Abstract
We discuss the use of urine electrolytes and urine osmolality in the clinical diagnosis of patients with fluid, electrolytes, and acid-base disorders, emphasizing their physiological basis, their utility, and the caveats and limitations in their use. While our focus is on information obtained from measurements in the urine, clinical diagnosis in these patients must integrate information obtained from the history, the physical examination, and other laboratory data.
Keywords: hyperkalemia, hypokalemia, hyponatremia, polyuria, renal tubular acidosis, urine electrolytes, urine osmolality
We discuss the use of urine electrolytes and urine osmolality (UOsm) in the clinical diagnosis of some disorders of fluid, electrolytes, and acid-base balance.1, 2, 3 Whereas there are usual ranges for the rates of excretion of water and electrolytes and the UOsm, there are no “normal” values. Data should be interpreted in the context of the expected renal response for the clinical situation. For example, a rate of potassium (K+) excretion of 60 mmol/d is within the usual range for K+ excretion in adults but indicates renal K+ wasting in a patient with hypokalemia and a defect in renal K+ excretion in a patient with hyperkalemia.
Patients With Potassium Disorders
In response to a low dietary K+ intake, the rate of K+ excretion in normal individuals fell to 10 to 15 mmol/d.4,5 The rate of K+ excretion rose to match K+ intake in normal individuals given a large K+ load (>200 mmol/d) on a long-term basis, with only a modest rise in the plasma K+ concentration (PK).6,7
The urine tests that have been commonly used to assess the renal response to hypokalemia or hyperkalemia are the calculation of the transtubular K+ concentration gradient (TTKG) and the ratio of the concentration of K+ and the concentration of creatinine in a spot urine sample (UK/UCreatinine).
The TTKG
The TTKG was proposed as a semiquantitative index of the driving force for K+ secretion in the aldosterone-sensitive distal nephron (ASDN), which includes the second part of the distal convoluted tubule, the connecting segment, and the cortical collecting duct (CCD).8,9 The TTKG is the ratio of the K+ concentration in the luminal fluid at the end of the CCD (KCCD), and the PK. To estimate KCCD, UK is adjusted for water reabsorption in the medullary collecting duct (MCD), by dividing UK by the ratio of UOsm to plasma osmolality (POsm), because the osmolality of the fluid at the end of the CCD should be equal to POsm when arginine vasopressin (AVP) acts.
The assumption in this calculation is that there is no appreciable reabsorption of osmoles in the MCD. Although this is largely correct for electrolytes, it is not for urea because of the process of intrarenal urea recycling.10,11 Extrapolating from micropuncture studies in rats, we estimate that in adult individuals consuming a typical Western diet, ∼600 mmol of urea per day is reabsorbed in the inner MCD and recycled back to the distal convoluted tubule.12,13 This adds 2 l/d to the volume of fluid at the end of the CCD (600 osm divided by an osmolality of 300 mosm/kg water [H2O]),which constitutes ∼40% of this volume (~5 l/d).13 Not accounting for urea recycling considerably overestimates KCCD because it considerably underestimates the flow rate in the terminal CCD. This error cannot be adjusted for because a number of factors affect the process of urea recycling, including protein intake, hormones (AVP, angiotensin II, aldosterone), cytotoxins, drugs (e.g., cyclosporine), medullary interstitial disease, and chronic renal dysfunction.14, 15, 16, 17, 18, 19, 20, 21
Because of this error, we do not use the TTKG in the clinical assessment of the patients with dyskalemias.13,22
UK/UCreatinine
Because creatinine is excreted at a nearly constant rate throughout the day, one can use the UK/UCreatinine to assess the rate of K+ excretion.13,23, 24, 25, 26 A caveat in this calculation, as in any calculation that uses the ratio of the concentration of a substance to the concentration of creatinine in a spot urine sample to estimate a 24-hour excretion rate, is that the rate of creatinine excretion, which depends on muscle mass, may be appreciably different in the patient from the usual rate of creatinine excretion (∼10 mmol/d [∼1 g/d]).
Patients With Hypokalemia
Hypokalemia could be due to the acute shift of K+ into cells or chronic loss of K+ via a renal or extrarenal route.
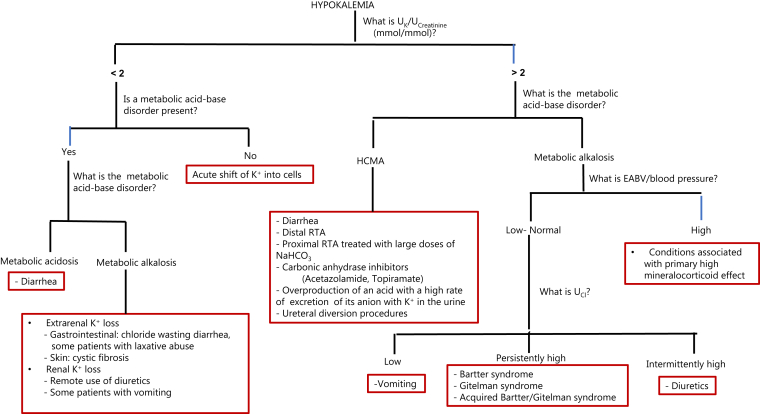
The expected UK/UCreatinine in patients with hypokalemia due to an intracellular shift of K+ is <2 mmol K+/mmol creatinine (<18 mmol K+/g creatinine).23,24 These low values, however, may be also observed in patients with hypokalemia due to extrarenal K+ loss (e.g., patients with diarrhea or laxative abuse) and in some patients with hypokalemia due to renal K+ loss (e.g., remote use of diuretics, some patients with chronic vomiting). These patients can be differentiated from those with hypokalemia due to an intracellular shift of K+ by the presence of a metabolic acid-base disorder. Patients with hypokalemia due to large-volume diarrhea frequently have hyperchloremic metabolic acidosis (HCMA), and patients with hypokalemia due to vomiting or the use of diuretics frequently have metabolic alkalosis (Figure 1). Patients with hypokalemia due to laxative abuse, however, commonly have no significant metabolic acid-base disorder or only a small increase in plasma bicarbonate (HCO3−) concentration.27
Figure 1.
Urine data in the clinical approach to the patients with hypokalemia. A urine potassium (UK)/urine creatinine (UCreatinine) <2 and the absence of a metabolic acid-base disorder suggest that the major cause of hypokalemia is an acute intracellular shift of K+. The UK/UCreatinine may be <2 in some patients with renal K+ loss (e.g., some patients with vomiting) and >2 in some patients with extrarenal K+ loss (some patients with diarrhea). The causes for renal K+ loss can be differentiated by the associated metabolic-acid base disorder. In patients with metabolic alkalosis, further differential diagnosis is based on assessment of the effective arterial blood volume (EABV) and the blood pressure. In the group with low/normal EABV and blood pressure, measurement of chloride concentration in the urine (UCl) provides useful clues to determine the cause of the renal K+ loss. Conditions associated with primary increased mineralocorticoid effect include primary hyperreninemic hyperaldosteronism (e.g., renal artery stenosis, renin-secreting tumor), primary hyperaldosteronism (e.g., adrenal adenoma, bilateral adrenal hyperplasia, glucocorticoid-remediable hyperaldosteronism), conditions in which cortisol acts as a mineralocorticoid (e.g., apparent mineralocorticoid excess syndrome, ingestion of compounds containing glycyrrhizic acid which inhibits 11 β hydroxysteroid dehydrogenase-2, adrenocorticotropic hormone-producing tumor), and constitutively active epithelial sodium channel (e.g., Liddle syndrome). RTA, renal tubular acidosis; HCMA, hyperchloremic metabolic acidosis; NaHCO3, sodium bicarbonate.
In patients with chronic hypokalemia, the first step is to examine the acid-base status in plasma.23,28, 29, 30, 31, 32 Patients with metabolic acidosis commonly have HCMA (Figure 1). Notwithstanding, although the hypokalemia in patients with diarrhea is largely due to the loss of K+ in diarrhea fluid, the UK/UCreatinine may be high if renal excretion of K+ is stimulated because of aldosterone released in response to low effective arterial blood volume (EABV), associated magnesium depletion and the effect of acidemia to decrease the reabsorption of Na+ and Cl− in the proximal convoluted tubule (PCT), the increased delivery of Na+ and Cl− to the ASDN may result in a higher rate of K+ excretion because of increased rate of electrogenic reabsorption of Na+ and increased flow rate in the ASDN.33,34
In patients with chronic hypokalemia and metabolic alkalosis, UK/UCreatinine <2 mmol K+/mmol creatinine suggests extrarenal K+ loss; for example, in sweat (patients with cystic fibrosis) or via the intestinal tract (patients with congenital or acquired chloride wasting diarrhea due to decreased activity of the colonic downregulated in adenoma anion exchanger). Hypokalemia in patients with vomiting is largely due to renal loss of K+ because of stimulation of K+ secretion in the ASDN by increased delivery of sodium bicarbonate (NaHCO3)35, 36, 37; the UK/UCreatinine may be low in these patients in the absence of increased distal delivery of NaHCO3.
Patients with metabolic alkalosis and UK/UCreatinine higher than stated above have renal K+ loss. The primary pathophysiology is an increased number of open epithelial Na channels in the luminal membrane of principal cells in the ASDN leading to a higher rate of electrogenic Na+ reabsorption.25,38 This could be due to two groups of disorders. Patients in the first group have low EABV causing the release of aldosterone. Patients in the second group have conditions associated with a primary high mineralocorticoid effect.
The use of chloride concentration in the urine (UCl) in the differential diagnosis in patients in the first group is shown in Figure 1. The diuretic effect in some patients may be due to an inherited disorder affecting NaCl reabsorption in the medullary thick ascending limb of the loop of Henle (i.e., Bartter syndrome) or the distal convoluted tubule (i.e., Gitelman syndrome).39,40 A clinical picture that mimics Bartter syndrome may result from activation of the calcium-sensing receptor in the medullary thick ascending limb of the loop of Henle by calcium in patients with hypercalcemia or by other cationic ligands (e.g., gentamicin, amikacin, cisplatin, and possibly cationic immunoglobulins).41, 42, 43, 44 Acquired Gitelman syndrome has been reported in some patients with autoimmune diseases.44, 45, 46
Measurement of UCl in multiple urine samples may help in differentiating patients with Bartter syndrome or Gitelman syndrome (persistently high UCl) from those with diuretic abuse (intermittently high UCl). An assay for diuretics in the urine, if required, should be performed in urine samples with high UCl. Hypocalciuria (UCalcium/UCreatinine <0.2 mmol/mmol) is usually present in patients with Gitelman syndrome.47,48
Patients With Hyperkalemia
Hyperkalemia caused by a shift of K+ out of cells is usually recognized by its acute onset and the clinical setting (e.g., rhabdomyolysis, tumor lysis syndrome, immediate postoperative period, diabetic ketoacidosis, hypoxic lactic acidosis). In a case report of hyperkalemia due to toad venom ingestion (contains bufadienolides, which akin to digitalis, inhibit the Na+/K+ adenosine 5ʹ-triphosphatase), the UK/UCreatinine was 15.9, indicating that hyperkalemia was not due to a defect in renal K+ excretion.49
The UK/UCreatinine is less useful in patients with chronic hyperkalemia. This is because to develop chronic hyperkalemia, there must be defect in the renal K+ excretion. Hence, the value of assessing K+ excretion rate in these patients is to determine whether a large dietary K+ intake is contributing to the degree of hyperkalemia. Because of the diurnal variation in K+ excretion, this requires a 24-hour urine collection.
A subgroup of patients with chronic hyperkalemia have disorders that cause a decreased number of open epithelial Na channels in the ASDN, leading to decreased rate of electrogenic Na+ reabsorption. This includes patients with primary hypoaldosteronism (e.g., Addison disease), patients with pseudohypoaldosteronism type I (e.g., due to molecular defects involving the aldosterone receptor or the epithelial Na channel), and a small subset of patients with hyporeninemic hypoaldosteronism who may have damage to the juxtaglomerular apparatus or a defect in converting prorenin to active renin.50 Patients with these disorders tend to have renal salt wasting, low EABV, and inappropriately high UNa and UCl. Of note, most patients with hyporeninemic hypoaldosteronism, who may also have hyperkalemia, have a clinical picture that mimics the syndrome of hypertension with hyperkalemia (Gordon syndrome or pseudohypoaldosteronism type II).51 The EABV is not low in these patients, and they do not have renal salt wasting.
Patients With Polyuria
Polyuria is commonly defined by a urine volume that is >3 l/d in adults.
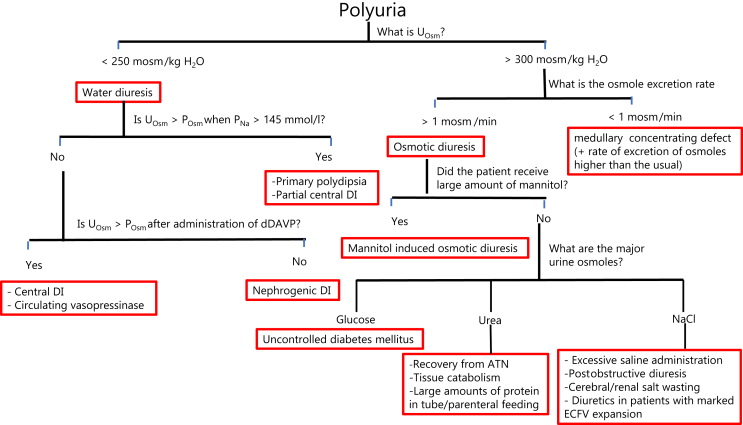
In patients suspected of having polyuria, a 24-hour urine collection should be obtained to measure urine volume, UOsm, creatinine, Na+, K+, Cl−, urea, and glucose and calculate the rate of excretion of osmoles.
A UOsm <250 mosm/kg H2O suggests a water diuresis. The absolute value of the UOsm depends on the rate of excretion of osmoles.52 A UOsm >300 mosm/kg H2O suggests an osmotic diuresis and/or a medullary interstitial disease impairing the process of concentrating the urine in the renal medulla.
Polyuria Due to a Water Diuresis
A water diuresis could be due to primary polydipsia or diabetes insipidus (DI).53,54 DI may be caused by:
-
(i)
Central DI: due to lesions affecting the neurohypophysis resulting in deficient synthesis and/or release of AVP. A subset of these patients retain the ability to release AVP in the presence of a higher PNa, hence described as partial central DI.55
-
(ii)
Nephrogenic DI: due to lesions that interfere with the binding of AVP to its V2 receptor (AVPR2), its effect is to cause trafficking and insertion of aquaporin water channel 2 (AQP2) in the luminal membrane of principal cells or a defect involving AQP2. Nephrogenic DI may be congenital due to mutations involving the genes encoding for AVPR2 or AQP2 or acquired (most commonly due to intake of lithium).
We prefer not to include patients with a medullary concentrating defect due to a medullary interstitial disease under the category of nephrogenic DI, but rather to characterize them as a separate group. This is because the pathophysiology of the polyuria in these patients is different, their UOsm is usually >300 mosm/kg H2O (because UOsm should be at least equal to the osmolality at the end of the CCD (i.e., ∼POsm), and from a management perspective, decreasing intake of osmoles is more likely to result in a lower urine volume in this group of patients.
-
(iii)
The presence of a circulating vasopressinase that breaks down AVP. This is commonly caused by its excess release from a large placenta (usually twin or multiple pregnancies) or its impaired degradation by the liver in patients with preeclampsia/HELLP (Hemolysis, Elevated Liver Enzymes, Lowered Platelets) syndrome.
Because of difficulties with the measurement of AVP due to its instability in drawn blood samples and inaccuracy of commercially available assays, and because measurements of copeptin are not widely available, clinicians rely on measurement of the change in UOsm in response to a sufficient osmotic stimulus (a rise in PNa to >145 mmol/L with water deprivation) and after the administration of 1-desamino-8-d-AVP (dDAVP) to determine the cause of a water diuresis.56,57
Chronic polyuria decreases the ability to concentrate the urine because of medullary washout.58, 59, 60 A larger volume of water is reabsorbed in the MCD during water diuresis than during antidiuresis.61,62 The resultant increase in flow in the ascending vasa recta impairs the efficiency of the countercurrent exchange between the ascending and the descending vasa recta and the surrounding medullary interstitium.63,64 Therefore, if there is a renal response to the release of AVP or the administration of dDAVP, the rise in UOsm may vary depending on the degree of this medullary washout but is expected to be at least to a value exceeding the osmolality at the end of the CCD (∼POsm).
A rise in UOsm to a value that is >POsm in response to a rise in PNa >145 mmol/l suggests that the cause of the water diuresis is primary polydipsia or partial central DI.65, 66, 67
dDAVP is administered if this response to a rise in PNa >145 mmol/l is not observed. A rise in UOsm to >POsm in response to the administration of dDAVP suggests that the cause of the water diuresis is complete central DI. Notwithstanding, although the UOsm of 3 of the 7 patients who were thought to have central DI in the study by Zerbe and Robertson65 more than doubled after the administration of AVP, it did not rise to a value >POsm.65 This could be interpreted to suggest downregulation of AQP2 during water diuresis. Of note however, UOsm was measured at 30 and 60 minutes after the administration of AVP; hence, mixing of urines with low and higher osmolality (especially if there is incomplete bladder emptying) may be suspected.
A rise in UOosm to a value > POsm after the administration of dDAVP is also expected in patients with water diuresis caused by breakdown of AVP by a circulating vasopressinase, because dDAVP is not degraded by these enzymes. These patients are expected not to respond to the administration of AVP. This investigation should not be attempted in pregnant women, because AVP has an oxytocic effect, unlike dDAVP which has been safely administered during pregnancy.68
If the UOsm fails to rise appropriately in response to a rise in PNa >145 mmol/L and the administration of dDAVP, the diagnosis is nephrogenic DI (Figure 2).
Figure 2.
Urine data in the clinical diagnosis of patients with polyuria. The first step is to determine whether the basis of polyuria is a water diuresis or an osmotic diuresis. A urine osmolality (UOsm) <250 mosm/kg H2O suggests a water diuresis. This could be due to diabetes insipidus (DI) or primary polydipsia. The cause of the water diuresis can be determined by examining the change in UOsm in response to a rise in plasma sodium concentration (PNa) to >145 mmol/l and the administration of 1-desamino-8-d-arginine vasopressin (dDAVP). A UOsm >300 mosm/kg H2O suggests that the polyuria is due to an osmotic diuresis or a medullary interstitial disease impairing the process of concentrating the urine in the renal medulla. These 2 disorders can be separated by calculating the rate of excretion of osmoles. The cause of the osmotic diuresis can be determined by measuring the individual osmoles in the urine (e.g., glucose, urea, and sodium chloride [NaCl]). A large amount of mannitol is not commonly given; hence, it is not likely to be the sole cause of a large and sustained osmotic diuresis. ATN, acute tubular necrosis; ECFV, extracellular fluid volume.
Polyuria Due to an Osmotic Diuresis
A UOsm >300 mosm/kg H2O suggests that the polyuria is caused by an osmotic diuresis and/or a low medullary interstitial osmolality due to a medullary interstitial disease. These 2 disorders can be differentiated by calculating the rate of excretion of osmoles in a timed urine collection (multiply the urine flow rate by the UOsm). The usual rate of excretion of osmoles in adults consuming a typical Western diet is ∼0.5 mosm/min. In patients with osmotic diuresis, the osmole excretion rate is usually >1 mosm/min. Conversely, if the osmole excretion rate is appreciably less than that, a medullary concentrating defect with a higher than usual rate of excretion of osmoles (e.g., in a patient who consumes a diet high in salt and animal protein) may be suspected.
An osmotic diuresis may be due to organic (mannitol, urea, or glucose) or NaCl osmoles, or a combination of these osmoles (Figure 2).
In patients with urea-induced osmotic diuresis, it is important to determine whether the source of urea is exogenous from the intake of proteins or endogenous from catabolism of tissue proteins. Because close to 16% of the weight of protein is nitrogen, 16 g of nitrogen will be produced when 100 g of protein is oxidized. The molecular mass of nitrogen is 14, and each molecule of urea contain 2 atoms of nitrogen; therefore, ∼570 mmol of urea is produced from the oxidation of 100 g of protein.
Patients With Acute Dysnatremia
Calculations of electrolyte free water (EFW) balance and tonicity balance are used to determine the basis for an acute change in PNa and the therapy needed to return the PNa to a normal value. These calculations can only be done in a hospital setting, where inputs and outputs are recorded and the concentrations of electrolytes in the urine are measured.
The difference between these 2 calculations is illustrated with case examples of 3 patients, in each of whom PNa rose from 140 mmol/l to 147 mmol/l (Table 1). Each patient excreted 3 L of urine with (UNa + UK) of 50 mmol/l, patient 1 received 3 L of isotonic saline (Na+ concentration 154 mmol/l), patient 2 received 4 L of isotonic saline, and patient 3 received no i.v. fluids. Total body water in each patient was 40 L.
Table 1.
Electrolyte free water balance versus tonicity balancea
| Patient | Output |
Input |
EFW balance | Tonicity balance |
|||
|---|---|---|---|---|---|---|---|
| H2O L | Na+ + K+ mmol | H2O L | Na+ + K+ mmol | H2O L | Na+ + K+ mmol | ||
| 1 | 3 | 150 | 3 | 450 | -2 L | 0 | +300 |
| 2 | 3 | 150 | 4 | 600 | -2 L | +1 | +450 |
| 3 | 3 | 150 | 0 | 0 | -2 L | −3 | −150 |
EFW, electrolyte free water.
Three case examples are described. In each patient, the plasma sodium (PNa) concentration rose from 140 mmol/L to 147 mmol/L. In all 3 examples, the urine volume was 3 L with a concentration of (Na+ + K+) of 50 mmol/L. Patient 1 received 3 L of isotonic saline (Na+ concentration 154 mmol/l), patient 2 received 4 L of isotonic saline, and patient 3 received no i.v. fluids. Calculation of the EFW balance shows that the basis of the rise in PNa is a negative balance of 2 L of EFW. Calculation of tonicity balance reveals that although the basis of the rise in PNa is a net negative balance of 2 L of EFW, this was the result of rather quite different balances for water and (Na+ + K+) in each of the 3 patients. Patient 1 has a positive balance of 300 mmol of Na+, patient 2 has a positive balance of 1 L of H2O and a positive balance of 450 mmol of Na+, and patient 3 has a deficit of 3 L of H2O and a deficit of 150 mmol of Na+. Hence, the design of the appropriate therapy to correct the hypernatremia and to return the volume and composition of the extracellular and intracellular fluid compartments to their normal values is different for each of them. It requires creating a negative balance for Na+ in patient 1, a negative balance for H2O and Na+ in patient 2, and a positive balance for H2O and Na+ in patient 3.
EFW Balance
Because urea is not an effective osmole in the body, calculation of EFW balance rather than osmole free water balance was suggested to determine the basis of a dysnatremia and its impact on cell volume.69 This calculation is based on determining how much water needs to be added to (or subtracted from) a solution to make its tonicity equal to the normal plasma tonicity. In the absence of hyperglycemia, tonicity of plasma water is approximated by the concentrations of (Na+ + K+) × 2 to account for accompanying anions (i.e., ∼300 mosm/kg plasma water, with a concentration of Na+ in plasma water ∼150 mmol/l). To calculate EFW balance, one needs to know the volumes and the concentrations of (Na+ + K+) of the input and the urine over the time period the PNa has changed.
Applying the calculation of EFW to patient 1, there is no EFW in the input because it has the same tonicity as plasma water. With regard to the output, this patient excreted the equivalent of 1 L of an isotonic solution and 2 L of EFW. Hence, this patient has a negative EFW balance of 2 L. Because total body water is down by 5% (2/40 L), the PNa rises by 5% to 147 mmol/l, and hence the administration of 2 L of EFW is required to return the PNa back to 140 mmol/l. Applying the EFW balance calculation to the other 2 patients gives the same result, a negative EFW balance of 2 L, and suggests that the appropriate therapy for each of them is the administration of 2 L of EFW.
Tonicity Balance
A tonicity balance refers to the balance of both water and of (Na+ + K+).70, 71, 72 To calculate a tonicity balance, one needs to know the volumes and the quantity of (Na+ + K+) of the input and the urine over the time period the PNa has changed.
Calculation of tonicity balance reveals that while it is true that the basis for the rise in PNa in all 3 patients is a net negative balance of 2 L of EFW, this is the result of rather quite different balances for water and for (Na+ + K+) in each of them (Table 1). Hence, the design of the appropriate therapy to return PNa to its normal value and restore the normal volume and composition of the ECF and ICF compartments is different for each one of them.
Patients With Low EABV
In response to decreased EABV, mechanisms that lead to retention of Na+ and Cl− by the kidney are activated. Because 24-hour urine collections to measure the excretion rates of Na+ and Cl− are not practical in clinical settings, UNa and UCl values of <15 mmol/l in spot urine samples are used instead to suggest decreased EABV. These are concentration terms that are also affected by the urine flow rate. The 24-hour excretion rate can be estimated by multiplying the ratio of UNa or UCl /UCreatinine by an estimate of the 24-hour creatinine excretion based on the patient’s muscle mass.
There are some caveats in using UNa and UCl to detect low EABV (Table 2).30,73 A low rate of excretion of Na+ and Cl− may reflect a low intake of NaCl rather than a low EABV. The UNa might not be low despite low EABV if the excretion of the cation Na+ is obligated by the excretion of an anion other than Cl− (e.g., HCO3− in a patient with recent vomiting [bicarbonaturia is suggested by the finding of an alkaline urine pH], anions of drugs such as piperacillin or carbenicillin). The UCl may not be low despite low EABV if the excretion of the anion Cl− is obligated by the excretion of another cation (e.g., NH4+ in patients with diarrhea, lithium in patients with lithium intoxication). Both UNa and UCl may not be low despite low EABV in patients with diuretic use/abuse, patients with Bartter syndrome or Gitelman syndrome, patients with adrenal insufficiency, patients with cerebral /renal salt wasting, and in a subset of patients with hyporeninemic hypoaldosteronism.
Table 2.
Urine Na+ and urine Cl−concentrations in patients with decreased effective arterial blood volumea
| Condition | Urine Na+ | Urine Cl− |
|---|---|---|
| Vomiting | ||
| Recent | High | Low |
| Remote | Low | Low |
| Diuretics | ||
| Recent | High | High |
| Remote | Low | Low |
| Diarrhea/some patients with laxative abuse | Low | High |
| Bartter syndrome/Gitelman syndrome/Bartter-like syndrome | High | High |
| Cerebral/renal salt wasting | High | High |
| Some patients with hyporeninemic hypoaldosteronism | High | High |
| Adrenal insufficiency | High | High |
High: urine concentration of Na+ or Cl− >15 mmol/l; low: urine concentration of Na+ or Cl− <15 mmol/L. Urine Cl− may be high in patients with laxative abuse if they have a high rate of excretion of NH4+ because of metabolic acidemia or because of hypokalemia, the latter is associated with a fall in pH in proximal convoluted tubule cells and increased production of NH4+. The UNa and UCl may not be high in patients with disorders associated with renal loss of Na+ and Cl−, if there is marked degree of decreased effective arterial blood volume as mechanisms to enhance Na+ and Cl− reabsorption in nephron segments other than those affected by the disorder are activated.
Patients With Hyponatremia
The clinical approach to the patients with hyponatremia centers on determining the cause of the release of AVP despite hypotonicity. In one group of patients, AVP release is due to decreased EABV. In contrast, in the group of patients with the syndrome of inappropriate secretion of antidiuretic hormone (SIADH) or the syndrome of inappropriate antidiuresis to include patients with mutations in AVPR2 causing it to be constitutively active, the release of AVP is not caused by low EABV.74,75 Notwithstanding, the degree of decreased EABV in some of the patients who are considered in the first group (e.g., some patients with thiazide-induced hyponatremia, patients with “tea and toast” hyponatremia) does not seem to be large enough to cause the release of AVP.76, 77, 78, 79 Reduced EFW excretion in these patients may be caused by a decreased volume of filtrate delivered to the distal nephron as a result of increased reabsorption in the PCT in response to a mild degree of EABV contraction, particularly in elderly patients with reduced glomerular filtration rate, and the presence of other mechanisms for water reabsorption in the distal nephron that are independent of AVP actions.79, 80, 81, 82
A mild degree of EABV contraction cannot be reliably detected by physical examination.83,84 laboratory tests that are based on the expected renal response to decreased EABV are suggested to help separate patients with decreased EABV from those with SIADH.85,86 The diagnostic accuracy of these tests is limited, particularly in elderly patients who more commonly have hyponatremia
A UNa >30 mmol/l and fractional excretion (FE) of Na+ (FENa; Equation 1) >0.5% are thought to be in keeping with euvolemia and the diagnosis of SIADH. The UNa may be <30 mmol/l, however, in some patients with SIADH if their salt intake is low, and >30 mmol/l in elderly patients who may have decreased ability to conserve salt despite decreased EABV and in the steady state excrete the salt they eat.87,88 The calculation of the FENa is also affected by the glomerular filtration rate (e.g., for the same rate of Na+ excretion, FENa will be twice as high in an individual whose glomerular filtration rate is reduced by 50% compared with another individual who has a normal glomerular filtration rate). A UNa >30 mmol/l was observed in 30% of the patients with hyponatremia who were thought to have hypovolemia, FENa was <0.5% in 40% of patients who were thought to have SIADH.
| (1) |
Reabsorption of urea and urate in the PCT is increased in response to decreased EABV. FE of urea (FEUrea) <50% was observed in 80% of patients who were thought to have hypovolemia, but also in 50% of patients who were thought to have SIADH. FE of urate (FEUrate) <10% was observed in only 60% of patients who were thought to have hypovolemia and also in 35% of patients who were thought to have SIADH.
A trial of EABV expansion with infusion of saline may be required to differentiate patients with SIADH from those with mildly contracted EABV. The occurrence of water diuresis is expected in patients with low EABV but not in patients with SIADH, who are also expected to rapidly excrete the salt load. Of note, a subset of patients (∼20%) with SIADH may have diminished baroreceptors sensitivity, mimicking the effect of decreased EABV. EABV expansion in this subset of patients may activate these stretch baroreceptors, leading to inhibition of the release of AVP and the excretion of a dilute urine.89
With discontinuation of thiazides and provision of salt, patients with thiazide-induced hyponatremia are expected to excrete a dilute urine, resulting in the correction of hyponatremia. Increasing salt intake may also help correct the hyponatremia in patients with SIADH, because the increase in the number of excreted effective osmoles increases the urine volume. These patients however, fail to excrete a dilute urine.
Hypouricemia and increased FEUrate in patients with SIADH are thought to be due increased renal clearance of urate because of EABV expansion due to water retention, an effect of V1 receptor activation by AVP and perhaps also an effect of chronic hyponatremia.90, 91, 92, 93, 94 Correction of hyponatremia in these patients with water restriction results in normalization of the FEUrate.91,92,95
Debate continues about the existence and true prevalence of the syndrome of cerebral salt wasting.96, 97, 98 A high FEUrate was observed in patients who were thought to have cerebral salt wasting and in a subset of patients with renal salt wasting who did not have an intracranial lesion.99, 100, 101, 102, 103 The suggested pathophysiology in these patients is the release of a yet unidentified circulating factor that inhibits the reabsorption of Na+ and of urate in the PCT.
In few reported cases, the FEUrate remained elevated in patients who were though to have cerebral/renal salt wasting after correction of hyponatremia with infusion of saline that resulted in the excretion of dilute urine. Hence, it was suggested that the FEUrate after correction of hyponatremia may differentiate patients with SIADH from those with cerebral salt wasting/renal salt wasting. Because this difference becomes only apparent after the correction of hyponatremia, it has limited utility in helping clinicians to decide what is the correct diagnosis and what is the appropriate therapy.
Patients With HCMA
There are 2 major pathophysiological mechanisms for the development of HCMA: the direct loss of NaHCO3 (i.e., Na+ and HCO3− are both lost via the same route) and the indirect loss of NaHCO3 (i.e., Na+ and HCO3− are lost via 2 different routes).
A direct loss of NaHCO3 may occur via the gastrointestinal tract. Because the capacity of the Cl−/HCO3− anion exchanger in the colon normally exceeds that of the Na+/H+ exchanger, NaHCO3 may be lost in stools if there is a large increase in the delivery of NaCl to the colon in a patient with diarrhea. If there is also increased production of organic acids in the colon in these patients , HCO3− may be titrated by H+ of these acids, and Na+ (or K+) ions are lost in the stool with the anions of these organic acids. In either case, there is a loss of NaHCO3.
A direct loss of NaHCO3 through the urine occurs in patients in the early phase of a disease process that causes proximal renal tubular acidosis (pRTA).
An indirect loss of NaHCO3 could be the result of 2 different groups of disorders. In the first group, there is overproduction of an acid and a high rate of excretion of its anion in the urine, either because these anions are filtered and also secreted by the PCT (e.g., hippurate anions of hippuric acid produced from toluene metabolism in people who sniff glue)104 or because the anions are filtered and an appreciable quantity is not reabsorbed in the PCT (e.g., β-hydroxybutyrate in some patients with diabetic ketoacidosis, and d-lactate in patients with d-lactic acidosis). Although the rate of NH4+ excretion is high in these patients, the rate of excretion of anions exceeds that of NH4+, and some of the anions are excreted in the urine with Na+ and/or K+. Hence, there is an indirect loss of NaHCO3: HCO3− is lost by titration with H+ of the added acid, and Na+ is lost by excretion in the urine with the anions of the acid.
In the second group, the major defect is a low rate of NH4+ excretion that is insufficient to generate enough HCO3− to replace HCO3− lost in buffering the daily acid load of sulfuric acid produced from the metabolism of sulfur-containing amino acids. This is another form of renal tubular acidosis, labeled as distal RTA.
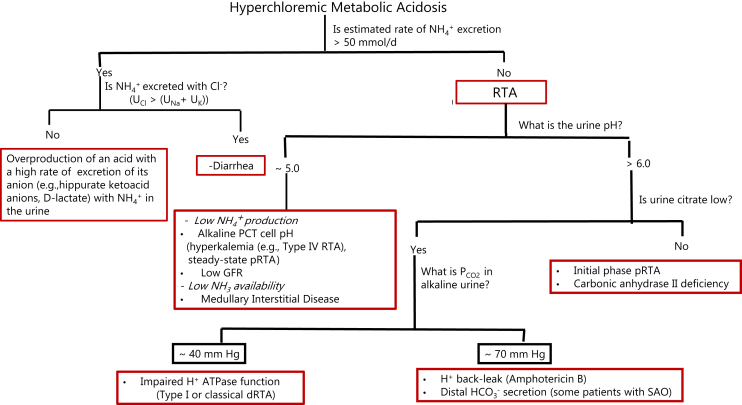
The initial step in the differential diagnosis in patients with HCMA is to assess the rate of NH4+ excretion, which is expected to be high in patients with gastrointestinal loss of HCO3− and in those with overproduction of an acid with a high rate of excretion of its anion in the urine, and to be low in patients with distal RTA (Figure 3). Of note, while pRTA in its initial phase is a NaHCO3 wasting disease, the metabolic acidemia in the chronic steady state of this disorder seems to be maintained by a low rate of NH4+ excretion. A study in patients with isolated autosomal-dominant pRTA showed that the rate of NH4+ excretion was “normal” in the steady state (in fact, low considering the presence of chronic acidemia), and failed to rise appropriately in response to ammonium chloride (NH4Cl) loading.105 It is postulated that the lesion that impairs the reabsorption of HCO3− in the PCT results in an alkaline intracellular pH, which inhibits the activity of some key enzymes in glutamine metabolism.106,107
Figure 3.
Urine data in the clinical diagnosis of the patients with hyperchloremic metabolic acidosis (HCMA). The concentration of ammonium (NH4+) in the urine can be estimated by dividing the urine (U) osmolal gap by 2, the rate of excretion of NH4+ can be calculated by multiplying the UNH4/UCreatinine by the estimated rate of excretion of creatinine in the patient.
A rate of excretion of NH4+ >50 mmol/d suggests that renal tubular acidosis (RTA) is not the cause of the HCMA. In patients with HCMA and a high rate of excretion of NH4+, one can determine whether an anion other than chloride is being excreted with NH4+ in the urine by comparing the concentrations of sodium and potassium in the urine (UNa + UK) versus that of chloride (UCl). The urine pH may provide a clue to suggest the pathophysiology causing a low rate of excretion of NH4+. A urine pH >6 suggests a defect in bicarbonate (HCO3−) reabsorption in the proximal convoluted tubule (early phase of proximal RTA [pRTA]) or a defect in H+ secretion in the distal nephron. Urine citrate excretion is not low in the former group. The pathophysiology causing decreased net H+ secretion in the distal nephron can be determined by measuring the Pco2 in alkaline urine (UPCO2). ATPase, adenosine 5ʹ-triphosphatase; dRTA, distal RTA; GFR, glomerular filtration rate; PCT, proximal convoluted tubule; SAO, Southeast Asian ovalocytosis.
Assess the Rate of NH4+ Excretion
The urine pH is not a reliable indicator for the rate of NH4+ excretion.108,109 In acute acidosis, distal H+ secretion is stimulated, but there is a lag period before NH4+ production is increased; the urine pH is low, but there is only a modest increase in NH4+ excretion. In chronic acidosis, NH4+ production and the transfer of NH3 into the lumen of the collecting duct are increased; a high rate of NH4+ excretion is achieved while the urine pH rises to ∼6 as more NH3 becomes available to titrate the H+ ions.
Because a direct assay for urine NH4+ is not often available in clinical settings, indirect tests are used to estimate the NH4+ excretion rate in patients with HCMA. Although these tests provide only semiquantitative estimates, this is adequate for clinical use because the information needed is whether the rate of NH4+ excretion is low enough that a defect in renal NH4+ excretion is the cause of the metabolic acidosis or whether it is sufficiently high that another cause of the HCMA should be considered. Normal individuals consuming a typical Western diet excrete 30 to 40 mmol of NH4+ per day, whereas NH4+ excretion rose to >200 mmol/d in normal individuals who were given an acid load of NH4Cl for several days.110,111
The Urine Anion Gap
The urine anion gap (UAnion gap), calculated as (UNa + UK − UCl), has been used to assess the rate of NH4+ excretion in patients with HCMA. The rationale behind this calculation is that if the rate of NH4+ excretion is high and NH4+ is excreted with Cl− (as is the case in patients with gastrointestinal loss of HCO3−), UCl will exceed (UNa + UK) (a negative value of the UAnion gap). In contrast, if there is a defect in NH4+ excretion, (UNa + UK) will exceed UCl (a positive value of the UAnion gap). There are 2 issues with using the UAnion gap that should be noted.
First, the equation that describes the relationship between the rate of NH4+excretion and the UAnion gap, which was based on measurements from 24-hour urine collections, had a constant of 82 (Equation 2).112
| (2) |
This constant represents the difference between the rates of excretion of other unmeasured anions and other unmeasured cations in the urine in individuals described as consuming a “normal” diet. Therefore, if UCl exceeds (UNa + UK), one assumes that NH4+ excretion is >82 mEq/d, and hence, RTA is not the cause of the metabolic acidosis. The validity of this assumption is rather questionable, because the rate of excretion of unmeasured anions in the urine may vary considerably depending on dietary intake.113
The use of the UAnion gap in spot urine samples was examined in a study of patients with RTA, patients with diarrhea, and normal individuals given an acid load of NH4Cl for 3 days.114 All patients with RTA had a positive value for the UAnion gap, and patients with diarrhea and normal individuals given NH4Cl had a negative value. The correlation between the value of the UAnion gap and the concentration of NH4+ in the urine (UNH4), however, was only 0.72. NH4+ excretion rates were not reported, but based on the data provided on UNH4 in a Figure in the paper, NH4+ excretion seemed to be lower than expected in some of the participants given NH4Cl and some patients with diarrhea. Three of the 7 normal participants given NH4Cl had UNH4 <30 mmol/l. Four of the 8 patients with diarrhea had UNH4 approximately or <30 mmol/l, and the UNH4 in 2 of these patients was in the range observed in the patients with RTA.
The second issue is that the UAnion gap detects NH4+ only if it is excreted with Cl−. Hence, if NH4+ is excreted with another anion (e.g., hippurate, β-hydroxy butyrate, d-lactate), the UAnion gap will fail to detect a high rate of NH4+ excretion, and these patients may be misdiagnosed as RTA.
The Urine Osmolal Gap
The urine osmolal gap (UOsmolal gap) may provide a better indirect test to assess the rate of NH4+ excretion in patients with HCMA.115, 116, 117, 118 The UOsmolal gap is calculated as the difference between the measured UOsm and the UOsm calculated from 2(UNa + UK) + UUrea + UGlucose (if hyperglycemia is present). Multiplying (UNa + UK) by 2, accounts for their accompanying anions. This overestimates the actual number of osmoles in the urine because some of these anions (e.g., SO42−, citrate2−, and perhaps some other organic anions) are not monovalent. On the other hand, the calculation of the UOsm underestimates the actual number of urine osmoles because it does not include calcium and magnesium and their accompanying anions.
Meregalli et al.119 in a study in normal individuals and Raphael and Xi120 in a study in kidney transplant recipients, a small subset of whom had HCMA, noted poor correlation between the value of the UOsmolal gap and the concentration of NH4+ in the urine. We emphasize that the purpose of the calculation of the UOsmolal gap is not to determine the actual concentration of NH4+ in the urine but rather to assess if the rate of excretion of NH4+ in a patient with HCMA is sufficiently high that a cause for the HCMA other than RTA should be considered.118
The UOsmolal gap detects NH4+ in the urine regardless of its accompanying anion.104,116 The UOsmolal gap is unreliable to assess the rate of NH4+ excretion if other osmoles (e.g., ethanol, methanol, ethylene glycol, mannitol) are present in the urine.
The concentration of NH4+ in the urine can be estimated by dividing the UOsmolal gap by 2, and the rate of NH4+ excretion can be calculated by multiplying the UNH4/UCreatinine by the estimated rate of excretion of creatinine in the patient. An estimated rate of NH4+ excretion >50 mmol/d suggests that RTA is not likely to be the cause of the HCMA.
Urinary Tests to Determine the Cause of Low NH4+ Excretion
The low rate of NH4+ excretion could be due to decreased availability of NH3 in the lumen of the collecting duct, because of decreased NH4+ production, a defect in NH4+ transfer to the medullary interstitium, or NH3 transfer to the lumen of the collecting duct via the nonerythroid Rhesus glycoproteins Rhbg and Rhcg, and/or decreased net rate of H+ secretion in the distal nephron.107,121,122
A low rate of NH4+ production may be due to alkalinization of PCT cells because of hyperkalemia or a genetic or an acquired disorder that compromises proximal H+ secretion or the exit of HCO3− from PCT cells, also causing reduced capacity to reabsorb HCO3− (i.e., pRTA). pRTA typically occurs in patients with Fanconi syndrome, in which other Na+-linked transport functions of PCT are affected, leading to renal glucosuria, aminoaciduria, citraturia, and increased fractional excretion rates of phosphate and urate. The common causes of Fanconi syndrome are paraproteinemia in adults and cystinosis in children.123
A low net rate of H+ secretion in the distal nephron could be due to an H+–adenosine 5ʹ-triphosphatase defect (e.g., autoimmune and hypergammaglobulinemic disorders, including Sjögren syndrome), back-leak of H+ (e.g., due to drugs such as amphotericin B), or a disorder associated with the distal secretion of HCO3− (e.g., in some patients with Southeast Asian ovalocytosis who have a second mutation in the Cl−/HCO3− anion exchanger causing it to be mistargeted to the luminal membrane of β-intercalated cells).
The following urinary tests may help to determine the basis of the low rate of NH4+excretion (Figure 3):
The Urine pH. The urine pH should be measured with a pH meter in a freshly collected urine sample, or in a urine sample collected under mineral oil, to minimize diffusion of CO2, if there will be a delay in performing the measurement. The basis for the low rate of NH4+ excretion may be deduced from the urine pH.107 A urine pH of ∼5 suggests a defect causing a low rate of NH4+ production or impairing the accumulation of NH4+ in the medullary interstitium/transfer of NH3 to the lumen of the MCD. A urine pH >6 suggests a defect in HCO3− reabsorption in the PCT (early phase of pRTA) or a defect in H+ secretion in the distal nephron.
The Rate of Citrate Excretion. The usual rate of citrate excretion is ∼2 mmol/d. The rate of citrate excretion provides a window into the pH in PCT cells.124, 125, 126 Patients with metabolic acidosis have hypocitraturia, partly because of the effect of the lower pH in PCT cells to stimulate the reabsorption of citrate.124,127, 128, 129 Citrate excretion however, is not low in patients with pRTA because of decreased citrate reabsorption as a result of an alkaline PCT cell pH and/or as a component of Fanconi syndrome.105,106 An alkaline urine pH in this setting suggests the diagnosis of pRTA in the initial phase. Citrate excretion was reported to be also not low in a patient with carbonic anhydrase II deficiency.130 Different from inhibition of luminal carbonic anhydrase IV by acetazolamide, which is associated with marked hypocitraturia, deficiency of the cytoplasmic carbonic anhydrase II leads to an alkaline pH in PCT cells, which decreases the metabolism of citrate in PCT cells and subsequently decreases citrate reabsorption. Carbonic anhydrase II deficiency involves also the distal nephron causing decreased distal H+ secretion.130,131
The Pco2 in Alkaline Urine. The urinary Pco2 is used to assess H+ secretion in the distal nephron.132, 133, 134 The patient is given a load of NaHCO3 to increase the filtered load of HCO3− and its delivery to the distal nephron. Secretion of H+ by the MCD leads to the formation of carbonic acid (H2CO3). Because there is no luminal carbonic anhydrase, H2CO3 is dehydrated slowly to CO2 + H2O. Urinary Pco2 close to 70 mm Hg suggests a normal rate of net H+ secretion in the distal nephron.
Despite a defect in distal H+ secretion, the urinary Pco2 is high in patients with a lesion that leads to H+ back-leak.135 This is because as more HCO3− is delivered distally, it traps H+ and prevents its back-leak. The urinary Pco2 is also high in patients with disorders that cause HCO3− secretion in the distal nephron (e.g., some patients with SOA).136,137 This is because secretion of HCO3− into the lumen of the distal nephron during HCO3− loading raises the luminal fluid pH, causing H+ to be released from monovalent phosphate (H2PO4−), these H+ react with luminal HCO3−and form H2CO3.
FE of HCO3−. The FE of HCO3− is used to assess H+ secretion by the PCT.138 NaHCO3 is given to raise the plasma HCO3 to the normal range. If there is a defect in H+ secretion in the PCT, the filtered load of HCO3− will exceed the capacity for its reabsorption, and HCO3− will be spilled in the urine; hence the urine pH becomes alkaline with values >7, and the FE of HCO3− exceeds 15%.
This test is usually not needed. These patients will be recognized clinically by failure to correct their metabolic acidemia despite large doses of NaHCO3.
Disclosure
The authors declared no competing interests.
Acknowledgements
The authors are grateful to Dr. Man Oh and Dr. Shiu-Hua Lin for very helpful critique and suggestions during the preparation of the manuscript.
References
- 1.Kamel K.S., Ethier J.H., Richardson R.M., Bear R.A., Halperin M.L. Urine electrolytes and osmolality: when and how to use them. Am J Nephrol. 1990;10:89–102. doi: 10.1159/000168062. [DOI] [PubMed] [Google Scholar]
- 2.Kamel K.S., Halperin M.L. Interpretation of electrolytes and acid base parameters in blood and urine. In: Skorecki K., Chertow G.M., Marsden P.A., editors. Brenner and Rector’s The Kidney. 11th ed. Elsevier; Philadelphia, PA: 2019. pp. 758–795. [Google Scholar]
- 3.Kamel K.S., Halperin M.L. Elsevier; Philadelphia, PA: 2017. Fluid, Electrolytes and Acid-Base Physiology: A Problem Based Approach. [Google Scholar]
- 4.Huth E.J., Squires R.D., Elkinton J.R. Experimental potassium depletion in normal human subjects. II. Renal and hormonal factors in the development of extracellular alkalosis during depletion. J Clin Invest. 1959;38:1149–1165. doi: 10.1172/JCI103891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Womersley R.A., Darragh J.H. Potassium and sodium restriction in the normal human. J Clin Invest. 1955;34:456–461. doi: 10.1172/JCI103094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talbott J.H., Schwab R.S. Recent advances in the biochemistry and therapeutics of potassium salts. N Engl J Med. 1940;222:585–590. [Google Scholar]
- 7.Brunner H.R., Baer L., Sealey J.E. The influence of potassium administration and of potassium deprivation on plasma renin in normal and hypertensive subjects. J Clin Invest. 1970;49:2128–2138. doi: 10.1172/JCI106430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.West M.L., Marsden P.A., Richardson R.M. New clinical approach to evaluate disorders of potassium excretion. Miner Electrolyte Metab. 1986;12:234–238. [PubMed] [Google Scholar]
- 9.Ethier J.H., Kamel K.S., Magner P.O. The transtubular potassium concentration in patients with hypokalemia and hyperkalemia. Am J Kidney Dis. 1990;15:309–315. doi: 10.1016/s0272-6386(12)80076-x. [DOI] [PubMed] [Google Scholar]
- 10.Fenton R.A., Yang B. Urea transporter knockout mice and their renal phenotypes. Subcell Biochem. 2014;73:137–152. doi: 10.1007/978-94-017-9343-8_9. [DOI] [PubMed] [Google Scholar]
- 11.Yang B., Bankir L. Urea and urine concentrating ability: new insights from studies in mice. Am J Physiol Renal Physiol. 2005;288:F881–F896. doi: 10.1152/ajprenal.00367.2004. [DOI] [PubMed] [Google Scholar]
- 12.Lassiter W.E., Gottschalk C.W., Mylle M. Micropuncture study of net transtubular movement of water and urea in nondiuretic mammalian kidney. Am J Physiol. 1961;200:1139–1147. doi: 10.1152/ajplegacy.1961.200.6.1139. [DOI] [PubMed] [Google Scholar]
- 13.Kamel K.S., Halperin M.L. Intrarenal urea recycling leads to a higher rate of renal excretion of potassium: an hypothesis with clinical implications. Curr Opin Nephrol Hypertens. 2011;20:547–554. doi: 10.1097/MNH.0b013e328349b8f9. [DOI] [PubMed] [Google Scholar]
- 14.Murdaugh H.V., Jr., Schmidt-Nielsen B., Doyle E.M., O’Dell R. Renal tubular regulation of urea excretion in man. J Appl Physiol. 1958;13:263–268. doi: 10.1152/jappl.1958.13.2.263. [DOI] [PubMed] [Google Scholar]
- 15.Bankir L.T., Trinh-Trang-Tan M.M. Renal urea transporters. Direct and indirect regulation by vasopressin. Exp Physiol. 2000;85 doi: 10.1111/j.1469-445x.2000.tb00029.x. Spec No:243S–252S. [DOI] [PubMed] [Google Scholar]
- 16.Kato A., Klein J.D., Zhang C., Sands J.M. Angiotensin II increases vasopressin-stimulated facilitated urea permeability in rat terminal IMCDs. Am J Physiol. Renal Physiol. 2000;279:F835–F840. doi: 10.1152/ajprenal.2000.279.5.F835. [DOI] [PubMed] [Google Scholar]
- 17.Gertner R.A., Klein J.D., Bailey J.L. Aldosterone decreases UT-A1 urea transporter expression via the mineralocorticoid receptor. J Am Soc Nephrol. 2004;15:558–565. doi: 10.1097/01.asn.0000113244.37857.ac. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt C., Hocherl K., Bucher M. Cytokine-mediated regulation of urea transporters during experimental endotoxemia. Am J Physiol. Renal Physiol. 2007;292:F1479–F1489. doi: 10.1152/ajprenal.00460.2006. [DOI] [PubMed] [Google Scholar]
- 19.Lim S.W., Li C., Sun B.K. Long-term treatment with cyclosporine decreases aquaporins and urea transporters in the rat kidney. Am J Physiol. Renal Physiol. 2004;287:F139–F151. doi: 10.1152/ajprenal.00240.2003. [DOI] [PubMed] [Google Scholar]
- 20.Trinh-Trang-Tan M.M., Geelen G., Teillet L., Corman B. Urea transporter expression in aging kidney and brain during dehydration. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1355–R1365. doi: 10.1152/ajpregu.00207.2003. [DOI] [PubMed] [Google Scholar]
- 21.Hu M.C., Bankir L., Michelet S. Massive reduction of urea transporters in remnant kidney and brain of uremic rats. Kidney Int. 2000;58:1202–1210. doi: 10.1046/j.1523-1755.2000.00275.x. [DOI] [PubMed] [Google Scholar]
- 22.Halperin M.L. Assessing the renal response in patients with potassium disorders: a shift in emphasis from the TTKG to the urine K+/creatinine ratio. Afr J Nephrol. 2017;20:22–24. [Google Scholar]
- 23.Lin S.H., Lin Y.F., Chen D.T. Laboratory tests to determine the cause of hypokalemia and paralysis. Arch Intern Med. 2004;164:1561–1566. doi: 10.1001/archinte.164.14.1561. [DOI] [PubMed] [Google Scholar]
- 24.Blanchard A., Bockenhauer D., Bolignano D. Gitelman syndrome: consensus and guidance from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2017;91:24–33. doi: 10.1016/j.kint.2016.09.046. [DOI] [PubMed] [Google Scholar]
- 25.Palmer B.F., Clegg D.J. Physiology and pathophysiology of potassium homeostasis: core curriculum 2019. Am J Kidney Dis. 2019;74:682–695. doi: 10.1053/j.ajkd.2019.03.427. [DOI] [PubMed] [Google Scholar]
- 26.Palmer B.F., Clegg D.J. The use of selected urine chemistries in the diagnosis of kidney disorders. Clin J Am Soc Nephrol. 2019;14:306–316. doi: 10.2215/CJN.10330818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gennari F.J., Weise W.J. Acid-base disturbances in gastrointestinal disease. Clin J Am Soc Nephrol. 2008;3:1861–1868. doi: 10.2215/CJN.02450508. [DOI] [PubMed] [Google Scholar]
- 28.Groeneveld J.H., Sijpkens Y.W., Lin S.H. An approach to the patient with severe hypokalaemia: the potassium quiz. QJM. 2005;98:305–316. doi: 10.1093/qjmed/hci046. [DOI] [PubMed] [Google Scholar]
- 29.Lin S.H., Davids M.R., Halperin M.L. Hypokalaemia and paralysis. QJM. 2003;96:161–169. doi: 10.1093/qjmed/hcg021. [DOI] [PubMed] [Google Scholar]
- 30.Kamel K.S., Ethier J., Levin A., Halperin M.L. Hypokalemia in the “beautiful people.”. Am J Med. 1990;88:534–536. doi: 10.1016/0002-9343(90)90436-h. [DOI] [PubMed] [Google Scholar]
- 31.Wu K.L., Cheng C.J., Sung C.C. Identification of the causes for chronic hypokalemia: importance of urinary sodium and chloride excretion. Am J Med. 2017;130:846–855. doi: 10.1016/j.amjmed.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 32.Lin S.H., Halperin M.L. Hypokalemia: a practical approach to diagnosis and its genetic basis. Curr Med Chem. 2007;14:1551–1565. doi: 10.2174/092986707780831050. [DOI] [PubMed] [Google Scholar]
- 33.Huang C.L., Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol. 2007;18:2649–2652. doi: 10.1681/ASN.2007070792. [DOI] [PubMed] [Google Scholar]
- 34.Wang T., Egbert A.L., Jr., Aronson P.S., Giebisch G. Effect of metabolic acidosis on NaCl transport in the proximal tubule. Am J Physiol. 1998;274:F1015–F1019. doi: 10.1152/ajprenal.1998.274.6.F1015. [DOI] [PubMed] [Google Scholar]
- 35.Kassirer J.P., Schwartz W.B. The response of normal man to selective depletion of hydrochloric acid. Factors in the genesis of persistent gastric alkalosis. Am J Med. 1966;40:10–18. doi: 10.1016/0002-9343(66)90182-3. [DOI] [PubMed] [Google Scholar]
- 36.Alexandridis G., Elisaf M. Renal potassium handling in a patient with surreptitious vomiting. Nephrol Dial Transplant. 2001;16:2275. doi: 10.1093/ndt/16.11.2275. [DOI] [PubMed] [Google Scholar]
- 37.Carlisle E.J., Donnelly S.M., Ethier J.H. Modulation of the secretion of potassium by accompanying anions in humans. Kidney Int. 1991;39:1206–1212. doi: 10.1038/ki.1991.152. [DOI] [PubMed] [Google Scholar]
- 38.Kamel K.S., Schreiber M., Halperin M.L. Renal potassium physiology: integration of the renal response to dietary potassium depletion. Kidney Int. 2018;93:41–53. doi: 10.1016/j.kint.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 39.Simon D.B., Lifton R.P. Ion transporter mutations in Gitelman’s and Bartter’s syndromes. Curr Opin Nephrol Hypertens. 1998;7:43–47. doi: 10.1097/00041552-199801000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Shaer A.J. Inherited primary renal tubular hypokalemic alkalosis: a review of Gitelman and Bartter syndromes. Am J Med. Sci. 2001;322:316–332. doi: 10.1097/00000441-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y.S., Fang H.C., Chou K.J. Gentamicin-induced Bartter-like syndrome. Am J Kidney Dis. 2009;54:1158–1161. doi: 10.1053/j.ajkd.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 42.Chrispal A., Boorugu H., Prabhakar A.T., Moses V. Amikacin-induced type 5 Bartter-like syndrome with severe hypocalcemia. J Postgrad Med. 2009;55:208–2010. doi: 10.4103/0022-3859.57407. [DOI] [PubMed] [Google Scholar]
- 43.Oronsky B., Caroen S., Oronsky A. Electrolyte disorders with platinum-based chemotherapy: mechanisms, manifestations and management. Cancer Chemother Pharmacol. 2017;80:895–907. doi: 10.1007/s00280-017-3392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casatta L., Ferraccioli G.F., Bartoli E. Hypokalaemic alkalosis, acquired Gitelman’s and Bartter’s syndrome in chronic sialoadenitis. Br J Rheumatol. 1997;36:1125–1128. doi: 10.1093/rheumatology/36.10.1125. [DOI] [PubMed] [Google Scholar]
- 45.Barathidasan G.S., Krishnamurthy S., Karunakar P. Systemic lupus erythematosus complicated by a Gitelman-like syndrome in an 8-year-old girl. CEN Case Rep. 2020;9:129–132. doi: 10.1007/s13730-019-00440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim Y.K., Song H.C., Kim Y.S., Choi E.J. Acquired Gitelman syndrome. Electrolyte Blood Press. 2009;7:5–8. doi: 10.5049/EBP.2009.7.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bettinelli A., Bianchetti M.G., Girardin E. Use of calcium excretion values to distinguish two forms of primary renal tubular hypokalemic alkalosis: Bartter and Gitelman syndromes. J Pediatr. 1992;120:38–43. doi: 10.1016/s0022-3476(05)80594-3. [DOI] [PubMed] [Google Scholar]
- 48.Ellison D.H. Divalent cation transport by the distal nephron: insights from Bartter’s and Gitelman’s syndromes. Am J Physiol Renal Physiol. 2000;279:F616–F625. doi: 10.1152/ajprenal.2000.279.4.F616. [DOI] [PubMed] [Google Scholar]
- 49.Cheng C.J., Lin C.S., Chang L.W., Lin S.H. Perplexing hyperkalaemia. Nephrol Dial Transplant. 2006;21:3320–3323. doi: 10.1093/ndt/gfl389. [DOI] [PubMed] [Google Scholar]
- 50.DuBose T.D., Jr. Hyperkalemic hyperchloremic metabolic acidosis: pathophysiologic insights. Kidney Int. 1997;51:591–602. doi: 10.1038/ki.1997.85. [DOI] [PubMed] [Google Scholar]
- 51.Kamel K.S., Schreiber M., Halperin M.L. Integration of the response to a dietary potassium load: a Paleolithic perspective. Nephrol Dial Transplant. 2014;29:982–989. doi: 10.1093/ndt/gft499. [DOI] [PubMed] [Google Scholar]
- 52.Bhasin B., Velez J.C. Evaluation of Polyuria: The roles of solute loading and water diuresis. Am J Kidney Dis. 2016;67:507–511. doi: 10.1053/j.ajkd.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 53.Christ-Crain M., Bichet D.G., Fenske W.K. Diabetes insipidus. Nat Rev Dis Primers. 2019;5:54. doi: 10.1038/s41572-019-0103-2. [DOI] [PubMed] [Google Scholar]
- 54.Verbalis J.G. Disorders of body water homeostasis. Best Pract Res Clin Endocrinol Metab. 2003;17:471–503. doi: 10.1016/s1521-690x(03)00049-6. [DOI] [PubMed] [Google Scholar]
- 55.Kamel K.S., Bichet D.G., Halperin M.L. A patient with partial central diabetes insipidus: clarifying pathophysiology and designing treatment. Am J Kidney Dis. 2001;37:1290–1293. doi: 10.1053/ajkd.2001.24537. [DOI] [PubMed] [Google Scholar]
- 56.Fenske W., Allolio B. Clinical review: current state and future perspectives in the diagnosis of diabetes insipidus: a clinical review. J Clin Endocrinol Metab. 2012;97:3426–3437. doi: 10.1210/jc.2012-1981. [DOI] [PubMed] [Google Scholar]
- 57.Miller M., Dalakos T., Moses A.M. Recognition of partial defects in antidiuretic hormone secretion. Ann Intern Med. 1970;73:721–729. doi: 10.7326/0003-4819-73-5-721. [DOI] [PubMed] [Google Scholar]
- 58.De Wardener H.E., Herxheimer A. The effect of a high water intake on the kidney’s ability to concentrate the urine in man. J Physiol. 1957;139:42–52. doi: 10.1113/jphysiol.1957.sp005873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Epstein F.H., Kleeman C.R., Hendrikx A. The influence of bodily hydration on the renal concentrating process. J Clin Invest. 1957;36:629–634. doi: 10.1172/JCI103462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harrington A.R., Valtin H. Impaired urinary concentration after vasopressin and its gradual correction in hypothalamic diabetes insipidus. J Clin Invest. 1968;47:502–510. doi: 10.1172/JCI105746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kiil F., Aukland K. Renal tubular localization of water and sodium reabsorption in antidiuresis and water diuresis. Scand J Clin Lab Invest. 1960;12:277–289. doi: 10.3109/00365516009062438. [DOI] [PubMed] [Google Scholar]
- 62.Jamison R.L., Buerkert J., Lacy F. A micropuncture study of collecting tubule function in rats with hereditary diabetes insipidus. J Clin Invest. 1971;50:2444–2452. doi: 10.1172/JCI106743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Levitin H., Goodman A., Pigeon G., Epstein F.H. Composition of the renal medulla during water diuresis. J Clin Invest. 1962;41:1145–1151. doi: 10.1172/JCI104567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zimmerhackl B.L., Robertson C.R., Jamison R.L. The medullary microcirculation. Kidney Int. 1987;31:641–647. doi: 10.1038/ki.1987.46. [DOI] [PubMed] [Google Scholar]
- 65.Zerbe R.L., Robertson G.L. A comparison of plasma vasopressin measurements with a standard indirect test in the differential diagnosis of polyuria. N Engl J Med. 1981;305:1539–1546. doi: 10.1056/NEJM198112243052601. [DOI] [PubMed] [Google Scholar]
- 66.Milles J.J., Spruce B., Baylis P.H. A comparison of diagnostic methods to differentiate diabetes insipidus from primary polyuria: a review of 21 patients. Acta Endocrinol (Copenh) 1983;104:410–416. doi: 10.1530/acta.0.1040410. [DOI] [PubMed] [Google Scholar]
- 67.Fenske W., Quinkler M., Lorenz D. Copeptin in the differential diagnosis of the polydipsia-polyuria syndrome—revisiting the direct and indirect water deprivation tests. J Clin Endocrinol Metab. 2011;96:1506–1515. doi: 10.1210/jc.2010-2345. [DOI] [PubMed] [Google Scholar]
- 68.Ray J.G. DDAVP use during pregnancy: an analysis of its safety for mother and child. Obstet Gynecol Surv. 1998;53:450–455. doi: 10.1097/00006254-199807000-00025. [DOI] [PubMed] [Google Scholar]
- 69.Rose B.D. New approach to disturbances in the plasma sodium concentration. Am J Med. 1986;81:1033–1040. doi: 10.1016/0002-9343(86)90401-8. [DOI] [PubMed] [Google Scholar]
- 70.Mallie J.P., Bichet D.G., Halperin M.L. Effective water clearance and tonicity balance: the excretion of water revisited. Clin Invest Med. 1997;20:16–24. [PubMed] [Google Scholar]
- 71.Carlotti A.P., Bohn D., Mallie J.P., Halperin M.L. Tonicity balance, and not electrolyte-free water calculations, more accurately guides therapy for acute changes in natremia. Intensive Care Med. 2001;27:921–924. doi: 10.1007/s001340100911. [DOI] [PubMed] [Google Scholar]
- 72.Mallie J.P. Hypernatraemia and tonicity balance. Nephrol Dial Transplant. 1999;14:1332–1333. doi: 10.1093/ndt/14.5.1332. [DOI] [PubMed] [Google Scholar]
- 73.Kamel K.S., Magner P.O., Ethier J.H., Halperin M.L. Urine electrolytes in the assessment of extracellular fluid volume contraction. Am J Nephrol. 1989;9:344–347. doi: 10.1159/000167991. [DOI] [PubMed] [Google Scholar]
- 74.Adrogue H.J., Madias N.E. Hyponatremia. N Engl J Med. 2000;342:1581–1589. doi: 10.1056/NEJM200005253422107. [DOI] [PubMed] [Google Scholar]
- 75.Ellison D.H., Berl T. Clinical practice. The syndrome of inappropriate antidiuresis. N Engl J Med. 2007;356:2064–2072. doi: 10.1056/NEJMcp066837. [DOI] [PubMed] [Google Scholar]
- 76.Goldsmith S.R., Francis G.S., Cowley A.W., Cohn J.N. Response of vasopressin and norepinephrine to lower body negative pressure in humans. Am J Physiol. 1982;243:H970–H973. doi: 10.1152/ajpheart.1982.243.6.H970. [DOI] [PubMed] [Google Scholar]
- 77.Robertson G.L. Vasopressin. In: Seldin D.W., Giebisch G., editors. The Kidney: Physiology and Pathophysiology. Lippincott; Philadelphia, PA: 2000. pp. 1132–1152. [Google Scholar]
- 78.Thaler S.M., Teitelbaum I., Berl T. “Beer potomania” in non-beer drinkers: effect of low dietary solute intake. Am J Kidney Dis. 1998;31:1028–1031. doi: 10.1053/ajkd.1998.v31.pm9631849. [DOI] [PubMed] [Google Scholar]
- 79.Filippone E.J., Ruzieh M., Foy A. Thiazide-associated hyponatremia: clinical manifestations and pathophysiology. Am J Kidney Dis. 2020;75:256–264. doi: 10.1053/j.ajkd.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 80.Kamel K.S., Halperin M.L. The importance of distal delivery of filtrate and residual water permeability in the pathophysiology of hyponatremia. Nephrol Dial Transplant. 2012;27:872–875. doi: 10.1093/ndt/gfr790. [DOI] [PubMed] [Google Scholar]
- 81.Ware J.S., Wain L.V., Channavajjhala S.K. Phenotypic and pharmacogenetic evaluation of patients with thiazide-induced hyponatremia. J Clin Invest. 2017;127:3367–3374. doi: 10.1172/JCI89812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oh M.S., Carroll H.J., Roy A. Chronic hyponatremia in the absence of ADH: possible role of decreased delivery of filtrate. J Am Soc Nephrol. 1997;8:108A. [Google Scholar]
- 83.Chung H.M., Kluge R., Schrier R.W., Anderson R.J. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med. 1987;83:905–908. doi: 10.1016/0002-9343(87)90649-8. [DOI] [PubMed] [Google Scholar]
- 84.Musch W., Thimpont J., Vandervelde D. Combined fractional excretion of sodium and urea better predicts response to saline in hyponatremia than do usual clinical and biochemical parameters. Am J Med. 1995;99:348–355. doi: 10.1016/s0002-9343(99)80180-6. [DOI] [PubMed] [Google Scholar]
- 85.Decaux G., Musch W. Clinical laboratory evaluation of the syndrome of inappropriate secretion of antidiuretic hormone. Clin J Am Soc Nephrol. 2008;3:1175–1184. doi: 10.2215/CJN.04431007. [DOI] [PubMed] [Google Scholar]
- 86.Musch W., Decaux G. Utility and limitations of biochemical parameters in the evaluation of hyponatremia in the elderly. Int Urol Nephrol. 2001;32:475–493. doi: 10.1023/a:1017586004688. [DOI] [PubMed] [Google Scholar]
- 87.Beck L.H. Changes in renal function with aging. Clin Geriatr Med. 1998;14:199–209. [PubMed] [Google Scholar]
- 88.Mallie J.P., Chevet D., Jeandel C. Defective post-diuretic antinatriuresis in aging kidney: a possible explanation for thiazide-induced hyponatremia in the elderly. Geriatr Nephrol Urol. 1995;4:153–160. [Google Scholar]
- 89.Fenske W.K., Christ-Crain M., Horning A. A copeptin-based classification of the osmoregulatory defects in the syndrome of inappropriate antidiuresis. J Am Soc Nephrol. 2014;25:2376–2383. doi: 10.1016/j.beem.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 90.Beck L.H. Hypouricemia in the syndrome of inappropriate secretion of antidiuretic hormone. N Engl J Med. 1979;301:528–530. doi: 10.1056/NEJM197909063011005. [DOI] [PubMed] [Google Scholar]
- 91.Dorhout Mees E.J., Blom van Assendelft P., Nieuwenhuis M.G. Elevation of uric acid clearance caused by inappropriate antidiuretic hormone secretion. Acta Med Scand. 1971;189:69–72. doi: 10.1111/j.0954-6820.1971.tb04340.x. [DOI] [PubMed] [Google Scholar]
- 92.Sonnenblick M., Rosin A. Increased uric acid clearance in the syndrome of inappropriate secretion of antidiuretic hormone. Isr J Med Sci. 1988;24:20–23. [PubMed] [Google Scholar]
- 93.Decaux G., Namias B., Gulbis B., Soupart A. Evidence in hyponatremia related to inappropriate secretion of ADH that V1 receptor stimulation contributes to the increase in renal uric acid clearance. J Am Soc Nephrol. 1996;7:805–810. doi: 10.1681/ASN.V75805. [DOI] [PubMed] [Google Scholar]
- 94.Decaux G., Prospert F., Soupart A., Musch W. Evidence that chronicity of hyponatremia contributes to the high urate clearance observed in the syndrome of inappropriate antidiuretic hormone secretion. Am J Kidney Dis. 2000;36:745–751. doi: 10.1053/ajkd.2000.17623. [DOI] [PubMed] [Google Scholar]
- 95.Weinberger A., Santo M., Solomon F. Abnormality in renal urate handling in the syndrome of inappropriate secretion of antidiuretic hormone. Isr J Med Sci. 1982;18:711–713. [PubMed] [Google Scholar]
- 96.Oh M.S., Carroll H.J. Cerebral salt-wasting syndrome. We need better proof of its existence. Nephron. 1999;82:110–114. doi: 10.1159/000045385. [DOI] [PubMed] [Google Scholar]
- 97.Singh S., Bohn D., Carlotti A.P., Cusimano M. Cerebral salt wasting: truths, fallacies, theories, and challenges. Crit Care Med. 2002;30:2575–2579. doi: 10.1097/00003246-200211000-00028. [DOI] [PubMed] [Google Scholar]
- 98.Verbalis J.G. The curious story of cerebral salt wasting: fact or fiction? Clin J Am Soc Nephrol. 2020;15:1666–1668. doi: 10.2215/CJN.00070120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maesaka J.K., Imbriano L.J., Miyawaki N. Determining fractional urate excretion rates in hyponatremic conditions and improved methods to distinguish cerebral/renal salt wasting from the syndrome of inappropriate secretion of antidiuretic hormone. Front Med (Lausanne) 2018;5:319. doi: 10.3389/fmed.2018.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Imbriano L.J., Mattana J., Drakakis J., Maesaka J.K. Identifying different causes of hyponatremia with fractional excretion of uric acid. Am J Med. Sci. 2016;352:385–390. doi: 10.1016/j.amjms.2016.05.035. [DOI] [PubMed] [Google Scholar]
- 101.Maesaka J.K., Imbriano L.J., Miyawaki N. High prevalence of renal salt wasting without cerebral disease as cause of hyponatremia in general medical wards. Am J Med. Sci. 2018;356:15–22. doi: 10.1016/j.amjms.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 102.Bitew S., Imbriano L., Miyawaki N. More on renal salt wasting without cerebral disease: response to saline infusion. Clin J Am Soc Nephrol. 2009;4:309–315. doi: 10.2215/CJN.02740608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maesaka J.K., Imbriano L.J., Miyawaki N. Evolution and evolving resolution of controversy over existence and prevalence of cerebral/renal salt wasting. Curr Opin Nephrol Hypertens. 2020;29:213–220. doi: 10.1097/MNH.0000000000000592. [DOI] [PubMed] [Google Scholar]
- 104.Carlisle E.J., Donnelly S.M., Vasuvattakul S. Glue-sniffing and distal renal tubular acidosis: sticking to the facts. J Am Soc Nephrol. 1991;1:1019–1027. doi: 10.1681/ASN.V181019. [DOI] [PubMed] [Google Scholar]
- 105.Brenes L.G., Sanchez M.I. Impaired urinary ammonium excretion in patients with isolated proximal renal tubular acidosis. J Am Soc Nephrol. 1993;4:1073–1078. doi: 10.1681/ASN.V441073. [DOI] [PubMed] [Google Scholar]
- 106.Halperin M.L., Kamel K.S., Ethier J.H., Magner P.O. What is the underlying defect in patients with isolated, proximal renal tubular acidosis? Am J Nephrol. 1989;9:265–268. doi: 10.1159/000167979. [DOI] [PubMed] [Google Scholar]
- 107.Kamel K.S., Briceno L.F., Sanchez M.I. A new classification for renal defects in net acid excretion. Am J Kidney Dis. 1997;29:136–146. doi: 10.1016/s0272-6386(97)90021-4. [DOI] [PubMed] [Google Scholar]
- 108.Richardson R.M., Halperin M.L. The urine pH: a potentially misleading diagnostic test in patients with hyperchloremic metabolic acidosis. Am J Kidney Dis. 1987;10:140–143. doi: 10.1016/s0272-6386(87)80047-1. [DOI] [PubMed] [Google Scholar]
- 109.Carlisle E.J., Donnelly S.M., Halperin M.L. Renal tubular acidosis (RTA): recognize the ammonium defect and pH or get the urine pH. Pediatr Nephrol. 1991;5:242–248. doi: 10.1007/BF01095965. [DOI] [PubMed] [Google Scholar]
- 110.Simpson D.P. Control of hydrogen ion homeostasis and renal acidosis. Medicine (Baltimore) 1971;50:503–541. doi: 10.1097/00005792-197111000-00002. [DOI] [PubMed] [Google Scholar]
- 111.Madison L.L., Seldin D.W. Ammonia excretion and renal enzymatic adaptation in human subjects, as disclosed by administration of precursor amino acids. J Clin Invest. 1958;37:1615–1627. doi: 10.1172/JCI103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Goldstein M.B., Bear R., Richardson R.M. The urine anion gap: a clinically useful index of ammonium excretion. Am J Med. Sci. 1986;292:198–202. doi: 10.1097/00000441-198610000-00003. [DOI] [PubMed] [Google Scholar]
- 113.Oh M., Carroll H.J. Value and determinants of urine anion gap. Nephron. 2002;90:252–255. doi: 10.1159/000049059. [DOI] [PubMed] [Google Scholar]
- 114.Batlle D.C., Hizon M., Cohen E. The use of the urinary anion gap in the diagnosis of hyperchloremic metabolic acidosis. N Engl J Med. 1988;318:594–599. doi: 10.1056/NEJM198803103181002. [DOI] [PubMed] [Google Scholar]
- 115.Dyck R.F., Asthana S., Kalra J. A modification of the urine osmolal gap: an improved method for estimating urine ammonium. Am J Nephrol. 1990;10:359–362. doi: 10.1159/000168150. [DOI] [PubMed] [Google Scholar]
- 116.Halperin M.L., Margolis B.L., Robinson L.A. The urine osmolal gap: a clue to estimate urine ammonium in “hybrid” types of metabolic acidosis. Clin Invest Med. 1988;11:198–202. [PubMed] [Google Scholar]
- 117.Kamel K.S., Halperin M.L. An improved approach to the patient with metabolic acidosis: a need for four amendments. J Nephrol. 2006;19(suppl 9):S76–S85. [PubMed] [Google Scholar]
- 118.Halperin M.L., Kamel K.S. Some observations on the clinical approach to metabolic acidosis. J Am Soc Nephrol. 2010;21:894–897. doi: 10.1681/ASN.2009080794. [DOI] [PubMed] [Google Scholar]
- 119.Meregalli P., Luthy C., Oetliker O.H., Bianchetti M.G. Modified urine osmolal gap: an accurate method for estimating the urinary ammonium concentration? Nephron. 1995;69:98–101. doi: 10.1159/000188374. [DOI] [PubMed] [Google Scholar]
- 120.Raphael K.L., Ix J.H. Correlation of urine ammonium and urine osmolal gap in kidney transplant recipients. Clin J Am Soc Nephrol. 2018;13:638–640. doi: 10.2215/CJN.13311117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Weiner I.D., Verlander J.W. Renal ammonia metabolism and transport. Compr Physiol. 2013;3:201–220. doi: 10.1002/cphy.c120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kurtz I. Renal tubular acidosis: H(+)/base and ammonia transport abnormalities and clinical syndromes. Adv Chronic Kidney Dis. 2018;25:334–350. doi: 10.1053/j.ackd.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Haque S.K., Ariceta G., Batlle D. Proximal renal tubular acidosis: a not so rare disorder of multiple etiologies. Nephrol Dial Transplant. 2012;27:4273–4287. doi: 10.1093/ndt/gfs493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Simpson D.P. Citrate excretion: a window on renal metabolism. Am J Physiol. 1983;244:F223–F234. doi: 10.1152/ajprenal.1983.244.3.F223. [DOI] [PubMed] [Google Scholar]
- 125.Hamm L.L. Renal handling of citrate. Kidney Int. 1990;38:728–735. doi: 10.1038/ki.1990.265. [DOI] [PubMed] [Google Scholar]
- 126.Hering-Smith K.S., Hamm L.L. Acidosis and citrate: provocative interactions. Ann Transl Med. 2018;6:374. doi: 10.21037/atm.2018.07.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dedmon R.E., Wrong O. The excretion of organic anion in renal tubular acidosis with particular reference to citrate. Clin Sci. 1962;22:19–32. [PubMed] [Google Scholar]
- 128.Wrong O., Davies H.E. The excretion of acid in renal disease. Q J Med. 1959;28:259–313. [PubMed] [Google Scholar]
- 129.Norman M.E., Feldman N.I., Cohn R.M. Urinary citrate excretion in the diagnosis of distal renal tubular acidosis. J Pediatr. 1978;92:394–400. doi: 10.1016/s0022-3476(78)80426-0. [DOI] [PubMed] [Google Scholar]
- 130.Nagai R., Kooh S.W., Balfe J.W. Renal tubular acidosis and osteopetrosis with carbonic anhydrase II deficiency: pathogenesis of impaired acidification. Pediatr Nephrol. 1997;11:633–636. doi: 10.1007/s004670050354. [DOI] [PubMed] [Google Scholar]
- 131.Ohlsson A., Cumming W.A., Paul A., Sly W.S. Carbonic anhydrase II deficiency syndrome: recessive osteopetrosis with renal tubular acidosis and cerebral calcification. Pediatrics. 1986;77:371–381. [PubMed] [Google Scholar]
- 132.Halperin M.L., Goldstein M.B., Haig A. Studies on the pathogenesis of type I (distal) renal tubular acidosis as revealed by the urinary PCO2 tensions. J Clin Invest. 1974;53:669–677. doi: 10.1172/JCI107604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.DuBose T.D., Jr. Hydrogen ion secretion by the collecting duct as a determinant of the urine to blood PCO2 gradient in alkaline urine. J Clin Invest. 1982;69:145–156. doi: 10.1172/JCI110425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.DuBose T.D., Jr., Caflisch C.R. Validation of the difference in urine and blood carbon dioxide tension during bicarbonate loading as an index of distal nephron acidification in experimental models of distal renal tubular acidosis. J Clin Invest. 1985;75:1116–1123. doi: 10.1172/JCI111805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Roscoe J.M., Goldstein M.B., Halperin M.L. Effect of amphotercin B on urine acidification in rats: implications for the pathogenesis of distal renal tubular acidosis. J Lab Clin Med. 1977;89:463–470. [PubMed] [Google Scholar]
- 136.Kaitwatcharachai C., Vasuvattakul S., Yenchitsomanus P. Distal renal tubular acidosis and high urine carbon dioxide tension in a patient with southeast Asian ovalocytosis. Am J Kidney Dis. 1999;33:1147–1152. doi: 10.1016/s0272-6386(99)70154-x. [DOI] [PubMed] [Google Scholar]
- 137.Jarolim P., Shayakul C., Prabakaran D. Autosomal dominant distal renal tubular acidosis is associated in three families with heterozygosity for the R589H mutation in the AE1 (band 3) Cl–/HCO3– exchanger. J Biol Chem. 1998;273:6380–6388. doi: 10.1074/jbc.273.11.6380. [DOI] [PubMed] [Google Scholar]
- 138.Elkinton J.R. The kidney and hydrogen ion metabolism. Bibl Paediatr. 1960;74:99–123. [PubMed] [Google Scholar]