Abstract
Camel’s milk is an important part of staple diet in several parts of the world, particularly in the arid and semi-arid zones. Camel’s milk is rich in health-beneficial substances, such as bioactive peptides, lactoferrin, zinc, and mono and polyunsaturated fatty acids. These substances could help in the treatment of some important human diseases like tuberculosis, asthma, gastrointestinal diseases, and jaundice. Camel’s milk composition is more variable compared to cow’s milk. The effects of feed, breed, age, and lactation stage on milk composition are more significant in camel. Region and season significantly change the ratio of compounds in camel’s milk. Camel’s whey protein is not only composed of numerous soluble proteins, but also has indigenous proteases such as chymotrypsin A and cathepsin D. In addition to their high nutritional value, these whey proteins have unique characteristics, including physical, chemical, physiological, functional, and technological features that are useful in the food application. The hydrolysis of camel’s milk proteins leads to the formation of bioactive peptides, which affect major organ systems of the body and impart physiological functions to these systems. The camel’s milk has antioxidant, antimicrobial, angiotensin-I-converting enzyme (ACE)-inhibitory peptides, antidiabetic as well as anticholesterol activities.
Keywords: Bacteria, Camel; Health; Medicine; Milk; Nutrition; Protein
1. Introduction
Camel belongs to the family Camelidae in the request Artiodactyla. There are two types of camels, the Bactrian two humped camel (Camelus bactrianus) and the Arabian or dromedary one humped camel (Camelus dromedarius) (El-Agamy et al., 1992). Camels play a significant role in the life way of numerous societies, especially those in dry zones in the Middle East and the Arabian territory (Kaskous, 2016). Camels can be adapted to various climatic conditions. They are utilized in transport, sport, wellspring of meat and milk; therefore, they contribute to raising the economy and food security for people (Suliman et al., 2019, Swelum et al., 2020).
Camels world population is approximately 29 million, based on the most recent food and agriculture organization (FAO) statistics, of which around 95% are dromedary (one humped) camels (Sikkema et al., 2019). The camels’ lactation period may vary from 9 to 18 months. The amount of obtained milk depends on many factors such as breed, animal health, stage of lactation, living conditions (Swelum et al., 2020). Camel’s milk yield is lower and unstable than cow’s milk yield, however, enhanced feed, water and veterinary practices may increase camel’s milk yield as the udder structure of camel is similar (Park & Haenlein, 2013).
Millions of people around the world are daily consuming milk due to its tremendous nutritional benefits such as the growth and development of bones in young children, as milk is a good source of calcium and vitamin D. It has also proven to be beneficial for older people, especially in menopausal women where calcium deficiency is a high-risk factor for the development of osteoporosis (El-Hatmi et al., 2015).
Milk is not only a source of nutrition, but its production also contributes to food security and income for most people in the developing countries. Around 150 million households are engaged in milk production across the globe (FAO, 2012). It is particularly beneficial for small scale producers because of quick cash turnouts. Camel’s milk provides the required human nutrition. In addition, it offers therapeutic benefits (Bai & Zhao, 2015).
The current review highlights the composition and the health benefits of camel’s milk as a natural source of bioactive components.
2. Camel’s milk
Camel’s milk is white opaque, with a slightly salty taste with pH ranges from 6.2 to 6.5 that is lower compared to that of cow’s milk (6.5–6.7) (El-Hatmi et al., 2015). Its fat content is very low, and has 96% triglycerides (Ereifej et al., 2011) and about 30 mg/100 g dry matter of cholesterol (Salwa & Lina, 2010). Its fat has less short chain fatty acids in comparison to cow’s milk (Ereifej et al., 2011). Furthermore, the fat globules’ average size is smaller compared to bovine, buffalo, and goat milk fat globules (Khalesi et al., 2017). Because camel’s milk is highly digestible (Meena et al., 2014); it may cause problems in the technological applications (Khalesi et al., 2017).
Camel’s milk is rich in vitamins including, B1, B2 and C (Ereifej et al., 2011). Vitamin C is three to five times higher than in cow’s milk, which makes it an important part of diet in arid areas where green foods have limited accessibility (Zhao et al., 2015, Kamal and Karoui, 2017).
It has been discovered that camel’s milk has antidiabetic, bactericidal activities, and hostile to hepatitis (Agrawal et al., 2009). To various degrees, it resists the contamination with microorganisms due to its characteristic inhibitory frameworks such as the lactoperoxidase/ thiocyanate/ hydrogen peroxide framework, lactoferrins, lysozyme, immunoglobulins and free greasy acids (EL-Fakharany et al., 2012).
3. Camel’s milk in comparison to Cow’s milk
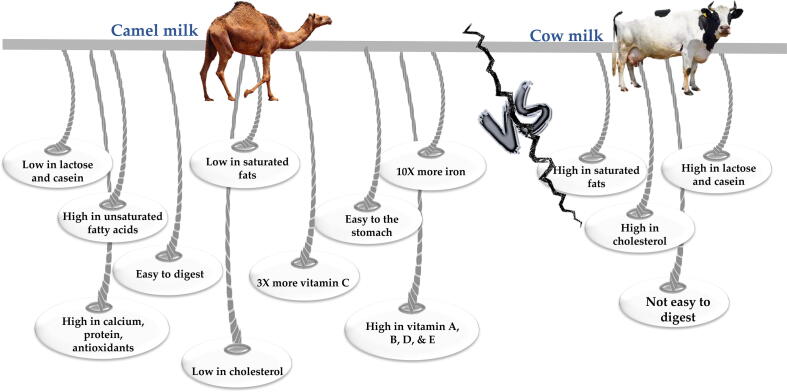
There are some important differences between camel’s and cow’s milk (Fig. 1). For instance, camel’s milk lacks β-lactoglobulin (β-LG), a major protein in cow’s milk that can trigger allergic reactions. Moreover, whey protein in camel’s milk has higher contents of antimicrobial agents, like lactoferrin, lysozyme, immunoglobulin, and lactoperoxidase than cow’s whey (Ahamad et al., 2017).
Fig. 1.
The important differences between camel’s and cow’s milk.
These differences in camel’s milk proteins might reveal variable biological activities upon hydrolysis such as mineral-binding properties and immunoglobulins (Farah and Atkins, 1992, Merin et al., 2001). Camel’s milk is whitish in color with a slightly salty aftertaste and its density is a bit lower than cow’s milk, with average value 1.029 g/cm3. The pH varies from 6.4 to 6.7. The water content changes from 87 to 90%, and the freezing point is ranging between −0.57 and −0.61 °C (Devendra et al., 2016).
The color of cow’s milk is opaque white with yellowish hue due to the presence of carotene and depends on the breed, type of feed and fat content (NPCS Board, 2012). The content of water in cow’s milk varies from 79 to 90% (Chandan & Kilara, 2010). The pH of cow’s milk varies from 6.4 to 6.6. Its density is about 1.030 g/cm3 and the freezing point is about −0.54 °C. Cow’s milk contains an average of 3.6% fat, 3.0% protein, and 4.6% lactose (Chandan & Kilara, 2010).
The composition of camel’s milk is more variable than cow’s milk. Region and season significantly affect the ratio of compounds in camel’s milk (El-Hatmi et al., 2015). Lactose content is stable in camel’s milk, and varies between 3.5 and 4.5% (Devendra et al., 2016). It is the main carbohydrate in camel’s milk. In addition, it contains a small number of different oligosaccharides which protect infants against pathogens, promote the formation of Bifidobacterium environment and help in developing the nervous system (Park & Haenlein, 2013).
Total protein of camel’s milk varies from 2.15 to 4.90%. Camel’s and cow’s milk have similar content of casein (αs1, αs2, β, and κ-casein), but they differ in the content of whey proteins. Thus, casein to whey proteins ratio in cow’s milk is higher than that of camel’s milk. This affects the firmness of coagulum, and camel’s milk forms softer gel than cow’s milk (Park & Haenlein, 2013).
Casein is the main protein in camel’s milk, and it represents about 52–87% of total proteins, while whey proteins contribute 20–25% (Devendra et al., 2016). Casein in camel’s milk has four fractions and accounts; the ratio of αs1 to αs2 to β to κ-casein significantly varies in camel’s milk, being 22:9.5:65:3.5 (Park & Haenlein, 2013). Camel’s milk has more β-casein than α-casein, 65 and 21% of total casein, respectively (Devendra et al., 2016).
Cow’s milk compared to camel’s milk contains approximately the same β-casein and α-casein percentages (36 and 38%, respectively) and higher content of κ-casein (13%), which is about four times lower in camel’s milk (3.47%) (Devendra et al., 2016). The β-casein is more digestible and less allergic for people, as it is more susceptible to peptic hydrolysis in the gut. The higher β-casein percentage makes camel’s milk beneficial for human health (Devendra et al., 2016).
Caseins micelles in camel’s milk have a wide range of sizes from 20 to 300 nm in diameter against 40–160 nm in cow’s milk (Park & Haenlein, 2013). Overall, the average diameter of casein micelles in camel’s milk is larger and its mineral charge is higher (Attia et al., 2001). The α-lactalbumin is the main whey protein of camel’s milk. It is more digestible and has higher antioxidant activity than α-lactalbumin from cow’s milk, which encourages using camel’s milk in the infant foods (Park & Haenlein, 2013).
The β-lactoglobulin is deficient in camel’s milk, which makes it less allergic, but the other whey proteins such as lactoferrin and immunoglobulins are present (Devendra et al., 2016). Lactoferrin is a glycoprotein that binds two ferric ions. Its content ranges from 0.02 to 2.1 g/L in camel’s milk (Park & Haenlein, 2013). It has antimicrobial, anti-inflammatory, immunomodulatory, and antitumor activities (Park & Haenlein, 2013).
Lysozyme, another milk antimicrobial agent, presents in camel’s milk with a concentration around 150 μg/L that is higher than in cow’s milk (70 μg/L). Immunoglobulins (IgG) are the whey proteins that play the major role in neonates’ passive immunity. The dominant immunoglobulin in camel’s milk is IgG. It is being secreted at a concentration around 100 g/L in the colostrum but rapidly decreases during lactation to less than 10 g/L as reported by Park & Haenlein (2013).
It is worth noting that the differences in protein profile can affect the composition of fermented camel and cow’s milk (Izadi et al., 2019). Fermented camel’s milk has more antioxidant peptides, probably, due to the structure of β-casein. So, β-casein in camel’s milk is shorter and contains more proline. Its hydrolysis results in the formation of bioactive peptides and release of amino acids such as phenylalanine and tryptophan with antioxidant properties (Izadi et al., 2019).
The fat content varies from 1.2 to 4.5% in camel’s milk (Devendra et al., 2016). However, Park & Haenlein (2013) reported that the content of fat in camel’s milk may reach up to 6.4%, and its profile is characterized with the presence of unsaturated and long chain fatty acids at higher amounts. This helps in lowering the level of lipids in human serum. The content of long-chain fatty acids is 92–99%, and the percentage of unsaturated acids is 35–50% (Izadi et al., 2019). These structural differences impart “waxy texture” to the camel’s milk fat. The lower content of carotene makes the color of camel’s milk whiter compared to cow’s milk (Devendra et al., 2016).
Mineral content of camel’s milk is similar to cow’s milk, especially; in Ca, P, Mg, Na, and K content (Kaskous, 2016). The main distinction is in the content of Zn, Cu, Fe, and Mn, as camel’s milk has higher concentrations of these minerals. Increased iron concentration in camel’s milk may be useful for the prevention of iron-deficiency anemia. In addition, lower concentration of citrate in camel’s milk than in cow’s milk increases lactoferrin antimicrobial activity, because it needs small levels of citrate to be beneficial (Park & Haenlein, 2013). The total mineral content of camel’s milk fluctuates from 0.60 to 0.90%. The salty taste of camel’s milk can be explained by the enhanced content of chloride obtained from the feed eaten by animals (Devendra et al., 2016).
In addition, the content of ascorbic acid is higher in camel’s milk. Therefore, it can extend the shelf-life of its products and increase its antioxidant and antiradical abilities (Izadi et al., 2019). The concentrations of mineral salts and vitamins in camel’s milk depend on breed, feed, water intake, and stage of lactation. Besides, camel’s milk contains higher concentration of vitamin C and niacin compared to cow’s milk. But it is deficient in B1, B2 and A vitamins, pantothenic acid and folic acid. Both camel’s and cow’s milk have almost the same content of vitamins B6 and B12 (Devendra et al., 2016).
On the other hand, camel’s milk has better heat stability than cow’s milk. The increase of camel’s milk temperature to 80 °C causes a break-down of 32–35% whey proteins, while the increase to 90 °C results in denaturation of 47–53% of its whey proteins (Izadi et al., 2019). The heat treatment of cow’s milk at 80 °C results in denaturation of 70% of whey proteins, while at 90 °C causes denaturation of 81% (Farah, 1986).
Camel’s milk has more inhibitory structures than cow’s milk, specifically, lysozyme and lactoferrins that are much higher than those of cow’s milk. Therefore, it could be stored at room temperature for a longer period (Korhonen and Pihlanto, 2001). Moreover, it contains peptides and proteins that have valuable impact on numerous bioprocesses like assimilation, ingestion, development and immunity (Yagil, 1987). A heterogeneous collection of proteins is included in camel’s whey, such as immunoglobulin, serum egg whites, α-lactalbumin, lactophorin and peptidoglycan (Omar & Eltinay, 2008).
4. Health benefits of Camel’s milk
For mammal’s newborns, milk is the most important source of nutrients. Milk has biologically active substances and compounds that are needed for the immunological protection and healthy growth. Camel’s milk has many beneficial nutritional and therapeutic characteristics, antibacterial, anticarcinogenic, antioxidant, anti-hypertensive, and anti-diabetic properties (Ayoub et al., 2018).
Peptides from dietary proteins have been extensively studied to investigate the health effects they may show in humans like antioxidant activity, mineral binding, reduction in blood pressure, immunomodulatory function and protective effects against various bacteria and viruses (Salami et al., 2010). Such peptides from milk proteins are widely acknowledged (Kitts & Weiler, 2003). Indigenous protease enzymes such as milk plasmin can hydrolyze proteins and lead to the release of bioactive peptide fragments during storage or processing (Mohanty et al., 2016). The bioactive peptides can also be obtained via enzymatic hydrolysis with microbial and digestive enzymes (Korhonen & Pihlanto, 2003). The activity of these peptides is based on their amino acid composition and sequence (Meisel & Fitzgerald, 2003).
The health-related bioactive properties of camel’s milk protein hydrolysates have recently been reported (Salami et al., 2010, Salami et al., 2011, Kumar et al., 2016a, Kumar et al., 2016b, Nongonierma et al., 2018, Nongonierma et al., 2017). These hydrolysates were obtained via enzymatic hydrolysis of milk proteins, which are susceptible to proteolysis (Salami et al., 2011). The enzymatic hydrolysis is known to improve the functional properties of milk proteins, in addition to the enhancement of the bioactive properties (Jrad et al., 2014b) However, camel’s milk proteins in their intact form have bioactive properties like anti-hypertensive, hypo-allergic, anti-cancer and anti-diabetic (Konuspayeva et al., 2009). Most of these properties have been demonstrated in the in vivo (human or rat model) for intact camel’s milk proteins. Recent research is being focused on generating bioactive hydrolysates from camel’s milk proteins and exploring their potential bioactive properties under in vitro and in vivo conditions (Mudgil et al., 2018).
Traditionally, camel’s milk has been used for the treatment of diseases like tuberculosis, asthma, dropsy, and jaundice owing to its content of natural bioactive components (Abdelgadir et al., 1998). Also, camel’s milk has better digestibility and nutritional value than cow’s milk, making it one of the alternative sources for human consumption (Salami et al., 2009). These bioactive components can be produced from milk proteins by the probiotic bacteria during fermentation process (Devendra et al., 2016). Camel’s milk can also be used in curing gastrointestinal disorders. It has a good effect on the stomach and the intestinal diseases due to its high level of anti-inflammatory proteins, polyunsaturated fatty acids and vitamins which increase carbohydrate metabolism (Kaskous, 2016).
Camel’s milk has antibacterial and antiviral properties due to the presence of lactoferrin, lysozyme, lactoperoxidase, hydrogen peroxide, and immunoglobulins. These compounds can suppress both Gram-positive and negative bacteria, e.g. Staphylococcus aureus, Listeria monocytogenes and Escherichia coli. The content of the antibacterial components in camel’s milk is higher than cow’s milk. However, the exposure of milk to 100 °C for 30 min completely inactivates their beneficial properties. Moreover, whey proteins of camel’s milk enhance the anti-rotaviruses functions to treat non-bacterial gastroenteritis (Devendra et al., 2016).
Lactoferrin and IgG of camel’s milk can inhibit the hepatitis C and B viruses and prevent their replication in cells. The IgG can recognize hepatitis C virus peptides in concentrations when human IgG does not detect the presence of virus. Moreover, camel’s milk can heal hepatitis B, as it increases the immune response and stops the DNA replication of the virus (Kaskous, 2016). The abundance of antimicrobial components in camel’s milk gives it a therapeutic effect against drug resistant tuberculosis. Thus, camel’s milk may relief symptoms such as cough, breathlessness and fever (Devendra et al., 2016).
Many food proteins contain angiotensin-I-converting enzyme (ACE)-inhibitory peptides in their primary structure, including milk proteins. These peptides are also present in the fermented camel’s milk. Probiotic bacteria, used in fermentation, break-down proteins into peptides and amino acids (Devendra et al., 2016).
The bioactive peptides in the fermented camel’s milk may have a positive effect on lowering the level of cholesterol (Devendra et al., 2016). Camel’s milk also has orotic acid, which is known to decrease cholesterol level in humans (Devendra et al., 2016). Raw camel’s milk and fermented dairy products are a source of probiotic strains. Species of Lactobacillus, Bifidobacterium, Enterococcus, and Streptococcus were isolated from camel’s milk and were used in the dairy industry (Shori & Baba, 2014).
Camel’s milk can be used for the treatment of diabetes type 1 and type 2, due to the presence of insulin and insulin-like substances as well as immunoglobulins in a small size (Devendra et al., 2016). The level of insulin in camel’s milk is high and comprises about 52 units/liter (Ayoub et al., 2018). Also, these components influence the pancreas and liver, leading to improvement of insulin secretion, so the required dose of insulin is reduced (Kaskous, 2016). Along with the application for the diabetes treatment, camel’s milk reduces blood sugar, decreases insulin resistance, and improves lipid profiles (Ayoub et al., 2018).
Another possible health benefit of camel’s milk is decreasing allergenicity, especially among children who are allergic to cow’s milk. Such allergy is caused due to the high content of α-casein and low content of hypoallergenic β-casein along with the presence of β- lactoglobulin. Especially, allergy to cow’s milk is of a big concern among infants, as in the most severe cases, cow’s milk consumption can cause anaphylaxis. Moreover, immunoglobulins of camel’s milk are similar to human’s milk, which make it safe for children to consume (Devendra et al., 2016, Izadi et al., 2019). In addition, individuals with lactose intolerance can safely consume camel’s milk. Camel’s milk has higher L-lactate content compared to cow’s milk which is rich in D-lactate. The L-lactate decreases milk allergenicity. The IgE of children allergic to cow’s milk does not react with camel’s milk; therefore, camel’s milk immunoglobulins decreases allergic symptoms (Kaskous, 2016).
Furthermore, camel’s milk has a potential positive effect on people with autism. In the intestines of patients with this autoimmune disease, the break-down of milk’s casein results in the formation of casomorphin, this is a strong opioid responsible for the brain damage. High level of β-casein content and β-lactoglobulin in cow’s milk make it more likely to form opioids (Devendra et al., 2016).
Moreover, camel’s milk has protective proteins (lactoferrin, lysozyme, and immunoglobulins) that may improve the development of brain (Devendra et al., 2016). Treating blood, lung, liver, and breast cancer is another camel’s milk benefit. It inhibits the proliferation of HepG2 and MCF7 cells as well as the stimulation of death receptors in cell lines and mechanisms caused by oxidative stress (Kaskous, 2016).
Camel’s milk improves the gut microbiota as its consumption helps to develop a higher abundance of Allobaculum, Akkermansia, and Bifidobacterium. The study by Wang et al., (2018) indicates that camel’s milk could enhance the abundance of Allobaculum, which may positively influence the physiological function of the organism. This genus produces short-chain fatty acids that improve colon health, prevent obesity, and decrease inflammations. Akkermansia, a mucin-degrading probiotic, is well-known for its beneficial effects on obesity, metabolic disorders, diabetes, and inflammation (Wang et al., 2018).
5. Protein composition in Camel’s milk
Protein content in camel’s milk varies due to many factors like breed and season. According to the study of Haddadin et al. (2008), it reaches 2.9%, the highest content, in December and 2.48%, the lowest content, in August. Camel’s milk whey protein is not only composed of numerous soluble proteins, but also indigenous proteases such as chymotrypsin A and cathepsin D (Alhaider et al., 2013). Thus, camel’s milk proteins may be bioactive by themselves or serve as precursors for bioactive peptides.
5.1. Casein proteins
Casein is the major protein in camel’s milk that constitutes 52–87% of total protein content (Khaskheli et al., 2005). It is composed of three main components: αs1-casein, αs2-casein, and β-casein, and a fourth minor fraction κ-casein. The major casein in camel’s milk is β-casein (65% of total casein), it is higher than that of cow’s milk (36%). β-casein is more easily hydrolyzed than αs-casein (El-Agamy et al., 2009). On the other hand, camel’s milk has lower αs1-casein (21%), in comparison to bovine αs1-CN (38%) (Khaskheli et al., 2005).
It has been reported that camel’s milk caseins have higher molecular masses in contrast to bovine caseins, as β-casein and α-casein which were found to be 28.6 kDa and 35 kDa, respectively. Whilst, in bovine it is 24 kDa for β-casein and 22–25 kDa for α-casein (Farah, 1986; Al Haj et al., 2018, El-Agamy, 2007, El-Hatmi et al., 2007). The κ-casein in camel’s milk is only 3.47% of total casein, whereas cow’s milk has 13% of κ–casein. Studies have shown that κ-casein is relatively harder to be detected because of its low concentrations in the camel’s milk (Farah & Atkins, 1992). The hydrolysis sites for κ-casein in both camel and cow’s milk differ; chymosin hydrolyzes κ-casein at the Phe97-Ile98 bond in camel’s milk, while bovine κ–casein is hydrolyzed at the Phe105-Met106 bond (Kappeler et al., 1998). Furthermore, Kappeler et al. (1998) observed an additional proline residue in κ-casein, which plays a critical role in the stability of camel’s milk. The capillary electrophoresis was used to determine the concentration of each casein component, except αS2-casein, which consisted of approximately 12.8 mg/mL of β-casein, 2.9 mg/mL of αS1-casein, and 1.7 mg/mL of κ-casein (Omar et al., 2016).
5.2. Whey proteins
Whey proteins represent about 30% of the total proteins in camel’s milk (Zhao et al., 2015). The α-lactalbumin (α-LA), lactophorin, immunoglobulins (Ig), lactoferrin (Lf), and serum albumin are fundamentally constructed (Merin et al., 2001, El-Hatmi et al., 2006).
The IgG is the main immunoglobulin in camel’s milk and the molecular weights of camel IgG differ to those of bovine, sheep, goat and human (Alavi et al., 2018). The α-LA and β – lactoglobulin (β-LG) are the major difference between camel’s and bovine’s whey. Camel’s whey lacks β–LG which is the main component in bovine whey (50%) (El-Agamy et al., 2009). The β–LG induces heat stability; hence the stability of camel’s milk is poor at temperatures up to 140 °C in contrast to cow’s milk (Farah and Atkins, 1992, Al-Saleh, 1996). The α-LA is the major component of camel’s whey, while bovine whey has only 25% of this protein (Farah, 1986, Farah and Atkins, 1992, Merin et al., 2001, Laleye et al., 2008). The molecular mass of α-LA in camel’s milk is 14.6 kDa with 123 residues, similar to bovine, goat and human milk (Beg et al., 1986). However, the amino acid sequence of camel’s milk α-LA largely differs from that of bovine, goat, and other species (Al Haj and Al Kanhal, 2010). Both camel and human milk are known to contain high contents of α-LA and lactoferrin (Lf) (Hinz et al., 2012).
Shortage of β-lactoglobulin in cow’s milk proteins makes camel’s milk a good substitute, with a potential to be used in infant formula. Whey proteins such as IgGs, Lf, lactoperoxidase, lysozyme, and other enzymes are potent antimicrobial components in camel’s milk (El-Agamy et al., 1992, Konuspayeva et al., 2005). The antimicrobial activities of camel’s milk are due to the high content of protective proteins in the whey fraction that are known to be more thermostable (El-Agamy et al., 2009). Therefore, camel’s whey proteins present a novel source of proteins which can generate bioactive peptides with potential health benefits.
6. Functional properties of Camel’s milk proteins
Whey proteins have unique characteristics (Parodi, 2007). Apart from their significance in nutrition, they have physical, chemical, physiological, functional, and technological features which could be useful in food applications (McIntosh et al., 1998).
Whey proteins have nutritional importance as they supply energy and essential amino acids and functional importance as they help in texture, structure modification and improvement of the overall appearance of food e.g., foam stability, gel formation and water retention (Panyam & Kilara, 1996). A study by Al Shamsi et al. (2018) produced camel’s milk protein hydrolysates using proteolytic enzymes alcalase, bromelain and papain. The techno-functional properties like emulsifying activity index, surface hydrophobicity, and protein solubility were investigated. It was found that these properties were higher in the protein hydrolysates compared to unhydrolyzed camel’s milk proteins. Also, their antioxidant potential was assessed in real food and in vitro systems (Al Shamsi et al., 2018). Thus, the potential application of camel’s milk protein hydrolysates as a functional food ingredient due to their enhanced techno-functional properties could be a novel approach.
7. Milk protein hydrolysates, bioactive peptides and their production
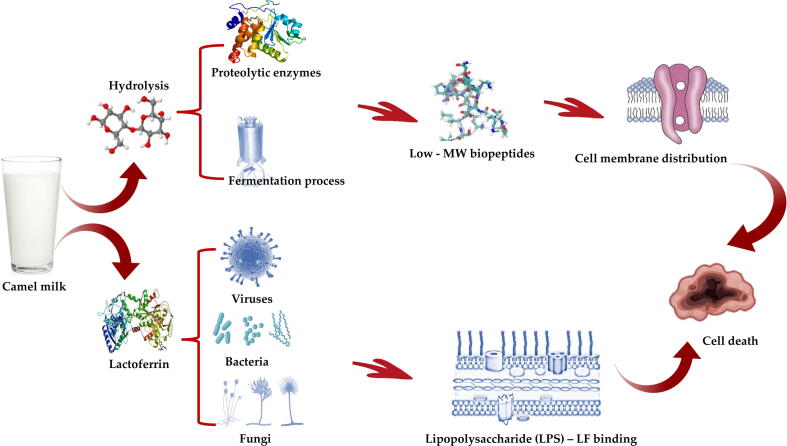
Milk proteins are great source of essential amino acids. In addition, research studies documented other functionalities and bioactive properties of milk protein’s biologically active peptides (Fig. 2). These peptides remain in an inactive state within the parent protein molecule and demonstrate bioactive potential only when released from the native protein (Khalesi et al., 2017).
Fig. 2.
Hydrolysis of camel milk proteins and its antimicrobial mechanism.
Bioactive peptides are defined as peptides that consist of specific protein fragments showing biological activity and may be beneficial in promoting health (Kitts & Weiler, 2003). There are various sources to get these peptides by hydrolysis (or breakdown) of proteins, but at present, milk-derived peptides are the most important source (Korhonen, 2009).
Many studies have reviewed the production and properties of peptides from milk proteins (Clare and Swaisgood, 2000, Korhonen and Pihlanto, 2003, Meisel, 2005, Silva and Malcata, 2005, Korhonen and Pihlanto, 2006). Several bioactive peptides have good health effects on digestive, immune, cardiovascular and nervous systems (Korhonen and Pihlanto, 2006, Hernández-Ledesma et al., 2011).
Three different ways can be used to produce biologically active peptides from camel’s milk, the first one is enzymatic hydrolysis (using digestive or microorganisms and plant derived enzymes), the second is fermentation (using proteolytic starter cultures), and the third is heating under alkali/acid conditions (Pihlanto-Leppälä, 2000, Muro Urista et al., 2011). Sometimes, the combination of the above methods also helps in obtaining highly potent peptide fractions with diverse bioactive properties (Korhonen, 2009).
7.1. In vitro enzymatic hydrolysis
The controlled enzymatic hydrolysis is critical in producing hydrolysates for three reasons: (i) to preserve the generated products properties, peptides and amino acids (Tavano, 2013), (ii) to avoid excessive hydrolysis of protein which can (a) result in bitter flavored peptides (Jung et al., 2005) and (b) hinder functionality, and (iii) to maintain the improved solubility in the protein as a result of the hydrolysis process (Tavano, 2013). Thus, by controlling the degree of hydrolysis, it will be possible to explain the potential bioactive and/or functional properties of the protein hydrolysates. In this process, whole protein molecule undergoes hydrolysis (or breakdown) by enzymes like pepsin, trypsin, and chymotrypsin. These enzymes can mimic gastrointestinal digestion effects on the food peptides (Tavano, 2013).
Other proteolytic enzymes such as alcalase, subtilisin, and thermolysin are used in conjunction with pepsin and trypsin to generate peptides with known biological activities (Agyei & Danquah, 2011). Liberation of peptides with a wide range of actions can thus be achieved using different enzymes (Tavano, 2013).
The bioactivity of peptides was extensively reported in literature, although the mechanism of actions is not well-understood. Few studies hypothesized it as a structure–activity relationship, while others suggested that the enzyme can be chosen to get the desired fragment and effect (Tavano, 2013). The milk protein hydrolysates can be produced from casein or whey proteins (Meisel and FitzGerald, 2003, Yamamoto et al., 2003). Casein hydrolysates have been reported to produce good angiotensin-I-converting enzyme (ACE)-inhibitory peptides (Otte et al., 2007), while whey peptides with sequence Ile-Leu-Pro Met-His-Ile-Arg from β-LG have also been identified to have strong antihypertensive activity (Maes et al., 2004).
7.2. Bioactive properties of whey protein hydrolysates
Whey is a good source of nutrients and important peptides (Kishawy et al., 2018, Ashour et al., 2019). To concentrate whey proteins and obtain nutritional value, several technologies like heating, drying, reverse osmosis, and membrane separation have been applied. Another approach is hydrolysis of whey to produce hydrolysates containing bioactive peptides. These peptides can be released by enzymatic hydrolysis in vitro as discussed previously. Thereafter, bioactive peptides from whey protein can act on major organ systems of the body and impart physiological functions to these systems (Sharma, 2014). Milk protein hydrolysates are known to possess various bioactive properties as following:
7.2.1. Antioxidant activity
Food oxidation is a major concern in food industry (Abd El-Hack et al., 2020a, Ashour et al., 2020). Lipid oxidation not only deteriorates the food quality, but also shortens the shelf life and generates free radicals that can in turn cause decomposition of fatty acids, which may decrease the safety and nutritional value of the food. Thus, it is critical to hinder lipid oxidation process and formation of free radicals (Peng et al., 2009).
Synthetic antioxidants have been incorporated into food products and prove to be cost-effective and efficient, but their potential toxic effects to human health have led manufacturers to seek natural antioxidants (Ito et al., 1985). Enzymatic hydrolysis of milk proteins and whey proteins, particularly those from bovine has been widely explored for generation of bioactive peptides with potential antioxidant properties. For example, antioxidant peptides from bovine αs-casein showed properties like free-radical scavenging and inhibition of enzymatic and non-enzymatic lipid peroxidation (Rival et al., 2001). In addition, hydrolysis of bovine whey proteins have resulted in peptides that may have antioxidant activity (Zhang et al., 2016).
The principal mechanism behind antioxidant activity of peptides is not very well understood, but studies have shown that they are free radical scavengers metal ions chelators and inhibitors of lipid peroxidation (Wu et al., 2003, Rajapakse et al., 2005, Moure et al., 2006, Qian et al., 2008). The peptides antioxidant effect are influenced by their structure, composition, hydrophobicity, and peptide sequence (Rajapakse et al., 2005). Other factors affecting antioxidant activity of bioactive peptides include the type of protease, peptide structure, degree of hydrolysis and peptide concentration (Peñta-Ramos and Xiong, 2002, Saito et al., 2003, Gibbs et al., 2004, Chen et al., 2007).
Several studies explored camel’s milk as a potential protein substrate for generating bioactive protein hydrolysates with antioxidant activities (Al-Saleh et al., 2014, Shori and Baba, 2014). Studies of Salami et al., 2011, Jrad et al., 2014a reported elevated antioxidant activity of camel’s milk casein hydrolysates when digested with gastrointestinal enzymes. The assays determining the antioxidant capacity can be categorized into two groups, based on the chemical reactions, the first group includes the methods based on electron transfer (ET), like ferric ion reducing antioxidant power (FRAP) and 2,2-diphenyl-1- picrylhydrazyl (DPPH) radical-scavenging assay (Jrad et al., 2014b). The second group includes the methods based on hydrogen atom transfer (HAT) like oxygen radical absorbance capacity (ORAC) and total radical trapping antioxidant parameter (TRAP) assay (Sarmadi & Ismail, 2010).
7.2.2. Antimicrobial activity
Contamination of food products by pathogenic and spoilage bacteria is of significant concern in the food industry (Abd El-Hack et al., 2020b; Abd El-Hack et al., 2021). To enhance the safety of food products and its shelf life several methods have been employed, which include use of natural antimicrobial and synthetic agents. Due to the detrimental impact of synthetic agents on the human health and the environment, manufacturers are incorporating more natural sources of antimicrobial agents in the food, but there is a need for novel antimicrobial agents (Brandelli et al., 2015, El-Saadony et al., 2019, Abdelnour et al., 2020a, Abdelnour et al., 2020b, Akl et al., 2020, El-Saadony et al., 2020, Reda et al., 2020, Sheiha et al., 2020).
Past literature showed a few strategies to improve the antimicrobial activities of proteins such as enzymatic hydrolysis (Korhonen and Pihlanto, 2006, Théolier et al., 2013). In addition, Hayes et al. (2006) studied the hydrolysis of casein proteins during fermentation with proteolytic bacterial strains to produce antimicrobial peptides. Furthermore, antimicrobial peptides have also been extracted from different cheese varieties (Rizzello et al., 2005, Lignitto et al., 2012). Whey proteins like lactoferrin, lysozyme, lactoperoxidase and immunoglobulins are the most studied till date (Chatterton et al., 2006).
Other whey proteins such as α-LA and β-LG also have antibacterial effects (Atanasova & Ivanova, 2010). The antimicrobial potential of whey protein hydrolysates and peptides were in vitro assessed through the identification of the peptide sequence which is then synthesized and tested against strains of bacteria to affirm the antimicrobial activity (Brandelli et al., 2015).
The antimicrobial efficacy of the bioactive peptides was influenced by several factors such as structural diversity, the load, specific amino acid composition, and hydrophobicity (Gennaro and Zanetti, 2000, Kustanovich et al., 2002). Meanwhile, the hydrolysates of goat whey were produced using alcalase from Bacillus licheniformis (Osman et al., 2016) and fractionated by size exclusion chromatography (SEC). The hydrolysates showed enhanced antibacterial activity when compared to unhydrolyzed goat whey. Another study on goat whey proteins used pepsin enzyme which produced peptides with considerable antibacterial activity (El-Zahar et al., 2004).
Théolier et al. (2013) assessed whey protein hydrolyzed by gastrointestinal enzymes. They concluded that trypsin and chymotrypsin hydrolysates did not show antimicrobial activity, while pepsin derived peptides exhibited considerable activity despite their weak degree of hydrolysis. Furthermore, goat whey proteins digested by gastric and duodenal juice were investigated and their inhibition against pathogenic bacteria was reported (Almaas et al., 2008).
Limited studies have been conducted on camel’s milk proteins and their hydrolysates for their antimicrobial activities. Camel caseins were enzymatically digested, and the digested sample were evaluated for its antibacterial activities against Gram-negative (Escherichia coli) and Gram-positive bacteria (Listeria monocytogenes, Bacillus cereus, and Staphylococcus aureus) (Kumar et al., 2016b). Casein hydrolysate produced by alcalase showed highest inhibitory activity (17.93 ± 0.82) against E. coli. Salami et al. (2010) reported that enzymatic hydrolysis of camel and bovine whey proteins improved the antimicrobial effects against E. coli. However, the degree of hydrolysis with chymotrypsin and trypsin was low, and thus the antimicrobial activity was not enhanced.
There is a need for in-depth exploration of antimicrobial properties of camel’s milk whey protein hydrolysates using different proteolytic enzymes and experimental conditions and test them against a wide range of pathogenic microorganisms in vitro as well as in vivo.
7.2.3. Angiotensin-I-converting enzyme (ACE)-inhibitory activity
Among the bioactive properties demonstrated by the milk bioactive peptides, ACE inhibitory activities were widely reported (Phelan & Kerins, 2011). A dipeptidyl carboxypeptidase, ACE catalyzes the conversion of inactive angiotensin I peptide into angiotensin II peptide which is a potent vasoconstrictor. Angiotensin II is responsible for increasing the salt levels which raises the blood pressure. The ACE inhibitor drugs are commonly prescribed in individuals suffering from hypertension or related cardiovascular disorders (Acharya et al., 2003).
Peptides that inhibit ACE have been reviewed and reported after enzymatic hydrolysis of milk proteins and after fermentation of milk with Lactobacillus sp. (Hernández-Ledesma et al., 2014). The most studied ACE-inhibitory peptides are valine-proline-proline [VPP; β-casein f (84e86)] and isoleucine-proline-proline [IPP; β-casein f (74e76)] which have been derived from bovine caseins following fermentation with Lactobacillus sp. (Solieri et al., 2015). Moreover, a bovine whey protein concentrate hydrolyzed with alcalase showed potent antihypertensive effect (da Costa et al., 2007).
Al Haj and Al Kanhal (2010) used two types of strains (Lactobacillus helveticus or Lactobacillus acidophilus with Streptococcus thermophilus) to ferment camel’s milk and determined the ACE activity of fermented and unfermented camel’s milk. Likely, Pihlanto et al. (2010) investigated the fermented camel’s milk inhibitory activity towards ACE. They used lactic acid bacteria (LAB) strains and found seven fermented camel’s milk samples that showed highest ACE inhibitory activity, along with a correlation between degree of hydrolysis and ACE inhibition. The ACE-inhibitory peptides can be identified using reversed-phase HPLC (RPHPLC) or be quantified using triple mass spectrometry (HPLCMS3) (Bütikofer et al., 2007).
7.2.4. Antidiabetic activity
Diabetes mellitus affects millions of people around the globe. It is a chronic condition in which the body is either unable to produce enough insulin, can not use the produced insulin or a combination of both. Clinical studies and murine models have demonstrated that consumption of camel’s milk by type 1 diabetes patients lowered the blood glucose level (Agrawal et al., 2005; Agrawal et al., 2007a). Although the mechanism is not fully understood, camel’s milk appeared to have an insulin-like protein that resists intestinal digestions, absorbs faster into blood, possesses larger lipid micelles and has different casein content. Agrawal et al., 2007a, Agrawal et al., 2007b showed that the weak coagulation of camel’s milk in the human stomach and the influence of small size immunoglobulins of camel’s milk on β-cells have also added to the possible hypoglycemic effect. A recent in vitro study reported a potentiating effect of camel’s milk proteins on insulin receptor activity expressed in HEK293 cells, which may be a possible mechanism of action (Abdulrahman et al., 2016).
The study of Jakubowicz and Froy (2013) suggested that whey proteins and their hydrolysates may stimulate the secretion of gut hormones in vivo by releasing bioactive peptides and amino acids, thereby helping mediate glycaemia. Furthermore, these peptides can act as dipeptidyl peptidase-4 (DPP-IV) inhibitors in vivo. Since, DPP-IV inhibition has helped in managing type-2 diabetes, several DPP-IV inhibitory peptides have been isolated and identified from bovine casein (Lacroix and Li-Chan, 2014b, Lacroix and Li-Chan, 2013, Lacroix and Li-Chan, 2012, Lacroix and Li-Chan, 2014a, Uenishi et al., 2012, Nongonierma and FitzGerald, 2013a, Nongonierma and FitzGerald, 2013b, Silveira et al., 2013) and caprine casein (Zhang et al., 2016).
Brandelli et al. (2015) concluded that DPP-IV inhibitory peptides usually have molecular masses below 2 kDa and most of them have hydrophobic amino acid residues (Lacroix & Li-Chan, 2012a). Much work has been done on diabetic rats treated with raw or fresh camel’s milk. There was significant reduction in plasma glucose levels (Agrawal et al., 2005a, Agrawal et al., 2005b, Kamal et al., 2007, Al-Numair and Alsaif, 2011). Camel’s milk whey protein caused a significant decrease in blood glucose levels from 411 ± 37 mg/dL to 261 ± 25.5 mg/dL in streptozotocin (STZ)-induced diabetic mice (Badr, 2013). The same study also reported that the treated group with camel’s milk whey protein showed higher levels of insulin compared to untreated diabetic mice.
Mahmoud et al. (2016) found that camel whey protein has protective effects on STZ-induced diabetic pregnant mice’s off-springs. It was found that when camel’s whey protein was orally administered as a supplement to diabetic pregnant mice, several postpartum complications were occurred to the offspring; like increased levels of pro-inflammatory cytokines, reactive oxygen, overexpression of activating transcription factors, and other immune-related functions were significantly reduced. Intact proteins from camel’s milk and camel whey have been reported to possess anti-diabetic property. However, there is no conducted study on bioactive peptides produced from camel whey proteins possessing anti-diabetic properties. The study of Nongonierma et al. (2017) identified novel DPP-IV inhibitory peptides (Leu-Pro-Val-Pro-Gln and Trp-Lys) from camel’s milk protein hydrolysates, which were not found in cow’s milk protein hydrolysate. An in-silico approach was undertaken where camel’s milk protein was hydrolyzed with trypsin to obtain potent DPP-IV inhibitory peptides (Nongonierma et al., 2017). Another study identified nine novel DPP-IV inhibitory peptides from trypsin digested camel’s milk proteins, of which two LPVP and MPVQA had IC50 values <100 μM (Nongonierma et al., 2018). These studies on camel’s milk protein hydrolysates provide a strong indication that the camel’s whey protein fraction upon hydrolysis might possess effective antidiabetic activities (Abd El-Hack et al., 2020b).
7.2.5. Anticholesterol activity
Studies on the dietary proteins derived from soybean and fish have been proposed to improve blood lipid profile in humans and animal experiments (Potter, 1995, Hori et al., 2001). Milk-derived bioactive peptides from whey were reported to have similar effects. For instance, Nagaoka et al. (2001) identified a novel hypocholesterolemic peptide from tryptic digestion of β-LG and tested it in Caco-2 cells and animal studies. Liver and serum cholesterol levels were markedly lower in rats administered with the tryptic hydrolysate. The inhibition of cholesterol micellar solubility was attributed to be the reason for declining cholesterol absorption. In another study, it was found that the water-soluble lactostatin was able to increase cholesterol mechanism by activating the transcription of cholesterol 7a-hydroxylase (CYP7A1) gene (Morikawa et al., 2007).
Previous studies reported that bovine casein can elevate blood cholesterol level due to its high lysine-arginine and methionine-glycine ratios, but the mechanism is not understood yet (Jacobucci et al., 2001). By comparing casein with other proteins like soy, fish and whey, it was suggested that these proteins could alter the plasma profile by decreasing atherogenesis and having a cardio-protective effect (Erdmann et al., 2008).
A study on the effects of fermented camel’s milk (gariss G) and gariss containing Bifidobacterium (G + Bb + 12) on plasma and liver cholesterol levels in rats was performed (Elayan et al., 2010). A decrease in plasma low-density lipoprotein (LDL) and plasma triglycerides was observed. Also, liver cholesterol levels were much lower in rats fed on (G) and (G + Bb + 12) diets as compared to rats fed the positive control (cholesterol-enrich) diets.
Few studies on fermented bovine and camel’s milk cholesterol-lowering activity have been reported. Damodharan et al. (2016) postulated that Lactobacillus helveticus strains (KII13 and KHI1. KII13) isolated from fermented cow’s milk displayed a greater cholesterol-lowering activity (47%) than KHI1 (28%) in vitro. The strain was then selected for in vivo study in atherogenic diet-fed hypercholesterolemic mice. Serum total cholesterol and LDL levels showed a decrease in mice fed with fermented cow’s milk (Damodharan et al., 2016).
In the same context, Abushelaibi et al. (2017) investigated selected some LAB strains from raw camel’s milk. They reported that among the isolated strains, Lactococcus lactis KX881768, Lactobacillus plantarum KX881772, Lactococcus lactis KX881782 and Lactobacillus plantarum KX881779 were found to be highly effective on cholesterol removing abilities.
8. Conclusion
Camel’s milk is rich in health-beneficial substances, for example, bioactive peptides, lactoferrin, zinc, and mono and polyunsaturated fatty acids. Whey proteins have nutritional importance as they supply energy and essential amino acids, and functional importance as they help in in texture, structure modification and improvement of the overall appearance of food. Camel’s milk whey is a good source of nutrients and important bioactive peptides. Antioxidant, antimicrobial, Angiotensin-Converting Enzyme (ACE) inhibitory, antidiabetic as well as anticholesterol activities are all representing the major value for these bioactive peptides.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors extend their gratitude to the Deputyship for Research & Innovation, “Ministry of Education” in Saudi Arabia for funding this research work through the project number IFKSURP-221.
Author contribution
All authors had equally shared in writing this review article.
Funding
Deputyship for Research & Innovation, “Ministry of Education” in Saudi Arabia funded this research through the project number IFKSURP-221.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Ayman A. Swelum, Email: aswelum@ksu.edu.sa.
Khaled A. El-Tarabily, Email: ktarabily@uaeu.ac.ae.
References
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Qattan S.Y., Batiha G.E., Khafaga A.F., Alagawany M. Probiotics in poultry feed: a comprehensive review. J. Anim. Physiol. Anim. Nutr. 2020;104:1835–1850. doi: 10.1111/jpn.13454. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Zabermawi N.M., Arif M., Batiha G.E., Al-Sagheer A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: a review. Int. J. Biol. Macromol. 2020;164:2726–2744. doi: 10.1016/j.ijbiomac.2020.08.153. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shehata A.M., Arif M., Paswan V.K., Batiha G.E.S., Elbestawy A.R. Approaches to prevent and control Campylobacter spp. colonization in broiler chickens: a review. Environ. Sci. Pollut. Res. 2021;28:4989–5004. doi: 10.1007/s11356-020-11747-3. [DOI] [PubMed] [Google Scholar]
- Abdelgadir W.S., Ahmed T.K., Dirar H.A. The traditional fermented milk products of the Sudan. Int. J. Food Microbiol. 1998;44:1–13. doi: 10.1016/s0168-1605(98)00090-7. [DOI] [PubMed] [Google Scholar]
- Abdelnour S.A., El-Saadony M.T., Saghirc S.A.M., Abd El-Hack M.E., Al-shargi O.Y.A., Al-Gabri N., Salama A. Mitigating negative impacts of heat stress in growing rabbits via dietary prodigiosin supplementation. Livest. Sci. 2020;240 [Google Scholar]
- Abdelnour S.A., Swelum A.A., Salama A., Al-Ghadie M.Q., Qattan S.Y.A., Abd El-Hack M.E., Khafaga A.F., Alhimaid A.R., Almutairi B.O., Ammari A.A., El-Saadony M.T. The beneficial impacts of dietary phycocyanin supplementation on growing rabbits under high ambient temperature. Italian J. Anim. Sci. 2020;19:1046–1056. [Google Scholar]
- Abdulrahman A.O., Ismael M.A., Al-Hosaini K., Rame C., Al-Senaidy A.M., Dupont J., Ayoub M.A. Differential effects of camel’s milk on insulin receptor signaling–toward understanding the insulin-like properties of camel’s milk. Front. Endocrinol. 2016;7(4):1–13. doi: 10.3389/fendo.2016.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abushelaibi A., Al-Mahadin S., El-Tarabily K., Shah N.P., Ayyash M. Characterization of potential probiotic lactic acid bacteria isolated from camel’s milk. LWT-Food Sci Technol. 2017;79:316–325. [Google Scholar]
- Acharya K.R., Sturrock E.D., Riordan J.F., Ehlers M.R. ACE revisited: a new target for structure-based drug design. Nat. Rev. Drug Discov. 2003;2:891–902. doi: 10.1038/nrd1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal R.P., Dogra R., Mohta N., Tiwari R., Singhal S., Sultania S. Beneficial effect of camel’s milk in diabetic nephropathy”. Acta Biomed. 2009;80:131–134. [PubMed] [Google Scholar]
- Agrawal R.P., Budania S., Sharma P., Gupta R., Kochar D.K. Zero prevalence of diabetes in camel’s milk consuming Raica community of northwest Rajasthan, India. Diabetes Res. Clin. Pract. 2007;76:290–296. doi: 10.1016/j.diabres.2006.09.036. [DOI] [PubMed] [Google Scholar]
- Agrawal R.P., Saran S., Sharma P., Gupta R., Kochar D.K., Sahani M.S. Effect of camel’s milk on residual b-cell function in recent onset type 1 diabetes. Diabetes Res. Clin. Pract. 2007;77:494–495. doi: 10.1016/j.diabres.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Agrawal R.P., Beniwal R., Sharma S., Kochar D.K., Tuteja F.C., Ghorui S.K., Sahani M.S. Effect of raw camel’s milk in type 1 diabetic patients: 1year randomised study. J. Camel Pract. Res. 2005;12:27–35. doi: 10.1016/j.diabres.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Agrawal R.P., Sahani M.S., Tuteja F.C., Ghouri S.K., Sena D.S., Gupta R., Kochar D.K. Hypoglycemic activity of camel’s milk in chemically pancreatectomized rats—an experimental study. Int. J. Diabetes Dev. Ctries. 2005;25:75–79. [Google Scholar]
- Agyei D., Danquah M.K. Industrial-scale manufacturing of pharmaceutical-grade bioactive peptides. Biotechnol. Adv. 2011;29:272–277. doi: 10.1016/j.biotechadv.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Ahamad S.R., Raish M., Ahmad A., Shakeel F. Potential health benefits and metabolomics of camel’s milk by GC-MS and ICP-MS. Biol. Trace Elem. Res. 2017;175:322–330. doi: 10.1007/s12011-016-0771-7. [DOI] [PubMed] [Google Scholar]
- Akl B.A., Nader M.H.M., El-Saadony M.T. Biosynthesis of silver nanoparticles by Serratia marcescens ssp sakuensis and its antibacterial application against some pathogenic bacteria. J. Agric. Chem. Biotech. 2020;11:1–8. [Google Scholar]
- Al Haj O.A., Al Kanhal H.A. Compositional, technological and nutritional aspects of dromedary camel’s milk. Int. Dairy J. 2010;20:811–821. [Google Scholar]
- Al Haj O.A., Metwalli A.A., Ismail E.A., Ali H.S., Al-Khalifa A.S., Kanekanian A.D. Angiotensin converting enzyme-inhibitory activity and antimicrobial effect of fermented camel’s milk (Camelus dromedarius) Int. J. Dairy Technol. 2018;71:27–35. [Google Scholar]
- Alavi F., Salami M., Emam-Djomeh Z., Mohammadian M. Nutraceutical properties of camel’s milk. In: Watson R.R., Collier R.J., Preedy V.R., editors. Nutrients in Dairy and their Implications on Health and Disease. Academic Press; 2018. pp. 451–468. [Google Scholar]
- Alhaider A., Abdelgader A.G., Turjoman A.A., Newell K., Hunsucker S.W., Shan B., Duncan M.W. Through the eye of an electrospray needle: Mass spectrometric identification of the major peptides and proteins in the milk of the one-humped camel (Camelus dromedarius) J. Mass Spectrom. 2013;48:779–794. doi: 10.1002/jms.3213. [DOI] [PubMed] [Google Scholar]
- Almaas H., Berner V., Holm H., Langsrud T., Vegarud G.E. Degradation of whey from caprine milk by human proteolytic enzymes, and the resulting antibacterial effect against Listeria monocytogenes. Small Rumin. Res. 2008;79:11–15. [Google Scholar]
- Al-Numair K.S., Alsaif M.A. Effect of camel’s milk on collagen abnormalities in streptozotocin-diabetic rats. Afr. J. Pharm. Pharmacol. 2011;5:238–243. [Google Scholar]
- Al-Saleh A.A. Heat coagulation of camel’s milk. J. King Saud Univ. 1996;8:107–117. [Google Scholar]
- Al-Saleh A.A., Metwalli A.A., Ismail E.A., Alhaj O.A. Antioxidative activity of camel’s milk casein hydrolysates. J. Camel Pract. Res. 2014;21(2):229–237. [Google Scholar]
- Al-Shamsi K.A., Mudgil P., Hassan H.M., Maqsood S. Camel’s milk protein hydrolysates with improved techno functional properties and enhanced antioxidant potential in in vitro and in food model systems. J. Dairy Sci. 2018;101:47–60. doi: 10.3168/jds.2017-13194. [DOI] [PubMed] [Google Scholar]
- Ashour E.A., Abd El-Hack M.E., Alagawany M., Swelum A.A., Osman A.O., Saadeldin I.M., Hussein E.S.O. Use of whey protein concentrates in broiler diets. J. Appl. Poult. Res. 2019;28:1078–1088. [Google Scholar]
- Ashour E.A., Abd El-Hack M.E., Shafi M.E., Alghamdi W.Y., Taha A.E., Swelum A.A., El-Saadony M.T. Impacts of green coffee powder supplementation on growth performance, carcass characteristics, blood indices, meat quality and gut microbial load in broilers. Agriculture. 2020;10:457. [Google Scholar]
- Atanasova J., Ivanova I. Antibacterial peptides from goat and sheep milk proteins. Biotechnol. Equip. 2010;24:1799–1803. [Google Scholar]
- Attia H., Kherouatou N., Dhouib A. Dromedary milk lactic acid fermentation: microbiological and rheological characteristics. J. Ind. Microbiol. Biotechnol. 2001;26:263–270. doi: 10.1038/sj.jim.7000111. [DOI] [PubMed] [Google Scholar]
- Ayoub M.A., Palakkott A.R., Ashraf A., Iratni R. The molecular basis of the antidiabetic properties of Camel’s milk. Diabetes. Res. Clin. Pract. 2018;146:305–312. doi: 10.1016/j.diabres.2018.11.006. [DOI] [PubMed] [Google Scholar]
- Badr G. Camel whey protein enhances diabetic wound healing in a streptozotocin-induced diabetic mouse model: the critical role of β-Defensin- 1, -2 and-3. Lipids Health Dis. 2013;12:1–11. doi: 10.1186/1476-511X-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y.H., Zhao D.B. The acid–base buffering properties of Alxa bactrian camel’s milk. Small Rumin. Res. 2015;123:287–292. [Google Scholar]
- Beg O.U., Bahr-Lindström H., Zaidi Z.H., Jörnvall H. A camel’s milk whey protein rich in half-cystine. Eur. J. Biochem. 1986;159:195–201. doi: 10.1111/j.1432-1033.1986.tb09852.x. [DOI] [PubMed] [Google Scholar]
- Brandelli A., Daroit D., Corrêa A. Whey as a source of peptides with remarkable biological activities. Food Res. Int. 2015;73:149–161. [Google Scholar]
- Bütikofer U., Meyer J., Sieber R., Wechsler D. Quantification of the angiotensin-converting enzyme-inhibiting tripeptides Val-Pro-Pro and Ile-ProPro in hard, semi-hard and soft cheeses. Int. Dairy J. 2007;17:968–975. [Google Scholar]
- Chandan R.C., Kilara A. Wiley; 2010. Dairy Ingredients for Food Processing. Retrieved from https://books.google.com/books?id=RnIpMHDjNc0C. [Google Scholar]
- Chatterton D.E., Smithers G., Roupas P., Brodkorb A. Bioactivity of β- lactoglobulin and α-lactalbumin technological implications for processing. Int. Dairy J. 2006;16:1229–1240. [Google Scholar]
- Chen G.W., Tsai J.S., Pan B.S. Purification of angiotensin I-converting enzyme inhibitory peptides and antihypertensive effect of milk produced by protease-facilitated lactic fermentation. Int. Dairy J. 2007;17:641–647. [Google Scholar]
- Clare D.A., Swaisgood H.E. Bioactive milk peptides: a prospectus. J. Dairy Sci. 2000;83:1187–1195. doi: 10.3168/jds.S0022-0302(00)74983-6. [DOI] [PubMed] [Google Scholar]
- da Costa E.L., da Rocha Gontijo J.A., Netto F.M. Effect of heat and enzymatic treatment on the antihypertensive activity of whey protein hydrolysates. Int. Dairy J. 2007;17:632–640. [Google Scholar]
- Damodharan K., Palaniyandi S.A., Yang S.H., Suh J.W. Functional probiotic characterization and in vivo cholesterol-lowering activity of Lactobacillus helveticus isolated from fermented cow’s milk. J. Microbiol. Biotechnol. 2016;26:1675–1686. doi: 10.4014/jmb.1603.03005. [DOI] [PubMed] [Google Scholar]
- Devendra K., Verma K.A., Chatli M.K., Singh R., Kumar P., Mehta N., Malav O.P. Camel’s milk: alternative milk for human consumption and its health benefits. Nutr. Food Sci. 2016;46:217–227. [Google Scholar]
- El-Agamy E.I., Ruppanner R., Ismail A., Champagne C.P., Assaf R. Antibacterial and antiviral activity of camel’s milk protective proteins. J. Dairy Res. 1992;59:169–175. doi: 10.1017/s0022029900030417. [DOI] [PubMed] [Google Scholar]
- El-Agamy E.I. The challenge of cow’s milk protein allergy. Small Rumin. Res. 2007;68:64–72. [Google Scholar]
- El-Agamy E.I., Nawar M., Shamsia S.M., Awad S., Haenlein G.F. Are camel’s milk proteins convenient to the nutrition of cow’s milk allergic children? Small Rumin. Res. 2009;82:1–6. [Google Scholar]
- Elayan A.A., Sulieman A.M.E., Saleh F.A. The hypocholesterolemic effect of gariss and gariss containing. Asian J. Biochem. 2010;5:205–209. [Google Scholar]
- EL-Fakharany E.M., Abedelbaky N., Haroun B.M., Sánchez L., Redwan N.A., Redwan E.M. Anti-infectivity of camel polyclonal antibodies against hepatitis C virus in Huh7.5 hepatoma. Virol. J. 2012;201:1–9. doi: 10.1186/1743-422X-9-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hatmi H., Girardet J.M., Gaillard J.L., Yahyaoui M.H., Attia H. Characterisation of whey proteins of camel (Camelus dromedarius) milk and colostrum. Small Rumin. Res. 2007;70:267–271. [Google Scholar]
- El-Hatmi H., Jrad Z., Salhi I., Aguibi A., Nadri A., Khorchani T. Comparison of composition and whey protein fractions of human, camel, donkey, goat and cow’s milk. Mljekarstvo/Dairy. 2015;65:159–167. [Google Scholar]
- El-Hatmi H., Levieux A., Levieux D. Camel (Camelus dromedarius) immunoglobulin G, α-lactalbumin, serum albumin and lactoferrin in colostrum and milk during the early post-partum period. J. Dairy Res. 2006;73:288–293. doi: 10.1017/S0022029906001713. [DOI] [PubMed] [Google Scholar]
- El-Saadony M.T., Elsadek M.F., Mohamed A.S., Taha A.E., Ahmed B.M., Saad A.M. Effects of chemical and natural additives on cucumber juice’s quality, shelf life, and safety. Foods. 2020;9:639. doi: 10.3390/foods9050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., El-Wafai N.A., El-Fattah H.I.A., Mahgoub S.A. Biosynthesis, optimization and characterization of silver nanoparticles using a soil isolate of Bacillus pseudomycoides MT32 and their antifungal activity against some pathogenic fungi. Adv. Anim. Vet. Sci. 2019;7:238–249. [Google Scholar]
- El-Zahar K., Sitohy M., Choiset Y., Metro F., Haertle T., Chobert J.M. Antimicrobial activity of ovine whey protein and their peptic hydrolysates. Milchwissenschaft. 2004;59:653–656. [Google Scholar]
- Erdmann K., Cheung B.W., Schröder H. The possible roles of foodderived bioactive peptides in reducing the risk of cardiovascular disease. J. Nutr. Biochem. 2008;19:643–654. doi: 10.1016/j.jnutbio.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Ereifej K.I., Alu'datt M.H., AlKhalidy H.A., Alli I., Rababah T. Comparison and characterization of fat and protein composition for camel’s milk from eight Jordanian locations. Food Chem. 2011;127:282–289. [Google Scholar]
- Farah Z. Effect of heat treatment on whey proteins of camel’s milk. Milchwissenschaft. 1986;41:763–765. [Google Scholar]
- Farah Z., Atkins D. Heat coagulation of camel’s milk. J. Dairy Res. 1992;59:229–231. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO), 2012. Gateway to dairy production and products. Retrieved November 23, 2017, from http://www.fao.org/dairy-production-products/production/en/.
- Gennaro R., Zanetti M. Structural features and biological activities of the cathelicidin-derived antimicrobial peptides. Pept. Sci. 2000;55:31–49. doi: 10.1002/1097-0282(2000)55:1<31::AID-BIP40>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Gibbs B.F., Zougman A., Masse R., Mulligan C. Production and characterization of bioactive peptides from soy hydrolysate and soy-fermented food. Food Res. Int. 2004;37:123–131. [Google Scholar]
- Haddadin M.S., Gammoh S.I., Robinson R.K. Seasonal variations in the chemical composition of camel’s milk in Jordan. J. Dairy Res. 2008;75:8–12. doi: 10.1017/S0022029907002750. [DOI] [PubMed] [Google Scholar]
- Hayes M., Ross R.P., Fitzgerald G.F., Hill C., Stanton C. Casein-derived antimicrobial peptides generated by Lactobacillus acidophilus DPC6026. Appl. Environ. Microbiol. 2006;72:2260–2264. doi: 10.1128/AEM.72.3.2260-2264.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Ledesma B., del Mar Contreras M., Recio I. Antihypertensive peptides: production, bioavailability and incorporation into foods. Adv. Colloid Interface Sci. 2011;165:23–35. doi: 10.1016/j.cis.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Hernández-Ledesma B., García-Nebot M.J., Fernández-Tomé S., Amigo L., Recio I. Dairy protein hydrolysates: Peptides for health benefits. Int. Dairy J. 2014;38:82–100. [Google Scholar]
- Hinz K., O’Connor P.M., Huppertz T., Ross R.P., Kelly A.L. Comparison of the principal proteins in bovine, caprine, buffalo, equine and camel’s milk. J. Dairy Res. 2012;79:185–191. doi: 10.1017/S0022029912000015. [DOI] [PubMed] [Google Scholar]
- Hori G., Wang M.F., Chan Y.C., Komatsu T., Wong Y., Chen T.H., Yamamoto K., Nagaoka S., Yamamoto S. Soy protein hydrolysate with bound phospholipids reduces serum cholesterol levels in hypercholesterolemic adult male volunteers. Biosci. Biotechnol. Biochem. 2001;65:72–78. doi: 10.1271/bbb.65.72. [DOI] [PubMed] [Google Scholar]
- Ito N., Fukushima S., Tsuda H. Carcinogenicity and modification of the carcinogenic response by BHA, BHT, and other antioxidants. CRC Crit. Rev. Toxicol. 1985;15:109–150. doi: 10.3109/10408448509029322. [DOI] [PubMed] [Google Scholar]
- Izadi A., Khedmat L., Mojtahedi S.Y. Nutritional and therapeutic perspectives of camel’s milk and its protein hydrolysates: a review on versatile biofunctional properties. J. Funct. Foods. 2019;60 [Google Scholar]
- Jacobucci H.B., Sgarbieri V.C., Dias N.F., Borges P., Tanikawa C. Impact of different dietary protein on rat growth, blood serum lipids and protein and liver cholesterol. Nutr. Res. 2001;21:905–915. [Google Scholar]
- Jakubowicz D., Froy O. Biochemical and metabolic mechanisms by which dietary whey protein may combat obesity and Type 2 diabetes. J. Nutr. Biochem. 2013;24:1–5. doi: 10.1016/j.jnutbio.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Jrad Z., El Hatmi H., Adt I., Girardet J.M., Cakir-Kiefer C., Jardin J., Degraeve P., Khorchani T., Oulahal N. Effect of digestive enzymes on antimicrobial, radical scavenging and angiotensin I-converting enzyme inhibitory activities of camel colostrum and milk proteins. Dairy Sci. Technol. 2014;94:205–224. [Google Scholar]
- Jrad Z., Girardet J.M., Adt I., Oulahal N., Degraeve P., Khorchani T., El Hatmi H. Antioxidant activity of camel’s milk casein before and after in vitro simulated enzymatic digestion. Mljekarstvo. 2014;64:287–294. [Google Scholar]
- Jung S., Murphy P.A., Johnson L.A. Physicochemical and functional properties of soy protein substrates modified by low levels of protease hydrolysis. J. Food Sci. 2005;70:180–187. [Google Scholar]
- Kamal A.M., Salama O.A., El-Saied K.M. Changes in amino acids profile and camel’s milk protein during the early lactation. Int. J. Dairy Sci. 2007;2:226–234. [Google Scholar]
- Kamal M., Karoui R. Monitoring of mild heat treatment of camel’s milk by front-face fluorescence spectroscopy. LWT- -Food Sci Technol. 2017;79:586–593. [Google Scholar]
- Kappeler S., Farah Z., Puhan Z. Sequence analysis of Camelus dromedaries milk caseins. J. Dairy Res. 1998;65:209–222. doi: 10.1017/s0022029997002847. [DOI] [PubMed] [Google Scholar]
- Kaskous S. Importance of camel’s milk for human health. Emir. J. Food Agric. (EJFA) 2016;28:158–163. doi: 10.9755/ejfa.2015-05-296. [DOI] [Google Scholar]
- Khalesi M., Salami M., Moslehishad M., Winterburn J., Moosavi-Movahedi A.A. Biomolecular content of camel’s milk: a traditional superfood towards future healthcare industry. Trends Food Sci. Technol. 2017;62:49–58. [Google Scholar]
- Khaskheli M., Arain M.A., Chaudhry S., Soomro A.H., Qureshi T.A. Physico-chemical quality of camel’s milk. J. Agric. Soc. Sci. 2005;2:164–166. [Google Scholar]
- Kishawy A.T., Amer S.A., Osman A., Elsayed S.A., Abd El-Hack M.E., Swelum A.A., Saadeldin I.M. Impacts of supplementing growing rabbit diets with whey powder and citric acid on growth performance, nutrient digestibility, meat and bone analysis, and gut health. AMB Exp. 2018;8:1–10. doi: 10.1186/s13568-018-0617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitts D., Weiler K. Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Curr. Pharm. Des. 2003;9:1309–1323. doi: 10.2174/1381612033454883. [DOI] [PubMed] [Google Scholar]
- Konuspayeva G., Faye B., Loiseau G. The composition of camel’s milk: a meta-analysis of the literature data. J. Food Compost. Anal. 2009;22:95–101. [Google Scholar]
- Konuspayeva G., Serikbayeva A., Loiseau G., Narmuratova M., Faye B. Lactoferrin of camel’s milk in Kazakhstan. In: Faye B., Esenov P., editors. Desertification Combat and Food Safety: The Value of Camel Producers. IOS Press; Amsterdam, The Netherlands: 2005. pp. 158–167. [Google Scholar]
- Korhonen H., Pihlanto A. Food-derived bioactive peptides opportunities for designing future foods”. Curr. Pharm. Des. 2001;9:1297–1308. doi: 10.2174/1381612033454892. [DOI] [PubMed] [Google Scholar]
- Korhonen H. Milk-derived bioactive peptides: From science to applications. J. Funct. Foods. 2009;1(2):177–187. [Google Scholar]
- Korhonen H., Pihlanto A. Food-derived bioactive peptides-opportunities for designing future foods. Curr. Pharm. Des. 2003;9:1297–1308. doi: 10.2174/1381612033454892. [DOI] [PubMed] [Google Scholar]
- Korhonen H., Pihlanto A. Bioactive peptides: production and functionality. Int. Dairy J. 2006;16:945–960. [Google Scholar]
- Kumar D., Chatli M.K., Singh R., Mehta N., Kumar P. Enzymatic hydrolysis of camel’s milk casein and its antioxidant properties. Dairy Sci Technol. 2016;96:391–404. [Google Scholar]
- Kumar D., Chatli M.K., Singh R., Mehta N., Kumar P. Antioxidant and antimicrobial activity of camel’s milk casein hydrolysates and its fractions. Small Rumin. Res. 2016;139:20–25. [Google Scholar]
- Kustanovich I., Shalev D.E., Mikhlin M., Gaidukov L., Mor A. Structural requirements for potent versus selective cytotoxicity for antimicrobial dermaseptin S4 derivatives. J. Biol. Chem. 2002;277:16941–16951. doi: 10.1074/jbc.M111071200. [DOI] [PubMed] [Google Scholar]
- Lacroix I.M., Li-Chan E.C. Overview of food products and dietary constituents with antidiabetic properties and their putative mechanisms of action: a natural approach to complement pharmacotherapy in the management of diabetes. Mol. Nutr. Food Res. 2014;58:61–78. doi: 10.1002/mnfr.201300223. [DOI] [PubMed] [Google Scholar]
- Lacroix I.M., Li-Chan E.C. Inhibition of dipeptidyl peptidase (DPP)-IV and α-glucosidase activities by pepsin treated whey proteins. J. Agric. Food Chem. 2013;61:7500–7506. doi: 10.1021/jf401000s. [DOI] [PubMed] [Google Scholar]
- Lacroix I.M., Li-Chan E.C. Dipeptidyl peptidase-IV inhibitory activity of dairy protein hydrolysates. Int. Dairy J. 2012;25:97–102. [Google Scholar]
- Lacroix I.M., Li-Chan E.C. Isolation and characterization of peptides with dipeptidyl peptidase-IV inhibitory activity from pepsin-treated bovine whey proteins. Peptides. 2014;54:39–48. doi: 10.1016/j.peptides.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Laleye L.C., Jobe B., Wasesa A.A.H. Comparative study on heat stability and functionality of camel and cow’s milk whey proteins. J. Dairy Sci. 2008;91:4527–4534. doi: 10.3168/jds.2008-1446. [DOI] [PubMed] [Google Scholar]
- Lignitto L., Segato S., Balzan S., Novelli E. Preliminary investigation on the presence of peptides inhibiting the growth of Listeria innocua and Listeria monocytogenes in Asiago d’Allevo cheese. Dairy Sci. Technol. 2012;92:297–308. [Google Scholar]
- Maes W., Van Camp J., Vermeirssen V., Huyghebaert A. Influence of the lactokinin Ala-Leu-Pro-Met-His-Ile-Arg (ALPMHIR) on the release of endothelin-1 by endothelial cells. Regul. Pept. 2004;118:105–109. doi: 10.1016/j.regpep.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Mahmoud M.H., Badr G., El Shinnawy N.A. Camel whey protein improves lymphocyte function and protects against diabetes in the offspring of diabetic mouse dams. Int. J. Immunopathol. Pharmacol. 2016;29:632–646. doi: 10.1177/0394632016671729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh G.H., Royle P.J., Le Leu R.K., Smithers G.W. Whey proteins as functional food ingredients. Int. Dairy J. 1998;8:425–434. [Google Scholar]
- Meena S., Rajput Y.S., Sharma R. Comparative fat digestibility of goat, camel, cow and buffalo milk. Int. Dairy J. 2014;35:153–156. [Google Scholar]
- Meisel H. Biochemical properties of peptides encrypted in Cow’s milk proteins. Curr. Med. Chem. 2005;12:1905–1919. doi: 10.2174/0929867054546618. [DOI] [PubMed] [Google Scholar]
- Meisel H., FitzGerald R.J. Biofunctional peptides from milk proteins: mineral binding and cytomodulatory effects. Curr. Pharm. Des. 2003;9:1289–1296. doi: 10.2174/1381612033454847. [DOI] [PubMed] [Google Scholar]
- Merin U., Bernstein S., Bloch-Damti A., Yagil R., van Creveld C., Lindner P., Gollop N. A comparative study of milk serum proteins in camel (Camelus dromedarius) and bovine colostrum. Livest. Prod. Sci. 2001;67:297–301. [Google Scholar]
- Mohanty D.P., Mohapatra S., Misra S., Sahu P.S. Milk derived bioactive peptides and their impact on human health–a review. Saudi J. Biol. Sci. 2016;23:577–583. doi: 10.1016/j.sjbs.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa K., Kondo I., Kanamaru Y., Nagaoka S. A novel regulatory pathway for cholesterol degradation via lactostatin. Biochem. Biophys. Res. Commun. 2007;352:697–702. doi: 10.1016/j.bbrc.2006.11.090. [DOI] [PubMed] [Google Scholar]
- Moure A., Domínguez H., Parajó J.C. Antioxidant properties of ultrafiltration-recovered soy protein fractions from industrial effluents and their hydrolysates. Process Biochem. 2006;41:447–456. [Google Scholar]
- Mudgil P., Kamal H., Yuen G.C., Maqsood S. Characterization and identification of novel antidiabetic and anti-obesity peptides from camel’s milk protein hydrolysates. Food Chem. 2018;259:46–54. doi: 10.1016/j.foodchem.2018.03.082. [DOI] [PubMed] [Google Scholar]
- Muro Urista C., Álvarez Fernández R., Riera Rodriguez F., Arana Cuenca A., Tellez Jurado A. Production and functionality of active peptides from milk. Food Sci. Technol. Int. 2011;17:293–317. doi: 10.1177/1082013211398801. [DOI] [PubMed] [Google Scholar]
- Nagaoka S., Futamura Y., Miwa K., Awano T., Yamauchi K., Kanamaru Y., Tadashi K., Kuwata T. Identification of novel hypocholesterolemic peptides derived from cow’s milk β-lactoglobulin. Biochem. Biophys. Res. Commun. 2001;281:11–17. doi: 10.1006/bbrc.2001.4298. [DOI] [PubMed] [Google Scholar]
- Nongonierma A.B., FitzGerald R.J. Inhibition of dipeptidyl peptidase IV (DPP-IV) by proline containing casein-derived peptides. J. Funct. Foods. 2013;5:1909–1917. [Google Scholar]
- Nongonierma A.B., FitzGerald R.J. Dipeptidyl peptidase IV inhibitory and antioxidative properties of milk protein-derived dipeptides and hydrolysates. Peptides. 2013;39:157–163. doi: 10.1016/j.peptides.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Nongonierma A.B., Paolella S., Mudgil P., Maqsood S., FitzGerald R.J. Identification of novel dipeptidyl peptidase IV (DPP-IV) inhibitory peptides in camel’s milk protein hydrolysates. Food Chem. 2018;244:340–348. doi: 10.1016/j.foodchem.2017.10.033. [DOI] [PubMed] [Google Scholar]
- Nongonierma A.B., Paolella S., Mudgil P., Maqsood S., FitzGerald R.J. Dipeptidyl peptidase IV (DPP-IV) inhibitory properties of camel’s milk protein hydrolysates generated with trypsin. J. Funct. Foods. 2017;34:49–58. [Google Scholar]
- NPCS Board, 2012. Detailed Project Profiles on Dairy & Dairy Products, secnd ed. NIIR Project Consultancy Services. Retrieved from https://books.google.com/books?id=8tlEAgAAQBAJ.
- Omar R.H., Eltinay A.H. Microbial quality of camel’s raw milk in central southern region of United Arab Emirates. Emir. J. Food Agric. 2008;20(1):76–83. [Google Scholar]
- Omar A., Harbourne N., Oruna-Concha M.J. Quantification of major camel’s milk proteins by capillary electrophoresis. Int. Dairy J. 2016;58:31–35. [Google Scholar]
- Osman A., Goda H., Abdel-Hamid M., Badran S., Otte J. Antibacterial peptides generated by Alcalase hydrolysis of goat whey. LWT - Food Sci. Technol. 2016;65:480–486. [Google Scholar]
- Otte J., Shalaby S.M., Zakora M., Pripp A.H., El-Shabrawy S.A. Angiotensin-converting enzyme inhibitory activity of milk protein hydrolysates: effect of substrate, enzyme and time of hydrolysis. Int. Dairy J. 2007;175:488–503. [Google Scholar]
- Panyam D., Kilara A. Enhancing the functionality of food proteins by enzymatic modification. Trends Food Sci. Technol. 1996;7:120–125. [Google Scholar]
- Park Y.W., Haenlein G.F.W. John Wiley & Sons; Chichester, West Sussex, UK: 2013. Milk and Dairy Products in Human Nutrition. https://doi.org/10.1002/9781118534168. [Google Scholar]
- Parodi P.W. A role for milk proteins and their peptides in cancer prevention. Curr. Pharm. Des. 2007;13:813–828. doi: 10.2174/138161207780363059. [DOI] [PubMed] [Google Scholar]
- Peng X., Xiong Y.L., Kong B. Antioxidant activity of peptide fractions from whey protein hydrolysates as measured by electron spin resonance. Food Chem. 2009;113:196–201. [Google Scholar]
- Peñta-Ramos E.A., Xiong Y.L. Antioxidant activity of soy protein hydrolysates in a liposomal system. J. Food Sci. 2002;67:2952–2956. [Google Scholar]
- Phelan M., Kerins D. The potential role of milk-derived peptides in cardiovascular disease. Food Funct. 2011;2:153–167. doi: 10.1039/c1fo10017c. [DOI] [PubMed] [Google Scholar]
- Pihlanto A., Virtanen T., Korhonen H. Angiotensin I converting enzyme (ACE) inhibitory activity and antihypertensive effect of fermented milk. Int. Dairy J. 2010;20:3–10. [Google Scholar]
- Pihlanto-Leppälä A. Bioactive peptides derived from bovine whey proteins: opioid and ace-inhibitory peptides. Trends Food Sci. Technol. 2000;11:347–356. [Google Scholar]
- Potter S.M. Overview of proposed mechanisms for the hypocholesterolemic. J. Nutr. 1995;125:606S–611S. doi: 10.1093/jn/125.3_Suppl.606S. [DOI] [PubMed] [Google Scholar]
- Qian Z.J., Jung W.K., Kim S.K. Free radical scavenging activity of a novel antioxidative peptide purified from hydrolysate of bullfrog skin, Rana catesbeiana Shaw. Bioresour. Technol. 2008;99:1690–1698. doi: 10.1016/j.biortech.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Rajapakse N., Mendis E., Jung W.K., Je J.Y., Kim S.K. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res. Int. 2005;38:175–182. [Google Scholar]
- Reda F.M., El-Saadony M.T., Elnesr S.S., Alagawany M., Tufarelli V. Effect of dietary supplementation of biological curcumin nanoparticles on growth and carcass traits, antioxidant status, immunity and caecal microbiota of Japanese quails. Animals. 2020;10:754. doi: 10.3390/ani10050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rival S.G., Boeriu C.G., Wichers H.J. Caseins and casein hydrolysates. 2. Antioxidative properties and relevance to lipoxygenase inhibition. J. Agric. Food Chem. 2001;49:295–302. doi: 10.1021/jf0003911. [DOI] [PubMed] [Google Scholar]
- Rizzello C.G., Losito I., Gobbetti M., Carbonara T., De Bari M.D., Zambonin P.G. Antibacterial activities of peptides from the water-soluble extracts of Italian cheese varieties. J. Dairy Sci. 2005;88:2348–2360. doi: 10.3168/jds.S0022-0302(05)72913-1. [DOI] [PubMed] [Google Scholar]
- Saito K., Jin D.H., Ogawa T., Muramoto K., Hatakeyama E., Yasuhara T., Nokihara K. Antioxidative properties of tripeptide libraries prepared by the combinatorial chemistry. J. Agric. Food Chem. 2003;51:3668–3674. doi: 10.1021/jf021191n. [DOI] [PubMed] [Google Scholar]
- Salami M., Moosavi-Movahedi A.A., Ehsani M.R., Pourtakdoost S. Improvement of the antimicrobial and antioxidant activities of camel and bovine whey proteins by limited proteolysis. J. Agric. Food Chem. 2010;58:3297–3302. doi: 10.1021/jf9033283. [DOI] [PubMed] [Google Scholar]
- Salami M., Moosavi-Movahedi A.A., Moosavi-Movahedi F., Haertlé T. Biological activity of camel’s milk casein following enzymatic digestion. J. Dairy Res. 2011;78:471–487. doi: 10.1017/S0022029911000628. [DOI] [PubMed] [Google Scholar]
- Salami M., Yousefi R., Ehsani M.R., Razavi S.H., Chobert J.M., Haertlé T., Saboury A.A., Atri M.S., Niasari-Naslaji A., Ahmad F., MoosaviMovahedi A.A. Enzymatic digestion and antioxidant activity of the native and molten globule states of camel α-lactalbumin: Possible significance for use in infant formula. Int. Dairy J. 2009;19:518–523. [Google Scholar]
- Salwa M.Q., Lina A.K. Antigenotoxic and anticytotoxic effect of camel’s milk in mice treated with cisplatin. Saudi J. Biol. Sci. 2010;17:159–166. doi: 10.1016/j.sjbs.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmadi B.H., Ismail A. Antioxidative peptides from food proteins: a review. Peptides. 2010;31:1949–1956. doi: 10.1016/j.peptides.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Sharma, S., 2014. Antivirulence activities of Bioactive Peptides produced by Lactobacillus helveticus and Lactobacillus acidophilus against Salmonella enterica serovar Typhimurium [e-thesis]. Master, University of Guelph, Ontario, Canada. Retrieved October 8, 2017, from The Atrium, https://atrium.lib.uoguelph.ca/xmlui/handle/10214/8476.
- Sheiha A.M., Abdelnour S.A., Abd El-Hack M.E., Khafaga A.F., Metwally K.A., Ajarem J.S., Maodaa S.N., Allam A.A., El-Saadony M.T. Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals. 2020;10:430. doi: 10.3390/ani10030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shori A.B., Baba A.S. Comparative antioxidant activity, proteolysis and in vitro α-amylase and α-glucosidase inhibition of Allium sativum-yogurts made from cow and camel’s milk. J. Saudi Chem. Soc. 2014;18:456–463. [Google Scholar]
- Sikkema R.S., Farag E.A.B.A., Islam M., Atta M., Reusken C.B.E.M., Al-Hajri M.M., Koopmans M.P.G. Global status of Middle East respiratory syndrome coronavirus in dromedary camels: a systematic review. Epidemiol. Infect. 2019;147:e84. doi: 10.1017/S095026881800345X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva S.V., Malcata F.X. Caseins as source of bioactive peptides. Int. Dairy J. 2005;15:1–15. [Google Scholar]
- Silveira S.T., Martínez-Maqueda D., Recio I., Hernández-Ledesma B. Dipeptidyl peptidase-IV inhibitory peptides generated by tryptic hydrolysis of a whey protein concentrate rich in β-lactoglobulin. Food Chem. 2013;141:1072–1077. doi: 10.1016/j.foodchem.2013.03.056. [DOI] [PubMed] [Google Scholar]
- Solieri L., Rutella G.S., Tagliazucchi D. Impact of non-starter lactobacilli on release of peptides with angiotensin-converting enzyme inhibitory and antioxidant activities during cow’s milk fermentation. Food Microbiol. 2015;51:108–116. doi: 10.1016/j.fm.2015.05.012. [DOI] [PubMed] [Google Scholar]
- Suliman G.M., Alowaimer A.N., Hussein E.O.S., Ali H.S., Abdelnour S.A., Abd El-Hack M.E., Swelum A.A. Chemical composition and quality characteristics of meat in three one-humped camel (Camelus dromedarius) breeds as affected by muscle type and post-mortem storage period. Animals. 2019;9:834. doi: 10.3390/ani9100834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swelum A.A., Saadeldin I.M., Abdelnour S.A., Ba-Awadh H., Abd El-Hack M.E., Sheiha A.M. Relationship between concentrations of macro and trace elements in serum and follicular, oviductal, and uterine fluids of the dromedary camel (Camelus dromedarius) Trop. Anim. Health Prod. 2020;52:1315–1324. doi: 10.1007/s11250-019-02137-0. [DOI] [PubMed] [Google Scholar]
- Tavano O.L. Protein hydrolysis using proteases: an important tool for food biotechnology. J. Mol. Catal. B: Enzymatic. 2013;90:1–11. [Google Scholar]
- Théolier J., Hammami R., Labelle P., Fliss I., Jean J. Isolation and identification of antimicrobial peptides derived by peptic cleavage of whey protein isolate. J. Funct. Foods. 2013;5:706–714. [Google Scholar]
- Uenishi H., Kabuki T., Seto Y., Serizawa A., Nakajima H. Isolation and identification of casein-derived dipeptidyl-peptidase 4 (DPP-4)-inhibitory peptide LPQNIPPL from gouda-type cheese and its effect on plasma glucose in rats. Int. Dairy J. 2012;22:24–30. [Google Scholar]
- Wang Z., Zhang W., Wang B., Zhang F., Shao Y. Influence of Bactrian camel’s milk on the gut microbiota. J. Dairy Sci. 2018;101:5758–5769. doi: 10.3168/jds.2017-13860. [DOI] [PubMed] [Google Scholar]
- Wu H.C., Chen H.M., Shiau C.Y. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus) Food Res. Int. 2003;36:949–957. [Google Scholar]
- Yagil R. Camel’s milk-a review. Int. J. Anim. Sci. 1987;2:81–99. [Google Scholar]
- Yamamoto N., Ejiri M., Mizuno S. Biogenic peptides and their potential use. Curr. Pharm. Des. 2003;9:1345–1355. doi: 10.2174/1381612033454801. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Chen R., Zuo F., Ma H., Zhang Y., Chen S. Comparison of dipeptidyl peptidase IV-inhibitory activity of peptides from bovine and caprine milk casein by in silico and in vitro analyses. Int. Dairy J. 2016;53:37–44. [Google Scholar]
- Zhao D.B., Bai Y.H., Niu Y.W. Composition and characteristics of Chinese bactrian camel’s milk. Small Rumin. Res. 2015;127:58–67. [Google Scholar]