Abstract
Oxidative stress, DNA damage, and unresolved inflammation are the predisposing factors of many chronic and degenerative diseases, including cancer. Stingless bee honey (SBH) is recognized to have high medicinal value by traditional medicine practitioners and has been used to treat various illnesses traditionally. This study aimed to determine the antioxidant, anti-inflammatory, and genoprotective effects of SBH by using in vitro cell culture models. The sugar content, total phenolic content, radical scavenging activity, and ferric reducing antioxidant power (FRAP) of SBH were determined in this study. Then, the protective effect of SBH against hydrogen peroxide (H2O2)-induced cell death and DNA damage was studied by using WIL2-NS human lymphoblastoid cell line, while the lipopolysaccharide (LPS)-induced RAW 264.7 murine macrophages cell line was used to study the anti-inflammatory effects of SBH. Results from this present study showed that the major sugar contents of SBH were fructose (19.39 + 0.01%) and glucose (14.03 ± 0.03%). Besides, the total phenolic content, the radical scavenging activity, and the FRAP value of SBH were 15.38 ± 0.02 mg GAE/100 g of honey, 34.04 ± 0.21%, and 206.77 + 1.76 μM AAE/100 g honey respectively. Pretreatment with SBH protected WIL2-NS cells from H2O2-induced cell death and DNA damage (p < 0.001). Moreover, SBH was also able to attenuate the production of nitric oxide by inhibiting the expression of inducible nitric oxide synthase in LPS-induced RAW 264.7 cells (p < 0.001). In conclusion, SBH is rich in total phenolic content and possesses strong antioxidant, anti-inflammatory, and genoprotective properties. Our current findings suggest that SBH might be useful in the prevention and treatment of many diseases caused by oxidative stress and inflammation assuming the observed effects are also achievable in vivo.
Keywords: Anti-inflammation, Antioxidant, DNA damage, Hydrogen peroxide, Lipopolysaccharide, Stingless bee honey
1. Background
Oxidative stress occurs due to the loss of balance between the cellular antioxidant defense mechanisms and the level of reactive oxygen species (ROS) (Pizzino et al., 2017). Excessive accumulation of ROS can cause damage to many major cellular components, including, lipid, protein, and DNA. It has been known that oxidative stress is one of the major causes of mutation and is involved in all stages of carcinogenesis during neoplasm formation (Kryston et al., 2011). Recent studies have shown that oxidative stress can cause genomic instability and gene silencing in cells, thus leading to the progression of degenerative diseases and speed up the aging process in the body (Lombard et al., 2005, Lu et al., 2004, Yaacob et al., 2017).
On the other hand, inflammation is a normal physiological response to fight against infections, tissue injuries, or irritations in the body (Ashley et al., 2012). In normal conditions, the inflammatory response eliminates infectious agents from the body and facilitates the tissue repairing process (Medzhitov, 2008). During acute inflammation, immune cells travel to injured tissue or site of pathogen invasion and become activated when they contact with the invading pathogens or via the actions of pro-inflammatory mediators. Activated immune cells then release toxic substances into the surrounding tissues to kill the invading pathogens, including the ROS and reactive nitrogen species (RNS) (Nathan, 2006). However, the toxic contents release by the activated immune cells not only kills the invading pathogens, but may damage the surrounding tissues as well. Hence, when the inflammatory responses become prolonged or unresolvable, pathological conditions may arise, such as autoimmunity, tissue fibrosis, or tumor growth (Serhan et al., 2020).
Honey is widely used as a sweetener in food. Honey contains a number of vitamins and minerals, such as vitamin B, vitamin C, sodium and calcium (Ajibola et al., 2012). Honey is also an antioxidant-rich natural product due to its high flavonoids and phenolic acids contents (Ajibola et al., 2012). Besides form being served as a food product, honey is used to treat various illnesses traditionally as well (Ediriweera and Premarathna, 2012). Honey has been shown to possess antibacterial (Jenkins et al., 2011, Nishio et al., 2016) and anti-fungal (Irish et al., 2006) effects. Furthermore, several studies showed that honey is capable to heal burn wounds, (Vandamme et al., 2013), chronic venous leg ulcers (Holland and Norris 2015), and foot ulcers in diabetic patients (Wang et al., 2019), suggesting that honey possesses high medicinal value.
Different types of honey bees can be found in Malaysia, such as Tualang bee, Jungle bee, Cerung or Cerang bee, and the Kelulut bee (Barakhbah et al., 2007). Kelulut bee (Trigona species) or better known as the stingless bee in Malaysia, is smaller in size and without having the sting (Boorn et al., 2010). The stingless bee is often found in the tropical and subtropical regions of the world. Honey produced by the stingless bee is more fluidic and diluted as compared to the other honey. In addition, it also possesses a sour like taste and aroma (Biluca et al., 2014). Stingless bee honey (SBH) is recognized to have medicinal value by traditional medicine practitioners (Rao et al., 2016, Yaacob et al., 2018). More recently, SBH has been shown to reduce the total number of aberrant crypt foci, aberrant crypts, and crypt multiplicity in the colon of azoxymethane-induced Sprague-Dawley rats, suggesting that SBH has chemopreventive properties in rats (Yazan et al., 2016). However, studies reporting on the biological activities of SBH remain limited. Hence, this study aimed to determine the antioxidant, anti-inflammatory, and genoprotective effects of SBH by using the in vitro cell culture model.
2. Materials and method
2.1. Honey samples
The SBH produced by stingless bee from Trigona itama species was supplied by Malaysia Agriculture Research and Development Institute (MARDI). The SBH was collected from a controlled agricultural ecosystem developed within the forest area by MARDI with approximately 120 species of rare plants and fruits and stingless bees as the main pollinator in this ecosystem.
2.2. Sugar analysis
The sugar content of SBH was analyzed by using a high-performance liquid chromatography (HPLC) system (Waters model 2707) coupled with the Waters RI-2414 refractive index detector and XBridge Amide column (250 × 4.6 mm, 3.5 μm particle size, Ireland), as reported previously with slight modification (Hussein et al., 2011). The SBH was diluted to 1 mg/mL by using deionized water. The diluted sample was filtered by using the 0.45 μm syringe filter (BT Lab, Malaysia) before injection into the HPLC system with a flow rate of 1.0 mL/min. Acetonitrile (75%) and deionized water (25%) with 0.2% of triethylamine were used as the mobile phase for this study.
2.3. Total phenolic content of SBH
The Folin-Ciocalteu method was used to study the total phenolic content of the SBH samples, as described previously with slight modification (Kek et al., 2014). The SBH was diluted in methanol to the concentration of 1 g/mL (Merck, Germany). Then, 0.5 mL of SBH sample was mixed with 2.5 mL of 0.2 N Folin-Ciocalteu reagents (Sigma Aldrich, USA) and was left at room temperature for 5 min before the addition of 2 mL of sodium carbonate solution (15% w/v; Sigma Aldrich, USA). After 2 h of incubation in dark conditions, the absorbance of the reaction mixture was measured at the wavelength of 798 nm by using a UV–VIS spectrophotometer. Gallic acid solutions (0 to 0.10 mg/mL) were used as the standard for this assay and the data were expressed as mg of Gallic acid equivalents per 100 g of honey sample (mg GAE/100 g honey).
2.4. The free radical-scavenging activity
The free radical-scavenging power of the SBH sample was measured by using the 2,2-diphenylpicrylhydrazyl (DPPH) assay, with slight modification from Aljadi and Kamaruddin (2004) method. Firstly, the concentration of SBH was adjusted to 0.1 g/mL by using distilled water. Then, the DPPH solution was prepared by dissolving 0.09 mg of DPPH (Sigma Aldrich, USA) in 1 mL of methanol. Subsequently, 0.75 mL of the diluted SBH sample was mixed with 1.5 mL of the DPPH reagent. After 90 min of incubation at room temperature in dark conditions, the absorbance was measured at the wavelength of 517 nm by using a UV–VIS spectrophotometer. The obtained data were expressed as the percentage of DPPH scavenging activity.
2.5. Ferric reducing antioxidant power (FRAP) assay
The FRAP assay was performed with slight modification from Benzie and Strain (1996) method. The concentration of SBH was adjusted to 0.1 g/mL by using distilled water. The FRAP reagent was prepared by mixing 1 mL of 20 mM ferric chloride (FeCl3) solution, 1 mL of 10 mM tripyridyltriazine (TPTZ) solution (0.31 g TPTZ dissolved in 100 mL 40 mM HCl) and 10 mL of acetate buffer (0.16 g sodium acetate dissolved in 100 mL of 0.28 M acetic acid; pH 3.6). The FRAP reagent was heated up to 37 °C before the experiment. Then, 25 μL of sample or standard solution was added into 175 μL FRAP reagents. After 4 min of incubation at 37 °C, the absorbance was measured at the wavelength of 593 nm by using a UV–VIS spectrophotometer. Ascorbic acid solutions with concentrations ranging from 0 to 1000 µM were used as the standard for this assay, and the FRAP value of the SBH sample was expressed as µM ascorbic acid equivalent per 100 g of honey sample (µM AAE/100 g honey).
2.6. Cell culture and SBH treatment
The WIL2-NS lymphoblastoid cell line and RAW 264.7 murine macrophages cell line were used to determine the protective effects of SBH against hydrogen peroxide (H2O2)-induced oxidative damage and the anti-inflammatory effects of SBH respectively. Both cell lines were obtained from American Type Culture Collection (ATCC, Rockville, MD, USA). Generally, WIL2-NS cells and RAW 264.7 cells were cultured following the protocol provided by ATCC. SBH was diluted in complete cell culture medium and was filter-sterilized using a 0.22 µm PES syringe filter (Millipore, USA) before each treatment. In general, the WIL2-NS cells (2 × 105 cells/mL) were treated with various concentrations of SBH (% v/v) for 3 days before challenging them with (30 µM) H2O2 (Sigma, USA) for 30 min. On the other hand, RAW 264.7 cells were plated at the initial density of 1 × 105 cells/mL for 18 h before being treated with different concentrations of SBH. After 2 h of incubation, lipopolysaccharide (LPS; 1 µg/mL; Sigma, USA) was added into the treatment medium and the cells were further incubated for another 22 h prior to testing for inflammation biomarkers.
2.7. MTT cell viability assay
Cytotoxic effects were determined by using the MTT cell viability assay, as described in the previous study (Ooi et al., 2014). Briefly, 20 μL of MTT solution (5 mg/mL dissolved in phosphate-buffered solution, Merck, Germany) was added into each well and the plate was incubated for 4 h in an incubator containing 5% carbon dioxide at 37 °C. Then, the supernatant was discarded and the remaining formazan was dissolved in 200 μL of dimethyl sulfoxide (DMSO). After 30 min of incubation, the absorbance of each well was measured at the wavelength of 570 nm by using the I-Mark™ microplate reader (Bio-Rad Laboratories, USA). The percentage of viable cells was calculated relative to the negative control group.
2.8. Alkaline comet assay
The alkaline comet assay was performed with some modification from the previous method (Ooi et al., 2020). First, the fully frosted slide was pre-coated with 100 µL of 0.6% (w/v) normal melting agarose (NMA; Sigma Aldrich, USA). Then, the harvested WIL2-NS cells were mixed with 0.6% (w/v) low melting point agarose (LMA; Sigma Aldrich, USA) and were pipetted on top of the solidified NMA layer. Once the LMA layer solidified, the slides were gently immersed in the lysis buffer solution (2.5 M NaCl, 100 mM EDTA, 10 mM Tris, and 1% Triton-X) at 4 °C for 12 h. Before electrophoresis, the slide was immersed in the pre-chilled electrophoresis buffer (0.3 N NaOH, 1 mM EDTA) for 20 min to unwind the DNA strands before running the electrophoresis for 20 min. Then, the slide was rinsed with neutralization buffer solution (400 mM Tris) 3 times. After staining with ethidium bromide solution (10 µg/mL; Sigma Aldrich, USA), the slide was covered with a glass slide and being observed by using the Olympus BX51 fluorescent microscope. The individual comet images were analyzed by using Comet Assay IV software.
2.9. Nitrite measurement
The Griess assay was performed as described in the previous study (Ooi et al., 2017). The Griess reagent was prepared freshly before the experiment by mixing the 1% (w/v) sulfanilamide solution [dissolved in 5% (v/v) phosphoric acid] with 0.1% (w/v) N-1-napthylethylenediamine dihydrochloride solution in 1:1 ratio. After treatment, 100 μL of the cell culture medium from each treatment was transferred into the designated well in a 96-well plate prior to the addition of 100 µL of Griess reagent into each well. The plate was protected from light and incubated at room temperature for 10 min before analyzed with an I-Mark™ microplate reader (Bio-Rad Laboratories, USA) at the wavelength of 570 nm. The nitrite concentrations in the culture supernatant were determined based on sodium nitrite as a standard reference.
2.10. Immunoblotting analysis
The harvested cells from each treatment were lysed by using 100 μL of radioimmunoprecipitation assay (RIPA) buffer (Sigma, USA). The protein concentration of each sample was measured by using a NanoPhotometer at the wavelength of 280 nm (IMPLEN P330, Germany) before adjusted to the concentration of 1 mg/mL by using RIPA buffer. The immunoblotting analysis was then performed by using the sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) coupled with the SNAP i.d. 2.0 protein detection system (Millipore, USA), as described by Ooi et al. (2014) with slight modification. The expression level of proteins of interest was detected by using the primary antibodies against poly(ADP-ribose) polymerase-1 (PARP-1), inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and β-actin (Cell Signaling Technology, USA) together with the horseradish peroxidase (HRP)-conjugated secondary antibody. The targeted protein bands were then visualized by using the Immobilon Forte Western HRP substrate (Millipore, USA) and the Fusion FX7 documentation system (Vilber Lourmat, Germany). The β-actin bands were used as the loading control for this study.
2.11. Statistical analysis
Three independent experiments (n = 3) were performed to answer each objective. Prism 7.0 software (GraphPad Inc., San Diego, CA) was used to perform the statistical analysis. Independent T-test and one-way analysis of variance (ANOVA) followed by Tukey's Post-Hoc test was used to analyze the data obtained from experiments. A p-value < 0.05 was considered statistically significant. All experimental results are expressed as the mean ± standard error (SE).
3. Results
3.1. The sugar content, total phenolic content, and antioxidant power of SBH
As shown in Table 1, the major sugar contents of SBH are fructose (19.39 ± 0.01 g/100 g honey) and glucose (14.03 ± 0.03 g/100 g honey). Both of them are monosaccharide sugars. Only a little amount of disaccharide sugars was detected in the SBH. The percentage of sucrose and maltose was 1.27 + 0.02 g/100 g honey and 0.84 ± 0.01 g/100 g honey respectively, with no lactose was detected in the samples. On the other hand, the total phenolic content, DPPH free radical scavenging activity, and FRAP value of SBH was 15.38 ± 0.02 mg GAE/100 g honey, 34.04 ± 0.21%, and 206.77 ± 1.76 μM AAE/100 g honey respectively (Table 1).
Table 1.
The sugar content, total phenolic content, DPPH radical scavenging activity, and FRAP value of SBH.
| Parameter | Value |
|---|---|
| Sugar content | |
| Glucose | 14.03 ± 0.03 g/100 g honey |
| Fructose | 19.39 + 0.01 g/100 g honey |
| Sucrose | 1.27 + 0.02 g/100 g honey |
| Maltose | 0.84 + 0.01 g/100 g honey |
| lactose | Not detected |
| Total phenolic content | 15.38 ± 0.02 mg GAE/100 g honey |
| DPPH radical scavenging activity | 34.04 ± 0.21%, |
| FRAP value | 206.77 ± 1.76 μM AAE/100 g honey |
Note: All results are expressed as mean ± SE (n = 3).
3.2. Effects of SBH on the viability of WIL2-NS cells
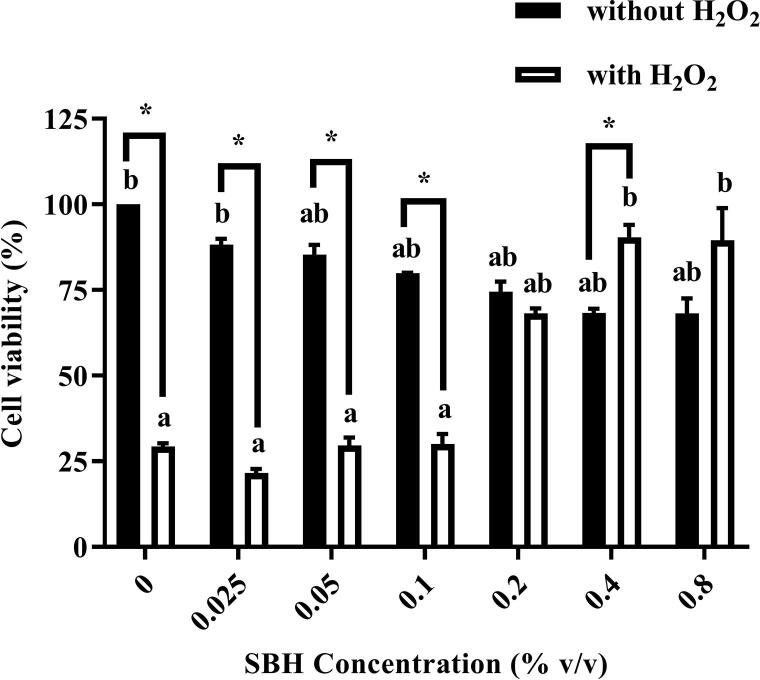
Based on Fig. 1, after treatment with SBH for 3 days, the viability of WIL2-NS cells reduced significantly with increasing concentration of SBH. Around 68.23 ± 4.28% of cells remained viable after treated with the highest concentration (0.8%) of SBH for 3 days. On the other hand, the viability of WIL2-NS cells dropped significantly from 100% in negative control to 29.41 ± 0.89% after H2O2 induction. In cells pretreated with SBH, significant increment in cell viability was detected at the concentrations of 0.2% (68.16 ± 1.45%, p < 0.01), 0.4% (90.41 ± 3.58%, p < 0.001) and 0.8% (89.49 ± 9.43%, p < 0.001) as compared to positive control group (cells induced with H2O2 only without SBH pretreatment).
Fig. 1.
The viability of WIL2-NS cells after treated with different concentrations of SBH with or without H2O2 induction (30 µM) for 30 min. aSignificant difference (p < 0.05) as compared to the negative control (cells without SBH and H2O2 treatment). bSignificant difference (p < 0.05) as compared to the positive control (cells treated with H2O2 only). *Significant difference (p < 0.05) between groups. All results are expressed as mean ± SE (n = 3).
3.3. SBH protects WIL2-NS cells from oxidative DNA damage
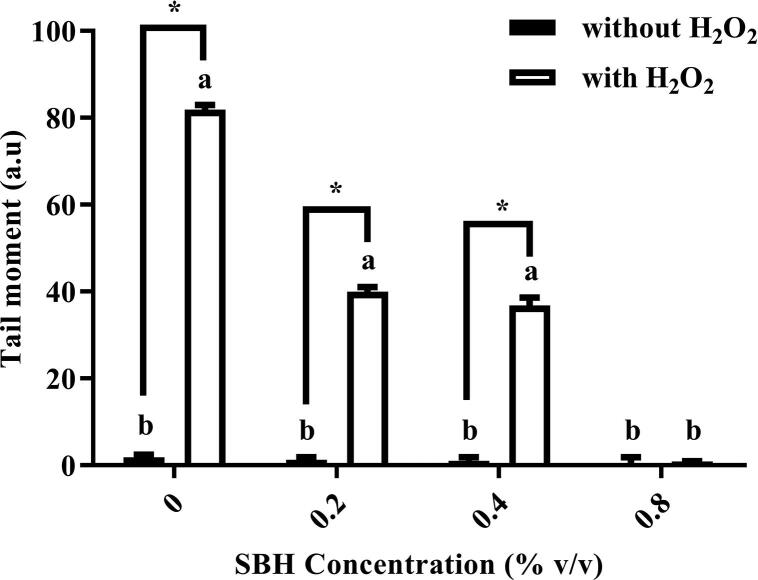
Fig. 2 showed that treatment with 0.2%, 0.4%, and 0.8% of SBH did not show any significant increment in the tail moment as compared to the negative control, suggesting that SBH itself is not genotoxic. After H2O2 induction, the tail moment increased significantly from 1.87 ± 0.60 a.u. in negative control to 81.87 ± 1.08 a.u. Pretreatment with SBH significantly reduced the tail moment values in a concentration-dependent manner. No significant difference in the tail moment value can be found between cells pretreated with 0.8% of SBH (0.86 ± 0.13 a.u.) and negative control group (1.87 ± 0.60 a.u.)..
Fig. 2.
Tail moment of WIL2-NS cells after treated with different concentrations of SBH with or without H2O2 induction (30 µM) for 30 min. aSignificant difference (p < 0.05) as compared to the negative control (cells without SBH and H2O2 treatment). bSignificant difference (p < 0.05) as compared to the positive control (cells treated with H2O2 only). *Significant difference (p < 0.05) between groups. All results are expressed as mean ± SE (n = 3).
3.4. Effects of SBH on PARP-1 expression in WIL2-NS cells
As shown in Fig. 3, SBH did not cause any significant changes in PARP-1 expression in the absence or presence of H2O2 induction, suggesting that the genoprotective effect of SBH was independent of PARP-1 expression.
Fig. 3.
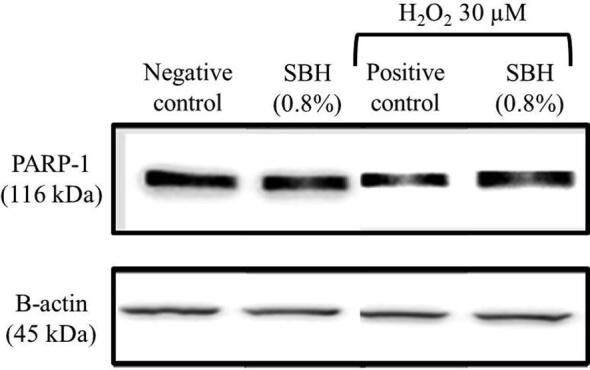
The immunoblot image representing PARP-1 expression level in WIL2- NS cells after treated with different concentrations of with or without H2O2 induction (30 µM) for 30 min.
3.5. Cytotoxicity of SBH against RAW 264.7 cells
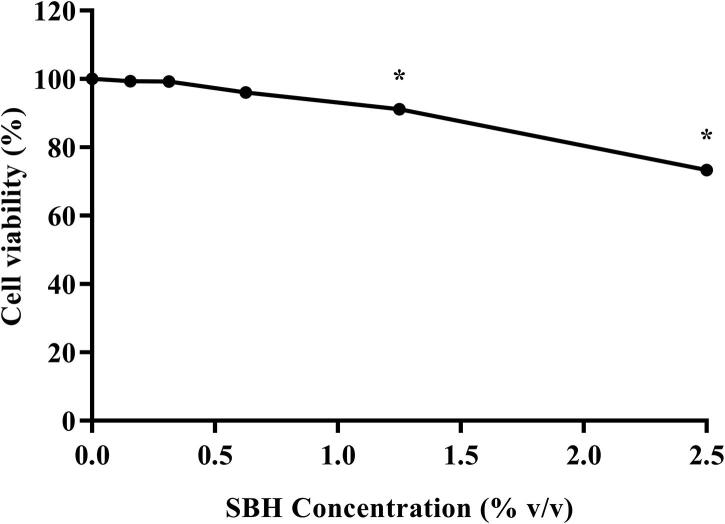
After 24 h of treatment, the viability of RAW 264.7 cells reduced significantly with increasing concentration of SBH. Significant reduction in cell viability was detected starting from cells treated with 1.25% of SBH (91.14 ± 1.10%), as compared to the negative control (100%). Hence, based on the cell viability curve (Fig. 4), the highest concentration used in the assessments of the anti-inflammatory potential of SBH was 1% (v/v) since this concentration caused less than 10% of cell death. This is because induction of cell death may cause many undesired effects that can jeopardize the validity of this in vitro anti-inflammatory model (Zitvogel et al., 2010).
Fig. 4.
Effects of SBH on the viability of RAW 264.7 cells. *Significant difference (p < 0.05) as compared to the negative control (cells without SBH and H2O2 treatment). All results are expressed as mean ± SE (n = 3).
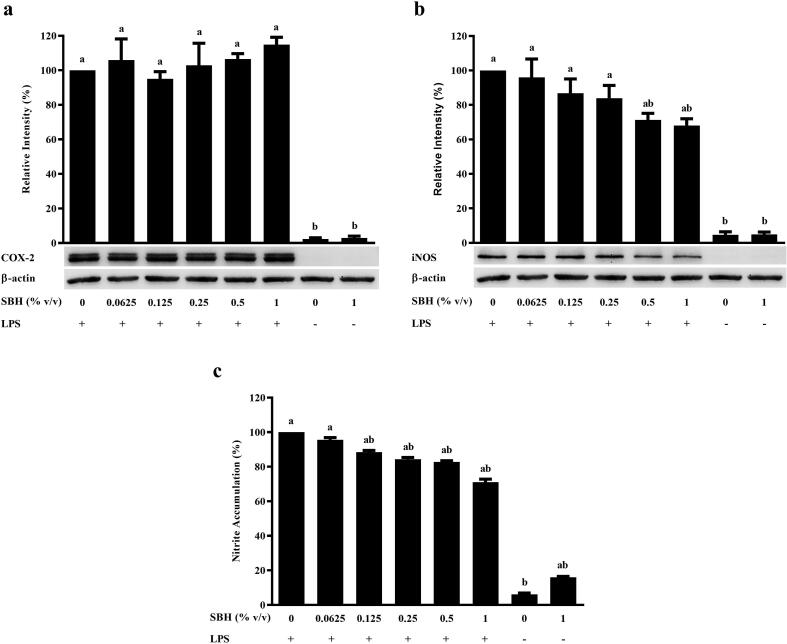
3.6. Effects of SBH on COX-2 expression, iNOS expression, and nitrite accumulation in RAW 264.7 cells
In the absence of LPS induction, pretreatment with 1% SBH did not induce production of NO or expression of iNOS and COX-2 in RAW 264.7 cells. After challenged with LPS, SBH did not suppress the expression of COX-2 in RAW 264.7 cells (Fig. 5a). However, iNOS expression and NO production were significantly inhibited by SBH pretreatment in a concentration-dependent manner (Fig. 5b & c). Significant suppression of iNOS expression (p < 0.05) was observed in RAW 264.7 cells pretreated with 0.5% (71.31 ± 3.82%) and 1% (68.11 ± 3.90%) of SBH, as compared to the positive control (cells induced with LPS only without SBH pretreatment; 100%). On the other hand, significant reduction in NO production was detected starting from 0.125% of SBH pretreatment (88.45 ± 1.02%; p < 0.001).
Fig. 5.
Effects of SBH on (a) COX-2 expression, (b) iNOS expression, and (c) nitric accumulation in LPS-induced RAW 264.7 cells. aSignificant difference (p < 0.05) as compared to the negative control (cells without SBH and H2O2 treatment). bSignificant difference (p < 0.05) as compared to the positive control (cells treated with H2O2 only). All results are expressed as mean ± SE (n = 3).
4. Discussion
The mono and disaccharide sugars found in honey play an essential and important role in cellular metabolism. Previous studies have shown that monosaccharide sugars such as fructose and glucose are among the many sugar components found in most stingless bees’ honey (Abd Jalil et al., 2017). In this present study, the amount of monosaccharide sugars was higher than the disaccharide sugars. Fructose and glucose are the major sugars found in the SBH, with the value of 19.39 + 0.01 g/100 g honey and 14.03 ± 0.03 g/100 g honey respectively. Previous studies have also reported that the average values of fructose and glucose levels in SBH produced by Trigona itama bee were 15.77 g/100 g honey and 9.22 g/100 g honey respectively (Kek et al., 2018). Similarly, another study on SBH showed that the fructose and glucose levels were 13.4 g/100 g of honey and 16.4 g/100 g of honey respectively (Se et al., 2018). The slight differences in the quantity of fructose and glucose levels in different stingless bee honey may be due to the differences in the type of nectar used by the bee and the geographical location of the beehives (Abd Jalil et al., 2017).
Besides that, it is noted that the total fructose and glucose content of the SBH as reported in this study and previous studies were less than the 60 g/100 g of honey and does not meet the Codex standard for honey (Codex Alimentarius Commission, 2001, Kek et al., 2018, Se et al., 2018). Similarly, another study conducted in Thailand showed that the total fructose and glucose content of SBH produced by Tetragonula laeviceps-pagdeni bee was 31.0 g/100 g of honey only (Chuttong et al., 2016). However, the SBH produced by other species of stingless bee in Brazil were able to meet the minimum requirement of the Codex standard (Biluca et al., 2016), suggesting that the SBH produced by certain genera of stingless bee have lower sugar contents, particularly the fructose and glucose contents.
On the other hand, a minimal amount of disaccharide sugars were present in the SBH sample which include sucrose and maltose. In agreement with our present findings, the study conducted by Se et al. (2018) showed an average sucrose content of 3.46 ± 2.49 g/100 g of honey, which meets the standard set by the Codex Alimentarius whereby the sucrose content of pure honey should not exceed 5%. The sucrose content of pure honey is usually small due to the presence of invertase enzyme that breakdown sucrose. However, previous studies have shown that the sucrose content in Malaysian SBH honey was 32.3 g/100 g of honey, which is much higher as compared to our current findings (Kek et al., 2018). The exact reason underlying such discrepancy remains unclear. However, high sucrose content is usually associated with overheating of the honey sample, which denatures the invertase enzyme and stopping the breakdown of sucrose into fructose and glucose (Chua et al., 2014). Besides that, the sucrose level of honey can also be increased if the honey is harvested before ripening since sucrose is broken down by the invertase enzyme during the ripening process (Belay et al., 2017).
The total phenolic content of our current SBH sample is slightly lower as compared to the previous findings, which were between 22.81 and 23.53 mg GAE/100 g honey (Ranneh et al., 2018). However, another study reported that the total phenolic content of SBH falls between the range of 79.15 ± 0.6 mg GAE/100 g honey and 105.88 ± 2.1 mg GAE/100 g honey (Kek et al., 2014). Many factors have been reported to cause variations in the total amount of phenolic content in SBH, such as the sources of nectar, the season of honey collection, storage condition, and harvesting technology (Kaškonienė and Venskutonis, 2010). In this present study, both DPPH radical scavenging activity and FRAP value of SBH were relatively lower as compared to the previously reported values (Alvarez-Suarez et al., 2018, Ng et al., 2017, Nweze et al., 2017). The main reason underlying such observation might be due to the discrepancy in the total phenolic content of the SBH since the antioxidant power of the SBH was positively correlated to the amount of phenolic content (Alvarez-Suarez et al., 2018, Nweze et al., 2017, Ranneh et al., 2018). Previously, high amount of phenolic content has also been reported to increase the antioxidant capacity of honey as well (Wilczyńska 2010).
Results from the MTT assay demonstrated that SBH alone can induce cell death in WIL2-NS cells and RAW 264.7 in a concentration-dependent manner. Previous studies have shown that the osmolality of honey is in parallel with the concentration of honey (Tan et al., 2014). As a result, more fluid will exit the cells when the concentration of the honey increases in order to achieve homeostasis with the cell's external environment. Eventually, this will cause cell death when the concentration of the honey is beyond the optimal concentration. Furthermore, the viability of the cells was also impacted by the acidity of the treatment medium since the medium became more acidic with an increasing concentration of SBH. Previously, it has been shown that treatment with Gelam honey can increase the rate of proliferation of corneal keratocytes (Yusof et al., 2016). This finding is in contrast with our current results since treatment with SBH did not stimulate proliferation in WIL2-NS cells. This may be due to the high glucose content found in Gelam honey, which may favor the proliferation of the cells since glucose is the main source of energy (Yusof et al., 2016).
In this present study, SBH was demonstrated to possess strong antioxidant capacity and was able to protect cells from H2O2-induced cytotoxicity and DNA damage. To the best of our knowledge, this is the first work describing the cytoprotective and genoprotective properties of SBH against oxidative damage. It is known that H2O2 can react with the ferrous ion to form hydroxyl radicals, which may damage the DNA strands and cause DNA strand breaks (Cheng et al., 2017). Hence, we postulated that the protective effect of SBH against oxidative DNA damage is probably due to the presence of high amount of phenolic content in the SBH. In agreement with our findings, previous study has demonstrated that honey and its phenolic extract was able to protect cells from H2O2-induced DNA damage due to its potent reducing power and radical scavenging activities (Cheng et al., 2017, Zhou et al., 2012). Besides, the expression level of PARP-1 was determined as well to study the mechanism underlying the genoprotective effects of SBH. PARP-1 is a DNA repairing enzyme that plays a crucial role in maintaining genomic integrity and stability (De Vos et al., 2012). In the occasion of DNA damage, PARP-1 immediately binds to the DNA lesion and catalyzes the poly(ADP-ribose) production, which promotes the recruitment of other DNA repairing and chromatin remodeling factors to the site of lesion (Langelier and Pascal 2013). However, pretreatment with SBH did not cause any significant changes in PARP-1 expression, suggesting that the genoprotective effect of SBH was independent of PARP-1 expression.
Results from this present study showed that SBH was capable of suppressing the production of NO in RAW 264.7 cells after LPS induction. NO is one of the important mediators of inflammation (Aktan 2004). The high amount of NO produced during inflammation is crucial in the elimination of various pathogens. However, overproduction of NO could be destructive because it may damage the surrounding tissue and leads to the development of various inflammatory diseases (Sharma et al., 2007). Thus, our present findings suggest that SBH could help to prevent the development of such complications. Besides that, pretreatment with SBH was also able to downregulate the expression of iNOS. Cells do not express iNOS in normal physiological conditions, and the induction of iNOS expression is usually initiated in response to several stimuli, such as the LPS. The induction of iNOS expression during the inflammatory process catalyzes the conversion of L-arginine into NO (Aktan, 2004). Taken together, we propose that the reduction of NO production in cells pretreated with SBH after LPS induction was due to the inhibitory effects of SBH on iNOS expression. Previously, several studies have also demonstrated that honey (Gelam honey and Tualang honey) was able to downregulate the iNOS expression and NO production (Ahmad et al., 2012, Hussein et al., 2012, Kassim et al., 2010, Kassim et al., 2012a, Kassim et al., 2012b).
COX-2 is the inducible isoform of cyclooxygenase which catalyzes the conversion of arachidonic acid to prostaglandins (Hashemi Goradel et al., 2019). At normal physiological conditions, COX-2 remains undetectable. However, various conditions such as pro-inflammatory stimulus greatly enhance the expression of COX-2. Overexpression of COX-2 is associated with several pathological conditions as well, such as rheumatoid arthritis and cancer (Tsatsanis et al., 2006). Previously, honey has been reported to suppress the induction of COX-2 expression (Ahmad et al., 2012, Hussein et al., 2012, Hussein et al., 2013). However, our present findings showed that SBH was unable to downregulate the expression of COX-2 after LPS induction. Although we do not know the actual reason underlying such discrepancy, however, it could be due to the variation in the composition of the honey tested in this present study and previous studies.
Besides, previous studies have demonstrated that honey can reduce the intracellular ROS level and inhibit the production of ROS from respiratory burst during inflammation (Ahmad et al., 2009, Ahmed Mesaik et al., 2008). Furthermore, it is known that oxidative stress can activate various signaling pathways and leads to inflammation (Reuter et al., 2010). Hence, the ability of honey to scavenge the intracellular ROS can stop the progression of inflammation. In this present study, we demonstrated that SBH was able to protect WIL2-NS cells from H2O2-induced oxidative damage due to its high phenolic content and antioxidant capacity, suggesting that the anti-inflammatory effects of SBH were partly due to its potent antioxidant properties.
Previous in vitro study also demonstrated that SBH was able to reduce the production of interleukin (IL)-6, tumor necrosis factor-α (TNF-α) and interferon production in LPS-stimulated macrophages (Ranneh et al., 2019). Besides, after being challenged with LPS, SBH was showed to improve the antioxidant status and suppress the NF-κB and MAPK signaling in various tissues of rats (Biluca et al., 2020). Taken together, we suggest that SBH might be useful in the management of various inflammatory diseases. Recently, Mustafa et al., (2020) suggested that SBH could be used as a functional food to complement the treatment of early COVID-19 infected patients due to its anti-inflammatory properties. The ability of SBH to suppress the production of IL-6 could help to relieve the severity of coronavirus infection since overexpression of IL-6 has been proposed to worsen the pathogenesis of the infection. However, further study is needed to support such postulation.
5. Conclusion
In conclusion, our current findings showed that SBH is rich in phenolic content, possesses potent cytoprotective and genoprotective effects against H2O2-induced oxidative damage, and was able to suppress the inflammatory process in LPS-induced RAW 264.7 cells. Our current findings suggested that SBH has high medicinal value and might be useful in the treatment and management of many diseases. Future in vivo intervention studies are warranted to further explore the bioavailability and bioefficacy of SBH bioactive compounds and beneficial health effects of SBH in humans when applied topically or consumed orally.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We would like to appreciate the Centre for Research and Instrumentation (CRIM), UKM, for providing the gel photo documentation system.
Funding
This work was supported by the Ministry of Higher Education Malaysia [FRGS/1/2016/SKK05/UKM/02/1].
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd Jalil M.A., Kasmuri A.R., Hadi H. Stingless bee honey, the natural wound healer: A review. Skin Pharmacol. Physiol. 2017;30:66–75. doi: 10.1159/000458416. [DOI] [PubMed] [Google Scholar]
- Ahmad A., Khan R.A., Mesaik M.A. Anti inflammatory effect of natural honey on bovine thrombin-induced oxidative burst in phagocytes. Phyther. Res. 2009;23:801–808. doi: 10.1002/ptr.2648. [DOI] [PubMed] [Google Scholar]
- Ahmad I., Jimenez H., Yaacob N.S., Yusuf N. Tualang honey protects keratinocytes from ultraviolet radiation-induced inflammation and DNA damage. Photochem. Photobiol. 2012;88:1198–1204. doi: 10.1111/j.1751-1097.2012.01100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Mesaik M., Kamran Azim M., Mohiuddin S. Honey modulates oxidative burst of professional phagocytes. Phyther. Res. 2008;22:1404–1408. doi: 10.1002/ptr.2509. [DOI] [PubMed] [Google Scholar]
- Ajibola A., Chamunorwa J.P., Erlwanger K.H. Nutraceutical values of natural honey and its contribution to human health and wealth. Nutr. Metab. 2012;9:61. doi: 10.1186/1743-7075-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75:639–653. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- Aljadi A.M., Kamaruddin M.Y. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chem. 2004;85:513–518. doi: 10.1016/S0308-8146(02)00596-4. [DOI] [Google Scholar]
- Alvarez-Suarez J.M., Giampieri F., Brenciani A., Mazzoni L., Gasparrini M., González-Paramás A.M., Santos-Buelga C., Morroni G., Simoni S., Forbes-Hernández T.Y., Afrin S., Giovanetti E., Battino M. Apis mellifera vs Melipona beecheii Cuban polifloral honeys: A comparison based on their physicochemical parameters, chemical composition and biological properties. LWT - Food Sci. Technol. 2018;87:272–279. doi: 10.1016/j.lwt.2017.08.079. [DOI] [Google Scholar]
- Ashley N.T., Weil Z.M., Nelson R.J. Inflammation: Mechanisms, costs, and natural variation. Annu. Rev. Ecol. Evol. Syst. 2012;43:385–406. doi: 10.1146/annurev-ecolsys-040212-092530. [DOI] [Google Scholar]
- Barakhbah A., Anisah S., Agil S. Honey in the Malay tradition. Malaysian J. Med. Sci. 2007;14:106. [Google Scholar]
- Belay A., Haki G.D., Birringer M., Borck H., Lee Y.C., Cho C.W., Kim K.T., Bayissa B., Baye K., Melaku S. Sugar profile and physicochemical properties of Ethiopian monofloral honey. Int. J. Food Prop. 2017;20:2855–2866. doi: 10.1080/10942912.2016.1255898. [DOI] [Google Scholar]
- Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Biluca F.C., Della Betta F., De Oliveira G.P., Pereira L.M., Gonzaga L.V., Costa A.C.O., Fett R. 5-HMF and carbohydrates content in stingless bee honey by CE before and after thermal treatment. Food Chem. 2014;159:244–249. doi: 10.1016/j.foodchem.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Biluca F.C., Braghini F., Gonzaga L.V., Costa A.C.O., Fett R. Physicochemical profiles, minerals and bioactive compounds of stingless bee honey (Meliponinae) J. Food Compos. Anal. 2016;50:61–69. doi: 10.1016/j.jfca.2016.05.007. [DOI] [Google Scholar]
- Biluca F.C., da Silva B., Caon T., Mohr E.T.B., Vieira G.N., Gonzaga L.V., Vitali L., Micke G., Fett R., Dalmarco E.M., Costa A.C.O. Investigation of phenolic compounds, antioxidant and anti-inflammatory activities in stingless bee honey (Meliponinae) Food Res. Int. 2020;129 doi: 10.1016/j.foodres.2019.108756. [DOI] [PubMed] [Google Scholar]
- Boorn K.L., Khor Y.Y., Sweetman E., Tan F., Heard T.A., Hammer K.A. Antimicrobial activity of honey from the stingless bee Trigona carbonaria determined by agar diffusion, agar dilution, broth microdilution and time-kill methodology. J. Appl. Microbiol. 2010;108:1534–1543. doi: 10.1111/j.1365-2672.2009.04552.x. [DOI] [PubMed] [Google Scholar]
- Cheng N., Wang Y., Cao W. The protective effect of whole honey and phenolic extract on oxidative DNA damage in mice lymphocytes using comet assay. Plant Foods Hum. Nutr. 2017;72:388–395. doi: 10.1007/s11130-017-0634-1. [DOI] [PubMed] [Google Scholar]
- Chua L.S., Adnan N.A., Abdul-Rahaman N.L., Sarmidi M.R. Effect of thermal treatment on the biochemical composition of tropical honey samples. Int. Food Res. J. 2014;21:773–778. [Google Scholar]
- Chuttong B., Chanbang Y., Sringarm K., Burgett M. Physicochemical profiles of stingless bee (Apidae: Meliponini) honey from South East Asia (Thailand) Food Chem. 2016;192:149–155. doi: 10.1016/j.foodchem.2015.06.089. [DOI] [PubMed] [Google Scholar]
- Codex Alimentarius Commission Codex alimentarius commission standards. Codex Stan. 2001;12–1981:1–8. [Google Scholar]
- De Vos M., Schreiber V., Dantzer F. The diverse roles and clinical relevance of PARPs in DNA damage repair: Current state of the art. Biochem. Pharmacol. 2012;84:137–146. doi: 10.1016/j.bcp.2012.03.018. [DOI] [PubMed] [Google Scholar]
- Ediriweera E.R.H.S.S., Premarathna N.Y.S. Medicinal and cosmetic uses of Bee′s Honey – A review. AYU (An Int Q. J. Res. Ayurveda) 2012;33:178. doi: 10.4103/0974-8520.105233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi Goradel N., Najafi M., Salehi E., Farhood B., Mortezaee K. Cyclooxygenase-2 in cancer: A review. J. Cell. Physiol. 2019;234:5683–5699. doi: 10.1002/jcp.27411. [DOI] [PubMed] [Google Scholar]
- Holland L.C., Norris J.M. Medical grade honey in the management of chronic venous leg ulcers. Int. J. Surg. 2015;20:17–20. doi: 10.1016/j.ijsu.2015.05.048. [DOI] [PubMed] [Google Scholar]
- Hussein S.Z., Mohd Yusoff K., Makpol S., Mohd Yusof Y.A. Gelam honey attenuates carrageenan-induced rat paw inflammation via NF-κB pathway. PLoS One. 2013;8 doi: 10.1371/journal.pone.0072365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein S.Z., Mohd Yusoff K., Makpol S., Mohd Yusof Y.A. Gelam honey inhibits the production of proinflammatory, mediators NO, PGE 2, TNF-α, and IL-6 in carrageenan-induced acute paw edema in rats. Evidence-based Complement. Altern. Med. 2012;2012 doi: 10.1155/2012/109636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein S.Z., Yusoff K.M., Makpol S., Yusof Y.A.M. Antioxidant capacities and total phenolic contents increase with gamma irradiation in two types of Malaysian honey. Molecules. 2011;16:6378–6395. doi: 10.3390/molecules16066378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish J., Carter D.A., Shokohi T., Blair S.E. Honey has an antifungal effect against Candida species. Med. Mycol. 2006;44:289–291. doi: 10.1080/13693780500417037. [DOI] [PubMed] [Google Scholar]
- Jenkins R., Burton N., Cooper R. Manuka honey inhibits cell division in methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2011;66:2536–2542. doi: 10.1093/jac/dkr340. [DOI] [PubMed] [Google Scholar]
- Kaškonienė V., Venskutonis P.R. Floral markers in honey of various botanical and geographic origins: a review. Compr. Rev. Food Sci. Food Saf. 2010;9:620–634. doi: 10.1111/j.1541-4337.2010.00130.x. [DOI] [PubMed] [Google Scholar]
- Kassim M., Achoui M., Mansor M., Yusoff K.M. The inhibitory effects of Gelam honey and its extracts on nitric oxide and prostaglandin E2 in inflammatory tissues. Fitoterapia. 2010;81:1196–1201. doi: 10.1016/j.fitote.2010.07.024. [DOI] [PubMed] [Google Scholar]
- Kassim M., Mansor M., Suhaimi A., Ong G., Yusoff K.M. Gelam honey scavenges peroxynitrite during the immune response. Int. J. Mol. Sci. 2012;13:12113–12129. doi: 10.3390/ijms130912113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassim M., Yusoff K.M., Ong G., Sekaran S., Yusof M.Y.B.M., Mansor M. Gelam honey inhibits lipopolysaccharide-induced endotoxemia in rats through the induction of heme oxygenase-1 and the inhibition of cytokines, nitric oxide, and high-mobility group protein B1. Fitoterapia. 2012;83:1054–1059. doi: 10.1016/j.fitote.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Kek S.P., Chin N.L., Yusof Y.A., Tan S.W., Chua L.S. Total phenolic contents and colour intensity of Malaysian honeys from the Apis spp. and Trigona spp. Bees. Agric. Agric. Sci. Procedia. 2014;2:150–155. doi: 10.1016/j.aaspro.2014.11.022. [DOI] [Google Scholar]
- Kek S.P., Chin N.L., Yusof Y.A., Tan S.W., Chua L.S. Classification of entomological origin of honey based on its physicochemical and antioxidant properties. Int. J. Food Prop. 2018;20:S2723–S2738. doi: 10.1080/10942912.2017.1359185. [DOI] [Google Scholar]
- Kryston T.B., Georgiev A.B., Pissis P., Georgakilas A.G. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat. Res. - Fundam. Mol. Mech. Mutagen. 2011;711:193–201. doi: 10.1016/j.mrfmmm.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Langelier M.F., Pascal J.M. PARP-1 mechanism for coupling DNA damage detection to poly(ADP-ribose) synthesis. Curr. Opin. Struct. Biol. 2013;23:134–143. doi: 10.1016/j.sbi.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard D.B., Chua K.F., Mostoslavsky R., Franco S., Gostissa M., Alt F.W. DNA repair, genome stability, and aging. Cell. 2005;120:497–512. doi: 10.1016/j.cell.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Lu T., Pan Y., Kao S.Y., Li C., Kohane I., Chan J., Yankner B.A. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Mustafa M.Z., Shamsuddin S.H., Sulaiman S.A., Abdullah J.M. Anti-inflammatory properties of stingless bee honey may reduce the severity of pulmonary manifestations in COVID-19 infections. Malaysian J. Med. Sci. 2020;27:165–169. doi: 10.21315/mjms2020.27.2.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Neutrophils and immunity: Challenges and opportunities. Nat. Rev. Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- Ng W.J., Chan Y.J., Lau Z.K., Lye P.Y., Ee K.Y. Antioxidant properties and inhibitory effects of Trigona honey against Staphylococcus Aureus Planktonic and biofilm cultures. Int. J. Geomate. 2017;13:28–33. doi: 10.21660/2017.37.2703. [DOI] [Google Scholar]
- Nishio, E.K., Ribeiro, J.M., Oliveira, A.G., Andrade, C.G.T.J., Proni, E.A., Kobayashi, R.K.T., Nakazato, G., 2016. Antibacterial synergic effect of honey from two stingless bees: Scaptotrigona bipunctata Lepeletier, 1836, and S. postica Latreille, 1807. Sci. Rep. 6, 21641. https://doi.org/10.1038/srep21641. [DOI] [PMC free article] [PubMed]
- Nweze J.A., Okafor J.I., Nweze E.I., Nweze J.E. Evaluation of physicochemical and antioxidant properties of two stingless bee honeys: A comparison with Apis mellifera honey from Nsukka, Nigeria. BMC Res. Notes. 2017;10:566. doi: 10.1186/s13104-017-2884-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi T.C., Chan K.M., Sharif R. Zinc L-carnosine suppresses inflammatory responses in lipopolysaccharide-induced RAW 264.7 murine macrophages cell line via activation of Nrf2/HO-1 signaling pathway. Immunopharmacol. Immunotoxicol. 2017;39:259–267. doi: 10.1080/08923973.2017.1344987. [DOI] [PubMed] [Google Scholar]
- Ooi T.C., Chan K.M., Sharif R. Protective effects of zinc L-carnosine against hydrogen peroxide-induced DNA damage and micronucleus formation in CCD-18co human colon fibroblast cells. Free Radic. Res. 2020;54:330–340. doi: 10.1080/10715762.2020.1763333. [DOI] [PubMed] [Google Scholar]
- Ooi T.C., Mohammad N.H., Sharif R. Zinc carnosine protects against hydrogen peroxide-induced DNA damage in WIL2-NS lymphoblastoid cell line independent of poly (ADP-Ribose) polymerase expression. Biol. Trace Elem. Res. 2014;162:8–17. doi: 10.1007/s12011-014-0153-y. [DOI] [PubMed] [Google Scholar]
- Pizzino G., Irrera N., Cucinotta M., Pallio G., Mannino F., Arcoraci V., Squadrito F., Altavilla D., Bitto A. Oxidative stress: harms and benefits for human health. Oxid. Med. Cell. Longev. 2017;2017:8416763. doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranneh Y., Akim A.M., Hamid H.A., Khazaai H., Fadel A., Mahmoud A.M. Stingless bee honey protects against lipopolysaccharide induced-chronic subclinical systemic inflammation and oxidative stress by modulating Nrf2, NF-κB and p38 MAPK. Nutr. Metab. 2019;16:15. doi: 10.1186/s12986-019-0341-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranneh Y., Ali F., Zarei M., Akim A.M., Hamid H.A., Khazaai H. Malaysian stingless bee and Tualang honeys: A comparative characterization of total antioxidant capacity and phenolic profile using liquid chromatography-mass spectrometry. LWT – Food Sci. Technol. 2018;89:1–9. doi: 10.1016/j.lwt.2017.10.020. [DOI] [Google Scholar]
- Rao P.V., Krishnan K.T., Salleh N., Gan S.H. Biological and therapeutic effects of honey produced by honey bees and stingless bees: A comparative review. Rev. Bras. Farmacogn. 2016;26:657–664. doi: 10.1016/j.bjp.2016.01.012. [DOI] [Google Scholar]
- Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Se K.W., Ibrahim R.K.R., Wahab R.A., Ghoshal S.K. Accurate evaluation of sugar contents in stingless bee (Heterotrigona itama) honey using a swift scheme. J. Food Compos. Anal. 2018;66:46–54. doi: 10.1016/j.jfca.2017.12.002. [DOI] [Google Scholar]
- Serhan C.N., Gupta S.K., Perretti M., Godson C., Brennan E., Li Y., Soehnlein O., Shimizu T., Werz O., Chiurchiù V., Azzi A., Dubourdeau M., Gupta S.S., Schopohl P., Hoch M., Gjorgevikj D., Khan F.M., Brauer D., Tripathi A., Cesnulevicius K., Lescheid D., Schultz M., Särndahl E., Repsilber D., Kruse R., Sala A., Haeggström J.Z., Levy B.D., Filep J.G., Wolkenhauer O. The Atlas of Inflammation Resolution (AIR) Mol. Aspects Med. 2020;74 doi: 10.1016/j.mam.2020.100894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma J.N., Al-Omran A., Parvathy S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. 2007;15:252–259. doi: 10.1007/s10787-007-0013-x. [DOI] [PubMed] [Google Scholar]
- Tan J.J., Azmi S.M., Yong Y.K., Cheah H.L., Lim V., Sandai D., Shaharuddin B. Tualang honey improves human corneal epithelial progenitor cell migration and cellular resistance to oxidative stress in vitro. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsatsanis C., Androulidaki A., Venihaki M., Margioris A.N. Signalling networks regulating cyclooxygenase-2. Int. J. Biochem. Cell Biol. 2006;38:1654–1661. doi: 10.1016/j.biocel.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Vandamme L., Heyneman A., Hoeksema H., Verbelen J., Monstrey S. Honey in modern wound care: A systematic review. Burns. 2013;39:1514–1525. doi: 10.1016/j.burns.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Wang C., Guo M., Zhang N., Wang G. Effectiveness of honey dressing in the treatment of diabetic foot ulcers: A systematic review and meta-analysis. Complement. Ther. Clin. Pract. 2019;34:123–131. doi: 10.1016/j.ctcp.2018.09.004. [DOI] [PubMed] [Google Scholar]
- Wilczyńska A. Phenolic content and antioxidant activity of different types of polish honey – A short report. Polish J. Food Nutr. Sci. 2010;60:309–313. [Google Scholar]
- Yaacob M., Rajab N.F., Shahar S., Sharif R. Stingless bee honey and its potential value: A systematic review. Food Res. 2018;2:124–133. doi: 10.26656/fr.2017.2(2).212. [DOI] [Google Scholar]
- Yaacob M., Stanis A.J., Rajab N.F., Shahar S., Sharif R. Current knowledge on honey and its derivatives with genomic stability: A mini review. J. Agric. Sci. 2017;9:145. doi: 10.5539/jas.v9n13p145. [DOI] [Google Scholar]
- Yazan L.S., Muhamad Zali M.F.S., Ali R.M., Zainal N.A., Esa N., Sapuan S., Ong Y.S., Tor Y.S., Gopalsamy B., Voon F.L., Syed Alwi S.S. Chemopreventive properties and toxicity of kelulut honey in sprague dawley rats induced with azoxymethane. Biomed Res. Int. 2016;2016:4036926. doi: 10.1155/2016/4036926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusof A.M., Abd Ghafar N., Kamarudin T.A., Hui C.K., Yusof Y.A.M. Gelam honey potentiates ex vivo corneal keratocytes proliferation with desirable phenotype expression. BMC Complement. Altern. Med. 2016;16:76. doi: 10.1186/s12906-016-1055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Li P., Cheng N., Gao H., Wang B., Wei Y., Cao W. Protective effects of buckwheat honey on DNA damage induced by hydroxyl radicals. Food Chem. Toxicol. 2012;50:2766–2773. doi: 10.1016/j.fct.2012.05.046. [DOI] [PubMed] [Google Scholar]
- Zitvogel L., Kepp O., Kroemer G. Decoding cell death signals in inflammation and immunity. Cell. 2010;140:798–804. doi: 10.1016/j.cell.2010.02.015. [DOI] [PubMed] [Google Scholar]