Abstract
Introduction
Albuminuric and nonalbuminuric pathways contribute to diabetic kidney disease. Proximal tubule and inflammation play important roles in these processes. Urinary biomarker(s) to detect early kidney damage and predict progression are needed.
Methods
Nine urinary biomarkers were measured at baseline in 400 patients with diabetes. Correlation and multivariate logistic and linear regression analyses were performed to assess the association of biomarkers with chronic kidney disease and progression.
Results
In the albumin/creatinine ratio (ACR) <3 cohort, the only biomarker significantly associated with estimated glomerular filtration rate < 60 ml/min was N-acetyl-β-d-glucosaminidase. A combination of ACR and monocyte chemoattractant protein 1 (MCP1) were significantly associated with stage 2 chronic kidney disease in this cohort. Logistic models showed that in patients with all levels of albuminuria, ACR, retinol binding protein (RBP), and MCP1 were associated with progression. A model including MCP1, interleukin 6, and neutrophil gelatinase-associated lipocalin showed significant association with progression to chronic kidney disease 3/4 in the ACR <3 cohort. Linear mixed-model regression analyses demonstrated MCP1, RBP, and ACR as significant proteins associated with progression to stage 3 or worse, whereas MCP1 was the only significant biomarker in the ACR <3 cohort. Time-to-event and Cox proportional hazard models confirmed significant hazard ratios for progression for ACR, RBP, and MCP1, with significant differences noted between quantiles of biomarkers for ACR, RBP, and MCP1.
Conclusion
In this study of diabetic patients with single baseline measurements of urinary biomarkers, albumin, RBP, and MCP1 were significantly associated with chronic kidney disease progression at all levels of albuminuria. Inflammatory cytokines, neutrophil gelatinase-associated lipocalin, and MCP1 were associated with progression in patients without albuminuria. N-acetyl-β-d-glucosaminidase demonstrated a significant association with an estimated glomerular filtration rate < 60 ml/min in the ACR <3 cohort.
Keywords: biomarker, CKD progression, diabetic kidney disease, fibrosis, inflammation, proximal tubular markers
Graphical abstract
See Commentary on Page 1200
Chronic kidney disease (CKD) occurs in 20% to 40% of diabetic patients, with prevalence increasing with age and duration of diabetes. Glomerular, tubulointerstitial, and vascular diseases contribute to the development and progression of CKD in diabetes (diabetic kidney disease). Atherosclerotic renovascular disease, prevalent in patients with diabetes, produces progressive renal hypoperfusion, tissue hypoxia, and inflammatory kidney injury, often described as ischemic nephropathy, which may or may not be associated with albuminuria.1,2
Currently, CKD is detected and monitored with the estimated glomerular filtration rate (eGFR) derived from creatinine-based equations (CKD-Epidemiology Collaboration or Modification of Diet in Renal Disease) and albuminuria (albumin/creatinine ratio [ACR]). Both have limitations for identifying early CKD and predicting CKD progression. A significant proportion of patients with type 2 diabetes and CKD have minimal or no albuminuria (ACR < 3 mg/mmol). A decline in GFR may predate proteinuria, and nonproteinuric pathways of kidney damage in diabetes are increasingly recognized.3
Although the glomerulus is thought to be the main site of injury and damage in diabetes, the proximal tubule is likely to play a key role at all stages of CKD.4 The role of tubular injury and inflammation in CKD progression has been highlighted in recent studies.5,6 The significant impact of sodium-glucose cotransporter 2 inhibitor, drugs targeting the proximal tubule in slowing down progression of diabetic kidney disease, has provided impetus for further studies to investigate the role of proximal tubule-derived biomarkers.7
Proximal tubule-derived proteins in urine and inflammatory cytokines could be the relevant biomarkers to assess the efficacy of drugs such as sodium-glucose cotransporter 2 inhibitors in addition to or instead of albumin and may be an appropriate end point to include in clinical trials involving these drugs and others designed to treat CKD. Previous studies have typically evaluated individual tubule injury markers, and a recent study assessed multiple biomarkers that reflect tubular injury and risk of CKD progression in hypertensive patients in the Systolic Blood Pressure Intervention Trial (SPRINT cohort).6 However, there is a paucity of literature on biomarkers in nonalbuminuric diabetic kidney disease.
We hypothesized that a panel of urinary biomarkers, including markers and mediators of renal disease, representing areas of renal injury (glomerulus, tubulointerstitium) and that reflect damage, inflammation, and fibrosis are required to assess whether they provide useful information in diabetic patients with and without albuminuria. We therefore designed a single-center prospective cohort study of patients with diabetes to test the utility of 9 biomarkers measured once at baseline in the detection of early kidney disease and progression in patients with diabetes.
Methods
A single-center prospective cohort study of 400 patients with diabetes was conducted. Appropriate ethics approval was obtained through the UK Heath Research Authority (HRA Approval ID: 08/H0806/8). Patients with diabetes mellitus attending outpatient clinics at Croydon University Hospital, South London, UK, between 2008 and 2013 were recruited. All consecutive patients were recruited without the requirement of biopsy specimen-proven diabetic kidney disease.
After basic demographics were recorded, random urine samples were collected in 20-ml sterile tubes. Samples were aliquoted immediately in 2-ml cryovials and transported to the laboratory on ice within 4 hours after collection. Samples were then stored at −80 °C for biomarker assays.
Exclusion criteria were patients with urinary tract infection, any systemic symptoms or signs of other infection, systemic inflammatory disorders, and patients on immunosuppressive medications. We excluded 12 of 400 patients because of very low urinary creatinine concentrations (<1 mmol/l) because this was too dilute to enable indexing of urinary biomarker to creatinine, and 388 patients were included in the analysis.
Baseline blood results (including eGFR, reported using the Modification of Diet in Renal Disease equation) from the samples taken at the time of urine collection or the most recent result available (within 3 months) were recorded. eGFR values reported as part of routine clinical care over 5-year period after recruitment were collected from patients’ electronic health records to analyze progression. Patients with at least 3 eGFR values over a follow-up period of 5 years were included for progression analysis.
Biomarkers
The urine samples were tested for baseline measures of following biomarkers: albumin (marker of glomerular damage), retinol binding protein (RBP), and N-acetyl-β-d-glucosaminidase (NAG), which are markers of proximal tubular injury; neutrophil gelatinase-associated lipocalin (NGAL), a marker of distal nephron injury and inflammation; monocyte chemotactic protein 1 (MCP1), interleukin (IL)-1β, IL-6, and tumor necrosis factor-α as inflammation markers; and transforming growth factor-β1, -2, and -3 as fibrosis markers. Further details of these assays are available in Supplementary File S1. Results were expressed as level of biomarker indexed to urinary creatinine. Random urine samples were also collected from 10 healthy volunteers for biomarker analysis.
Statistical Analysis
Data are described as mean (SD) or median and 25th–75th percentiles (interquartile range [IQR]). Because the distribution of each biomarker was positively skewed, the log transformation was used to conform with the assumptions necessary for regression analysis. The relationship of individual biomarkers with eGFR categories was explored by nonparametric analysis of variance (Kruskal-Wallis test) and the Jonckheere-Terpstra test. In subset of 20 patients and 10 healthy controls, comparisons between eGFR groups were done by the Kruskal-Wallis test with the Dunn multiple comparison test. Comparisons between 2 groups were done by the Mann-Whitney U test. Associations between individual biomarkers were tested with Spearman correlation analyses. Because most of the IL-1β values were below the detection limit of the assay, we have reported only the results of models that include MCP1 or IL-6 as inflammatory markers.
Multivariate logistic regression analysis was done to test the association of biomarkers with the presence of CKD stage 3 or worse (eGFR < 60 ml/min at baseline) or CKD stage 2 (eGFR 60–89 ml/min) and whether a patient was a “progressor” (progression to CKD3 or from CKD3 to CKD4/5). For a biomarker or a combination of biomarkers showing significant association in logistic models, the receiver operating characteristic (ROC) area under curve (AUC) results were obtained to assess its sensitivity and specificity to detect CKD stage 3 or worse (eGFR < 60 ml/min) or to detect a progressor, as defined above. When the P value is ≤0.05, the estimated odds ratio (OR) for a unit increase in the log of the biomarker and its 95% confidence interval (CI) is reported. Further details of statistical methodology of these logistic models are given in Supplementary File S2.
We also performed Cox proportional hazard model analysis with an event defined as a drop in eGFR to ≥30% from baseline value (the progressor) during the follow-up period of 5 years. Using same definition of event (to define progressive CKD) we performed time-to-event analysis. The biomarkers were grouped in 20% quantiles, and Kaplan-Meier plots were drawn to assess probability of survival to within 70% of the baseline eGFR during the follow-up period of 5 years, and the curves were compared with log-rank tests. The Cox proportional hazard models were used to estimate biomarker hazard ratios with CIs after adjustment for sex, age, ethnicity, glycated hemoglobin, and systolic blood pressure.
We performed linear (mixed-model) regression analysis using all eGFR values available from each patient. A mixed model was used to explore the association of biomarkers with patients’ trajectories of eGFR over a 5-year period. We used backwards elimination to investigate the best subset of biomarkers. The first model included 7 biomarkers (ACR, IL-1β, IL-6, MCP1, NAG, NGAL, and RBP). Those with nonsignificant (at the 5% level) fixed effects were removed one-by-one to reduce the model to one where each biomarker had a significant fixed effect on the gradient of eGFR over time in years, and predictive models were developed. The results also provide the β-value where we find a significant association. The β-value is an estimate for the increase in the natural log of eGFR each year when the natural log of the biomarker increases by 1 while other variables are held constant.
Next, we developed predictive models for CKD progression. For the whole cohort, using 1243 observations of eGFR over 5 years, we arrived at models where the eGFR gradient against time in years is associated with baseline measures of biomarkers. Models with the same numbers of observations were compared using the Akaike information criterion goodness-of-fit measure.
Statistical analyses were performed using SAS 9.4 (SAS Institute Inc, Cary, NC), GraphPad Prism 8.4 (GraphPad Software Inc, San Diego, CA), and R 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria) software.
Results
Baseline Characteristics, Descriptive, and Comparative Statistics
Table 1 describes baseline patient characteristics (N = 400). Mean duration of diabetes was 12.8 (SD, 7.7) years, median 11 years (IQR, 7–18 years) for type 2 diabetic patients, and mean 21 (SD, 12.5) years, median 20 years (IQR, 11–33 years) for type 1 diabetic patients. Medications included angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers in 66% of patients and a statin in 56%. Hypertension was documented in 85%. Of 388 patients included in the biomarker analysis, approximately 50% of patients had an eGFR of >60 ml/min and 50% at <60 ml/min: 42 (11%) were stage 4/5 (G4/5) CKD (eGFR < 30 ml/min), 142 (37%) were stage 3 (G3) CKD, and 204 (52%) had an eGFR of >60 ml/min (eGFR 60–89.9 ml/min, 131 [34%]; eGFR ≥ 90 ml/min, 73 [18.6%]).
Table 1.
Baseline patient characteristics along with comparisons between ACR <3 and ACR >3 groups
| Covariate | Overall |
ACR <3 |
ACR >3 |
P value |
|---|---|---|---|---|
| (N = 400) | (n = 198) | (n = 202) | ||
| Age, y | ||||
| Mean (SD) | 61.2 (15.2) | 60 | 62.4 | 0.11 |
| Median(IQR) | 64 (52.8-73) | |||
| Range | 17–85 | |||
| Weight, kg | ||||
| Mean (SD) | 83.4 (18.3) | 83.2 | 83.6 | 0.84 |
| Median (IQR) | 80.6 (71.1–92.6) | |||
| Range | 48–144 | |||
| Systolic BP, mm Hg | ||||
| Mean (SD) | 135.1 (16.5) | 133.3 | 137.0 | 0.026 |
| Median (IQR) | 137 (124–144) | |||
| Range | 100–198 | |||
| Diastolic BP, mm Hg | ||||
| Mean (SD) | 75.7 (9.8) | 74.9 | 76.3 | 0.15 |
| Median (IQR) | 75 (70–82) | |||
| Range | 50–108 | |||
| HbA1c , mmol/mol | ||||
| Mean (SD) | 66.1 (9.8) | 63.9 | 68.3 | 0.019 |
| Median (IQR) | 61.7 (54.1–73.8) | |||
| Range | 34.4–148 | |||
| Sex, No. | 0.91 | |||
| Male | 251 | 125 | 126 | |
| Female | 149 | 73 | 76 | |
| Diabetes type, No. | 0.99 | |||
| Type 1 | 66 | 32 | 34 | |
| Type 2 | 334 | 165 | 169 | |
| Ethnicity, No. | 0.045 | |||
| Asian | 100 | 41 | 59 | |
| Black | 71 | 31 | 40 | |
| White | 229 | 125 | 104 |
HbA1c, systolic BP, and ethnicity were significantly different between the 2 groups.
ACR, albumin/creatinine ratio; BP, blood pressure; HbA1c, glycated hemoglobin; IQR, interquartile range.
In our cohort of 388 patients selected for biomarker analysis, there was approximately a 50:50 distribution of patients with and without albuminuria (ACR): 202 (52%) had an ACR of ≥3 and 186 (48%) had an ACR of <3. There was significant difference in glycated hemoglobin, systolic blood pressure, and ethnicity between the 2 groups. Regression models included adjustment for these variables.
Tables 2 and 3 describe urinary biomarker levels in patients (n = 388, Table 2) and healthy controls (n = 10, Table 3). The levels IL-1β and IL-6 were not detectable in many patients (74% for IL-1β and 46% for IL-6), with values close to 0 (below detection limits of the assay), and therefore, results involving these covariates, in particular IL-1β, are unlikely to be of clinical significance with current assays. Transforming growth factor-β1, -2, and -3 and tumor necrosis factor-α were not detectable and, therefore, are excluded from analysis.
Table 2.
Levels of urinary biomarkers obtained at baseline from patients (N = 388)
| Statistic | RBP/Cre |
NAG/Cre |
IL-1β/Cre |
IL-6/Cre |
MCP-1/Cre |
NGAL/Cre |
ACR |
|---|---|---|---|---|---|---|---|
| (mg/mmol) | (μmol/h/mmol) | (pg/mmol) | (pg/mmol) | (pg/mmol) | (pg/mmol) | (mg/mmol) | |
| Valid, No. | 388 | 388 | 388 | 388 | 388 | 388 | 388 |
| Mean | 132.67 | 98.13 | 3.85 | 0.42 | 19.24 | 1029.22 | 30.71 |
| SD | 411.53 | 76.64 | 22.88 | 1.44 | 23.51 | 2661.24 | 87.9 |
| Minimum | 1.66 | 7.77 | 0 | 0 | 1.046 | 54.12 | 0.268 |
| Maximum | 4581.61 | 655.2 | 355.1 | 24.06 | 351.72 | 1884.42 | 711.89 |
| Percentiles | |||||||
| 25 | 10.79 | 52.43 | 0.01 | 0 | 9.09 | 487.04 | 1.25 |
| 50 | 21.105 | 74.99 | 0.076 | 0.146 | 13.24 | 717.12 | 2.94 |
| 75 | 63.16 | 121.26 | 0.54 | 0.366 | 21.88 | 1166 | 14.47 |
ACR, albumin/creatinine ratio; Cre, creatinine; IL, interleukin; MCP-1, monocyte chemotactic protein-1; NAG, N-acetyl-β-d-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; RBP, retinol binding protein.
Table 3.
Levels of urinary biomarkers obtained at baseline from healthy controls (n = 10)a
| Statistic | IL-1β/Cre |
IL-6/Cre |
MCP1/Cre |
RBP/Cre |
NAG/Cre |
|---|---|---|---|---|---|
| (pg/mmol) | (pg/mmol) | (pg/mmol) | (mg/mmol) | (μmol/h/mmol) | |
| Minimum | 0 | 0 | 4.44 | 6.55 | 2.71 |
| 25% percentile | 0 | 0 | 5.94 | 9.713 | 21.83 |
| Median | 0 | 0.01 | 8.75 | 13.57 | 29.58 |
| 75% percentile | 0.045 | 0.0425 | 9.755 | 21.13 | 39.69 |
| Maximum | 0.33 | 0.27 | 18.41 | 23.37 | 66.88 |
| Range | 0.33 | 0.27 | 13.97 | 16.82 | 64.17 |
| Mean | 0.051 | 0.041 | 8.881 | 14.87 | 30.78 |
| SD | 0.1132 | 0.08266 | 3.854 | 6.102 | 17.09 |
Cre, creatinine; IL, interleukin; MCP-1, monocyte chemotactic protein-1; NAG, N-acetyl-β-d-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; RBP, retinol binding protein.
The healthy controls (n = 10), 5 men and 5 women, were a mean age of 56 (SD, 4.24) years.
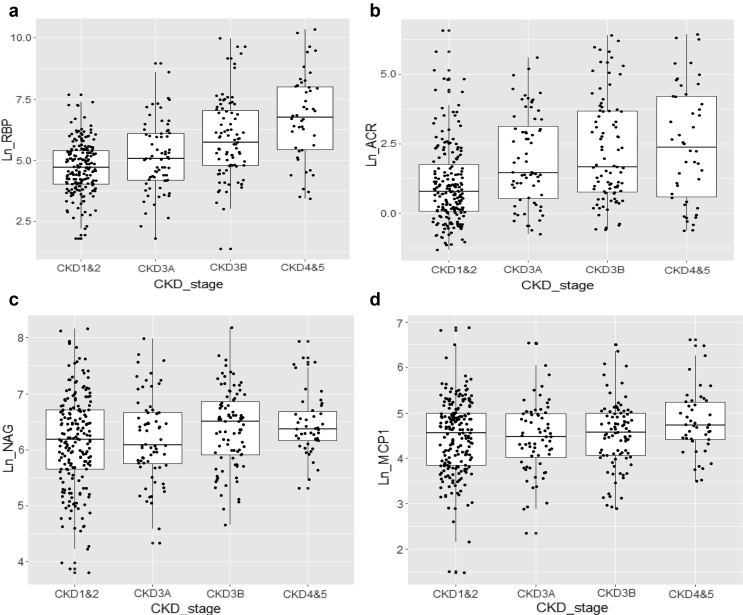
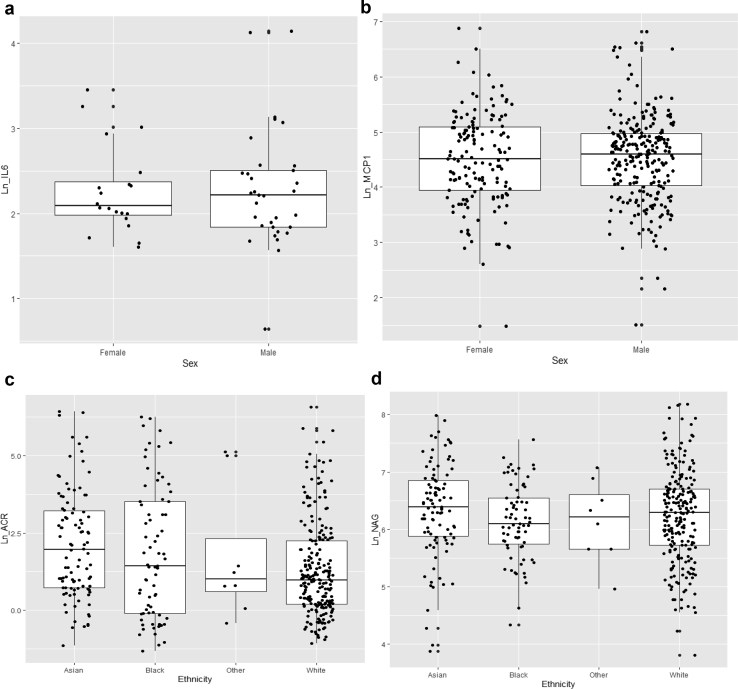
Comparison of biomarker levels between eGFR categories showed significant differences in the levels of urinary RBP and albumin with increasing CKD stages (Figure 1a and b) and to a lesser extent NAG (Figure 1c) but not MCP1 (Figure 1d). Urinary levels of inflammatory cytokines (IL-6 and MCP1) were higher in men compared to women (Figure 2a and b). Asian and Black ethnic groups had higher levels of urinary albumin compared with patients of White ethnicity. NAG levels in Asians were higher compared with Whites, but patients of Black ethnicity had lower NAG levels (Figure 2c and d); however, pairwise comparisons between the groups were nonsignificant.
Figure 1.
Urinary biomarker levels in various chronic kidney disease (CKD) stages. Significant difference in biomarker levels with increasing CKD stages was noted for (a) retinal binding protein (RBP), (b) the albumin/creatinine ratio (ACR), and to a lesser extent (c) N-acetyl-β-d-glucosaminidase (NAG), but (d) not for monocyte chemotactic protein 1 (MCP1). (a and b) Kruskal-Wallis test, P < 0.001; Jonckheere-Terpstra test for trend in the ordinal estimated glomerular filtration rate (eGFR) categories, P < 0.001. (c) Kruskal-Wallis test, P = 0.061; Jonckheere-Terpstra test for trend in the ordinal eGFR categories, P = 0.008. (d) Kruskal-Wallis test, P = 0.104; Jonckheere-Terpstra test for trend in the ordinal eGFR categories, P = 0.280. The line in the middle of each box indicates the median; the top and bottom borders of the box mark the 75th and 25th percentiles, respectively; and the vertical lines mark the 90th and 10th percentiles.
Figure 2.
Sex and ethnicity and urinary biomarker levels. Significant differences in the levels of inflammatory cytokines (a) interleukin 6 (IL-6) and (b) monocyte chemotactic protein 1 (MCP1) were seen in men compared with women, with higher levels seen in men. The levels of urinary (c) albumin (albumin/creatinine ratio [ACR]) and (d) and N-acetyl-β-d-glucosaminidase (NAG) were higher in Asians, and people of Black ethnicity had lower NAG levels, but pairwise comparisons were nonsignificant. (a and b) P < 0.01 by Mann-Whitney U test; (c) P = 0.007 and (d) P = 0.001 by Kruskal-Wallis test. The line in the middle of each box indicates the median; the top and bottom borders of the box mark the 75th and 25th percentiles, respectively; and the vertical lines mark the 90th and 10th percentiles.
Correlation Analysis
The correlation heat map was generated to assess correlations between individual biomarkers, and Table 4 describes relevant findings. Strong positive correlations were found between inflammatory cytokines IL-1β, IL-6, and MCP1 (R = 0.8 between MCP1 and IL6), moderate positive correlation between RBP and ACR (R = 0.61), a weak positive correlation between NAG and NGAL with ACR, and a weak correlation between markers of tubular injury and inflammatory cytokines (Table 4).
Table 4.
The “heat” map summarizing the pair-wise correlations among biomarkersa
| Biomarker | RBP/Cre | NAG/Cre | NGAL/Cre | IL1β/Cre | IL6/Cre | MCP1/Cre | ACR |
|---|---|---|---|---|---|---|---|
| RBP/Cre | 0.21 | 0.07 | 0.01 | 0.04 | 0.19 | 0.61 | |
| NAG/Cre | 0.21 | 0.06 | 0.07 | 0.09 | 0.24 | 0.23 | |
| NGAL/Cre | 0.07 | 0.06 | 0.15 | 0.04 | 0.01 | 0.08 | |
| IL1β/Cre | 0.01 | 0.07 | 0.15 | 0.86 | 0.65 | 0.03 | |
| IL6/Cre | 0.04 | 0.09 | 0.04 | 0.86 | 0.8 | 0.06 | |
| MCP1/Cre | 0.19 | 0.24 | 0.01 | 0.65 | 0.8 | 0.18 | |
| ACR | 0.61 | 0.23 | 0.08 | 0.03 | 0.06 | 0.18 |
ACR, albumin/creatinine ratio; Cre, creatinine; IL, interleukin; MCP-1, monocyte chemotactic protein-1; NAG, N-acetyl-β-d-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; RBP, retinol binding protein.
The R values in bold show a strong correlation, R values in italic show a moderate correlation, R values in black show a weak correlation.
Testing Association of Biomarkers with CKD Stage 3+ (eGFR < 60 at baseline): Multivariate Logistic Regression Analysis at All Levels of ACR
There was a significant association between ACR (OR, 1.6; 95% CI, 1.4–1.9; P = 0.001), log RBP (OR, 2.1; 95% CI, 1.7–2.5; P = 0.001), log NAG (OR, 1.62; 95% CI, 1.2–2.3; P = 0.005), and to a lesser extent, log NGAL (OR, 1.23; 95% CI, 102–1.47; P = 0.03) with odds for being CKD 3 or worse but not for other biomarkers tested.
Testing Association of Biomarkers With Stage 3+ (eGFR < 60 ml/min) and Stage 2 CKD: Multivariate Logistic Regression Analysis in ACR <3 Cohort
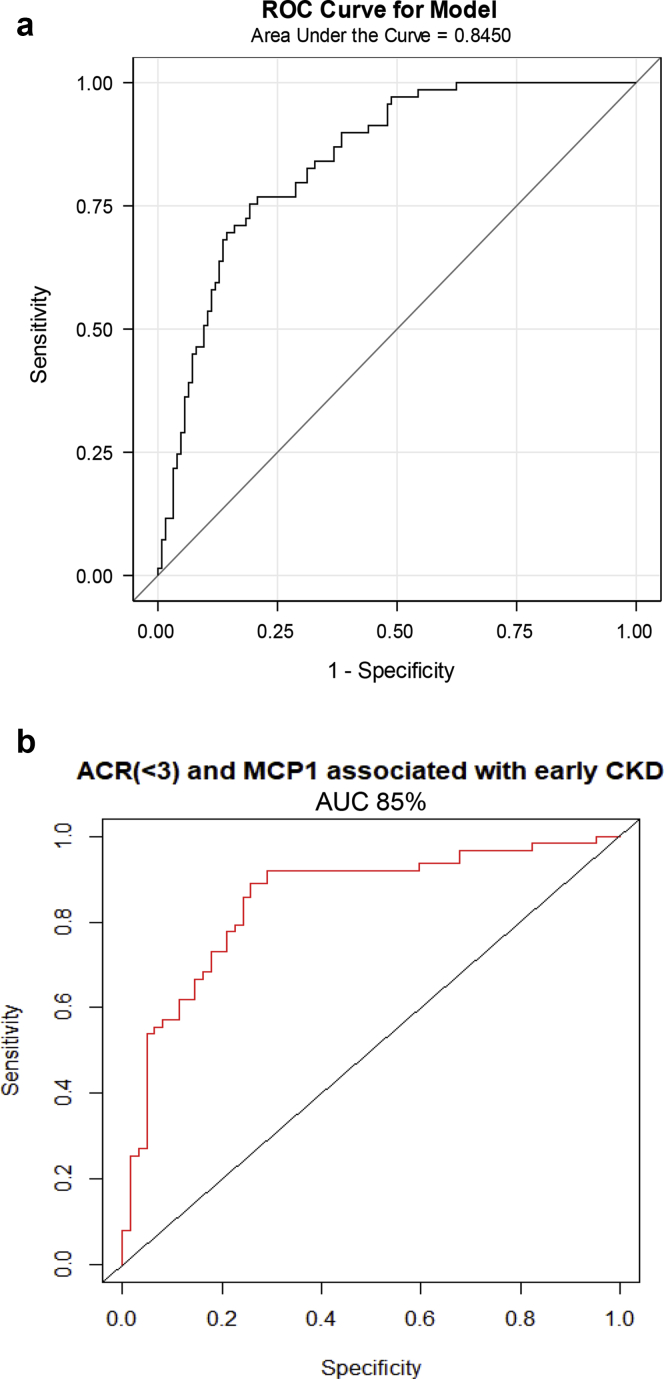
To test association of biomarkers with CKD in patients with ACR <3, we used a logistic model restricted to patients with ACR <3 using186 individuals’ observations for predicting CKD 3+ status. In this analysis, the only biomarker that showed significant association was NAG (log NAG OR, 2.3; 95% CI, 1.2-4.3; P = 0.009) with a ROC AUC of 85% (Table 5 and Figure 3a). The addition of log MCP1 to log RBP modestly improved the ability of RBP to predict CKD 3+ (OR, 1.5; P = 0.04), but this or any other combination of biomarkers did not demonstrate superior ROC AUCs compared with NAG alone. These results are summarized in Table 5 along with ROC AUCs for each relevant biomarker. We then performed logistic regression analyses to test association of biomarkers with early CKD (eGFR 60-89 ml/min). There were 131 participants in this group with ACR <3. Log ACR and log MCP1 were significantly associated with stage 2 CKD with an OR of 5.4 (95% CI, 1.6–27; interaction P = 0.02) and a ROC AUC of 0.85 (Table 6, Figure 3b).
Table 5.
Testing association of biomarkers with CKD stage 3+ (eGFR <60): Multivariate logistic regression analysis in patients with ACR <3a
| Biomarker | P value | Odds ratio (95% CI) | ROC AUC, % |
|---|---|---|---|
| Log NAG/Cre | 0.009 | 2.3(1.2–4.3) | 85 |
| Log NGAL/Cre | 0.88 | ||
| Log MCP1/Cre | 0.36 | ||
| Log RBP/Cre | 0.36 | ||
| Log MCP1/Cre + Log RBP/Cre | 0.04 | 1.5 (1–2.1) | 85 |
ACR, albumin/creatinine ratio; AUC, area under the curve; CI, confidence interval; CKD, chronic kidney disease; Cre, creatinine; eGFR, estimated glomerular filtration rate; MCP-1, monocyte chemotactic protein-1; NAG, N-acetyl-β-d-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; RBP, retinol binding protein; ROC, receiver operating characteristic curve.
The table describes the P value for the estimated odds ratio for the association between that biomarker (or a combination of biomarkers) and the odds for being CKD 3 or worse along with its ROC AUC as a percentage. NAG and a combination of RBP and MCP1 showed significant odds ratio for association with CKD3 or worse with ROC AUC of 85%. The models are adjusted for age, sex, and ethnicity.
Figure 3.
(a) Testing association of biomarkers with chronic kidney disease (CKD) stage 3 or worse (estimated glomerular filtration rate [eGFR] < 60 ml/min). Logistic regression analysis in patients with an albumin/creatinine ratio (ACR) <3, receiver operating characteristic curve (ROC) curve for log N-acetyl-β-d-glucosaminidase (NAG)/creatinine. NAG demonstrated ROC area under the curve (AUC) of 84% for association with eGFR< 60 ml/min in patients with ACR <3. (b) Testing association of biomarkers with CKD stage 2 (eGFR 60-89 ml/min). Logistic regression analysis in patients with ACR <3, ROC curve for log ACR + log monocyte chemotactic protein-1 (MCP1). A combination of ACR and MCP1 demonstrated a ROC AUC of 85% for association with eGFR of 60-89 ml/min in patients with ACR <3.
Table 6.
Testing association of biomarkers with CKD stage 2 (eGFR 60-89 ml/min): Multivariate logistic regression analysis in patients with ACR <3a
| Biomarker | P value | Odds ratio (95% CI) | ROC AUC, % |
|---|---|---|---|
| Log NAG/Cre | 0.69 | ||
| Log NGAL/Cre | 0.51 | ||
| Log MCP1/Cre | 0.16 | ||
| Log RBP/Cre | 0.29 | ||
| Log ACR | 0.66 | ||
| Log MCP1/Cre + Log ACR | 0.021 (with interaction) | 5.4 (1.6–27) | 85 |
ACR, albumin/creatinine ratio; AUC, area under the curve; BP, blood pressure; CI, confidence interval; CKD, chronic kidney disease; Cre, creatinine; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; MCP-1, monocyte chemotactic protein-1; NAG, N-acetyl-β-d-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; odds ratio; RBP, retinol binding protein; ROC, receiver operating characteristic curve.
The table describes the P value for the estimated odds ratio for the association between that biomarker (or a combination of biomarkers) and the odds for being CKD 2 along with its ROC AUC as a percentage. A combination of ACR and MCP1 showed significant odds ratio for association with CKD 2 with ROC AUC of 85%. The models are adjusted for age, sex, ethnicity, HbA1c, and systolic BP.
Urinary Levels of Biomarkers in Patients With Early CKD and No CKD Compared With Controls
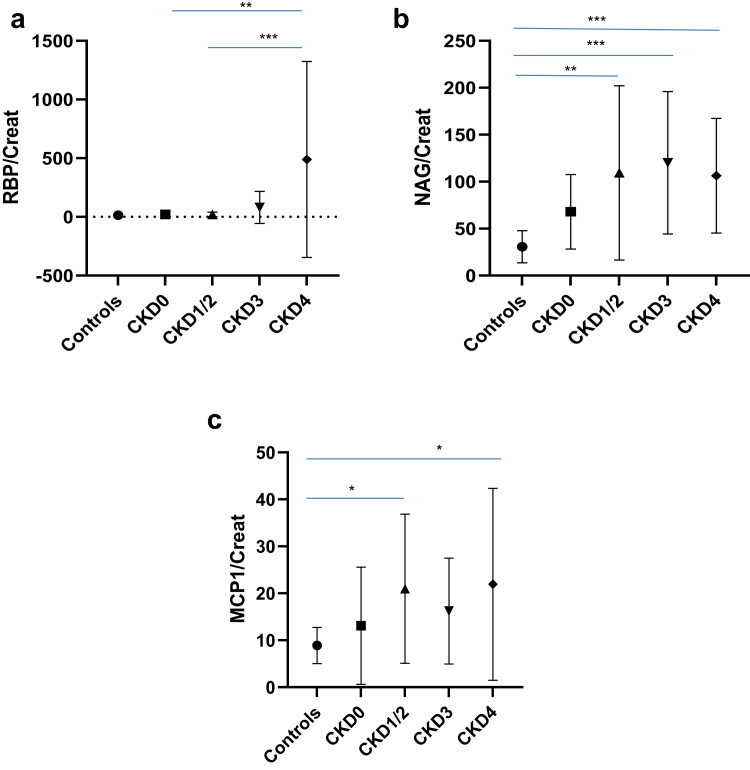
To evaluate selected urinary biomarkers (RBP, NAG, and MCP1) in early CKD, we analyzed urine samples from 20 patients from each stage of CKD and compared the levels with those from 10 healthy controls and 10 patients with no CKD (CKD 0). The tubular markers RBP and NAG showed a different pattern. RBP levels increased from stage 3 onward. In contrast, higher levels of NAG were seen in early CKD and in diabetic patients with CKD0 (eGFR > 90 ml/min, ACR <3) compared with healthy controls (Figure 4).
Figure 4.
Urinary levels of biomarkers in patients with early chronic kidney disease (CKD) and no CKD compared with controls. CKD 0 (estimated glomerular filtration rate [eGFR] ≥ 90 ml/min, albumin/creatinine ratio [ACR] < 3), CKD 2 (eGFR 60-89 ml/min), CKD 3 (eGFR 30-59 ml/min), and CKD4 (eGFR < 30 ml/min). Controls and CKD 0, n = 10; all other groups, n = 20. The urinary levels of biomarkers tested were higher in patients compared with controls. The proximal tubule markers (a) retinol binding protein (RBP) and (b) N-acetyl-β-d-glucosaminidase (NAG) showed different patterns, with RBP rising in later stages of CKD and NAG trending upward in early CKD and in diabetic patients with no CKD compared with healthy controls. (c) The levels of NAG and monocyte chemotactic protein 1(MCP1) were significantly higher in patients with early diabetic kidney disease (eGFR > 60 ml/min) compared with healthy controls. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. Each datapoint represents mean and the range bars represent SD.
Testing Association of Biomarkers With Progression to CKD Stage 3 or Worse (eGFR < 60 ml/min): Logistic and Linear (Mixed-Model) Regression Analysis at All Levels of ACR and ACR <3
Progression data were available in 302 patients, of which 68 progressed to stage 3 or worse (22.5% progression rate) over a period of 5 years. Among progressors, 45 of 68 (66%) had albuminuria at baseline and 23 of 68 (34%) had no albuminuria. Among the biomarkers tested, ACR (OR, 1.2; P = 0.02), MCP1 (OR, 2; P = 0.002), and RBP (OR, 1.2; P = 0.05) demonstrated significant ORs for association with progression but with poor ROC AUCs of 62% to 65%. A model with combination of IL-6, MCP1, and NGAL improved the OR for progression to 3.5 (P = 0.005), but the ROC AUC remained poor at 71% (Table 7). None of the biomarkers tested showed an association with progression in patients with an ACR <3 on their own; however, a model consisting of inflammatory cytokines IL-6, MCP1, and NGAL yielded a ROC AUC of 79% for progression (OR, 1.2; 95% CI, 1.1-1.5; P = 0.05).
Table 7.
Testing association of biomarkers with progression to CKD stage 3 or 4: Multivariate Logistic Regression Analysis at all levels of ACRa
| Biomarker | P value | Odds ratio (95% CI) | ROC AUC, % |
|---|---|---|---|
| Log NAG/Cre | 0.28 | ||
| Log NGAL/Cre | 0.81 | ||
| Log MCP1/Cre | 0.002 | 2 (1.3–3) | 65 |
| Log RBP/Cre | 0.05 | 1.2 (1–1.4) | 61 |
| Log ACR | 0.02 | 1.2 (1–1.4) | 62 |
| Log IL6/Cre + NGAL/Cre + MCP1/Cre | 0.005 | 3.5 (1.5–8.2) | 71 |
ACR, albumin/creatinine ratio; AUC, area under the curve; BP, blood pressure; CI, confidence interval; CKD, chronic kidney disease; Cre, creatinine; eGFR, estimated glomerular filtration rate; IL, interleukin; MCP-1, monocyte chemotactic protein-1; NAG, N-acetyl-β-d-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; odds ratio; RBP, retinol binding protein; ROC, receiver operating characteristic curve.
The table describes the P value for the estimated odds ratio (OR) for the association between that biomarker (or a combination of biomarkers) and the odds for being a progressor. ACR, RBP, and MCP1 showed significant ORs for association with CKD progression to stage 3 or 4 but with poor ROC AUCs of 61% to 65%. A combination of IL6, NGAL, and MCP1 demonstrated an OR of 3.5 but the ROC AUC, although better compared to single biomarkers, remained suboptimal at 71%.
In linear regression mixed-model multivariate analysis, log ACR (β = 0.013; P = 0.006,), log RBP/creatinine (β = 0.00003; P = 0.02), and log MCP1/creatinine (β = 0.03; P = 0.003) showed significant association with progression as defined previously (change in CKD stage), with RBP levels at baseline demonstrating strongest association based on β-value. In the ACR <3 cohort, only log MCP1/creatinine showed a significant association with progression (β = 0.05; P = 0.003). In contrast to the previously described results obtained with logistic regression, NGAL and IL-6 did not show significance in both cohorts.
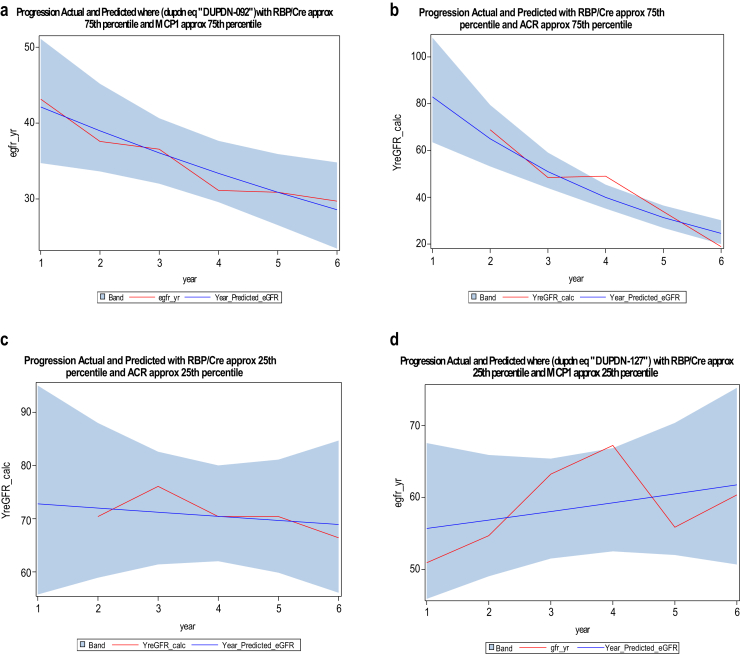
Next, we derived 2 predictive models for CKD progression, one with RBP and ACR as covariates (Akaike information criterion = 246.6) and 1 with RBP and MCP1 as covariates (Akaike information criterion = 216.4). Representative graphs from these 2 models (with biomarkers in 25th and 75th percentiles) showing actual eGFRs and values predicted by the model are shown in Figure 5.
Figure 5.
Linear regression mixed-model multivariate analysis, development of predictive model for chronic kidney disease (CKD) progression. The 2 predictive models were developed using 1243 observations of estimated glomerular filtration rate (eGFR) during the years 2008 to 2013 with best (lowest) Akaike information criterion (AIC) values. Model 1 with retinol binding protein (RBP) and albumin/creatinine ratio (ACR) as covariates (AIC = 246.6) and model 2 with RBP and MCP1 as covariates (AIC = 216.4) as covariates. (a–d) Representative graphs from these 2 models (with biomarkers in 25th and 75th percentiles) showing actual eGFRs (red line) and values predicted by the model (blue). The blue bands represent 95% confidence intervals.
Time-to-Event (eGFR Drop by ≥30%)/Cox Proportional Hazards Model Analysis
Progression data were available for 143 patients in the ACR <3 cohort. Biomarker(s) association with the 34 events where a patient’s eGFR value reduced ≥30% from baseline (23.7% progression) was analyzed. None of the individual or a combination of biomarkers was associated with the event of ≥30% eGFR decline. In the entire cohort with all levels of albuminuria, progression data were available in 302 patients. Biomarker(s) association with 100 events where a patient’s eGFR value reduced ≥30% from baseline (33% progression) was analyzed. Log RBP (hazard ratio, 1.3; 95% CI, 1.1–1.4; P < 0.001), ACR (hazard ratio, 1.2; 95% CI, 1.1–1.4; P < 0.001), and log MCP1 (hazard ratio, 1.6; 95% CI, 1.2–2.2; P = 0.0015) were significantly associated with progression. The rest of the biomarkers tested (NAG, IL-6, IL-1β, and NGAL) were nonsignificant. A combination of biomarkers did not improve the strength of the association (Table 8).
Table 8.
Testing association of biomarkers with 30% or more eGFR decline: Cox proportional regression model analysis at all levels of ACRa
| Biomarker | P value | Hazard ratio (95% CI) |
|---|---|---|
| RBP/Cre | <0.001 | 1.3 (1.1–1.4) |
| ACR | <0.001 | 1.2 (1.1–1.4) |
| MCP1/Cre | 0.0015 | 1.6 (1.2–2.2) |
| NGAL/Cre | 0.51 | |
| NAG/Cre | 0.13 |
ACR, albumin/creatinine ratio; BP, blood pressure; CI, confidence interval; Cre, creatinine; eGFR, estimated glomerular filtration rate; HbA1c glycated hemoglobin; HR, hazard ratio; IL, interleukin; MCP1, monocyte chemotactic protein-1; NAG, N-acetyl-β-d-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; RBP, retinol binding protein.
In this analysis of entire cohort with all levels of albuminuria, progression data was available in 302 patients. Biomarker(s) association with 100 events where a patient’s eGFR value reduced ≥30% from baseline (33% progression) was analyzed. Log RBP (P < 0.001; HR, 1.3; 95% CI, 1.1–1.4), ACR (P < 0.001; HR, 1.2; 95% CI, 1.1–1.4), and log MCP1 (P = 0.0015; HR, 1.6; 95% CI, 1.2–2.2) were significantly associated with progression. The rest of the biomarkers tested (NAG, IL6, IL1B, NGAL) were nonsignificant. Combination of biomarkers did not improve the strength of association. The models are adjusted for age, sex, ethnicity, HbA1c and systolic BP.
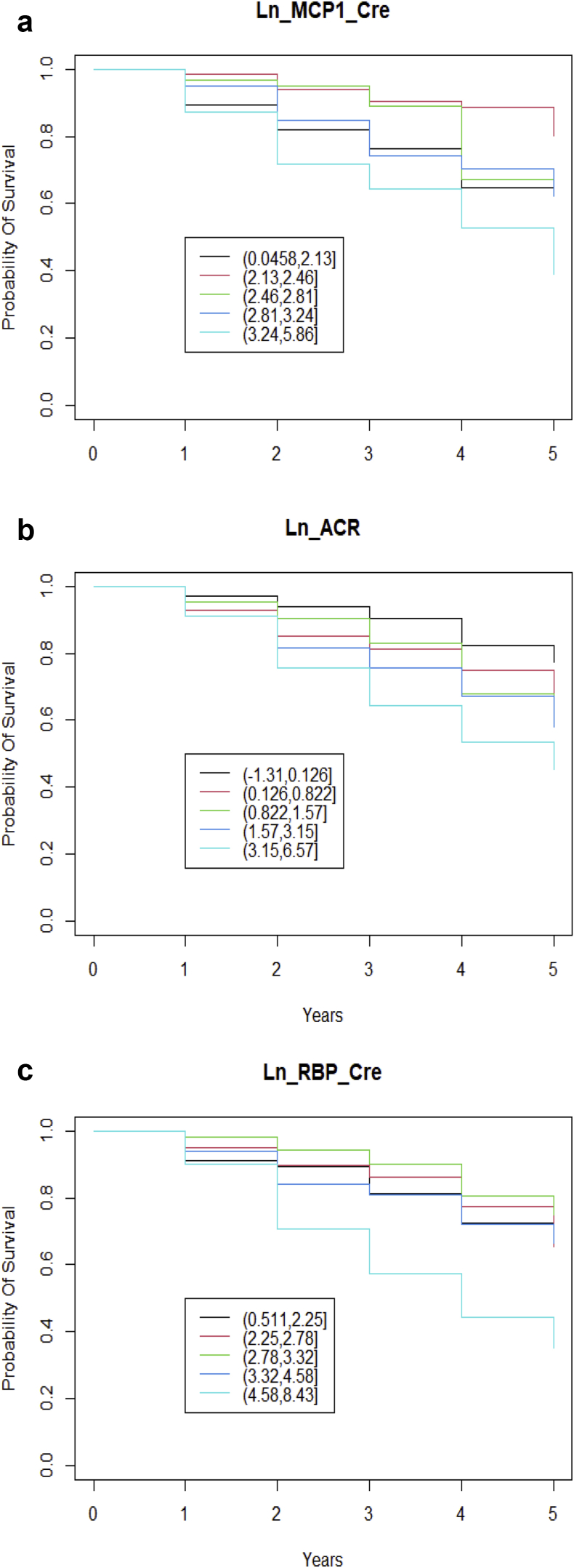
Survival curves for log MCP1, log RBP, and log ACR showed significant differences, but no significant differences were found in the curves for the other biomarkers tested (Figure 6).
Figure 6.
Time-to-event analysis and Kaplan-Meier survival curves. The biomarkers (log transformed) were grouped in 20% quantiles, and Kaplan-Meier plots were drawn to assess the probability of survival, which is defined as estimated glomerular filtration rate (eGFR) remaining within 70% of the baseline eGFR during the follow-up period of 5 years, and the curves were compared with log-rank tests. Each colored line represents a quantile of the biomarker. The log-rank test comparisons revealed significant differences between the curves for (a) log (Ln) monocyte chemotactic protein-1 (MCP1) (P < 0.01), (b) log albumin/creatinine ratio (ACR) (P = 0.004), and (c) log retinol binding protein (RBP) (P < 0.01). The rest of the biomarkers tested showed no significant difference between the curves (log N-acetyl-β-d-glucosaminidase, P = 0.1; log neutrophil gelatinase-associated lipocalin, P = 0.4; log interleukin 6, P = .6). Cre, creatinine.
Discussion
In diabetic patients with various stages of CKD and low levels of albuminuria (ACR <3), among the 7 biomarkers tested, urinary NAG showed strong association with stage 3 CKD. A combination of urinary ACR and MCP1 was significantly associated with early CKD (stage 2). NAG levels increased early in diabetic patients without any other evidence of renal damage, and therefore, it could be a biomarker of choice for detection of early kidney damage. Among tubular markers, RBP correlated well with albuminuria but not NAG, and inflammatory cytokines correlated well with each other but not with albuminuria. A combination of inflammatory cytokines and NGAL demonstrated stronger association with progression compared with other biomarkers in patients with and without albuminuria in logistic models, but the linear regression mixed-models identified RBP, ACR, and MCP1 as significant biomarkers associated with progression to stage 3 or 4. In this model, MCP1 was the only significant biomarker associated with progression to stage 3 or worse in the ACR <3 cohort. The Cox proportional hazard model analysis identified ACR, RBP, and MCP1 as relevant biomarkers for progression, defined as a ≥30% decline in eGFR, and this was confirmed further by survival analysis.
We were able to detect all of the biomarkers in urine except for tumor necrosis factor-α and transforming growth factor -β1, -2, and -3. Among the inflammatory cytokines, levels of IL-1β and IL-6 were below the detection limit of the assay in a large number of patients, and therefore, these cytokines are unlikely to be of utility with the current methodology, in particular IL-1β, which was below the limit of detection in 74% of patients. Groupwise comparisons revealed significant differences in biomarker levels in various CKD stages with the exception of MCP1. IL-6 and MCP1 levels were higher in men compared with women, suggesting sex differences in the contribution of inflammatory pathways in CKD. There was a trend toward higher ACR and NAG levels in Asians compared with patients of White ethnicity, and these observations need further exploration.
The correlation analysis demonstrated a strong correlation between inflammatory cytokines but a weak correlation between ACR and inflammatory cytokines, suggesting divergence of albuminuric and inflammatory pathways in diabetic kidney disease. Correlation between tubular markers (RBP and NAG) was weak, suggesting different mechanisms for the appearance of these proteins in the urine. RBP showed a moderate correlation with ACR but not with NAG. This suggests common mechanisms behind albuminuria and excretion of RBP in urine or the interdependency of these 2 proteins. RBP is a 21-kDa small-molecular-weight protein, freely filtered by the glomerulus and almost completely reabsorbed by the proximal tubule.8 Proximal tubular damage or dysfunction results in impaired endocytosis and excretion of RBP and albumin in the urine. Our data showed increasing urinary RBP during the later stages of CKD, as is the case with albuminuria, which further supports the hypothesis that excretion of these 2 proteins in the urine is driven by common mechanisms and correlate with each other. Therefore, it is unlikely that RBP would add value to ACR in albuminuric patients.
In contrast to RBP, urinary NAG seems to be independent of albuminuria, and this was the only single biomarker associated with stage 3 CKD in patients with ACR of <3. In a smaller subset of patients, higher NAG levels were seen in early CKD, and compared with healthy controls, higher levels were seen diabetic patients with no evidence of kidney disease. These observations suggest that NAG could be an early marker of kidney disease in patients with diabetes. NAG has been previously reported to be a useful marker of early diabetic kidney disease.9,10 Our data add to the published literature on role of NAG as a biomarker of early diabetic kidney disease and provide novel evidence for NAG as a biomarker for nonalbuminuric diabetic kidney disease.
Dissimilar patterns of urinary excretion of 2 proximal tubular biomarkers, RBP and NAG, suggest specific pathologic processes driving excretion of these proteins at different stages of diabetic kidney disease. Previous studies investigating RBP and NGAL have shown increasing levels of these proteins in urine with increasing albuminuria11,12 and an ability of RBP to predict CKD.13 Consistent with these studies, our results confirmed the ability of RBP and NGAL to predict CKD when analyzed in patients with all levels of albuminuria and not in nonalbuminuric patients. We also found significant association of ACR and MCP1 with early CKD (eGFR 60–89 ml/min) with an interaction between these 2 biomarkers. This observation supports relevance of low-grade albuminuria in diabetic kidney damage and provides evidence for its association with inflammatory pathways within the kidney.
Among all the biomarkers tested with logistic regression (progression to stage 3 or stage 4 CKD), ACR, MCP1, and RBP showed significant correlations but the ROC AUCs of these biomarkers to predict progression was suboptimal. A model consisting of NGAL, IL-6, and MCP1 demonstrated better but still suboptimal ROC AUC (71%). In patients without albuminuria, no biomarker on its own could predict progression, but a combination of inflammatory cytokines (IL-6 and MCP1) and NGAL demonstrated an ROC AUC of 79%. These results suggest a role for inflammatory cytokines in progression of diabetic CKD and are consistent with studies demonstrating the role of inflammation in diabetic kidney disease.14
Lin et al.15 showed upregulation of Toll-like receptor 4 in renal tubules driving tubulointerstitial inflammation, and Yiu et al.16 demonstrated increased expression of complement component C5a (a chemoattractant stimulus for macrophages) in proximal tubules of patients with diabetic nephropathy driving inflammation in progressive diabetic CKD. In diabetic patients, NGAL produced in tubules could be released in the urine or may reflect proximal tubule dysfunction leading to urinary leak of filtered plasma NGAL, which, as recently shown, is produced in hepatocytes driven by IL-6.17 In nondiabetic CKD, a recent study on autosomal-dominant polycystic kidney disease patients found MCP1 as a strong predictor of progression.18
Our linear regression mixed models using a larger number of observations demonstrated significant associations between ACR, RBP, and MCP1 and CKD progression (to stage 3 or 4) in the whole cohort and MCP1 in the ACR <3 cohort but not NGAL or IL-6, as shown in logistic regression models. This disparity could be due to the difference in the number of observations used for these 2 analyses. Also, it must be noted that IL-6 was not measurable with confidence in >40% of our patients, and therefore, results from models using this biomarker may not be reliable. MCP1 was significant in both of these analyses. The predictive models derived from linear mixed-model regression included ACR, RBP, and MCP1 as significant covariates, and we intend to test these models in prospective validation cohorts. The time-to-event and Cox proportional hazard model analyses defining progression as a drop in eGFR by ≥30% demonstrated the utility of ACR, RBP, and MCP1 for predicting CKD progression but could not identify biomarkers useful in the ACR <3 cohort, which is likely to be due to small number of events in this cohort.
Our study has some limitations. We collected only 1 baseline random urine sample, and it is likely that repeated measurements and trend in biomarker levels would improve our ability to predict progression. Random samples are likely to add to the variability but are more practical to obtain. In addition, frozen storage might have resulted in reductions in some of the biomarker levels, as shown before.19 We have shown statistically significant associations and patterns without drawing cutoff values for the biomarkers, and we will attempt to do this in our future work with separate derivation and validation cohorts.
We did not assess the effect of baseline CKD status on progression. Although this would likely have an independent effect on progression of CKD, our objective was to identify useful biomarkers of diagnostic and prognostic utility in diabetic kidney disease and also identify potential targets for clinical trials and therapy.
Conclusion
In this study of diabetic patients with single measurements of urinary biomarkers at baseline, we have derived predictive models for CKD progression based on urinary biomarkers ACR, RBP, and MCP1 in patients with all levels of albuminuria and identified MCP1 and NGAL as useful biomarkers for progressive CKD in nonalbuminuric patients, suggesting important roles of proximal tubule and inflammation in progression of DKD. Urinary NAG, and MCP1 levels were elevated in early CKD, and these are likely to be markers and mediators of renal disease in diabetes from the early stages.
Disclosures
BMH reports personal fees from Travere Therapeutics, Gilead Sciences, AstraZeneca, ViiV Healthcare, and Otsuka, all outside the submitted work. All the other authors declared no competing interests
Acknowledgements
The authors acknowledge all of the patients who participated in this study and the diabetes team at Croydon University Hospital, UK, the staff at SW Thames Institute for Renal Research, with special thanks to Dr Marta Lapsley for her contribution to the institute and the development of RBP assays, and the SW Thames Kidney Fund (The Kidney Fund). The study was funded by Johnson & Johnson and a SEEDA (South East England Development Agency) Innovation Acceleration Award.
Footnotes
Supplementary File S1: Biomarker assays
Supplementary File S2: Logistic models–statistical methodology
Supplementary Material
Supplementary File S1: Biomarker assays
Supplementary File S2: Logistic models–statistical methodology
References
- 1.Fine L.G., Bandyopadhay D., Norman J.T. Is there a common mechanism for the progression of different types of renal diseases other than proteinuria? Towards the unifying theme of chronic hypoxia. Kidney Int Suppl. 2000;75:S22–S26. [PubMed] [Google Scholar]
- 2.Textor S.C., Lerman L. Renovascular hypertension and ischemic nephropathy. Am J Hypertens. 2010;23:1159–1169. doi: 10.1038/ajh.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porrini E., Ruggenenti P., Mogensen C.E. Non-proteinuric pathways in loss of renal function in patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2015;3:382–391. doi: 10.1016/S2213-8587(15)00094-7. [DOI] [PubMed] [Google Scholar]
- 4.Zeni L., Norden A.G.W., Cancarini G., Unwin R.J. A more tubulocentric view of diabetic kidney disease. J Nephrol. 2017;30:701–717. doi: 10.1007/s40620-017-0423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenberg J.H., Abraham A.G., Xu Y. Plasma biomarkers of tubular injury and inflammation are associated with CKD progression in children. J Am Soc Nephrol. 2020;31:1067–1077. doi: 10.1681/ASN.2019070723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malhotra R., Katz R., Jotwani V. Urine markers of kidney tubule cell injury and kidney function decline in SPRINT trial participants with CKD. Clin J Am Soc Nephrol. 2020;15:349–358. doi: 10.2215/CJN.02780319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannon C.P., Perkovic V., Agarwal R. Evaluating the effects of canagliflozin on cardiovascular and renal events in patients with type 2 diabetes mellitus and chronic kidney disease according to baseline HbA1c, including those with HbA1c <7%: results from the CREDENCE Trial. Circulation. 2020;141:407–410. doi: 10.1161/CIRCULATIONAHA.119.044359. [DOI] [PubMed] [Google Scholar]
- 8.Lapsley M., Akers K., Norden A.G. Sensitive assays for urinary retinol-binding protein and beta-2-glycoprotein-1 based on commercially available standards. Ann Clin Biochem. 1998;35:115–119. doi: 10.1177/000456329803500116. [DOI] [PubMed] [Google Scholar]
- 9.Mocan Z., Erem C., Yildirim M., Telatar M., Deger O. Urinary beta 2-microglobulin levels and urinary N-acetyl-beta-D-glucosaminidase enzyme activities in early diagnosis of non-insulin-dependent diabetes mellitus nephropathy. Diabetes Res. 1994;26:101–107. [PubMed] [Google Scholar]
- 10.Omozee E.B., Okaka E.I., Edo A.E., Obika L.F. Urinary N-acetyl-beta-d-glucosaminidase levels in diabetic adults. J Lab Physicians. 2019;11:1–4. doi: 10.4103/JLP.JLP_164_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbasi F., Moosaie F., Khaloo P. Neutrophil gelatinase-associated lipocalin and retinol-binding protein-4 as biomarkers for diabetic kidney disease. Kidney Blood Press Res. 2020;45:222–2232. doi: 10.1159/000505155. [DOI] [PubMed] [Google Scholar]
- 12.Titan S.M., Vieira J.M., Jr., Dominguez W.V. Urinary MCP-1 and RBP: independent predictors of renal outcome in macroalbuminuric diabetic nephropathy. J Diabetes Complications. 2012;26:546–553. doi: 10.1016/j.jdiacomp.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Qin Y., Zhang S., Shen X. Evaluation of urinary biomarkers for prediction of diabetic kidney disease: a propensity score matching analysis. Ther Adv Endocrinol Metab. 2019;10 doi: 10.1177/2042018819891110. 2042018819891110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li A., Yi B., Liu Y. Urinary NGAL and RBP are biomarkers of normoalbuminuric renal insufficiency in type 2 diabetes mellitus. J Immunol Res. 2019;2019:5063089. doi: 10.1155/2019/5063089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin M., Yiu W.H., Wu H.J. Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. J Am Soc Nephrol. 2012;23:86–102. doi: 10.1681/ASN.2010111210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yiu W.H., Li R.X., Wong D.W.L. Complement C5a inhibition moderates lipid metabolism and reduces tubulointerstitial fibrosis in diabetic nephropathy. Nephrol Dial Transplant. 2018;33:1323–1332. doi: 10.1093/ndt/gfx336. [DOI] [PubMed] [Google Scholar]
- 17.Skrypnyk N.I., Gist K.M., Okamura K. IL-6-mediated hepatocyte production is the primary source of plasma and urine neutrophil gelatinase-associated lipocalin during acute kidney injury. Kidney Int. 2020;97:966–979. doi: 10.1016/j.kint.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Messchendorp A.L., Meijer E., Boertien W.E. Urinary biomarkers to identify autosomal dominant polycystic kidney disease patients with a high likelihood of disease progression. Kidney Int Rep. 2018;3:291–301. doi: 10.1016/j.ekir.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nauta F.L., Bakker S.J., Lambers Heerspink H. Effect of frozen storage on urinary concentration of kidney damage markers. Am J Kidney Dis. 2012;59:586–589. doi: 10.1053/j.ajkd.2011.11.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.