Key Points
Question
What is the long-term (68-week) efficacy of baricitinib, 4 and 2 mg, in patients with moderate to severe atopic dermatitis who were treatment responders or partial responders?
Findings
In this long-term extension study of 2 randomized clinical trials, the proportions of 70 responders/partial responders to baricitinib, 4 mg, in the original studies who achieved a validated Investigator Global Assessment for Atopic Dermatitis score of 0 or 1 during continuous treatment were 45.7% at week 16 and 47.1% at week 68. Corresponding proportions for 54 patients receiving baricitinib, 2 mg, were 46.3% at week 16 and 59.3% at week 68.
Meaning
In this study, baricitinib demonstrated sustained long-term efficacy, suggesting that baricitinib may be a longer-term treatment option for moderate to severe atopic dermatitis.
Abstract
Importance
Baricitinib, an oral selective Janus kinase inhibitor, improved the clinical signs and symptoms of moderate to severe atopic dermatitis in the 16-week, phase 3 monotherapy studies, BREEZE-AD1 and BREEZE-AD2. Long-term efficacy has not yet been examined.
Objective
To evaluate the long-term (68-week) efficacy of baricitinib in adults with moderate to severe atopic dermatitis who were treatment responders or partial responders in BREEZE-AD1 and BREEZE-AD2.
Design, Setting, and Participants
Patients completing BREEZE-AD1/BREEZE-AD2 entered the ongoing, multicenter, double-blind, long-term extension study BREEZE-AD3. The study was initiated on March 28, 2018. Data were analyzed on December 13, 2019.
Interventions
Responders and partial responders (patients achieving validated Investigator Global Assessment for Atopic Dermatitis [vIGA-AD] score of 0 or 1 [0,1], or 2) at BREEZE-AD1/BREEZE-AD2 completion remained on originally assigned treatment for 52 weeks (68 total weeks of continuous therapy).
Main Outcomes and Measures
The primary end point was the proportion of patients achieving a vIGA-AD score of 0,1 at weeks 16, 36, and 52 of BREEZE-AD3. Secondary end points included the proportion of patients achieving 75% or more improvement in the Eczema Area and Severity Index [EASI75] score and 4-point or more improvement in the itch numeric rating scale (NRS), using originating study baseline data. Itch data were collected during the first 16 weeks in BREEZE-AD3. The last originating study visit was the first BREEZE-AD3 visit; therefore, data are presented for continuous weeks of therapy, including the 16-week originating study period. Missing data were imputed by last observation carried forward. Modified intention-to-treat analysis was used.
Results
Of the responder/partial responder population, the proportion of patients treated with baricitinib, 4 mg (n = 70) (mean [SD] age, 36.7 [15.5] years; 42 [60%] were men), achieving vIGA-AD (0,1) at week 16 was 45.7% (BREEZE-AD3 baseline) and, at week 68, 47.1%. Improvement of 75% or more in the EASI score was 70.0% at week 16 and 55.7% at week 68. The proportion of patients achieving an itch NRS improvement greater than or equal to 4 points at week 16 was 52.5% and, at week 32, 45.9%. Of the responder/partial responder population, the proportion of patients treated with baricitinib, 2 mg (n = 54) (mean [SD] age, 32.8 [12.7] years; 28 [51.9%] were men), achieving vIGA-AD (0,1) at week 16 was 46.3% and, at week 68, 59.3%. Improvement in the EASI75 score was 74.1% at week 16 and 81.5% at week 68. The proportion of patients achieving an itch NRS improvement greater than or equal to 4 points at week 16 was 44.2% and, at week 32, 39.5%.
Conclusions and Relevance
In this long-term double-blind extension study of 2 randomized clinical trials, baricitinib, 4 and 2 mg, demonstrated sustained long-term efficacy in patients with moderate to severe atopic dermatitis.
Trial Registration
ClinicalTrials.gov Identifier: NCT03334435
This extension study of 2 randomized clinical trials examines the continuous use of baricitinib in patients with atopic dermatitis from the originating studies to 68 weeks of treatment.
Introduction
Atopic dermatitis (AD), or atopic eczema, is a common chronic inflammatory skin disease that can be debilitating for patients.1 Patients who do not respond to emollients and topical corticosteroids may require systemic therapy2,3; however, current systemic therapies for moderate to severe AD are not effective in all patients and may have adverse effects.4,5,6,7,8
Baricitinib is an oral selective Janus kinase 1 and 2 inhibitor indicated in the European Union and Japan for the treatment of moderate to severe AD in adults who are candidates for systemic therapy. Findings from 3 placebo-controlled phase 3 trials have been reported demonstrating that baricitinib significantly improved the clinical signs and symptoms of moderate to severe AD within 16 weeks of treatment in patients with an inadequate response or intolerance to topical corticosteroids when given as monotherapy (BREEZE-AD1/BREEZE-AD29) or combined with topical corticosteroids (BREEZE-AD710).
BREEZE-AD3 is an ongoing phase 3, double-blind, long-term extension study evaluating the maintenance of efficacy in patients who responded to baricitinib treatment in BREEZE-AD1, BREEZE-AD2, or BREEZE-AD7 and the effect of baricitinib dose uptitration in patients who did not respond to baricitinib treatment. Herein, we report 52-week efficacy outcomes (week 68 of continuous therapy) for patients originating from monotherapy studies (BREEZE-AD1/BREEZE-AD2).
Methods
Study Design
BREEZE-AD3 is a phase 3, multicenter, double-blind, long-term extension study at sites in Europe, Asia, Latin America, and Australia. This ongoing study started on March 28, 2018, is being performed in accordance with ethical principles of the Declaration of Helsinki11 and Good Clinical Practice guidelines, and was approved by institutional review boards/ethics committees at each site. Data were analyzed on December 13, 2019. All patients provided written informed consent; patients received financial compensation. Further details regarding the study design and methods can be found in the study protocol (Supplement 1) and eMethods in Supplement 2. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
On entry, patients were classified into groups based on their response to treatment in the originating studies (BREEZE-AD1/BREEZE-AD2): responders, defined as those who achieved a validated Investigator Global Assessment for AD (vIGA-AD) score of 0 or 1 (0,1) and never received rescue therapy during the originating study; partial responders, defined as those who achieved a vIGA-AD score of 2 and never received rescue therapy during the originating study; and nonresponders, defined as those who achieved a vIGA-AD score of 3 or 4 or received rescue therapy during the originating study. During the originating studies, investigators could provide rescue therapy for patients experiencing unacceptable or worsening of AD symptoms. Initial rescue therapy comprised triamcinolone, 0.1%, cream and/or hydrocortisone, 2.5%, ointment. Because all patients reported in this analysis previously completed a 16-week placebo-controlled study and the last visit of BREEZE-AD1/BREEZE-AD2 at week 16 was the first visit of BREEZE-AD3, efficacy analyses are reported based on the week of continuous therapy. For example, baseline of study BREEZE-AD3 is equivalent to 16 weeks of continuous therapy and week 52 of BREEZE-AD3 is equivalent to 68 weeks of continuous therapy.
Patients who were initially randomized to baricitinib, 4 or 2 mg, and were classified as responders or partial responders at the start of BREEZE-AD3 remained on their original doses (eFigure in Supplement 2). Patients initially randomized to placebo or baricitinib, 1 mg, who were classified as responders or partial responders at the start of BREEZE-AD3 received rescue therapy of baricitinib, 4 or 2 mg (1:1 rerandomization), during BREEZE-AD3 if they experienced worsening of their AD (vIGA-AD score of 3 or 4). Thus, long-term efficacy data from patients treated with placebo and baricitinib, 1 mg, are not included in this article owing to a decrease in sample size in these groups over time. Nonresponders receiving placebo; baricitinib, 1 mg; or baricitinib, 2 mg, at completion of the originating study were rerandomized in a blind-fashion 1:1 to baricitinib, 4 or 2 mg. Nonresponders receiving baricitinib, 4 mg, at originating study completion remained on the same dose. Data from nonresponders rerandomized to baricitinib, 4 or 2 mg, are not included in the analyses described herein.
Efficacy End Points
Efficacy end points evaluated in responders and partial responders are summarized in eTable 1 in Supplement 2. The primary end point was the proportion of patients with vIGA-AD (0,1) at weeks 32, 52, and 68 of continuous therapy. Secondary end points included the proportion of patients achieving 75% improvement from baseline in the Eczema Area and Severity Index score (EASI75) and itch numeric rating scale (NRS) improvement of 4 points or more from baseline. Exploratory end points included the proportion of patients achieving skin pain NRS improvement of 4 points or more from baseline and Atopic Dermatitis Sleep Scale (ADSS) item 2 improvement of 1.5 points or more from baseline. Baseline disease severity assessments used the originating study baseline value (at initial randomization). Patient-reported outcome data were collected using daily diaries (completed during the first 16 weeks of BREEZE-AD3 only; week 32 of continuous therapy). Weekly mean scores were used for analyses. vIGA-AD (0,1), EASI75, and itch NRS end points were also evaluated in all patients (responders, partial responders, and nonresponders) who received continuous baricitinib, 4 mg, in the originating study through BREEZE-AD3.
Statistical Analysis
Efficacy analyses were performed using the modified intention-to-treat principle, including patients who received 1 or more dose of the investigational product in BREEZE-AD3. To evaluate maintenance of response, analyses were performed on patients who were initially randomized to baricitinib, 4 or 2 mg, and entered BREEZE-AD3 as responders or partial responders (ie, patients who continued their originally assigned treatment). To evaluate durability of response, analyses were performed on all patients (responders, partial responders, and nonresponders combined) who received continuous baricitinib, 4 mg, in the originating studies (BREEZE-AD1/BREEZE-AD2) through BREEZE-AD3. The intention-to-treat population was used for BREEZE-AD1/BREEZE-AD2 (weeks 0-16) and the modified intention-to-treat population was used for BREEZE-AD3. Results for patients originating from BREEZE-AD7 are not reported in this article because the 68-week efficacy follow-up data were not available.
Data accruing after permanent study drug discontinuation were treated as missing. For maintenance analyses on responders and partial responders, last observation carried forward (LOCF) was used for missing data imputation as the primary approach. Nonresponder imputation (NRI) was also performed. Response rates based on LOCF and NRI are summarized descriptively over time. For analyses of all patients (responders, partial responders, and nonresponders) who received continuous baricitinib, 4 mg, in the originating study through BREEZE-AD3, response rates were based on NRI.
Analyses were performed using SAS, version 9.4 or higher (SAS Institute Inc). The statistical analysis plan is provided in Supplement 1.
Results
Disposition and Baseline Characteristics
Of the 1239 patients enrolled in BREEZE-AD1/BREEZE-AD2, 1081 (87.2%) enrolled in BREEZE-AD3. A total of 221 patients enrolled from BREEZE-AD1/BREEZE-AD2 were classified as responders and partial responders at the start of BREEZE-AD3 and maintained their originating study treatment (Figure 1). At the time of this analysis, 33.3% (15/45) of patients who received baricitinib, 1 mg, and 50.0% (26/52) of those who received placebo initially classified as responders or partial responders at the start of BREEZE-AD3 had experienced loss of response and were given rescue therapy with baricitinib, 4 or 2 mg. Therefore, efficacy results are shown only for baricitinib, 4 and 2 mg. At the completion of BREEZE-AD1/BREEZE-AD2 (week 16), there were an additional 45 patients receiving baricitinib, 4 mg, and 65 patients receiving baricitinib, 2 mg, who had achieved a vIGA-AD score of 0,1 or 2 at week 16 but received rescue therapy during BREEZE-AD1/BREEZE-AD2 and, thus, were considered nonresponders at the start of BREEZE-AD3. These patients were not included in the present analyses. Baseline characteristics for baricitinib, 4 mg and 2 mg, responders and partial responders who enrolled in BREEZE-AD3 are reported in the Table.
Figure 1. CONSORT Diagram for Patients Who Entered BREEZE-AD3 as Responders or Partial Responders.
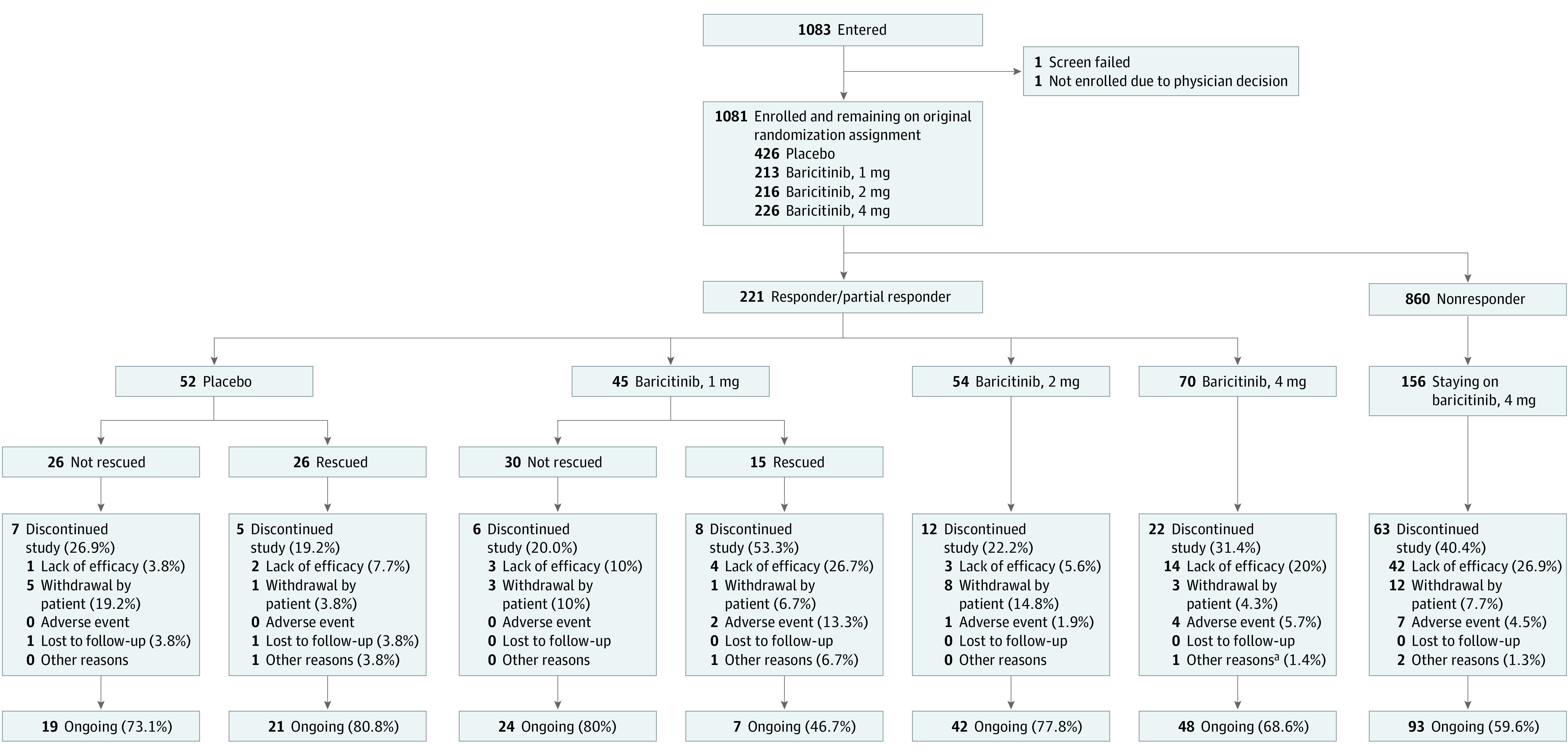
Patients who had a validated Investigator Global Assessment for Atopic Dermatitis score of 0 or 1 were considered responders; those with a score of 2 were considered partial responders. No rescue therapy was used in these groups during the originating study (BREEZE-AD1/BREEZE-AD2).
aFor baricitinib, 4 mg, the other reason was the investigator’s decision owing to the patient’s work commitments.
Table. Baseline Demographics and Disease Characteristics for Patients Who Entered BREEZE-AD3 as Responders or Partial Respondersa.
| Characteristic | Baricitinib, 2 mg (n = 54) | Baricitinib, 4 mg (n = 70) |
|---|---|---|
| Originating study baselineb | ||
| Age, mean (SD), y | 32.8 (12.7) | 36.7 (15.5) |
| Female, No. (%) | 26 (48.1) | 28 (40.0) |
| Race, No. (%) | ||
| White | 45 (83.3) | 47 (67.1) |
| Asian | 7 (13.0) | 18 (25.7) |
| Black | 0 | 1 (1.4) |
| Other | 2 (3.7) | 4 (5.7) |
| Time since AD diagnosis, mean (SD), y | 19.2 (11.8) | 23.2 (16.8) |
| Weight, mean (SD), kg | 77.3 (18.5) | 74.2 (15.2) |
| BMI, mean (SD) | 26.5 (6.3) | 25.4 (4.2) |
| Geographic region, No. (%) | ||
| Europe | 30 (55.6) | 41 (58.6) |
| Japan | 1 (1.9) | 6 (8.6) |
| Other | 23 (42.6) | 23 (32.9) |
| vIGA-AD score 4, No. (%)c | 18 (33.3) | 22 (31.4) |
| EASI score, mean (SD)d | 24.9 (8.7) | 28.1 (10.6) |
| SCORAD score, mean (SD)e | 62.2 (12.0) | 63.4 (12.3) |
| Itch NRS score, mean (SD)f | 6.1 (2.2) | 6.5 (2.1) |
| Skin pain NRS score, mean (SD)g | 5.8 (2.5) | 5.9 (2.5) |
| ADSS item 2 score, mean (SD)h | 1.7 (3.7) | 2.2 (3.5) |
| BREEZE-AD3 baseline | ||
| vIGA-AD score, No. (%)c | ||
| 0,1 | 25 (46.3) | 32 (45.7) |
| 2 | 29 (53.7) | 38 (54.3) |
| EASI score, mean (SD)d | 4.5 (4.5) | 5.2 (5.3) |
| SCORAD score, mean (SD)e | 23.9 (12.9) | 21.8 (13.1) |
| BSA affected, mean (SD), % | 9.2 (12.9) | 11.0 (13.4) |
| Itch NRS score, mean (SD)f | 2.7 (2.5) | 2.4 (2.0) |
| Skin pain NRS score, mean (SD)g | 2.3 (2.4) | 2.1 (1.9) |
| ADSS item 2 score, mean (SD)h | 0.4 (0.7) | 0.4 (0.8) |
| DLQI score, mean (SD)i | 3.4 (3.7) | 3.1 (3.6) |
Abbreviations: AD, atopic dermatitis; ADSS, Atopic Dermatitis Sleep Scale; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BSA, body surface area; DLQI, Dermatology Life Quality Index; EASI, Eczema Area and Severity Index; NRS, numeric rating scale; SCORAD, Scoring Atopic Dermatitis; vIGA-AD, validated Investigator Global Assessment for Atopic Dermatitis.
Responders and partial responders had vIGA-AD scores of 0,1 or 2, respectively, at entry into BREEZE-AD3 and never received rescue therapy during the originating study (BREEZE-AD1/BREEZE-AD2).
Combined data from BREEZE-AD1/BREEZE-AD2.
Responders were defined as those who achieved a vIGA-AD score of 0 or 1 and never received rescue therapy during the originating study; partial responders, those who achieved a vIGA-AD score of 2 and never received rescue therapy during the originating study; and nonresponders were defined as those who achieved a vIGA-AD score of 3 or 4 or received rescue therapy during the originating study.
Scores range from 0 to 72, with higher scores indicating greater severity.
Combined score of investigator-reported disease severity and affected body surface area and patient-reported symptoms of itch and sleep dysfunction; scores range from 0 to 103, with higher scores indicating greater disease severity.
Scores range from 0 (no itch) to 10 (worst itch imaginable).
Scores range from 0 (no pain) to 10 (worst pain imaginable).
Assesses the frequency of nighttime awakenings attributable to itch the previous night on a scale of 0 to 29, with higher scores indicating a greater number of awakenings owing to itch each night.
Evaluates health-related quality of life on a scale of 0 to 30, with higher scores indicating a greater effect on a patient's life.
Efficacy End Points
Clinical Signs
The proportion of baricitinib, 4 mg, responders and partial responders (n = 70) (mean [SD] age, 36.7 [15.5] years; 42 [60%] were men) achieving or maintaining vIGA-AD (0,1) was stable throughout BREEZE-AD3: 45.7% at baseline (week 16 of continuous therapy) and 47.1% at week 68 of continuous therapy (LOCF) (Figure 2A). Results for EASI75 response showed a slight decrease over time (week 16, 70.0%; week 68, 55.7%) (LOCF) (Figure 2A).
Figure 2. Validated Investigator Global Assessment for Atopic Dermatitis score of 0 or 1 (vIGA-AD [0,1]), 75% Improvement From Originating Study Baseline in Eczema Area and Severity Index (EASI75), and Mean EASI Change From Baseline Responses Through 68 Weeks of Continuous Treatment for Patients Who Entered BREEZE-AD3 as Responders or Partial Responders.
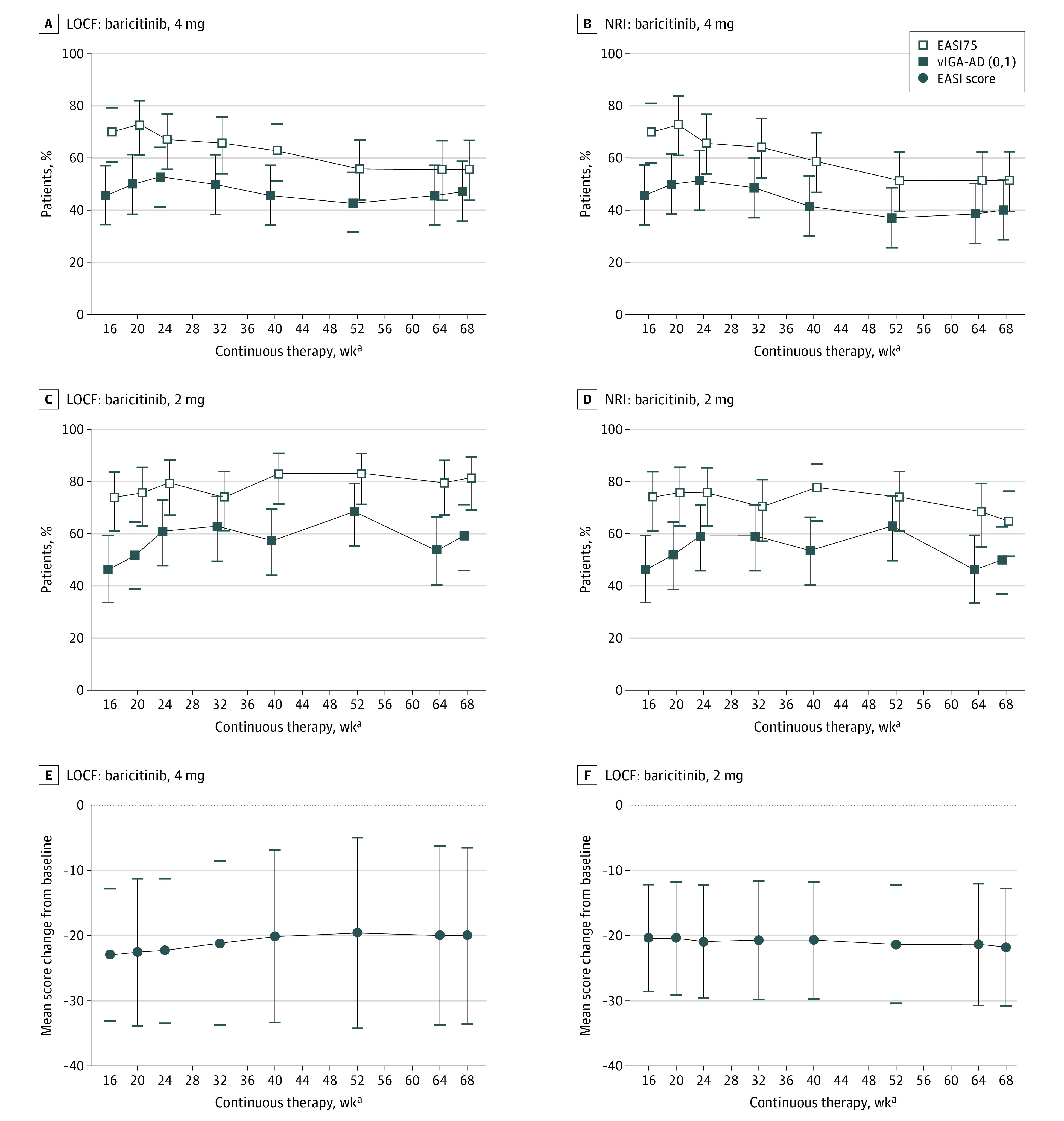
Patients who had a vIGA-AD score of 0 or 1 were considered responders; those with a score of 2 were considered partial responders. No rescue therapy was used in these groups during the originating study (BREEZE-AD1/BREEZE-AD2). LOCF indicates last observation carried forward; NRI, nonresponder imputation. Error bars indicate 95% CI (A-D) or SD (E,F).
aData for the modified intent-to-treat population are shown as weeks of continuous therapy, which includes the 16-week treatment period in the originating studies.
The proportion of baricitinib, 2 mg, responders and partial responders (n = 54) (mean [SD] age, 32.8 [12.7] years; 28 [51.9%] were men) achieving or maintaining vIGA-AD (0,1) was mostly stable to slightly increased (week 16, 46.3%; week 68, 59.3%) (LOCF) (Figure 2C). Results for EASI75 response were stable to slightly increased (week 16, 74.1%; week 68, 81.5%) (LOCF) (Figure 2C).
Results for clinical signs assessment using itch NRI were consistent with findings from the LOCF analysis (Figure 2B, D). The EASI total score change from baseline of originating study in responders and partial responders also showed sustained efficacy throughout BREEZE-AD3. For baricitinib, 4 mg, the mean change from baseline was –22.9 at week 16 and –20.0 at week 68 of continuous therapy (Figure 2E). For baricitinib, 2 mg, changes from baseline were –20.4 at week 16 and –21.7 at week 68 (Figure 2F). Change from baseline results for other available harmonizing outcome measures for eczema are given in eTable 2 in Supplement 2.
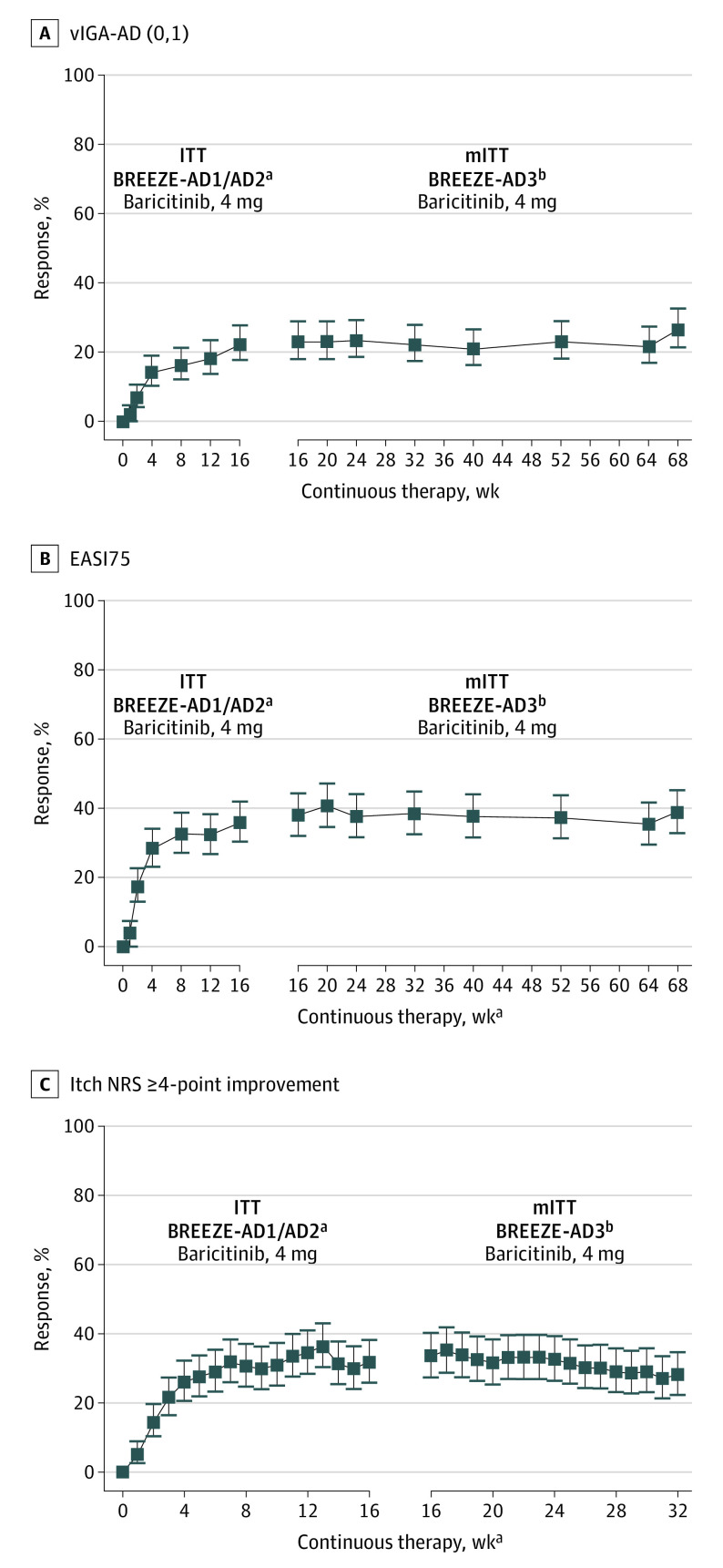
Figure 3 shows the persistence of response for clinical signs (vIGA-AD [0,1] and EASI75) for all patients (responders, partial responders, and nonresponders) who received continuous baricitinib, 4 mg, through BREEZE-AD3 (NRI). Owing to the study design, all patients who did not respond to baricitinib, 2 mg, in the originating study (nonresponders) were rerandomized 1:1 to baricitinib, 4 or 2 mg, in BREEZE-AD3. Therefore, persistence of response (continuous efficacy from weeks 0-68) can be presented only for patients initially randomized to baricitinib, 4 mg, owing to a significant change in sample size for the baricitinib, 2 mg, group at the start of BREEZE-AD3. Overall, response rates were maintained for the 68-week duration of treatment. Only NRI analysis is shown for these data because of the more conservative imputation analysis used in this study.
Figure 3. Improvement for All Patients Who Received Continuous Baricitinib, 4 mg, Through BREEZE-AD1/BREEZE-AD2 and BREEZE-AD3.
A, Validated Investigator Global Assessment for Atopic Dermatitis score of 0 or 1 (vIGA-AD [0,1]). B, 75% Improvement in Eczema Area and Severity Index (EASI75). C, Itch numeric rating scale greater than or equal to 4-point (NRS ≥4) improvement. Missing data were imputed using nonresponder imputation (NRI). Error bars indicate 95% CI; ITT, intent-to-treat; and mITT, modified ITT.
aResponse during weeks 0 to 16 was censored only at the time of the drug discontinuation; ITT population included all patients as randomized during the study. Data after study treatment discontinuation were imputed as NRI.
bmITT in BREEZE-AD3 included all patients receiving 1 or more dose of baricitinib in BREEZE-AD3.
Patient-Reported Outcomes
Responses to baricitinib, 4 mg, remained relatively stable throughout BREEZE-AD3 for responders and partial responders for itch: NRS 4 points or greater improvement (week 16, 52.5%; week 32, 45.9%), skin pain NRS 4 points or greater improvement (week 16, 61.8%; week 32, 54.5%), and ADSS item 2 1.5 points or greater improvement (week 16, 75.0%; week 32, 71.4%) (LOCF) (Figure 4A).
Figure 4. Patient-Reported Outcomes Through 32 Weeks of Continuous Treatment for Patients Who Entered BREEZE-AD3 as Responders or Partial Responders.
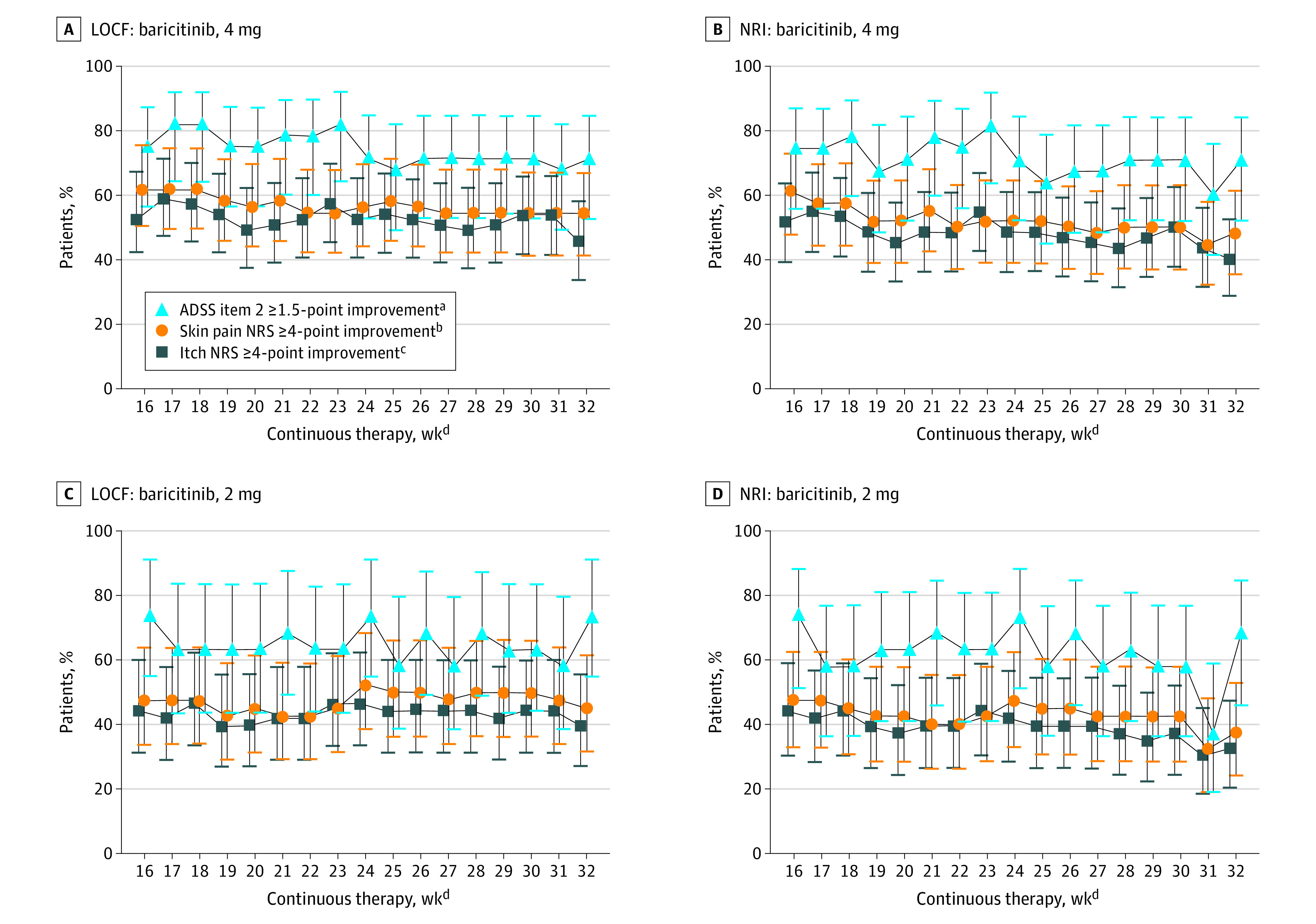
Responders and partial responders were patients who had a validated Investigator Global Assessment for Atopic Dermatitis score of 0,1 or 2 and never received rescue therapy during the originating study (BREEZE-AD1/BREEZE-AD2). Error bars indicate 95% CIs. Findings shown for Atopic Dermatitis Sleep Scale (ADSS) item 2 improvement of 1.5 points or more; itch numeric rating scale (NRS) improvement of 4 points or more, and skin pain NRS improvement of 4 points or more. A, Last observation carried forward (LOCF) for baricitinib, 4 mg: ADSS item 2, n = 28; itch NRS, n = 61; skin pain NRS, n = 55. B, Nonresponder imputation (NRI) for baricitinib, 4 mg: ADSS, n = 28; itch NRS, n = 61; skin pain NRS, n = 55. C, LOCF for baricitinib, 2 mg: ADSS, n = 19; itch NRS, n = 43; NRS, skin pain, n = 40. D, NRI for baricitinib, 2 mg: ADSS, n = 19; itch NRS, n = 43; skin pain NRS, n = 40.
aIn patients with an originating study baseline ADSS item 2 score greater than or equal to 1.5.
bIn patients with an originating study baseline skin pain NRS score greater than or equal to 4.
cIn patients with an originating study baseline itch NRS score greater than or equal to 4.
dData for the modified intent-to-treat population are shown as weeks of continuous therapy, which includes the 16-week treatment period in the originating studies.
Responses with baricitinib, 2 mg, remained relatively stable through BREEZE-AD3 for itch: NRS 4 points or greater improvement (week 16, 44.2%; week 32, 39.5%), skin pain NRS 4 points or greater improvement (47.5% and 45.0%), and ADSS item 2 1.5 points or greater improvement (73.7% and 73.7%) (LOCF) (Figure 4C). Similar to clinical signs, NRI and LOCF analyses showed consistent results (Figures 4B, D), with LOCF noting smaller variability from week to week.
Persistence of response in patients originally randomized to baricitinib, 4 mg, was also assessed for patient-reported outcomes. For all patients (responders, partial responders, and nonresponders) who received continuous baricitinib, 4 mg, in the originating study through BREEZE-AD3, the itch NRS 4 points or greater improvement response remained stable from weeks 16 to 32 (NRI) (Figure 3C). Topical corticosteroid use is summarized in eTable 3 in Supplement 2.
Discussion
To our knowledge, BREEZE-AD3 is the first study to evaluate the long-term efficacy of baricitinib in patients with moderate to severe AD. Herein, we described how patients who entered BREEZE-AD3 as responders or partial responders to treatment with baricitinib, 4 or 2 mg, maintained improvements in clinical signs (vIGA-AD [0,1] and EASI75) through 68 weeks of continuous treatment, despite use of small amounts of topical corticosteroids.
In this study, baricitinib, 4 mg, showed a slight decrease in EASI75 response among responders and partial responders, whereas baricitinib, 2 mg, showed a mostly stable response between weeks 16 and 68. This seeming loss of EASI75 response with baricitinib, 4 mg, was, however, not observed with the more stringent end point of clear/almost clear skin (vIGA-AD [0,1]). Similarly, persistence of response analysis for vIGA-AD (0,1) and EASI75, including all patients treated with baricitinib, 4 mg, showed sustained long-term efficacy on both end points. The shift in EASI75 response was associated with only a modest change in absolute EASI scores during the same period (approximately 3-point change between weeks 16 and 68). Thus, both the discrepancy observed between EASI75 and vIGA-AD maintenance and response data and the disproportionate loss of EASI75 responders compared with the observed EASI score change likely reflects the distribution of EASI responses in this group, with patients who achieved responses marginally better than EASI75 likely shifting to EASI improvements slightly below EASI75.
Consistent with the clinical signs findings, we found that improvements in patient-reported outcomes were maintained through 32 weeks of continuous treatment. Although small decreases over time were observed in itch and skin pain severity, these decreases likely reflect patients resetting their baseline after 8 months from initiation of therapy, with patients experiencing a possible change in their perspective of symptom severity, as has been observed in other studies.12 Sleep disturbance due to itch, which reports the number of nighttime awakenings instead of severity of symptoms, showed the least change over time. We also evaluated efficacy in all patients who received continuous baricitinib, 4 mg, in the originating study through BREEZE-AD3. Consistent with the responder/partial responder analyses, we found that improvements in itch NRS among these patients were maintained through 32 weeks of continuous treatment. Taken together, these findings indicate that baricitinib, 4 and 2 mg, showed sustained long-term efficacy in patients with moderate to severe AD.
A recent publication provided an integrated summary of the safety of baricitinib, 4 and 2 mg, in AD,13 with pooled data from 8 randomized clinical trials, including BREEZE-AD3; hence, a separate evaluation of safety was not performed in this smaller data set. The integrated analysis revealed a low (<2.5%) frequency of serious adverse events, which was no higher than observed incident rates in placebo-treated patients. Nasopharyngitis, headache, creatine kinase level elevations, and diarrhea were the most common treatment-emergent adverse events with baricitinib. An initial increase in herpes simplex infections compared with placebo was apparent in the first 16 weeks, but there was no evidence of an increased incidence of herpes infection with prolonged treatment and no increases in skin infections requiring antibiotic treatment, major adverse cardiovascular events, or conjunctival disorders with baricitinib treatment.
Limitations
This study has additional limitations not already mentioned. These include most patients being White, the relatively small patient numbers for some end points assessed, and patient-reported outcomes not assessed beyond week 32 of continuous treatment.
Conclusions
The findings of this study demonstrate that baricitinib, 4 and 2 mg, have long-term efficacy (up to 68 weeks) in adults with moderate to severe AD. Overall, these findings provide support that baricitinib may be a longer-term treatment option for patients with moderate to severe AD.
Trial Protocol
eMethods. Supplementary Methods
eTable 1. End Points Evaluated in Responders and Partial Responders
eTable 2. Mean Change From Baseline Responses in Patient-Reported Harmonizing Outcome Measures for Eczema (DLQI, POEM) at Weeks 16, 32, and 68 of Continuous Treatment for Patients Who Entered BREEZE-AD3 as Responders or Partial Responders
eTable 3. Topical Corticosteroid Use During the 52-Week Treatment Period of BREEZE-AD3 for Patients Who Entered BREEZE-AD3 as Responders or Partial Responders
eFigure. BREEZE-AD3 Study Design
Supplement 3. Data Sharing Statement
References
- 1.Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396(10247):345-360. doi: 10.1016/S0140-6736(20)31286-1 [DOI] [PubMed] [Google Scholar]
- 2.Johnson BB, Franco AI, Beck LA, Prezzano JC. Treatment-resistant atopic dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:181-192. doi: 10.2147/CCID.S163814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wollenberg A, Christen-Zäch S, Taieb A, et al. ; European Task Force on Atopic Dermatitis/EADV Eczema Task Force . ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol. 2020;34(12):2717-2744. doi: 10.1111/jdv.16892 [DOI] [PubMed] [Google Scholar]
- 4.Sidbury R, Davis DM, Cohen DE, et al. ; American Academy of Dermatology . Guidelines of care for the management of atopic dermatitis: section 3—management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71(2):327-349. doi: 10.1016/j.jaad.2014.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renert-Yuval Y, Guttman-Yassky E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann Allergy Asthma Immunol. 2020;124(1):28-35. doi: 10.1016/j.anai.2019.10.005 [DOI] [PubMed] [Google Scholar]
- 6.Wollenberg A, Barbarot S, Bieber T, et al. ; European Dermatology Forum (EDF), the European Academy of Dermatology and Venereology (EADV), the European Academy of Allergy and Clinical Immunology (EAACI), the European Task Force on Atopic Dermatitis (ETFAD), European Federation of Allergy and Airways Diseases Patients’ Associations (EFA), the European Society for Dermatology and Psychiatry (ESDaP), the European Society of Pediatric Dermatology (ESPD), Global Allergy and Asthma European Network (GA2LEN) and the European Union of Medical Specialists (UEMS) . Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32(6):850-878. doi: 10.1111/jdv.14888 [DOI] [PubMed] [Google Scholar]
- 7.Frampton JE, Blair HA. Dupilumab: a review in moderate-to-severe atopic dermatitis. Am J Clin Dermatol. 2018;19(4):617-624. doi: 10.1007/s40257-018-0370-9 [DOI] [PubMed] [Google Scholar]
- 8.Halling AS, Loft N, Silverberg JI, Guttman-Yassky E, Thyssen JP. Real-world evidence of dupilumab efficacy and risk of adverse events: a systematic review and meta-analysis. J Am Acad Dermatol. 2021;84(1):139-147. doi: 10.1016/j.jaad.2020.08.051 [DOI] [PubMed] [Google Scholar]
- 9.Simpson EL, Lacour JP, Spelman L, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183(2):242-255. doi: 10.1111/bjd.18898 [DOI] [PubMed] [Google Scholar]
- 10.Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(12):1333-1343. doi: 10.1001/jamadermatol.2020.3260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 12.Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287-2303. doi: 10.1016/S0140-6736(17)31191-1 [DOI] [PubMed] [Google Scholar]
- 13.Bieber T, Thyssen JP, Reich K, et al. Pooled safety analysis of baricitinib in adult patients with atopic dermatitis from 8 randomized clinical trials. J Eur Acad Dermatol Venereol. 2021;35(2):476-485. doi: 10.1111/jdv.16948 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods. Supplementary Methods
eTable 1. End Points Evaluated in Responders and Partial Responders
eTable 2. Mean Change From Baseline Responses in Patient-Reported Harmonizing Outcome Measures for Eczema (DLQI, POEM) at Weeks 16, 32, and 68 of Continuous Treatment for Patients Who Entered BREEZE-AD3 as Responders or Partial Responders
eTable 3. Topical Corticosteroid Use During the 52-Week Treatment Period of BREEZE-AD3 for Patients Who Entered BREEZE-AD3 as Responders or Partial Responders
eFigure. BREEZE-AD3 Study Design
Supplement 3. Data Sharing Statement