Abstract
The current study was undertaken to investigate anticancer activity of coumestrol phytoestrogen against human skin cancer. MTT assay was performed for cell viability assessment and clonogenic assay for cell colony formation assessment. Apoptosis was analysed by Annexin V/FITC staining, AO/EB staining and western blotting assays. Effects on the m-TOR/PI3K/AKT signalling pathway were investigated by western blotting. Results indicated that coumestrol induced significant toxicity in human skin cancer cells in contrast to mouse skin cancer cells. The proliferation rate in normal skin cells remained almost intact. Annexin V-FITC and AO/EB staining assays indicated coumestrol induced cytotoxicity in skin cancer cells is mediated through apoptosis stimulation. The apoptosis in skin cancer cells was mediated through caspase-activation. Cell migration and invasion was inhibited by coumestrol in human skin cancer cells via inhibition of MMP-2 and MMP-9 expressions. Moreover, m-TOR/PI3K/AKT signalling pathway in SKEM-5 cells was blocked by coumestrol.
Keywords: Skin cancer, Phytoestrogens, Coumestrol, Mitochondrial apoptosis, Cell cycle
1. Introduction
In recent years, dietary compounds are involved in one of the chemotherapeutic approaches to tackle cancer with higher efficacy (Alice, 2002). Natural products (polyphenolic compounds in particular) have become an essential part of cancer therapy and prevention as alternative medicine (Foye, 2008, Rachael et al., 1992, Scalbert and Williamson, 2000, Le Marchand, 2002). Phytoestrogens belong to polyphenolic compounds and have been classified into stilbenes (like resveratrol), coumestans (like coumestrol), lignans (like enterodiol) and isoflavones (like genistein). Phytoestrogens show protective effects over different human ailments including diabetes, menopausal symptoms, heart diseases, osteoporosis and hormone associated carcinogenesis including prostrate, ovaries, cervix and breast cancers. Phytoestrogens operate through anti/pro-estrogenic collaborations with ESR1 (estrogen receptor alpha and ESR2 (estrogen receptor beta) (Patisaul and Jefferson, 2010). Consumption of soy and soy containing products have been found to lower the occurrence of prostrate, gastrointestinal and breast cancers (Kumar et al., 2011, Koo et al., 2015, Tse and Eslick, 2016, Lesinski et al., 2015). Coumestrol (major constituent of soy) is an active member of phytoestrogens and possess an array of biological and medicinal applications. Coumestrol has been reported as anticancer, osteoblastic differentiation stimulant, and neuro-protective agent (Wu et al., 2009, Canal Castro et al., 2012, Malar et al., 2020a, Vijayakumar et al., 2020). In addition to this, it initiates senescence via protein kinase CKII activation, which enhances the ROS (reactive oxygen species) production, in colon and breast cancers (Lee et al., 2013). For instances, coumestrol induces apoptotic cell death in breast cancer and supresses the hypoxia PC3 prostate cancer via inhibition of sphingosine kinase 1 and hypoxia inducible factor-1 (Obiorah et al., 2014, Cho et al., 2014, Mitchell et al., 2000). Skin cancer (SC) is the most frequent malignant distortion found predominantly in Caucasians (D’Orazio et al., 2013). Each year approximately over one million cases of SC are registered globally. SC bears different subtypes differentiated on the basis of clinical behaviour and the cells from which the disease actually originates. Squamous cell carcinoma and basal cell carcinoma are the most common types of SC and together they are referred as MM (malignant melanoma) and NMSC (non-melanocytic skin cancer) (Sim, 2015). Most of the skin cancer cases in humans are due NMSC and its incidences are amplifying yearly in Australia, United States, Canada and Europe (Narayanan et al., 2010). The etiological factors responsible for SC development include ionizing radiation, UV light, and exposure to chemical carcinogens. Current treatment strategies for SC management include surgery, “basic” pharmacological therapy, immunotherapy target therapy and chemotherapy. Lack of effective SC curbing chemopreventives and treatment strategies generates a need for novel drugs that can tackle SC with better results. Therefore, the current study was designed to investigate anti-skin cancer effects of naturally occurring coumestrol. Its effects of inducing mitochondrial-mediated apoptosis, cell cycle arrest, inhibition of cell migration and invasion and modulation of m-TOR/PI3K/AKT signalling pathway, were examined as well.
2. Materials and methods
2.1. Cell culture and conditions
Present study involves mouse skin B16F1 cancer, human skin SKMEL-5 cancer and normal human Detroit 551 fibroblast cell lines, which were procured from Cancer Research Institute of Beijing (Beijing, China). Procured cell lines were cultured and maintained in DMEM (Dulbecco’s modified Eagle’s medium) Invitrogen Life Technologies, United States. DMEM was supplemented with fetal bovine serum (10%) and 100 U/ml each of streptomycin and penicillin G, (Himedia, Pennsylvania, United States of America). Afterwards, media with procured cells was placed in CO2 (5%) humidified incubator at 37 °C of temperature.
2.2. Cell viability determination
The antiproliferative efficacy of coumestrol molecule was estimated through MTT (Roche, United States) assay (a colorimetric assay). MTT gets reduced to an insoluble formazan complex in a living cell due to the presence of succinate dehydrogenase in mitochondria. Cytotoxicity of coumestrol was testified against B16F1 (mouse skin cancer cell line) and SKMEL-5 (human skin cancer cell line) cancer cell lines and normal Detroit 551 fibroblast cell line. Briefly, all the three cell lines at a concentration of 1 × 105 cells/well were precultured in 96-well plates for 24 h. Thereafter, each cell line was separately exposed to varying coumestrol doses viz control, 10, 20, 40, 80 and 160 μM, for 24 h in a humidified 5% CO2 incubator at 37 °C. After treatment, each cell line was subjected to PBS washing twice followed by MTT exposure (100 µl) with incubation for 60 min. ELISA plate reader (ELX 800; Bio-Tek Instruments, USA) was used to record absorbance for estimation of OD (optical density) at 490 nm.
2.3. Clonogenic assay
For clonogenic analysis, human SKMEL-5 skin cancer cells were harvested and then numbered with a haemocytometer. 200 cells/well were seeded in each well of 6-well plate with incubation for 24 h. These cells are left to attach so that a complete monolayer of cells is formed. Afterwards, cells were treated with several coumestrol doses viz control, 20, 80 AND 160 ΜM, and subjected to additional incubation for 10 days. Thereafter, cells were washed with PBS and colonies were fixed in methanol. Finally, cells were stained with crystal violet for about 20 min and numbered under a light microscope.
2.4. AO/EB staining assay for apoptosis investigation
To execute AO/EB staining assay, SKMEL-5 cells were harvested from 6-well plates at a concentration of 0.5 × 105 cells per well. Subsequently, cultured SKMEL-5 cells were subjected to variant coumestrol drug doses viz control, 20, 80 and 160 μM, for 24 h. Treated SKMEL-5 cells were then fixed in formaldehyde (5%) Thereafter, glass slides were prepared for loading of treated SKMEL-5 cells for staining with 10 μl of AO/EB solution for 10 min. Finally, fluorescence microscope (BioTek Instruments, Inc., Winooski, VT, USA,) was used for apoptosis analysis of coumestrol treated SKMEL-5 cells.
2.5. Annexin V-FITC assay for apoptosis assessment
The effects of coumestrol on cell apoptosis was determined via Annexin V-FITC assay (Sigma-Aldrich). Briefly, human skin SKMEL-5 cancer cells were cultured in 6-well plates with each well containing 2 × 106 cells. Cells were subjected to incubation for 12 h followed by coumestrol treatment at variant doses viz control, 20, 80 and 160 μM, for 48 h. Coumestrol treated cells were trypsinized and thereafter washed two times with PBS. Afterwards, trypsinized cells were resuspended followed by the addition of binding buffer (250 μl) to each well. Binding buffer bearing Annexin V-FITC (20 μl) and propidium iodide (20 μl). Finally, cells were placed in dark for further incubation for 30 min and finally apoptosis assessment was performed through flow cytometry (BD Biosciences).
2.6. Analysing cell cycle in SKMEL-5 cells
Flow cytometric analysis was performed for determination of cell cycle phase distribution. SKMEL-5 cancer cells were seeded with a concentration of 2 × 105 cells/ml and subjected to preculturing overnight with incubation. Afterwards, medium was drawn off and replaced by fresh DMEM carrying varying doses of coumestrol drug viz control, 20, 80 and 160 μM. After 48 h of incubation, treated cells and untreated controls were subjected to trypsinisation followed by cold PBS washing two times. Moreover, cells were fixed in methanol (70%) for half an hour and then again washed in PBS. Washed cells were then PI (propidium iodide) stained followed by addition of 10 μg/ml RNase A for 30 min. Finally, data collected from FACSCalibur flow cytometry (BD Biosciences) was processed through Modifit 2.0 cell cycle analysis software.
2.7. Cell migration and invasion examination
The impact of coumestrol drug on cell migration and invasion tendency of SKMEL-5 cells was analysed by transwell chamber assay. Target cells were seeded at 1 × 104 cells per ml of density in upper chambers of transwell containing cultural media, 10% FBS (fetal bovine serum) and varying coumestrol doses viz control, 20, 80 and 160 μM. Polycarbonate filters with 8 μm pore size were used to grow these cells followed by transfer of chambers to 24-well plate and incubated for 24 h at 37 °C. Afterwards, swabbing was done to eliminate the un-migrated cells and migrated cells were stained with 0.5% crystal violet for about 25 min. Cells were then washed with PBS and finally subjected to microscopic analysis under a light microscope. For cell invasion analysis, similar procedure was obeyed except transwell chambers were coated with Matrigel (Sigma Aldrich, USA).
2.8. Western blotting analysis
For western blotting assay, SKMEL-5 cells were harvested and then subjected to washing using ice-cold PBS twice. On ice cells were treated with phosphate and protease inhibitor carrying RIPA buffer for extraction of proteins. Quantification of proteins was accomplished with BCA assay and equal amounts were separated through electrophoresis by SDS-PAGE. Afterwards, proteins were transferred to PVDF membranes and blocked using BCA (5%) for 1 h. Thereafter, blocked membranes were exposed to primary antibodies (antibodies against Bax, Bcl-2, caspase-3, caspase-8, caspase-9, MMP-2, MMP-9, PI3K, AKT and m-TOR (Cell Signaling Technology, USA)) overnight at 4 °C. After primary antibody treatment, membranes were washed with TBST and then subjected to secondary antibody (HRP-conjugated) treatment with incubation for 1 h. Finally, bands were visualised through ECL kit (enhanced chemiluminescence kit).
2.9. Statistical analysis
From three independent experiments data was expressed as mean ± SEM. GraphPad Prism 5.01 (California, USA) was used to examine significant differences and data was analyzed by ANOVA. p < 0.05 was considered as statistically significant.
3. Results
3.1. Cytotoxicity and anti-clonogenic effects of coumestrol
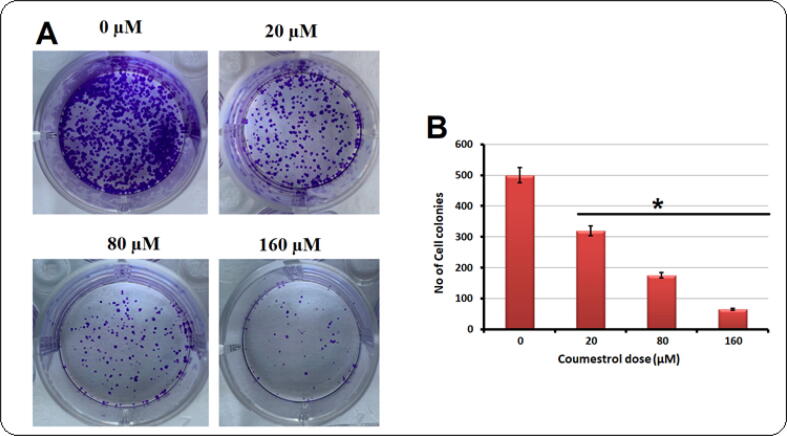
Coumestrol has been shown to inhibit several human malignancies including cancer (Fig. 1A). Therefore, its anticancer effects against human skin SKEM-5, mouse skin B16F1 cancer cells and normal Detroit 551 fibroblasts were investigated. All the three cell lines were exposed to coumestrol drug for 24 h at variant concentrations (0–160 μM). Cell proliferation rates were monitored by MTT assay. Results portrayed that the proliferation rate of these cells was significantly supressed with a drug concentration-reliant manner, except normal fibroblasts. The proliferation rate in SKEM-5 cells was more susceptible to coumestrol exposure in comparison to B16F1 cells (Fig. 1B). In case of coumestrol treatment to normal fibroblasts, the proliferation rate remained almost intact (Fig. 1C). Further investigations were carried out on human skin cancer cells as high cytotoxicity was recorded in case of human SKEM-5 cells. Clonogenic assay indicated that the SKEM-5 cell colonies growth was inhibited in a concentration-reliant fashion after coumestrol exposure for 10 days (Fig. 2A). The number of colonies declined from 500 to almost 50 with higher drug doses (0–160 μM) (Fig. 2B).
Fig. 1.
Coumestrol and its activity. A. Structure of Coumestrol. B. Viability of B16F1 (mouse skin cancer cell line) and SKMEL-5 (human skin cancer cell line) cancer cell lines through MTT assay after coumestrol exposure. C). Viability of normal Detroit 551 fibroblasts through MTT assay after coumestrol exposure. All the three cell lines were exposed to varying coumestrol doses for 24 h. Values were represented as means ± SD (n = 3). *P < 0.05.
Fig. 2.
(A). SKEM-5 cells were exposed to coumestrol drug at indicate doses for 10 days. The number of cell colonies were then numbered under a light microscope with crystal violet staining. (B). Graphical representation of results from clonogenic assay after coumestrol exposure for 10 days. Values were represented as means ± SD (n = 3). *P < 0.05.
3.2. Induction of apoptosis by coumestrol in SKEM-5 cells
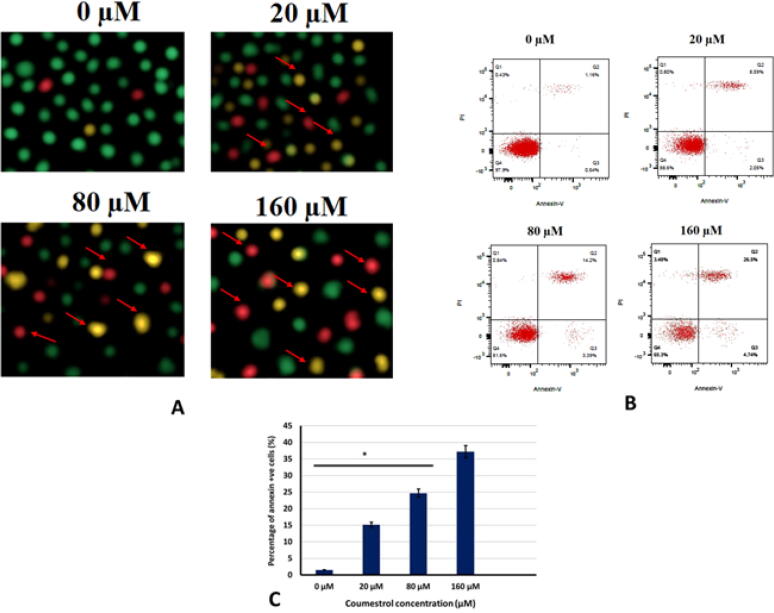
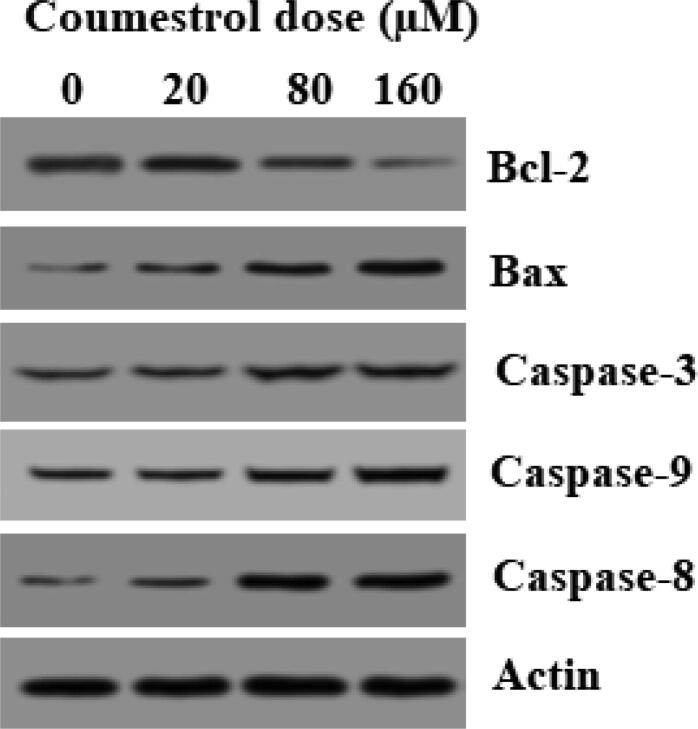
To examine whether the coumestrol growth inhibitory effects were apoptosis mediated in SKEM-5 cancer cells AO/EB staining was performed. Results presented that coumestrol treatment changed the cellular morphology and apoptosis indicative changes were observed. Fluorescence microscopy indicated nuclear fragmentation and condensation, membrane blebbing and plasma membrane rupture. Early apoptotic, late apoptotic and necrotic cell percentage increased with amplifying drug doses (Fig. 3A). Next, Annexin V/FITC assay was executed for apoptosis quantification. A significant rise in the SKEM-5 apoptotic cell percentage was observed upto 65% (0–160 μM) (Fig. 3B,3C). The number of annexin + ve cells also increased from almost 0% to 35% (0–160 μM). Further, the apoptotic cell death was supported by western blotting assay. The activity of pro-apoptotic proteins including Bax and caspases (-3, −8 and −9) was boosted by coumestrol exposure of SKEM-5 cells. In contrast to this, anti-apoptotic (Bcl-2) protein expression was retarded by coumestrol drug (Fig. 4). Therefore, the fact that coumestrol induced apoptotic cell death in SKEM-5 cells was supported by the results collectively from AO/EB staining, Annexin V/FITC and western blotting assays.
Fig. 3.
(A). To inspect cell morphology of coumestrol treated skin cancer SKEM-5 cells, AO/EB staining assay was accomplished. Results revealed apoptotic cell morphology as represented by arrows like necrotic, early apoptotic and late apoptotic cells. 4B). Flow cytometric investigation of coumestrol treated skin cancer SKEM-5 cells after execution of annexin V/PI staining assay. Results representing increasing number of apoptotic cell percentage with increasing drug doses. 4C). Graphical representation of Annexin + ve cell percentage after coumestrol exposure at indicated doses. Values were represented as means ± SD (n = 3). *P < 0.05.
Fig. 4.
Results demonstrating the activity caspases (-3, −8 and −9) and pro and anti-apoptotic proteins (Bax and Bcl-2, respectively. Values were represented as means ± SD (n = 3). *P < 0.05.
3.3. Inhibition of cell migration and invasion by coumestrol drug
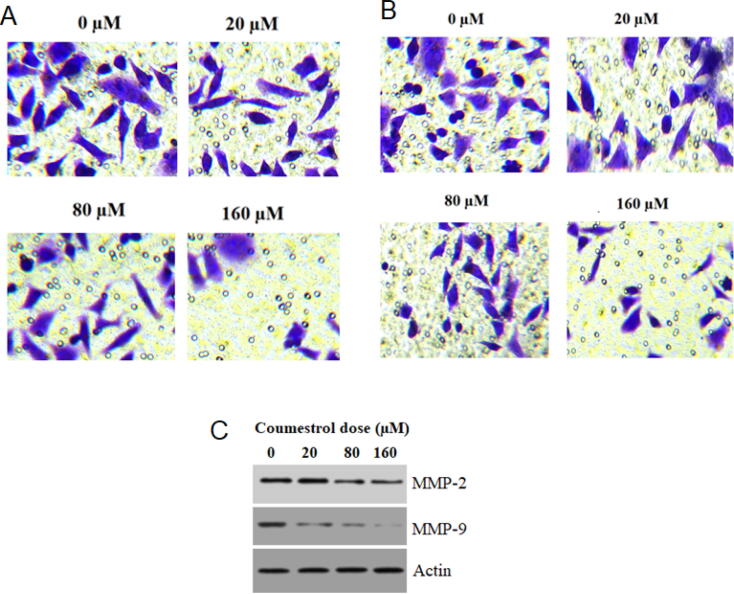
Cell migration and invasion in SKEM-5 cells was studied through transwell chambers assay. It was observed that in case of untreated controls the number of migrated cells were seen with no change. On application of coumestrol drug, the migration was retarded and on higher drug doses (0–160 μM) migration was limited to minimum (Fig. 5A). The transwell chambers were coated with Matrigel in case of cell invasion investigation. Results indicated that in comparison to untreated controls, treated SKEM-5 cells showed reduced and minimum invaded number of cells (Fig. 5B). Western blotting assay further supported the anti-migratory effects of coumestrol as the expressions of MMP-2 and MMP-9 (migration regulatory proteins) reduced with its application (Fig. 5C).
Fig. 5.
(A). Transwell chambers assay featuring reduced rate of migration after coumestrol treatment to skin cancer SKEM-5 cells at indicated doses. (B). Transwell chambers assay highlighting the condensed rate of invasion after coumestrol treatment to skin cancer SKEM-5 cells at indicated doses. (C). Western blotting revealing the activity of pro cell migratory proteins after coumestrol exposure at indicated doses. n = 3.
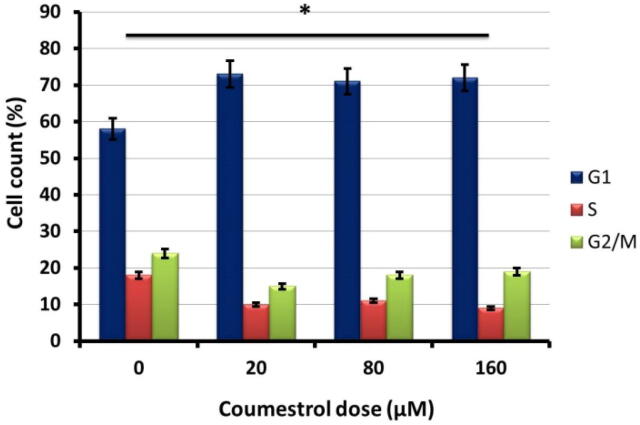
3.4. Inhibition of cell cycle by coumestrol
To investigate different phases of cell cycle flow cytometry through PI-staining was executed. It was observed that after coumestrol exposure the number of G1-phase SKEM-5 cells amplified as compared to untreated controls (Fig. 6). The reduced number of S-phase and G2/M−phase was also accompanied with increased G1-phase cells. Therefore signifying blocking of cell cycle at G1 check point.
Fig. 6.
Results from flow cytometric analysis after PI staining of coumestrol treated SKEM-5 cells. Representation of different cell populations after coumestrol exposure at different check points of cell cycle. Values were represented as means ± SD (n = 3). *P < 0.05.
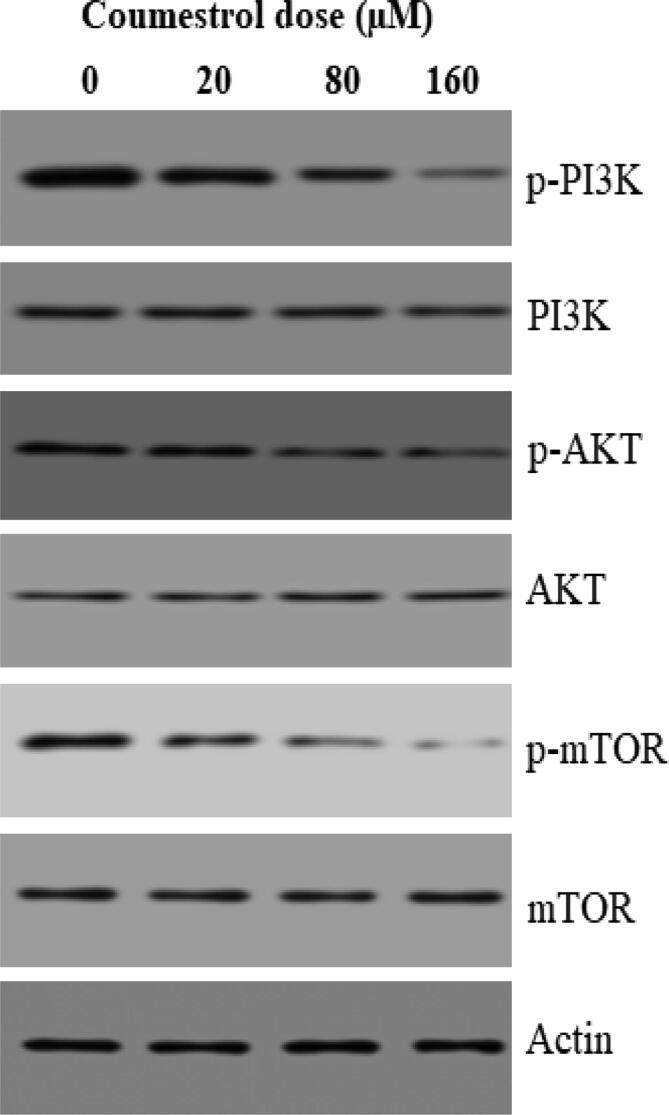
3.5. Coumestrol modulated the m-TOR/PI3K/AKT signalling pathway
m-TOR/PI3K/AKT signalling pathway is an important cell survival regulatory mechanism. The effect of coumestrol on m-TOR/PI3K/AKT signalling pathway in skin cancer SKEM-5 cells was monitored with western blotting assay. The activity of allied phosphorylated proteins (p-mTOR, p-PI3K and p-AKT) was inhibited in a dose reliant fashion in comparison to non-phosphorylated. In case of non-phosphorylated m-TOR, PI3K and AKT, activity remained almost intact (Fig. 7). Thus, it was clear that coumestrol blocked the m-TOR/PI3K/AKT signalling pathway in SKEM-5 cells.
Fig. 7.
Protein expression of m-TOR/PI3K/AKT signalling pathway in SKMEL-5 cells after exposure to designated doses of coumestrol drug. Individual experiments were repeated three times.
4. Discussion
Natural products are continuously playing a decisive role in the field of anticancer drug discovery and a number of natural products are currently involved in cancer chemotherapy (Lippman et al., 1994). Naturally occurring or semi-synthetic/synthetic compounds that act as chemopreventives inhibit carcinogenesis at advanced stage or at initiation stage of cancer. The process of carcinogenesis is stopped, inhibited or reversed by the application of these chemopreventives (Shukla and Gupta, 2005, Guptam, 2007). Several in vitro and in vivo investigations regarding plant-based compounds, like alkaloids, xanthones, terpenoids, coumarins, flavonoids, anthocyanins as well as phytoestrogens, have reported anticancer cancer behaviour against a wide range of cancers in experimental models. A broad spectrum of their therapeutic targets have also been reported like cell cycle, apoptosis, angiogenesis and cell proliferation. Moreover, these phytochemicals target several growth regulatory and other vital cancer cell survival pathways including PI3K/Akt/m-TOR pathway (Ng et al., 2011, Li et al., 2007, Cummings et al., 2004, Wattenberg, 1996, Liu et al., 2015). Apoptosis and cell cycle arrest are considered as potential strategies to inhibit carcinogenesis by stopping the uncontrolled cell growth. Apoptosis in particular performs as a defensive mechanism that eliminates impaired or harmful cells preceding to the appearance of malignancy. Akt/mTOR signalling is a well rated mechanism that regulates both autophagy as well as apoptosis in sequence or simultaneously. Against this backdrop, the current investigated aimed to assess the anticancer effects of coumestrol phytoestrogen against skin cancer cell lines viz B16F1 (mouse skin cancer cell line) and SKMEL-5 (human skin cancer cell line) cancer cell lines and normal Detroit 551 cell line. The effects of the drug on colony formation, apoptosis, cell cycle phases, cell migration and invasion and m-TOR/PI3K/AKT signalling pathway, were also studied. The cytotoxic effects of coumestrol were testified by MTT colorimetric assay on B16F1 and SKMEL-5 cancer cells and normal human Detroit 551 fibroblast skin cells (Arasu et al., 2019, Valsalam et al., 2019, Venkatadri et al., 2020, Al-Dhabi et al., 2020). Results displayed that coumestrol reduced viability remarkably in human SKMEL-5 cells in comparison to mouse B16F1 cells and the viability of Detroit 551 skin cells remained almost intact. Further studies were carried out on human skin SKMEL-5 cancer cells as coumestrol displayed high cytotoxicity against them. Clonogenic assay indicated significant colony suppression of SKMEL-5 cells after coumestrol drug treatment. Further, apoptosis investigations revealed that coumestrol induced cytotoxicity was due its apoptosis inducing potential in SKMEL-5 cells. The levels of apoptosis correlated proteins Bax and caspases, enhanced with increased coumestrol doses (Malar et al., 2020b, Al-Ansari et al., 2020, Atif et al., 2020, Kalaiyarasi et al., 2020). Thus caspase dependent apoptosis was observed in coumestrol treated SKMEL-5 cells. Next, flow cytometry examination revealed that coumestrol inhibited cell cycle at G0-phase of cell cycle. Thereafter, effect on cell migration and cell invasion capability of skin cancer cells was evaluated via transwell chambers assay. Results indicated coumestrol dose relative inhibition of both cell migration and invasion along with inhibition of MMP-2 and MMP-9. Finally, the m-TOR/PI3K/AKT signalling pathway in SKMEL-5 cells was blocked by coumestrol treatment.
5. Conclusion
Collectively, the outcomes of the present study signified that coumestrol possesses chemotherapeutic effects against growth and progression of human skin cancer. Moreover, it induced mitochondrial-mediated apoptosis, cell cycle arrest, inhibition of cell migration and invasion and modulation of m-TOR/PI3K/AKT signalling pathway. Thus, coumestrol can prove a lead drug in curbing skin cancer provided preclinical in vivo studies are required to identify its toxicity, pharmacokinetics and bioavailability.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Ansari M., Kalaiyarasi M., Almalki M.A., Vijayaraghavan P. Optimization of medium components for the production of antimicrobial and anticancer secondary metabolites from Streptomyces sp. AS11 isolated from the marine environment. J. King Saud. Univ. – Sci. 2020;32(3):1993–1998. [Google Scholar]
- Al-Dhabi N.A., Esmail G.A., Ghilan A.K.M., Arasu M.V., Duraipandiyan V., Ponmurugan K. Chemical constituents of Streptomyces sp. strain Al-Dhabi-97 isolated from the marine region of Saudi Arabia with antibacterial and anticancer properties. J Infect Public health. 2020;13(2):235–243. doi: 10.1016/j.jiph.2019.09.004. [DOI] [PubMed] [Google Scholar]
- Alice. C., 2002. 1. Natural Products“, Foye's Principles of Medicinal Chemistry., 24.
- Arasu M.V., Madankumar A., Theerthagiri J., Salla S., Prabu S., Kim H.S., Al-Dhabi N.A., Arokiyaraj S., Duraipandiyan V. Synthesis and characterization of ZnO nanoflakes anchored carbon nanoplates for antioxidant and anticancer activity in MCF7 cell lines. Material Sci Eng C. 2019;102:536–540. doi: 10.1016/j.msec.2019.04.068. [DOI] [PubMed] [Google Scholar]
- Atif M., Ilavenil S., Devanesan S., AlSalhi M.S., Choi K.C., Vijayaraghavan P., Alfuraydi A.A., Alanazi N.F. Essential oils of two medicinal plants and protective properties of jack fruits against the spoilage bacteria and fungi. Ind Crop Prod. 2020;147 [Google Scholar]
- Canal Castro C., Pagnussat A.S., Orlandi L., Worm P., Moura N., Etgen A.M., Alexandre Netto C. Coumestrol has neuroprotective effects before and after global cerebral ischemia in female rats. Brain Res. 2012;1474:82–90. doi: 10.1016/j.brainres.2012.07.025. [DOI] [PubMed] [Google Scholar]
- Cho S.Y., Cho S., Park E., Kim B., Sohn E.J., Oh B., Lee E.O., Lee H.J., Kim S.H. Coumestrol suppresses hypoxia inducible factor 1alpha by inhibiting ROS mediated sphingosine kinase 1 in hypoxic PC-3 prostate cancer cells. Bioorg. Med. Chem. Lett. 2014;24:2560–2564. doi: 10.1016/j.bmcl.2014.03.084. [DOI] [PubMed] [Google Scholar]
- Cummings J., Ward T.H., Ranson M., Dive C. Apoptosis pathway-targeted drugs from the bench to the clinic. BBA. 2004;1705:3–66. doi: 10.1016/j.bbcan.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Foye, W.O., 2008. Foye's principles of medicinal chemistry‖, Eds. Thomas L. Lemke, and David A. Williams. Lippincott Williams & Wilkins, 2008.
- Guptam S. Prostate cancer chemoprevention: current status and future prospects. Toxicol. Appl. Pharmacol. 2007;224:369–376. doi: 10.1016/j.taap.2006.11.008. [DOI] [PubMed] [Google Scholar]
- J. D’Orazio, S., Jarrett, A., Amaro-Ortiz, Scott, T., 2013. UV radiation and the skin. Int J Mol Sci. 14(6), 12222–12248. [DOI] [PMC free article] [PubMed]
- Kalaiyarasi M., Ahmad P., Vijayaraghavan P. Enhanced production antibiotics using green gram husk medium by Streptomyces sp. SD1 using response surface methodology. J King Saud Uni-Sci. 2020;32(3):2134–2141. [Google Scholar]
- Koo J., Cabarcas-Petroski S., Petrie J.L., Diette N., White R.J., Schramm L. Induction of proto-oncogene BRF2 in breast cancer cells by the dietary soybean isoflavone daidzein. BMC Cancer. 2015;15:905. doi: 10.1186/s12885-015-1914-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Verma V., Jain A., Jain R.K., Maikhuri J.P., Gupta G. Synergistic chemoprotective mechanisms of dietary phytoestrogens in a select combination against prostate cancer. J. Nutr. Biochem. 2011;22:723–731. doi: 10.1016/j.jnutbio.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Le Marchand L. Cancer preventive effects of flavonoids-a review‖. Biomed. Pharmacother. 2002;56:296–301. doi: 10.1016/s0753-3322(02)00186-5. [DOI] [PubMed] [Google Scholar]
- Lee Y.H., Yuk H.J., Park K.H., Bae Y.S. Coumestrol induces senescence through protein kinase CKII inhibition-mediated reactive oxygen species production in human breast cancer and colon cancer cells. Food Chem. 2013;141:381–388. doi: 10.1016/j.foodchem.2013.03.053. [DOI] [PubMed] [Google Scholar]
- Lesinski G.B., Reville P.K., Mace T.A., Young G.S., Ahn-Jarvis J., Thomas-Ahner J., Vodovotz Y., Ameen Z., Grainger E., Riedl K., Schwartz S., Clinton S.K. Consumption of soy isoflavone enriched bread in men with prostate cancer is associated with reduced proinflammatory cytokines and immunosuppressive cells. Cancer Prev Res. 2015;8:1036–1044. doi: 10.1158/1940-6207.CAPR-14-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Cheung H.Y., Zhang Z., Chan G.K.L., Fong W.F. Andrographolide induces cell cycle arrest at G2/M phase and cell death in HepG2 cells via alteration of reactive oxygen species. Eur. J. Pharmacol. 2007;568:31–44. doi: 10.1016/j.ejphar.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Lippman S.M., Benner S.E., Hong W.K. Cancer chemoprevention. J. Clin. Oncol. 1994;12:851–873. doi: 10.1200/JCO.1994.12.4.851. [DOI] [PubMed] [Google Scholar]
- Liu J.J., Zhang L., Lou J.M., Wu C.Y. Chalcone derivative, chana 1 induces inhibition of cell proliferation and prevents metastasis of pancreatic carcinoma. Adv Biomed Pharma. 2015;2:115–119. [Google Scholar]
- Malar T.J., Antonyswamy J., Vijayaraghavan P., Kim Y.O., Al-Ghamdi A.A., Elshikh M.S., Hatamleh A.A., Al-Dosary M.A., Na S.W., Kim H.J. In-vitro phytochemical and pharmacological bio-efficacy studies on Azadirachta indica A. Juss and Melia azedarach Linn for anticancer activity. Saudi K Biol Sci. 2020;27(2):682–688. doi: 10.1016/j.sjbs.2019.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malar, TRJ Jeba, J. Antonyswamy, Ponnuswamy Vijayaraghavan, Young Ock Kim, Abdullah A. Al-Ghamdi, Mohamed S. Elshikh, Ashraf A. Hatamleh, Monerah A. Al-Dosary, Sae Won Na, and Hak-Jae Kim. “In-vitro phytochemical and pharmacological bio-efficacy studies on Azadirachta indica A. Juss and Melia azedarach Linn for anticancer activity.” Saudi journal of biological sciences 27, no. 2 (2020): 682-688. [DOI] [PMC free article] [PubMed]
- Mitchell J.H., Duthie S.J., Collins A.R. Effects of phytoestrogens on growth and DNA integrity in human prostate tumor cell lines: PC-3 and LNCaP. Nutr. Cancer. 2000;38:223–228. doi: 10.1207/S15327914NC382_12. [DOI] [PubMed] [Google Scholar]
- Narayanan D.L., Saladi R.N., Fox J.L. Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010;49(9):978–986. doi: 10.1111/j.1365-4632.2010.04474.x. [DOI] [PubMed] [Google Scholar]
- Ng W.K., Yazan L.S., Ismail M. Thymoquinone from Nigella sativa was more potent than cisplatin in eliminating of SiHa cells via apoptosis with down-regulation of Bcl-2 protein. Toxicol Vitro. 2011;25:1392–1398. doi: 10.1016/j.tiv.2011.04.030. [DOI] [PubMed] [Google Scholar]
- Obiorah I.E., Fan P., Jordan V.C. Breast cancer cell apoptosis with phytoestrogens is dependent on an estrogen-deprived state. Cancer Prev Res. 2014;7:939–949. doi: 10.1158/1940-6207.CAPR-14-0061. [DOI] [PubMed] [Google Scholar]
- Patisaul H.B., Jefferson W. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 2010;31:400–419. doi: 10.1016/j.yfrne.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachael M.A., Martin J.S., Dudley H.W. The evolutionary role of secondary metabolites—a review. Gene. 1992;115(1–2):151–157. doi: 10.1016/0378-1119(92)90553-2. [DOI] [PubMed] [Google Scholar]
- Scalbert A., Williamson G. Dietary intake and bioavailability of polyphenols‖. J. Nutr. 2000;130:2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- Shukla S., Gupta S. Dietary agents in the chemoprevention of prostate cancer. Nutr. Cancer. 2005;53:18–32. doi: 10.1207/s15327914nc5301_3. [DOI] [PubMed] [Google Scholar]
- Simoes, M.C.F., Sousa, J.J.S., Pais, A.A.C.C., 2015. Skin cancer and new treatment perspectives: a review. Cancer Lett. 357(1), 8–42. [DOI] [PubMed]
- Tse G., Eslick G.D. Soy and isoflavone consumption and risk of gastrointestinal cancer: A systematic review and meta-analysis. Eur. J. Nutr. 2016;55:63–73. doi: 10.1007/s00394-014-0824-7. [DOI] [PubMed] [Google Scholar]
- Valsalam S., Agastian P., Arasu M.V., Al-Dhabi N.A., Ghilan A.K.M., Kaviyarasu K., Ravindran B., Chang S.W., Arokiyaraj S. Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. J Photochem Photobiol B: Biol. 2019;191:65–74. doi: 10.1016/j.jphotobiol.2018.12.010. [DOI] [PubMed] [Google Scholar]
- Venkatadri B., Shanparvish E., Rameshkumar M.R., Arasu M.V., Al-Dhabi N.A., Ponnusamy V.K., Agastian P. Green synthesis of silver nanoparticles using aqueous rhizome extract of Zingiber officinale and Curcuma longa: In-vitro anti-cancer potential on human colon carcinoma HT-29 cells. Saudi J Biol Sci. 2020;27(11):2980–2986. doi: 10.1016/j.sjbs.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar M., Priya K., Ilavenil S., Janani B., Vedarethinam V., Ramesh T., Arasu M.V., Al-Dhabi N.A., Kim Y.O., Kim H.J. Shrimp shells extracted chitin in silver nanoparticle synthesis: Expanding its prophecy towards anticancer activity in human hepatocellular carcinoma HepG2 cells. Int. J. Biol. Macromol. 2020;165:1402–1409. doi: 10.1016/j.ijbiomac.2020.10.032. [DOI] [PubMed] [Google Scholar]
- Wattenberg L.W. Chemoprevention of cancer. Prev. Med. 1996;1996(25):44–45. doi: 10.1006/pmed.1996.0015. [DOI] [PubMed] [Google Scholar]
- Wu X.T., Wang B., Wei J.N. Coumestrol promotes proliferation and osteoblastic differentiation in rat bone marrow stromal cells. J. Biomed. Mater. Res. B Appl. Biomater. 2009;90:621–628. doi: 10.1002/jbm.b.31326. [DOI] [PubMed] [Google Scholar]