Key Points
Question
What is the frequency of adverse events among individuals carrying truncating variants of the gene encoding filamin C (FLNCtv) vs the gene encoding TTN (TTNtv)?
Findings
In this cohort study of 167 consecutive FLNCtv carriers, 29 (17%) reached the primary end point of malignant ventricular arrhythmia (MVA) or end-stage heart failure. There was no significant difference in freedom from MVA among FLNCtv carriers with mild to moderate vs severely impaired left ventricular ejection fraction (LVEF), but FLNCtv carriers with baseline impaired LVEF had reduced freedom from an MVA end point compared with TTNtv carriers with similar LVEF.
Meaning
Higher LVEF values than those currently recommended should be considered for a prophylactic implantable cardioverter-defibrillator in FLNCtv carriers.
Abstract
Importance
Truncating variants in the gene encoding filamin C (FLNCtv) are associated with arrhythmogenic and dilated cardiomyopathies with a reportedly high risk of ventricular arrhythmia.
Objective
To determine the frequency of and risk factors associated with adverse events among FLNCtv carriers compared with individuals carrying TTN truncating variants (TTNtv).
Design, Setting, and Participants
This cohort study recruited 167 consecutive FLNCtv carriers and a control cohort of 244 patients with TTNtv matched for left ventricular ejection fraction (LVEF) from 19 European cardiomyopathy referral units between 1990 and 2018. Data analyses were conducted between June and October, 2020.
Main Outcomes and Measures
The primary end point was a composite of malignant ventricular arrhythmia (MVA) (sudden cardiac death, aborted sudden cardiac death, appropriate implantable cardioverter-defibrillator shock, and sustained ventricular tachycardia) and end-stage heart failure (heart transplant or mortality associated with end-stage heart failure). The secondary end point comprised MVA events only.
Results
In total, 167 patients with FLNCtv were studied (55 probands [33%]; 89 men [53%]; mean [SD] age at baseline evaluation, 43 [18] years). For a median follow-up of 20 months (interquartile range, 7-60 months), 29 patients (17.4%) reached the primary end point (19 patients with MVA and 10 patients with end-stage heart failure). Eight (44%) arrhythmic events occurred among individuals with baseline mild to moderate left ventricular systolic dysfunction (LVSD) (LVEF = 36%-49%). Univariable risk factors associated with the primary end point included proband status, LVEF decrement per 10%, ventricular ectopy (≥500 in 24 hours) and myocardial fibrosis detected on cardiac magnetic resonance imaging. The LVEF decrement (hazard ratio [HR] per 10%, 1.83 [95% CI, 1.30-2.57]; P < .001) and proband status (HR, 3.18 [95% CI, 1.12-9.04]; P = .03) remained independent risk factors on multivariable analysis (excluding myocardial fibrosis and ventricular ectopy owing to case censoring). There was no difference in freedom from MVA between FLNCtv carriers with mild to moderate or severe (LVEF ≤35%) LVSD (HR, 1.29 [95% CI, 0.45-3.72]; P = .64). Carriers of FLNCtv with impaired LVEF at baseline evaluation (n = 69) had reduced freedom from MVA compared with 244 TTNtv carriers with similar baseline LVEF (for mild to moderate LVSD: HR, 16.41 [95% CI, 3.45-78.11]; P < .001; for severe LVSD: HR, 2.47 [95% CI, 1.04-5.87]; P = .03).
Conclusions and Relevance
The high frequency of MVA among patients with FLNCtv with mild to moderate LVSD suggests that higher LVEF values than those currently recommended should be considered for prophylactic implantable cardioverter-defibrillator therapy in FLNCtv carriers.
This cohort study determines the frequency of adverse events and assesses the association of these events with the severity of left ventricular systolic dysfunction among individuals carrying filamin C truncating variants vs titin truncating variants.
Introduction
Dilated cardiomyopathy (DCM) has an estimated population prevalence ratio of 1:250 and is the commonest cause for heart transplant (HTx) worldwide.1,2 Pathogenic truncating variants in the gene encoding filamin C, a large actin–cross-linking protein located at the myofibrillar intercalated Z-disk, account for up to 4% of DCM cases, which are characterized by an arrhythmogenic phenotype with extensive left ventricular myocardial fibrosis.3,4 The aim of the present study was to determine the frequency of adverse events and to assess the association of these adverse events with the severity of left ventricular systolic dysfunction (LVSD) in a large international cohort of carriers of the heterozygous FLNC truncating variant (FLNCtv) (OMIM 102565) compared with a control population of patients with DCM having truncating variants in the TTN gene (TTNtv) (OMIM 188840), the commonest genetic subtype of DCM.
Methods
Study Design and Cohort Composition
This was a multicenter, longitudinal cohort study of patients with FLNCtv recruited from 19 European cardiomyopathy units between 1990 and 2018. The study conformed to the principles of the Declaration of Helsinki.5 The authors from each participating center guaranteed the integrity of data from their institution and received approval for anonymized patient data collation and analysis granted by local ethics committees and institutional review boards. All participating patients provided written informed consent for genetic testing. No one received compensation or was offered any incentive for participating in this study.
Genetic Testing
Probands were defined as an index patient with an FLNCtv and either left ventricular ejection fraction (LVEF) lower than 50% or unexplained left ventricular dilatation, irrespective of the presence of a family history of sudden cardiac death (SCD) or DCM (eMethods in the Supplement). Genetic testing in probands was undertaken using next-generation targeted panels or whole-exome sequencing at participating institutions or at a regional accredited genetics laboratory. Novel or rare FLNCtvs (minor allele frequency <0.01% in control populations using gnomAD browser6) were prioritized and classified using the American College of Medical Genetics and Genomics criteria. Only pathogenic or likely pathogenic variants were included (eMethods in the Supplement).7 Patients with additional pathogenic gene variants associated with cardiomyopathy were excluded from the analysis. Sanger sequencing was used for cascade screening of relatives. The FLNCtvs were defined as nonsense, frameshift, or canonical splice site variants (eMethods in the Supplement). Identical methods were used for the control cohort of carriers of TTNtv.
Baseline Assessment
Demographic characteristics, symptoms, 12-lead electrocardiogram (ECG), transthoracic echocardiogram (TTE), and, where available, Holter and cardiac magnetic resonance (CMR) data were collected from clinical records. Data were collected independently at each participating center by using uniform methods. Race/ethnicity information was obtained from hospital databases by patient self-identification provided to local health care clinicians and was reported to assess whether any associations may be generalized across races/ethnicities.
We defined LVSD as LVEF lower than 50% on TTE or CMR imaging. Mild to moderate and severe LVSD were defined as LVEF values of 36% to 49% and 35% or lower, respectively. Frequent ventricular ectopy (VE) was defined as a VE burden of 500 or more in 24 hours.3 Abnormal T-wave inversion, atrial tachyarrhythmia, left ventricular (LV) dilatation, and nonsustained ventricular tachycardia (NSVT) were defined per international standards (eMethods in the Supplement).8,9,10 A mild nondiagnostic phenotype was defined as a minor cardiac abnormality suggestive of genetic expression on clinical evaluation (atrial fibrillation, abnormal T-wave inversion on ECG, LV dilatation, NSVT, frequent VE, or late gadolinium enhancement [LGE]) in patients with preserved LVEF of 50% or higher.
Study End Points
All patients had planned reviews every 12 to 18 months or more frequently if clinically indicated. The follow-up for each patient was calculated from the date of the first evaluation at a participating center to the occurrence of a study end point, death from another cause, or the date of the most recent evaluation. An arbitrary minimum follow-up duration of 1 day was applied to censor adverse events presenting at baseline evaluation.
The primary end point was a composite of malignant ventricular arrhythmia (MVA) (SCD, aborted SCD, appropriate implantable cardioverter-defibrillator [ICD] shock, sustained ventricular tachycardia, or antitachycardia pacing for ventricular tachycardia) or end-stage heart failure (ESHF) (HTx or mortality associated with ESHF). A secondary end point of only MVA end point events was also evaluated (eMethods in the Supplement). Patients were censored at the time of their first end point event during follow-up or at their last evaluation, whichever occurred first. Only events occurring during follow-up at participating centers were included.
Carriers of an FLNCtv with baseline LVSD were compared with a control cohort of 244 carriers of TTNtv with DCM at baseline evaluation from the same centers to assess for differences in freedom from the arrhythmic secondary end point for individuals with mild to moderate or severe LVSD. Carriers of TTNtv were used as a control cohort given that they represent the commonest cause of genetic DCM.11
Statistical Analysis
All data were anonymized, and statistical analyses were performed from June to October 2020 using SPSS, version 25.0 (SPSS Inc) and Stata, version 12 (StataCorp). A 2-sided P < .05 defined statistical significance. Baseline demographic characteristic, symptom, ECG, echocardiogram, Holter, and CMR variables were expressed as numbers (percentages), mean (SD) values, or median values (interquartile range [IQR]) as appropriate. Either χ2 analysis or the Fisher exact test was used to compare categorical data for sex differences in FLNCtv carriers and to compare differences between the TTNtv and FLNCtv cohorts. The independent samples t test and the Mann-Whitney test were used to compare normally and nonnormally distributed continuous data from Holter, TTE, and CMR, respectively, between the different FLNCtv sexes and between the different genetic groups.
Univariable Cox regression was used to assess the association of baseline variables with the composite primary end point after confirmation that the proportional hazards assumption remained valid. Risk factors with P < .05 on univariable analysis were incorporated into a multivariable Cox regression model to calculate the associated hazard ratios (HRs) and 95% CIs to enable identification of baseline clinical variables that were associated with adverse events during follow-up.
Kaplan-Meier plots were used to display the cumulative probability of the occurrence of the primary and secondary end points stratified according to the presence and severity of LVSD, and the log-rank test was used to compare survival against individuals with LVEF within reference ranges at baseline evaluation. Kaplan-Meier plots were also used to compare freedom from an arrhythmic secondary end point between FLNCtv and TTNtv carriers with DCM and similar severities of LVSD at baseline evaluation.
Results
Cohort Composition
In total, 175 patients with FLNCtv were identified; 8 patients (6 men) were excluded because they lacked baseline antemortem evaluation or follow-up data owing to index presentation with a SCD event or identification of an FLNCtv after HTx. The final cohort comprised 167 individuals (55 probands [33%] from 61 families; 89 men [53%]) with a median family size of 1 (IQR, 1-4), comprising 49 distinct FLNCtvs (13 nonsense, 23 frameshift, and 10 canonical splice-site variants and 3 deletions) (eFigure 1, eTable 1, and eTable 2 in the Supplement).
Baseline Characteristics
The baseline characteristics of FLNCtv carriers are summarized in Table 1. The mean (SD) age at baseline evaluation was 43 (18) years (mean [SD] age of probands, 46 [16] years; mean age [SD] of relatives, 42 [19] years; P = .11). Dyspnea was the commonest presenting symptom, followed by palpitations.
Table 1. Baseline Demographic and Clinical Characteristics of 167 Patients Positive for FLNC Truncating Variants and With Follow-up Data, Stratified by Sex.
| Variable | No. of patients evaluated from cohort | Patients, No. (%) | P value | ||
|---|---|---|---|---|---|
| Overall | Male (n = 89) | Female (n = 78) | |||
| Demographic characteristic | |||||
| Proband | 167 | 55 (32.9) | 36 (40.4) | 19 (24.4) | .03 |
| White race/ethnicity | 164 | 150 (91.5) | 81 (93.1) | 69 (89.6) | .83 |
| FH of cardiomyopathya | 55 | 35 (63.6) | 21 (58.3) | 14 (73.7) | .26 |
| FH of SCDa | 55 | 30 (54.5) | 19 (52.8) | 11 (57.9) | .72 |
| FH of multiple SCDa | 55 | 16 (29.1) | 8 (22.2) | 8 (42.1) | .12 |
| Baseline clinical evaluation | |||||
| Age, mean (SD), y | 167 | 43 (18) | 45 (17) | 42 (19) | .25 |
| Aborted SCD prior to evaluation | 167 | 9 (5.4) | 8 (9.0) | 1 (1.3) | .04 |
| Dyspnea | 166 | 39 (23.5) | 20 (22.5) | 19 (24.7) | .74 |
| NYHA class III or IV | 17 (10.2) | 10 (11.2) | 7 (9.1) | ||
| Chest pain | 165 | 22 (13.3) | 15 (17.0) | 7 (9.1) | .17 |
| Syncope | 165 | 11 (6.7) | 5 (5.7) | 6 (7.8) | .76 |
| Palpitations | 165 | 36 (21.8) | 22 (25.0) | 14 (18.2) | .29 |
| Baseline ECG evaluation | |||||
| ECG rhythm | 164 | .56 | |||
| Sinus rhythm | 157 (95.7) | 84 (96.6) | 73 (94.8) | ||
| AF | 6 (3.7) | 3 (3.4) | 3 (3.9) | ||
| PR interval, mean (SD), ms | 151 | 155 (28) | 158 (26) | 151 (29) | .15 |
| LBBB QRS morphology | 156 | 7 (4.5) | 3 (3.6) | 4 (5.5) | .74 |
| QRS duration, mean (SD), ms | 155 | 97 (20) | 99 (16) | 94 (23) | .14 |
| Abnormal T-wave inversion | 159 | 38 (23.9) | 24 (28.8) | 14 (18.3) | .53 |
| Low QRS voltage limb leads | 148 | 33 (22.3) | 14 (17.5) | 19 (27.9) | .13 |
| Baseline TTE evaluation, mean (SD) | |||||
| Maximum wall thickness, mm | 131 | 9.5 (2.6) | 10.1 (2.9) | 8.6 (1.9) | .002 |
| Left atrium size PLAX view, mm | 111 | 35.8 (6.2) | 37.9 (6.2) | 33.2 (5.2) | <.001 |
| LV end-diastolic diameter, mm | 137 | 53.5 (9.7) | 55.7 (10.2) | 50.7 (8.2) | .003 |
| LV ejection fraction, % | 162 | 50.6 (15.2) | 49.8 (14.4) | 51.4 (16.1) | .51 |
| RV TAPSE, mm | 77 | 21.3 (3.7) | 21.4 (3.7) | 21.2 (3.7) | .87 |
| Baseline 24-h Holter evaluation | |||||
| NSVT first Holter | 104 | 33 (31.7) | 20 (35.1) | 13 (27.7) | .42 |
| VE burden per 24 h, median (IQR) | 64 | 197 (3-1216) | 400 (5-1343) | 161 (1-688) | .50 |
| Frequent VE (≥500 per 24 h) | 90 | 36 (40.0) | 24 (49.0) | 12 (29.3) | .06 |
| Baseline CMR evaluation, mean (SD) | |||||
| LV end-diastolic volume, mL | 71 | 185 (56) | 208 (54) | 152 (41) | <.001 |
| Ejection fraction, % | |||||
| LV | 89 | 50 (15) | 50 (15) | 50 (16) | .95 |
| RV | 66 | 55 (11) | 54 (9) | 57 (13) | .39 |
| Late gadolinium enhancement | 91 | 50 (54.9) | 37 (64.9) | 13 (38.2) | .01 |
| Extra-cardiac phenotype baseline evaluation | |||||
| CK, median (IQR), U/L | 56 | 79 (59-124) | 85 (64-146) | 73 (57-103) | .09 |
Abbreviations: AF, atrial fibrillation; CK, creatine kinase; CMR, cardiac magnetic resonance; ECG, electrocardiogram; FH, family history; IQR, interquartile range; LBBB, left bundle branch block; LV, left ventricular; NSVT, nonsustained ventricular tachycardia; NYHA, New York Heart Association; PLAX, parasternal long axis; RV, right ventricular; SCD, sudden cardiac death; TAPSE, tricuspid annular plane systolic excursion; TTE, transthoracic echocardiogram; VE, ventricular ectopy.
SI conversion factor: To convert CK to microkatals per liter, multiply by 0.0167.
Probands evaluated only for family history.
Men were more likely than women to be probands (36 [40.4%] vs 19 [24.4%]; P = .03), have an aborted SCD or surrogate prior to baseline evaluation (8 [9.0%] vs 1 [1.3%]; P = .04) and larger mean (SD) left atrial (37.9 [6.2] mm vs 33.2 [5.2] mm; P < .001) and left ventricular (55.7 [10.2] vs 50.7 [8.2]; P = .003) dimensions on TTE and were more likely to have fibrosis detected on CMR imaging (37 [64.9%] vs 13 [38.2%]; P = .01) (Table 1). The mean (SD) baseline LVEF on TTE was lower in probands than in relatives (37.3% [12.9%] vs 57.2% [11.5%]; P < .001); 23 relatives (21%) had LVSD at baseline evaluation (eTable 3 in the Supplement).
Overall, 70 patients (42%) had impaired LVEF at baseline evaluation (37 [52%] with mild to moderate LVSD and 33 [48%] with severe LVSD); 1 patient (1%) had arrhythmogenic cardiomyopathy (with right ventricular microaneurysms and dilatation in the absence of impaired LVEF), and 47 patients (28%) had a normal phenotype; 1 patient lacked baseline LVEF assessment but, together with 48 other patients (29%), showed evidence of a mild nondiagnostic phenotype (Figure 1).
Figure 1. Flowchart Showing the Proportion of Patients With FLNC Truncating Variants (FLNCtvs) Who Had Impaired Left Ventricular Ejection Fraction (LVEF) and Arrhythmogenic Cardiomyopathy (AC), Normal Phenotype, or Mild Nondiagnostic Criteria at Baseline and Final Evaluation.
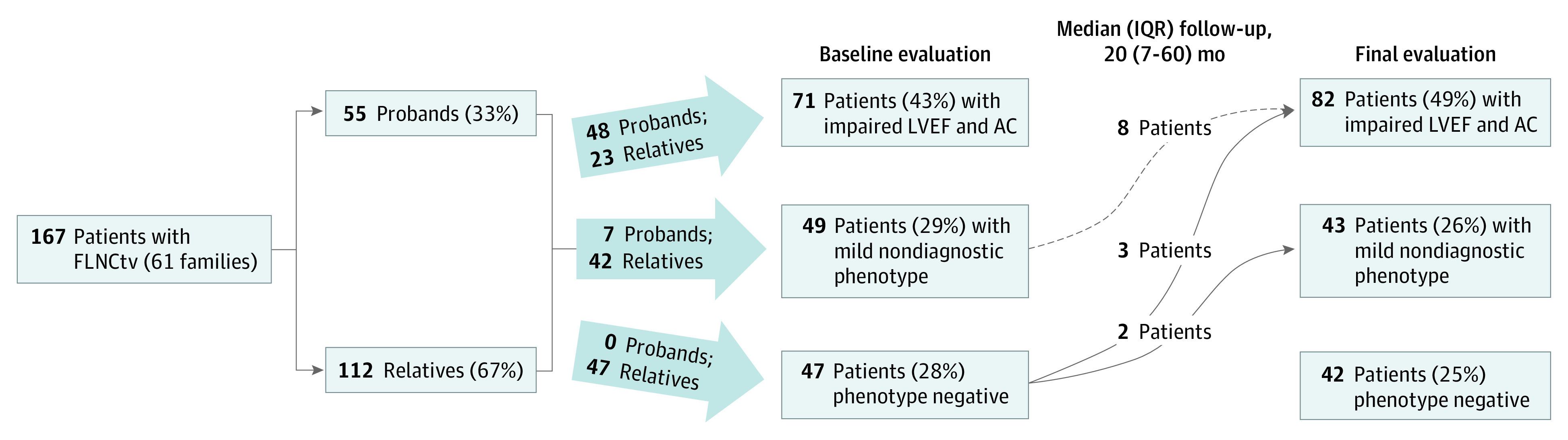
Mild nondiagnostic criteria included isolated left or right ventricular dilation on transthoracic echocardiogram or cardiac magnetic resonance imaging, atrial fibrillation, nonsustained ventricular tachycardia, frequent ventricular ectopy, late gadolinium enhancement on cardiac magnetic resonance imaging, and T-wave inversion on electrocardiogram with LVEF 50% or higher. IQR indicates interquartile range.
At baseline, 6 of 164 individuals (4%) had atrial fibrillation, 36 of 90 individuals (40%) had frequent VE (median, 197 [IQR, 3-1216] VEs in 24 hours), and 33 of 104 individuals (32%) had NSVT. The VE burden increased with progressive LVSD (VEs in 24 hours: median for LVEF within reference ranges, 48 [IQR, 0-479]; median for mild to moderate LVSD, 1142 [IQR, 575-1933]; median for severe LVSD, 1025 [IQR, 339-5625]; P = .001) (eFigure 2 in the Supplement). Among patients with CMR data, 56 of 96 (58%) had LGE (20 [39%] with preserved LVEF, 24 [80%] with mild to moderate LVSD, and 10 [83%] with severe LVSD; P < .001); 8 patients had LVSD without LGE. The presence of LGE at baseline was associated with proband status (85% vs 38%; P < .001), lower LVEF (mean, 45% [15%] vs 56% [12%]; P < .001) and increased VE burden (median, 992 [IQR, 164-2105] vs 1 [IQR, 0-86] VEs in 24 hours; P < .001).
Follow-up
Median follow-up was 20 months (IQR, 7-60 months) with a total of 540 patient-years. By the end of follow-up, 54 patients (32%) had an ICD, of which 12 implants (22%) were for secondary prevention; 3 patients (2%) had received cardiac resynchronization therapy.
Thirteen patients (8%) developed new cardiac abnormalities resulting in a change from baseline diagnostic classification (Figure 1). At final evaluation, 82 patients (49%) had LVSD and arrhythmogenic cardiomyopathy, 43 patients (26%) had a mild nondiagnostic phenotype, and 42 patients (25%) remained phenotype negative.
Twenty-two patients (13%) had a heart failure admission requiring intravenous diuretics. By final evaluation, we identified atrial fibrillation in 19 of 164 patients (12%), frequent VE in 41 of 92 patients (45%), and NSVT in 48 of 112 patients (43%).
Composite Primary End Point
Twenty-nine patients (17.4%) reached the primary end point: MVA in 19 patients (66%) and ESHF in 10 patients (34%). The most frequent MVA end point was SCD (n = 8), followed by aborted SCD (n = 4), appropriate ICD shock (n = 4), and sustained ventricular tachycardia (n = 3; 2 of whom had appropriate antitachycardia pacing). The ESHF end points were most commonly HTx (n = 9) followed by mortality associated with ESHF (n = 1) (Figure 2). The median duration from listing for advanced heart failure therapies to undergoing HTx was 76 days (IQR, 32-195 days).
Figure 2. Flowchart Showing the Outcomes of Patients With FLNC Truncating Variants (FLNCtvs) During Follow-up With Respect to the Composite Primary End Point (Malignant Ventricular Arrhythmia and End-stage Heart Failure End Points).
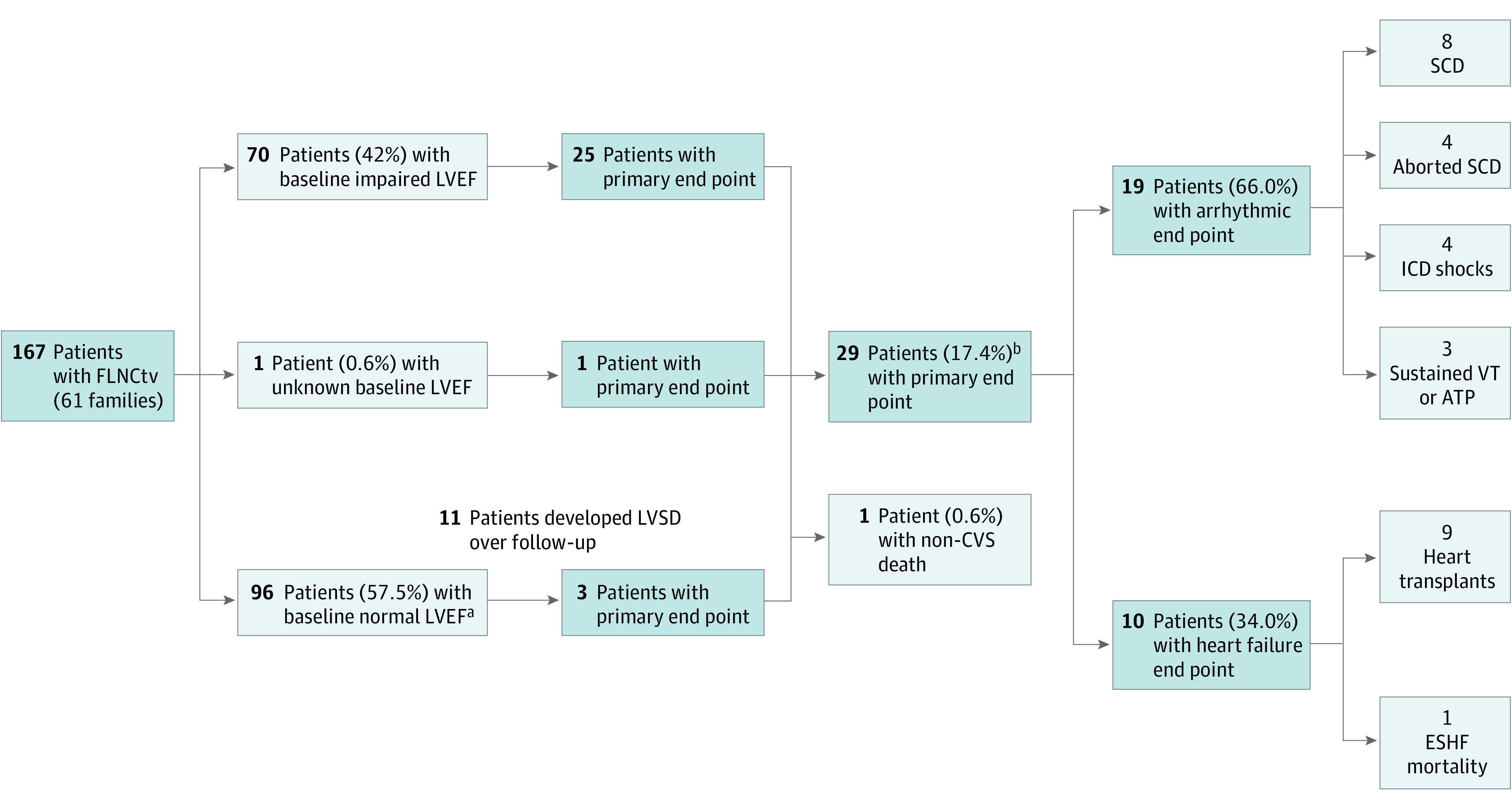
ATP indicates antitachycardia pacing; CVS, cardiovascular; ESHF, end-stage heart failure; LVEF, left ventricular ejection fraction; LVSD, left ventricular systolic dysfunction; ICD, implantable cardioverter-defibrillator; SCD, sudden cardiac death; VT, ventricular tachycardia.
aIncludes 1 patient with arrhythmogenic cardiomyopathy and LVEF within reference ranges.
bFive patients (3%) had multiple malignant ventricular arrhythmia or end-stage heart failure events during follow-up.
Five patients (3%) had multiple MVA, ESHF, or both end point events during follow-up. Six relatives (5.4%) met the primary end point during follow-up.
All end point events occurred in patients with LVSD or LV dilatation, and extensive myocardial structural changes were observed post mortem. The age groups at which the first MVA or ESHF event occurred, including patients presenting with SCD without antemortem evaluation, are shown in eFigure 3 in the Supplement.
All patients with an ESHF end point had severe LVSD at baseline evaluation. Patients with any severity of LVSD at baseline evaluation had decreased survival from the primary end point compared with those with preserved LVEF (HR, 9.35 [95% CI, 2.80-31.21]; P < .001) (Figure 3A). Three patients having LVEF in reference ranges at baseline evaluation went on to reach the primary end point. One was lost to follow-up and presented 10 years later with SCD and had biventricular dilatation and fibrosis on post mortem. The second patient developed severe LVSD during follow-up, followed by SCD. The third patient developed hemodynamically stable, sustained ventricular tachycardia and was subsequently identified to have basal lateral epicardial fibrosis and LV dilatation with preserved LVEF on CMR imaging.
Figure 3. Kaplan-Meier Survival Analyses.
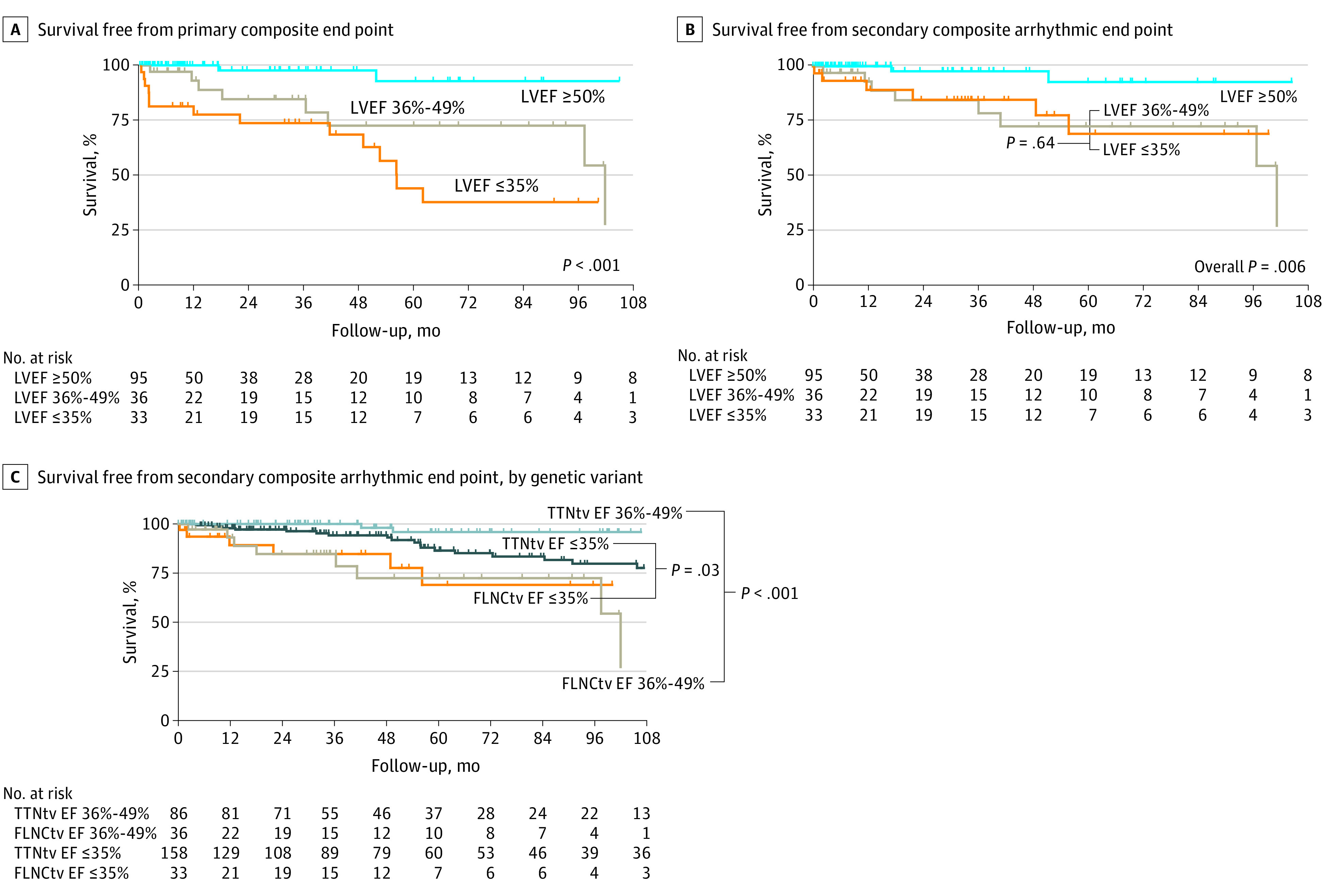
A, Kaplan-Meier survival analysis from baseline evaluation for the primary end point stratified according to the presence of left ventricular ejection fraction (LVEF) within reference ranges (LVEF ≥50%), mild to moderate left ventricular systolic dysfunction (LVSD) (LVEF 36%-49%), or severe LVSD (LVEF ≤35%) on transthoracic echocardiogram (TTE) or cardiac magnetic resonance (CMR) imaging for patients with FLNC truncating variants (FLNCtvs). B, Kaplan-Meier survival analysis from baseline evaluation for the arrhythmic secondary end point stratified according to the presence of LVEF within reference ranges, mild to moderate LVSD, or severe LVSD on TTE or CMR imaging for patients with FLNCtv. C, Kaplan-Meier survival analysis from baseline evaluation for the arrhythmic secondary end point stratified according to genetic variant (for TTN or FLNC) and the presence of baseline mild to moderate LVSD or severe LVSD on TTE or CMR imaging.
Arrhythmic Secondary End Point
Nineteen patients (11.4%) reached the arrhythmic secondary end point. The frequency of arrhythmic secondary end point events among patients having values within references ranges was 3 of 95 (3.2%), and it was 8 of 36 (22.2%) among patients with mild to moderate LVSD at baseline and 7 of 33 (21.2%) among patients with severe LVSD at baseline (Figure 3B). There was no significant difference in freedom from MVA between patients with mild to moderate and patients with severely impaired LVEF (HR, 1.29 [95% CI, 0.45-3.72]; P = .64). The presence of any degree of LVSD at baseline evaluation was associated with a significantly increased risk of MVA events (mild to moderate LVSD vs LVEF within reference ranges: HR, 6.80 [95% CI, 1.76-26.35]; P = .006; severe LVSD vs LVEF within reference ranges: HR, 5.08 [95% CI, 1.30-19.90]; P = .02).
LVEF at Time of Arrhythmic Events
A summary of LVEF contemporary to censored arrhythmic events during follow-up as well as excluded events occurring prior to baseline evaluation is given in eTable 4 in the Supplement. Those findings show that, for 12 patients (52% of patients with MVA events and with clinical evaluation), the first MVA event occurred in the presence of only mild to moderate LVSD.
TTN Control Group Comparison
We compared 69 patients with FLNCtv who had impaired LVEF at baseline evaluation against 244 patients from the same centers with TTNtv and DCM. The baseline demographic and clinical characteristics of the 2 genetic cohorts are given in Table 2. Patients with TTNtv were more likely than patients with FLNCtv to be male (69.3% vs 56.5%; P = .048), have larger left atrial dimensions (mean [SD], 41.2 [7.7] mm vs 37.5 [7.3] mm; P = .004), and have lower LVEF on TTE (mean [SD], 30.6% [11.3%] vs 36.2% [11.1%]; P < .001). Conversely, patients who were FLNCtv carriers with DCM were more likely than patients who were TTNtv carriers with DCM to have a family history of SCD (24 [52.2%] vs 47 [25.0%]; P < .001), lower QRS voltage on ECG (20 [33.9%] vs 29 [12.6%]; P < .001), frequent VE on 24-hour Holter monitor (24 [70.6%] vs 40 [41.2%]; P = .003), and fibrosis on baseline CMR imaging (35 [83.3%] vs 65 [57.0%]; P = .002).
Table 2. Baseline Demographic and Clinical Characteristics of 313 Patients With DCM (69 With FLNCtv and 244 With TTNtv) and With Impaired LVEF at Baseline Evaluation.
| Characteristic | No. of patients evaluated from cohort (n = 313) | Patients, No. (%) | P value | ||
|---|---|---|---|---|---|
| Overall | FLNCtv (n = 69) | TTNtv (n = 244) | |||
| Demographic data | |||||
| White race/ethnicity | 310 | 276 (89.0) | 61 (92.4) | 215 (88.1) | .50 |
| Proband | 313 | 234 (74.8) | 46 (66.7) | 188 (77.0) | .08 |
| Male sex | 313 | 208 (66.5) | 39 (56.5) | 169 (69.3) | .048 |
| FH of cardiomyopathya | 234 | 131 (56.0) | 30 (65.2) | 101 (53.7) | .16 |
| FH of SCDa | 234 | 71 (30.3) | 24 (52.2) | 47 (25.0) | <.001 |
| FH of multiple SCDa | 234 | 24 (10.3) | 13 (28.3) | 11 (5.9) | <.001 |
| Baseline clinical evaluation | |||||
| Age, mean (SD), y | 313 | 48 (16) | 49 (18) | 48 (15) | .76 |
| Dyspnea | 307 | 205 (66.8) | 35 (51.5) | 170 (71.1) | .002 |
| NYHA class III or IV | 104 (33.9) | 16 (23.5) | 88 (36.8) | ||
| Chest pain | 307 | 52 (16.9) | 15 (22.1) | 37 (15.5) | .20 |
| Syncope | 307 | 16 (5.2) | 5 (7.4) | 11 (4.6) | .37 |
| Palpitations | 307 | 95 (30.9) | 24 (35.3) | 71 (29.7) | .38 |
| Baseline ECG evaluation | |||||
| ECG rhythm | 305 | .03 | |||
| Sinus rhythm | 265 (86.9) | 63 (92.6) | 202 (85.2) | ||
| AF | 39 (12.8) | 4 (5.9) | 35 (14.8) | ||
| PR interval, mean (SD), ms | 225 | 169 (33) | 154 (32) | 173 (32) | <.001 |
| LBBB QRS morphology | 300 | 28 (9.3) | 7 (10.6) | 21 (9.0) | .69 |
| QRS duration, mean (SD), ms | 250 | 103 (22) | 108 (20) | 102 (22) | .08 |
| Abnormal T-wave inversion | 293 | 114 (38.9) | 31 (45.6) | 83 (36.9) | .20 |
| Low QRS voltage limb leads | 290 | 49 (16.9) | 20 (33.9) | 29 (12.6) | <.001 |
| Baseline TTE evaluation, mean (SD) | |||||
| Maximum wall thickness, mm | 261 | 9.5 (1.8) | 9.3 (1.8) | 9.6 (1.8) | .28 |
| Left atrium size PLAX view, mm | 213 | 40.4 (7.7) | 37.5 (7.3) | 41.2 (7.7) | .004 |
| LV end-diastolic diameter, mm | 254 | 60.1 (8.9) | 60.2 (9.8) | 60.0 (8.7) | .92 |
| LV ejection fraction TTE, % | 313 | 31.9 (11.5) | 36.2 (11.1) | 30.6 (11.3) | <.001 |
| RV TAPSE, mm | 146 | 18.2 (4.9) | 19.8 (4.0) | 17.6 (5.0) | .01 |
| Baseline 24-h Holter evaluation | |||||
| NSVT first Holter | 199 | 100 (50.3) | 22 (47.8) | 78 (51.0) | .71 |
| VE burden per 24 h, median (IQR) | 113 | 469 (32-2419) | 1142 (488-2105) | 250 (19-2513) | .08 |
| Frequent VE (≥500 per 24 h) | 131 | 64 (48.9) | 24 (70.6) | 40 (41.2) | .003 |
| Baseline CMR evaluation, mean (SD) | |||||
| LV end-diastolic volume | 124 | 231 (81) | 217 (60) | 236 (87) | .19 |
| Ejection fraction, % | |||||
| LV CMR | 156 | 36 (13) | 37 (12) | 36 (13) | .47 |
| RV | 112 | 49 (14) | 50 (14) | 49 (14) | .62 |
| Late gadolinium enhancement | 156 | 100 (64.1) | 35 (83.3) | 65 (57.0) | .002 |
| Extra-cardiac phenotype baseline evaluation | |||||
| CK, median (IQR), U/L | 88 | 88 (61-132) | 80 (58-114) | 100 (73-175) | .08 |
Abbreviations: AF, atrial fibrillation; CK, creatine kinase; CMR, cardiac magnetic resonance; DCM, dilated cardiomyopathies; ECG, electrocardiogram; FH, family history; FLNCtv, FLNC truncating variants; IQR, interquartile range; LBBB, left bundle branch block; LV, left ventricular; LVEF, left ventricular ejection fraction; NSVT, nonsustained ventricular tachycardia; NYHA, New York Heart Association; PLAX, parasternal long axis; RV, right ventricular; TAPSE, tricuspid annular plane systolic excursion; TTE, transthoracic echocardiogram; TTNtvs, TTN truncating variants; VE, ventricular ectopy.
SI conversion factor: To convert CK to microkatals per liter, multiply by 0.0167.
Probands evaluated only for family history.
Among patients with mild to moderate LVSD at baseline evaluation, 36 FLNCtv carriers and 86 TTNtv carriers had similar ages (mean [SD], 48 [17] years vs 46 [16] years; P = .39) and baseline LVEF (mean [SD], 42% [4%] for both; P = .79). Among patients with severe LVSD at baseline evaluation, 33 FLNCtv carriers and 158 TTNtv carriers had similar ages (mean [SD], 49 [19] years vs 50 [14] years; P = .97) and baseline LVEF (mean [SD], 25% [8%] vs 23% [7%]; P = .16).
The composite primary end point of MVA or ESHF events was reach by 71 patients (47 of 244 [19.3%] TTNtv carriers vs 24 of 69 [34.8%] FLNCtv carriers; P < .001]). The arrhythmic secondary end point was reached by 39 patients (24 of 244 [9.8%] TTNtv carriers vs 15 of 69 [21.7%] FLNCtv carriers; P < .001]).
With respect to arrhythmic end point events, FLNCtv carriers with impaired LVEF at baseline evaluation had decreased freedom from an MVA end point compared with the control cohort of TTNtv carriers with similar baseline LVEF (for patients with mild to moderate LVSD: HR, 16.41 [95% CI, 3.45-78.11], P < .001; for patients with severe LVSD: HR, 2.47 [95% CI, 1.04-5.87]; P = .03) (Figure 3C).
Risk Factors Associated With the Primary End Point
Univariable Cox regression analysis identified baseline risk factors associated with the composite primary end point to be proband status (HR, 4.48 [95% CI, 1.79-11.16]; P < .001), LVEF decrement (from LVEF 50%) (HR per 10%, 2.08 [95% CI, 1.54-2.81]; P < .001), frequent VE (HR, 5.05 [95% CI, 1.09-23.40]; P = .04), and LGE (HR, 10.38 [95% CI, 1.36-79.02]; P = .02) (eTable 5 in the Supplement). Significant baseline variables (P < .05) as well as age at baseline evaluation (to reflect age-related penetrance) were incorporated into a multivariable model, with the exception of LGE and frequent VE, because fewer individuals underwent complete baseline Holter or CMR evaluation, reflecting different institutional practices. These variables were excluded to minimize the risk of data reduction by case censoring of incomplete data (use of baseline frequent VE and LGE would allow for incorporation of only 68 cases and 9 events in the multivariable model). Multivariable modeling incorporating 157 patients (94%) with 27 primary end point events showed LVEF decrement (from LVEF 50%) (HR per 10%, 1.83 [95% CI, 1.30-2.57]; P < .001) and proband status (HR, 3.18 [95% CI, 1.12-9.04]; P = .03) to be independent risk factors associated with the primary end point (eTable 5 in the Supplement).
Discussion
This study shows that FLNCtv is associated with an arrhythmogenic DCM and that conventional severe LVEF-based thresholds for SCD risk estimation and ICD implantation may underserve this cohort of patients given that ventricular arrhythmias occurred frequently in patients with mild to moderate LVSD. Asymptomatic carriers of FLNCtv with an equivocal clinical phenotype developed cardiomyopathy during follow-up, emphasizing the importance of long-term follow-up.
Association of FLNCtv With MVA
Emerging evidence from cohort studies shows that disease etiology is strongly associated with the natural history and, by implication, treatment of different forms of cardiomyopathy.11,12,13,14 The present study shows that cardiomyopathy among FLNCtv carriers was associated with frequent ventricular arrhythmia, with more than 40% of carriers showing evidence of NSVT or frequent VE and a high risk of MVA and progression to ESHF. We showed that the risk of MVA is substantial throughout early adulthood and that this risk persists through the middle decades of adult life. Progressive heart failure becomes increasingly common from the age of 40 years onward.
Current practice guidelines advocate the use of severe LVSD as the main stratifier for primary-prevention ICD among patients with DCM. However, real-world data have shown that adverse arrhythmic events frequently occur in patients with an LVEF higher than 35%. In this study, we found no difference in the frequency of ventricular arrhythmic events between patients with mild to moderate LVSD and patients with severe LVSD, which suggests that patients who are FLNCtv carriers may be miscategorized as low risk when using conventional guideline-based ejection fraction thresholds.15 Patients who were FLNCtv carriers with mild to moderate LVSD or severe LVSD had significantly more arrhythmic end point events compared with patients who were TTNtv carriers with DCM, despite similar severities of LVSD. This highlights the arrhythmogenicity of FLNCtv and the increased risk of adverse arrhythmic events during follow-up even in individuals with LVEF higher than 35% compared with the commonest cause of genetic DCM.
Disease in Relatives
We identified LVSD in 21% of relatives at baseline evaluation (more than half of whom were asymptomatic) and a change in diagnostic classification during longitudinal follow-up in 8% of the cohort. This finding highlights the importance of familial cascade genetic screening in families affected by FLNCtv and the role of ongoing clinical surveillance for asymptomatic pathogenic variant carriers.
Association of Sex With Disease Expression
As observed for other genetic cardiomyopathies, we detected sex differences in baseline clinical phenotypes, with men showing a higher incidence of hemodynamically unstable ventricular tachycardia or aborted SCD prior to baseline evaluation as well as SCD at presentation. However, survival analysis from baseline evaluation and Cox regression analysis did not identify sex to be an independent risk factor associated with the primary end point. This may be explained in part by similar presenting LVEF on TTE and CMR imaging for both sexes at baseline evaluation, reinforcing the importance of LVEF in causing adverse events. More data are needed with larger cohorts to understand whether this may represent a survival bias, with more men presenting with SCD prior to baseline evaluation.
Myocardial Fibrosis in FLNCtv Carriers
Myocardial fibrosis detected on CMR imaging has been shown to be an important risk factor for MVA in patients with DCM. In patients with FLNCtv, extensive fibrosis is a relatively common finding of postmortem studies,3 and in the present study, we showed that more than 80% of patients with a DCM and arrhythmogenic cardiomyopathy phenotype had LGE detected on CMR imaging. Moreover, more than a third of patients with LVEF within reference ranges also had myocardial fibrosis. Although LGE was not incorporated into the multivariable model to minimize case censoring, univariable analysis indicated that LGE detected on CMR imaging was associated with an increased risk of events. Larger data sets with longer longitudinal follow-up are needed to assess the role of these other variables in estimating outcomes.
Limitations
The participating centers were all referral units, and a majority of patients were of White race/ethnicity (92%); thus, the results of the study may not be generalizable in all clinical scenarios and across all races/ethnicities. Baseline data for patients presenting with SCD were not available. Because probands were identified following clinical presentation, there is a potential for lead-time and length-time bias of various baseline risk factors associated with adverse events. A majority of end point events occurred in probands; therefore, the clinical course identified for nonprobands may be attenuated and less severe. Incomplete data on Holter monitor and CMR imaging, including right ventricular function, limits the ability to identify other arrhythmic or imaging markers associated with outcomes. Body surface area data were not collected, and therefore absolute volumes and dimensions were used for TTE and CMR assessments of ventricular and atrial dimensions. Data were not routinely collected on the presence of heart failure or antiarrhythmic medications, which were managed per routine institutional practice in participating centers in accordance with European Society of Cardiology guidelines. Although strict criteria for pathogenicity were instituted prior to incorporation into the cohort, there were limited cosegregation data to further support the pathogenicity of individual variants.
Conclusions
This study shows that DCM in carriers of FLNCtv is associated with a high incidence of MVA even in the presence of LVEF higher than 35%. This finding supports consideration of higher values of LVEF for ICD implants in this group of patients.
eMethods. Supplementary Definitions
eFigure 1. Structure of Filamin C
eFigure 2. Box-Plot Demonstrating the VE-Burden /24 Hours on Baseline Holter Assessment
eFigure 3. Bar Chart Demonstrating the Spectrum of First MVA or ESHF End Point From Birth in the Entire Cohort of 174 FLNCtv Carriers for Different Age Groups
eTable 1. List of Mutations in FLNC Incorporated into the Cohort and Predicted to Result in Protein Truncation
eTable 2. List of splice Site Mutations in FLNC Identified
eTable 3. Baseline Demographic and Clinical Data of 167 Genotype-Positive FLNCtv Patients With Follow-up Data Stratified According to Proband Status
eTable 4. Table Summarizing Baseline and Follow-up LVEF including Contemporaneous LVEF (Where Available) of Any Arrhythmic End Points
eTable 5. Univariable and Multivariable Predictors of the Composite Primary End Point Using Baseline Clinical Data Obtained From Clinical Evaluation, TTE, CMR, and Holter
eReferences
References
- 1.Towbin JA, Bowles NE. The failing heart. Nature. 2002;415(6868):227-233. doi: 10.1038/415227a [DOI] [PubMed] [Google Scholar]
- 2.Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nat Rev Cardiol. 2013;10(9):531-547. doi: 10.1038/nrcardio.2013.105 [DOI] [PubMed] [Google Scholar]
- 3.Ortiz-Genga MF, Cuenca S, Dal Ferro M, et al. Truncating FLNC mutations are associated with high-risk dilated and arrhythmogenic cardiomyopathies. J Am Coll Cardiol. 2016;68(22):2440-2451. doi: 10.1016/j.jacc.2016.09.927 [DOI] [PubMed] [Google Scholar]
- 4.Begay RL, Graw SL, Sinagra G, et al. Filamin C truncation mutations are associated with arrhythmogenic dilated cardiomyopathy and changes in the cell-cell adhesion structures. JACC Clin Electrophysiol. 2018;4(4):504-514. doi: 10.1016/j.jacep.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 6.gnmoAD browser. Genome aggregation database. Accessed March 28, 2021. https://gnomad.broadinstitute.org
- 7.Richards S, Aziz N, Bale S, et al. ; ACMG Laboratory Quality Assurance Committee . Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. doi: 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ponikowski P, Voors AA, Anker SD, et al. ; Authors/Task Force Members; Document Reviewers . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC): developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891-975. doi: 10.1002/ejhf.592 [DOI] [PubMed] [Google Scholar]
- 9.Pinto YM, Elliott PM, Arbustini E, et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC working group on myocardial and pericardial diseases. Eur Heart J. 2016;37(23):1850-1858. doi: 10.1093/eurheartj/ehv727 [DOI] [PubMed] [Google Scholar]
- 10.Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J. 2010;31(7):806-814. doi: 10.1093/eurheartj/ehq025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akhtar MM, Lorenzini M, Cicerchia M, et al. Clinical phenotypes and prognosis of dilated cardiomyopathy caused by truncating variants in the TTN gene. Circ Heart Fail. 2020;13(10):e006832. doi: 10.1161/CIRCHEARTFAILURE.119.006832 [DOI] [PubMed] [Google Scholar]
- 12.van Rijsingen IA, Arbustini E, Elliott PM, et al. Risk factors for malignant ventricular arrhythmias in lamin A/C mutation carriers a European cohort study. J Am Coll Cardiol. 2012;59(5):493-500. doi: 10.1016/j.jacc.2011.08.078 [DOI] [PubMed] [Google Scholar]
- 13.Japp AG, Gulati A, Cook SA, Cowie MR, Prasad SK. The diagnosis and evaluation of dilated cardiomyopathy. J Am Coll Cardiol. 2016;67(25):2996-3010. doi: 10.1016/j.jacc.2016.03.590 [DOI] [PubMed] [Google Scholar]
- 14.Domínguez F, Cuenca S, Bilińska Z, et al. ; European Genetic Cardiomyopathies Initiative Investigators . Dilated cardiomyopathy due to BLC2-associated athanogene 3 (BAG3) mutations. J Am Coll Cardiol. 2018;72(20):2471-2481. doi: 10.1016/j.jacc.2018.08.2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar S, Baldinger SH, Gandjbakhch E, et al. Long-term arrhythmic and nonarrhythmic outcomes of lamin A/C mutation carriers. J Am Coll Cardiol. 2016;68(21):2299-2307. doi: 10.1016/j.jacc.2016.08.058 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplementary Definitions
eFigure 1. Structure of Filamin C
eFigure 2. Box-Plot Demonstrating the VE-Burden /24 Hours on Baseline Holter Assessment
eFigure 3. Bar Chart Demonstrating the Spectrum of First MVA or ESHF End Point From Birth in the Entire Cohort of 174 FLNCtv Carriers for Different Age Groups
eTable 1. List of Mutations in FLNC Incorporated into the Cohort and Predicted to Result in Protein Truncation
eTable 2. List of splice Site Mutations in FLNC Identified
eTable 3. Baseline Demographic and Clinical Data of 167 Genotype-Positive FLNCtv Patients With Follow-up Data Stratified According to Proband Status
eTable 4. Table Summarizing Baseline and Follow-up LVEF including Contemporaneous LVEF (Where Available) of Any Arrhythmic End Points
eTable 5. Univariable and Multivariable Predictors of the Composite Primary End Point Using Baseline Clinical Data Obtained From Clinical Evaluation, TTE, CMR, and Holter
eReferences


