Abstract
The radiofrequency electromagnetic radiation emitted by smart phones on biological systems has wide media coverage and public concern in recent years. The aim of this study was to explore the effects of fourth-generation cell phone radiation exposure on hematological (Total leukocyte count, Total erythrocyte count, and hemoglobin %), biochemical (Serum creatinine) parameters, and histopathological changes in the kidney and testis of Swiss albino mice. A total of 30 male Swiss albino mice weighing 45–65 g was randomly divided into three groups (n = 10). The first group A was the control group, the second group B, was exposed to 40 minutes of mobile phone radiation daily, the third group C was exposed to 60 minutes of radiation daily from two 2400 Megahertz fourth-generation connected mobile phones for 60 days, respectively. The electromagnetic radiation frequency radiometer measured the frequency of electromagnetic radiation emitted from cell phones. The specific absorption rate was calculated as 0.087 W/kg. The control group was kept under similar conditions, but the electromagnetic field was not given for the same period. All the mice were sacrificed at the end of the experiment. The blood samples were collected for hematobiochemical study, and then kidney and testis tissues were collected for histopathological study. Results of the study showed that the body weight and total erythrocyte count values were significantly (p < 0.05) decreased while total leukocyte count, hemoglobin %, and serum creatinine values were significantly (p < 0.05) increased in both the radiation exposure groups relative to the control group. Histopathological observation showed the kidney of 60 minutes exposed mice interstitial inflammation that causes marked mononuclear cellular infiltration compared to the 40 minutes and control mice. Compared to control mice, histopathological examinations of testicular tissue from the exposed mice, showed irregular in shapes and non-uniform sizes and fewer spermatogenic cells layer that leads to the larger lumen in the seminiferous tubules. It is concluded that fourth-generation cell phone radiation exposure may affect blood hemostasis and inflammation of mice's kidney and testis tissue. Based on these studies, it is important to increase public consciousness of potential adverse effects of mobile phone radiofrequency electromagnetic radiation exposure.
Keywords: Mobile phone radiation, Haematology, Serum creatinine, Kidney, Testis
1. Introduction
Mobile phone use has dramatically increased over the last decade. Researchers had recommended that electromagnetic fields (EMF) released into the atmosphere from a mobile phone has deleterious effects on human beings (Azab, 2017, Elmas, 2016). At present mobile phones have started using fourth generation (4G) wireless communication technology (2200–2400 Megahertz (MHz)) that offer a very high Internet speed. The adverse effects of EMF are a global public concern at present. Numerous adverse effects of cell phone use have been reported on different body organs such as the brain (Hardell et al., 2011, Kesari et al., 2011), ear (Colletti et al., 2011), and reproductive organs (Erogul et al., 2006, Falzone et al., 2011, Khillare and Behari, 1998). Many other studies have confirmed that the electromagnetic frequencies from mobile phone can cause numerous deleterious effects on molecular and cellular levels, such as DNA injury, various forms of cancer, oxidative stress, lipid peroxidation, increased free radicals, and chromosomal abnormalities (Akdag et al., 2016, Çam and Seyhan, 2012, Chauhan et al., 2017, Dasdag et al., 2015, Deshmukh et al., 2013, Megha et al., 2015). Blood parameters are the most significant way of assessing the state of health of animal models (Alghamdi and El-Ghazaly, 2012). EMF exposure from cell phones to humans contributes to reduced blood cells and an imbalance in plasma enzymes (Alghamdi and El-Ghazaly, 2012, Hasan and Issmer, 2014). In animal studies, long-term exposures to EMF can have detrimental effects on the liver, blood cells, and other activities. Hoverer, varying degrees of EMF have a conflicting influence on the blood and blood formation in animals (Sani et al., 2018, Usman et al., 2011). The impact of EMF in rats causes decreased hemoglobin oxygen-binding capacity; i.e., hypoxia does not exclude kidney damage contributions. It also has to be supposed that radiation influences hematopoiesis in the blood and bone marrow in the animals (Singh et al., 2004).
EMF has many biochemical consequences, such as the degradation of large cell molecules and ionic equilibrium imbalances. EMF exposure is associated with increased amounts of reactive oxygen species (ROS) formation (Kivrak et al., 2017). These ROS may cause injury to cellular constituents, including lipids, proteins, and DNA. Free radical generation may happen in various ways, such as UV light, immune response, radioactivity, stress, cigarettes, and physiological redox (Okano, 2008). It has been shown that electromagnetic radiation influences their impact on living organisms by producing or rising ROS. ROS leads to various physiological influences, such as DNA damage. Exposure to cell phone radiation oxidative stress (OS) has been increased in hematopoietic sites (Moustafa et al., 2001). The kidneys could absorb EMF from 900-MHz cell phones as, most of the time, they are carried by the belt (Oktem et al., 2005). Current concerns on mobile phone exposure are mainly focused on the kidney and testis. Because the mobile phone is usually kept in a pocket that is very close to the urogenital organ. The 2400 MHz 4G radiofrequency electromagnetic radiation (RF-EMR) has been exposed to male mice over a long period in this study.
The exposure of wireless electromagnetic radiation may injure both the hepatic, renal, and splenic tissues. Hasan and Islam (2020) reported that mononuclear cellular aggregates surrounding the bile duct and hepatic artery with congestion in the portal vein and the central vein of the liver after daily exposure of 4G connected 2400 MHz mobile phone radiation to mice whole-body for a month. With EMF exposure time, the amount of harm increased. The extent of potential harm expanded with the duration of EMF exposure (Lee et al., 2010).
Studies show that exposure to toxic chemicals, ionizing radiation, radiofrequency (RF) radiation, and other environmental nuisances are mainly related to male infertility (Bin-Meferij and El-Kott, 2015). In contrast, many studies have found that mobile phones have harmful effects on the male reproductive system (Kesari et al., 2011). EMF has probable harmful effects on male reproductive functions, specially Leydig cells, seminiferous tubules, and spermatozoa. The harmful effects of EMF on the male reproductive system based on the wave frequency and duration of exposure are the main factors (Kesari et al., 2011, Kesari et al., 2010). Many cross-sectional experiments have already shown that cellular device usage may be related to semen quality, and increase cell phone use may cause male infertility (Ozguner et al., 2005, Pareek et al., 2005). (Lee et al. (2010) examined testicular histological changes in rats exposed to 848.5 MHz radiofrequency radiation (RFR) for 12 weeks. The possible adverse effects of mobile phone exposure on male fertility have been widely studied in the past decade by several researchers who have documented notable adverse effects of radiation on testis and seminal parameters, including motility, concentration and changes in testicular morphology, increased permeability of the blood-testis barrier, decreased sperm count, and percent normal morphology (Agarwal et al., 2008, Aitken et al., 2005, Fejes et al., 2005). Most mobile phone users bring their mobile phone in their pant pocket throughout the day that is very close to their genitals (Lavranos et al., 2012). Experiment results evaluated to identify testicular damages caused by low-intensity RF indicate contradictory findings (Dasdag et al., 1999, Ozguner et al., 2005, Ribeiro et al., 2007, Saunders and Kowalczuk, 1981, Yan et al., 2007). The leading cause associated with these adverse consequences was increased oxidative stress inside the reproductive organs (De Iuliis et al., 2009, Kesari et al., 2010, Mailankot et al., 2009, Naziroǧlu et al., 2013, Qin et al., 2012). There has been a marked decrease in male fertility (Sepehrimanesh et al., 2014, Yildirim et al., 2015). Therefore, it is essential to evaluate the impact of RF-EMR on male fertility more reliably because men frequently carry mobile phones in their pockets, which is close to their reproductive organs.
Much of the previous research had already been conducted using second generation (2G) or third generation (3G) wireless mobile radiation exposure causes many physiological changes; however, no study has been undertaken using the 4G cell phone radiation (2200–2400 MHz) exposure in animal model. Besides this, mobile phone radiation can be consumed more by pelvic organs, particularly the kidney and testis, than many other inner organs. Therefore this experiment aimed to evaluate the impact of a 4G cell phone radiation exposure on hematobiochemical and possible histopathological effects on the kidney and testis of the Swiss albino mice model.
2. Materials and methods
2.1. Statement of the experiment
The research work was conducted in the Department of Anatomy and Histology, Faculty of Veterinary Science, Bangladesh Agricultural University, Mymensingh-2202, Bangladesh during the period from August 2018 to December 2019 to assess the impacts of 4G cell phone radiation on hematological (Total leukocyte count, Total erythrocyte count, and hemoglobin %), biochemical (Serum creatinine) parameters and histopathological changes in the kidney and testis of Swiss albino mice.
2.2. Ethical approval
As per the standardized instruction, all of the animals received human care. These research interventions have been permitted and carried out in compliance with animal welfare recommendations and use as defined by the Animal Welfare and Experimental Ethics Committee, Bangladesh Agricultural University, Mymensingh, Bangladesh (Protocol Number: AWEEC/BAU/2019-56).
2.3. Animals and experimental procedures
Swiss albino male mice (6 weeks old) (45 ± 65 g) have been obtained from the International Center for Diarrheal Disease Research (ICDDRB), Mohakhali, Dhaka. All the mice appeared to have good health and lacked any visible deformities. In the temperature-controlled environment, the mice were kept 52 cm × 36 cm × 25 cm in plastic cages. Daily diet and drink water ad libitum were given. After one week of acclimatization, the mice were haphazardly categorized into three equivalent groups; each group includes ten mice. The group named were A, B, and C. Individual weights of all mice were documented before radiation exposure. Among all of the three groups, group A has been considered controlled without radiation exposure. Group B was exposed to 40 min of radiation, and group C was exposed to 60 min of radiation from 4G cell phone connected handsets. Both experimental and operational requirements were designed to comply with the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health.
2.4. The electromagnetic radiation exposure system
Two 2400 Megahertz (MHz) 4G connected mobile phones (Huawei GR5 2017) have been used for the generation of the radiofrequency electromagnetic radiation (RF-EMR) with different duration of exposure every day for 60 days (2 months). The cell phone with auto-answer mode was centrally installed on the roof of the mice cage from the inside for unified radiation exposure during the exposure time, trying to avoid contact with both animals and phones (Kumar et al., 2009, Narayanan et al., 2009). At first, we confirmed that there was no other source of electromagnetic fields (EMF). The temperature and humidity of the exposure room and the mouse cage were measured regularly during the RF-EMR application using a digital thermometer. When the mice were exposed to radiation, the frequency of RF-EMR emitted from cell phones was first measured by the electromagnetic radiation frequency radiometer (ED-78S Electrosmog frequency meter) in an active call using 2400 MHz range and assure that radiation exposure is uniformly distributed on the mice. The Specific absorption rate (SAR) was calculated as 0.087 W/kg. When the exposure begins, a call was made to the cell phone placed in the mice cage by another 4G connected 2400 MHz mobile phone and kept in answer mode for 40 and 60 min, respectively. During RF-EMR exposure, the mobile phone was held in the loudspeaker mode. Group B was exposed for 40 min per day hourly from 10.00 AM to 10.40 AM; Group C was exposed for 60 min, from 10.00 AM to 11.00 AM per day. The control group A was placed away from the radiofrequency source in the plastic cage. However, it was not receiving any exposure to radiation. This is similar to the exposure method used in previous studies for the 900-MHz RF-EMR application (Baş et al., 2013, Keleş et al., 2018, Odac et al., 2016, Odaci et al., 2015). That whole exposure time has been continued for two months.
2.5. Bodyweight
Each mouse's body weight was weighed using a digital scale at the beginning of the exposure to radiation and on the day of sacrificing.
2.6. Hematological analysis
The blood samples were obtained by sacrificing the mice at the end of the experiment. Approximately 1 ml of blood from the syringe was collected for hematological tests in the test tube containing anticoagulant Ethylene diamine tetraacetic acid (EDTA). The remaining amount of syringe blood has been used to obtain the serum. The below hematological variables were tested using Sysmex automated CBC analyzers: Total erythrocyte count (TEC), Hemoglobin (Hb) percentage (%), and Total leukocyte count (TLC).
2.7. Biochemical analysis
Approximately 2 ml of blood was obtained in a sterile glass test tube. The blood containing syringe was held upright at room temperature for 6 h. The tubes were then incubated in the refrigerator (4°C) overnight. Serum samples were isolated and centrifuged to eliminate excess blood cells where possible. Serum samples were deposited in the capped tube at −20°C for analysis of serum creatinine by using a CS-T240 auto-chemistry analyzer.
2.8. Sample collection for gross and histological study
Following blood collection, the kidney and testis were obtained for gross and histopathological inspection. Both kidneys and testis of different groups of mice were collected for the gross study. Parameters such as color and weight were taken into consideration for gross observation. The weight of the kidney and testis in the control group and the exposed group of mice were measured on a graded scale. After gross examination, samples were stored in 10% formaldehyde for 48 h. After processing to make paraffin blocks, 7 mm sections were cut using a rotary microtome (Leica RM2135) and stained with Haematoxylin & Eosin (H & E) stain for routine histological examination as per the proper procedure described by the (Zare et al., 2007).
2.9. Statistical analysis
The statistical analysis has been carried out using Graph pad prism software version 7.0 (GraphPad Software Inc., San Diego, CA). For statistical analysis One-way ANOVA followed by Turkeys Multiple Comparison Test was performed. A p-value <0.05 was considered to be statistically significant. Data were expressed as mean ± standard error (mean ± SEM).
3. Results
3.1. Effects on body weight
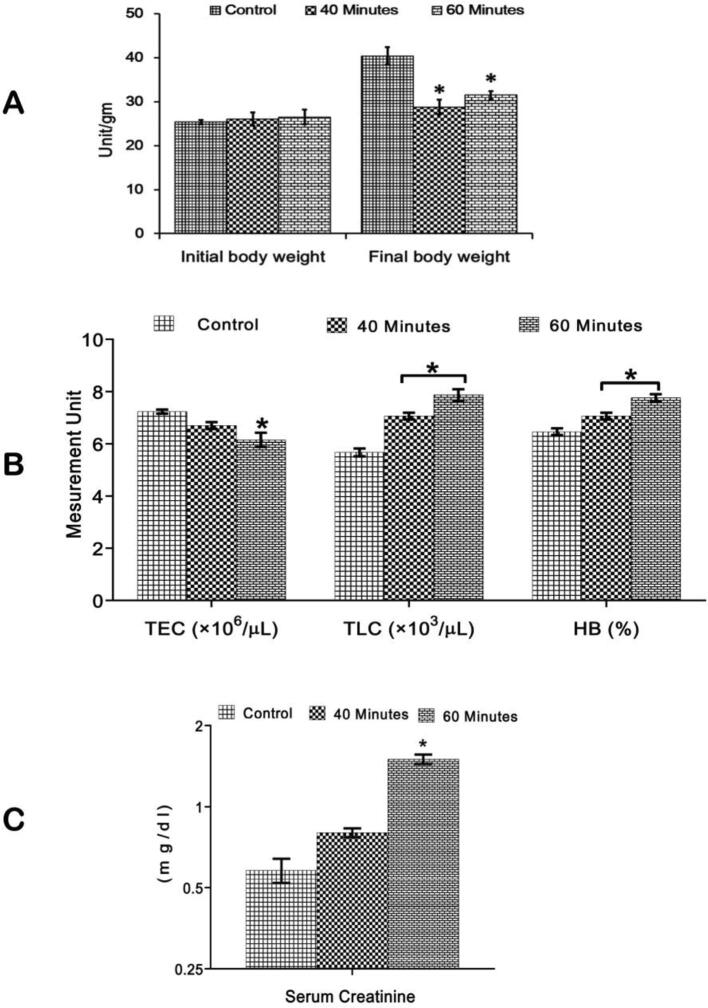
The mean body weight of mice in different groups has been presented in (Fig. 1A). The initial and final body weight of control mice was 25.36 ± 0.48 and 40.30 ± 0.21 g, respectively. In 40- and 60-minutes exposed mice, the mean body weight at the beginning of exposure and the end of the experiment were 26.47 ± 1.53, 28.74 ± 1.67 and 27.66 ± 1.65, 31.49 ± 0.89 g, respectively. After the end of the exposure, the body weight was significantly (p < 0.05) decreased in both exposed mice compared to the control mice.
Fig. 1.
(A) Initial and final body weight of different groups of mice. (B) Effects of mobile phone radiation on Total Erythrocyte Count (TEC), Total Leukocyte Count (TLC) and Hemoglobin (HB) % in mice. (C) Effects of mobile phone radiations on Serum creatinine in mice. Values were given as mean ± SE. *= Significant at 5% (p < 0.01) level of probability.
3.2. Effects on hematological and biochemical parameters
The effects of mobile phone radiations on different hematological parameters, i.e., Total leukocyte count (TEC), Total erythrocyte count (TLC), and hemoglobin percentage (Hb%) in mice, were presented in (Fig. 1B). In the control mice, the mean value of TEC, TLC and Hb% were 7.23 ± 0.08, 5.81 ± 0.29 and 6.46 ± 0.13. The values of TLC and Hb% were increased significantly (p < 0.05) in 40 min (7.06 ± 0.13, 7.13 ± 0.31) and 60 min (7.87 ± 0.22, 7.76 ± 0.14) exposed mice compared to the control mice. In comparison to the 40 min exposed (6.70 ± 0.13) and control (7.23 ± 0.08) mice the values of TEC decreased significantly (p < 0.05) in 60 min (6.15 ± 0.27) exposed mice. The results of mobile phone radiations on Serum creatinine in different groups of mice were presented in (Fig. 1C). There was a significant increase (p < 0.05) in serum creatinine value in 40 min (0.80 ± 0.03) and 60 min (1.50 ± 0.06) exposed mice compared to the control mice (0.58 ± 0.06). Serum levels of creatinine indirectly reflect the kidney injury.
3.3. Gross architectural change of the kidney and testis
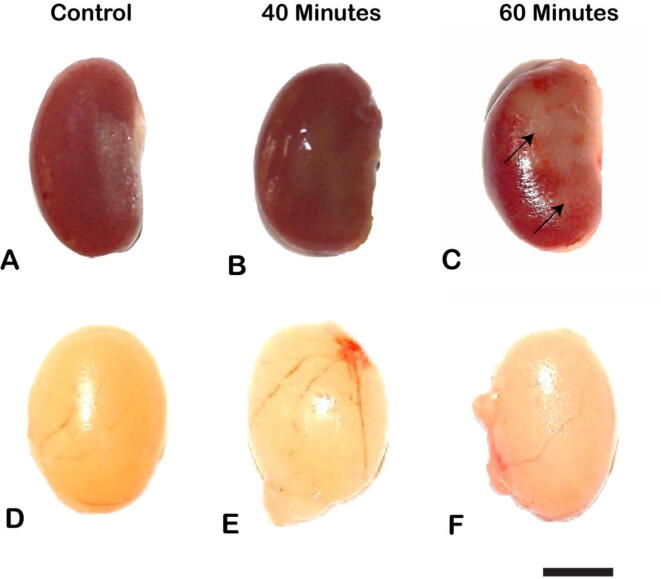
The kidney of control and 40 min exposed mice were reddish-brown with a smooth and shiny surface (Fig. 2A-B). No anatomical changes were found in these groups of mice while 60 min of the exposed mice showed pale colour on the kidney surface (Fig. 2C). The testis was white in colour, oval in shape in control and both exposed groups of mice. There were no morphological changes observed in control and exposed groups of mice (Fig. 2D-F).
Fig. 2.
Gross observation of the kidney of control (A), 40 min (B), and 60 min (C) exposed mice. Healthy appearance of the kidney was observed in control and 40 min exposed mice wherein 60 min exposed mice showed pale color (arrow) on the surface of the kidney; Gross observation of the testis of control (D), 40 min (E) and 60 min (F) exposed mice. Healthy morphological appearance of testis was found in control and exposed mice. Scale bar stands for 50 mm.
3.4. Histopathological study
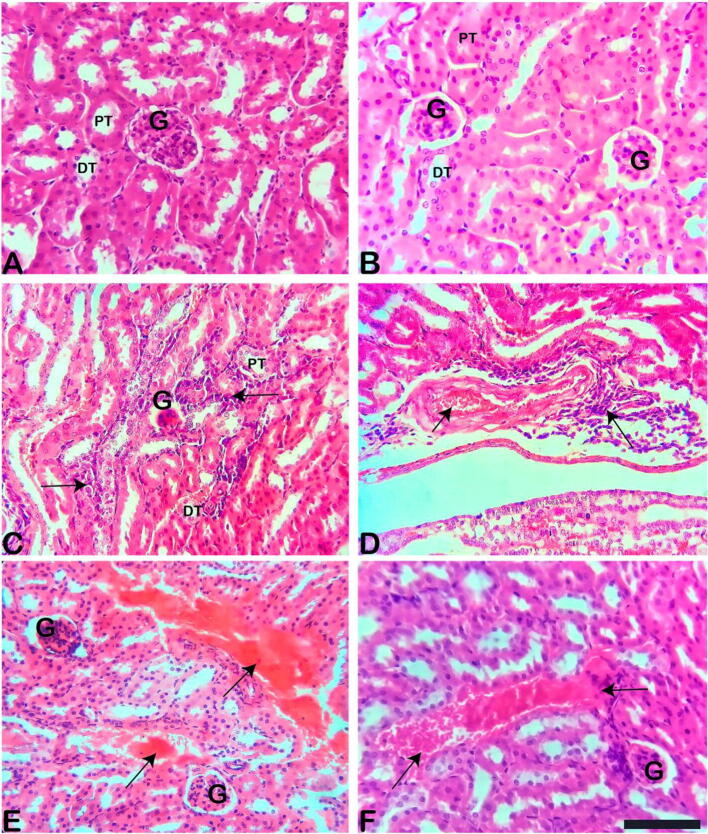
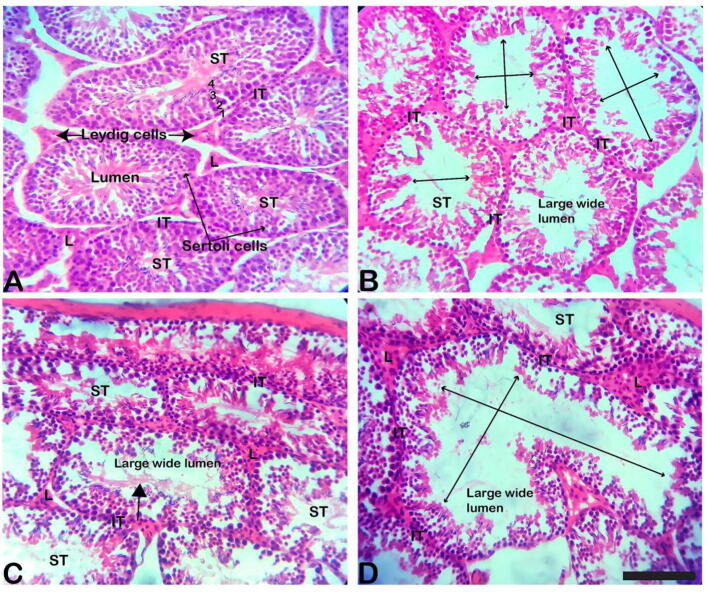
Examination of H & E-stained sections of the kidney collected from the control and 40 min exposed mice group revealed the normal histological architecture (Fig. 3A-B). Regarding the histopathological changes, the kidney of 60 min exposed mice showed interstitial inflammation that causes marked mononuclear cellular infiltration with severe vascular congestion in comparison to the control and 40 min exposed mice (Fig. 3C-F). Histopathological observations of the testes of control mice revealed the normal architecture of the seminiferous tubule. The seminiferous tubules were hexagonal or rounded and separated by a thin intertubular interstitial connective tissue. In the seminiferous tubules, the spermatogenic cells and the Sertoli cells were regularly arranged. Interstitium tissue contained the interstitial cells (Leydig cells). The Leydig cells seemed to be regular in form and size throughout the interstitial connective tissue between the seminiferous tubules. The germinal epithelia are formed of normal spermatogenic layers represented by spermatogonia, primary and secondary spermatocytes, spermatids and sperms. The spermatids are seen external to the spermatocytes. The spermatids then further develop into spermatozoa. They usually lie in groups with their heads projecting between the deeper cells and are connected with one of the Sertoli cells of the lining epithelium. The spermatogenic cells lines throughout different phases of growth and development were regularly arranged (Fig. 4A). The testis of 40 min exposed mice showed testicular atrophy and degenerative changes in spermatogenic cells lining the seminiferous tubules, with incomplete spermatogenesis. The seminiferous tubules were in a less regular shape. There was an alternation of the lining epithelium and reduced spermatogenic cells with a large lumen in the seminiferous tubules (Fig. 4B). The testis section of 60 min exposed mice revealed irregular shapes and non-uniform sizes seminiferous tubules. There were reduced spermatogenic cells with accumulation of spermatozoa in some of the seminiferous tubules, which were not seen in the control group. Occlusion of the lumen, as well as an increase in the size of seminiferous tubules, was evident (Fig. 4C-D). The mean height ofthe germinal epithelium was significantly decreased in both exposed groups. Testis lesions were more prominent in 60 min exposed mice in comparison with the testis of 40 min exposed mice (Fig. 4B-D).
Fig. 3.
Histological observation of the kidney of control (A), 40 min (B) & 60 min (C) exposed mice kidney sections stained with H & E stain. (A-B) showing control & 40 min exposure mice: no congestion, hemorrhage and normal architecture of renal corpuscles with their glomeruli and renal tubules were found in this group of mice; (C-F) exposure to 60 min: showing marked mononuclear cellular infiltration (arrow) with severe vascular congestion (arrow). Scale bar stands for 100 μm. G = Glomerulus, PT = Proximal Tubule & DT = Distal Tubule.
Fig. 4.
Histological observation of testis of control (A), 40 min (B) & 60 min (C) exposed mice testis sections stained with H & E stain. The transverse section of the testis of (A) control mice showed normal seminiferous tubule. The seminiferous tubule has well-developed interstitial tissue, seminiferous tubules lined with numerous Sertoli cells, and different stages of spermatogenic cells; (1) spermatogonia, (2) primary spermatocytes, (3) round secondary spermatocytes or early spermatids, and (4) spermatids. The Leydig cells were present between seminiferous tubules and seemed to be appropriate in both size and structure in the interstitial connective tissue. The testis of 40 min (B) exposed mice showing seminiferous tubules with irregular outlines, lost normal distribution of the epithelial lining, and fewer spermatogenic cells, which lead to the empty large wider tubule (large arrow) in the seminiferous tubules. The interstitium contains few Leydig cells compared to the control. (C-D) Testes of mice treated with 60 min EMR showed irregular shapes and non-uniform sizes of seminiferous tubules. There were reduced spermatogenic cells in the seminiferous tubules and accumulation of spermatozoa with exudate in the tubular lumen (arrowhead). The spermatogenic cells appeared pyknotic nuclei of some spermatogonia with degenerated spermatogenic cells, widening of the seminiferous tubular lumen (large arrow), and spermatozoa absence within the lumen. Scale bar stands for 100 μm. ST = Seminiferous Tubule, = Interstitial Tissue, L = Leydig cell.
4. Discussion
The harmful effects of mobile phone radiation exposure are poorly elucidated in vital organs such as the kidney and the testis. In the present study, therefore, we evaluated the effects of EMF on the hematobiochemical parameters and the potentially harmful effects of EMF on the kidneys and testis of mice model. Mobile phone users in various countries and continents are exposed to different frequencies. EMF exposure depends on the frequency of the cell phone (Meo et al., 2011). Many histological and biological experiments have been performed to assess the harmful effects of electromagnetic radiation on public health concerning the central nervous system, tumors, the renal system, fertility, growth, and immune function. The mice were exposed to 2400 MHz Radiofrequency Electromagnetic Radiation (RF-EMR) in this experiment because 2200–2400 MHz frequency 4G connected cell phones are extensively used in Bangladesh well as many other parts of the world. The present study results showed that the bodyweight of exposed mice was significantly reduced compared to the control mice, which agrees with the results of Wilson et al. (1999). The results (Shabat and Shahwan, 2017) noted that the mean weight of 3hrs and 5hrs high electromagnetic radiation (EMR) exposed groups decreases the initial mean weight. Likewise, (Marino et al., 1976) observed that mice exposed to low electric fields (150 V/cm) at 60 Hz for one month had reduced body weight and decreased water intake. Although (Lee et al., 2004) identified no significant weight difference in rats exposed to the magnetic field. (Gerardi et al., 2008) noted that the bodyweight increases as rats are exposed to electromagnetic radiation at a very well-defined frequency for a long-term duration.
Changes in antioxidant, biochemical, enzymological, and hematological levels indicate weakening animal homeostasis leading to stress and a reduction in functional ability (Gecit et al., 2014, Saravanan et al., 2012). Assessments of blood parameters are the most important means to determine the health status of experimental animals (Sud and Sekhon, 1989). It helps in evaluating and recognizing the effects of EMF induced hazardous alterations to humans. The present study results revealed that mobile phone radiations affected the hematological profile of the test animals remarkably. There were significant increases TLC and Hb% in radiation-exposed groups compared to the control group. In this background, there is an increase in the concentration of Hb, RBC, WBC, and platelet numbers in rats following 1 h/day EMF exposure for 30 consecutive days has been shown (Amara et al., 2006, Salem et al., 2005). Previous epidemiological studies have shown an increased risk of leukemia due to both electric and magnetic fields (Bastuji-Garin et al., 1990, Garland et al., 1990). Our findings were similar to previous research in where shown that exposure to EMF has led to a noticeable rise in the number of WBCs and differential WBC counts (Alghamdi and El-Ghazaly, 2012, Amara et al., 2006, Aziz et al., 2010, Chater et al., 2006, Sisodia et al., 2013). The increase in TLC may be related to electromagnetic field exposure, which triggers the hemopoietic mechanism to discharge more white blood cells, which increases its number in blood circulation (Aziz et al., 2010, Sisodia et al., 2013). Our results comply with previous research done by Forgács et al. (2006) and Roberts et al. (1986) in which reports indicate that many hematological parameters such as white blood cell count, differential white blood cell count, platelet levels, red blood cell count, mitotic index of hematopoietic stem-cells, as well as hematocrit, hemoglobin and bone marrow megakaryocytes are susceptible to exposure to RF/Megawatt (MW). This suggests that these blood components are broken due to irradiation by electromagnetic radiation from cell phones. The TEC values of 60 min exposed mice were decreased significantly in compared to the control mice. Previous research studies stated that exposure to EMF in experimental models induced a reduction in RBCs' count and their correlations (Alghamdi and El-Ghazaly, 2012, Ghadhban and Mhaibes, 2018, Marzook et al., 2016, Sisodia et al., 2013). The reduction of the hematological parameters after exposure to EMF can be related to direct damage and excess production of reactive oxygen species (ROS) by association with electromagnetic radiation (Eid et al., 2015, Sisodia et al., 2013). The free radicals affect the red blood cell membrane and cytoskeleton and leading to the leakage of hemoglobin from the cells (Creang et al., 2009, Eid et al., 2015). Red blood cell hemolysis reflects the degradation of cell integrity that can contribute to intracellular hemoglobin leakage (Eid et al., 2015). Spleen hyperfunction enhances red blood cell, leukocyte, and platelet breakdown rate. Due to hyperfunction of the spleen, the hematological variation has been happened in rats due to exposure to EMF was reported by Osbakken et al. (1986). The results revealed that the EMR waves damage the blood cell walls, leading to a decrease in the TLC count.
Besides, we also have investigated kidney dysfunction after exposure to EMF. Our study results indicated that the Serum creatinine value was significant increases in radiation exposed mice. Similarly, Tsuji et al. (1996) reported the increased blood urea nitrogen and creatinine levels in mice exposed to EMFs (5 T) for 48 h. Creatinine is generally created in muscles and exists freely in blood plasma and urine. Its higher serum levels act as an indicator of impaired renal function. This may be due to renal failure correlated with congestion in some tubules and focal leukocytic infiltration by pathological examination. Increased Serum creatinine levels in mice also agree with the findings of Asgari et al. (2014), who stated that Serum creatinine levels increased significantly in rats exposed to 3 h of mobile phone radiation a day compared to the control rats (P < 0.01). Studies have found that urea and creatinine increase dramatically when microwaving exposure duration increases (Morelli et al., 2005). Hepatic, renal, and splenic tissues were damaged due to exposure to electromagnetic radiation from cell phones (Al-Glaib et al., 2008). Damage to these tissues can lead to an increase in serum creatinine levels. There is a correlation between cell phone radiation and cellular damage that may contribute to elevated creatinine serum concentrations (Al-Glaib et al., 2008). Kidneys could mainly absorb the 900-MHz radiation from cell phones because they are often carried in the belt (Oktem et al., 2005), which may influence serum creatinine level.
There is a relation between mobile phone radiation and cellular damage that can lead to higher serum creatinine levels (Grundler et al., 1992). Grossly the kidney of control and 40 min exposed groups of mice were reddish-brown with a smooth and shiny surface. However, in 60 min exposed group showed pale color on the surface of the kidney. Our findings agreed with (Zare et al., 2007) that the morphology of the kidney of electromagnetic field exposed group mice was congested and pale. The testis was white in color and oval in shape in control and exposed mice.
The histopathological effects of 4G electromagnetic field radiation released from a cell phone device have been evaluated in this study by assessing the presence of pathological lesions in the kidney and testis. In the present study, there was no histological changes in the kidney of the control and 40 min exposed mice. In histopathological alterations, the 60-minute exposed mice reveals mononuclear cellular aggregates and intense venous congestion compared to the control mice. Similar findings were made by (Bayazit, 2009, Jaya et al., 2015, Makker et al., 2009, Ozguner et al., 2006) reporting that electromagnetic radiation (EMR) exposure induces many atrophied glomeruli, infiltration of leucocytes between the tubules of the kidney, and vacuolation of some tubules. Previous research found similar tissue changes using low frequencies EMR (Attia and Yehia, 2002, Forgács et al., 2004). Chauhan et al. (2017) stated that microwave radiation exposure causes shrunken glomeruli and irregular kidney tubules. Damage to these tissues may result in a rise in serum creatinine levels. EMR and high-power waves cause increased temperature rises. These waves interacted together and produced free radicals that cause increased lipid peroxidation and exhibit their damaging effects on cells like by ionizing rays. Free radicals damage the lipids of the cell and modify their composition and break protein boundary cause cell death. The oxidative stress induced by reactive oxygen species (ROS) is an essential element for tissue injury due to exposure to radiation (Al-Glaib et al., 2008, Markov, 2013).
Radiation is a potent toxicant, and exposure to the whole body may affect the animal's overall physiology and can have an effect on the normal histology and physiopathology of the testes (Sepehrimanesh et al., 2014). Radiation also has effects on steroidogenic and spermatogenic activity by the production of oxidative stress, antioxidant suppression mechanism, and various molecular processes included in decision-making about the death and life of germ cells that later changed normal testicular structure (Peltola et al., 1992). In the present experiment, histological examination of seminiferous tubules of irradiated mice showed marked pathological alterations in the form of shrinkage of tubules, distortion of cellular arrangement. These findings are in close agreement with the earlier report of (Goyal et al., 2011, Pareek et al., 2005), which documented the distorted architecture of seminiferous tubules in the form of shrunken tubules, pycnotic nuclei, necrotic cells, and degenerative effects of gamma rays on spermatogenesis in lethally irradiated mice.
In the present investigation, histological observation of testis of 40- and 60-minutes exposed mice showed that the seminiferous tubules were less regular in shape and alternation of the epithelium lining and fewer spermatogenic cells layer that led to the large wide lumen in the seminiferous tubules. There was an accumulation of spermatozoa and occlusion of the lumen in some of the seminiferous tubules. Similar findings were reported by Esa et al. (2018), who stated that the testicular tissue in mice exposed to a 3 kV EMF shows that the seminiferous tubules had almost the same size but less regular shape. EMF has caused noticeable changes in the seminiferous tubules in the testes of mice exposed to 5 kV contributing to uneven shapes and dimensions. Similar to the present study, Chauhan et al. (2017) stated that radiation-exposed group mice showed alternation of the epithelium lining of seminiferous tubules and occlusion of the lumen and size of seminiferous tubules was reduced with cell population. Several studies have shown that testis size and seminiferous tubules diameter were decreased following exposure to microwave radiation (Falzone et al., 2011, Ozguner et al., 2002). The excess free radical formation leads to histological and morphological changes in the testis and spermatogenic cell morphology. The ability of EMF to cause oxidative stress in the testes strongly suggests that the testis is a vulnerable tissue that is strongly dependent on oxygen for spermatogenesis and yet highly sensitive to the toxic effects of reactive oxygen enzymes (Chauhan et al., 2017, Dasdag et al., 1999). (Ozlem Nisbet et al., 2012) noticed that when rat exposure to electromagnetic radiation of 900–1800 MHz resulted in vacuolar degeneration, severe necrosis, and seminiferous epithelium desquamation. Long time exposure to 900 MHz EMF induced apoptosis of spermatogenic cells and responsible for reduced Leydig cell number as well assignificantly decreased mature spermatogenic cells and testosterone amount stated by many authors (Kim et al., 2014, Kim et al., 2009, Sepehrimanesh et al., 2014). The present study revealed similar findings that there was a significantly decreased spermatogenic cell layer in radiation-exposed mice.
5. Conclusion
Cell phones are a fundamental part of our social lives. However, people are still worried about the potential effects of smartphones. In this study, we tended to focus mostly on the potentially harmful effects of 4G electromagnetic radiation from mobile phones. This study indicated that long-time exposure to mobile phone radiation could lead to degenerative changes on specific hematobiochemical parameters, including kidney and testis histology. The results indicate that kidney and testis were oxidative. Most reports concerning Electromagnetic effects with low frequency were concentrated at a specific organ or process. This study recommends that different vital organs are equally affected by similar bloodstream during high frequencies of EMF exposure due to excessive smartphone use. This research gives an insight into mobile emitted radiation that might induce alterations in the blood and the kidney and testis tissue. However, the study alerts us all to the potentially detrimental effects of long-term electromagnetic radiation exposure. To escape these harmful effects of cell phone radiation, excessive mobile phone use should be avoided. Hopefully, comprehensive long-term studies at the molecular level are undoubtedly required in order to draw definitive conclusions.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We thank the Bangladesh Agricultural University Research System (BAURES) and University Grants Commission (UGC) of Bangladesh for providing financial support to conduct the research.
Funding
This work has been funded by the Bangladesh Agricultural University Research System (BAURES) Project (No. 2018/558AU-GC).
Author contributions
The experiment was planned and designed by MRI and IH. MRI has set up all the research facilities and supervised the entire research. IH conducted the entire laboratory works. IH participated in the histological analysis. IH with TA, MRA and MRI interpreted the findings of the statistical research activities and also engaged in the manuscript draft and carefully reviewed and revised the manuscript. The final version of the manuscript is read and agreed upon by all authors. Each author also guarantees that this contentor related information has not really been submitted to or will not be published anywhere.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Imam Hasan, Email: imamhasan@bau.edu.bd.
Mohammad Rafiqul Islam, Email: rafiqul.islam@bau.edu.bd.
References
- Agarwal A., Deepinder F., Sharma R.K., Ranga G., Li J. Effect of cell phone usage on semen analysis in men attending infertility clinic: an observational study. Fertil. Steril. 2008;89:124–128. doi: 10.1016/j.fertnstert.2007.01.166. [DOI] [PubMed] [Google Scholar]
- Aitken R.J., Bennetts L.E., Sawyer D., Wiklendt A.M., King B.V. Impact of radio frequency electromagnetic radiation on DNA integrity in the male germline. Int. J. Androl. 2005;28:171–179. doi: 10.1111/j.1365-2605.2005.00531.x. [DOI] [PubMed] [Google Scholar]
- Akdag M.Z., Dasdag S., Canturk F., Karabulut D., Caner Y., Adalier N. Does prolonged radiofrequency radiation emitted from Wi-Fi devices induce DNA damage in various tissues of rats? J. Chem. Neuroanat. 2016;75:116–1122. doi: 10.1016/j.jchemneu.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Al-Glaib B., Al-Dardfi M., Al-Tuhami A., Elgenaidi A., Dkhil M. A technical report on the effect of electromagnetic radiation from a mobile phone on mice organs. Libyan J. Med. 2008;3:8–9. doi: 10.4176/080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghamdi M.S., El-Ghazaly N.A. Effects of exposure to electromagnetic field on of some hematological parameters in mice. Open J. Med. Chem. 2012;2:30–42. doi: 10.4236/ojmc.2012.22005. [DOI] [Google Scholar]
- Amara S., Abdelmelek H., Ben Salem M., Abidi R., Sakly M. Effects of static magnetic field exposure on hematological and biochemical parameters in rats. Brazilian Arch. Biol. Technol. 2006;49:889–895. doi: 10.1590/s1516-89132006000700005. [DOI] [Google Scholar]
- Asgari M., Ahmadi R., Gohari A. The effects of noise pollution on serum levels of testosterone in male rats. Int. Con. Earth, Environ. Life Sci. 2014;23–24 [Google Scholar]
- Attia A.A., Yehia M.A. Histological, ultrastructural and immunohistochemical studies of the low frequency electromagnetic field effect on thymus, spleen and liver of albino swiss mice. Pakistan J. Biol. Sci. 2002;5:931–937. doi: 10.3923/pjbs.2002.931.937. [DOI] [Google Scholar]
- Azab A.E. Exposure to electromagnetic fields induces oxidative stress and pathophysiological changes in the cardiovascular system. J. Appl. Biotechnol. Bioeng. 2017;4:1–7. doi: 10.15406/jabb.2017.04.00096. [DOI] [Google Scholar]
- Aziz I.A., El-Khozondar H.J., Shabat M., Elwasife K., Mohamed-Osman A. Effect of electromagnetic field on body weight and blood indices in albino rats and the therapeutic action of vitamin C or E. Rom. J. Biophys. 2010;20:235–244. [Google Scholar]
- Baş O., Sönmez O.F., Aslan A., Ikinci A., Hanci H., Yildirim M., Kaya H., Akça M., Odaci E. Pyramidal cell loss in the cornu ammonis of 32-day-old female rats following exposure to a 900 megahertz electromagnetic field during prenatal days 13–21. NeuroQuantology. 2013;11:591–599. doi: 10.14704/nq.2013.11.4.701. [DOI] [Google Scholar]
- Bastuji-Garin S., Richardson S., Zittoun R. Acute leukaemia in workers exposed to electromagnetic fields. Eur. J. Cancer Clin. Oncol. 1990;26:1119–1120. doi: 10.1016/0277-5379(90)90266-V. [DOI] [PubMed] [Google Scholar]
- Bayazit V. Evaluation of the potential carcinogenic Effects of Electromagnetic Fields (EMF) on tissue and organs. Aust. J. Basic Appl. Sci. 2009;3:1043–1059. [Google Scholar]
- Bin-Meferij M.M., El-Kott A.F. The radioprotective effects of moringa oleifera against mobile phone electromagnetic radiation-induced infertility in rats. Int. J. Clin. Exp. Med. 2015;8:12487–12497. [PMC free article] [PubMed] [Google Scholar]
- Çam S.T., Seyhan N. Single-strand DNA breaks in human hair root cells exposed to mobile phone radiation. Int. J. Radiat. Biol. 2012;88:420–424. doi: 10.3109/09553002.2012.666005. [DOI] [PubMed] [Google Scholar]
- Chater S., Abdelmelek H., Pequignot J.M., Sakly M., Ben Rhouma K. Effects of sub-acute exposure to static magnetic field on hematologic and biochemical parameters in pregnant rats. Electromagn. Biol. Med. 2006;25:135–144. doi: 10.1080/15368370600860135. [DOI] [PubMed] [Google Scholar]
- Chauhan P., Verma H.N., Sisodia R., Kesari K.K. Microwave radiation (2.45 GHz)-induced oxidative stress: whole-body exposure effect on histopathology of Wistar rats. Electromagn. Biol. Med. 2017;36:20–30. doi: 10.3109/15368378.2016.1144063. [DOI] [PubMed] [Google Scholar]
- Colletti V., Mandalà M., Manganotti P., Ramat S., Sacchetto L., Colletti L. Intraoperative observation of changes in cochlear nerve action potentials during exposure to electromagnetic fields generated by mobile phones. J. Neurol. Neurosurg. Psychiatry. 2011;82:766–771. doi: 10.1136/jnnp.2010.222737. [DOI] [PubMed] [Google Scholar]
- Creang D.E., Culea M., Ndejde C., Oancea S., Curecheriu L., Racuciu M. Magnetic nanoparticle effects on the red blood cells. J. Phys. Conf. Ser. 2009 doi: 10.1088/1742-6596/170/1/012019. [DOI] [Google Scholar]
- Dasdag S., Akdag M.Z., Erdal M.E., Erdal N., Ay O.I., Ay M.E., Yilmaz S.G., Tasdelen B., Yegin K. Effects of 2.4 GHz radiofrequency radiation emitted from Wi-Fi equipment on microRNA expression in brain tissue. Int. J. Radiat. Biol. 2015;91:555–561. doi: 10.3109/09553002.2015.1028599. [DOI] [PubMed] [Google Scholar]
- Dasdag S., Ketani M.A., Akdag Z., Ersay A.R., Sari I., Demirtas Ö.C., Celik M.S. Whole-body microwave exposure emitted by cellular phones and testicular function of rats. Urol. Res. 1999;27:219–223. doi: 10.1007/s002400050113. [DOI] [PubMed] [Google Scholar]
- De Iuliis G.N., Newey R.J., King B.V., Aitken R.J. Mobile phone radiation induces reactive oxygen species production and DNA damage in human spermatozoa in vitro. PLoS ONE. 2009;4:e6446. doi: 10.1371/journal.pone.0006446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh P.S., Megha K., Banerjee B.D., Ahmed R.S., Chandna S., Abegaonkar M.P., Tripathi A.K. Detection of low level microwave radiation induced deoxyribonucleic acid damage vis-à-vis genotoxicity in brain of fischer rats. Toxicol. Int. 2013;20:19. doi: 10.4103/0971-6580.111549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid F.A., El-Gendy A.M., Zahkouk S.A., El-Tahway N.A., El-Shamy S.A. Ameliorative effect of two antioxidants on the liver of male albino rats exposed to electromagnetic field. Egypt. J. Hosp. Med. 2015;58:74–93. doi: 10.12816/0009363. [DOI] [Google Scholar]
- Elmas O. Effects of electromagnetic field exposure on the heart: a systematic review. Toxicol. Ind. Health. 2016;32:76–82. doi: 10.1177/0748233713498444. [DOI] [PubMed] [Google Scholar]
- Erogul O., Oztas E., Yildirim I., Kir T., Aydur E., Komesli G., Irkilata H.C., Irmak M.K., Peker A.F. Effects of electromagnetic radiation from a cellular phone on human sperm motility: an in vitro study. Arch. Med. Res. 2006;37:840–843. doi: 10.1016/j.arcmed.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Esa P.D., Suryandari D.A., Sari P. Effect of extremely low frequency electromagnetic fields on the diameter of seminiferous tubules in mice, in. J. Phys. Conf. Ser. 2018:062043. doi: 10.1088/1742-6596/1073/6/062043. [DOI] [Google Scholar]
- F. Ghadhban, R., Mhaibes, A., 2018. Study effect electromagnetic field (emf) and mobile phone radiation on some hematological, biochemical and hormonal parameters in female rats. Basrah J. Vet. Res. 17, 155–164. 10.33762/bvetr.2018.144948. [DOI]
- Falzone N., Huyser C., Becker P., Leszczynski D., Franken D.R. The effect of pulsed 900-MHz GSM mobile phone radiation on the acrosome reaction, head morphometry and zona binding of human spermatozoa. Int. J. Androl. 2011;34:20–26. doi: 10.1111/j.1365-2605.2010.01054.x. [DOI] [PubMed] [Google Scholar]
- Fejes I., Závaczki Z., Szöllosi J., Koloszár S., Daru J., Kovács L., Pál A. Is there a relationship between cell phone use and semen quality? Arch. Androl. 2005;51:385–393. doi: 10.1080/014850190924520. [DOI] [PubMed] [Google Scholar]
- Forgács Z., Somosy Z., Kubinyi G., Bakos J., Hudák A., Surján A., Thuróczy G. Effect of whole-body 1800 MHz GSM-like microwave exposure on testicular steroidogenesis and histology in mice. Reprod. Toxicol. 2006;22:111–117. doi: 10.1016/j.reprotox.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Forgács Z., Somosy Z., Kubinyi G., Sinay H., Bakos J., Thuróczy G., Surján A., Hudák A., Olajos F., Lázár P. Effects of whole-body 50-Hz magnetic field exposure on mouse Leydig cells. ScientificWorldJournal. 2004;4:83–90. doi: 10.1100/tsw.2004.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland F.C., Shaw E., Gorham E.D., Garland C.F., White M.R., Sinsheimer P.J. Incidence of leukemia in occupations with potential electromagnetic field exposure in United States navy personnel. Am. J. Epidemiol. 1990;132:293–303. doi: 10.1093/oxfordjournals.aje.a115658. [DOI] [PubMed] [Google Scholar]
- Gecit İ., Kavak S., Yüksel M.B., Basel H., Bektaş H., Gümrükçüoğlu H.A., Meral İ., Demir H. Effect of short-term treatment with levosimendan on oxidative stress in renal tissues of rats. Toxicol. Ind. Health. 2014;30:47–51. doi: 10.1177/0748233712451773. [DOI] [PubMed] [Google Scholar]
- Gerardi G., De Ninno A., Prosdocimi M., Ferrari V., Barbaro F., Mazzariol S., Bernardini D., Talpo G. Effects of electromagnetic fields of low frequency and low intensity on rat metabolism. Biomagn. Res. Technol. 2008;6:3. doi: 10.1186/1477-044X-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal, P.K., Sharma, P., Parmar, J., Verma, P., 2011. Radiation-induced testicular injury and its amelioration by tinospora cordifolia (an Indian medicinal plant) extract. Evidence-based Complement. Altern. Med. 10.1155/2011/643847. [DOI] [PMC free article] [PubMed]
- Grundler W., Kaiser F., Keilmann F., Walleczek J. Mechanisms of electromagnetic interaction with cellular systems. Naturwissenschaften. 1992;79:551–559. doi: 10.1007/BF01131411. [DOI] [PubMed] [Google Scholar]
- Hardell L., Carlberg M., Mild K.H. Pooled analysis of case-control studies on malignant brain tumours and the use of mobile and cordless phones including living and deceased subjects. Int. J. Oncol. 2011;38:1465–1474. doi: 10.3892/ijo.2011.947. [DOI] [PubMed] [Google Scholar]
- Hasan H.R., Issmer A.H. Effect of emitted radiation from mobile phones and its base station antennas on some biochemical parameters in human red blood cells. Int. J. Sci. Eng. Res. 2014;5:965–970. [Google Scholar]
- Hasan I., Islam M.R. Biochemical and histopathological effects of mobile phone radiation on the liver of Swiss albino mice. Eur. J. Anat. 2020;24:257–261. [Google Scholar]
- Jaya P., Kataria S.K., Raichandani L., Raichandani S. A study of histological effects of chronic exposure to a 2G Cellphone Radiations (900–1900 MHz) on kidneys of albino rats. Sch. J. Appl. Med. Sci. 2015;3:257–260. [Google Scholar]
- Keleş A.İ., Yıldırım M., Gedikli Ö., Çolakoğlu S., Kaya H., Baş O., Sönmez O.F., Odacı E. The effects of a continuous 1-h a day 900-MHz electromagnetic field applied throughout early and mid-adolescence on hippocampus morphology and learning behavior in late adolescent male rats. J. Chem. Neuroanat. 2018;94 doi: 10.1016/j.jchemneu.2018.08.006. [DOI] [PubMed] [Google Scholar]
- Kesari K.K., Kumar S., Behari J. Effects of radiofrequency electromagnetic wave exposure from cellular phones on the reproductive pattern in male Wistar rats. Appl. Biochem. Biotechnol. 2011;164:546–559. doi: 10.1007/s12010-010-9156-0. [DOI] [PubMed] [Google Scholar]
- Kesari K.K., Kumar S., Behari J. Mobile phone usage and male infertility in wistar rats. Indian J. Exp. Biol. 2010;48:987–992. [PubMed] [Google Scholar]
- Khillare B., Behari J. Effect of amplitude-modulated radiofrequency radiation on reproduction pattern in rats. Electromagn. Biol. Med. 1998;17:43–55. [Google Scholar]
- Kim H.S., Park B.J., Jang H.J., Ipper N.S., Kim S.H., Kim Y.J., Jeon S.H., Lee K.S., Lee S.K., Kim N., Ju Y.J., Gimm Y.M., Kim Y.W. Continuous exposure to 60Hz magnetic fields induces duration- and dose-dependent apoptosis of testicular germ cells. Bioelectromagnetics. 2014;35:100–107. doi: 10.1002/bem.21819. [DOI] [PubMed] [Google Scholar]
- Kim Y.W., Kim H.S., Lee J.S., Kim Y.J., Lee S.K., Seo J.N., Jung K.C., Kim N., Gimm Y.M. Effects of 60 Hz 14 μT magnetic field on the apoptosis of testicular germ cell in mice. Bioelectromagnetics. 2009;30:66–72. doi: 10.1002/bem.20448. [DOI] [PubMed] [Google Scholar]
- Kivrak E., Yurt K., Kaplan A., Alkan I., Altun G. Effects of electromagnetic fields exposure on the antioxidant defense system. J. Microsc. Ultrastruct. 2017;5:167–176. doi: 10.1016/j.jmau.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, R.S., Sareesh, N.N., Nayak, S., Mailankot, M., 2009. Hypoactivity of wistar rats exposed to mobile phone on elevated plus maze. Indian J. Physiol. Pharmacol. [PubMed]
- Lavranos, G., Balla, M., Tzortzopoulou, A., Syriou, V., Angelopoulou, R., 2012. Investigating ROS sources in male infertility: a common end for numerous pathways. Reprod. Toxicol. 10.1016/j.reprotox.2012.06.007. [DOI] [PubMed]
- Lee H.J., Pack J.K., Kim T.H., Kim N., Choi S.Y., Lee J.S., Kim S.H., Lee Y.S. The lack of histological changes of CDMA cellular phone-based radio frequency on rat testis. Bioelectromagnetics. 2010;31:528–534. doi: 10.1002/bem.20589. [DOI] [PubMed] [Google Scholar]
- Lee J.S., Ahn S.S., Jung K.C., Kim Y.W., Lee S.K. Effects of 60 Hz electromagnetic field exposure on testicular germ cell apoptosis in mice. Asian J. Androl. 2004;6:29–34. [PubMed] [Google Scholar]
- Mailankot M., Kunnath A.P., Jayalekshmi H., Koduru B., Valsalan R. Radio frequency electromagnetic radiation (RF-EMR) from GSM (0.9/1.8GHZ) mobile phones induces oxidative stress and reduces sperm motility in rats. Clinics. 2009;64:561–565. doi: 10.1590/S1807-59322009000600011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makker K., Varghese A., Desai N.R., Mouradi R., Agarwal A. Cell phones: modern man’s nemesis? Reprod. Biomed. Online. 2009;18:148–157. doi: 10.1016/S1472-6483(10)60437-3. [DOI] [PubMed] [Google Scholar]
- Marino A.A., Berger T.J., Austin B.P., Becker R.O., Hart F.X. Evaluation of electrochemical information transfer system: I. Effect of electric fields on living organisms. J. Electrochem. Soc. 1976;123:1199. doi: 10.1149/1.2133035. [DOI] [Google Scholar]
- Markov M.S. Effects of electromagnetic fields on biological systems. Electromagn. Biol. Med. 2013;32:121–122. doi: 10.3109/15368378.2013.776333. [DOI] [PubMed] [Google Scholar]
- Marzook E.A., Marzook F.A., Atomic E., Authority E. Glutathione enhancer protects some biochemical and haematological parameters from the effect of electromagnetic field. Egypt. J. Radiat. Sci. Appl. 2016;29:33–48. doi: 10.21608/ejrsa.2016.1577. [DOI] [Google Scholar]
- Megha K., Deshmukh P.S., Banerjee B.D., Tripathi A.K., Ahmed R., Abegaonkar M.P. Low intensity microwave radiation induced oxidative stress, inflammatory response and DNA damage in rat brain. Neurotoxicology. 2015;51:158–165. doi: 10.1016/j.neuro.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Meo S.A., Arif M., Rashied S., Husain S., Khan M.M., Vohra M.S., Usmani A.M., Imran M.B., Al-Drees A.M. Hypospermatogenesis and spermatozoa maturation arrest in rats induced by mobile phone radiation. J. Coll. Physicians Surg. Pakistan. 2011;21:262–265. [PubMed] [Google Scholar]
- Morelli A., Ravera S., Panfoli I., Pepe I.M. Effects of extremely low frequency electromagnetic fields on membrane-associated enzymes. Arch. Biochem. Biophys. 2005;441:191–198. doi: 10.1016/j.abb.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Moustafa Y.M., Moustafa R.M., Belacy A., Abou-El-Ela S.H., Ali F.M. Effects of acute exposure to the radiofrequency fields of cellular phones on plasma lipid peroxide and antioxidase activities in human erythrocytes. J. Pharm. Biomed. Anal. 2001;26:605–608. doi: 10.1016/S0731-7085(01)00492-7. [DOI] [PubMed] [Google Scholar]
- Narayanan S.N., Kumar R.S., Potu B.K., Nayak S., Mailankot M. Spatial memory performance of Wistar rats exposed to mobile phone. Clinics (Sao Paulo) 2009;64:231–234. doi: 10.1590/s1807-59322009000300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naziroǧlu M., Yüksel M., Köse S.A., Özkaya M.O. Recent reports of Wi-Fi and mobile phone-induced radiation on oxidative stress and reproductive signaling pathways in females and males. J. Membr. Biol. 2013 doi: 10.1007/s00232-013-9597-9. [DOI] [PubMed] [Google Scholar]
- Odac E., Hanc H., Yuluʇ E., Türedi S., Aliyazcoʇlu Y., Kaya H., Çolakoʇlu S. Effects of prenatal exposure to a 900 MHz electromagnetic field on 60-day-old rat testis and epididymal sperm quality. Biotech. Histochem. 2016;91:9–19. doi: 10.3109/10520295.2015.1060356. [DOI] [PubMed] [Google Scholar]
- Odaci E., Ünal D., Mercantepe T., Topal Z., Hanci H., Türedi S., Erol H.S., Mungan S., Kaya H., Çolakoʇlu S. Pathological effects of prenatal exposure to a 900 MHz electromagnetic field on the 21-day-old male rat kidney. Biotech. Histochem. 2015;90:93–101. doi: 10.3109/10520295.2014.947322. [DOI] [PubMed] [Google Scholar]
- Okano H. Effects of static magnetic fields in biology: role of free radicals. Front. Biosci. 2008;13:610–625. doi: 10.2741/3141. [DOI] [PubMed] [Google Scholar]
- Oktem F., Ozguner F., Mollaoglu H., Koyu A., Uz E. Oxidative damage in the kidney induced by 900-MHz-emitted mobile phone: protection by melatonin. Arch. Med. Res. 2005;36:350–355. doi: 10.1016/j.arcmed.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Osbakken M., Griffith J., Taczanowsky P. A gross morphologic, histologic, hematologic, and blood chemistry study of adult and neonatal mice chronically exposed to high magnetic fields. Magn. Reson. Med. 1986;3:502–517. doi: 10.1002/mrm.1910030404. [DOI] [PubMed] [Google Scholar]
- Ozguner F., Bardak Y., Comlekci S. Protective effects of melatonin and caffeic acid phenethyl ester against retinal oxidative stress in long-term use of mobile phone: a comparative study. Mol. Cell. Biochem. 2006;282:83–88. doi: 10.1007/s11010-006-1267-0. [DOI] [PubMed] [Google Scholar]
- Ozguner I.F., Dindar H., Yagmurlu A., Savas C., Gokcora I.H., Yucesan S. The effect of electromagnetic field on undescended testis after orchiopexy. Int. Urol. Nephrol. 2002;33:87–93. doi: 10.1023/A:1014473407519. [DOI] [PubMed] [Google Scholar]
- Ozguner M., Koyu A., Cesur G., Ural M., Ozguner F., Gokcimen A., Delibas N. Biological and morphological effects on the reproductive organ of rats after exposure to electromagnetic field. Saudi Med. J. 2005;26:405–410. [PubMed] [Google Scholar]
- Ozlem Nisbet H., Nisbet C., Akar A., Cevik M., Onder Karayigit M. Effects of exposure to electromagnetic field (1.8/0.9GHz) on testicular function and structure in growing rats. Res. Vet. Sci. 2012;93:1001–1005. doi: 10.1016/j.rvsc.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Pareek T.K., Rimpu K., Dev P.K., Goyal P.K. Modulation of radiation-induced lesions in testes of Swiss Albino mice by vitamin E. J. Cell Tissue Res. 2005;5:471. [Google Scholar]
- Peltola V., Huhtaniemi I., Ahotupa M. Antioxidant enzyme activity in the maturing rat testis. J. Androl. 1992;13:450–455. doi: 10.1002/j.1939-4640.1992.tb03343.x. [DOI] [PubMed] [Google Scholar]
- Qin F., Zhang J., Cao H., Yi C., Li J.X., Nie J., Chen L.L., Wang J., Tong J. Effects of 1800-MHz radiofrequency fields on circadian rhythm of plasma melatonin and testosterone in male rats. J. Toxicol. Environ. Heal. - Part A Curr. Issues. 2012;75:1120–1128. doi: 10.1080/15287394.2012.699846. [DOI] [PubMed] [Google Scholar]
- Ribeiro E.P., Rhoden E.L., Horn M.M., Rhoden C., Lima L.P., Toniolo L. Effects of subchronic exposure to radio frequency from a conventional cellular telephone on testicular function in adult rats. J. Urol. 2007;177:395–399. doi: 10.1016/j.juro.2006.08.083. [DOI] [PubMed] [Google Scholar]
- Roberts, N.J., Michaelson, S.M., Lu, S.T., 1986. The biological effects of radiofrequency radiation: A critical review and recommendations. Int. J. Radiat. Biol. 10.1080/095530086145i50841. [DOI] [PubMed]
- Salem A., Hafedh A., Rached A., Mohsen S., Khémais B.R. Zinc prevents hematological and biochemical alterations induced by static magnetic field in rats. Pharmacol. Reports. 2005;57:616–622. [PubMed] [Google Scholar]
- Sani A., Labaran M.M., Dayyabu B. Effects of electromagnetic radiation of mobile phones on hematological and biochemical parameters in male albino rats. Eur. J. Exp. Biol. 2018;8:11. doi: 10.21767/2248-9215.100052. [DOI] [Google Scholar]
- Saravanan M., Devi K.U., Malarvizhi A., Ramesh M. Effects of Ibuprofen on hematological, biochemical and enzymological parameters of blood in an Indian major carp, Cirrhinus mrigala. Environ. Toxicol. Pharmacol. 2012;34:14–22. doi: 10.1016/j.etap.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Saunders R.D., Kowalczuk C.I. Effects of 2·45 GHz microwave radiation and heat on mouse spermatogenic epithelium. Int. J. Radiat. Biol. 1981;40:623–632. doi: 10.1080/09553008114551611. [DOI] [PubMed] [Google Scholar]
- Sepehrimanesh M., Saeb M., Nazifi S., Kazemipour N., Jelodar G., Saeb S. Impact of 900 MHz electromagnetic field exposure on main male reproductive hormone levels: a Rattus norvegicus model. Int. J. Biometeorol. 2014;58:7–13. doi: 10.1007/s00484-013-0771-7. [DOI] [PubMed] [Google Scholar]
- Shabat M.M., Shahwan O.A. Analysis of the biochemical parameters of liver, kidney functions and thyroid stimulated hormone in children after exposure to mobile phone base station radiation and therapeutic action of olive oil. IUG J. Nat. Stud. 2017:79–84. [Google Scholar]
- Singh M., Singh U.P., Singh K.P., Mishra A. Effect of 50-Hz powerline exposed magnetized water on rat kidney. Electromagn. Biol. Med. 2004;23:241–249. doi: 10.1081/JBC-200045091. [DOI] [Google Scholar]
- Sisodia R., Rifat F., Sharma A., Srivastava P., Sharma K.V. Effects of 10-GHz microwaves on hematological parameters in swiss albino mice and their modulation by Prunus avium. J. Environ. Pathol. Toxicol. Oncol. 2013;32 doi: 10.1615/JEnvironPatholToxicolOncol.2013007891. [DOI] [PubMed] [Google Scholar]
- Sud V.K., Sekhon G.S. Blood flow through the human arterial system in the presence of a steady magnetic field. Phys. Med. Biol. 1989;34:795. doi: 10.1088/0031-9155/34/7/001. [DOI] [PubMed] [Google Scholar]
- Tsuji Y., Nakagawa M., Suzuki Y. Five-tesla static magnetic fields suppress food and water consumption and weight gain in mice. Ind. Health. 1996;34:347–357. doi: 10.2486/indhealth.34.347. [DOI] [PubMed] [Google Scholar]
- Usman A.D., Wan Ahmad W.F., Ab Kadir M.Z.A., Mokhtar M., Ariffin R. Microwave effect of 0.9GHz and 1.8GHz CW frequencies exposed to unrestrained Swiss albino mice. Prog. Electromagn. Res. B. 2011;36:69–87. doi: 10.2528/PIERB11072709. [DOI] [Google Scholar]
- Wilson B.W., Matt K.S., Morris J.E., Sasser L.B., Miller D.L., Anderson L.E. Effects of 60 Hz magnetic field exposure on the pineal and hypothalamic-pituitary-gonadal axis in the Siberian hamster (Phodopus sungorus) Bioelectromagnetics. 1999;20:224–232. doi: 10.1002/(SICI)1521-186X(1999)20:4<224::AID-BEM3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Yan J.G., Agresti M., Bruce T., Yan Y.H., Granlund A., Matloub H.S. Effects of cellular phone emissions on sperm motility in rats. Fertil. Steril. 2007;88:957–964. doi: 10.1016/j.fertnstert.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Yildirim M.E., Kaynar M., Badem H., Cavis M.I., Karatas O.F., Cimentepe E.I. What is harmful for male fertility: cell phone or the wireless internet? Kaohsiung. J. Med. Sci. 2015;31:480–484. doi: 10.1016/j.kjms.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zare, S., Alivandi, S., Ebadi, A., 2007. Histological studies of the low frequency electromagnetic fields effect on liver, testes and kidney in guinea pig. World Appl. Sci. J.