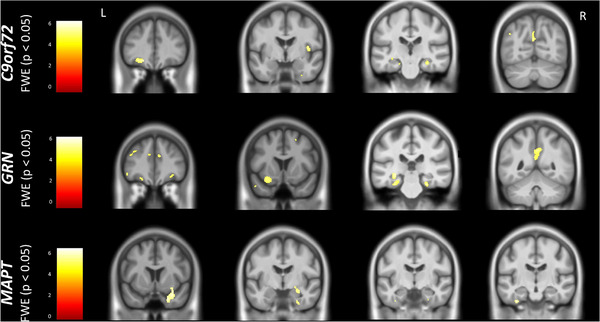
Neuroanatomical correlates of performance on the FCSRT immediate free recall. Results are shown on a study‐specific T1‐weighted magnetic resonance imaging template in Montreal Neurological Institute space and at P < 0.05 family‐wise error corrected. Color bars represent T‐values. Abbreviations: C9orf72, chromosome 9 open reading frame 72; FCSRT, Free and Cued Selective Reminding Test; FWE, family‐wise error; GRN, progranulin; L, left; MAPT, microtubule‐associated protein tau; R, right
FIGURE 2.
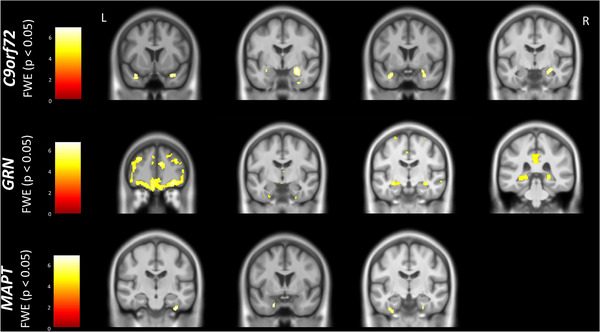
Neuroanatomical correlates of performance on the FCSRT immediate total recall. Results are shown on a study‐specific T1‐weighted magnetic resonance imaging template in Montreal Neurological Institute space and at P < 0.05 family‐wise error corrected. Color bars represent T‐values. C9orf72, chromosome 9 open reading frame 72; FCSRT, Free and Cued Selective Reminding Test; FWE, family‐wise error; GRN, progranulin; L, left; MAPT, microtubule‐associated protein tau; R, right
4. DISCUSSION
This study demonstrates the presence of memory impairment in genetic FTD, including in the presymptomatic period of MAPT and C9orf72 mutation carriers, and with differential underlying neural correlates in different genetic groups. Results showed that all symptomatic mutation carriers had lower performance than controls and presymptomatic mutation carriers. Presymptomatic MAPT mutation carriers performed lower on all four FCSRT scores compared to controls, and presymptomatic C9orf72 mutation carriers performed lower than controls on all scores except delayed total recall. The strongest associations between the FCSRT and cognitive tasks were with measures of executive function as well as memory and language in C9orf72 and GRN mutation carriers but mainly with memory and naming tests for MAPT mutation carriers. Neural correlates varied between genetic groups, with frontal and temporal as well as subcortical involvement in C9orf72 and GRN mutation carriers, but almost exclusively temporal areas being implicated in the MAPT group. Interestingly, a difference in frontal versus temporal involvement was seen in free versus total recall measures in C9orf72 mutation carriers. Together these results indicate that the FCSRT is a sensitive test in the presymptomatic period of C9orf72‐ and MAPT‐associated FTD, and provides important additional insight into the underlying basis of memory impairment in different forms of FTD.
All symptomatic mutation carriers had impaired memory as measured by the FCSRT compared to controls and presymptomatic mutation carriers, whereas only MAPT‐ and C9orf72‐associated FTD were impaired presymptomatically. This is in line with previous studies investigating cognitive functioning in people with genetic FTD, demonstrating memory impairment in C9orf72‐, 18 , 22 , 33 , 34 GRN‐, 19 , 22 , 35 and MAPT‐ 22 related FTD, earlier (and presymptomatically) in C9orf72 36 and MAPT 21 , 37 , 38 mutations, and only in the later symptomatic stages in GRN‐related FTD. 17 , 22 Some of these studies interpreted memory impairment as a distinctive characteristic of the specific gene mutation involved, but our results suggest that, although all (symptomatic) genetic groups were impaired, the underlying cause of memory impairment might differ between the genetic groups. This is illustrated by the finding of lower immediate free, total, and delayed free recall in presymptomatic C9orf72 mutation carriers, while presymptomatic MAPT carriers performed worse on all four tests, including delayed total recall, compared to controls and presymptomatic GRN carriers. According to the classical view, the FCSRT total scores are assumed to represent a “true” form of memory consolidation due to the cued format and the free recall scores are believed to be more dependent on executive functioning as well. 5 In light of this theory, our results indicate that lower performance in MAPT mutation carriers might be the result of a pure memory impairment, that starts in the presymptomatic stage, whereas memory performance in C9orf72 mutation carriers is initially influenced by executive dysfunction resulting in an ineffective encoding and/or retrieval strategy. This theory is further corroborated by our finding that in the C9orf72 group there were significant associations between the FCSRT and executive tests such as the D‐KEFS Color‐Word Interference Test in particular. In contrast, although there were moderate associations between the FCSRT and executive tests in the presymptomatic MAPT group as well, the FCSRT was exclusively associated with tests for visual and semantic memory in the symptomatic group, indicating a stronger underlying temporal component in this group. This is not surprising given that semantic impairment has been associated with anteromedial temporal lobe atrophy and is a common symptom in the later disease stages of people with a MAPT mutation. 22 , 39 , 40 As such, semantic impairment might also have influenced performance on the FCSRT. In GRN mutation carriers, memory processes appear to become affected at a later, symptomatic, stage of the disease possibly due to increasing cognitive impairment in executive function or language domains affecting memory performance as well. 41 GRN mutation carriers performed better than the other mutation carrier groups on the FCSRT in the presymptomatic stage, whereas they performed significantly worse than C9orf72 mutation carriers in the symptomatic stage. This is in line with previous studies showing that there is minimal cognitive decline in presymptomatic GRN mutation carriers, with often rapidly progressive cognitive decline after symptom onset, 21 , 22 , 35 , 41 whereas in C9orf72‐related FTD cognitive decline already starts at an early stage, and then may progress relatively slowly for several years after symptom onset. 18 , 22 , 33 , 34 , 36
Although the mean and standard deviation of FCSRT scores in the presymptomatic MAPT mutation carriers are similar to the entire control group (Table 1), this group is significantly younger than the overall control group, and the adjusted mean differences seen in Table 2 approximate to the difference between the mean of the presymptomatic MAPT mutation carriers and that of a younger control group (Table A.3). For example, the mean for immediate free recall in this group was 31.6 with a mean age within this group of 39.8, while in the age 30 to 40 younger controls (Table A.3) the mean score was 34.0, 2.4 points higher than the presymptomatic MAPT mutation carriers.
The VBM analysis revealed that for MAPT mutation carriers both free and total recall were correlated almost exclusively with temporal lobe areas, including parts of the medial temporal lobe memory system (e.g., entorhinal and parahippocampal cortices). 42 , 43 Although this memory network, including the hippocampus, amygdala, and fusiform gyrus, was implicated in C9orf72 and GRN mutation carriers as well, there was additional involvement of the frontal cortices, thalamus, and insula in these groups, areas that are involved with executive processes such as inhibitory control, initiative, planning of behavior, and attention. 44 , 45 , 46 , 47 , 48 , 49 Interestingly, this executive network was not implicated in the total recall measures in C9orf72 mutation carriers reducing it to exclusively memory‐related areas. This suggests that in C9orf72‐related FTD, although frontal/executive processes influence free recall performance, temporal/memory processes affect performance on total recall measures. On the other hand, in GRN‐related FTD frontal/executive processes appear to influence performance on both free and cued memory recall formats. These results are consistent with previous neuroimaging studies showing progressive deterioration of the brain areas that were correlated to FCSRT performance in each genetic group. 17 , 18 , 19 , 23 , 36 , 50 For example, a previous GENFI study revealed hippocampal loss followed by temporal lobe atrophy in presymptomatic MAPT mutation carriers from, respectively, 15 to 10 years before estimated symptom onset, whereas the insula and parietal areas were the earliest affected areas in GRN and the thalamus in C9orf72. 23 Overall, the neuroanatomical correlates were more extensive for the immediate than delayed recall scores. A possible explanation for this might be that there is a larger variance in the distribution of scores in immediate recall with a maximum score of 48, compared to delayed recall with a maximum score of 16, and therefore less sensitivity to detect a change in gray matter volume.
A major strength of this study is the use of a large cohort of genetic FTD patients and presymptomatic mutation carriers, allowing not only gene‐specific analyses, but also the use of a matched control group of mutation‐negative family members. However, despite the large sample size, the MAPT mutation carrier group was still smaller than the other groups, which might have influenced particularly the power of VBM analyses, in which we did not find significant correlations with delayed recall test scores after FWE correction. Another limitation of this study is that bulbar/motor symptoms of patients with FTD‐ALS or severe language difficulties in patients with PPA might have affected performance on the FCSRT or other cognitive tests, although these groups were in the minority compared to those with a primary diagnosis of bvFTD, and furthermore, instructions for test administration include example items for most cognitive tests to check if instructions are understood and if a patient is too severely affected the test is discontinued according to the judgment of an experienced neuropsychologist. Future research studies might investigate the loss of information over the delay between the immediate and delayed recall phases; however, this data was not available in this study.
To summarize, we demonstrated significant episodic memory impairment in genetic FTD, beginning in the presymptomatic period of MAPT and C9orf72. Presymptomatic C9orf72 mutation carriers were not impaired in delayed total recall (i.e., free + cued recall), and FCSRT free recall was more strongly associated with tests for executive functioning. This suggests that lower FCSRT free recall might initially be the result of an ineffective retrieval strategy, rather than a “true” memory impairment. On the other hand, presymptomatic MAPT mutation carriers performed, for their overall younger age, worse than controls on both immediate and delayed total recall, with strong associations with memory tests, suggesting that “true” memory processes affect performance on the FCSRT in this group. In contrast, FCSRT performance is only impaired at the symptomatic stage of GRN mutation carriers. These findings were corroborated by demonstrating an exclusive temporal/memory network association with FCSRT performance in MAPT mutation carriers, whereas areas important for executive functioning were also correlated with FCSRT performance in GRN and C9orf72 mutation carriers. Only temporal memory‐related areas were associated with total recall in C9orf72, suggesting that there is a pure memory component implicated in this group as well, possibly only at the symptomatic stage when the temporal lobes become affected. Together, these results demonstrate that memory deficits are an integral part of the clinical spectrum in MAPT and C9orf72 mutation carriers. It suggests that comprehensive memory tasks that can delineate executive function and memory processes such as the FCSRT should be incorporated in the standard diagnostic work‐up. In addition, they can potentially serve as a useful outcome measure in upcoming clinical trials that target specific pathologies.
FUNDING INFORMATION
The Dementia Research Centre is supported by Alzheimer's Research UK, Alzheimer's Society, Brain Research UK, and The Wolfson Foundation. This work was supported by the NIHR UCL/H Biomedical Research Centre, the Leonard Wolfson Experimental Neurology Centre (LWENC) Clinical Research Facility, and the UK Dementia Research Institute, which receives its funding from UK DRI Ltd, funded by the UK Medical Research Council, Alzheimer's Society, and Alzheimer's Research UK. J. D. Rohrer is supported by an MRC Clinician Scientist Fellowship (MR/M008525/1) and has received funding from the NIHR Rare Disease Translational Research Collaboration (BRC149/NS/MH). This work was also supported by the MRC UK GENFI grant (MR/M023664/1); the Bluefield Project; the JPND GENFI‐PROX grant (2019‐02248); the Dioraphte Foundation (grant numbers 09‐02‐00); the Association for Frontotemporal Dementias Research Grant 2009; The Netherlands Organization for Scientific Research (NWO; grant HCMI 056‐13‐018); ZonMw Memorabel (Deltaplan Dementie, project numbers 733 050 103 and 733 050 813); JPND PreFrontAls consortium (project number 733051042). J. M. Poos is supported by a Fellowship award from Alzheimer Nederland (WE.15‐2019.02). This work was conducted using the MRC Dementias Platform UK (MR/L023784/1 and MR/009076/1). Several authors of this publication are members of the European Reference Network for Rare Neurological Diseases ‐ Project ID No 739510.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
Supporting information
Supporting information
ACKNOWLEDGMENTS
We thank the research participants and their families for their contribution to the study.
List of GENFI Consortium Authors
Martin N. Rossor MD FRCP1, Nick C. Fox MD FRCP1, Jason D. Warren PhD FRACP1, Katrina Moore PhD1, Rhian Convery MSc1, Imogen J. Swift MSc1, Rachelle Shafei MRCP1, Carolin Heller BSc1, Emily Todd MSc1, Arabella Bouzigues BSc MSc1, David Cash PhD1, Ione Woollacott PhD1, Henrik Zetterberg1, Annabel Nelson BSc1, Jennifer Nicholas PhD2, Rita Guerreiro PhD3, Jose Bras PhD3, David L. Thomas PhD4, Simon Mead PhD5, Lieke Meeter MD6, Jessica Panman MSc6, Rick van Minkelen PhD7, Myriam Barandiaran PhD8,9, Begoña Indakoetxea MD8,9, Alazne Gabilondo MD9, Mikel Tainta MD9, Ana Gorostidi PhD9, Miren Zulaica BSc9, Alina Díez MSc9, Jorge Villanua MD PhD10, Sergi Borrego‐Ecija MD11, Jaume Olives MSc11, Albert Lladó PhD11, Mircea Balasa PhD11, Anna Antonell PhD11, Nuria Bargallo PhD12, Enrico Premi MD13, Stefano Gazzina MD14, Roberto Gasparotti MD15, Silvana Archetti MBiolSci16, Sandra Black MD17, Sara Mitchell MD17, Ekaterina Rogaeva PhD18, Morris Freedman MD19, Ron Keren MD20, David Tang‐Wai MD21, Hakan Thonberg MD22, Linn Öijerstedt MD22,23, Christin Andersson PhD24, Vesna Jelic MD25, Andrea Arighi MD26,27, Chiara Fenoglio PhD26,27, Elio Scarpini MD26,27, Giorgio Fumagalli MD26,27, Thomas Cope MRCP28, Carolyn Timberlake BSc28, Timothy Rittman MRCP28, Christen Shoesmith MD29, Robart Bartha PhD30,31, Rosa Rademakers PhD32, Carlo Wilke MD33,34, Hans‐Otto Karnarth MD35, Benjamin Bender MD36, Rose Bruffaerts MD PhD37, Philip Vandamme MD PhD38, Mathieu Vandenbulcke MD PhD39,40, Catarina B. Ferreira MSc41, Gabriel Miltenberger PhD42, Carolina Maruta MPsych PhD43, Ana Verdelho MD PhD44, Sónia Afonso BSc45, Ricardo Taipa MD PhD46, Paola Caroppo MD PhD47, Giuseppe Di Fede MD PhD47, Giorgio Giaccone MD47, Sara Prioni PsyD47, Veronica Redaelli MD47, Giacomina Rossi MSc47, Pietro Tiraboschi MD47, Diana Duro NPsych48, Maria Rosario Almeida PhD48, Miguel Castelo‐Branco MD PhD48, Maria João Leitão BSc49, Miguel Tabuas‐Pereira MD50, Beatriz Santiago MD50, Serge Gauthier MD51, Pedro Rosa‐Neto MD PhD52, Michele Veldsman PhD53, Paul Thompson PhD54, Tobias Langheinrich MD54, Catharina Prix MD55, Tobias Hoegen MD55, Elisabeth Wlasich Mag. rer. nat.55, Sandra Loosli MD55, Sonja Schonecker MD55, Sarah Anderl‐Straub Dr.hum.biol Dipl.Psych56, Jolina Lombardi56, Nuria Bargalló MD PhD57, Alberto Benussi MD58, Valentina Cantoni58, Maxime Bertoux PhD59,60, Anne Bertrand MD PhD61, Alexis Brice MD PhD61, Agnès Camuzat61, Olivier Colliot PhD61, Sabrina Sayah61, Aurélie Funkiewiez61,62, Daisy Rinaldi61,62, Gemma Lombardi62, Benedetta Nacmias62, Dario Saracino61,62,63, Valentina Bessi64, Camilla Ferrari64, Marta Cañada65, Vincent Deramecourt66, Gregory Kuchcinski66, Thibaud Lebouvier66, Sebastien Ourselin67, Cristina Polito68, Adeline Rollin69.
Affiliations
1Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK; 2Department of Medical Statistics, London School of Hygiene and Tropical Medicine, London, UK; 3Center for Neurodegenerative Science, Van Andel Institute, Grand Rapids, Michigan, Michigan, USA; 4Division of Neuroscience and Experimental Psychology, Wolfson Molecular Imaging Centre, University of Manchester, Manchester, UK; 5MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK; 6Department of Neurology, Erasmus Medical Center, Rotterdam, the Netherlands; 7Department of Clinical Genetics, Erasmus Medical Center, Rotterdam, the Netherlands; 8Cognitive Disorders Unit, Department of Neurology, Donostia University Hospital, San Sebastian, Gipuzkoa, Spain; 9Neuroscience Area, Biodonostia Health Research Insitute, San Sebastian, Gipuzkoa, Spain; 10OSATEK, University of Donostia, San Sebastian, Gipuzkoa, Spain; 11Alzheimer's Disease and Other Cognitive Disorders Unit, Neurology Service, Hospital Clínic, Barcelona, Spain; 12Imaging Diagnostic Center, Hospital Clínic, Barcelona, Spain; 13Stroke Unit, ASST Brescia Hospital, Brescia, Italy; 14Neurology, ASST Brescia Hospital, Brescia, Italy; 15Neuroradiology Unit, University of Brescia, Brescia, Italy; 16Biotechnology Laboratory, Department of Diagnostics, ASST Brescia Hospital, Brescia, Italy; 17Sunnybrook Health Sciences Centre, Sunnybrook Research Institute, University of Toronto, Toronto, Canada; 18Tanz Centre for Research in Neurodegenerative Diseases, University of Toronto, Toronto, Canada; 19Baycrest Health Sciences, Rotman Research Institute, University of Toronto, Toronto, Canada; 20The University Health Network, Toronto Rehabilitation Institute, Toronto, Canada; 21The University Health Network, Krembil Research Institute, Toronto, Canada; 22Center for Alzheimer Research, Division of Neurogeriatrics, Department of Neurobiology, Care Sciences and Society, Bioclinicum, Karolinska Institutet, Solna, Sweden; 23Unit for Hereditary Dementias, Theme Aging, Karolinska University Hospital, Solna, Sweden; 24Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden; 25Division of Clinical Geriatrics, Karolinska Institutet, Stockholm, Sweden; 26Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Neurodegenerative Diseases Unit, Milan, Italy; 27University of Milan, Centro Dino Ferrari, Milan, Italy; 28Department of Clinical Neuroscience, University of Cambridge, Cambridge, UK; 29Department of Clinical Neurological Sciences, University of Western Ontario, London, Canada; 30Department of Medical Biophysics, The University of Western Ontario, London, Canada; 31Centre for Functional and Metabolic Mapping, Robarts Research Institute, The University of Western Ontario, London, Canada; 32Department of Neurosciences, Mayo Clinic, Jacksonville, Florida, USA; 33Department of Neurodegenerative Diseases, Hertie‐Institute for Clinical Brain Research and Center of Neurology, University of Tübingen, Tübingen, Germany; 34Center for Neurodegenerative Diseases (DZNE), Tübingen, Germany; 35Division of Neuropsychology, Hertie‐Institute for Clinical Brain Research and Center of Neurology, University of Tübingen, Tübingen, Germany; 36Department of Diagnostic and Interventional Neuroradiology, University of Tübingen, Tübingen, Germany; 37Laboratory for Cognitive Neurology, Department of Neurosciences, KU Leuven, Leuven, Belgium; 38Neurology Service, University Hospitals Leuven, Belgium; Laboratory for Neurobiology, VIB‐KU Leuven Centre for Brain Research, Leuven, Belgium; 39Geriatric Psychiatry Service, University Hospitals Leuven, Leuven, Belgium; 40Neuropsychiatry, Department of Neurosciences, KU Leuven, Leuven, Belgium; 41Laboratory of Neurosciences, Institute of Molecular Medicine, Faculty of Medicine, University of Lisbon, Lisbon, Portugal; 42Faculty of Medicine, University of Lisbon, Lisbon, Portugal; 43Laboratory of Language Research, Centro de Estudos Egas Moniz, Faculty of Medicine, University of Lisbon, Lisbon, Portugal; 44Department of Neurosciences and Mental Health, Centro Hospitalar Lisboa Norte ‐ Hospital de Santa Maria & Faculty of Medicine, University of Lisbon, Lisbon, Portugal; 45Instituto Ciencias Nucleares Aplicadas a Saude, Universidade de Coimbra, Coimbra, Portugal; 46Neuropathology Unit and Department of Neurology, Centro Hospitalar do Porto ‐ Hospital de Santo António, Oporto, Portugal; 47Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy; 48Faculty of Medicine, University of Coimbra, Coimbra, Portugal; 49Centre of Neurosciences and Cell Biology, Universidade de Coimbra, Coimbra, Portugal; 50Neurology Department, Centro Hospitalar e Universitario de Coimbra, Coimbra, Portugal; 51Alzheimer Disease Research Unit, McGill Centre for Studies in Aging, Department of Neurology & Neurosurgery, McGill University, Montreal, Canada; 52Translational Neuroimaging Laboratory, McGill Centre for Studies in Aging, McGill University, Montreal, Canada; 53Nuffield Department of Clinical Neurosciences, Medical Sciences Division, University of Oxford, Oxford, UK; 54Division of Neuroscience and Experimental Psychology, Wolfson Molecular Imaging Centre, University of Manchester, Manchester, UK; 55Neurologische Klinik, Ludwig‐Maximilians‐Universität München, Munich, Germany; 56Department of Neurology, University of Ulm, Ulm, Germany; 57Imaging Diagnostic Center, Hospital Clínic, Barcelona, Spain; 58Centre for Neurodegenerative Disorders, Department of Clinical and Experimental Sciences, University of Brescia, Brescia, Italy; 59Inserm 1172, Lille, France; 60CHU, CNR‐MAJ, Labex Distalz, LiCEND Lille, France; 61Sorbonne Université, Paris Brain Institute – Institut du Cerveau – ICM, Inserm U1127, CNRS UMR 7225, AP‐HP ‐ Hôpital Pitié‐Salpêtrière, Paris, France; 62Centre de référence des démences rares ou précoces, IM2A, Département de Neurologie, AP‐HP ‐ Hôpital Pitié‐Salpêtrière, Paris, France; 63Inria, Aramis project‐team, F‐75013, Paris, France; 64Department of Neuroscience, Psychology, Drug Research and Child Health, University of Florence, Florence, Italy; 65CITA Alzheimer, San Sebastian, Gipuzkoa, Spain; 66University of Lille, France; 67School of Biomedical Engineering & Imaging Sciences, King's College London, London, UK; 68Department of Biomedical, Experimental and Clinical Sciences “Mario Serio,” Nuclear Medicine Unit, University of Florence, Florence, Italy; 69CHU, CNR‐MAJ, Labex Distalz, LiCEND, Lille, France.
Poos JM, Russell LL, Peakman G, et al. Impairment of episodic memory in genetic frontotemporal dementia: A GENFI study. Alzheimer's Dement. 2021;13:e12185. 10.1002/dad2.12185
Contributor Information
Jackie M. Poos, Email: j.m.poos@erasmusmc.nl.
Jonathan D. Rohrer, Email: j.rohrer@ucl.ac.uk.
the Genetic FTD Initiative, GENF:
Martin N. Rossor, Nick C. Fox, Jason D. Warren, Katrina Moore, Rhian Convery, Imogen J. Swift, Rachelle Shafei, Carolin Heller, Emily Todd, Arabella Bouzigues, David Cash, Ione Woollacott, Henrik Zetterberg, Annabel Nelson, Jennifer Nicholas, Rita Guerreiro, Jose Bras, David L. Thomas, Simon Mead, Lieke Meeter, Jessica Panman, Rick van Minkelen, Myriam Barandiaran, Begoña Indakoetxea, Alazne Gabilondo, Mikel Tainta, Ana Gorostidi, Miren Zulaica, Alina Díez, Jorge Villanua, Sergi Borrego‐Ecija, Olives Jaume, Albert Lladó, Mircea Balasa, Anna Antonell, Nuria Bargallo, Enrico Premi, Stefano Gazzina, Roberto Gasparotti, Silvana Archetti, Sandra Black, Sara Mitchell, Ekaterina Rogaeva, Morris Freedman, Ron Keren, David Tang‐Wai, Hakan Thonberg, Linn Öijerstedt, Christin Andersson, Vesna Jelic, Andrea Arighi, Chiara Fenoglio, Elio Scarpini, Giorgio Fumagalli, Thomas Cope, Carolyn Timberlake, Timothy Rittman, Christen Shoesmith, Robart Bartha, Rosa Rademakers, Carlo Wilke, Hans‐Otto Karnarth, Benjamin Bender, Rose Bruffaerts, Philip Vandamme, Mathieu Vandenbulcke, Catarina B. Ferreira, Gabriel Miltenberger, Carolina Maruta, Ana Verdelho, Sónia Afonso, Ricardo Taipa, Paola Caroppo, Giuseppe Di Fede, Giorgio Giaccone, Sara Prioni, Veronica Redaelli, Giacomina Rossi, Pietro Tiraboschi, Diana Duro, Maria Rosario Almeida, Miguel Castelo‐Branco, Maria João Leitão, Miguel Tabuas‐Pereira, Beatriz Santiago, Serge Gauthier, Pedro Rosa‐Neto, Michele Veldsman, Paul Thompson, Tobias Langheinrich, Catharina Prix, Tobias Hoegen, Elisabeth Wlasich, Sandra Loosli, Sonja Schonecker, Sarah Anderl‐Straub, Jolina Lombardi, Nuria Bargalló, Alberto Benussi, Valentina Cantoni, Maxime Bertoux, Anne Bertrand, Alexis Brice, Agnès Camuzat, Olivier Colliot, Sabrina Sayah, Aurélie Funkiewiez, Daisy Rinaldi, Gemma Lombardi, Benedetta Nacmias, Dario Saracino, Valentina Bessi, Camilla Ferrari, Marta Cañada, Vincent Deramecourt, Gregory Kuchcinski, Thibaud Lebouvier, Sebastien Ourselin, Cristina Polito, and Adeline Rollin
REFERENCES
- 1. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(Pt 9):2456‐2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gorno‐Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006‐1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Poos JM, Jiskoot LC, Papma JM, van Swieten JC, van den Berg E. Meta‐analytic review of memory impairment in behavioral variant frontotemporal dementia. J Int Neuropsychol Soc. 2018;24(6):593‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hornberger M, Wong S, Tan R, et al. In vivo and post‐mortem memory circuit integrity in frontotemporal dementia and Alzheimer's disease. Brain. 2012;135(Pt 10):3015‐3025. [DOI] [PubMed] [Google Scholar]
- 5. Bertoux M, Ramanan S, Slachevsky A, et al. So close yet so far: executive contribution to memory processing in behavioral variant frontotemporal dementia. J Alzheimers Dis. 2016;54(3):1005‐1014. [DOI] [PubMed] [Google Scholar]
- 6. Fernandez‐Matarrubia M, Matias‐Guiu JA, Cabrera‐Martin MN, et al. Episodic memory dysfunction in behavioral variant frontotemporal dementia: a clinical and FDG‐PET study. J Alzheimers Dis. 2017;57(4):1251‐1264. [DOI] [PubMed] [Google Scholar]
- 7. Pasquier F, Grymonprez L, Lebert F, Van der Linden M. Memory impairment differs in frontotemporal dementia and Alzheimer's disease. Neurocase. 2001;7(2):161‐171. [DOI] [PubMed] [Google Scholar]
- 8. Buschke H. Cued recall in amnesia. J Clin Neuropsychol. 1984;6(4):433‐440. [DOI] [PubMed] [Google Scholar]
- 9. Bertoux M, Flanagan EC, Hobbs M, et al. Structural anatomical investigation of long‐term memory deficit in behavioral frontotemporal dementia. J Alzheimers Dis. 2018;62(4):1887‐1900. [DOI] [PubMed] [Google Scholar]
- 10. Basely M, Ceccaldi M, Boyer L, Mundler O, Guedj E. Distinct patterns of medial temporal impairment in degenerative dementia: a brain SPECT perfusion study in Alzheimer's disease and frontotemporal dementia. Eur J Nucl Med Mol Imaging. 2013;40(6):932‐942. [DOI] [PubMed] [Google Scholar]
- 11. Bertoux M, de Souza LC, Corlier F, et al. Two distinct amnesic profiles in behavioral variant frontotemporal dementia. Biol Psychiatry. 2014;75(7):582‐588. [DOI] [PubMed] [Google Scholar]
- 12. Teichmann M, Epelbaum S, Samri D, et al. Free and Cued Selective Reminding Test ‐ accuracy for the differential diagnosis of Alzheimer's and neurodegenerative diseases: a large‐scale biomarker‐characterized monocenter cohort study (ClinAD). Alzheimers Dement. 2017;13(8):913‐923. [DOI] [PubMed] [Google Scholar]
- 13. Bertoux M, Cassagnaud P, Lebouvier T, et al. Does amnesia specifically predict Alzheimer's pathology? A neuropathological study. Neurobiol Aging. 2020;95:123‐130. [DOI] [PubMed] [Google Scholar]
- 14. Papma JM, Seelaar H, de Koning I, et al. Episodic memory impairment in frontotemporal dementia; a (9)(9)mTc‐ HMPAO SPECT study. Curr Alzheimer Res. 2013;10(3):332‐339. [DOI] [PubMed] [Google Scholar]
- 15. de Souza LC, Chupin M, Bertoux M, et al. Is hippocampal volume a good marker to differentiate Alzheimer's disease from frontotemporal dementia?. J Alzheimers Dis. 2013;36(1):57‐66. [DOI] [PubMed] [Google Scholar]
- 16. Lashley T, Rohrer JD, Mead S, Revesz T. An update on clinical, genetic and pathological aspects of frontotemporal lobar degenerations. Neuropathol Applied Neurobiol. 2015;41(7):858‐881. [DOI] [PubMed] [Google Scholar]
- 17. Rohrer JD, Ridgway GR, Modat M, et al. Distinct profiles of brain atrophy in frontotemporal lobar degeneration caused by progranulin and tau mutations. Neuroimage. 2010;53(3):1070‐1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mahoney CJ, Downey LE, Ridgway GR, et al. Longitudinal neuroimaging and neuropsychological profiles of frontotemporal dementia with C9ORF72 expansions. Alzheimers Res Ther. 2012;4(5):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Le Ber I, Camuzat A, Hannequin D, et al. Phenotype variability in progranulin mutation carriers: a clinical, neuropsychological, imaging and genetic study. Brain. 2008;131(Pt 3):732‐746. [DOI] [PubMed] [Google Scholar]
- 20. Lima M, Tábuas‐Pereira M, Duro D, et al. Neuropsychological features of progranulin‐associated frontotemporal dementia: a nested case‐control study. Neural Regen Res. 2021;16(5):910‐915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jiskoot LC, Panman JL, van Asseldonk L, et al. Longitudinal cognitive biomarkers predicting symptom onset in presymptomatic frontotemporal dementia. J Neurol. 2018;265(6):1381‐1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Poos JM, Jiskoot LC, Leijdesdorff SMJ, et al. Cognitive profiles discriminate between genetic variants of behavioral frontotemporal dementia. J Neurol. 2020;267(6):1603‐1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rohrer JD, Nicholas JM, Cash DM, et al. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the genetic frontotemporal dementia initiative (GENFI) study: a cross‐sectional analysis. Lancet Neurol. 2015;14(3):253‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brooks BR, Miller RG, Swash M, Munsat TL. World Federation of Neurology Research Group on Motor Neuron D. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293‐299. [DOI] [PubMed] [Google Scholar]
- 25. Miyagawa T, Brushaber D, Syrjanen J, et al. Utility of the global CDR® plus NACC FTLD rating and development of scoring rules: data from the ARTFL/LEFFTDS Consortium. Alzheimers Dement. 2020;16(1):106‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189‐198. [DOI] [PubMed] [Google Scholar]
- 27. Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and Cognitive Variables and Descriptive Data From Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20(4):210‐216. [DOI] [PubMed] [Google Scholar]
- 28. Corrigan JD, Hinkeldey NS. Relationships between parts A and B of the Trail Making Test. J Clin Psychol. 1987;43(4):402‐409. [DOI] [PubMed] [Google Scholar]
- 29. Delis DC, Kaplan E, Kramer J, den Buysch HO, Noens ILJ, Berckelaer‐Onnes IA. D‐KEFS: Delis‐Kaplan Executive Function System: Color‐Word Interference Test: Handleiding. Amsterdam: Pearson; 2008.</bib> [Google Scholar]
- 30. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95‐113. [DOI] [PubMed] [Google Scholar]
- 31. Ridgway GR, Omar R, Ourselin S, Hill DL, Warren JD, Fox NC. Issues with threshold masking in voxel‐based morphometry of atrophied brains. Neuroimage. 2009;44(1):99‐111. [DOI] [PubMed] [Google Scholar]
- 32. Malone IB, Leung KK, Clegg S, et al. Accurate automatic estimation of total intracranial volume: a nuisance variable with less nuisance. Neuroimage. 2015;104:366‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Suhonen N‐M, Haanpää RM, Korhonen V, et al. Neuropsychological profile in the C9ORF72 associated behavioral variant frontotemporal dementia. J Alzheimers Dis. 2017;58(2):479‐489. [DOI] [PubMed] [Google Scholar]
- 34. Lulé DE, Müller H‐P, Finsel J, et al. Deficits in verbal fluency in presymptomatic C9orf72 mutation gene carriers—a developmental disorder. J Neurol Neurosurg Psychiatry. 2020;91:1195‐1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Van Deerlin VM, Wood EM, Moore P, et al. Clinical, genetic, and pathologic characteristics of patients with frontotemporal dementia and progranulin mutations. Arch Neurol. 2007;64(8):1148‐1153. [DOI] [PubMed] [Google Scholar]
- 36. Bertrand A, Wen J, Rinaldi D, et al. Early cognitive, structural, and microstructural changes in presymptomatic C9orf72 carriers younger than 40 years. JAMA Neurol. 2018;75(2):236‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cheran G, Wu L, Lee S, et al. Cognitive indicators of preclinical behavioral variant frontotemporal dementia in MAPT carriers. J Int Neuropsychol Soc. 2019;25(2):184‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jiskoot LC, Dopper EG, Heijer T, et al. Presymptomatic cognitive decline in familial frontotemporal dementia: a longitudinal study. Neurology. 2016;87(4):384‐391. [DOI] [PubMed] [Google Scholar]
- 39. Rohrer JD, Warren JD. Phenotypic signatures of genetic frontotemporal dementia. Curr Opin Neurol. 2011;24(6):542‐549. [DOI] [PubMed] [Google Scholar]
- 40. Tolboom N, Koedam EL, Schott JM, et al. Dementia mimicking Alzheimer's disease owing to a tau mutation: cSF and PET findings. Alzheimer Dis Assoc Disord. 2010;24(3):303‐307. [DOI] [PubMed] [Google Scholar]
- 41. Jiskoot LC, Panman JL, Meeter LH, et al. Longitudinal multimodal MRI as prognostic and diagnostic biomarker in presymptomatic familial frontotemporal dementia. Brain. 2018;142(1):193‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Squire LR, Wixted JT. The cognitive neuroscience of human memory since H.M. Annu Rev Neurosci. 2011;34:259‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Squire LR, Zola‐Morgan S. The medial temporal lobe memory system. Science. 1991;253(5026):1380‐1386. [DOI] [PubMed] [Google Scholar]
- 44. Schmahmann JD. The cerebellum and cognition. Neurosci Lett. 2019;688:62‐75. [DOI] [PubMed] [Google Scholar]
- 45. Van der Werf YD, Witter MP, Uylings HB, Jolles J. Neuropsychology of infarctions in the thalamus: a review. Neuropsychologia. 2000;38(5):613‐627. [DOI] [PubMed] [Google Scholar]
- 46. Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72(5):341‐372. [DOI] [PubMed] [Google Scholar]
- 47. Lanciego JL, Luquin N, Obeso JA. Functional neuroanatomy of the basal ganglia. Cold Spring Harb Perspect Med. 2012;2(12):a009621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55(1):11‐29. [DOI] [PubMed] [Google Scholar]
- 49. Pan J, Sawyer K, McDonough E, Slotpole L, Gansler D. Cognitive neuroanatomical, and genetic predictors of executive function in healthy children and adolescents. Dev Neuropsychol. 2018;43(7):535‐550. [DOI] [PubMed] [Google Scholar]
- 50. Panman JL, Jiskoot LC, Bouts M, et al. Gray and white matter changes in presymptomatic genetic frontotemporal dementia: a longitudinal MRI study. Neurobiol Aging. 2019;76:115‐124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


