Abstract
Cancer is the second leading cause of mortality accounting for one in every six deaths globally. Plant secondary metabolites, among them polyphenols, represent an effective and much safer alternative approach to the currently available medications. In this work, utilizing LC-MS/MS, we characterized the constituents of S. yapa leaves extract and evaluated its antioxidant and anticancer properties. In total, 34 secondary metabolites, mainly flavonoids (Tricin, luteolin, and apigenin and their glucosides as well as sulfated derivatives) were identified. The extract manifested substantial antioxidant activity in DPPH assay, and high total phenolic content determined by Folin Ciocalteu method. The extract was safe up to 4800 mg/kg b.wt. when administered orally in mice and neither affected the hematological parameters nor the liver enzyme levels at the studied dose (LD50, 480 mg, kg b.wt.). In the treated animals, the extract surpassed the reference drug (5-flouro uracil) and significantly reduced the tumor volume and weight by 71.50 and 85.46%, respectively, increased the median survival time to 53.2 days and the lifespan by 116%. The extract improved all the hematological parameters, where it increased the hemoglobin (Hb) concentration, red blood cell (RBC) count, packed cell volume (PVC) and platelets by 58.21, 8.98, 9.89 and 120%, respectively, compared to the untreated EAC bearing animals. Additionally, the extract significantly declined the elevated levels of ALT and AST enzymes by 29.18% and 59.88%, respectively. In molecular docking, the annotated flavonoids displayed appreciable binding affinities to the active sites of VEGFR1 and VEGFR2. In conclusion, Saba yapa is a promising plant that can be introduced to further advanced clinical studies for the development of novel anticancer drugs with lower side effects.
Keywords: Sabal yapa, Ehrlich ascites carcinoma, Sulfated polyphenols, Vascular endothelial growth factor B receptor
1. Introduction
Following cardiovascular diseases, cancer comes as the second leading cause of mortality accounting for one in every six deaths globally. According to recent statistics, cancer resulted in over 9 million death cases during 2018, 70% of which were recorded in low- and middle-income countries (Bray et al., 2018). The disease is characterized by rapid uncontrolled formation of abnormal cell masses that grow beyond the usual boundaries. These malicious cells can then invade other adjoining parts of the body and spread to distant organs in a process known as metastases (Hanahan and Weinberg, 2000).
Cancer etiology involves a series of mutations occurring successively in the proto-oncogenes that regulate and control the cell cycle phases including cell growth, division, and proliferation. Carcinogens such as different hazardous chemicals, pollutants, pathogens, and radiations stand as the main culprit of the cancer-initiating gene mutations (Seto et al., 2010).
Apart from the primary surgical procedure and radiotherapy, which are limited to certain tumor types and patient’s conditions, the systematic drug approach comprising either standard or metronomic chemotherapy is the most common and widespread cancer treatment strategy. However, this approach is associated with several adverse effects that, in many cases, limit the continuation of the therapy (Baskar et al., 2012). In this regard, some isolated plant secondary metabolites could represent an effective and much safer alternative.
Flavonoids represent one of the most renowned classes of polyphenols and found in high concentrations in the edible plant parts of many vegetables and fruits (Symonowicz and Kolanek, 2012). Based on their chemical structure, flavonoids are classified into the major classes flavanols, flavonols, flavanones, flavones, isoflavones, and anthocyanins in addition to many subclasses. A wide array of pharmacological activities has been reported for this class of compounds including anti-inflammatory, antioxidant, anti-aging, antimicrobial, as well as anti-cancer and cytotoxic properties (Brodowska, 2017). The flavonoids apigenin, genistein and 3-hydroxyflavone were reported to act as chemopreventive agents as they in vitro inhibited angiogenesis and consequently suppress the proliferation of tumor and endothelial cells (Kim, 2003).
The genus Sabal, family Arecaceae, comprises 17 species and is endemic to the new world; however, it is currently cultivated worldwide. Sabal species demonstrated different biological activities. For instance, S. serrulatum is used for managing and preventing prostate hyperplasia and nonbacterial prostatitis (Olennikov et al., 2013). In a study by (Ibrahim et al., 2017), S. palmetto extract and its fractions demonstrated substantial anti-inflammatory and anti-cancer activities.
Sabal yapa C. Wright ex Becc. is an evergreen palm that is cultivated in Egypt. Successive solvent extracts from the fruits exhibited promising antiandrogenic activities in castrated rats in which the non-polar extract reduced the prostate weight and plasma testosterone level and did not change the plasma levels of creatinine, aspartate transaminase and alanine transaminase. These activities were attributed the presence of several fatty acids, among them lauric, oleic and linolenic acids (Ammar et al., 2013). Another study explored anti-cancer activities of three sabal species from Egypt against glioblastoma and prostate cancer cell lines and profiled their chemical composition utilizing LC-MS (El-Hawary et al., 2020a).
In the present work, the chemical composition of a hydroalcoholic extract from S. yapa leaves was explored using HPLC-MS/MS. In addition, the antioxidant properties and the anticancer activities were investigated in vivo using Ehrlich ascites carcinoma (EAC). Several parameters including tumor volume and weight, viable and nonviable tumor cell count, median survival time and percentage increase in lifespan, liver enzymes activities and hematological parameters were investigated. Furthermore, an in-silico docking study was used to evaluate the potential of the mainly identified flavonoids in the extract to interfere with angiogenesis via blocking the vascular endothelial growth factor B receptors; VEGFR1 and VEGFR2.
2. Materials and methods
2.1. Plant material and extraction
Sabal yapa fresh leaves were collected during April 2016 from El-Orman botanical garden, Cairo, Egypt. The leaves (200 g) were air-dried at room temperature, coarsely powdered and extracted using 70% ethanol (3 × 1 L). The combined extracts were concentrated under reduced vacuum and lyophilized yielding a dry fine powder (16 g) with an extraction yield of 8%. The lyophilized powder was kept at −20 °C for biological and phytochemical evaluations.
2.2. LC-MS/Ms
Phytochemical analysis of S. yapa extract was performed using a ThermoFinnigan LC system (ThermoElectron Corporation, Austin, TX, USA) that was controlled by Xcalibur software (Xcalibur™ 2.0.7, Thermo Scientific, Waltham, MA, USA). MS operating parameters in the negative mode were set up as described in El-Hawary et al. (2020b).
2.3. Total phenolic content (TPC) and radical scavenging potential
TPC was amounted using Folin Ciocalteu method as described in Ghareeb et al. (2018) and the radical α, α-diphenyl-β-picrylhydrazyl (DPPH) scavenging potential was evaluated as detailed in Ghareeb et al. (2018).
2.4. In vivo anti-tumor activity
2.4.1. Ethicals statement
Animal experiments were carried out with strict adherence to the ethical policies and procedures approved by the Medical Research Ethics Committee for animal care and use, National Research Centre, Egypt, registration No. 6/014.
2.4.2. Acute toxicity study
Female albino mice (n = 8) were treated orally with different doses of S. yapa extract. The dosing pattern started with 500 mg/kg body weight (b.wt.) and increased, at a rate of 500 mg/ kg, up to a maximum dose of 6000 mg/kg b.wt. The control group received only normal saline (Bruce, 1985). The LD50 value was calculated using BioStat program (BioStat 2009 Build 5.8.4.3) and was recorded to be 4800 mg/kg b.wt. Therefore, the selected dose to study the in-vivo antitumor activity of the extract was 480 mg/kg b.wt./day as the 1/10th of the LD50 according to (Ghosh, 1984).
2.4.3. Anti-tumor activity of the extract in Ehrlich ascites carcinoma model
Ehrlich ascites carcinoma (EAC) cells were used at a concentration of 2 × 106 cell/ mouse and were obtained from National Cancer Institute, Cairo, Egypt. Female albino mice (20 to 25 g) were obtained from the animal house of National Research Centre, Cairo, Egypt. Animals were kept in standard polypropylene cages, fed on standard diet and water ad libitum, maintained under normal room conditions (temperature of 25–30 °C and 60–65% relative humidity) for one week before experimental period for adaptation. Mice were classified into five groups, 14 animals each.
Group I: mice received the vehicle (saline solution) orally for 10 consecutive days and served as the negative control group. Group II: mice treated orally with the extract at a dose of 480 mg/kg b.wt. for 10 consecutive days and served as the extract control group. Group III: mice injected with EAC (2 × 106 cell/ mouse), incubated for 24 h, then received saline orally for 10 consecutive days and served as the tumor-bearing group. Group IV: mice injected with EAC (2 × 106 cell/ mouse), incubated for 24 h, then received orally the reference drug, 5-fluorouracil in the recommended dose (20 mg/kg b.wt./day) for 10 consecutive days. Group V: mice injected with EAC (2 × 106 cell/ mouse), incubated for 24 h, then received orally S. yapa extract at a dose of 480 mg/kg b.wt. for 10 consecutive days and served as the extract treated group. At the end of the experiment and after fasting overnight, mice were anesthetized by an injection of ketamine (87 mg/kg b.wt.) and xylazine (13 mg/kg b.wt.). The two drugs were dissolved in normal saline, and each mouse received 0.2 mL/100 g body weight (Van Pelt, 1977). Animals were sacrificed after anesthesia, blood samples were collected from eye blood vein from six mice/ group only for estimation of hematological parameters. Eight mice were kept alive to check the increase in lifespan of the tumor-bearing hosts (Sivakumar et al., 2005).
2.4.4. Determination of tumor volume and weight
Ascitic fluids from the animals’ peritoneal cavity were collected in a graduated centrifuge tube and the fluids’ volume and weight were measured immediately (Karmakar et al., 2013).
2.4.5. Estimation of viable and nonviable tumor cell count
The ascitic fluid was placed in white blood cell pipette and diluted 100 times. A drop of the diluted suspension was placed in the Neubauer counting chamber to stain the cells with trypan blue (0.4% in normal saline) dye for distinguishing the viable cells (that were not stained) from the nonviable ones. Both cell counts were estimated by the following equation (Karmakar et al., 2013):
| Cell Count = Number of cell × dilution∕Area × film thickness |
2.4.6. Determination of percentage increase in lifespan and median survival time
The percentage increase in the lifespan and median survival time of the treated animals were recorded for monitoring of mortality, by the following formula:
Increase in lifespan% = (T − C/C) × 100; Where T is the number of days survived by the treated animals, and C is the number of days control mice survived (Karmakar et al., 2013).
2.4.7. Determination of solid tumor volume
The solid tumor in mice was estimated according to Kuttan et al., (1990). The tumor mass was measured starting the 11th day following the tumor induction. The measurement was carried out every five days for 30 days. The volume of tumor mass was estimated using the following formula:
| V = 4/3 × πr2 |
where r is the mean of r1 and r2, which are independent radii of the tumor mass.
2.4.8. Measurement of hematological parameters
Collected blood samples were used for determining the hematological parameters according to Dacie and Lewis (1975), including hemoglobin, total leukocyte count, red blood cell count, packed cell volume, platelet count, and differential white blood cell.
2.4.9. Estimation of liver enzymes activities
The liver enzymes AST and ALT activities were spectrophotometrically measured in sera according to Reitman and Frankel (1957).
2.4.10. Molecular docking
The major identified flavonoids in S. yapa extract were docked to the vascular epithelial growth factor B receptors; VEGFR1 and VEGFR2 that are associated with the activation and regulation mechanisms of the angiogenesis. The compounds’ chemical scaffolds were drawn using the builder facility of molecular operating environment (MOE), 2013.08; Chemical Computing Group Inc., Montreal, QC, Canada, H3A 2R7, 2016. The compounds were set to their ionized state, and finally energy minimized by the aid of the molecular mechanic force-field mmff94x. The crystal structures of VEGFR1 (pdb ID: 3HNG) and VEGFR2 (pdb ID: 3EWH) with the corresponding bound inhibitor were downloaded from the protein data bank (www.pdb.org). The protein structures were prepared by adding the hydrogen atoms, assigning their protonation state and geometry. Running the docking rounds was done using the default triangle matcher placement method and London dG scoring function. Docking poses were refined by force-field energy minimization using London dG scoring function. The best three docking poses showing the minimal estimated binding free energy were virtually analyzed in terms of the interactions afforded between the amino acid residues in the binding site and the docked compounds to judge their ability to iterate the reported interactions of the reference inhibitors.
2.4.11. Statistical analysis
Data was recorded as mean ± SD and analyzed by the one-way ANOVA using IBM SPSS statistics program (version 23). P < 0.05 was considered as significant difference.
3. Results
3.1. LC-MS/MS analysis of S. Yapa leaves crude extract
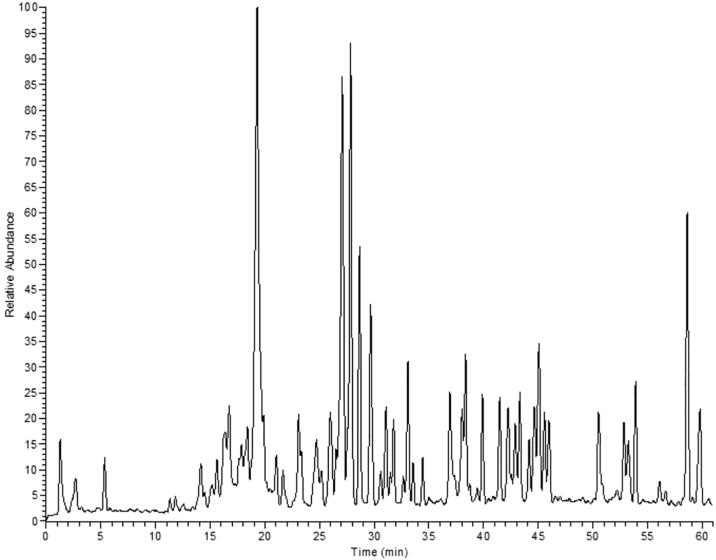
Altogether, thirty-four compounds, mainly flavonoids and their sulphated derivatives as well as flavolignans, were identified in the hydroalcoholic extract of S. yapa leaves. The identification was based on molecular weight, MS2 fragmentation, in-house library, available authentic compounds and literature (Table 1 and Fig. 1).
Table 1.
Tentative identification of polyphenolics of S. yapa extract.
| No. | Rt | [M−H]- | MS2 (m/z) | Tentatively Identified Compounds | References |
|---|---|---|---|---|---|
| 1 | 1.47 | 191 | Quinic acid | El-Hawary et al. (2020b) | |
| 2 | 2.21 | 133 | Malic acid | Sobeh et al. (2017) | |
| 3 | 5.32 | 287 | 125, 241 | Flavanol | |
| 4 | 11.33 | 137 | 93 | p-hydroxybenzoic acid | Țigu et al. (2021) |
| 5 | 14.16 | 353 | 191, 179 | Chlorogenic acid | Țigu et al. (2021) |
| 6 | 15.18 | 289 | 245, 205, 179 | Epicatechin | Sobeh et al. (2017) |
| 7 | 16.69 | 515 | 357, 405 | Pinoresinol derivative | |
| 8 | 19.08 | 503 | 423, 305, 197 | (epi)Gallocatechin phenylacetyl sulphate | |
| 9 | 21.13 | 289 | 245, 205, 179 | Catechin | Sobeh et al. (2017) |
| 10 | 21.67 | 449 | 287, 151, 135 | Eriodictyol 7-O-glucoside | Kachlicki et al. (2008) |
| 11 | 21.83 | 593 | 473, 413, 383, 353 | Apigenin 6,8-di-C-glucoside (Vicenin-2) | Ahmed et al. (2019) |
| 12 | 23.08 | 503 | 423, 305, 197 | (epi)Gallocatechin phenylacetyl sulphate | |
| 13 | 24.70 | 643 | 563, 515, 443 | Schaftoside sulphate* | |
| 14 | 25.78 | 563 | 503, 413, 383, 353 | Apigenin 6-C-glucosyl-8-C-xyloside (Schaftoside) | Sun, et al. (2013) |
| 15 | 26.53 | 527 | 447, 429, 357, 327 | Orientin sulphate | Ahmed et al. (2019) |
| 16 | 27.79 | 447 | 357, 327, 285 | Luteolin 8-C-glucoside (Orientin) | Ahmed et al. (2019) |
| 17 | 29.58 | 563 | 443, 383, 353 | Apigenin 6,8-di-C-glucoside (Vicenin-1) | Ahmed et al. (2019) |
| 18 | 31.08 | 431 | 431, 341, 311, 269 | Apigenin 6-C-glucoside (Isovitexin) | Ahmed et al. (2019) |
| 19 | 32.84 | 463 | 343, 327, 301 | Quercetin 3-O-glucoside | Sobeh et al. (2017) |
| 20 | 33.00 | 447 | 357, 327, 285 | Luteolin 7-O-glucoside | Ahmed et al. (2019) |
| 21 | 34.43 | 461 | 371, 341 | Diosmentin 8-C-glucoside | Ahmed et al. (2019) |
| 22 | 37.15 | 497 | 417 | Naringenin rhamnoside sulphate | Kachlicki et al. (2008) |
| 23 | 37.93 | 571 | 491, 329, 371 | Tricin 7-O-glucoside-5-sulphate | Ahmed et al. (2019) |
| 24 | 38.18 | 477 | 315, 271, 151 | Isorhamnetin 3-O-glucoside | El-Hawary et al. (2020b) |
| 25 | 39.91 | 491 | 371, 329 | Tricin 7-O-glucoside | Ahmed et al. (2019) |
| 26 | 41.46 | 737 | 657, 567, 495, 329 | Tricin 4′-O-(erythro-β-4 hydroxyphenylglyceryl ether) 7-O-glucoside sulphate | |
| 27 | 42.86 | 767 | 687, 567, 525, 329 | Tricin 4′-O-(erythro-β-4 guaiacylglyceryl ether) 7-O- glucoside sulphate | Ahmed et al. (2019) |
| 29 | 43.29 | 767 | 687, 567, 525, 329 | Tricin 4′-O-(threo-β-4 guaiacylglyceryl ether) 7-O- glucoside sulphate | Ahmed et al. (2019) |
| 30 | 44.17 | 687 | 525, 329 | Tricin 4′-O-(erythro-β-4 guaiacylglyceryl ether) 7-O-glucopyranoside | |
| 31 | 44.66 | 737 | 657, 567, 495, 329 | Tricin 4′-O-(threo-β-4-hydroxyphenylglyceryl ether) 7-O-glucoside sulphate | |
| 32 | 46.59 | 687 | 525, 329 | Tricin 4′-O-(threo-β-4 guaiacylglyceryl ether) 7-O-glucopyranoside | |
| 33 | 50.52 | 409 | 329, 311, 295 | Tricin -O- sulphate* | |
| 34 | 58.59 | 329 | 314, 299, 285 | Tricin | El-Hawary et al. (2020b) |
Fig. 1.
LC -MS profile of the hydroalcoholic extract of S. yapa leaves.
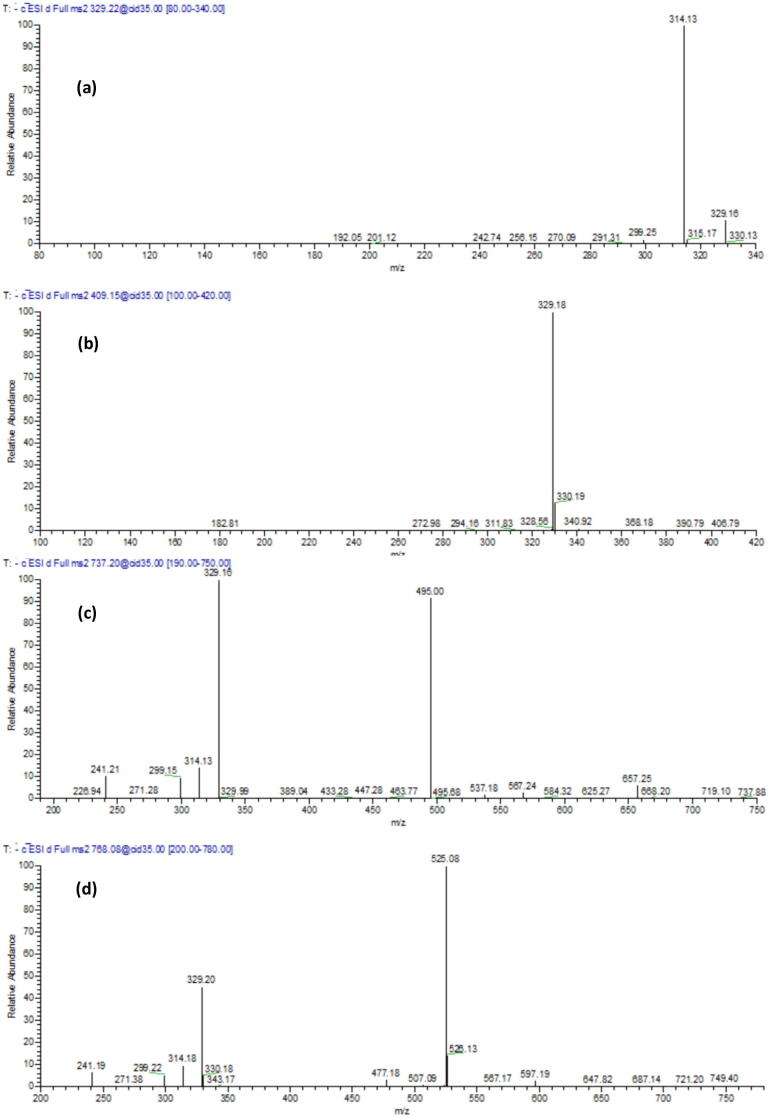
The O-methylated flavone, tricin, along with 9 hexosides and sulphated derivatives were characterized in the extract (Table 1). They showed [M−H]- at m/z 767, 737, 678, 571, 491, 409 and a molecular ion peak at 329; they were annotated as tricin 4′-O-(erythro-β-4 guaiacylglyceryl ether) 7-O- glucoside sulphate, tricin 4′-O-(erythro-β-4 hydroxyphenylglyceryl ether) 7-O-glucoside sulphate, tricin 4′-O-(erythro-β-4 guaiacylglyceryl ether) 7-O- glucopyranoside, tricin 7-O-glucoside-5-sulphate, tricin 7-O-glucoside and tricin O-sulphate. The corresponding retention times and fragmentation patterns are presented in Table 1 and some representative MS/MS spectra are shown in Fig. 2
Fig. 2.
MS/MS spectra of some identified compounds in the extract; (a) Tricin, (b) Tricin O-sulphate, (c) Tricin 4′-O-(erythro-β-4 hydroxyphenylglyceryl ether) 7-O-glucoside sulphate, (d) Tricin 4′-O-(erythro-β-4 guaiacylglyceryl ether) 7-O- glucoside sulphate.
Several luteolin and apigenin derivatives were also characterized along with p-hydroxybenzoic acid, chlorogenic acid, catechin and its isomer epicatechin, malic and quinic acids, their retention times and fragmentation pattern are displayed in Table 1. Moreover, two compounds showed a molecular ion peak at [M–H]- m/z 503 and three daughter ions at 197, 305, and 423; they were tentatively assigned as (epi) gallocatechin phenylacetyl sulphate, Table 1.
3.2. Antioxidant properties
The hydroalcoholic extract of S. yapa leaves demonstrated considerable antiradical potential against DPPH radicals (IC50 = 6.25 µg/mL) when compared to the reference compound, vitamin C (IC50 = 3.12 µg/mL). This efficacy could be attributed to its high phenolic content, which was assayed by Folin Ciocalteu method; it represented 312 mg gallic acid equivalents/ g extract. The obtained results come in agreement with numerous plant extracts rich in polyphenols (Sobeh et al., 2019, Sobeh et al., 2020, El-Hawary et al., 2020a).
3.3. Anti-tumor properties of S. Yapa leaves extract
3.3.1. Tumor volume and weight, median survival time and lifespan
Following the carcinoma cell injection, the tumor volume and weight were significantly increased. Consequently, the lifespan of the tumor bearing mice was decreased to 20.00 ± 0.18 days. The extract significantly reduced the tumor volume and tumor weight by 71.50 and 85.46%, respectively, which in turn increased the median survival time to 53.2 day, which represents 116% increase in the lifespan. This is considered a significant increment compared to the reference drug, 5-flouro uracil, Table 2.
Table 2.
Effects of S. yapa leaves extract on survival parameters of bearing mice.
| Parameters | Groups |
||
|---|---|---|---|
| Tumor bearing | FU-treated (20 mg/kg/ day) | Extract treated (480 mg/kg/day) | |
| Tumor volume (cm3) | 3.00 ± 0.28 | 1.52 ± 0.16* | 0.855 ± 0.028* |
| % change | −49.33% | −71.50% | |
| Tumor weight | 3.59 ± 0.66 | 0.73 ± 0.15* | 0.522 ± 0.066* |
| % change | −79.67% | −85.46% | |
| MST (day) | 20 ± 0.18 | 41 ± 0.84* | 53.2 ± 0.142* |
| Increase in lifespan % | – | 105 ± 0.45 | 116 ± 0.817 |
Data are presented as mean of 5 animals ± SD followed with increasing or decreasing percentage as compared to tumor bearing mice. MST: median survival time. One-way ANOVA was used for data analysis followed by post Hoc test for multiple comparisons (n = 5, p < 0.05). Treated groups were compared to bearing group (*).
3.3.2. Viable and nonviable tumor cells count
After 10 days, the extract significantly reduced the carcinoma cells count in the tumor-bearing group by 71.50% with more pronounced efficiency than that of the reference drug (51.40%). These observed effects were reflected by the great suppression of the viable cells’ total number by 91.83% and the rise of the nonviable cells’ count by 154.87%, Table 3.
Table 3.
Effects S. yapa leaves extract on viable and non-viable tumor cell count.
| Parameter | Groups |
||
|---|---|---|---|
| Tumor bearing | FU-treated | Extract treated | |
| Total cell count (x 107 cell/ml) | 10 ± 1.13 | 4.86 ± 0.87* | 2.85 ± 0.50* |
| Change (%) | −51.40% | −71.50% | |
| Viable cell count (x 107 cell/ml) | 9.18 ± 1.10 | 1.41 ± 0.69* | 0.75 ± 0.39* |
| Change (%) | −84.64% | −91.83% | |
| Non-viable cell count (x 107 cell/ml) | 0.82 ± 0.45 | 3.45 ± 0.61* | 2.10 ± 0.53* |
| Change (%) | + 320.73% | 154.87% | |
| Viable cell % | 97.23 ± 1.64 | 18.00 ± 0.71* | 26.32 ± 0.56* |
| Non-viable cell % | 2.77 ± 0.95 | 82.00 ± 0.68* | 73.68 ± 0.78* |
Data are presented as mean ± SD followed with decreasing or increasing percentage. One-way ANOVA was used for data analysis followed by post Hoc test for multiple comparisons (n = 5, p < 0.05). *Significantly different as compared to bearing mice.
3.3.3. Hematological parameters
The ameliorative effects of S. yapa extract on tumor cell count was also confirmed by the measured hematological parameters, where the extract significantly reduced the deleterious effects of carcinoma cells on all these parameters. The extract significantly increased hemoglobin concentration (Hb), red blood cell count (RBC's count), packed cell volume (PVC) and platelets by 58.21, 8.98, 9.89 and 120%, respectively, compared to the tumor-bearing mice group, Table 4.
Table 4.
Potential effect of S. yapa leaves extract on hematological parameters in Ehrlich ascites carcinoma bearing mice.
| Parameter | Hb conc | RBC's Count | PCV | Platelets count | WBC x 1000 mm3 | Deferential white blood cells |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| mg/dl | X million/mm3 | mm | X 1000/mm3 | Neutrophil | Lymphocytes | Monocytes | Eosinophil | Basophiles | ||
| Normal groups | ||||||||||
| Normal control | 11.5 ± 1.10 | 11.12 ± 1.00 | 41.12 ± 1.25 | 300 ± 1.41 | 7 ± 0.98 | 25.63 ± 0.69 | 65 ± 1.02 | 3.41 ± 1.24 | 4 ± 0.78 | 1.96 ± 0.30 |
| S. yapa control group | 13 ± 1.11 | 10.65 ± 1.16 | 46 ± 0.97 | 296.67 ± 0.79 | 6.8 ± 0.28 | 28 ± 1.17 | 65 ± 1.75 | 3 ± 0.21 | 2 ± 0.10 | 2 ± 0.14 |
| Ehrlich ascites carcinoma bearing mice | ||||||||||
| Tumor bearing mice | 6.53 ± 0.96** | 7.24 ± 1.11** | 31.32 ± 1.52** | 100 ± 1.61** | 11.41 ± 1.00** | 60 ± 0.84** | 29 ± 0.67** | 5.50 ± 0.13** | 3 ± 0.09**a | 2.5 ± 0.14** |
| 5-Flourouracil | 7.11 ± 1.06* | 7.53 ± 0.97a | 38.54 ± 2.16* | 185 ± 1.86* | 9.24 ± 1.08* | 36.33 ± 2.11* | 56.24 ± 1.46* | 4.16 ± 0.74* | 2.27 ± 0.85*a | 1.0 ± 0.04 |
| % change | +8.88% | +4.01% | +23.05% | +85% | −19.02% | −39.45% | +93.93% | −24.36% | −24.33% | −60% |
| S. yapa treated mice | 10.07 ± 1.01*a | 7.89 ± 0.90a | 34.42 ± 1.28* | 220.87 ± 1.39* | 6.65 ± 0.92* | 42.75 ± 0.65* | 49.40 ± 1.75* | 2.90 ± 0.25* | 2.95 ± 0.05* | 2.0 ± 0.03* |
| % change | +54.21% | +8.98% | +9.89% | +120% | −41.71% | −28.75% | +70.34%) | −47.27% | −1.6% | −20% |
Data are presented as mean ± SD followed with increasing or decreasing percentage as compared to tumor bearing mice. ANOVA one-way was used for data analysis followed by post Hoc test for multiple comparisons (n = 5, p < 0.05). Bearing mice were compared to –ve control group (**) while treated groups were compared to bearing group (*). Hb; hemoglobin, RBC's; red blood cell, PCV; packed cell volume, WBC; white blood cell. Percentages of increasing or decreasing was calculated as compared to bearing mice.
The extract’s recovery effects were also extended to improve the deferential white blood cells status, especially neutrophils and lymphocytes. The extract reduced the elevated neutrophils by 28.75% and increased the lymphocytes by 70.34%, Table 4. The same assessments were also conducted for the positive control group that received the extract only to determine any regression effect on animals as a part of safety profile. All recorded parameters proved a good safety margin for the extract (Table 4).
3.3.4. Liver function parameters
Because of the toxic effect of the carcinoma cells injection, the liver enzymes (AST and ALT) were significantly elevated in the tumor-bearing mice. Administration of the extract significantly reduced the elevated enzyme levels by 29.18% and 59.88% for ALT and AST, respectively. Similar effects were obtained from the standard drug 5-fluorouracil. Noteworthy, S. yapa extract did not affect liver enzymes in the extract positive control group with respect to the negative control group (Table 5).
Table 5.
Efficacy of S. yapa extract on the liver function of tumor bearing mice in Ehrlich model.
| Groups | ALT |
AST |
AST/ALT ratio |
|---|---|---|---|
| U/L | |||
| Normal groups | |||
| Negative control | 29.33 ± 1.02 | 25.83 ± 1.11 | 0.88 ± 0.21 |
| Extract control group | 29 ± 1.71 | 26.31 ± 1.31 | 0.91 ± 0.13 |
| Ehrlich Ascites carcinoma bearing mice | |||
| Tumor bearing mice | 110 ± 0.05** | 450 ± 2.14** | 4.09 ± 1.32** |
| FU treated group | 90 ± 1.51* | 192 ± 1.05* | 1.92 ± 0.31* |
| % change | −18.18% | −57.33% | −53.06% |
| Extract treated group | 77.9 ± 0.199* | 180.5 ± 0.437* | 2.194 ± 0.95* |
| % change | −29.18% | −59.88% | −46.45% |
Data are presented as mean of five animals ± SD. One-way ANOVA was used for data analysis followed by post Hoc for multiple comparisons (n = 5, p < 0.05). Bearing mice and + ve controls were compared to –ve control group (**) while treated groups were compared to bearing group (*). FU; flouro uracil treated group, ALT; Glutamic- pyruvic transaminase, AST; Glutamic- oxaloacetic transaminase.
3.3.5. Molecular docking
Generation of blood vessels through pathological angiogenesis is a critical event for sustaining chronic inflammation and tumor progression. Induction of angiogenesis is mediated through the vascular endothelial growth factor (VEGF) family of cytokines, which exert their biological roles through activation of three tyrosine kinase receptors; namely VEGFR1, VEGFR2, and VEGFR3 (Otrock et al. 2007). Some flavonoids such as genistein, apigenin, and jaceidin were reported to block the angiogenesis cascade via interfering with VEGF signaling (Kim et al. 2003, Elhady et al., 2020). In the current work, the flavonoids identified S. yapa extract were docked to VEGFR1 and VEGFR2 proteins, whose structures were co-crystallized with bound chemical inhibitors, to evaluate their inhibitory potential on VEGF receptors. Docking poses of these flavonoids revealed their ability to bind to the active sites of VEGFR1 and VEGFR2 with appreciable estimated free binding energy reflected by lower docking score values relative to that of the co-crystalized inhibitor (Table 6).
Table 6.
Docking scores (kcal/mol) of the major compounds identified in S. yapa extract upon docking into VEGFR1 and VEGFR2 active sites.
| Compound name | Docking score (kcal/mol) |
|
|---|---|---|
| VEGFR1 | VEGFR2 | |
| Tricin | −13.87 | −15.38 |
| Tricin 7-O-glucoside | −17.28 | −21.07 |
| Tricin 7-O-glucoside-5-sulphate | −18.77 | −26.13 |
| Apigenin | −13.87 | −14.30 |
| Apigenin 6-C-glucoside (Isovitexin) | −16.34 | −20.27 |
| Apigenin 6,8-di-C-glucoside (Vicenin 2) | −19.37 | −23.28 |
| Apigenin 6-C-glucosyl-8-C-xyloside (Schaftoside) | −17.93 | −22.09 |
| Apigenin 6-C-glucosyl-8-C-xyloside-7-sulphate | −20.99 | −28.85 |
| Eriodictyol | −14.10 | −15.69 |
| Eriodictyol 7-O-glucoside | −17.64 | −20.08 |
| Quercetin | −14.83 | −15.46 |
| Quercetin 3-O-glucoside | −15.16 | −20.52 |
| Diosmetin | −13.97 | −15.37 |
| Diosmentin 8-C-glucoside | −17.20 | −20.92 |
| Luteolin | −15.18 | −15.36 |
| Luteolin 7-O-glucoside | −17.33 | −20.09 |
| Luteolin 8-C-glucoside (Orientin) | −17.20 | −24.12 |
| Luteolin 8-C-glucoside sulphate | −17.74 | −26.93 |
| Co-crystallized inhibitor | −13.18 | −16.29 |
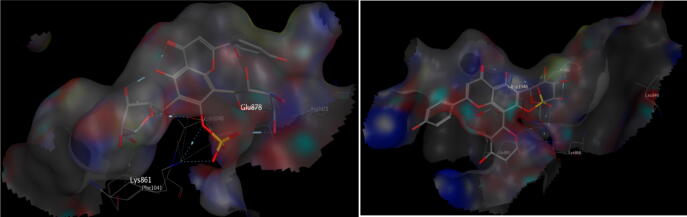
In general, flavonoids of S. yapa showed better binding to VEGFR2, which is reflected by lower binding energy values (Table 6). O- and C-glucoside derivatives showed to be more efficient towards both receptors relative to their free aglycone congeners, most probably due to higher number of hydroxyl groups engaged in more H-bonding interactions with amino acid residues of the active sites. Vicenin 2 showed the best binding to VEGFR1 with an estimated binding energy of –19.37 kcal/mol. Orientin, on the other hand, showed the least binding energy (–24.12 kcal/mol) upon binding to VEGFR2. The sulfated derivatives, however, surpassed all the other docked compounds towards both receptors, where the sulphate group was involved in extra interactions with Lys861 in the active site of VEGFR1 and Lys868 in that of VEGFR2. The most efficient member was schaftoside sulfate that scored a free binding energy of –20.99 kcal/mol and –28.85 on VEGFR1 and VEGFR2, respectively. It iterated the major amino acid interactions reported for the co-crystallized inhibitors of both target proteins; namely the interactions with Asp1040, Glu878, and Leu882 in VEGFR1 and Asp1046 and Glu885 in VEGFR2 active sites. (Fig. 3).
Fig. 3.
3D poses of schaftoside sulphate docked to VEGFR1 (left) and VEGFR2 (right).
4. Discussion
The polyphenolic constituents of S. yapa were comprehensively characterized for the first time yielding thirty-four compounds. They included flavonoids, flavonoid C-glycosides, flavonoid O-glycosides, flavolignans and flavonoid sulphate derivatives. The studied S. yapa extract exhibited antioxidant activities in vitro and anti-tumor properties in Ehrlich ascites carcinoma (EAC) model. Ehrlich ascites carcinoma is a spontaneous murine mammary adenocarcinoma. It lacks H-2 histocompatibility antigens; therefore, it proliferates rapidly in most mouse hosts and stands as good model to evaluate anti-tumor activity of newly developed drug candidates.
The extract prolonged the lifespan, ameliorated the WBC count, and showed a noticeable reduction of the viable EAC cells in the treated tumor bearing mice, which all points out its role in reducing the nutritional fluid volume and the source of tumor growth. In addition, the extract demonstrated protective effects on the hematopoietic system by resorting the hemoglobin content, RBC cells count, and other hematological parameters to their normal values.
The anti-tumor activities of the extract might be attributed to the presence of flavonoids, flavonoid C-glycosides and their sulphated derivatives, hence, C2 = C3 double bond significantly contributes to the molecular planarity and the conjugation between rings C and A/B, which is essential for potent tumor inhibition (Chidambara Murthy et al., 2012). Many sulphated phenolic compounds were identified in S. yapa leaves extract in the present work, they include schaftoside sulphate, orientin sulphate, tricin 7-glucoside sulphate, tricin 4′-O-(erythro-β-4 hydroxyphenylglyceryl ether) 7-O-glucoside sulphate isomers, tricin 4′-O-(erythro-β-4 guaiacylglyceryl ether) 7-O-glucoside sulphate isomers and tricin -O- sulphate. This sulphation renders polyphenols more hydrophilic, increases their bioavailability, and affects their interactions with other biomolecules (Manach et al., 2004).
Jaceidin, a flavonoid from Chiliadenus montanus, attenuated EAC tumor progression in mice and interfered with angiogenesis by lowering VEGF levels (Elhady et al., 2020). Our molecular docking study revealed the strong potential of S. yapa flavonoids to bind to the inhibitor’s site of VEGFR1 and VEGFR2, thus hindering the receptors activation by VEGF cytokines and consequently could interfere with angiogenesis. Among the identified compounds, orientin; it exhibited solid cytotoxic properties against several cancer cell lines, and its apoptotic effects were explained by their interference with caspases 3/7 and caspase-9 pathways (Law et al., 2014).
The identified phenolic p- hydroxybenzoic acid was reported to possess antimicrobial, estrogenic, cytotoxic effects as well as antioxidant properties (Pugazhendhi et al., 2005). Chlorogenic, ferulic and caffeic acids were also proved as potent anticancer and antimutagenic substances (Cai et al., 2004). Quercetin identified in this extract as well, demonstrated substantial antiproliferative properties, activated apoptosis in many human cancer cell lines (HT-29, LNCaP, HCT and MCF7) and induced cell cycle arrest (Rahman et al., 2011, Plaumann et al., 1996). Another promising anticancer flavonoid, tricin, characterized in the S. yapa leaves extract is used to treat colorectal cancer. Moreover, it competitively inhibits the cytosolic sulfotransferases, thus increases the flavonoids’ bioavailability in tissues and blood (Wen et al., 2017). Vitexin identified in our extract, was reported for its antineoplastic effect in PC12 and U937 cells due to induction of apoptosis via interference with Bcl-2, caspase-3 and caspase-9 pathways (Choi et al., 2006).
The identified flavonoids, luteolin, luteolin- 7-O-glucoside, and luteolin-4-O-glucoside that were previously isolated from Olea Europaea showed antiproliferative effect on cancer and endothelial cell lines with IC50 value between 3 and 50 μM for luteolin (Seelinger et al., 2008, Goulas et al., 2009). Similar cytotoxic activities were reported from several extracts rich in flavonoids and polyphenols. For instance, a study by Islam et al. (2012) reported antineoplastic activity against Ehrlich ascites carcinoma by Eucalyptus extracts. Another study by Rahman et al. (2017) documented solid antioxidant and anticancer properties of Aponogeton undulates extracts.
5. Conclusions
The phytochemical analysis of a hydroalcoholic extract from Sabal yapa leaves revealed thirty-four compounds among which different flavonoids along with their sulfated derivatives and C- and O-glucoside derivatives prevailed the extract. In the biological assays, the extract turned to be safe to experimental animals up to a dose as high as 4800 mg/kg b.wt. It revealed high phenolic content and showed substantial antioxidant potential. The extract showed a solid anticancer potential against EAC through reducing tumor weight and volume, increasing the animals lifespan, improving various hematologic parameters, and restoring a normal liver enzymes level. Molecular docking showed that the identified flavonoids were able to block VEGFR1 and VEGFR2 and thus might be able to interfere with angiogenesis. In conclusion, S. yapa leaves extract could be considered as a safe anticancer regimen that is worth to be a subject for further studies to prove its therapeutic potential.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank National Research Centre for the work financial support and thank Dr. Mohammed El-Gebali for identifying the plant.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mohamed A. El Raey, Email: elraiy@gmail.com.
Mansour Sobeh, Email: mansour.sobeh@um6p.ma.
References
- Ahmed R., Elkhrisy E., EL-kashak W.A., El Raey M., Nassar M., Aboutabl E.S. Structural Characterization of Polyphenolics in Livistona chinensis Using HPLC-PDA-MS. J. Adv. Pharm. Res. 2019;1(3):23–29. [Google Scholar]
- Ammar N.M., Hefnawy M.S., Mohamed D.A., Agoor F.S., Afifi A.H. Antiandrogenic and phytochemical evaluation of solvents and supercritical CO2 extracts of Sabal yapa fruit cultivated in Egypt. Rivista Italiana Delle Sostanze Grasse. 2013;90:229–235. [Google Scholar]
- Baskar R., Lee K.A., Yeo R., Yeoh K.W. Cancer and radiation therapy: current advances and future directions. Int. J. Med. Sci. 2012;9:193. doi: 10.7150/ijms.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J. Clinicians. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Brodowska K.M. Natural flavonoids: classification, potential role, and application of flavonoid analogues. Eur. J. Biol. Res. 2017;7:108–123. [Google Scholar]
- Bruce R.D. An up-and-down procedure for acute toxicity testing. Fundam Appl Toxicol. 1985;5:151–157. doi: 10.1016/0272-0590(85)90059-4. [DOI] [PubMed] [Google Scholar]
- Cai Yizhong, Luob Qiong, Sunc Mei, Corke Harold. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidambara Murthy K.N., Kim J., Vikram A. Differential inhibition of human colon cancer cells by structurally similar flavonoids of citrus. Food Chem. 2012;132(1):27–34. doi: 10.1016/j.foodchem.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Choi H.J., Eun J.S., Kim B.G., Kim S.Y., Jeon H., Soh Y. Vitexin, an HIF-1alpha inhibitor, has anti-metastatic potential in PC12 cells. Mol. Cell. 2006;22(3):291–299. [PubMed] [Google Scholar]
- Dacie, J.V., Lewis, S.M., 1975. Laboratory investigation in hemolytic anemia in practical haematology. 5th ed. New York: Churchill Livingstone, Elsevier. p. 40. ISBN: 978-0-7020-6696.
- Elhady S.S., Eltamany E.E., Shaaban A.E., Bagalagel A.A., Muhammad Y.A., El-Sayed N.M., Ayyad S.N., Ahmed A.AM., Elgawish M.S., Ahmed S.A. Jaceidin flavonoid isolated from chiliadenus montanus attenuates tumor progression in mice via VEGF inhibition: in vivo and in silico studies. Plants. 2020;8:1031. doi: 10.3390/plants9081031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hawary S.S., Saber F.R., Abd Almaksoud H.M., Elimam H., Sayed A.M., Abdelmohsen U.R. Cytotoxic potential of three Sabal species grown in Egypt: a metabolomic and docking-based study. Nat. Prod. Res. 2020;19:1–6. doi: 10.1080/14786419.2020.1851228. [DOI] [PubMed] [Google Scholar]
- El-Hawary S.S., Sobeh M., Badr W.K., Abdelfattah M.AO., Ali Z.Y., El-Tantawy M.E., Rabeh M.A., Wink M. HPLC-PDA-MS/MS profiling of secondary metabolites from Opuntia ficus-indica cladode, peel and fruit pulp extracts and their antioxidant, neuroprotective effect in rats with aluminum chloride induced neurotoxicity. Saudi J. Biol. Sci. 2020;27:2829–2838. doi: 10.1016/j.sjbs.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghareeb M.A., Mohamed T., Saad A.M., Refahy L.A., Sobeh M., Wink M. HPLC-DAD-ESI-MS/MS analysis of fruits from Firmiana simplex (L.) and evaluation of their antioxidant and antigenotoxic properties. J. Pharmacy Pharmacol.. 2018;70(1):133–142. doi: 10.1111/jphp.12843. [DOI] [PubMed] [Google Scholar]
- Ghosh M.N. Scientific Book Agency; Calcutta: 1984. Satistical analysis, fundamentals of experimental pharmacology; pp. 189–190. [Google Scholar]
- Goulas Vlassios, Exarchou Vassiliki, Troganis Anastassios N., Psomiadou Eleni, Fotsis Theodoros, Briasoulis Evangelos, Gerothanassis Ioannis P. Phytochemicals in olive-leaf extracts and their antiproliferative activity against cancer and endothelial cells. Mol. Nutr. Food Res. 2009;53:600–608. doi: 10.1002/mnfr.200800204. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Ibrahim Abeer Y., El-Newary Samah A., El-Raey Mohamed A. Evaluation of the antioxidant, anti-inflammatory, and antitumor properties of Sabal grown in Egypt. Egyptian Pharm. J. 2017;16:168–183. [Google Scholar]
- Islam Farhadul, Khatun Hasina, Ghosh Soby, Ali M.M., Khanam J.A. Bioassay of Eucalyptus extracts for anticancer activity against Ehrlich ascites carcinoma (EAC) cells in Swiss albino mice. Asian Pac. J. Trop. Biomed. 2012;5:2394–2398. doi: 10.1016/S2221-1691(12)60063-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachlicki P., Einhorn J., Muth D., Kerhoas L., Stobiecki M. Evaluation of glycosylation and malonylation patterns in flavonoid glycosides during LC/MS/MS metabolite profiling. J. Mass Spectrom. 2008;43:572–586. doi: 10.1002/jms.1344. [DOI] [PubMed] [Google Scholar]
- Karmakar I., Dolai N., Kumar R.B.S., Kar B., Roy S.N., Haldar P.K. Antitumor activity and antioxidant property of Curcuma caesia against Ehrlich’s ascites carcinoma bearing mice. Pharm. Biol. 2013;5:753–759. doi: 10.3109/13880209.2013.764538. [DOI] [PubMed] [Google Scholar]
- Kim M.H. Flavonoids inhibit VEGF/bFGF-induced angiogenesis in vitro by inhibiting the matrix-degrading proteases. J. Cell. Biochem. 2003;89:529–538. doi: 10.1002/jcb.10543. [DOI] [PubMed] [Google Scholar]
- Kuttan G., Vasudevan D.M., Kuttan R. Effect of a preparation from Viscum album on tumor development in vitro and in mice. J. Ethnopharmacol. 1990;29:35–41. doi: 10.1016/0378-8741(90)90095-b. [DOI] [PubMed] [Google Scholar]
- Law B.N.T., Ling A.P.K., Koh R.Y., Chye S.M., Wong Y.P. Neuroprotective effects of orientin on hydrogen peroxide-induced apoptosis in SH-SY5Y cells. Mol. Med. Rep. 2014;9(3):947–954. doi: 10.3892/mmr.2013.1878. [DOI] [PubMed] [Google Scholar]
- Manach C., Scalbert A., Morand C., Remesy C., Jimenez L. Polyphenols: Food sources and bioavailability. A. J. Clin. Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- Olennikov D.N., Zilfikarov I.N., Khodakova S.E. Phenolic compounds from Serenoa repens fruit. Chem. Nat. Comp. 2013;49:526–529. [Google Scholar]
- Otrock Z.K., Makarem J.A., Shamseddine A.I. Vascular endothelial growth factor family of ligands and receptors. Blood Cells Mol. Dis. 2007;38:258–268. doi: 10.1016/j.bcmd.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Plaumann B., Fritsche M., Rimpler H., Brandner G., Hess R.D. Flavonoids activate wild-type p53. Oncogene. 1996;13:1605–1614. [PubMed] [Google Scholar]
- Pugazhendhi D., Pope G.S., Darbre P.D. Oestrogenic activity of phydroxybenzoic acid (common metabolite of paraben esters) and methylparaben in human breast cancer cell lines. J. Appl. Toxicol. 2005;25:301–309. doi: 10.1002/jat.1066. [DOI] [PubMed] [Google Scholar]
- Rahman Mohammad Saifur, Alam Mohammad Badrul, Choi Yun Hee, Yoo Jin Cheol. Anticancer activity and antioxidant potential of Aponogeton undulatus against Ehrlich ascites carcinoma cells in Swiss albino mice. Oncol. Lett. 2017;14(3):3169–3176. doi: 10.3892/ol.2017.6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman Shahedur, Salehin Faizus, Iqbal Asif. In vitro antioxidant and anticancer activity of young Zingiber officinale against human breast carcinoma cell lines. BMC Complement. Alternat. Med. 2011;11:76. doi: 10.1186/1472-6882-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Reitman S., Frankel S.A. Colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- Seelinger Günter, Merfort Irmgard, Wölfle Ute, Schempp Christoph M. Anti-carcinogenic effects of the flavonoid luteolin. Molecules. 2008;13:2628–2651. doi: 10.3390/molecules13102628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto M., Honma K., Nakagawa M. Diversity of genome profiles in malignant lymphoma. Cancer Sci. 2010;101 doi: 10.1111/j.1349-7006.2009.01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakumar T., Sambathkumar R., Perumal P., Vamsi M.L.M., Kanagasabai R. Antitumor and antioxidant activities of Bryonia laciniosa against Ehrlich’s ascites carcinoma bearing Swiss albino mice. Oriental Phar. Exp. Med. 2005;5:322–330. [Google Scholar]
- Sobeh Mansour, Hamza Marwa S., Ashour Mohamed L., Elkhatieb Mona, El Raey Mohamed A., Abdel-Naim Ashraf B., Wink Michael. A polyphenol-rich fraction from Eugenia uniflora exhibits antioxidant and hepatoprotective activities in vivo. Pharmaceuticals. 2020;13(5):84. doi: 10.3390/ph13050084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobeh M., Hassan S.A., El Raey M.A., Khalil W.A., Hassan M.A., Wink M. Polyphenolics from Albizia harveyi exhibit antioxidant activities and counteract oxidative damage and ultra-structural changes of cryopreserved bull semen. Molecules. 1993;2017:22. doi: 10.3390/molecules22111993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobeh M., El-Raey M., Rezq S., Abdelfattah M.A., Petruk G., Osman S., El-Shazly A.M., El-Beshbishy H.A., Mahmoud M.F., Wink M. Chemical profiling of secondary metabolites of Eugenia uniflora and their antioxidant, anti-inflammatory, pain killing and anti-diabetic activities: A comprehensive approach. J. Ethnopharmacol. 2019;240 doi: 10.1016/j.jep.2019.111939. [DOI] [PubMed] [Google Scholar]
- Sun D., Dong L., Guo P., Yan W., Wang C., Zhang Z. Simultaneous determination of four flavonoids and one phenolic acid in rat plasma by LC–MS/MS and its application to a pharmacokinetic study after oral administration of the Herba Desmodii Styracifolii extract. J. Chromatogr. B. 2013;1(932):66–73. doi: 10.1016/j.jchromb.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Symonowicz M., Kolanek M. Flavonoids and their properties to form chelate complexes. Biotechnol. Food Sci. 2012;76:35–41. [Google Scholar]
- Țigu A.B., Moldovan C.S., Toma V.A., Farcaș A.D., Moț A.C., Jurj A., Fischer-Fodor E., Mircea C., Pârvu M. Phytochemical analysis and in vitro effects of Allium fistulosum L. and Allium sativum L. extracts on human normal and tumor cell lines: a comparative study. Molecules. 2021;26(3):574. doi: 10.3390/molecules26030574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pelt L.F. Ketamine and xylazine for surgical anesthesia in rats. J. Am. Vet. Med. Assoc. 1977;171:842–844. [PubMed] [Google Scholar]
- Wen Lingrong, Jiang Yueming, Yang Jiali, Zhao Yupeng, Tian Miaomiao, Yang Bao. Structure, bioactivity, and synthesis of methylated. Flavonoids. Annals of the New York Academy of Sciences. 2017 doi: 10.1111/nyas.13350. [DOI] [PubMed] [Google Scholar]