Key Points
Question
Is an intrathoracic or cervical anastomosis the preferable location of the anastomosis after a transthoracic, minimally invasive esophagectomy, in terms of anastomotic leakage requiring reintervention?
Findings
In this randomized clinical trial of 245 patients, anastomotic leakage necessitating reintervention occurred in 15 of 122 patients (12.3%) with intrathoracic anastomosis and 39 of 123 patients (31.7%) with cervical anastomosis.
Meaning
In this study, intrathoracic anastomosis resulted in better outcome for patients treated with transthoracic minimally invasive esophagectomy for midesophageal to distal esophageal or gastroesophageal junction cancer.
This open, multicenter randomized clinical trial compares intrathoracic and cervical anastomosis approaches for patients with resectable midesophageal to distal esophageal or gastroesophageal junction cancer.
Abstract
Background
Transthoracic minimally invasive esophagectomy (MIE) is increasingly performed as part of curative multimodality treatment. There appears to be no robust evidence on the preferred location of the anastomosis after transthoracic MIE.
Objective
To compare an intrathoracic with a cervical anastomosis in a randomized clinical trial.
Design, Setting, and Participants
This open, multicenter randomized clinical superiority trial was performed at 9 Dutch high-volume hospitals. Patients with midesophageal to distal esophageal or gastroesophageal junction cancer planned for curative resection were included. Data collection occurred from April 2016 through February 2020.
Intervention
Patients were randomly assigned (1:1) to transthoracic MIE with intrathoracic or cervical anastomosis.
Main Outcomes and Measures
The primary end point was anastomotic leakage requiring endoscopic, radiologic, or surgical intervention. Secondary outcomes were overall anastomotic leak rate, other postoperative complications, length of stay, mortality, and quality of life.
Results
Two hundred sixty-two patients were randomized, and 245 were eligible for analysis. Anastomotic leakage necessitating reintervention occurred in 15 of 122 patients with intrathoracic anastomosis (12.3%) and in 39 of 123 patients with cervical anastomosis (31.7%; risk difference, −19.4% [95% CI, −29.5% to −9.3%]). Overall anastomotic leak rate was 12.3% in the intrathoracic anastomosis group and 34.1% in the cervical anastomosis group (risk difference, −21.9% [95% CI, −32.1% to −11.6%]). Intensive care unit length of stay, mortality rates, and overall quality of life were comparable between groups, but intrathoracic anastomosis was associated with fewer severe complications (risk difference, −11.3% [−20.4% to −2.2%]), lower incidence of recurrent laryngeal nerve palsy (risk difference, −7.3% [95% CI, −12.1% to −2.5%]), and better quality of life in 3 subdomains (mean differences: dysphagia, −12.2 [95% CI, −19.6 to −4.7]; problems of choking when swallowing, −10.3 [95% CI, −16.4 to 4.2]; trouble with talking, −15.3 [95% CI, −22.9 to −7.7]).
Conclusions and Relevance
In this randomized clinical trial, intrathoracic anastomosis resulted in better outcome for patients treated with transthoracic MIE for midesophageal to distal esophageal or gastroesophageal junction cancer.
Trial Registration
Trialregister.nl Identifier: NL4183 (NTR4333)
Introduction
In the Western world, the incidence of esophageal cancer is increasing; it is the sixth cause of cancer-associated death.1 Transthoracic esophagectomy is considered the cornerstone of curative treatment by many surgeons because it allows for adequate thoracic lymph node dissection. Transthoracic esophagectomy is most often performed in combination with neoadjuvant chemoradiotherapy or perioperative chemotherapy.2,3 Transthoracic esophagectomy can be performed with either intrathoracic4 or cervical5 anastomosis. In open esophagectomy, intrathoracic anastomosis is associated with a clinically relevant lower anastomotic leak rate, although the evidence is of limited quality.6 However, this difference in anastomotic leak rate may be important, because anastomotic leakage is a severe complication associated with considerable morbidity, decreased quality of life, a mortality rate of 2% to 12%, and decreased long-term survival.6,7,8,9
In the last decade, minimally invasive esophagectomy (MIE) has been shown to be superior compared with open esophagectomy regarding postoperative outcomes, without compromising oncologic safety.10,11,12 Although not all surgeons are convinced of the benefits of MIE (eg, MIE has also been associated with increased complication rates in registries), it has led to many surgeons implementing transthoracic MIE with cervical anastomosis, because minimally invasive creation of an intrathoracic anastomosis is considered more challenging.13,14 To our knowledge, no randomized clinical trial has compared the outcome of intrathoracic anastomosis vs cervical anastomosis after transthoracic MIE. Although some nonrandomized studies have shown lower anastomotic leak rates after intrathoracic anastomosis, other studies failed to show a difference.15,16,17,18 However, these studies were of limited quality and likely to be flawed by confounding by indication. As a consequence, transthoracic MIE with intrathoracic anastomosis and cervical anastomosis are equally favored. The aim of this study was to compare transthoracic MIE with intrathoracic anastomosis with transthoracic MIE with cervical anastomosis, in terms of anastomotic leakage necessitating reintervention and other postoperative morbidity and mortality outcomes in patients with potentially curable esophageal or gastroesophageal junction cancer.
Methods
Trial Design
This open randomized clinical superiority trial was performed in 9 high-volume hospitals in the Netherlands, including 5 university medical centers and 4 teaching hospitals. Dutch centers that had performed more than 50 total cases of transthoracic MIE with intrathoracic anastomosis, more than 50 total cases of transthoracic MIE with cervical anastomosis, and more than 30 cases of transthoracic MIE per year were invited to participate. Prior to participation, operative videos and outcomes of the centers from the last 2 years were reviewed in a study group meeting, in which expert consensus was achieved on whether centers were suitable for participation. The study protocol was approved by the institutional review board of the Radboud University Medical Center and all participating centers. All patients provided written informed consent. The ICAN trial is registered in the Dutch trial register (NL4183 [NTR4333]), and the protocol has been published previously.19
Participants
Adult patients with histologically proven primary esophageal adenocarcinoma or squamous cell carcinoma were screened for eligibility. Patients were eligible for participation in the study if the tumor was resectable (cT1b-4a, N0-3, and M0) and located in the midesophagus (from the level of the carina to the distal esophagus) or distal esophagus or at the level of the gastroesophageal junction (ie, Siewert levels I to II).20 Patients with a second, prognosis-determining malignant condition and patients who had undergone previous major gastric or major thoracic surgery were excluded. All patients were screened for malnutrition by dieticians and received preoperative supplemental enteral nutrition, if necessary, according to local protocols.
Interventions
According to national guidelines, patients received neoadjuvant chemoradiotherapy3 or perioperative chemotherapy,2 unless this was contraindicated. All patients were subsequently scheduled to undergo either a transthoracic MIE with intrathoracic anastomosis or a transthoracic MIE with cervical anastomosis, by either a hybrid minimally invasive approach (ie, laparoscopy and thoracotomy) or totally minimally invasive approach (ie, laparoscopy and thoracoscopy). Originally, this trial was designed to compare intrathoracic anastomosis with cervical anastomosis for thoracolaparoscopic MIE. Since the results of the French MIRO trial11 were presented during the trial, the trial steering committee recognized the interest to additionally include patients undergoing transthoracic hybrid MIE, because this might increase generalizability of the study. Therefore, we allowed 1 high-volume hospital that only performed transthoracic hybrid MIE resections to include patients, on the conditions that (1) patients undergoing transthoracic hybrid MIE would not be counted in the sample size calculation and (2) results of patients with transthoracic hybrid MIE and transthoracic total MIE were also reported separately, in addition to a pooled analysis. Transthoracic total MIE consisted of a laparoscopic and thoracoscopic approach, and transthoracic hybrid MIE consisted of a laparoscopic and open thoracic approach. A 2-field lymph node dissection was performed in all included patients, irrespective of the location of the anastomosis. Anastomotic techniques were chosen as preferred by the operating surgeon to ensure that surgeons used their most-used technique and had extensive experience with the performed type of anastomosis. In all patients, an omental wrap around the anastomosis was performed. In the case of a cervical anastomosis, a neck drain was routinely left in 6 of 9 hospitals. To evaluate surgical quality of the trial, the operation videos of 1 in 5 randomized patients per center were assessed (eMethods 1 in the Supplement). Pyloric drainage procedures were not routinely performed, and feeding jejunostomy tubes were routinely placed in 7 of 9 participating hospitals.
Sample Size
The sample size calculation was based on an incidence of anastomotic leakage requiring endoscopic, radiologic, or surgical reintervention of 10% after transthoracic total MIE with intrathoracic anastomosis and 25% after transthoracic total MIE with cervical anastomosis (based on literature6,21,22). A sample size of 200 (100 per group) was needed to achieve 80% power to detect a clinically relevant difference of 15% between the intrathoracic anastomosis and cervical anastomosis groups at 5% (2-sided) significance level.
Randomization
Patients were enrolled by their treating surgeons or research staff and randomly assigned at the outpatient clinic 1 to 6 weeks before surgery, in a 1:1 ratio either to transthoracic MIE with intrathoracic anastomosis or transthoracic MIE with cervical anastomosis. Patients were randomized by an online randomization service (http://www.castoredc.com), which was used by the coordinating investigators and/or principal investigator (F.v.W., M.H.P.V., and C.R.) to assign the patients. This online system ensured allocation concealment and stratified patients by treatment site using random, permuted blocks of 2, 4, 6, or 8.23 Allocation and block size were concealed to all investigators.
Outcomes
The primary end point was anastomotic leakage within 30 days after esophagectomy for which endoscopic, radiologic, or surgical reintervention was needed. This corresponds to the definition of the Esophagectomy Complications Consensus Group of anastomotic leakage types 2 and 3.24 Anastomotic leakage was defined as clinical suspicion confirmed (1) by a computed tomography scan with intravenous and oral contrast, (2) by an endoscopy, (3) by drainage of ingested materials or saliva into the chest tube or at the cervical wound, (4) during reintervention, or (5) at autopsy. Diagnostic investigations were performed on indication. Predefined secondary end points included the incidence of postoperative complications, recurrent laryngeal nerve palsy (defined as vocal cord palsy on laryngoscopy), tumor-free resection margin rate, number of examined lymph nodes, hospital and intensive care unit length of stay, intensive care unit readmission rate, and mortality (in-hospital, 30-day, and 90-day). This predefined set of outcome parameters and their definitions corresponds to the internationally defined standardized template for data collection after esophagectomy.24 Quality of life was measured using the cancer-specific European Organization for Research and Treatment of Cancer Quality of Life Questionnaire25 (EORTC QLQ) C30 and the esophagogastric cancer–specific EORTC QLQ-OG25 at baseline (1 to 4 weeks prior to esophagectomy) and 6 weeks postoperatively via mail, email, or telephone. Predetermined end points were overall quality-of-life scores and the specific subdomains.26,27 In keeping with previously published literature, a difference in mean scores of more than 10 points was considered clinically relevant.28,29,30
Data Collection, Storage, Validation, and Sharing
Data collection occurred from April 2016 through February 2020. Data were recorded on a daily basis in a secure electronic case report form with online logbook functionalities.23 Data validation was performed by checking the case report forms with the medical records of all patients in the trial on the primary outcome and main secondary outcome parameters by the study coordinator (M.H.P.V.). Discrepancies were discussed by the data verification committee, including the coordinating investigator (M.H.P.V.), principal investigator (C.R.), and lead investigators (D.L.v.d.P., J.H., E.A.K., G.A.P.N., S.S.G., J.W.H., and J.J.B.v.L.). In addition, the data verification committee checked all records on the primary outcome parameter and associated grading (ie, Esophagectomy Complications Consensus Group grading and Clavien-Dindo classification) and reinterventions.
Statistical Analysis and Reporting
Statistical analysis was in line with the previously published trial protocol,19 and an overview of the analysis and the syntax that was used are shown in eMethods 2 in the Supplement. Trial data was reported according to the CONSORT statement. Data analysis was completed with SPSS version 25 (IBM) and RStudio version 3.6.2 (RStudio).
Results
Patients and Surgical Procedures
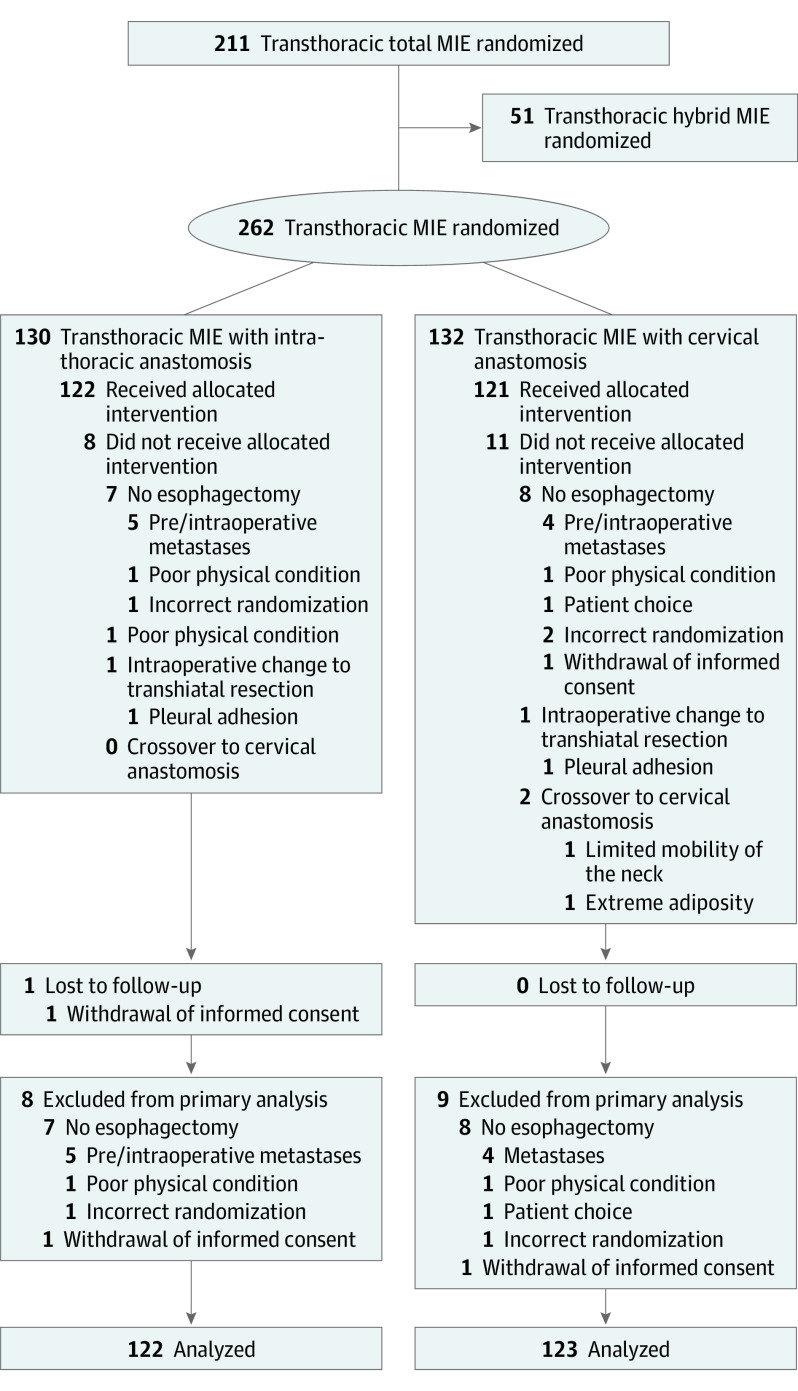
Between April 2016 and October 2019, a total of 262 patients (median [interquartile range] age: those with intrathoracic anastomosis, 67 [5.1] years vs those with cervical anastomosis, 68 [9.2] years; male patients: those with intrathoracic anastomosis, 98 of 122 [80.3%] vs those with cervical anastomosis, 92 of 123 [74.8%]) were randomly assigned to transthoracic MIE with intrathoracic anastomosis (n = 130) or transthoracic MIE with cervical anastomosis (n = 132). Seventeen patients were excluded from analysis, mainly because they were found to have metastatic disease just before or during surgery, and 4 patients received a different procedure than allocated (Figure). The baseline characteristics of the participants are presented in Table 1. Using a structured analysis of the videos, no relevant differences in surgical quality were demonstrated between the groups (eResults in the Supplement).
Figure. CONSORT Flow Diagram.
MIE indicates minimally invasive esophagectomy.
Table 1. Baseline Characteristics.
| Characteristics | Transthoracic minimally invasive esophagectomy, No. (%) | |
|---|---|---|
| With intrathoracic anastomosis (n = 122) | With cervical anastomosis (n = 123) | |
| Age, median (interquartile range), y | 67 (5.1) | 68 (9.2) |
| Male | 98 (80.3) | 92 (74.8) |
| Female | 24 (19.7) | 31 (25.2) |
| American Society of Anesthesiologists classification | ||
| 1 | 12 (10.2) | 14 (11.7) |
| 2 | 80 (67.8) | 83 (69.2) |
| 3 | 25 (21.2) | 23 (19.2) |
| 4 | 1 (0.8) | 0 (0.0) |
| Charlson Comorbidity Index score | ||
| 0 | 81 (66.4) | 80 (65.0) |
| 1 | 22 (18.0) | 21 (17.1) |
| 2 | 9 (7.4) | 10 (8.1) |
| 3 | 7 (5.7) | 7 (5.7) |
| 4 | 2 (1.6) | 2 (1.6) |
| 5 | 0 | 2 (1.6) |
| 6 | 1 (0.8) | 1 (0.8) |
| 7 | 0 | 0 |
| Tumor type | ||
| Adenocarcinoma | 105 (86.1) | 114 (92.3) |
| Squamous cell carcinoma | 12 (9.8) | 7 (5.7) |
| Other | 5 (4.1) | 2 (1.6) |
| Tumor location | ||
| Intrathoracic midesophagus | 6 (4.9) | 3 (2.4) |
| Intrathoracic distal esophagus | 105 (86.1) | 106 (86.2) |
| Gastroesophageal junction | 11 (9.0) | 14 (11.4) |
| cT stage | ||
| T1 | 2 (1.6) | 4 (3.2) |
| T2 | 23 (18.9) | 19 (15.4) |
| T3 | 68 (55.7) | 65 (52.8) |
| T4 | 1 (0.8) | 2 (1.6) |
| Tx | 28 (23.0) | 33 (26.8) |
| cN stage | ||
| N0 | 53 (43.4) | 60 (48.8) |
| N1 | 45 (36.9) | 39 (31.7) |
| N2 | 16 (13.1) | 19 (15.4) |
| N3 | 3 (2.5) | 1 (0.8) |
| N+ | 3 (2.5) | 3 (2.4) |
| Nx | 2 (1.6) | 1 (0.8) |
| Neoadjuvant treatment | ||
| Yes | ||
| Chemoradiotherapy | 118 (96.7) | 117 (95.1) |
| Chemotherapy | 2 (1.6) | 3 (2.4) |
| None | 2 (1.6) | 3 (2.4) |
| Operation type | ||
| Total minimally invasive esophagectomy | 97 (79.5) | 100 (81.3) |
| Hybrid minimally invasive esophagectomy | 25 (20.5) | 23 (18.7) |
| Configuration of anastomosis | ||
| End to end | 3 (2.5) | 46 (37.4) |
| End to side | 42 (34.4) | 2 (1.6) |
| Side to side | 77 (63.1) | 75 (61.0) |
| Anastomosis technique | ||
| Handsewn | 4 (3.3) | 108 (87.8) |
| Stapled | 118 (96.7) | 15 (12.2) |
Primary Outcome
Anastomotic leakage for which endoscopic, radiologic, or surgical reintervention was performed, occurred in 15 patients in the intrathoracic anastomosis group (12.3%) and 39 patients in the cervical anastomosis group (31.7%; risk difference, −19.4% [95% CI, −29.5% to −9.3%]) (Table 2), and details of reinterventions are shown in eTable 1 in the Supplement. Post hoc correction for center did not substantially change this result (estimated difference, −18.4% [95% CI, −28.6% to −8.2%]). Additional per-protocol analyses did not alter the results regarding the primary outcome (eTable 2 in the Supplement). The predefined subgroup analysis for anastomosis configuration showed no significant differences in the primary end point. Analysis of both total MIEs and hybrid MIEs showed comparable results regarding the primary outcome (eTables 3 and 4 in the Supplement).
Table 2. Primary and Secondary Outcome Parameters.
| Characteristic | Transthoracic minimally invasive esophagectomy, No. (%) | Difference in pooled mean scores (95% CI) | P value | |
|---|---|---|---|---|
| With intrathoracic anastomosis (n = 122) | With cervical anastomosis (n = 123) | |||
| Anastomotic leakage | ||||
| Requiring reintervention | 15 (12.3) | 39 (31.7) | −19.4 (−29.5 to −9.3) | <.001 |
| Total | 15 (12.3) | 42 (34.1) | −21.9 (−32.1 to −) | <.001 |
| Gastric conduit | ||||
| Leakage | 1 (0.8) | 0 | 0.8 (−1.4 to 3.1) | .47 |
| Necrosis | 1 (0.8) | 2 (1.6) | −0.8 (−3.6 to 1.9) | .57 |
| Pulmonary complications | ||||
| Pneumonia | 14 (11.5) | 23 (18.4) | −7.2 (−16.1 to 1.7) | .11 |
| Pneumothorax requiring drainage | 3 (2.5) | 7 (5.7) | −3.2 (−8.2 to 1.7) | .20 |
| Pleural effusion requiring drainage | 12 (9.8) | 26 (21.1) | −11.3 (−20.2 to −2.4) | .01 |
| Empyema requiring drainage | 4 (3.3) | 6 (4.9) | −1.6 (−6.5 to 3.3) | .53 |
| Tracheobronchial defect | 0 | 1 (0.8) | −0.8 (−3.0 to 1.4) | .47 |
| Respiratory failure requiring reintubation | 10 (8.2) | 13 (10.6) | −2.4 (−9.7 to 4.9) | .52 |
| Mediastinal fluid collection | 1 (0.8) | 6 (4.9) | −4.1 (−8.2 to 0.1) | .05 |
| Cardiac complications | ||||
| Supraventricular arrhythmia | 16 (13.1) | 26 (21.1) | −8.0 (−17.4 to 1.4) | .09 |
| Ventricular arrythmia | 2 (1.6) | 1 (0.8) | 0.8 (−1.9 to 3.6) | .57 |
| Cardiac decompensation | 1 (0.8) | 2 (1.6) | −0.8 (−3.6 to 1.9) | .57 |
| Myocardial infarction | 0 | 0 | 0.0 (−1.6 to 1.6) | 1.0 |
| Chyle leakage | 9 (7.4) | 11 (8.9) | −1.6 (−0.8 to 0.1) | .65 |
| Recurrent laryngeal nerve palsy | 0 | 9 (7.3) | −7.3 (−12.1 to −2.5) | .003 |
| Severe complication with Clavien-Dindo level ≥3b | 13 (10.7) | 27 (22.0) | −11.3 (−20.4 to −2.2) | .02 |
| Comprehensive complication index, mean (SD) | 22.9 (21.6) | 19.2 (23.0) | −3.8 (−9.4 to 1.8) | .19 |
| Length of stay, median (IQR), d | ||||
| Hospital | 10.0 (7) | 11.5 (9) | 0.19a | .003 |
| Intensive care unit | 2 (1) | 2 (2) | 0.10a | .12 |
| Intensive care unit readmission | 11 (9.0) | 22 (17.9) | −8.9 (−17.3 to −0.4) | .04 |
| Mortality | ||||
| In-hospital | 3 (2.5) | 1 (0.8) | 1.6 (−1.5 to 4.8) | .31 |
| 30-d | 3 (2.5) | 1 (0.8) | 1.6 (−2.2 to 5.5) | .31 |
| 90-d | 4 (3.3) | 2 (1.6) | 1.7 (−2.2 to 5.5) | .40 |
| Conversion | ||||
| To laparotomy | 0 | 7 (5.7) | −5.7 (−10.0 to −1.3) | .01 |
| To thoracotomy | 3 (2.5) | 2 (1.6) | 0.8 (−2.7 to 4.4) | .64 |
| Operating time, median (IQR), min | 267 (100) | 272 (90) | 0.12a | .06 |
| Blood loss, median (IQR), mL | 100 (200) | 100 (198) | 0.07a | .31 |
| Lymph nodes, median (IQR) | ||||
| Retrieved | 22 (11) | 22 (11) | 0.04a | .55 |
| Positive | 0 (1.5) | 0 (1) | 0.03a | .62 |
| R0 resection | 121 (99.2) | 121 (98.4) | 0.8 (−1.9 to 3.6) | .57 |
Abbreviations: IQR, interquartile range; R0, radical.
Effect size is given here as r = Z/√N, without 95% CIs.
Secondary Outcome Parameters
Secondary outcome parameters for all patients are presented in Table 2. Overall anastomotic leak rate (ie, ECCG grades 1, 2, and 3) was 12.3% after transthoracic MIE with intrathoracic anastomosis and 34.1% after transthoracic MIE with cervical anastomosis (risk difference, −21.9% [95% CI, −32.1% to −11.6%]). The incidence of recurrent laryngeal nerve palsy (risk difference, −7.3% [95% CI, −12.1% to −2.5%]) and severe complications (risk difference, −11.3% [−20.4% to −2.2%]) was lower and median hospital length of stay (median [interquartile range], 10.0 [7] days vs 11.5 [9] days; P = .003) was shorter in the intrathoracic group. Mortality rates were comparable between the groups.
In the subgroup of patients with anastomotic leakage, the severity of cervical vs intrathoracic anastomotic leakage was similar (Table 3). In addition to the predefined set of outcome parameters, 3 patients underwent a reoperation. Indications for reoperations were deviation of the trachea because of a bulky omentum (in a patient in the cervical group), herniation of lung through the ribs (in a patient in the intrathoracic group), and iatrogenic damage to the anastomosis by a nasogastric tube (in a patient randomized for cervical anastomosis who crossed over to intrathoracic anastomosis). Separate outcomes for hybrid MIE and total MIE are presented in eTables 3 and 4 in the Supplement.
Table 3. Detailed Outcomes of Patients With Anastomotic Leakage.
| Characteristic | Anastomotic leakage, No. (%) | |
|---|---|---|
| After intrathoracic anastomosis (n = 15) | After cervical anastomosis (n = 42) | |
| Anastomotic leakage by Esophagectomy Complications Consensus Group classification, grade | ||
| I | 0 | 3 (7.1) |
| II | 11 (73.3) | 35 (83.3) |
| III | 4 (26.7) | 4 (9.5) |
| Anastomotic leakage by Clavien-Dindo classification, grade | ||
| I | 0 | 5 (11.9) |
| II | 0 | 8 (19.0) |
| IIIa | 9 (60) | 15 (35.7) |
| IIIb | 0 | 4 (9.5) |
| IVa | 4 (26.7) | 10 (23.8) |
| IVb | 1 (6.7) | 0 |
| V | 1 (6.7) | 0 |
| Total number of reinterventions, No. | ||
| Radiologic | 8 | 6 |
| Endoscopic | 16 | 41 |
| Reoperation | 4 | 7 |
| Hospital admission | ||
| Hospital length of stay, median (IQR), d | 30.5 (19.8) | 19.0 (20.0) |
| Hospital readmission | 3 (20.0) | 11 (26.2) |
| Intensive care unit admission | ||
| Length of stay, median (IQR), d | 1.0 (5.0) | 2.0 (4.8) |
| Readmission | 4 (26.7) | 15 (35.7) |
| Mortality | ||
| In-hospital | 1 (6.7) | 1 (2.4) |
| 30-d | 1 (6.7) | 1 (2.4) |
| 90-d | 1 (6.7) | 2 (4.8) |
Abbreviation: IQR, interquartile range.
Quality of Life
Six weeks after transthoracic MIE, patients with intrathoracic anastomosis reported fewer problems of dysphagia compared with patients with cervical anastomosis (mean difference, −12.2 [95% CI, −19.6 to −4.7]). In addition, patients with intrathoracic anastomosis experienced fewer problems of choking when swallowing (mean difference, −10.3 [95% CI, −16.4 to 4.2]) and trouble with talking (mean difference, −15.3 [95% CI, −22.9 to −7.7]). An overview of all quality-of-life domains is shown in Table 4.
Table 4. Quality-of-Life Outcomes After Multiple Imputation at 6 Weeks After Esophagectomya.
| Characteristic | Transthoracic minimally invasive esophagectomy, mean (SD) | Difference in pooled mean scores (95% CI) | |
|---|---|---|---|
| With intrathoracic anastomosis (n = 122) | With cervical anastomosis (n = 123) | ||
| QLQ-C30 | |||
| Global health status | 65.6 (18.6) | 62.0 (20.6) | 3.7 (−1.5 to 8.2) |
| Functional scales | |||
| Physical | 70.7 (20.3) | 64.5 (23.7) | 6.3 (0.4 to 12.2) |
| Role | 55.6 (30.3) | 51.1 (29.2) | 4.5 (−3.4 to 12.4) |
| Emotional | 82.5 (19.3) | 81.2 (20.0) | 1.3 (−3.8 to 6.4) |
| Cognitive | 86.3 (18.8) | 84.0 (22.0) | 2.4 (−3.2 to 7.8) |
| Social | 72.4 (26.6) | 67.4 (28.0) | 5.0 (−2.2 to 12.1) |
| Symptom scales | |||
| Fatigue | 43.9 (24.2) | 47.6 (24.4) | −3.6 (−10.1 to 2.8) |
| Nausea and vomiting | 21.4 (25.2) | 21.8 (26.9) | −0.4 (−7.4 to 6.7) |
| Pain | 22.6 (22.1) | 23.4 (26.5) | −0.79 (−7.4 to 5.8) |
| Dyspnea | 32.0 (27.9) | 33.0 (30.0) | −1.03 (−8.7 to 6.7) |
| Insomnia | 32.2 (28.7) | 33.7 (30.4) | −1.5 (−9.4 to 6.5) |
| Appetite loss | 42.4 (35.0) | 41.0 (33.5) | −1.4 (−7.67 to 10.4) |
| Constipation | 10.8 (20.5) | 9.9 (20.5) | 0.97 (−4.4 to 6.4) |
| Diarrhea | 22.3 (27.1) | 23.7 (29.5) | −1.4 (−9.3 to 6.4) |
| Financial difficulties | 5.4 (15.3) | 7.5 (15.8) | −2.1 (−6.2 to 1.9) |
| QLQ-C30 summary score | 72.7 (14.8) | 71.6 (15.8) | 1.1 (−2.9 to 5.1) |
| QLQ-OG25 | |||
| Symptom scale | |||
| Dysphagia | 22.9 (24.5) | 35.0 (30.9) | −12.2 (−19.6 to −4.7) |
| Eating | 42.9 (28.4) | 49.6 (29.2) | −6.7 (−14.5 to 1.0) |
| Reflux | 13.8 (21.5) | 14.4 (25.0) | −0.5 (−6.8 to 5.7) |
| Odynophagia | 14.8 (19.9) | 20.5 (25.3) | −5.8 (−12.2 to 0.7) |
| Pain and discomfort | 13.1 (16.9) | 16.2 (23.1) | −3.1 (−8.5 to 2.4) |
| Anxiety | 31.0 (23.7) | 36.1 (27.8) | −5.1 (−11.9 to 1.6) |
| Eating with others | 14.8 (26.2) | 20.1 (30.9) | −5.3 (−13.3 to 2.6) |
| Dry mouth | 27.0 (30.0) | 33.4 (33.6) | −6.3 (−14.7 to 2.0) |
| Trouble with taste | 28.6 (34.2) | 28.3 (33.6) | 0.4 (−8.7 to 9.5) |
| Body image | 14.5 (23.3) | 23.0 (30.4) | −8.5 (−15.8 to −1.2) |
| Trouble swallowing saliva | 10.9 (21.7) | 19.1 (30.4) | −8.2 (−15.4 to −1.0) |
| Choking when swallowing | 10.1 (17.7) | 20.4 (25.1) | −10.3 (−16.4 to −4.2) |
| Trouble with coughing | 48.7 (28.9) | 58.0 (28.1) | −9.3 (−17.0 to −1.6) |
| Trouble with talking | 14.1 (23.1) | 29.4 (33.1) | −15.3 (−22.9 to −7.7) |
| Weight loss | 28.7 (28.7) | 24.1 (27.4) | 4.6 (−2.8 to 12.0) |
| Hair loss | 14.1 (23.0) | 12.3 (20.9) | 1.7 (−4.6 to 8.1) |
Abbreviations: QLQ-C30, Quality of Life Questionnaire C30 (cancer-specific questionnaire); QLQ-OG25, Quality of Life Questionnaire OG25 (esophagogastric cancer–specific questionnaire).
Outcomes are displayed as pooled mean scores with SD in parentheses.
Discussion
This randomized clinical trial showed that transthoracic MIE with intrathoracic anastomosis resulted in a lower anastomotic leak rate compared with transthoracic MIE with cervical anastomosis. In addition, an intrathoracic anastomosis was associated with a lower recurrent laryngeal nerve palsy rate, a lower rate of severe complications, shorter hospital length of stay, and a better quality of life at 6 weeks postoperatively regarding dysphagia, choking when swallowing, and trouble with talking. No differences were observed in intensive care unit length of stay and mortality rates.
Strengths
The major strength of our study is that it is a high-quality randomized clinical trial giving insight in the important question whether transthoracic MIE with intrathoracic or cervical anastomosis should be preferred. Stringent trial participation criteria and video and outcome assessment ensured that only surgical teams that were proficient in both techniques participated in this study. The use of a standardized outcome set for reporting complications,24 real-time data collection, and an extensive data validation process contributed to ensuring reproducibility and robustness of data.
Limitations
Some limitations should also be discussed. First, patients and outcome assessors were not blinded, since this was considered not to be feasible at the time of design of the trial. Given the results of a more recent study, blinding of patients and outcome assessors might have been possible.31 However, our primary outcome parameter definition enabled objective assessment, and this was verified in all patients by the data verification committee, making it less likely that blinding would have led to different results. Second, data on the number of patients screened were not reliably retrieved during the study, and so unknown selection bias cannot be ruled out. Third, although patients were randomized and stratification by treatment site was performed, we did not correct for confounders and correction for within-site correlation was performed only post hoc. Fourth, it may be argued that differences in intervention, such as various techniques to create intrathoracic or cervical anastomoses (eg, configuration, handsewn or stapled), influenced trial outcome. However, we did not find any substantial differences between different anastomotic techniques in a predefined subgroup analysis. The pragmatic trial design was chosen to ensure the trial would reflect nationwide practice, and therefore some heterogeneity of interventions was allowed. Fifth, although nearly all videos were retrieved for structured quality analysis, some video material was not available. However, we do believe surgical quality was adequate, since assessment showed good scores in both groups. In addition, the comparably good anastomotic scores make it less likely that bias has occurred, because surgeons were not using their preferred technique. Moreover, we applied strict qualitative and quantitative entry rules for centers that participated in the study. In fact, these strict rules for participation may have resulted in less generalizability of the trial results, because not all surgeons may have the same level of experience as the surgeons in this trial. We aim to investigate this in a future study. Finally, even though strict trial participation rules were used, we cannot rule out that the outcomes were affected by a learning curve. This may particularly be important for the intrathoracic group, since this was the newer intervention in the study, and it has been described that learning curve effects are important for this procedure.32 However, even if a learning curve influenced the results of this trial, it would have resulted in an even larger difference in our primary outcome parameter. We are therefore confident that our findings are robust.33,34,35,36,37,38,39
Although the anastomotic leak rates in both groups are higher than reported in other studies,10,40,41 we believe that the comparison between both techniques is valid, since similar rates have been reported in other randomized clinical trials and in the Dutch national registry.3,17,18,42 We did not find any evidence to support hypotheses that the high leak rates could be explained by surgical technique (eResults in the Supplement) or case mix. The inclusive definition and careful data registration may have contributed to a comprehensive and complete reporting of anastomotic leak rate.
The lower anastomotic leak rate, shorter hospital length of stay, and lower incidence of recurrent laryngeal nerve palsy after intrathoracic anastomosis is in agreement with other nonrandomized studies.17,18,22 For the hybrid MIE subgroup, we were unable to formally establish whether anastomotic leakage is lower after intrathoracic anastomosis, because we did not power for this analysis. In our opinion, however, it is likely that intrathoracic anastomosis has a similar beneficial effect, given the found effect size in this study and similar effects in other trials comparing intrathoracic vs cervical anastomosis in open esophagectomy.6 The lower anastomotic leak rate in the intrathoracic group may be explained by relatively less ischemia at the tip of the shorter gastric tube in esophagectomy with intrathoracic anastomosis. In addition to the incidence of anastomotic leakage, it is also important to appreciate the severity of anastomotic leakage. Many surgeons believe that intrathoracic anastomotic leakage is more severe than cervical anastomotic leakage, although the evidence is scarce. Following this train of thought, a higher incidence of anastomotic leakage after cervical anastomosis may not outweigh more severe sequelae of intrathoracic anastomotic leakage, although severe intrathoracic consequences of anastomotic leakage may develop in cervical anastomotic leakage, too.43 In the subgroup of patients that experienced anastomotic leakage, no important differences were observed in patients with intrathoracic vs cervical anastomotic leakage in terms of reoperation rate, intensive care unit length of stay, and mortality rates, but hospital length of stay was longer for patients with an intrathoracic leak. Overall, however, patients had a significantly shorter length of stay after intrathoracic anastomosis, and it must be kept in mind that the present study was not powered to assess differences in outcome in the subgroup of patients with leakages.
The results of this trial support implementation of intrathoracic anastomosis in patients undergoing minimally invasive esophagectomy, although the choice for anastomotic location should be individualized for each patient and each surgeon. The technical challenge of creating a minimally invasive intrathoracic anastomosis, which is specific to transthoracic total MIE (as opposed to transthoracic hybrid MIE, in which the creation of the anastomosis is performed by means of open surgery), could hamper broad implementation. This is supported by a previous study from our group, which showed a significant learning curve and learning associated morbidity of transthoracic total MIE with intrathoracic anastomosis, even for surgeons who were experienced in MIE with cervical anastomosis.39 These findings underline the importance of safe implementation, which may be facilitated by structured training programs, proctorship, feedback with validated competency assessment tools, and learning the procedure in a high-volume center.44
Conclusions
In conclusion, intrathoracic, as opposed to cervical, anastomosis resulted in better outcome for patients treated with transthoracic MIE for midesophageal to distal esophageal or gastroesophageal junction cancer. Future research will be needed to evaluate to what extent broad implementation of transthoracic MIE with intrathoracic anastomosis will lead to improved patient outcomes and assess long-term functional and oncological outcome between patients with intrathoracic and cervical anastomosis after transthoracic MIE.
eMethods 1. Methods for surgical quality assessment
eMethods 2. Statistical analysis and reporting
eResults. Results of surgical quality assessment: mean scores of assessed videos
eTable 1. Detailed data of reinterventions of anastomotic leakage patients
eTable 2. Results of per protocol analysis of primary and secondary outcome parameters
eTable 3. Primary and secondary outcomes for total MIE group
eTable 4. Primary and secondary outcomes for hybrid MIE group
Trial Protocol
Data Sharing Statement
Nonauthor Collaborators
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Al-Batran SE, Homann N, Pauligk C, et al. ; FLOT4-AIO Investigators . Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393(10184):1948-1957. doi: 10.1016/S0140-6736(18)32557-1 [DOI] [PubMed] [Google Scholar]
- 3.van Hagen P, Hulshof MC, van Lanschot JJ, et al. ; CROSS Group . Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074-2084. doi: 10.1056/NEJMoa1112088 [DOI] [PubMed] [Google Scholar]
- 4.Lewis I. The surgical treatment of carcinoma of the oesophagus; with special reference to a new operation for growths of the middle third. Br J Surg. 1946;34:18-31. doi: 10.1002/bjs.18003413304 [DOI] [PubMed] [Google Scholar]
- 5.McKeown KC. Total three-stage oesophagectomy for cancer of the oesophagus. Br J Surg. 1976;63(4):259-262. doi: 10.1002/bjs.1800630403 [DOI] [PubMed] [Google Scholar]
- 6.Biere SS, Maas KW, Cuesta MA, van der Peet DL. Cervical or thoracic anastomosis after esophagectomy for cancer: a systematic review and meta-analysis. Dig Surg. 2011;28(1):29-35. doi: 10.1159/000322014 [DOI] [PubMed] [Google Scholar]
- 7.Alanezi K, Urschel JD. Mortality secondary to esophageal anastomotic leak. Ann Thorac Cardiovasc Surg. 2004;10(2):71-75. [PubMed] [Google Scholar]
- 8.Goense L, Meziani J, Ruurda JP, van Hillegersberg R. Impact of postoperative complications on outcomes after oesophagectomy for cancer. Br J Surg. 2019;106(1):111-119. doi: 10.1002/bjs.11000 [DOI] [PubMed] [Google Scholar]
- 9.Markar S, Gronnier C, Duhamel A, et al. ; FREGAT (French Eso-Gastric Tumors) working group, FRENCH (Fédération de Recherche EN CHirurgie), and AFC (Association Française de Chirurgie) . The impact of severe anastomotic leak on long-term survival and cancer recurrence after surgical resection for esophageal malignancy. Ann Surg. 2015;262(6):972-980. doi: 10.1097/SLA.0000000000001011 [DOI] [PubMed] [Google Scholar]
- 10.Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. 2012;379(9829):1887-1892. doi: 10.1016/S0140-6736(12)60516-9 [DOI] [PubMed] [Google Scholar]
- 11.Mariette C, Markar SR, Dabakuyo-Yonli TS, et al. ; Fédération de Recherche en Chirurgie (FRENCH) and French Eso-Gastric Tumors (FREGAT) Working Group . Hybrid minimally invasive esophagectomy for esophageal cancer. N Engl J Med. 2019;380(2):152-162. doi: 10.1056/NEJMoa1805101 [DOI] [PubMed] [Google Scholar]
- 12.Straatman J, van der Wielen N, Cuesta MA, et al. Minimally invasive versus open esophageal resection: three-year follow-up of the previously reported randomized controlled trial: the TIME trial. Ann Surg. 2017;266(2):232-236. doi: 10.1097/SLA.0000000000002171 [DOI] [PubMed] [Google Scholar]
- 13.Haverkamp L, Seesing MF, Ruurda JP, Boone J, V Hillegersberg R. Worldwide trends in surgical techniques in the treatment of esophageal and gastroesophageal junction cancer. Dis Esophagus. 2017;30(1):1-7. [DOI] [PubMed] [Google Scholar]
- 14.Seesing MFJ, Gisbertz SS, Goense L, et al. A propensity score matched analysis of open versus minimally invasive transthoracic esophagectomy in the Netherlands. Ann Surg. 2017;266(5):839-846. doi: 10.1097/SLA.0000000000002393 [DOI] [PubMed] [Google Scholar]
- 15.Brown AM, Pucci MJ, Berger AC, et al. A standardized comparison of peri-operative complications after minimally invasive esophagectomy: Ivor Lewis versus McKeown. Surg Endosc. 2018;32(1):204-211. doi: 10.1007/s00464-017-5660-4 [DOI] [PubMed] [Google Scholar]
- 16.van Workum F, Berkelmans GH, Klarenbeek BR, Nieuwenhuijzen GAP, Luyer MDP, Rosman C. McKeown or Ivor Lewis totally minimally invasive esophagectomy for cancer of the esophagus and gastroesophageal junction: systematic review and meta-analysis. J Thorac Dis. 2017;9(suppl 8):S826-S833. doi: 10.21037/jtd.2017.03.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Workum F, Slaman AE, van Berge Henegouwen MI, et al. Propensity score-matched analysis comparing minimally invasive Ivor Lewis versus minimally invasive Mckeown esophagectomy. Ann Surg. 2020;271(1):128-133. doi: 10.1097/SLA.0000000000002982 [DOI] [PubMed] [Google Scholar]
- 18.Gooszen JAH, Goense L, Gisbertz SS, Ruurda JP, van Hillegersberg R, van Berge Henegouwen MI. Intrathoracic versus cervical anastomosis and predictors of anastomotic leakage after oesophagectomy for cancer. Br J Surg. 2018;105(5):552-560. doi: 10.1002/bjs.10728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Workum F, Bouwense SA, Luyer MD, et al. Intrathoracic versus cervical anastomosis after minimally invasive esophagectomy for esophageal cancer: study protocol of the ICAN randomized controlled trial. Trials. 2016;17(1):505. doi: 10.1186/s13063-016-1636-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rüdiger Siewert J, Feith M, Werner M, Stein HJ. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg. 2000;232(3):353-361. doi: 10.1097/00000658-200009000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Workum F, van den Wildenberg FJ, Polat F, de Wilt JH, Rosman C. Minimally invasive oesophagectomy: preliminary results after introduction of an intrathoracic anastomosis. Dig Surg. 2014;31(2):95-103. doi: 10.1159/000358812 [DOI] [PubMed] [Google Scholar]
- 22.van Workum F, van der Maas J, van den Wildenberg FJ, et al. Improved functional results after minimally invasive esophagectomy: intrathoracic versus cervical anastomosis. Ann Thorac Surg. 2017;103(1):267-273. doi: 10.1016/j.athoracsur.2016.07.010 [DOI] [PubMed] [Google Scholar]
- 23.Castor EDC . The scalable decentralized and hybrid clinical trial platform: castor electronic data capture 2019. Published August 27, 2019. Accessed April 7, 2021. http://www.castoredc.com.
- 24.Low DE, Alderson D, Cecconello I, et al. International consensus on standardization of data collection for complications associated with esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg. 2015;262(2):286-294. doi: 10.1097/SLA.0000000000001098 [DOI] [PubMed] [Google Scholar]
- 25.Fayers P, Aaronson N, Bjordal K, Groenvold M, Curran D, Bottomley A; EORTC Quality of Life Group . The EORTC QLQ-C30 scoring manual. Published 2001. Accesssed April 7, 2021. https://www.eortc.org/app/uploads/sites/2/2018/02/SCmanual.pdf
- 26.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365-376. doi: 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 27.Lagergren P, Fayers P, Conroy T, et al. ; European Organisation for Research Treatment of Cancer Gastrointestinal and Quality of Life Groups . Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-OG25, to assess health-related quality of life in patients with cancer of the oesophagus, the oesophago-gastric junction and the stomach. Eur J Cancer. 2007;43(14):2066-2073. doi: 10.1016/j.ejca.2007.07.005 [DOI] [PubMed] [Google Scholar]
- 28.Musoro JZ, Bottomley A, Coens C, et al. ; EORTC Melanoma Group and EORTC Quality of Life Group . Interpreting European Organisation for Research and Treatment for Cancer Quality of Life Questionnaire Core 30 scores as minimally importantly different for patients with malignant melanoma. Eur J Cancer. 2018;104:169-181. doi: 10.1016/j.ejca.2018.09.005 [DOI] [PubMed] [Google Scholar]
- 29.Musoro JZ, Coens C, Fiteni F, et al. ; EORTC Breast and Quality of Life Groups . Minimally important differences for interpreting EORTC QLQ-C30 scores in patients with advanced breast cancer. JNCI Cancer Spectr. 2019;3(3):pkz037. doi: 10.1093/jncics/pkz037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139-144. doi: 10.1200/JCO.1998.16.1.139 [DOI] [PubMed] [Google Scholar]
- 31.Metcalfe C, Avery K, Berrisford R, et al. Comparing open and minimally invasive surgical procedures for oesophagectomy in the treatment of cancer: the ROMIO (Randomised Oesophagectomy: Minimally Invasive or Open) feasibility study and pilot trial. Health Technol Assess. 2016;20(48):1-68. doi: 10.3310/hta20480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Claassen L, van Workum F, Rosman C. Learning curve and postoperative outcomes of minimally invasive esophagectomy. J Thorac Dis. 2019;11(suppl 5):S777-S785. doi: 10.21037/jtd.2018.12.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo W, Zou YB, Ma Z, et al. One surgeon’s learning curve for video-assisted thoracoscopic esophagectomy for esophageal cancer with the patient in lateral position: how many cases are needed to reach competence? Surg Endosc. 2013;27(4):1346-1352. doi: 10.1007/s00464-012-2614-8 [DOI] [PubMed] [Google Scholar]
- 34.Lin J, Kang M, Chen C, et al. Thoracolaparoscopy oesophagectomy and extensive two-field lymphadenectomy for oesophageal cancer: introduction and teaching of a new technique in a high-volume centre. Eur J Cardiothorac Surg. 2013;43(1):115-121. doi: 10.1093/ejcts/ezs151 [DOI] [PubMed] [Google Scholar]
- 35.Mu JW, Gao SG, Xue Q, et al. Updated experiences with minimally invasive McKeown esophagectomy for esophageal cancer. World J Gastroenterol. 2015;21(45):12873-12881. doi: 10.3748/wjg.v21.i45.12873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramage L, Deguara J, Davies A, et al. Gastric tube necrosis following minimally invasive oesophagectomy is a learning curve issue. Ann R Coll Surg Engl. 2013;95(5):329-334. doi: 10.1308/003588413X13629960045751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song SY, Na KJ, Oh SG, Ahn BH. Learning curves of minimally invasive esophageal cancer surgery. Eur J Cardiothorac Surg. 2009;35(4):689-693. doi: 10.1016/j.ejcts.2008.11.014 [DOI] [PubMed] [Google Scholar]
- 38.Tapias LF, Morse CR. Minimally invasive Ivor Lewis esophagectomy: description of a learning curve. J Am Coll Surg. 2014;218(6):1130-1140. doi: 10.1016/j.jamcollsurg.2014.02.014 [DOI] [PubMed] [Google Scholar]
- 39.van Workum F, Stenstra MHBC, Berkelmans GHK, et al. Learning curve and associated morbidity of minimally invasive esophagectomy: a retrospective multicenter study. Ann Surg. 2019;269(1):88-94. doi: 10.1097/SLA.0000000000002469 [DOI] [PubMed] [Google Scholar]
- 40.Briez N, Piessen G, Bonnetain F, et al. Open versus laparoscopically-assisted oesophagectomy for cancer: a multicentre randomised controlled phase III trial—the MIRO trial. BMC Cancer. 2011;11:310. doi: 10.1186/1471-2407-11-310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fransen LFC, Berkelmans GHK, Asti E, van Berge Henegouwen MI, Berlth F, Bonavina L, et al. The effect of postoperative complications after minimally invasive esophagectomy on long-term survival: an international multicenter cohort study. Ann Surg. 2020. doi: 10.1097/SLA.0000000000004293 [DOI] [PubMed] [Google Scholar]
- 42.Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347(21):1662-1669. doi: 10.1056/NEJMoa022343 [DOI] [PubMed] [Google Scholar]
- 43.van Heijl M, van Wijngaarden AK, Lagarde SM, Busch OR, van Lanschot JJ, van Berge Henegouwen MI. Intrathoracic manifestations of cervical anastomotic leaks after transhiatal and transthoracic oesophagectomy. Br J Surg. 2010;97(5):726-731. doi: 10.1002/bjs.6971 [DOI] [PubMed] [Google Scholar]
- 44.van Workum F, Fransen L, Luyer MD, Rosman C. Learning curves in minimally invasive esophagectomy. World J Gastroenterol. 2018;24(44):4974-4978. doi: 10.3748/wjg.v24.i44.4974 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Methods for surgical quality assessment
eMethods 2. Statistical analysis and reporting
eResults. Results of surgical quality assessment: mean scores of assessed videos
eTable 1. Detailed data of reinterventions of anastomotic leakage patients
eTable 2. Results of per protocol analysis of primary and secondary outcome parameters
eTable 3. Primary and secondary outcomes for total MIE group
eTable 4. Primary and secondary outcomes for hybrid MIE group
Trial Protocol
Data Sharing Statement
Nonauthor Collaborators