Key Points
Question
What are the clinical course and histopathologic examination findings for delayed injection-site reactions to the Moderna coronavirus disease 2019 (COVID-19) vaccine?
Findings
The Moderna COVID-19 vaccine may cause a delayed localized hypersensitivity reaction with a median latency to onset of 7 days after vaccine administration. This pruritic and variably tender reaction has a median duration of 5 days, but may persist for up to 21 days, and may occur again and sooner after the second vaccine dose; no serious adverse events were observed in association with this cutaneous reaction to the Moderna COVID-19 vaccine.
Meaning
Self-limited localized delayed hypersensitivity reactions to the Moderna COVID-19 vaccine may occur, and in contrast with immediate hypersensitivity reactions, these delayed hypersensitivity reactions are not a contraindication to subsequent vaccination.
This case-series study describes the clinical course and histopathologic examination findings for delayed localized cutaneous injection-site reactions to the Moderna COVID-19 vaccine.
Abstract
Importance
In response to the coronavirus disease 2019 (COVID-19) pandemic, 2 mRNA vaccines (Pfizer-BioNTech and Moderna) received emergency use authorization from the US Food and Drug Administration in December 2020. Some patients in the US have developed delayed localized cutaneous vaccine reactions that have been dubbed “COVID arm.”
Objective
To describe the course of localized cutaneous injection-site reactions to the Moderna COVID-19 vaccine, subsequent reactions to the second vaccine dose, and to characterize the findings of histopathologic examination of the reaction.
Design, Setting, and Participants
This retrospective case series study was performed at Yale New Haven Hospital, a tertiary medical center in New Haven, Connecticut, with 16 patients referred with localized cutaneous injection-site reactions from January 20 through February 12, 2021.
Main Outcomes and Measures
We collected each patient’s demographic information, a brief relevant medical history, clinical course, and treatment (if any); and considered the findings of a histopathologic examination of 1 skin biopsy specimen.
Results
Of 16 patients (median [range] age, 38 [25-89] years; 13 [81%] women), 14 patients self-identified as White and 2 as Asian. The delayed localized cutaneous reactions developed in a median (range) of 7 (2-12) days after receiving the Moderna COVID-19 vaccine. These reactions occurred at or near the injection site and were described as pruritic, painful, and edematous pink plaques. None of the participants had received the Pfizer-BioNTech vaccine. Results of a skin biopsy specimen demonstrated a mild predominantly perivascular mixed infiltrate with lymphocytes and eosinophils, consistent with a dermal hypersensitivity reaction. Of participants who had a reaction to first vaccine dose (15 of 16 patients), most (11 patients) developed a similar localized injection-site reaction to the second vaccine dose; most (10 patients) also developed the second reaction sooner as compared with the first-dose reaction.
Conclusions and Relevance
Clinical and histopathologic findings of this case series study indicate that the localized injection-site reactions to the Moderna COVID-19 vaccine are a delayed hypersensitivity reaction. These reactions may occur sooner after the second dose, but they are self-limited and not associated with serious vaccine adverse effects. In contrast to immediate hypersensitivity reactions (eg, anaphylaxis, urticaria), these delayed reactions (dubbed “COVID arm”) are not a contraindication to subsequent vaccination.
Introduction
The coronavirus disease-2019 (COVID-19) outbreak was declared a pandemic by the World Health Organization on March 11, 2020, and development of a safe, effective COVID-19 vaccine rapidly became a global priority.1 In the US, the Pfizer-BioNTech and Moderna mRNA COVID-19 vaccines were granted emergency use authorization in December 2020, with more than 48 million doses administered nationwide to date.2 As vaccine administration increases, recognition and understanding of these novel vaccines’ adverse effects are essential. In this Brief Report, we present a series of localized injection-site reactions to the Moderna COVID-19 vaccine that are consistent with clinical and histopathologic examination findings for delayed-type hypersensitivity reactions.
Methods
A retrospective case-series study was performed at Yale New Haven Hospital in New Haven, Connecticut, to assess clinical and histopathologic features of injection-site reactions to COVID-19 vaccines. The Yale University Institutional Review Board approved the study, and informed consent was waived because data were retrospective and deidentified.
All 16 patients referred to Yale New Haven Hospital’s Dermatology Services from January 20 through February 12, 2021, were included. We collected each patient’s demographic information, vaccine indication and manufacturer, medical history, medications, allergies, prior vaccine reactions, latency and duration of injection-site reactions, other symptoms, and treatment. We also reviewed clinical photographs of 13 of the 16 patients and histopathologic examination findings for 1 skin biopsy specimen from 1 of the 16 patients.
Results
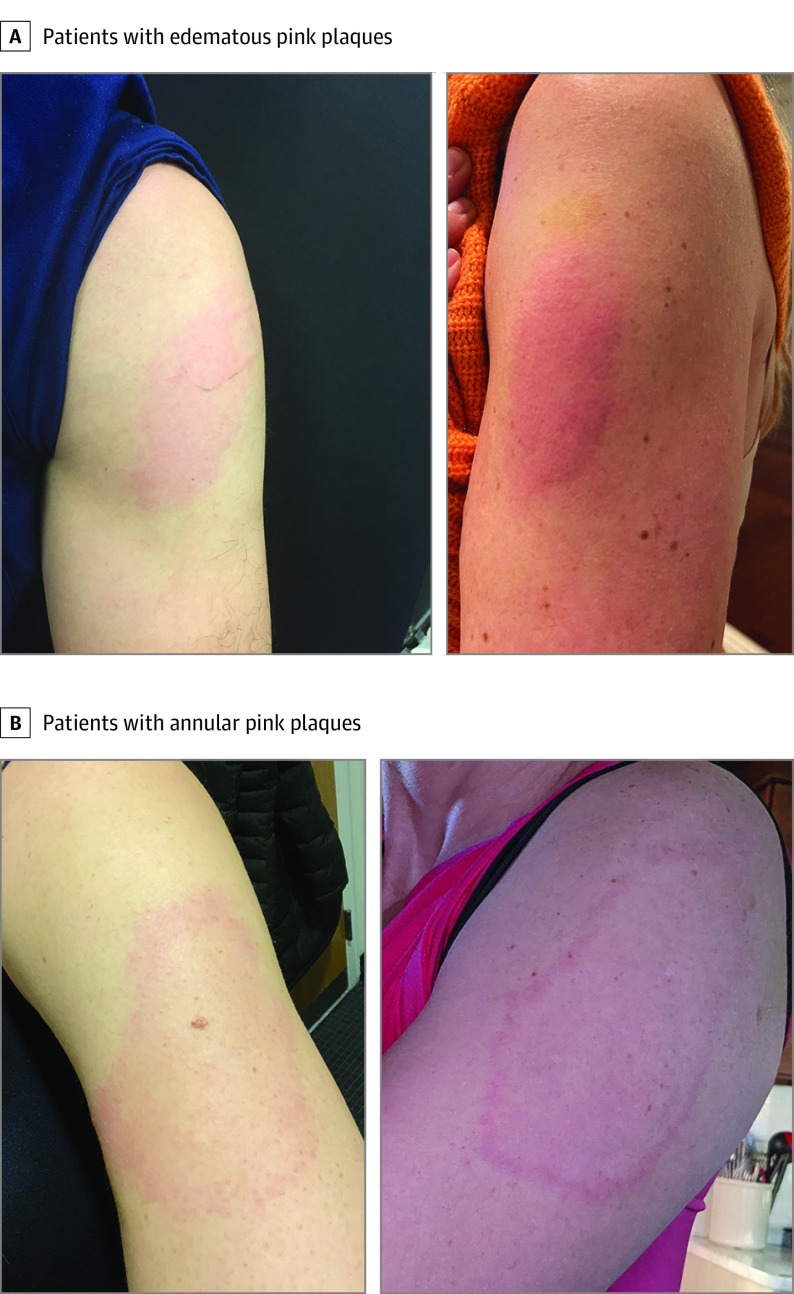
Of 16 patients, (median [range] age, 38 [25-89] years; 13 [81%] women) 14 patients self-identified as White and 2 as Asian. The main characteristics of these patients with delayed cutaneous hypersensitivity reactions are shown in the Table. All 16 participants received the Moderna COVID-19 vaccine, and only 1 reported a prior localized vaccine reaction (mild reaction to an influenza vaccine). Most (13 of 16) of the patients were health care workers; for the others (3 of 16), vaccine indication was for older age. Of the 16 patients, 15 developed localized cutaneous reactions after the first dose; 1 patient developed a reaction only after the second dose. After the first dose, pruritic and variably painful erythematous reactions near the injection site developed in a median (range) of 7 (2-12) days after vaccine administration. The pink plaques were variably edematous or indurated and were typically homogenous (Figure 1, A and B) or less commonly annular (Figure 1, C and D). Treatments included topical steroids, oral antihistamines, and cool compresses; 1 patient had received cephalexin for presumed cellulitis. Reactions to the first vaccine dose had a median (range) duration of 5 (1-21) days.
Table. Main Characteristics of Patients With Delayed Localized Cutaneous Hypersensitivity Reactions to the Moderna Coronavirus Disease 2019 Vaccine.
| Patient No. | Prior allergies | First-dose reaction | Second-dose reaction | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Onset, d | Duration, d | Other symptoms | Treatment | Onset, d | Duration, d | Other symptoms | Treatment | ||
| 1 | Amoxicillin/clavulanic acid, TMP-SMX | 9 | 15 | None | None | No reaction | NA | Nausea, chills, myalgias, headache, sore arm | NA |
| 2 | None | 2 | 14 | Fevers, chills, arthralgias, myalgias, headache, fatigue | None | No reaction | NA | Fatigue, myalgias, headaches | NA |
| 3 | None | 8 | 5 | Sore arm | Clobetasol ointment | 1 | 3 | Headache, myalgias, chills, sore arm | Clobetasol ointment |
| 4 | Seasonal allergies | 12 | 1 | None | None | 3 | 2 | Myalgias, chills | None |
| 5 | Seasonal allergies | 7 | 6 | None | None | 0 | 2 | Headache, fatigue, sore arm | Antihistamine |
| 6 | None | 7 | 2 | None | Antihistamine | 5 | 1 | Headache, chills, joint pain | None |
| 7 | None | 7 | 3 | None | Clobetasol ointment | 1 | 2 | Malaise, fevers, fatigue, headache | Clobetasol ointment |
| 8 | Cephalexin | 8 | 7 | Sore arm | Clobetasol ointment and antihistamine | 1 | 3 | Fatigue, decreased appetite, sore arm | Clobetasol ointment and antihistamine |
| 9 | Amoxicillin/clavulanic acid, TMP-SMX | 3 | 4 | Sore arm | None | 1 | 4 | Headache, fatigue, chills | None |
| 10 | Seasonal and food allergies | 7 | 5 | None | Cephalexin | No reaction | NA | Fever, headache, fatigue | NA |
| 11 | None | 7 | 4 | Fatigue | None | 2 | 2 | Fever, headache, neck pain, arthralgias | None |
| 12 | TMP-SMX | 7 | 21 | Sore arm | Antihistamine | 2 | 5 | Lethargy | None |
| 13 | None | 6 | 7 | None | Cool compresses | 2 | 5 | None | None |
| 14 | Seasonal and food allergies | No reaction | NA | Sore arm | NA | 2 | 4 | Fevers, chills, myalgias, rigors | None |
| 15 | None | 2 | 5 | Fatigue | Hydrocortisone cream | 2 | 5 | Fever, headache, fatigue | Hydrocortisone cream |
| 16 | None | 9 | 2 | Sore arm | None | No reaction | NA | Sore arm | NA |
Abbreviations: NA, not applicable; TMP-SMX, trimethoprim-sulfamethoxazole.
Figure 1. Delayed Localized Cutaneous Hypersensitivity Reactions After the Moderna Coronavirus Disease 2019 Vaccine.
A, Edematous pink plaques. B, Annular pink plaques.
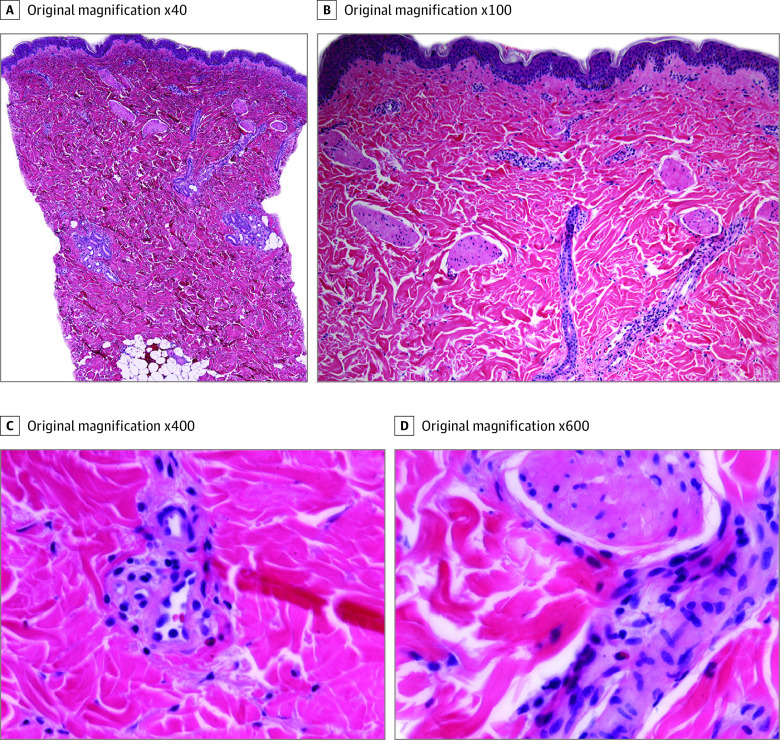
Of the 16 patients, 12 developed injection-site reactions to the second dose, with a median (range) onset of 2 (0-5) days after vaccine administration; 1 of these 12 patients had no reaction after the first dose. Among the 15 patients who had experienced a reaction after the first dose, most (11 patients) developed a reaction to the second dose. Of these, most (10 patients) developed the second-dose reaction sooner as compared with their first-dose reaction. Second-dose reactions had a median (range) duration of 3 (1-5) days and were clinically similar to the first-dose reactions. Findings of an histologic examination of a skin punch-biopsy specimen of a second-dose reaction demonstrated mild predominantly perivascular and focal interstitial mixed infiltrate with lymphocytes and eosinophils consistent with a dermal hypersensitivity reaction (Figure 2).
Figure 2. Histopathologic Features of a Delayed Localized Hypersensitivity Reaction to the Moderna Coronavirus Disease 2019 Vaccine.
A and B, Punch biopsy specimen demonstrating mild predominantly perivascular and focal interstitial mixed infiltrate with lymphocytes and eosinophils (hematoxylin-eosin). C and D, Perivascular eosinophils (hematoxylin-eosin).
Discussion
We report on 16 cases of delayed localized cutaneous reactions to the Moderna mRNA COVID-19 vaccine, dubbed “COVID arm,” which were consistent with clinical and histopathologic examinations findings for delayed-type hypersensitivity reactions. This delayed reaction is distinctly different from the local pain, redness, and swelling observed on average 1 day after either dose with a median duration of 2 to 3 days, as reported in the Moderna COVID-19 vaccine trial.3 Instead, COVID arm represents a delayed injection-site reaction characterized by erythema, pruritus, induration, and tenderness. In this case series, the median (range) onset of the reactions was 7 (2-12) days after the first dose, and the reaction had a median duration of 5 days.
Similarly, COVID arm symptoms reported in the Moderna trial4 appeared on or after day 8 following the first dose (244 of 30 351 total trial participants; 0.8%) or the second dose (68 of 30 351 total trial participants; 0.2%) and resolved after 4 to 5 days. Delayed localized reactions may have been underreported by the Moderna trial because local reactions were actively solicited only until day 7, with unsolicited adverse events collected thereafter. In this case series, the second vaccine reactions developed more quickly in 10 of 11 patients who experienced reactions to both vaccine doses, with a median onset of 2 days after the second dose. This may have been captured by the increased frequency of erythema within 7 days of the second dose of the mRNA vaccine (1257 of 14 677 participants; 8.5%) vs the first dose (430 of 15 168 participants; 2.8%) in the Moderna trial.
In the present case-series study, the timing and histopathologic examination findings of the reaction suggest cell-mediated immunity associated with delayed-type hypersensitivity reactions. These observations are consistent with a recent case-series study by Blumenthal and colleagues5 of 12 delayed localized cutaneous reactions to the Moderna vaccine. The present case-series supports and expands on the findings of those authors by reporting on cases among patients with a broader age range (25-89 years) and additional histopathologic examination findings. Similar to the findings of the present study, results of a skin biopsy specimen in the prior study5 demonstrated perivascular and interstitial inflammatory infiltrate with lymphocytes, eosinophils, and minimal epidermal change. At our institution, we too have observed these findings in the results of 3 additional biopsy specimens of delayed localized Moderna vaccine reactions from patients not among the 16 described in this Brief Report.
The histopathologic findings observed in the present case series are characteristic of dermal hypersensitivity reactions, which may be seen in response to medications.6 Such medication-associated delayed hypersensitivity reactions are T-cell mediated,7 and similarly, we hypothesize that delayed localized cutaneous reactions to Moderna COVID-19 vaccine may be associated with T-cell responses to a vaccine excipient, lipid nanoparticle, or mRNA component. Polyethylene glycol, present in both the Moderna and the Pfizer vaccine, has been implicated in some immediate hypersensitivity reactions,8 but its role in delayed-type hypersensitivity reactions remains unknown. Patch testing of patients with COVID arm using vaccine components may be informative.
All of the patients in this case-series study had received the Moderna vaccine. Although similar amounts of the Moderna and Pfizer vaccines have been allocated to Connecticut,9,10 it is possible that the New Haven area may have received more of the Moderna vaccine. To determine the incidence of COVID arm for each vaccine, we suggest that the Centers for Disease Control and Prevention add questions about this delayed reaction to the v-safe health checker (https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafe.html), and we encourage health care professionals to submit cases of cutaneous COVID-19 vaccine reactions to the American Academy of Dermatology registry (https://www.aad.org/member/practice/coronavirus/registry).
Most (14 of 16) of the patients in this series were White, possibly because of Connecticut’s demographic composition and the national distribution trends of COVID-19 vaccine.11 Another reason for the predominantly White cohort is that erythema may be overlooked or not as obvious on darker phototype skin. Most (13 of 16) of the patients were also women. Women have received more (59.2%) of the COVID-19 vaccines in the US to date.11 Women may also be more prone to developing COVID arm, just as women are more likely to develop anaphylaxis, immediate hypersensitivity, and injection-site reactions to other vaccines.12,13 Finally, it is possible that women may be more likely to report symptoms or to seek medical care for such reactions.
As COVID-19 vaccine administration increases, clinicians and the public should be aware of COVID arm. In this case-series cohort, the clinical characteristics and the findings of histopathologic examinations were consistent with delayed-type hypersensitivity reactions, and no serious vaccine adverse events occurred in association with these cutaneous reactions. It is critical that health care professionals distinguish these delayed-type reactions from immediate-type hypersensitivity reactions and from cellulitis. The Centers for Disease Control and Prevention currently recommends14 that patients who experience immediate hypersensitivity reactions, including urticaria, within 4 hours of receiving a COVID-19 vaccine postpone the second dose until after consulting an allergist-immunologist. In contrast, the delayed localized hypersensitivity reaction we describe in this case-series study is not a contraindication to subsequent vaccination and patients and health care professionals should be aware that this type of reaction may develop more rapidly after the second vaccine dose.
Limitations
This case series was from a single center during a short period of time. Most of the patients were health care workers, which may limit generalizability.
Conclusions
In this case series, we characterize delayed localized injection-site reactions to the Moderna COVID-19 vaccine, dubbed COVID arm, which we suggest renaming “COVID vaccine arm.” These cutaneous reactions occur near the injection site and are benign and self-limited. These reactions appear a median of 7 days after the first vaccine dose and 2 days after the second dose. In contrast to immediate hypersensitivity reactions (eg, anaphylaxis and urticaria) that present within 4 hours of vaccine administration, these delayed localized hypersensitivity reactions are not a contraindication to subsequent vaccination.
References
- 1.Jin Y, Yang H, Ji W, et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12(4):372. doi: 10.3390/v12040372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention . COVID Data Tracker. Accessed February 12, 2021. http://covid.cdc.gov/covid-data-tracker/#global-counts-rates
- 3.Centers for Disease Control and Prevention . Local reactions, systemic reactions, adverse events, and serious adverse events: Moderna COVID-19 vaccine. Accessed February 12, 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html
- 4.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumenthal KG, Freeman EE, Saff RR, et al. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med. 2021;384(13):1273-1277. doi: 10.1056/NEJMc2102131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fung MA. The clinical and histopathologic spectrum of “dermal hypersensitivity reactions,” a nonspecific histologic diagnosis that is not very useful in clinical practice, and the concept of a “dermal hypersensitivity reaction pattern.” J Am Acad Dermatol. 2002;47(6):898-907. doi: 10.1067/mjd.2002.120908 [DOI] [PubMed] [Google Scholar]
- 7.Copaescu A, Gibson A, Li Y, Trubiano JA, Phillips EJ. An updated review of the diagnostic methods in delayed drug hypersensitivity. Front Pharmacol. 2021;11:573573. doi: 10.3389/fphar.2020.573573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabanillas B, Akdis C, Novak N. Allergic reactions to the first COVID-19 vaccine: a potential role of Polyethylene glycol? Allergy. 2020. doi: 10.1111/all.14711 [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention . COVID-19 vaccine distribution allocations by jurisdiction–Moderna. Accessed February 12, 2021. https://data.cdc.gov/Vaccinations/COVID-19-Vaccine-Distribution-Allocations-by-Juris/b7pe-5nws
- 10.Centers for Disease Control and Prevention . COVID-19 vaccine distribution allocations by jurisdiction–Pfizer. Accessed February 12, 2021. https://data.cdc.gov/Vaccinations/COVID-19-Vaccine-Distribution-Allocations-by-Juris/saz5-9hgg
- 11.Centers for Disease Control and Prevention . Demographic characteristics of people receiving COVID-19 vaccines in the United States. Accessed February 12, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccination-demographic
- 12.McNeil MM, DeStefano F. Vaccine-associated hypersensitivity. J Allergy Clin Immunol. 2018;141(2):463-472. doi: 10.1016/j.jaci.2017.12.971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook IF. Sex differences in injection site reactions with human vaccines. Hum Vaccin. 2009;5(7):441-449. doi: 10.4161/hv.8476 [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention . Interim clinical considerations for use of mRNA COVID-19 vaccines currently authorized in the United States. Accessed February 13, 2021. http://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html#contraindications