Abstract
Introduction
Containment measures implemented to minimize the spread of coronavirus disease 2019 (COVID‐19) are reported to be negatively affecting mental health, diet, and alcohol consumption. These factors, as well as poor cardiometabolic health and insufficient physical and cognitive activity, are known to increase the risk of developing dementia. COVID‐19 “lockdown” measures may have exacerbated these dementia risk factors among people in mid‐to‐later life.
Methods
We compared longitudinal data from before (October 2019) and during (April‐June 2020) the first COVID‐19 lockdown period in Tasmania, Australia. Participants (n = 1671) were 50+ years of age and engaged in a public health program targeting dementia risk reduction, with one‐third participating in the Preventing Dementia Massive Open Online Course (PD‐MOOC). Regression models were used to assess changes in smoking, alcohol use, body mass index (BMI), diet, physical exercise, cognitive and social activity, anxiety and depression, and management of cholesterol, diabetes, and blood pressure. Where significant changes were noted, the moderating influence of being in current employment, living with others, and completing the PD‐MOOC was tested.
Results
Although friend networks contracted marginally during lockdown, no detrimental effects on modifiable dementia risk factors were noted. Anxiety levels and alcohol consumption decreased, there was no change in depression scores, and small but significant improvements were observed in cognitive and physical activity, smoking, diet, and BMI. Stronger improvements in cognitive activity were observed among people who were cohabiting (not living alone) and both cognitive activity and adherence to the MIND diet (Mediterranean‐DASH diet Intervention for Neurological Delay) improved more for people who participated in the PD‐MOOC.
Discussion
Longitudinal data did not show widespread negative effects of COVID‐19 lockdown on modifiable dementia risk factors in this sample. The results counter the dominant narratives of universal pandemic‐related distress and suggest that engaging at‐risk populations in proactive health promotion and education campaigns during lockdown events could be a protective public health strategy.
Keywords: COVID‐19, dementia, lockdown, longitudinal, modifiable risk‐factors
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) pandemic has profoundly impacted people of all ages and nationalities. In countries such as Australia, with low infection rates (≈0.1% population in September 2020), only a fraction of individuals experienced health issues directly due to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection. However, many Australians have experienced “lockdown” periods, in which restrictions on activity outside the home were instituted to prevent the virus from spreading. 1 For individuals and communities to recover from their experiences and manage challenges ahead, understanding of the widespread health impacts of the pandemic‐related response measures is vital.
Trends in COVID‐19 case data show that older people are at the greatest risk of death and serious illness due to this virus. 2 , 3 Whether healthy or living with complex health issues, it is likely that support from family, friends, and health services would have been constrained during lockdown. 4 , 5 This presents a particular risk for older people who are retired, living alone, or living remotely, since social isolation and loneliness are recognized as important health risk factors. 6 Indeed, containment during COVID‐19 lockdown may have profound, long‐lasting impacts on people in mid‐to‐later life by increasing dementia risk.
Depression, hypertension, diabetes, smoking, physical inactivity, obesity, high alcohol consumption, social isolation, air pollution, hearing loss, traumatic brain injury, and low educational attainment are recognized as modifiable factors that collectively account for ≈40% of the population attributable fraction of dementia risk. 7 , 8 In addition, meta‐analytic evidence indicates that unmanaged stress is an additional risk factor for Alzheimer's disease (AD). 9 According to the World Health Organization, regular physical exercise, smoking cessation, a Mediterranean‐like diet, reducing hazardous drinking, cognitive training, and management of obesity, hypertension, dyslipidaemia, and diabetes can help reduce risk of cognitive decline and dementia. 10 Emerging evidence points to detrimental changes in psychological health, 11 , 12 , 13 , 14 , 15 , 16 alcohol use, 17 , 18 , 19 , 20 , 21 , 22 , 23 and eating patterns 24 , 25 in the initial stages of the COVID‐19 pandemic, but it is unclear how these and other dementia risk behaviors have been affected in middle‐aged and older Australian adults.
Tasmania, an island state of Australia with ≈540,000 residents, has experienced low community transmission of COVID‐19 (149 locally acquired cases as of August 30, 2020). Most cases occurred in the north‐west region, with only 11 cases reported in the more populous parts of the state. Given the low prevalence of COVID‐19, this Tasmanian population (excluding the north‐west) presents an opportunity to investigate the impacts of lockdown measures employed after COVID‐19 was declared a pandemic by the World Health Organization on March 11, 2020. The Tasmanian Government instigated border restrictions that stopped entry by non‐essential travellers, and enforced quarantine for people potentially exposed to the disease. Tasmanian residents were advised to cease all but essential visits to retail and service businesses, and to stay home wherever possible. Visits to aged‐care facilities and attendance at social, sporting, and cultural events were prohibited. By March 30, work‐from‐home and home‐schooling were implemented, and beaches, parks, and reserves were closed. Residents with flu‐like symptoms were strongly advised to access cost‐free COVID‐19 testing at clinics in the state's major metropolitan centers. Post‐test quarantine was strictly required until negative results were returned. If the result was positive, contact tracing was conducted and all potentially exposed people were required to undergo testing. As of August 30, 2020, a total of 87,292 tests had been carried out. The stringent stage 1 lockdown restrictions were in place for 6 weeks and led to a steady decline in new positive cases across Tasmania. Restrictions were eased after May 11 (stage 2) when constraints on travel and social contact remained but funeral attendance and some aged care visits were permitted, students returned to school, and parks and reserves re‐opened. Restrictions were further eased on June 26 after a month without new cases (stage 3) to allow gatherings of 20 people in private homes and 80 people in public places. Meanwhile, the escalating rate and impact of COVID‐19 infections in other parts of Australia and the world received constant media coverage and speculation.
The Island Study Linking Ageing and Neurodegenerative Disease (ISLAND) is a large‐scale, long‐term, prospective public health research program aiming to reduce modifiable dementia risk at population level in Tasmania. Data collected from ISLAND participants prior to and then during COVID‐19 lockdown provided a unique opportunity to examine longitudinal change, spanning the onset and lifting of lockdown in Tasmania, on dementia risk factors in a sample of middle‐aged and older Tasmanian residents.
We hypothesized that our sample would report detrimental changes from October 2019 to April‐June 2020 in dementia risk factors that are psychosocial (anxiety, depression, social networks) and lifestyle related (physical exercise, cognitive activity, alcohol consumption, smoking). We also hypothesized that these effects would vary by demographic factors, such as work and living (cohabitation) status. Finally, we hypothesized that exposure to dementia risk reduction education would exert a protective effect on areas of risk that are amenable to behavior change.
2. METHODS
2.1. Participants and data collection
Recruitment into ISLAND research was via an Expression of Interest register, promoted via online, print, and broadcast media, community talks, information booths flyers and posters. Participation was open to anyone living in Tasmania who were 50+ years of age. Data collection commenced for ISLAND in October 2019 and people who had registered interest were invited to provide consent and complete baseline surveys. Residents of north‐west Tasmania were invited to join a separate study, so only ISLAND registrants from the rest of the state were invited. Participants in the ISLAND study completed baseline (T1) via online surveys during October 2019 (n = 4282). After T1, participants received a personal dementia‐risk profile based on their survey responses and were encouraged to undertake the 4‐week Preventing Dementia Massive Open Online Course (PD‐MOOC), which was run in November 2019 and in May 2020. The PD‐MOOC aims to build knowledge of dementia risk, self‐efficacy in managing modifiable risk factors, and an ability to appraise relevant evidence (https://www.utas.edu.au/wicking/preventing‐dementia). Invitations to repeat the surveys relating to dementia risk behaviors and some questions about social activity during lockdown were sent to ISLAND participants 6 months from baseline, and responses were provided between April 23 and June 5, 2020 (T2). The T2 data were thus collected before lockdown measures were substantially eased, on June 26. Participants who provided T1 and T2 data for the Hospital Anxiety and Depression Scale (HADS) and the Lubben Social Network Scale (LSNS), and who had not undertaken the PD‐MOOC prior to October 2019, were included in this study (n = 1671). Baseline surveys were conducted at a time when no environmental disasters or socio‐cultural catastrophes were recorded in Tasmania. Thus T1 data were unaffected by COVID‐19 and unlikely to be confounded by other major social disruptions, whereas T2 data were collected after several weeks of stringent COVID‐19 lockdown restrictions. Although community transmission was low (n = 11) in the majority of Tasmania, it is possible that some participants may have had family/friends diagnosed with COVID‐19 in north‐west Tasmania (138 locally acquired cases) or elsewhere. However, data on whether participants or their family members had been diagnosed with COVID‐19 were not collected. All procedures involving human participants were approved by the University of Tasmania Health and Medical Human Research Ethics Committee (H0018264) and written informed consent was provided by all participants.
HIGHLIGHTS
We examined modifiable dementia risk factors: mental health, lifestyle and behaviors
Data were obtained before coronavirus disease 2019 (COVID‐19) emergence and during “lockdown” in adults 50+ years of age
Lockdown had no detrimental impact on the examined factors, small improvements were observed
Involvement in an online dementia risk education campaign (Island Study Linking Ageing and Neurodegenerative Disease [ISLAND]) appears protective
RESEARCH IN CONTEXT
Systematic review: Literature review (eg, PubMed) indicated that research on the impacts of coronavirus disease 2019 (COVID‐19) containment measures on modifiable dementia risk factors is limited. Available cross‐sectional studies report increased depression, anxiety, alcohol use, and food intake.
Interpretation: This study is among the first to longitudinally examine change in key dementia risk factors in people 50+ years of age during the COVID‐19 pandemic. In a sample with low virus exposure but subject to “lockdown” measures, we observed no change or small improvements in risk‐related behavior. Involvement in an online dementia risk‐reduction education campaign may protect against reported detrimental impacts of lockdown.
Future directions: Additional research on the determinants of resilience of middle‐aged and older people during pandemic containment lockdown is warranted, as the widely reported detrimental effects are not universal. This should include the implementation and evaluation of accessible preventive health campaigns, which may help offset the exacerbation of dementia risk.
2.2. Assessments
Online self‐report surveys were used to measure anxiety and 11 domains recognized as modifiable dementia risk factors 8 , 9 : depression, alcohol consumption, and smoking; management of blood pressure, blood glucose, and cholesterol; diet and body mass index (BMI); social networks; and cognitive and physical activity. Methods for quantitative assessment of dementia‐related risk factors are outlined in Table 1. Demographic data included age, gender, cohabitation status, work status, and residential remoteness. Participation in the PD‐MOOC between T1 and T2 survey completion was coded to determine if this influenced and/or moderated changes in the above domains. PD‐MOOC exposure was defined as completing at least the first of four course modules. At T2, participants were also asked to report the frequency of their social and cultural activities under usual circumstances, and during lockdown.
TABLE 1.
Assessment of dementia risk‐related factors
| Risk factor | Assessment |
|---|---|
| Continuous variables a | |
| Alcohol | Number of standard drinks per drinking occasion x drinking frequency per week |
| BMI | Weight in kilograms divided by height in meters squared |
| MIND diet | Adherence to the Mediterranean‐DASH Intervention for Neurodegenerative Delay (MIND) diet excluding wine consumption 52 ; 0, 0.5, or 1 point was given to each category; total MIND score (range 0‐14) calculated by adding all sub‐scores |
| LSNS Totalb | The 18‐item Lubben Social Networks Scale (LSNS‐18; 53 ). Equally weighted responses from three subscales summed to produce an overall score (range 0‐90) |
| ‐LSNS Friendsb | LSNS‐18 subscale assessing size and supportiveness of friends network (range 0‐30) |
| ‐LSNS Relativesb | LSNS‐18 subscale assessing size and supportiveness of relatives network (range 0‐30) |
| ‐LSNS Neighborsb | LSNS‐18 subscale assessing size and supportiveness of neighbors network (range 0‐30) |
| Cognitive activity | Frequency of 11 different cognitive activities (range 0‐55): reading, participating in craft or similar activities, playing games, writing, socializing, using online social networks, participating in “brain training” activities, visiting a library/museum/gallery/exhibition/talk, learning new music or dance, attending a concert/play/musical, and undertaking study or courses 54 |
| Depressionb | The Hospital Anxiety and Depression Scale (HADS). 55 Provides normative cut‐points for normal (0‐7), borderline (8‐10), and high (11‐21) risk of clinical anxiety and depression |
| Anxietyb,c | HADS (as above) |
| Physical activity | Minutes per week of walking, moderate, and vigorous activity were assessed (at least 10 minutes at a time). A score of 3.3, 4, and 8 metabolic equivalent of tasks (METs) was given to each minute of walking, moderate, and vigorous activity respectively per week to calculate sub‐scores. 56 Total physical activity score was calculated by summing the sub‐scores. |
| Categorical variables a | |
| Inattention to Cholesterol | Diagnosis of high cholesterol, having check‐ups, and management were assessed. Low risk was assigned for participants reporting no diagnosis and regular check‐ups, or those with diagnosis but managing the condition; high risk was assigned to participants with diagnosis but no regular check‐ups and/or insufficient management, or participants without diagnosis but no regular check‐ups. |
| Inattention to Diabetes | Diagnosis of diabetes, having check‐ups, and management were assessed. Low and high risk were assigned as above (cholesterol) |
| Smoking | Frequency of smoking was assessed. Low risk was assigned to participants who do not smoke; high risk was assigned for participants who smoke (any frequency). |
| Inattention to Blood pressure | Diagnosis of hypertension, having check‐ups, and management were assessed. Low and high risk were assigned as above (cholesterol) |
All data are self‐reported.
Validated survey instrument.
Anxiety is not a recognized dementia risk factor, but is associated with stress and is an outcome of interest in the current context.
As part of a related project, participants who completed the PD‐MOOC for the first time in May‐June 2020 were invited to semi‐structured interviews with one author (HF). These participants (n = 7) were asked to retrospectively describe changes in their social networks and communication habits during the lockdown period. The experiences described in these interviews are included in this paper to help explain some of the patterns observed in quantitative results.
2.3. Statistical analysis
Some variables (alcohol use, physical activity, and depression) were loge(x+1)‐transformed to improve the normality of residuals (verified using Q‐Q plots). Scale dependent variables were analyzed using multiple linear regression, and multiple logistic regression was applied for dichotomous outcomes. Because each participant was surveyed twice and as our interest was in the population‐level effects of lockdown, P‐values and 95% confidence intervals were calculated using robust standard errors. 26 Analyses were conducted in the R statistical computing environment, version 3.4.3. 27
Model 1 assessed the unadjusted effects of time on alcohol consumption, BMI, diet, social, cognitive, and physical activity, management of blood pressure, cholesterol and blood glucose levels, smoking, and depression and anxiety. Model 2a estimated the effects of time adjusted for covariates: age, gender, work status (retired, employed), cohabitation (alone, with others), and residential remoteness (inner regional, outer regional/remote). Because restrictions were partially eased during the period of T2 survey completion, a supplementary analysis (model 2b) assessed the effect of time adjusting for a main effect of T2 restriction stage and an interaction of time by T2 restriction stage, as well as the other covariates in model 2a. Finally, when significant effects of time were observed, model 3 was fitted to test the interactions between time and potential effect moderators: work status, cohabitation and PD‐MOOC status (exposed, unexposed). The standardized mean difference (SMD) effect estimates should be interpreted as small when SMD = 0.2, medium when SMD = 0.5, and large when SMD = 0.8. 28
2.4. Qualitative analysis
Two authors (HF and MF) independently identified sections of each semi‐structured interview where impacts of the COVID‐19 lockdown were discussed, and reached consensus decisions on which sections to include in this analysis. The identified sections were then inductively coded into themes and sub‐themes by one author (HF) with coding verified by a second author (MF).
3. RESULTS
The characteristics of the analyzed sample were comparable, with the characteristics of all ISLAND participants who provided baseline data in October 2019 (Table 2). The median age was 63 years. Participants were predominantly female (73%), living with another person or “cohabiting” (81%), university qualified (70%), and living in inner‐regional locations (79%). About half were employed (46%). The PD‐MOOC was undertaken between T1 and T2 by 32% of participants.
TABLE 2.
Participant characteristics
| Analysis sample | Population sample | |
|---|---|---|
| n = 1671 | n = 4282 | |
| Gender (Female, n (%)) | 1218 (72.9%) | 3096 (72.3%) |
| Age | ||
| ‐ Mean (SD) | 63.4 (7.17) | 63.2 (7.61) |
| ‐ Median [min, max] | 63.0 [50.0, 88.0] | 63.0 [50.0, 92.0] |
| ‐ 65 years or over, n (%) | 737 (44.1%) | 1817 (42.4%) |
| ‐ Under 65 years, n (%) | 934 (55.9%) | 2465 (57.6%) |
| Living alone, n (%) | 310 (18.6%) | 356 (8.3%) |
| Currently employed, n (%) | 772 (46.2%) | 1990 (46.5%) |
| Educational attainment, n (%) | ||
| ‐ University qualification | 1162 (69.5%) | 2870 (67.0%) |
| ‐ Vocational qualification | 163 (9.8%) | 464 (10.8%) |
| ‐ School only | 280 (16.8%) | 759 (17.7%) |
| Residential remoteness, n (%) | ||
| ‐ Inner regional Australia | 1323 (79.2%) | 3295 (77.0%) |
| ‐ Outer regional/remote Australia | 335 (20.0%) | 955 (22.3%) |
Marked lifestyle changes during lockdown were reported by participants when surveyed at T2. They highlighted reduced engagement in social club meetings, visits and outings with family and friends, and attendance at cultural/ entertainment activities (going to the movies, the theatre, sporting clubs or events, dancing, dining out, or music performances) during the COVID‐19 lockdown period (Appendix A, Table A1).
For each modifiable dementia risk factor investigated, means for each time point and the main effect of time adjusted for covariates (model 2a) are presented in Table 3 and illustrated in Figure 1.
TABLE 3.
Main results: effects of time on dementia risk factors from October 2019 to April‐June 2020 adjusted for age, gender, work status, cohabitation, and residential remoteness (model 2a)
| T1 (October 2019) | T2 (April‐May 2020) | Model 2aa | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Continuous variables | n | M | 95% CI | n | M | 95% CI | SMD | P | ||
| Alcoholc | 1474 | 3.05 | 2.85 | 3.27 | 1571 | 3.34 | 3.11 | 3.57 | ‐0.07 | <0.001 |
| BMIc | 1506 | 26.24 | 26.02 | 26.47 | 1659 | 26.38 | 26.14 | 26.62 | ‐0.03 | 0.008 |
| MIND diet | 1653 | 9.46 | 9.38 | 9.53 | 1284 | 9.81 | 9.72 | 9.89 | 0.22 | <0.001 |
| LSNS Total | 1671 | 44.83 | 44.25 | 45.42 | 1671 | 43.95 | 43.95 | 45.09 | ‐0.03 | 0.075 |
| ‐LSNS Friends network | 1671 | 16.75 | 16.48 | 17.01 | 1671 | 16.33 | 16.07 | 16.59 | ‐0.08 | <0.001 |
| ‐LSNS Relatives network | 1671 | 18.28 | 18.01 | 18.54 | 1671 | 18.32 | 18.08 | 18.56 | 0.01 | 0.568 |
| ‐LSNS Neighbors Network | 1671 | 9.81 | 9.53 | 10.09 | 1671 | 9.87 | 9.59 | 10.15 | 0.01 | 0.527 |
| Cognitive activity | 1659 | 33.29 | 33.03 | 33.55 | 1646 | 33.75 | 33.49 | 34.00 | 0.09 | <0.001 |
| Depressionc | 1671 | 2.07 | 1.97 | 2.17 | 1671 | 2.05 | 1.95 | 2.16 | ‐0.01 | 0.593 |
| Anxiety | 1671 | 5.56 | 5.39 | 5.73 | 1671 | 4.88 | 4.72 | 5.04 | ‐0.20 | <0.001 |
| Physical activityc | 1570 | 1634.87 | 1563.11 | 1709.93 | 1668 | 1934.93 | 1858.43 | 2014.57 | 0.19 | <0.001 |
| Categorical variablesb | n | % | 95% CI | n | % | 95% CI | SMD | p | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Cholesterol management | 1653 | 17.00 | 15.25 | 18.90 | 1665 | 14.05 | 12.45 | 15.82 | ‐0.12 | 0.002 |
| Diabetes management | 1609 | 16.09 | 14.39 | 17.95 | 1628 | 10.44 | 9.04 | 12.02 | ‐0.27 | <0.001 |
| Smoking | 1628 | 3.12 | 2.37 | 4.09 | 1629 | 2.52 | 1.85 | 3.41 | ‐0.12 | 0.020 |
| Blood pressure management | 1621 | 6.49 | 5.39 | 7.79 | 1625 | 5.72 | 4.69 | 6.96 | 0.07 | 0.275 |
aModel 2a:adjusted main effect of time.
bProportion of sample meeting criteria for high‐risk behavior.
cln(x+1) transformed.
Variables: Alcohol, number of standard drinks per week; BMI, body mass index = [weight (kilograms)/height (meters) 2]; MIND diet, adherence to the Mediterranean‐DASH diet Intervention for Neurodegenerative Delay (MIND) diet excluding wine consumption, sum of scores (0, 0.5, or 1) for each category; LSNS, Lubben Social Networks Scale and subscales; Cognitive activity, summed frequency of 11 different cognitive activities; Anxiety and Depression, Hospital Anxiety and Depression Scale; Physical activity, sum of metabolic equivalent of tasks (METs) for walking, moderate, and vigorous activity; Inattention to cholesterol, low risk behavior = no diagnosis and regular check‐ups, or with diagnosis that is monitored and managed; high risk behavior = diagnosed but no regular check‐ups and/or insufficient management, or no diagnosis but no regular check‐ups; Inattention to diabetes, diagnosis of diabetes, having check‐ups, and management were assessed. Low and high‐risk behavior were assigned as for cholesterol; Smoking, low risk = do not smoke, high risk = smokers (any frequency); Inattention to blood pressure, diagnosis of hypertension, having check‐ups, and management were assessed. Low and high‐risk behavior were assigned as for cholesterol.
FIGURE 1.
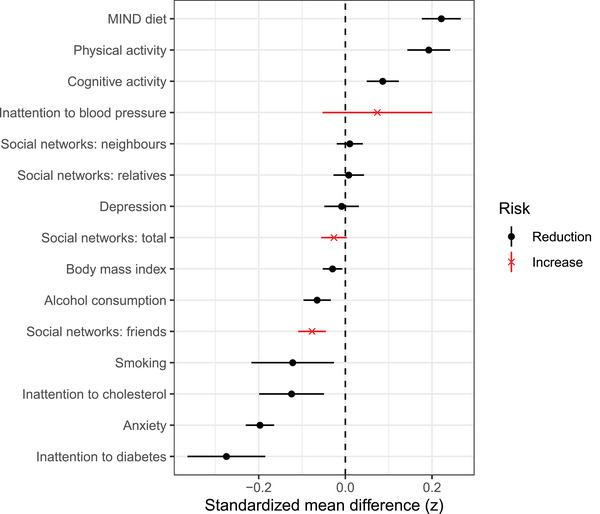
Standardized mean difference with 95% CI from T1 to T2 for each of the assessed variables (model 2a)
HADS anxiety scores were lower at T2 than T1 (SMD = −0.20, 95% confidence interval [CI] −0.23 to −0.16), whereas physical activity increased at T2 (SMD = 0.19, 95% CI 0.14 to 0.24). Participants also reported stronger adherence at T2 to the MIND diet (SMD = 0.22, 95% CI 0.18 to 0.27) and the proportion of participants in the high‐risk category for attention to diabetes reduced (SMD = −0.27, 95% CI −0.36 to −0.18). Cognitive activity improved (SMD = 0.09, 95% CI 0.05 to 0.12) and improvements were observed in the proportion of participants in the high‐risk category for attention to cholesterol (SMD = −0.12, 95% CI −0.20 to −0.05) and smoking (SMD = −0.12, 95% CI −0.22 to −0.03). BMI (SMD = −0.3, 95% CI −0.05 to −0.01) and alcohol consumption (SMD = −0.07, 95% CI −0.10 to −0.03) decreased from T1 to T2. The only detrimental effect observed was a decrease in social contact with friends (SMD = −0.08, 95% CI −0.11 to −0.04). Although these effects were all significant, the magnitude of change ranged from small to trivial. No significant changes from T1 to T2 were evident for depression, attention to blood pressure, or social networks (other than friends). When controlling for T2 restriction stage in model 2b (Appendix A, Table A2), improvements between T1 and T2 in anxiety, physical activity, diet, attention to diabetes, cognitive activity, and alcohol consumption were still evident, but although the direction was stable, the differences between T1 and T2 in BMI, cholesterol management, and smoking were no longer significant.
Figure 2 illustrates results from our tests of interaction between the effects of time and PD‐MOOC exposure, cohabitation, and employment status (model 3). Compared with unexposed participants, those exposed to the PD‐MOOC increased adherence to the MIND diet (SMD = 0.21, 95% CI 0.12 to 0.31, P < .001) and cognitive activity (SMD = 0.16, 95% CI 0.08 to 0.24, P < .001) during lockdown (Figure 2A and 2B). Compared with living alone, cohabiting also positively affected cognitive activity (SMD = 0.14, 95% CI 0.05 to 0.23, P = .002; Figure 2C). The magnitude of effects was very small for each of the significant interactions.
FIGURE 2.
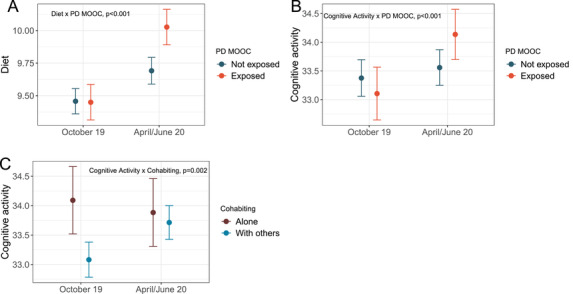
Interactions of time with PD‐MOOC exposure and cohabitation status at T2. Error bars‐ 95% CI. PD‐MOOC‐ Preventing Dementia Massive Open Online Course. Unadjusted means for Diet‐ adherence to the Mediterranean‐DASH Intervention for Neurodegenerative Delay (MIND) diet excluding wine consumption, sum of scores (0, 0.5, or 1) for each category; Cognitive activity‐ summed frequency of 11 different cognitive activities
In semi‐structured interviews, participants described a range of explanations for the impacts of the COVID‐19 lockdown on their modifiable dementia risk behavior (Appendix A, Table A3). These participant's personal accounts were consistent with and provide insight into the results of our quantitative analysis. For example, they reported using alternative communication methods during lockdown and variable adherence to lockdown restrictions.
4. DISCUSSION
This study compared longitudinal data collected routinely as part of the ISLAND Project in October 2019 and April‐June 2020. These data provided a unique opportunity to evaluate the impact of COVID‐19 lockdown measures on dementia risk factors in an Australian sample of adults 50+ years of age. Surprisingly, in contrast to our hypotheses, we saw no increase in dementia risk‐related states and behaviors across the study period other than marginally reduced social engagement with friends.
We observed a small reduction in anxiety scores and no change in depression scores. Adherence to the MIND diet showed a small improvement, as did attention to diabetes and participation in physical activity. Changes in BMI, cognitively stimulating activity, attention to cholesterol, alcohol consumption, and smoking were all significant and positive, albeit very small in magnitude. Although the adjusted effects of time on BMI, attention to cholesterol, and smoking lost significance when controlling for the staged easing of lockdown restrictions, no detrimental results were observed. These observations do not imply that the lockdown period was beneficial for dementia risk behaviors, but they do challenge prevailing narratives about generalized detrimental changes in psychological health, alcohol use, and eating patterns during the COVID‐19 pandemic. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24
The data collected in qualitative interviews offer some plausible explanations for the absence of detrimental dementia risk outcomes as a result of COVID‐19 lockdown in Tasmania (Appendix A, Table A3). For example, the quantitative finding that cognitive activity increased across the cohort may be partially explained by interview respondents’ reports of having more time during lockdown to participate in educational opportunities like the PD‐MOOC. Similarly, the increase in physical activity levels may be partially explained by interview respondents’ reports of using exercise as a way to connect with friends (visiting each other's homes was not permitted but going for a walk together was). In addition, the quantitative finding that total social network size was not significantly affected by the COVID‐19 lockdown may be partially explained by interview respondents’ reports of exercising with friends, using alternate communication methods, and only partially adhering to lockdown restrictions.
The Tasmania Project (https://www.utas.edu.au/tasmania‐project) 29 was a separate, concurrent cross‐sectional study of experiences during the pandemic in a sample with similar characteristics to the ISLAND cohort (relatively highly educated, living mostly in southern Tasmania). The Tasmania Project findings from April 27 to May 10 suggest that participants 65+ years of age were more concerned about COVID‐19 than younger people but felt well informed about symptoms and how to respond to them. They were less concerned than younger people about social isolation impacting their physical and mental health. Other studies have found higher anxiety in younger people during the pandemic. 12 , 16 , 31 Furthermore, The Tasmania Project results showed that anxiety levels were inversely correlated with self‐reported knowledge about how to protect oneself from, and respond to, COVID‐19. 30 It is therefore plausible that the reduced anxiety observed in the current study was due to ISLAND participants having confidence in their health management and capacity to cope with societal changes. The lack of substantive community transmission of COVID‐19 in Tasmania may also have played a role.
The decrease in anxiety during lockdown observed in this study is at odds with other findings in the literature. During the early stages of the pandemic in China, most participants surveyed using the Depression, Anxiety, and Stress Scale (DASS‐21) self‐reported moderate or severe psychological impacts. 11 Similarly, when Australian adults of all ages were asked to estimate the change in their anxiety levels, comparing early April to before the pandemic, they described increased subjective levels of anxiety. 13 , 32 Since then, many cross‐sectional studies in different populations and countries have reported that the pandemic has had detrimental effects on mental health using instruments such as the HADS, Generalized Anxiety Disorder Scale‐7 (GAD‐7), DASS‐21, Patient Health Questionnaire‐2, 4 (PHQ‐2, 4), and the Edinburgh Depression Scale (EPDS). 12 , 13 , 14 , 15 , 16 , 31 , 33 , 34 , 35 , 36 , 37 , 38 Other studies which used the HADS, as we did, reported increased anxiety and depression, 37 , 38 suggesting that differences between survey instruments were not responsible for the divergence of our findings. Some studies compared prevalence data with standardized data from analogous pre‐pandemic cohorts (eg, 15 , 16 , 38 ). Other studies asked participants to retrospectively estimate the change in their anxiety levels (eg, 13 , 36 ). Our study is the first, to our knowledge, to use longitudinal data and find that anxiety was not exacerbated during the lockdown period.
Weight gain and increased snacking during COVID‐19 lockdown (or “covibesity”) has been reported, 24 , 25 but our participants slightly improved adherence to the MIND diet during lockdown and did not increase their BMI. It is feasible that this contrary finding may be due to involvement in the ISLAND Project and PD‐MOOC, in which adherence to the MIND diet is promoted for reducing dementia risk. Indeed, participants exposed to the PD‐MOOC demonstrated a significantly greater positive change in their MIND diet scores. We did not assess calorie intake or eating patterns, so it is unclear whether levels of snacking changed. However, we did observe increased physical activity, which, combined with improved diet, could explain the BMI result. Qualitative data suggest that some participants used exercise to maintain contact with others during lockdown (Appendix A, Table A3), as exercising outdoors was permitted in Tasmania in this period. Runners, joggers, walkers, and cyclists were unimpacted, whereas others may have found exercise more difficult as public pools, beaches, and gyms closed. It is possible that increased physical activity may have contributed to the observed decrease in anxiety, as has been reported in a study of Spanish adults. 39
Cognitive activity increased from T1 to T2. Although the change was very small (SMD < 0.1), stronger increases were observed for participants who undertook the PD‐MOOC, and for people who were not living alone. The impact of the PD‐MOOC on cognitive activity may be circular, since people inclined to fill their available time by doing the course will have increased their cognitive activity. People cohabiting had lower levels of cognitive activity at T1 than those living alone, but increased this by T2. The influence of cohabitation on cognitive activity could be due to there being people available with whom to play, read, or learn in company; one participant described completing jigsaw puzzles while at home with their partner during lockdown (Appendix A, Table A3). However, we found no evidence of a negative effect of living alone on depression or anxiety. Although unexpected, this result supports prior research that shows that the links between social isolation and mental health are not clear‐cut. 40
Alcohol use and smoking decreased to a very small but significant extent from T1 to T2. Although increased alcohol consumption is a frequently reported consequence of lockdown in COVID‐19 research, 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 33 , 41 , 42 , 43 , 44 the current study is among the first to quantify alcohol consumption longitudinally during lockdown. Lockdown is a risk factor for people with coping‐related alcohol use disorders, 45 contributing to relapse for people who are abstinent. 20 Given the potential impact of alcohol‐related harms 41 and the influence of excessive alcohol on dementia risk 8 it is important that longitudinal, case‐matched data are used to assess trends in consumption. As with the anxiety findings, our result for alcohol also challenges common narratives about impacts of the pandemic behaviors observed during COVID‐19 lockdown. 19
Sustained increases in alcohol use and smoking have been observed following past pandemics. 46 , 47 However, data from The Tasmania Project show that younger people (18 to 44 years) were more likely than older people (65+ years) to report increased alcohol consumption at home. 30 Other literature indicates that the risk of increased drinking during COVID‐19 is higher for people who are younger, have a lower level of education, and higher level of perceived stress, anxiety, or depression. 18 , 21 , 23 , 33 , 42 It is important to note that given the older, well‐educated, and healthy profile of current study participants, the observed changes in smoking and alcohol use are unlikely to be representative of the Tasmanian population. Instead, our result may be illustrative of the protective benefits of underlying motivations to maintain healthy behaviors, indicated by active engagement with the ISLAND dementia risk reduction project. Regardless, our results suggest that during lockdown, study participants did not increase either smoking or alcohol intake.
Societal changes during COVID‐19 have led to decreases in some forms of social interaction, and, in our study, this was reflected in the changes noted in friend networks, the frequency of community meetings and cultural pursuits, and changes reported in interviews in the way people communicate with friends and family. Older people were believed to be at greater risk of loneliness and social isolation during lockdown, 1 , 6 and our results may partially support this. Because social and cultural activity in later life is associated with cognitive function and dementia risk, 8 initiatives to facilitate health behaviors and alternative means of social engagement are warranted for those facing extended periods of social isolation. This recommendation is also proposed in response to findings from a cross‐sectional analysis of retrospective data collected in June 2020 from participants in the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability. 48 , 49
The cohort participating in this ISLAND sub‐study may differ in important ways from cohorts in other studies. Most of the studies identified in our literature review were cross‐sectional, with limited sensitivity to changes across time. Many also included younger participants, who have been shown to respond more acutely to lockdown. 12 , 16 , 30 , 31 , 33 In addition, many were conducted in countries with greater community transmission such as the United States, Germany, and the United Kingdom. It is likely that these and other factors underpinned the divergence of our findings from others in the literature.
This study has some limitations. It is not appropriate to claim a causal link between the lockdown measures associated with COVID‐19 and the behavioral trends observed in this study. There are likely a range of factors influencing the changes, as indicated in the interview data (Appendix A, Table A3). Furthermore, the ISLAND project provides participants with feedback on their modifiable dementia risk factors, tips for achieving low‐risk status, and the opportunity to do the PD‐MOOC, exposures that may have independently led to behavior modification. The study was conducted online, so a selection bias toward people with high computer literacy and cognitive functioning is acknowledged. People with limited online access are underrepresented in this cohort and may have experienced lockdown differently from those in our study. Self‐reported behaviors (for example, exercise, alcohol, smoking) are subject to responder bias, and changes in work circumstances and associated financial strain were not assessed. The clinical significance of the small positive changes in risk reduction behaviors we observed is not clear. Small changes at population level can yield an important public health impact. However, such changes are most meaningful for people above thresholds for high risk, and analyses of these subgroups in our (generally healthy) sample were not conducted. We acknowledge that our sample is relatively stable and less urban, and may have higher levels of social capital, which can help support resilience to major community challenges and disasters. 50 Finally, we did not include exposure to the COVID‐19 virus in our investigation.
In summary, this longitudinal study found that the early stages of COVID‐19 lockdown did not lead to a universal increase in dementia risk‐related behaviors in adults 50+ years of age in Tasmania. Apart from a minor attenuation of social engagement with friends, our participants’ dementia risk behaviors were either maintained or improved. These results counter the dominant narratives of pandemic‐related distress and suggest that engaging at‐risk populations in proactive health promotion and education campaigns during lockdown scenarios could be a protective public health strategy.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The contributions made by our study participants, the Wicking Centre data managers (Alex Kitsos and Timothy Saunder), and the ISLAND Project Team (Helen Douglas, Justine Keay and Dr Adam Kane) are gratefully acknowledged. This was a sub‐study of the ISLAND Project, which is supported by the Medical Research Futures Fund—Keeping Tasmanians out of Hospital, the University of Tasmania, St Lukes Health, and the Masonic Centenary Medical Research Foundation.
Bartlett L, Brady JJR, Farrow M, et al. Change in modifiable dementia risk factors during COVID‐19 lockdown: The experience of over 50s in Tasmania, Australia. Alzheimer's Dement. 2021;7:e12169. 10.1002/trc2.12169
DATA AVAILABILITY STATEMENT
Scripts used to prepare the models and conduct analyses can be accessed online. 51 The data can be made available under a transfer agreement. Direct requests to the corresponding author.
REFERENCES
- 1. Beck MJ, Hensher DA. Insights into the impact of COVID‐19 on household travel and activities in Australia–The early days under restrictions. Transport Policy. 2020;96:76‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID‐19 cases: a systematic literature review and meta‐analysis. J Infect. 2020;81:16‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rimmer A. Covid‐19: GPs can stop health checks for over 75s and routine medicine reviews. BMJ. 2020;368:m1157. [DOI] [PubMed] [Google Scholar]
- 5. Alexander GC, Qato DM. Ensuring access to medications in the US during the COVID‐19 pandemic. JAMA. 2020;324:31‐32. [DOI] [PubMed] [Google Scholar]
- 6. Courtin E, Knapp M. Social isolation, loneliness and health in old age: a scoping review. Health Soc Care Community. 2017;25:799‐812. [DOI] [PubMed] [Google Scholar]
- 7. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673‐2734. [DOI] [PubMed] [Google Scholar]
- 8. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. 2020:413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu JT, Xu W, Tan CC, et al. Evidence‐based prevention of Alzheimer's disease: systematic review and meta‐analysis of 243 observational prospective studies and 153 randomised controlled trials. J Neurol Neurosurge Psychiat. 2020;91:1201‐1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prince MJ, Wimo A, Guerchet M, Ali G‐C, Wu Y‐T, Prina M. World Alzheimer's Report 2019: The Global Impact of Dementia. An Analysis of Prevalence, Incidence, Cost and Trends. London: Alzheimer's Disease International; 2015. [Google Scholar]
- 11. Wang C, Pan R, Wan X, et al. Immediate psychological responses and associated factors during the initial stage of the 2019 Coronavirus disease (COVID‐19) epidemic among the general population in China. Int J Environment Res Public Health. 2020;17:1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang Y, Zhao N. Generalized anxiety disorder, depressive symptoms and sleep quality during COVID‐19 outbreak in China: a web‐based cross‐sectional survey. Psychiatry Res. 2020;288:112954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Newby JM, O'Moore K, Tang S, Christensen H, Faasse K. Acute mental health responses during the COVID‐19 pandemic in Australia. PLoS One. 2020;15:e0236562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barzilay R, Moore TM, Greenberg DM, et al. Resilience, COVID‐19‐related stress, anxiety and depression during the pandemic in a large population enriched for healthcare providers. Translational Psychiatry. 2020;10:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lebel C, MacKinnon A, Bagshawe M, Tomfohr‐Madsen L, Giesbrecht G. Elevated depression and anxiety symptoms among pregnant individuals during the COVID‐19 pandemic. J Affect Disord. 2020;277:5‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bäuerle A, Teufel M, Musche V, et al. Increased generalized anxiety, depression and distress during the COVID‐19 pandemic: a cross‐sectional study in Germany. J Public Health (Oxf). 2020;42:672‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Biddle N, Edwards B, Gray M, Sollis K. Alcohol Consumption During the COVID‐19 Period. ANU Centre for Social Research & Methods; 2020. [Google Scholar]
- 18. Tran TD, Hammarberg K, Kirkman M, Nguyen HTM, Fisher J. Alcohol use and mental health status during the first months of COVID‐19 pandemic in Australia. J Affect Disord. 2020;277:810‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Colbert S, Wilkinson C, Thornton L, Richmond R. COVID‐19 and alcohol in Australia: industry changes and public health impacts. Drug Alcohol Rev. 2020;39:435‐440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim JU, Majid A, Judge R, et al. Effect of COVID‐19 lockdown on alcohol consumption in patients with pre‐existing alcohol use disorder. Lancet Gastroenterol Hepatol. 2020;5:886‐887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koopmann A, Ekaterini G, Falk K, Hillemacher T. Did the general population in Germany drink more alcohol during the COVID‐19 pandemic lockdown?. Alcohol Alcoholism. 2020;55:698‐699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lechner WV, Laurene KR, Patel S, Anderson M, Grega C, Kenne DR. Changes in alcohol use as a function of psychological distress and social support following COVID‐19 related University closings. Addict Behav. 2020;110:106527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Neill E, Meyer D, Toh WL, et al. Alcohol use in Australia during the early days of the COVID‐19 pandemic: initial results from the COLLATE project. Psychiatry Clin Neurosci. 2020:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sidor A, Rzymski P. Dietary choices and habits during COVID‐19 lockdown: experience from Poland. Nutrients. 2020;12:1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khan MA, Moverley Smith JE. “Covibesity,” a new pandemic. Obesity Med. 2020;19:100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harrell FE, rms: Regression Modeling Strategies. R package version 6.0‐1. 2020.
- 27. R Core Team . R: A Language and environment for statistical computing. https://cran.r‐project.org/. 2017.
- 28. Cohen J. Statistical Power and Analysis for the Behavioral Sciences. NJ: Lawrence: Hillsdale; 1988. [Google Scholar]
- 29. Lester L, Steel R, Denny L, et al. Preliminary report on survey and interview findings from The Tasmania Project. http://blogs.utas.edu.au/isc/2020/05/19/the‐tasmania‐project‐initial‐findings/. 2020.
- 30. Brady JJ, Sinclair D, How are older Tasmanians experiencing the pandemic? http://blogs.utas.edu.au/isc/2020/06/04/how‐are‐older‐tasmanians‐experiencing‐the‐pandemic‐report‐12/2020.
- 31. Smith L, Jacob L, Yakkundi A, et al. Correlates of symptoms of anxiety and depression and mental wellbeing associated with COVID‐19: a cross‐sectional study of UK‐based respondents. Psychiatry Res. 2020;291:113138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Van Rheenen TE, Meyer D, Neill E, et al. Mental health status of individuals with a mood‐disorder during the COVID‐19 pandemic in Australia: initial results from the COLLATE project. J Affect Disord. 2020;275:69‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stanton R, To QG, Khalesi S, et al. Depression, anxiety and stress during COVID‐19: associations with changes in physical activity, sleep, tobacco and alcohol use in Australian adults. Int J Environment Res Public Health. 2020;17:4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shi L, Lu ZA, Que JY, et al. Prevalence of and risk factors associated with mental health symptoms among the general population in China during the Coronavirus disease 2019 Pandemic. JAMA. 2020;3:e2014053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Raj A, Johns NE, Barker KM, Silverman JG. Time from COVID‐19 shutdown, gender‐based violence exposure, and mental health outcomes among a state representative sample of California residents. EClinicalMedicine. 2020;26:100520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Van Rheenen TE, Meyer D, Neill E, et al. Mental health status of individuals with a mood‐disorder during the COVID‐19 pandemic in Australia: initial results from the COLLATE project. J Affect Disord. 2020;275:69‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Özdin S, Bayrak Özdin Ş. Levels and predictors of anxiety, depression and health anxiety during COVID‐19 pandemic in Turkish society: the importance of gender. Int J Soc Psychiatry. 2020;66:504‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu M, Han H, Lin T, et al. Prevalence and risk factors of mental distress in China during the outbreak of COVID‐19: a national cross‐sectional survey. Brain Behav. 2020;10:e01818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. López‐Bueno R, Calatayud J, Ezzatvar Y, et al. Association between current physical activity and current perceived anxiety and mood in the initial phase of COVID‐19 confinement. Front Psychiatry. 2020;11:729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cornwell EY, Waite LJ. Social disconnectedness, perceived isolation, and health among older adults. J Health Soc Behav. 2009;50:31‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ramalho R. Alcohol consumption and alcohol‐related problems during the COVID‐19 pandemic: a narrative review. Australas Psychiatry. 2020:1039856220943024. [DOI] [PubMed] [Google Scholar]
- 42. Rodriguez LM, Litt DM, Stewart SH. Drinking to cope with the pandemic: the unique associations of COVID‐19‐related perceived threat and psychological distress to drinking behaviors in American men and women. Addict Behav. 2020;110:106532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sun Y, Li Y, Bao Y, et al. Brief report: increased addictive internet and substance use behavior during the COVID‐19 pandemic in China. Am J Addict. 2020;29:268‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yehudai M, Bender S, Gritsenko V, Konstantinov V, Reznik A, Isralowitz R. COVID‐19 fear, mental health, and substance misuse conditions among University social work students in Israel and Russia. Int J Mental Health Addict. 2020:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wardell JD, Kempe T, Rapinda KK, et al. Drinking to cope during COVID‐19 pandemic: the role of external and internal factors in coping motive pathways to alcohol use, solitary drinking, and alcohol problems. Alcoholism: Clin Experiment Res. 2020:1‐11. [DOI] [PubMed] [Google Scholar]
- 46. Esterwood E, Saeed SA. Past epidemics, natural disasters, COVID19, and mental health: learning from history as we deal with the present and prepare for the future. Psychiatr Q. 2020;91:1121‐1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu P, Liu X, Fang Y, et al. Alcohol abuse/dependence symptoms among hospital employees exposed to a SARS outbreak. Alcohol Alcoholism. 2008;43:706‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at‐risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255‐2263. [DOI] [PubMed] [Google Scholar]
- 49. Lehtisalo J, Palmer K, Mangialasche F, Solomon A, Kivipelto M, Ngandu T. Changes in lifestyle, behaviors, and risk factors for cognitive impairment in older persons during the first wave of the Coronavirus disease 2019 pandemic in Finland: results from the FINGER Study. Front Psychiat. 2021;12:624125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aldrich DP, Meyer MA. Social capital and community resilience. Am Behav Sci. 2014;59:254‐269. [Google Scholar]
- 51. Bartlett L, Brady JJR, Farrow M, et al. Data From: Change in Modifiable Dementia Risk Factors During COVID‐19 Lockdown: the Experience of Over 50s in Tasmania. Australia: University of Tasmania; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Morris MC, Tangney CC, Wang Y, et al. MIND diet slows cognitive decline with aging. Alzheimer's Dement. 2015;11:1015‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lubben J, Gironda M. Centrality of Social Ties to the Wellbeing of Older Adults. In: Berkman B, Harootyan L, eds. Social Work and Health Care in an Aging Society: Education, Policy, Practice and Research. New York: Springer; 2003:319‐350. [Google Scholar]
- 54. Anstey KJ, Cherbuin N, Herath PM. Development of a new method for assessing global risk of Alzheimer's disease for use in population health approaches to prevention. Prev Sci. 2013;14:411‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361‐370. [DOI] [PubMed] [Google Scholar]
- 56. Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12‐country reliability and validity. Med Sci Sports Exercise. 2003;35:1381‐1395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
Scripts used to prepare the models and conduct analyses can be accessed online. 51 The data can be made available under a transfer agreement. Direct requests to the corresponding author.


