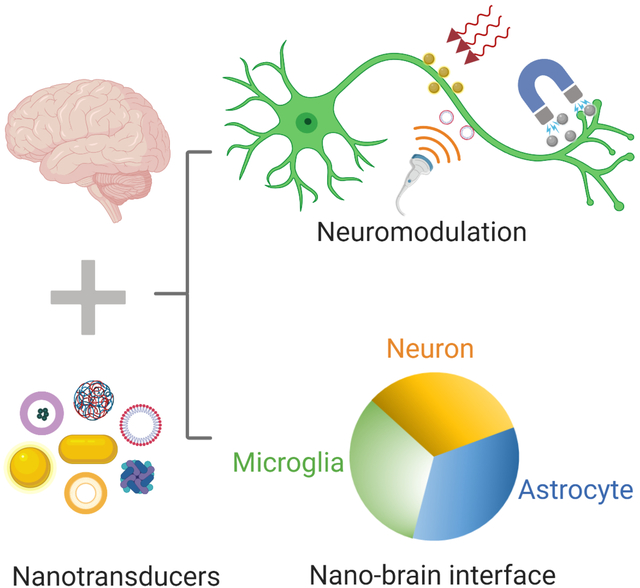
Summary
Understanding the signal transmission and processing within the central nervous system (CNS) is a grand challenge in neuroscience. The past decade has witnessed significant advances in the development of new tools to address this challenge. Development of these new tools draws diverse expertise from genetics, materials science, electrical engineering, photonics and other disciplines. Among these tools, nanomaterials have emerged as a unique class of neural interfaces due to their small size, remote coupling and conversion of different energy modalities, various delivery methods, and mitigated chronic immune responses. In this review, we will discuss recent advances in nanotransducers to modulate and interface with the neural system without physical wires. Nanotransducers work collectively to modulate brain activity through optogenetic, mechanical, thermal, electrical and chemical modalities. We will compare important parameters among these techniques including the invasiveness, spatiotemporal precision, cell-type specificity, brain penetration, and translation to large animals and humans. Important areas for future research include a better understanding of the nanomaterials-brain interface, integration of sensing capability for bidirectional closed-loop neuromodulation, and genetically engineered functional materials for cell-type specific neuromodulation.
Keywords: neuromodulation, optical stimulation, magnetic stimulation, ultrasound modulation, nanotransducers
Graphical Abstract
eTOC blurb
Miniaturization creates new opportunities to interface with the neural system and reduces the long-term impact on the brain environment. Towards this end, nanoscale transducers or nanotransducers, have emerged as a promising platform for wireless neuromodulation and sensing in the brain. This review provides an overview of the state-of-the-art nanotransducers and current limitations. We provide perspectives for future research in better understanding the nano-brain interface, and next-generation nanotransducers with sensing and capability for bidirectional communication.
A grand challenge in neuroscience is to understand signal transmission and processing in the nervous system. This has attracted significant interest of researchers from diverse disciplines such as genetics, materials science, engineering, and imaging, and has led to significant advances in developing new tools to address this challenge in the past decades. These advances include making smaller and more flexible electrodes to implant in local brain regions1–2, developing optogenetics to optically address individual neurons3, and creating pharmacological approaches such as caged compounds to selectively stimulate signaling in defined cell populations4. These techniques come with their unique advantages and disadvantages, such as cell specific neuron stimulation or inhibition but limited light penetration for optogenetics and caged compounds, reliable electrical modulation of local brain circuits but immune response and scar formation from large metal electrodes5, and the requirement of genetically-encoded non-native proteins for optogenetics. An ideal brain modulation technique would allow noninvasive control of neuron activities in target areas of the brain with high spatiotemporal resolution, and it should be able to translate into safe and effective use in large mammals, non-human primates and humans4, 6. Such neuromodulation techniques with high spatiotemporal resolution, deep brain penetration, minimal invasiveness and negligible inflammatory response are still highly desired.
Nanomaterial transducers (nanotransducers) have emerged as a unique neuromodulation interface with the brain in the past 5–10 years, in order to overcome limitations of current neuromodulation techniques. The major advantage of nanotransducers is their small size and promise to greatly reduce immune response compared with large electrodes7. Nanotransducers have the capability to wirelessly transduce external electric fields, light, magnetic fields, or ultrasound waves into a local signal (light, thermal, mechanical, electrical, or chemical) in the region of interest (Figure 1A). For example, light can be converted into chemical signal8, heat9, mechanical force10, and electric current or voltage by optical nanotransducers11, such as semi-conducting polymer nanoparticles. Similarly, magnetic nanotransducers can locally convert a magnetic field into electric current or voltage12, heat13 or mechanical force14. The local stimulation leads to changes in the neuron activity through various mechanisms including stimuli-responsive ion channels, G-protein coupled receptors (GPCRs), or transient membrane capacitance changes. The capability of nanotransducers-enabled neuromodulation is largely dependent on the nanotransducer properties and the interaction of nanotransducers with the brain (nano-brain interface).
Figure 1. Working principles and evolution of nanotransducers enabled wireless neuromodulation.
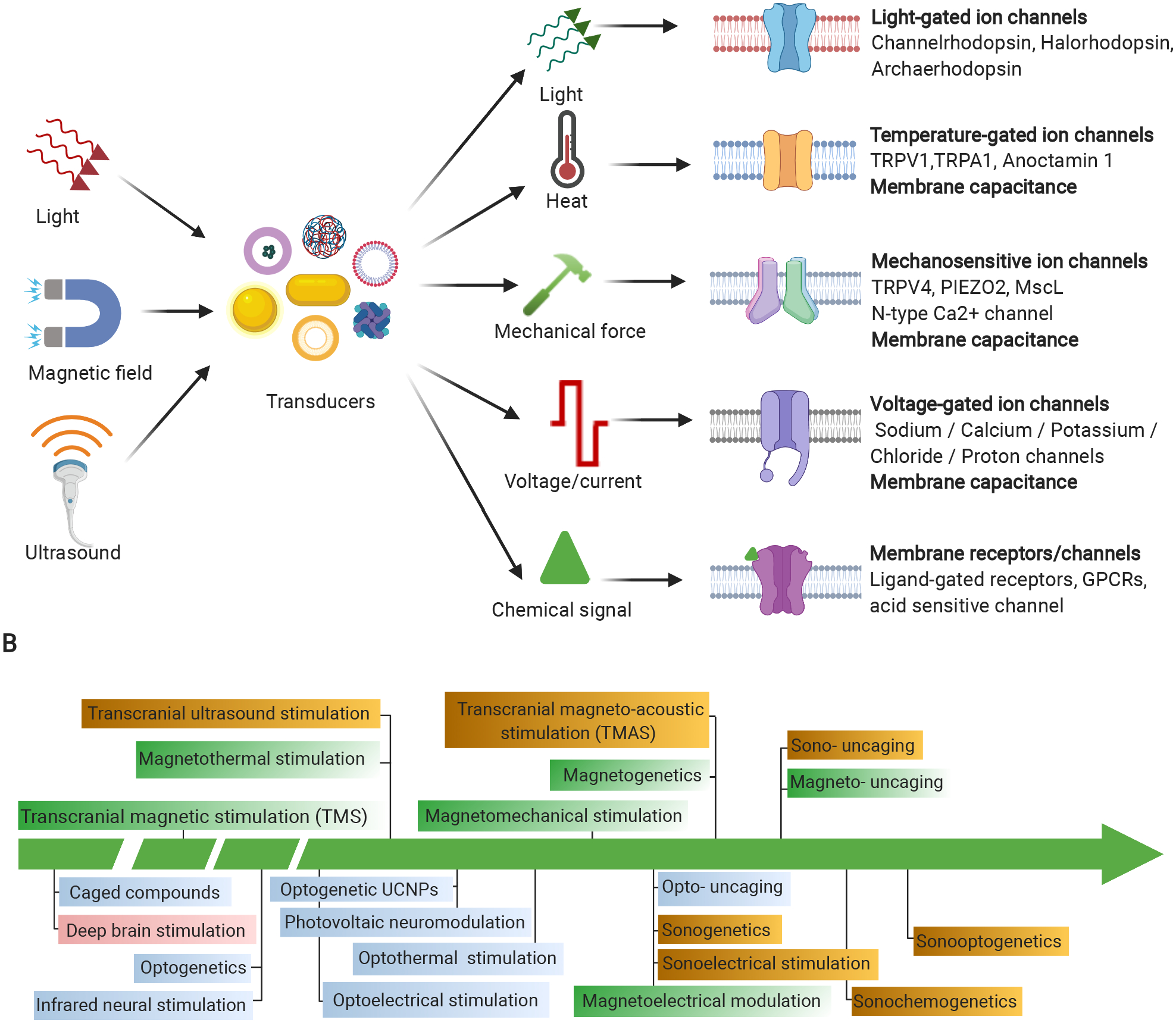
(A) working principles of nanotransducers for neuromodulation and (B) representative developments in nanotransducer-based and related neuromodulation techniques. Yellow for sono-neuromodulation, green for magneto-neuromodulation, cyan for optical neuromodulation, and pink for electrical neuromodulation. References for each technique: Caged compounds15; Deep brain stimulation16; Transcranial magnetic stimulation (TMS)17; Optogenetics18–19; Infrared neural stimulation20; Optoelectrical stimulation21; Magnetothermal modulation22; Transcranial ultrasound stimulation (TUS)23; Optogenetics using upconversion nanoparticles (UCNPs)24; Photovoltaic neuromodulation25; Optothermal stimulation26; Magnetomechanical stimulation27; Opto-uncaging; Sonogenetics28; Sonoelectrical stimulation29; Magnetoelectrical modulation12; Magnetogenetics30–31; Transcranial magneto-acoustic stimulation (TMAS)32; Magneto- uncaging33; Sono-uncaging34; Sonochemogenetics35; Sonooptogenetics36.
In this review, we provide an overview for nanotransducers-enabled neuromodulation by focusing on two important aspects: innovations in nanotransducers for wireless neuromodulation and the nano-brain interface. Briefly, we will summarize recent advances in nanotransducers-enabled neuromodulation, mainly including optogenetic, mechanical, thermal, electrical and chemical neuromodulation. The nanotransducer design, the working principle, and pros and cons of each approach will be covered. We will also discuss molecular and nanomaterials sensors for neurotransmitters and imaging of nanotransducers in the brain. Next, we will discuss the nano-brain interface, including approaches for delivery of nanomaterials to the CNS, the interaction between nanomaterials and neurons/glial cells, and nanomaterials transport and clearance in the CNS. Finally, we will outline current challenges toward developing the next generation of neuromodulation techniques and discuss some promising new nanotransducers for neuromodulation. Advances in nanotransducers-enabled neuromodulation techniques will contribute to understanding the signaling transmission and processing within the CNS and treatment of CNS disorders.
Innovations in nanotransducers for wireless neuromodulation
There are five main working mechanisms for nanotransducers-enabled neuromodulation, including optogenetic, thermal, mechanical, electrical and chemical neuromodulation (Figure 1 and Tables 1–2). The overarching idea is that nanomaterials transduce external energy in the form of light, magnetic field, or ultrasound, into local energy that may be either the same or different modality as the external energy input. The transduced energy directly interfaces with intrinsic or engineered cellular mechanisms to produce a cellular response and modulate neuron activity. These mechanisms include light-gated ion channels for optogenetic modulation, temperature-gated ion channels or temperature-dependent membrane capacitance change for thermal modulation, mechanosensitive ion channels or altered membrane capacitance for mechanical modulation, voltage-gated ion channels for electrical modulation, and ligand-gated ion channels and GPCRs for chemical modulation. For each mechanism, we will discuss the working principle and nanotransducers that have been reported in the last 5–10 years. We will compare the nanotransducer design, invasiveness, spatiotemporal precision, cell-type specificity, brain penetration, and potential translation to large animals and humans. We will also discuss a closely related topic on molecular and nanomaterials sensors for neurotransmitters and imaging of nanotransducers in the brain.
Table 1.
Summary of recent advances in nanotransducers for wireless neuromodulation. (N.A.: not applicable)
| External stimulus | Local stimulus | Brain interface | Nanotransducers | Pros (+) and cons (−) | Refs | |
|---|---|---|---|---|---|---|
| Light | Light | Ectopic opsins | UCNPs | (+) high spatiotemporal resolution (+) improved tissue penetration (−) required genetically-encoded non-native proteins |
24, 40, 43 | |
| Ectopic opsins | Mechanoluminescent NPs | (+) high spatiotemporal resolution (+) through intact scalp and skull (+) deep tissue penetration |
36 | |||
| Heat | Membrane capacitance | Targeted GNPs, Au nanorods (NRs), 3D fuzzy graphene | (+) doesn’t require genetically-encoded non-native proteins | (+) high spatiotemporal resolution (−) limited tissue penetration |
44−45 | |
| TRPV1 channel | Targeted Au NRs, semiconducting polymer nanobioconjugates | (−) requires genetically-encoded non-native proteins | 9, 46−47 | |||
| PAR-2 | Targeted GNPs | (+) doesn’t require genetically-encoded non-native proteins (+) nanoscale |
48 | |||
| Mechanical force | Membrane capacitance | Azobenzene compound (Ziapin2) | (+) high spatiotemporal resolution (+) doesn’t require genetically-encoded non-native proteins |
10 | ||
| Electrical signal | Voltage-gated sodium channel | QDs, metal and semiconducting organic nanocrystals, organic electrolytic photocapacitors, Semiconducting polymer NPs, GNP-decorated titania nanowire arrays, silicon | (+) high spatiotemporal resolution (+) doesn’t require genetically-encoded non-native proteins (−) limited tissue penetration |
11, 58−60 | ||
| Chemical signal | Neurotransmitter/Neuropeptide receptors | Gold nanoshell tethered liposomes, DNA nanocages, polypyrrole | (+) high spatiotemporal resolution (+) doesn’t require genetically-encoded non-native proteins (+) can be extended to different biomolecules (−) passive cargo leak in vivo |
65 | ||
| Inositol trisphosphate receptor (IP3R) | GNP-coated liposomes and mechanosensitive nanovesicles | 8, 66 | ||||
| Magnetic field | Heat | TRPV1 | Superparamagnetic ferrite NPs, MNPs, | (+) deep tissue penetration (+) allows for chronic stimulation (−) limited spatiotemporal resolution (−) potential tissue damage upon long-term exposure (−) slower temporal dynamics compared with optothermal modulation (−) restricted motion of the animals due to magnetic coil |
7, 13, 22, 50 | |
| TMEM16A | Membrane-bound MNPs | 51 | ||||
| Mechanical force | TRPV4/ PIEZO2 | magnetite nanodiscs, ferritin protein fused to TRPV1/TRPV4 | (+) deep tissue penetration (+) non-invasiveness (−) limited spatiotemporal resolution (−) restricted motion of the animals due to magnetic coil |
30, 54–55, 57 | ||
| N-type mechano sensitive Ca2+ | fMNPs | 14 | ||||
| Electrical signal | Ca2+ and Na+ voltage-gated channels | CoFe2O4–BaTiO3 NPs | (+) deep tissue penetration (+) non-invasiveness (+) doesn’t require genetically-encoded non-native proteins (−) limited spatiotemporal resolution |
12 | ||
| Chemical signal | Dopamine D2 receptor | Magnetic hydrogels | (+) lower dose of nanotransducers compared with magnetothermal modulation (+) deep tissue penetration (+) doesn’t require genetically-encoded non-native proteins (−) slower release of cargo compared with |
67 | ||
| TRPV1 / acid-sensing ion | Iron oxide MNPs, Magnetoliposomes | 33, 68,69 | ||||
| Ultrasound | Mechanical force | Engineered TRPV4, Prestin | N. A | (+) deep tissue penetration (+) moderate spatiotemporal resolution (−) requires head-mounted ultrasound transducer (−) May induce neuronal activity change via indirect mechanism |
28, 72−74 | |
| TRPV4 | Semiconducting polymer NPs | 52 | ||||
| Electrical signal | Ca2+ and Na+ voltage-gated channels | Piezoelectric barium titanate NPs (BTNPs), | (+) deep tissue penetration (+) doesn’t require genetically-encoded non-native proteins (+) non-invasiveness (+) moderate spatiotemporal resolution |
29, 64 | ||
| Chemical signal | GABAA receptor | PFCs containing nanoemulsions, nanodroplets with microbubble contrast agent and PFB gas | (+) deep tissue penetration (+) doesn’t require genetically-encoded non-native proteins (+) non-invasiveness (−) requires head-mounted ultrasound transducer (−) slower release of cargo compared with opto-uncaging |
4, 35, 75 | ||
Table 2.
Quantifying nanotransducer-based neuromodulation
| Main category | Parameters | Quantity | Refs | |||
|---|---|---|---|---|---|---|
| Nano-trans ducers | Size | Liposomes | 100~800 nm | 4, 8, 66, 68 | ||
| GNPs/GNRs | 10~80 nm | 9, 49, 76 | ||||
| Polymeric NPs | 25~800 nm | 52, 77 | ||||
| Magnetic NPs | 10~1000 nm | 7, 13, 51, 78–79 | ||||
| Microbubbles/nanodroplets | 200~2000 nm | 28, 75 | ||||
| Quantum dots | 2~10 nm | 58 | ||||
| Diffusion coefficient | 0.3% agarose | cortex | 80 | |||
| 35 nm QD | 1.9×10−7 cm2/s | 1.7×10−9 cm2/s | ||||
| 70 kDa dextran (~14 nm) | 4.7×10−7 cm2/s | 6.5×10−8 cm2/s | ||||
| 3 kDa dextran (~3 nm) | 2.22×10−6 cm2/s | 5.3×10−7 cm2/s | ||||
| Retention time | Polystyrene NPs | >3 weeks | 81 | |||
| Magnetic NPs | 1 month | 7 | ||||
| Energy | Upconversion quantum efficiency | UCNPs: 0.1–9% | 41 | |||
| Penetration depth in brain | Light | Blue-green light: 0.5 mm; red light: 1.5 mm; near infrared: 3.5 mm |
82 | |||
| Magnetic field | >1 cm, frequency dependent | 83 | ||||
| Ultrasound | > 1 cm, frequency dependent | 84 | ||||
| Brain interface | Synapse | Number (human) | ~ 1013–1015 (≈ 109/mm3) | 85 | ||
| Synaptic vesicle volume) | ~10−5 μm3 | 85 | ||||
| Width of synaptic cleft | 20 nm | 86 | ||||
| Membrane thickness | ~4 nm | 85 | ||||
| CSF | Volume | Human | 125 ~ 150 mL | 87 | ||
| Mouse | 0.035 mL | 88 | ||||
| Turnover time | Human | 4.8 h | 88 | |||
| Mouse | 1.8 h | 88 | ||||
| ECS | Space | 50–1000 nm, heterogeneous | 89 | |||
| Main composition | Hyaluronan, heparan sulphate, chrondroitin sulphate, collagen, fibronectin, laminin, tenascin-R | 90 | ||||
| Optogenetics | Opsin | Activation wavelength | 470 ~ 630 nm | 91 | ||
| Activation threshold | 0.4~1 mW/mm2 | 91 | ||||
| Time to ON (ms) | 50–200 ms | 38 | ||||
| Time to peak (ms) | 300~2000 ms | 38 | ||||
| Transducer concentration per dose | NaYF4:Yb/Tm@SiO2 UC NPs | 200 mg/mL (5×1011 particle/dose) | 92 | |||
| ZnS:Ag,Co@ZnS NPs | 8 mg/mL (1013 particle/dose) | 92 | ||||
| β-NaYF4/Yb/Er@β-NaY F4 UCNPs | 25 mg/mL (5×1011 particle/dose) | 92 | ||||
| Thermal modulation | TRPV1 | Activation threshold | ~40 °C | 71 | ||
| Rise time | 0.75 ms | 71 | ||||
| Transducer dose | Superparamagnetic NPs | 10 nM | 79 | |||
| Magnetic NPs | 8×1011 particle/dose | 7 | ||||
| Ano1/TMEM16A | Activation threshold | ~ 29 °C | 70 | |||
| Time to ON | 250 ms | 93 | ||||
| Transducer dose | Magnetic NPs | 10 μg/mL | 51 | |||
| Mechanical modulation | TRPV4 | Activation time | 0.1~1 s | 52 | ||
| Transducer dose | Photoacoustic NPs | 1 mg/mL | 52 | |||
| PIEZO2 | Activation threshold | 5–10 mmHg | 94 | |||
| Inactivation time | ~10 ms | 95 | ||||
| MscL | Activation threshold | ~97 mmHg | 73 | |||
| Electrical modulation | Transient voltage | 20 ~ 110 mV | 96 | |||
| Time constant | 50 μs to 5 ms | 96 | ||||
| Chemical modulation | Release time | <1 ms to mins | 8 | |||
| Chemical dose | GABAA receptor agonist (propofol) | 1mg/mL (8×1015 particle/dose) | 4 | |||
| DREADD agonist (clozapine N-oxide, or CNO) | 5 mg/mL | 68 | ||||
| D1 receptor antagonist (SCH-23390) | 2.5 mg/mL | 68 | ||||
| Intracellular second messenger (IP3) | 0.31 mg/mL (concentration inside liposomes) | 8 | ||||
| GABAA receptor agonist (pentobarbital) | 0.022 ± 0.03 mg/mL | 75 | ||||
Optogenetic neuromodulation
Working principle:
First demonstrated in 2005, optogenetics has since transformed neuroscience research and entered clinical translation18. Optogenetics uses light to control genetically engineered neurons that express light-sensitive ion channels and pumps (e.g., channelrhodopsin, halorhodopsin, and archaerhodopsin). These light-sensitive channels and pumps allow charged ions to flow across the cell upon illumination in order to stimulate or inhibit neuron activity. Taking advantage of genetic techniques to transfect specific cell types, optogenetics has allowed cell-specific modulation in freely moving mammals. Optogenetics also offers high spatiotemporal resolution, where light can be focused onto specific cells using one photon or two-photon techniques with millisecond control of neuron stimulation or inhibition3. Despite advances in the development of light-sensitive ion channels and pumps, such as the development of red-shifted opsins37, the potential of optogenetics is limited by the tissue penetration of visible light that is required to activate the engineered opsins. Opsins vary in light sensitivity (~ 1 mW/mm2 38) and kinetics (millisecond), and mutations that increase the light sensitivity of opsins often negatively affect the kinetics of the channel39. For example, channelrhodopsin-2 shows peak responses with a light intensity of 1.10 mW/mm2, almost 5 times lower than another channelrhodopsin variant, ChETA. However, the channelrhodopsin-2 engineered channels open within 1.21 ms, slower than ChETA engineered channels which open within 0.86 ms39.
Nanotransducers:
Recently, two promising nanotransducers have been developed to overcome the limitations of visible light penetration into tissue (Figure 2A and Figure 3). The first technique involves the use of upconversion nanoparticles (UCNPs), which convert infrared or near-infrared (NIR) wavelengths into the visible light spectrum. NIR light encounters reduced scattering and absorption in tissue compared to visible light, enabling deeper tissue penetration. UCNP-enabled neuromodulation was first proposed in 201124 and was later used to stimulate neurons in culture and in living animals. Chen et al. reported that channelrhodopsin-2 (ChR2) expressing neurons released dopamine in the ventral tegmental area (~ 4 mm depth), upon near-infrared (NIR) irradiation of NaYF4 nanocrystals doped with Yb3+/Tm3+ 40. While inducing theta oscillations via activating inhibitory neurons in the medial septum, the released dopamine also silenced seizure through inhibiting hippocampal excitatory cells. However, several limitations of UCNPs remain. The upconversion quantum efficiency remains low (0.1–9%)41 and often requires a high NIR laser fluence. This high laser fluence may introduce additional effects such as brain heating that may alter neural activity42.
Figure 2. Collection of recently reported nanotransducers for neuromodulation.
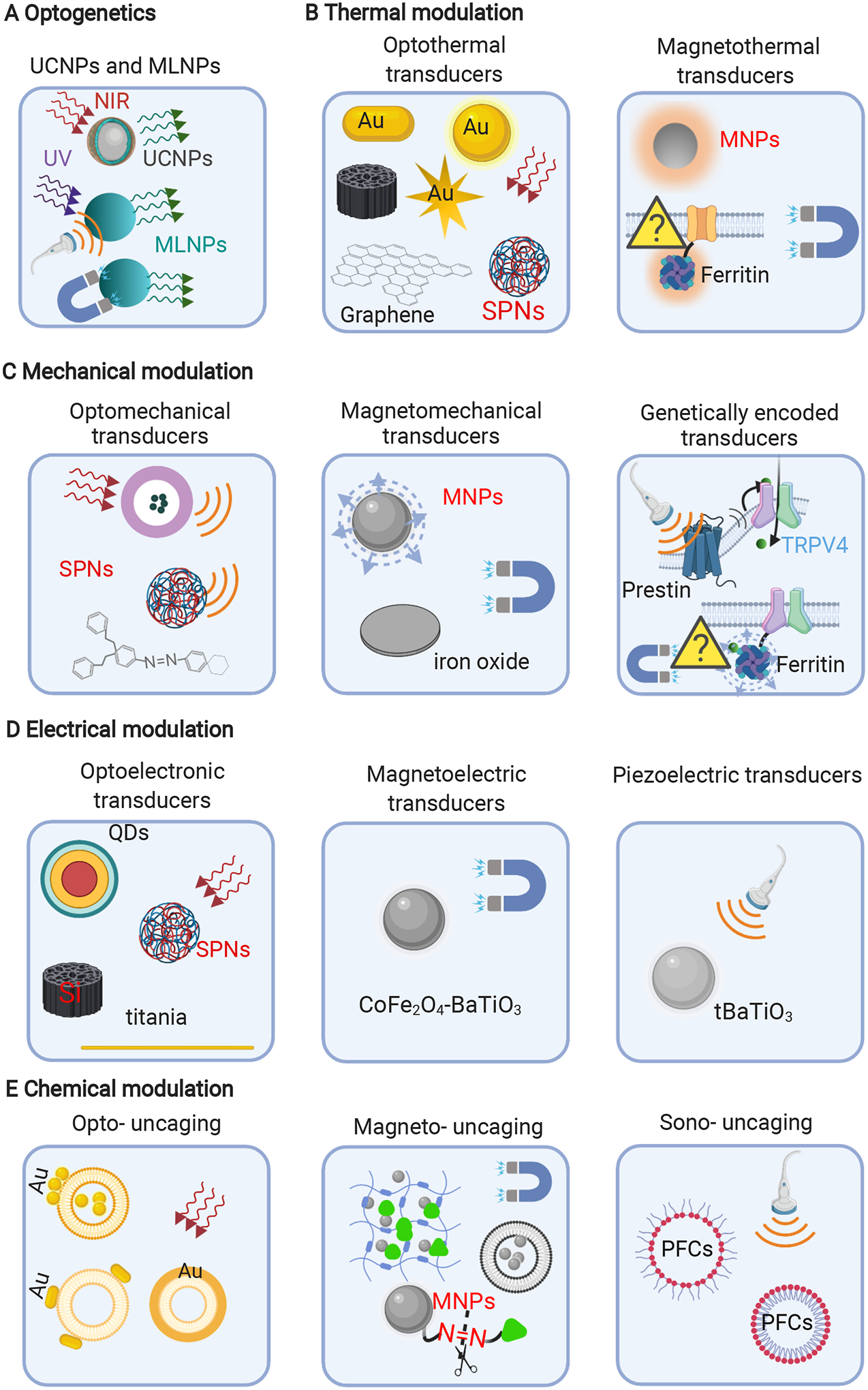
(A) Nanotransducers for optogenetics40, 43; (B) Optical9, 44–49 and magnetic transducers7, 13, 22, 50–51 for thermal modulation; (C) Nanotransducers for mechanical modulation, Left, optomechanical transducers10, 52–53; Middle, Magnetomechanical transducers14, 54; right, genetically encoded transducers30–31, 55–57. (D) Nanotransducers for electrical modulation; Left, optoelectronic transducers11, 58–62; Middle, Magnetoelectric transducers12, 63; Right, Piezoelectric transducers29, 64; (E) Nanotransducers for chemical modulation. Left, transducers for opto-uncaging8, 65–66; transducers for magneto- uncaging33, 67–69; transducers for Sono-uncaging4, 34–35. MLNPs, mechanoluminescent nanoparticles; SPNs, semiconducting polymer nanoconjugates, MNPs, magnetic nanoparticles; QDs, quantum dots; PFCs, fluorocarbons, e.g. perfluorobutane (PFB) and perfluoropentane (PFP).
Figure 3. Light delivery for neuromodulation.
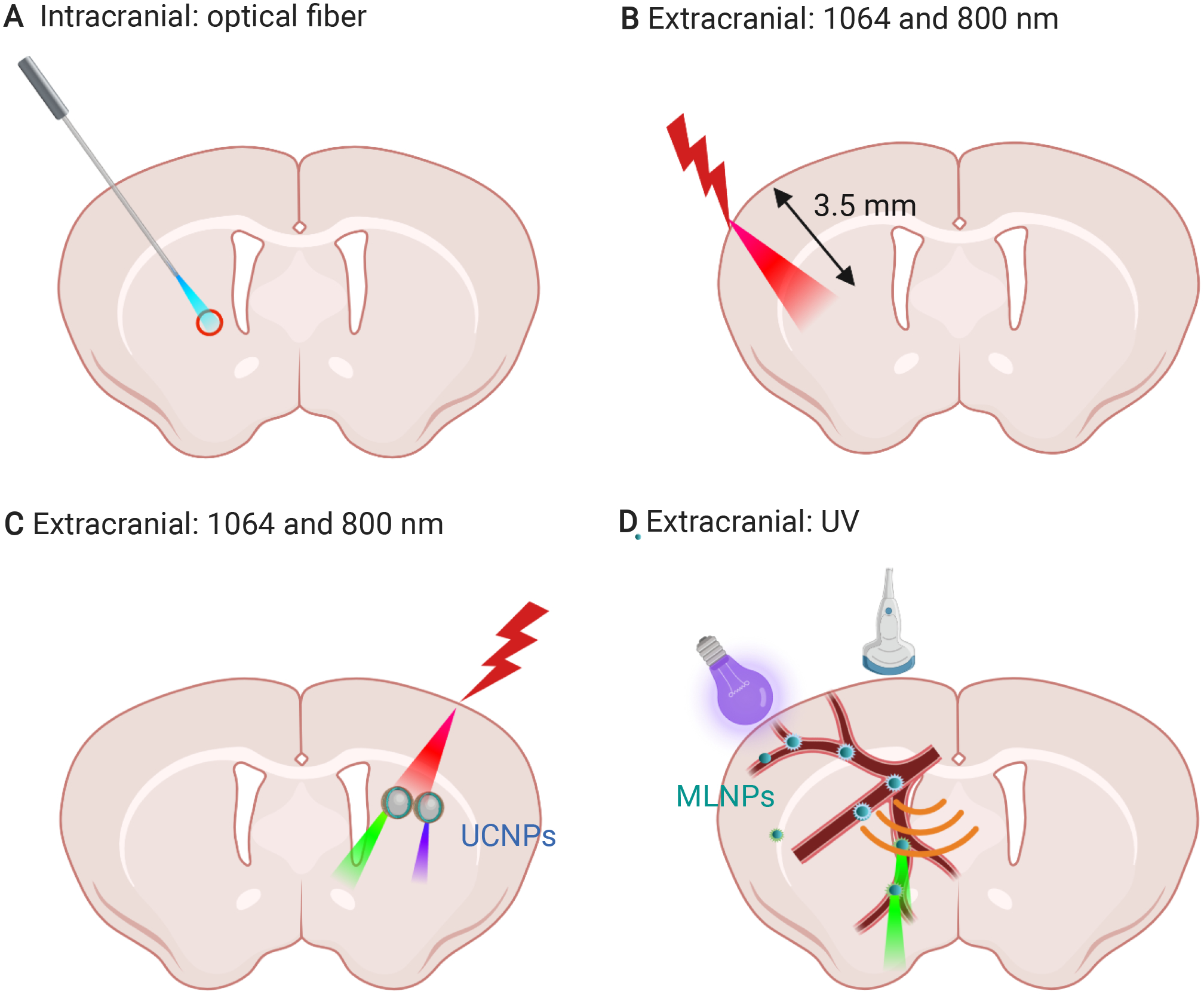
(A) intracranial light delivery via optical fiber; (B) extracranial delivery of near-infrared light, the penetration depth of near-infrared light in the brain: ~3.5 mm102; (C) upconversion nanoparticles (UCNPs) convert near-infrared light to visible light; (D) mechanoluminescent nanoparticles (MLNPs) converts UV light into green light upon ultrasound activation.
Another advance for non-invasive optogenetic neuromodulation is the development of mechanoluminescent nanoparticles (MLNPs). Mechanoluminescent nanoparticles use tissue-penetrating focused ultrasound to trigger light emission and subsequent optogenetic modulation, coined as “sono-optogenetics”36. These nanoparticles can be injected systemically and “charged” by 400 nm light during circulation in superficial blood vessels. Afterwards, nanoparticles can be activated by 1.5 MHz focused ultrasound stimulation to emit 470-nm light repetitively in the brain for optogenetic modulation. This approach combines the non-invasiveness and the tissue penetration of ultrasound stimulation with the high spatiotemporal control of optogenetics. With further work in the materials development for mechanoluminescence, this concept may provide a clinically viable route for noninvasive neuromodulation in deep brain regions. Specifically, by preparing mechanoluminescent materials with a sensitive response to higher-frequency ultrasound, spatially precise sono-optogenetic neuromodulation with a higher resolution may be achieved. In addition, materials with different wavelengths of mechanoluminescence may provide multiplexed and spatiotemporally resolved excitation and inhibition patterns in the same animal’s brain via a noninvasive interface.
Thermal neuromodulation
Working principle:
Local temperature changes can modulate neuron activity by changing membrane capacitance or activating temperature-gated ion channels, such as the transient receptor potential cation channel subfamily V member 1 (TRPV1), the transient receptor potential cation channel ankyrin 1 (TRPA1) and the temperature-gated chloride channel anoctamin 1 (TMEM16A). These temperature-gated ion channels change their open probabilities within different temperature ranges to detect temperature from 29°C to 40 °C (Table 2)70. TRPV1 responds to temperature change within milliseconds and can be modeled by a two-state model71.
Nanotransducers:
Nanotransducers have been reported to generate thermal energy from external light and magnetic fields (Figure 2B). The first type involves optothermal nanotransducers that convert light into heat, such as plasmonic nanoparticles26, semiconducting polymer nanoconjugates77, 97–98 and silicon nanomaterials. The stimulation mechanism of optothermal nanotransducers can be categorized into three different groups. The first mechanism involves thermally-sensitive ion channels, such as TRPV1. There are several reports that show heating neurons by gold particles will inhibit/excite neurons9, 49, 76. Surface-engineered plasmonic nanoparticles, such as gold nanorods, can activate single neuronal cells upon light illumination9. Highly localized heat generated by gold nanoparticles (GNPs) induced Ca2+ influx by activating TRPV1, a thermosensitive channel. Other than NIR-responsive plasmonic nanoparticles, organic materials with higher biocompatibility, e.g., semiconducting polymer-based nanobioconjugates77, have also been explored for thermal neuromodulation. Semiconducting polymers have large delocalized π-conjugated backbones that efficiently absorb NIR light with high optothermal conversion efficiency. Lyu et al. developed a series of NIR-responsive semiconducting polymer nanobioconjugates with higher optothermal conversion efficiency than that of gold nanorods46. When excited by an 808-nm laser, targeted SPNsbc specifically and rapidly activated the TRPV1 in a hybrid mouse neuroblastoma/rat dorsal root ganglion neuron cell line (ND7/23 cells), inducing significant intracellular Ca2+ influx. Built upon the semiconducting polymer nanobioconjugates, macromolecular infrared nanotransducers for deep-brain stimulation have been demonstrated to enable through-scalp neuromodulation in freely moving mice99. Owing to the minimized tissue attenuation of 1064-nm near-infrared-II (NIR-II) irradiation, MINDS have enabled neuromodulation in the motor cortex and ventral tegmental area of naturally behaving mice with wide-field NIR-II illumination at a low power density, in contrast to a fiber-tethered interface required for conventional optogenetic neuromodulation. This approach can remotely apply light to stimulate individual animals in the same arena, such as the IntelliCage, thus may enable simultaneous neuromodulation of multiple socially interacting animals. In addition to exciting neurons, optothermal transducers can also inhibit neurons via a transmembrane thermosensitive potassium channel, e.g., TREK-1. Thermal stimuli activates TREK-1 to allow hyperpolarizing K+ currents, thus reducing neuron excitability100,. Light irradiation of gold nanoparticles generated heat, and inhibited the electrical activity, which was fully restored when stimulus light was removed. The degree of inhibition was precisely modulated by tuning the laser intensity76. Ye et al. showed that neuron-targeted gold nanorods inhibited the neural activity of the left stellate ganglion, alleviating myocardial ischemia-induced ventricular arrhythmias in a canine model44, 76. This approach is promising for restoring normal firing of a hyperactive neuronal network and providing therapeutic effects for some brain disorders, such as epilepsy and Parkinson’s disease.
The second stimulation mechanism involves thermally-driven membrane capacitance change. Shapiro et al. demonstrated that rapid infrared heating of water can excite cells by altering the electrical capacitance of cell membrane and generating depolarizing capacitive currents101. This mechanism leads to infrared neural stimulation in vivo, which similarly uses pulsed infrared light (1400–2000 nm) to create transient temperature increase in neurons and induce neuron firing102. Several applications of infrared neural stimulation have been reported in recent years, including altering GABAergic neurotransmission103 and activating visual cortex104 and auditory neurons105. In addition to leveraging the heating of water alone, optothermal transducers that convert pulsed light into heat (such as gold nanoparticles) can also change membrane capacitance, depolarizing the cell and eliciting an action potential. fuzzy graphene45 has also been explored as organic optothermal transducers for nongenetic stimulation. When used for thermal neuromodulation, silicon nanowire-templated 3D fuzzy graphene requires activation laser energies lower than 100 nanojoules45. Although more in vivo studies are needed to validate the efficiency of these organic optothermal nanotransducers for nongenetic thermal neuromodulation, these studies demonstrate promising alternative nanotransducers for optothermal neuromodulation.
The last mechanism involves a new technique called molecular hyperthermia, which uses plasmonic gold nanoparticles to target specific proteins such as endogenous membrane receptors and generates intense nanoscale heating upon nanosecond pulse excitation to inactivate targeted protein molecules. As a test case, molecular hyperthermia was demonstrated to transiently inactivate protease-activated receptor 2 (PAR2), an important G-protein coupled receptor for chronic pain sensitization48. Molecular hyperthermia with high spatiotemporal resolution can selectively and remotely manipulate protein activity and cellular behavior. This technique with a time scale of nanoseconds and a length scale of nanometer is different from traditional hyperthermia and did not induce global tissue heating. The photo-inactivation of membrane receptors is transient and lasts 6–8 hours, because cells have a mechanism to recycle inactivated receptors and synthesize new membrane receptors.
In addition to optothermal nanotransducers, the second type of thermal neuromodulation involves magnetothermal nanotransducers that generate heat when exposed to alternating magnetic fields78. Magnetic hyperthermia has been studied to remotely treat cancer by local injection of magnetic nanoparticles and stimulation using external magnetic fields since the 1960s and several companies including MagForce are actively pursuing clinical translation. The idea of magnetothermal stimulation of neurons expressing TRPV1 was first demonstrated in 201022 and subsequently shown to allow wireless deep brain stimulation in behaving mice13. Short 10 s magnetic field stimulation pulses heated magnetic particles, enabling a rapid rise in median temperature up to 43 °C with a maximum increase to 45 °C. During the subsequent 50 s rest time, the tissue cooled back down to 37 °C49. This short intermittent exposure to a magnetic field induced neural activation and prevented harmful extended heating. Magnetic nanoparticles bound to TRPV1 on the neuronal membrane activated neurons expressing TRPV1 upon alternating magnetic field stimulation with high temporal control13. The magnetothermal stimulation applied in the striatum resulted in rotation around the body-axis in freely moving mice13. The duration of the behavior was highly correlated with the duration of alternating magnetic field application. The same technique has also been used to silence targeted neurons in vitro with a chloride channel Anoctamin 151. These studies suggest that magnetothermal nanotransducers are promising in vivo tools for remote, transient neuronal silencing for both therapeutic application and fundamental research. It is worth noting that magnetothermal modulation involves collective heating of injected magnetic nanoparticles that result in local brain tissue heating, rather than localized heating of individual nanoparticles, as demonstrated in multiple physical analyses106.
To further advance thermal modulation into the next stage of development and translation, there are several key questions to be addressed. First, it remains unclear whether the repeated heating up to 43°C ~ 45°C, although short in duration (several seconds), causes any deleterious effects on neurons and local brain tissue for long-term use. As there is a small temperature window for physiological function and there are many processes dependent on temperature (e.g., blood flow, protein function), potential deleterious effects must be carefully examined. Second, off-target heating may occur as a result of heat diffusion, which may trigger associated circuit (off-target) neural responses42. Therefore, this technique requires methods to confine the heat in the localized regions.
Mechanical neuromodulation
Working principle:
Local mechanical force can modulate membrane capacitance or activate mechanosensitive channels, including transient receptor potential cation channel subfamily V member 4 (TRPV4), piezo-type mechanosensitive ion channel component (PIEZO) channels, N-type mechanosensitive Ca2+ channel, and mechanosensitive channel of large conductance (MscL) (Figure 2C). PIEZO1 can be activated in about 10 ms95 with a threshold of 97 mmHg73, while TRPV4 responses to mechanical stimuli within 0.1–10 s52 (Table 2).
Nanotransducers:
Generation of local mechanical force can be introduced by external magnetic, ultrasound, and optical energy. Transcranial activation of photoacoustic transducers is an emerging strategy for neuromodulation53. Huang et al., used targeted photoacoustic nanotransducers based on semiconducting polymer nanoparticles to stimulate neural activities52. Nanosecond laser pulse in the second NIR region (3 ns, 3.3 kHz, 21 mJ/cm2) of photoacoustic nanotransducers induced localized acoustic waves to activate neurons. Upon light stimulation, photoacoustic nanotransducers were able to modulate brain activities in freely behaving mice upon injection into brain cortex. This technique exhibited a temporal resolution in the millisecond range, a spatial resolution in the sub-millimeter and no harmful temperature accumulation.
External magnetic field stimulation can generate mechanical forces on nanoparticles (magnetomechanical transducers). This mechanical force in the pN range is strong enough to activate mechanosensitive channels, including TRPV4 and PIEZO2 in primary dorsal root ganglion neurons107. Nanomagnetic force (0.1–1 nN) stimulation of ferromagnetic nanoparticles opened N-type mechanosensitive Ca2+ channels, inducing Ca2+ influx within in vitro grown cortical neural networks14. The approach was then further applied for chronic stimulation of a fragile X syndrome (FXS) neural network model14. FXS is the most common cause of intellectual disability and autism, and it shows an off-balance ratio of excitatory to inhibitory ion channels/receptors, including increased N-type Ca2+ channels and decreased GABA receptors. Chronic magnetic stimulation lowered the expression of N-type Ca2+ channels in FXS neurons to normal level and increased the expression of GABAA receptors, thus restoring the ion channel equilibrium. Magnetic nanotransducers may chronically modulate the expression of endogenous ion channels in neural circuits to investigate pathological mechanism of many CNS diseases. Compared with other technologies, magneto-mechanical neurostimulation is less likely to create deleterious responses, such as tissue heating during long exposure time, and is thus promising for long-term applications.
Genetically encoded transducers can link directly with mechanosensitive channels and can be stimulated externally with magnetic field or ultrasound. A major development in the area is referred to as magnetogenetics, i.e. use of the paramagnetic protein ferritin fused to TRPV4 for non-invasive neuromodulation30 (coined as Magneto). TRPV4 channel could be opened and closed by activating ferritin fusion protein tethered to TRPV4 with an external magnetic field. Wheeler et al. reported that Magneto remotely controlled neural firing rates and behavior on a rapid and physiologically relevant time scale upon magnetic stimulation in both zebrafish and mice30. While a few studies by other authors have validated this approach using the same or similar actuators111–112, at least three independent studies showed that magnetic stimulation failed to electrophysiologically activate neurons expressing this magnetogenetic actuator55, 57, 113. Although different stimulation efficiency of Magneto may result from experimental conditions, including alternating magnetic field versus constant magnetic field, different virus used and different virus expression levels56, the efficiency of magnetogenetic actuator is still controversial. The underlying reason is that the proposed mechanisms, either heating or magnetic force introduced by individual ferritin protein, are 5 to 10 orders of magnitude lower than the threshold to activate the TRPV1 and TRPV4 channels114. Recently, it has been suggested that reactive oxygen species (ROS) may play an important role110. Specifically, radio-frequency waves activate ferritin tagged channels via iron-induced lipid oxidation, suggesting a biochemical mechanism109–110 (Figure 4).
Figure 4. Proposed working mechanisms of magnetogenetics.
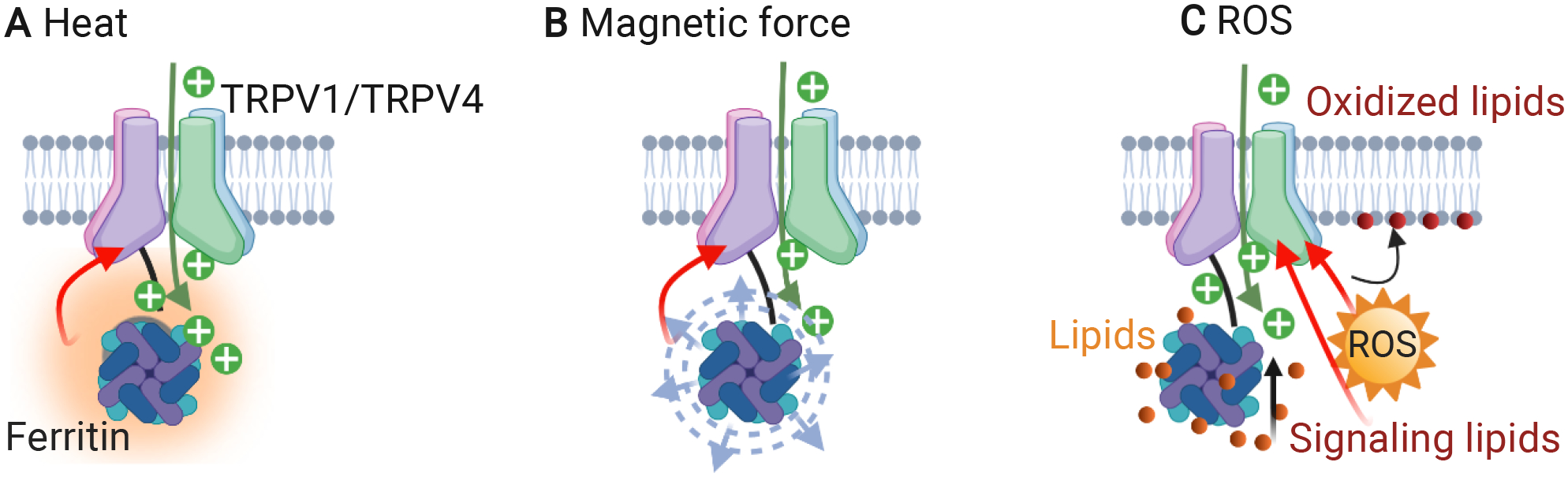
Magnetic field stimulation of the ferritin protein directly coupled to TRPV1/TRPV4 induces calcium transients through a heat-108(A), force-30–31, 108(B) or reactive oxygen species (ROS)-109–110(C) based mechanism.
Ultrasound can directly stimulate mechanosensitive ion channels to modulate neuronal activities. Transcranial focused ultrasonic stimulation is a clinically used neuromodulation technique based on this mechanism. This technique combines noninvasiveness with high spatial resolution even in deep brain regions115, enabling exploration of the role of specific brain regions in behaviors and neurological disorders. Compared with transcranial magnetic or electric stimulation, transcranial ultrasound stimulation can reach deeper brain regions, and shows a higher spatial precision. However, the mechanism of ultrasonic stimulation is not yet fully understood. Several proposed mechanisms include modulation of mechanosensitive ion channels to mediate transmembrane currents116 and opening of temperature-gated ion channel TRPA1117. Besides activating the endogenous ion channels with ultrasound, sonogenetics involves the delivery of transgenes into neurons for expressing mechanosensitive ion channels, followed by ultrasonic stimulation of these neurons. Huang et al. designed an ultrasound-responsive prestin (mPrestin) to modulate cellular activities28, 72. Focused ultrasound stimulation (0.5 MHz FUS, 0.5 MPa, 10 Hz PRF, 3 s duration) activated calcium signaling in neurons transfected with mPrestin, even in deep brain. Other mechanosensitive channels, such as MscL73, 118 and sound-responsive opsin protein119, have also been explored to enable sonogenetics in neurons. Ultrasound stimulation can activate cortical activity via indirectly activating auditory pathways instead of directly modulating neurons at the ultrasound stimulation sites120–121. This indirect auditory mechanism for ultrasonic neuromodulation suggests that careful consideration is required when developing new ultrasonic neuromodulation techniques for brain research.
Azobenzene structures can convert light to molecular conformation changes or mechanical force to modulate neuronal activities. Recently, a plasma membrane bound light-sensitive azobenzene compound (Ziapin2) was developed to modulate neuronal activity at high spatial-temporal resolution10. Trans-dimerization of Ziapin2 in the dark thins the plasma membrane, increasing membrane capacitance at steady state. Millisecond pulses of visible light triggered trans→cis isomerization of Ziapin2, thickened the plasma membrane, decreased the membrane capacitance, and then induced action potential firing without affecting ion channels or local temperature. Action potentials can be evoked for up to 7 days, suggesting that Ziapin2 is promising for long-term applications.
Electrical neuromodulation
Working principle:
Nanotransducers convert light, ultrasound and magnetic field into a localized electric voltage or current, which can modulate voltage-gated ion channels, including sodium, potassium, calcium, chloride and proton channels (Figure 2D). These channels respond to electrical stimulus within microseconds to milliseconds and operate at mV ranges96.
Nanotransducers:
Three classes of nanotransducers generate electrical output upon external stimulation, including optical, ultrasound, and magnetic energy. Optoelectronic transducers convert light into voltage or current and have been explored to remotely control neuron electrical signals. Semiconductor quantum dots (QD) are of great interest for electrical neuromodulation because of their broad absorption, narrow emission spectra and large extinction coefficients58. Jalali et al. incorporated a type-II indium phosphide/zinc oxide core/shell QDs into a photoelectrode structure. Upon visible light exposure, this electrode induced a hyperpolarizing bioelectrical current and triggered the neuron firing58. Visible light at 4 μW mm−2, 26-fold lower than the ocular safety limit for continuous exposure, is strong enough to activate the electrode. Organic pigment photocapacitors59 and silicon based materials61 have also been explored as nanotransducers for electrical neuromodulation. Jiang et al. developed a series of silicon-based materials that can behave as freestanding devices to modulate brain activities and simple animal behaviors62. These silicon-based materials use light as a trigger; therefore, excessive wiring is not required, and the location of stimulation is determined by the location of light. Thus, optical stimulation of silicon-based materials has high flexibility and spatial resolution, and it can implement multiplexed and patterned stimulations. In another example, silicon nanowires with atomic gold on their surfaces elicit action potentials in neurons through a primarily atomic gold-enhanced photoelectrochemical process122. An exciting and emerging application for nanotransducers-enabled electrical neuromodulation is vision restoration. Conjugated polymer nanoparticles injected into the subretinal space mediated light-evoked stimulation of retinal neurons and persistently rescued visual functions in a rat model of retinitis pigmentosa11. No effects on photoreceptor degeneration or retinal inflammation were observed upon light stimulation.
Piezoelectric transducers, such as barium titanate nanoparticles, also show potential for wireless electrical neuromodulation29, 64. Piezoelectric nanotransducers can efficiently generate electricity upon ultrasound stimulation to activate voltage-gated membrane channels, induce Ca2+ influx, and thus wirelessly modulate neuronal activity deep in the brain.
Magnetoelectric transducers have also been explored for wireless electrical neuromodulation. Magnetoelectric nanoparticles are composed of multiferroic materials that can efficiently convert external magnetic fields into local electric fields for modulating cell activities. Magnetoelectric nanoparticles were first proposed as a technique to non-invasively stimulate the brain of a patient with Parkinson’s disease in a computational study63, and further work is required to demonstrate the feasibility of this approach. Guduru et al. showed that CoFe2O4-BaTiO3 30-nm nanoparticle can be excited by external magnetic field to modulate neuronal activities in the deep brain regions12, and further characterizations are needed to better understand this mechanism.
Chemical neuromodulation
Working principle:
Chemical neuromodulation uses neuromodulatory agents such as drugs or neurotransmitters that bind to ionotropic or metabotropic receptors and modulate neuron activity. This allows receptor-specific actions in local brain regions. These neuromodulatory agents can modulate either endogenous membrane receptors or channels (ligand gated channels, GPCRs, and acid sensitive channels), or designer receptors exclusively activated by designer drugs (DREADDs), a class of chemogenetically-engineered proteins that allow spatial and temporal control of G protein signaling in vivo.
Nanotransducers:
Three types of nanotransducers release molecules to modulate brain activity in local regions. First, neuromodulators can be released locally in the brain by light stimulation (opto-uncaging), offering high spatiotemporal resolution to modulate brain activity. NIR picosecond laser pulses induce transient nanobubbles around gold nanoparticle-coated liposomes. The collapse of those nanobubbles resulted in nanomechanical stress that rapidly ejected the encapsulated compound within 0.1 ms8. This ultrafast release speed crosses the critical biological threshold and enables studies on fast cell signaling processes, such as neurotransmission. Recently, ultra-light-sensitive nanotransducers were developed based on gold coated mechanoresponsive nanovesicles for releasing molecules in the deep brain. NIR picosecond laser pulses illumination induced nanomechanical stress to trigger cargo release in sub-seconds. The laser energy threshold of gold coated mechanosensitive nanovesicles is 40 times lower than that of gold coated traditional liposomes. The technique enable release of calcein in deep brain region (4 mm)66, suggesting its potential for deep brain neuromodulation.
Secondly, magnetic fields can remotely heat nanotransducers to release packaged molecules (magneto-uncaging). Neuromodulatory compounds can either be conjugated to magnetic nanoparticles via a thermally-labile linker33 or loaded in magnetic nanoparticles containing hydrogel67. Rao et al. applied an alternating magnetic field to heat magnetic nanoparticles and subsequently release encapsulated molecules from thermally sensitive lipid vesicles (20 s latency) to provide non-invasive molecular control of neural circuits. The released small molecules activated both genetically engineered and endogenously expressed receptors with high temporal and spatial precision68. When combined with polymeric scaffolds, magnetic transducers can also convert magnetic fields into protons in physiological environments. Upon magnetic field stimulation, the magnetothermal effect caused by magnetic nanoparticles triggered the hydrolytic degradation of surrounding polyanhydride or polyester to release protons into the extracellular space, open acid-sensing ion channels, and induce Ca2+ influx in neurons69.
The third mechanism involves ultrasonic release of molecules (sono-uncaging). Several “sono-transducers” including perfluorocarbons (PFCs) containing microbubbles, nanoemulsions and nanodroplets, have been explored for chemical neuromodulation35, 75. Ultrasonic nanotransducer-enabled chemical neuromodulation is spatially and temporally controlled by the size of the ultrasound focus, the timing of sonication, and the pharmacokinetics of the neuromodulatory agents4. Wang et al. showed that ultrasonic uncaging of propofol can lead to brain activity changes in brain regions that are anatomically distinct from and functionally connected to the stimulated region. This has allowed for non-invasive mapping of the network connectivity in the brain under pharmacological activation of specific targets4. Airan et al. reported that transcranial stimulation of propofol-loaded emulsions silenced seizures in an acute rat seizure model. No brain parenchymal damage or blood-brain barrier opening associated with their use were observed34. These studies suggest that further development of ultrasonic nanotransducers will offer new tools for non-invasive, spatiotemporally precise neuromodulation, which may find a variety of biological applications.
Engineered neuromodulator sensors
In parallel with the developments of nanotransducers for chemical neuromodulation, there have been significant interest in new sensors to report and image neuromodulator release locally in the brain. Two categories of sensors were reported. The first category involves genetically-encoded sensors for neuromodulators based on fluorescent proteins. Two dopamine (DA) sensors, dLight1 and GRABDA, were first reported by Tian’s group123 and Li’s group124, respectively. These genetically-encoded sensors show a large fluorescence increase in response to extracellular DA release and allow high spatiotemporal resolution imaging using advanced microscopies such as two-photon microscopy. These sensors exhibit nanomolar-to-sub-micromolar affinity and have been validated with various pharmacological, electrophysiological and optogenetic stimulations125–126. This type of sensor has proven to be a versatile platform and been extended for other neuromodulators, such as acetylcholine (GRABAch127), serotonin or 5-hydroxytryptamine (5-HT, GRAB5-HT128), norepinephrine123, 129, opioid neuropeptide123, 130. Other genetically-encoded sensors for neuropeptides are also being developed such as for oxytocin131 or neuropeptide release reporters132.
The second category involves fluorescent sensors based on synthetic nanomaterials133–135, such as carbon nanotube. Landry and co-workers reported a series of developments in using carbon nanotubes functionalized with single-stranded DNA (ssDNA) to generate a unique class of nanosensors. These nanosensors can detect dopamine and norepinephrine133–134, 136, and serotonin135. They exhibit fluorescent emission in the infrared range (1000 to 1300 nm), and have shown promise in monitoring the release of neuromodulators in acute brain slices upon electrically or optogenetically evoked release. The infrared emission provides advantages for imaging the brain tissue. Further discussions on brain imaging are available in other review articles and won’t be discussed further here137.
Nano-Brain Interface
Understanding the interaction between nanomaterials and the brain (nano-brain interface) is critical for the rational design and safe application of nanotransducers for neuromodulation. Nano-brain interface includes nanomaterials delivery to the brain, interaction of nanomaterials with different types of cells in the brain (i.e. cellular tropism), nanomaterials transport (extracellular, intracellular, and intercellular), the immune response following nanomaterials administration, and the brain clearance of nanomaterials (Figure 5 and Table 2). We will discuss and highlight recent advances in these areas.
Figure 5. Nano-brain interface.
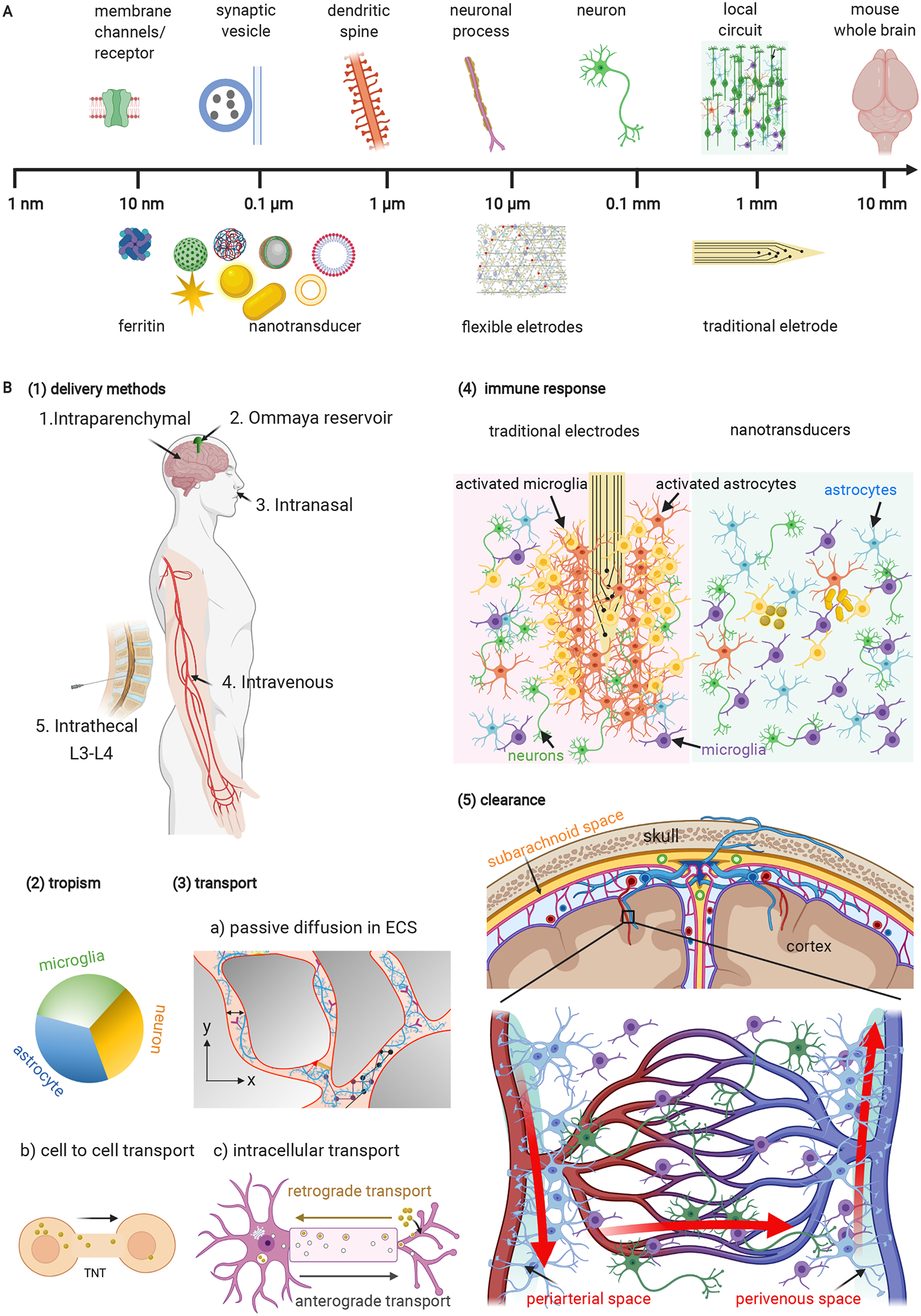
(A) Multi-scale brain-materials interface including nanotransducers. Modified from138. Schematic for flexible electrodes1. (B) Aspects of nano-brain interface: (1) Delivery of nanotransducers to the brain. (2) Cellular tropism of nanomaterials in the brain. (3) Passive diffusion of nanomaterials in extracellular space and active transport of nanomaterials in the brain. (4) The immune response following traditional electrode implantation and nanomaterials. (5) Brain clearance of nanomaterials via the perivascular pathway. L3-L4, lumbar segment 3–4; ECS, extracellular space; TNT, tunneling tube.
Delivery of nanomaterials to the brain
To enable nanotransducer-mediated neuromodulation, nanotransducers must be delivered into targeted brain regions. There are four main routes for nanomaterials delivery to the brain, including intraparenchymal, systemic, intrathecal, and intranasal administration. The major drawback of systemic administration is the limited accessibility of the nanomaterials to the brain from the blood. This is largely due to the presence of blood brain barrier (BBB), which blocks most nano/micro particles from entering into the brain.
Most studies so far directly inject nanomaterials into local brain regions (intraparenchymal injection). Intraparenchymal injection bypasses the BBB but requires stereotactic insertion of an invasive injection needle. Direct administration to the CNS via intraparenchymal injection can achieve high local concentration near the injection site with relatively low dose. While this works well for proof-of-concept studies and may be adapted for large animals and humans, the invasive procedure may pose a challenge, especially if it requires repeated injection over time. The approach is promising for selectively modulating specific brain regions, but it is less suitable for a large brain region, since injected particles can be restricted to the area surrounding the injection site139. Systemic administration of nanomaterials has been used for cases that do not require nanomaterials entry into the brain or nanomaterials can transport across BBB. For example, mechanoluminescent nanoparticles for sono-optogenetics36 can be infused into blood circulation to allow remote ultrasound activation. Moreover, blood circulation provides an endogenous mechanism for recharging the mechanoluminescent nanoparticles in superficial blood vessels and transporting the recharged energy to the brain for ultrasound-triggered localized light emission. Furthermore, ultrasound responsive nanodroplets/nanoemulsions were systemically delivered to release pentobarbital that crosses the BBB for targeted neuromodulation4, 75. Nance et al. reported that systemically delivered dendrimers (size ~3–12 nm) can across the impaired BBB to diffuse efficiently within the brain parenchyma and target activated microglia and astrocytes in regions of injury140.
Other routes of administration are less defined for neuromodulation by nanotransducers. Intrathecal injection relies on administering substance into the cerebrospinal fluid (CSF) that surrounds the brain and spinal cord. While intrathecal injection circumvents the BBB, there is limited evidence that nanoparticles can penetrate into the brain parenchyma via the perivascular space. Studies show that 100 nm PEGylated- polystyrene NPs rapidly distributed through the subarachnoid space along the entire neuraxis following injection into cisterna magna, with NPs showing some preference for ventral surfaces and minimal penetration into the parenchyma81. This is in contrast to studies that show widespread expression of injected antisense oligonucleotide (ASO) to treat amyotrophic lateral sclerosis and other neurological diseases141. Intrathecal injection of adeno-associated virus (AAV) also led to broad distribution throughout the brain and spinal cord parenchyma142. Further work is required to establish the feasibility of this route for neuromodulation. Ommaya reservoir, a subcutaneous device directly inserted in the lateral ventricles, also offers a route for CNS penetration. Drugs delivered via Ommaya reservoir directly reach ventricular CSF, and then homogenously distributed in the subarachnoid space. This technology has been used in clinic for improving brain delivery of chemotherapeutic agents and adopted to enhance nanoparticles transport. However, Ommaya reservoirs may be ineffective for delivering nanoparticle to parenchyma since agents can only diffuse within millimeters from the ependymal surface143. Nasal to CNS drug delivery is another approach to bypass the BBB, which has demonstrated its potential in clinical trials for the treatment of pain144 and recurrent glioblastoma. This pathway also has been explored to deliver nanomaterials. Studies show that solid lipid nanoparticles modified with brain targeting peptide (mApoE) can be delivered to the brain via pulmonary administration145.
Cellular tropism of nanomaterials
The interaction of nanomaterials with different cell types in the brain is an important and basic question to understand the nano-brain interface. Jenkins et al. studied the interaction of nanoparticles with different cell types in cell culture, and showed that nanoparticles with polyethylene glycol (PEG) modification showed modest reduction in cell uptake for all cell types (microglia, astrocytes, oligodendrocyte progenitor cells, neural stem cells, neurons) compared with nanoparticles with bio-adhesive end-groups (carboxymethyl dextran, or CMX)146. Song et al. investigated the cellular tropism of poly(lactic acid) nanoparticles with different surface chemistries (bare, PEG coating, hyperbranched glycerol (HPG), and aldehyde (-CHO) modified HPG)147. ‘Stealth’ properties (PEG, HPG) mostly reduced internalization by all cell types, while bio-adhesive end-groups (-CHO) enhanced cellular uptake. Furthermore, the measured rates of uptake in vitro correlate with uptake of NPs in specific cell types in vivo. Dante et al. demonstrated that surface charge of nanoparticles played a critical role in their neuronal interaction148. Anionic nanoparticles interact with membrane of neurons and locate at the synaptic cleft, while no neutral and cationic nanoparticles were observed on neurons. The anionic particles selectively bound to excitable neurons, but did not interact with non-excitable glial cells. However, these studies do not have the sufficient resolution (e.g., by electron microscopy) to examine the nanoscale details of nanoparticle interactions with the brain parenchyma.
Extracellular, intracellular, and intercellular transport of nanomaterials
Nanoparticles can passively diffuse in the extracellular space upon injection into the brain. The diffusion of nanoparticles is significantly reduced in the brain compared with in free medium, due to the tortuosity and narrow gap of the extracellular space. Several seminal works in this area have investigated the diffusion of quantum dots in the extracellular space and characterized the diffusion coefficient (Table 2). Recently, Cognet and co-workers have developed a nanoscale imaging technique to track the movement of individual nanotubes in the extracellular space, and use this as a tool to locally map the geometry and rheology in local brain regions149. The results show that the extracellular space is highly heterogeneous in the dimension (down to 40 nm). Hyaluronan is the major diffusion barrier and local tissue organizer and undergoes significant changes in neurodegenerative conditions150. The group also reported photoswitchable single-walled carbon nanotubes for super-resolution imaging in the near-infrared wavelength (>1 μm)151. A hybrid nanomaterial was created by covalently linking photoswitching molecules on the carbon nanotube. The photoswitching molecules control the intrinsic emission of the carbon nanotubes to generate a photoswitchable carbon nanotube with controllable blinking for localization and super-resolution imaging.
Active transport of nanoparticles in the brain involves the retrograde axonal transport and anterograde transport. Retrograde axonal transport conveys materials from axon to cell body and travels long distances (millimeters) along neuronal projections. The nanomaterial surface charge and endosome uptake have an effect on the intracellular transport152. In primary mouse cortical neurons, negatively charged polystyrene NPs smaller than 100 nm undergo axonal retrograde transport upon uptake by the axons and accumulate in the soma. In cortical neurons, negatively charged polystyrene nanoparticles inside lysosomes and 40 nm positively charged polystyrene nanoparticles undergo slow axonal transport, while negatively charged free polystyrene nanoparticles outside lysosomes undergo fast axonal transport mediated by dynein. Anterograde transport of exogenous particles was firstly reported in 1980s. Particles with size up to 500 nm, e.g., polystyrene nanoparticles, travelled rapidly along the axon in the anterograde direction after microinjection into crab axons. Studies in squid axons shown that green fluorescent protein-labeled herpes simplex virus underwent anterograde axonal transport at an average speed of 0.9 μm/sec, four times faster than that of mitochondria and ten times faster than negative fluorescence beads153. The motor protein kinesin mediates the anterograde axonal transport of vesicles, organelles and particles (Figure 5B)154. Nanoparticles can enter nerve terminals and undergo axonal transport to neuron cell bodies after intrapulmonary155, intramuscular or intradermal administration156.
In glial and neuronal cells, nanoparticle intercellular transport is mediated by membrane nanotubes, i.e., tunneling-nanotube (TNT)-like structures. TNTs mediate the intercellular transport of various cellular components by generating membrane continuity between cells, a process facilitated by forming membranous F-actin rich structures between cells. Studies show QDs are actively transported via membrane nanotubes between cardiac myocytes with a mean velocity of 1.23 μm/s157. Surface modification of nanoparticles with neural cell adhesion molecule 1 (NCAM1) and CD44 antibodies was not found to change the cell-to-cell transport mechanism in neurons158.
The immune response of nanomaterials
Mechanical insertion of bulky implants and subsequent micromotions within the skull leads to immune activation and glial scar formation that encapsulates the implants. This can displace neurons of interest, decrease the overall performance, and remodel the structure and function of the neural network surrounding the implant5. Lessons learned from minimizing electrode implants suggest that implants smaller than individual cell bodies or nerve fibers may overcome the limitations of traditional electrodes159, without being recognized by the immune system. Compared with bulk implants, nanotransducers can elicit less immune response due to their small size. McKenzie et al. reported that nanoscale carbon fibers with diameter less than or equal to 100 nm inhibited the adhesion of astrocytes (glial scar tissue-forming cells) and decreased astrocyte proliferation compared with fibers larger than 100 nm160. In addition, this study also showed that surface coating of carbon fibers with some polymers, such as polycarbonate urethane, can also effectively inhibit astrocyte adhesion and decrease astrocyte proliferation, thus, leading to decreased glial scar tissue formation. In addition to formation of glia scar, exogenous nanomaterials may also induce transcriptomic changes in microglia. Yang et al reported that single-walled carbon nanotubes upregulated genes specific to the immune response and induced morphological changes in SIM-A9 microglial cells in vitro161. The transcriptomic and morphological changes in microglia were mitigated by surface coating with PEGylated phospholipids.
Nanomaterials clearance
Understanding the retention and clearance of nanomaterials in the brain is important for the design and application of nanotransducers-enabled neuromodulation. Firstly, the retention and clearance time varies significantly for different nanomaterials. While one study suggests a long retention of magnetic nanoparticles for weeks7, another study showed that two organic nanoparticles, reconstituted high density lipoprotein and poly(ethylene glycol)-b-poly(lactic acid) nanoparticles, were cleared rapidly from the brain following intraparenchymal administration, with half-life less than 5 hours162. The rapid clearance may facilitate applications for transient neuromodulation while the long retention time is beneficial for chronic neuromodulation. Secondly, it was suggested that microglia-mediated transport mediates the nanoparticle clearance via the glymphatic pathway162. Lastly, an impaired glymphatic system such as in Alzheimer’s disease can significantly slow down the clearance of interstitial solutes and nanoparticles162. This suggests the need to take the brain disease into account when analyzing brain clearance of nanotransducers.
Conclusion and outlook
Approaches for non-invasive and remote control of neuronal activity are important for interrogation of neural systems and treatment of many neurological diseases. There has been significant interest to develop wireless neuromodulation with nanotransducers and it has shown significant potential in terms of non-invasiveness, spatiotemporal precision, cell-type specificity, deep brain penetration, and translation to large animals and humans. The capability to transduce various energy modalities into local stimuli and interface with the neural system opens up new possibilities to modulate neural activity. New innovations in combining the energy modalities can overcome the limitations of single energy modality and may continue to drive the field to the next phase of development. For example, sono-optogenetics36 utilizes mechanoluminescent nanoparticles and takes advantage of the deep penetration of ultrasound with the high spatiotemporal control of optogenetic modulation.
A better understanding of the nanomaterials-brain interface is critical for the success of nanotransducers for neuromodulation. However, limited work has been done in this area. Future research should develop minimally invasive approaches to deliver nanomaterials in local brain regions, and gain a better understanding of the immune response, cellular interaction, and retention and clearance of the nanomaterials in the brain. This is particularly important for applications requiring long-term utility of modulation.
One important consideration is the size and the complexity of the energy sources, which may limit broader dissemination and the clinical translation of nanotransducers-enabled neuromodulation techniques. For clinical use, the energy sources, such as light sources, magnetic and ultrasound system, have to be small and/or portable. Recently, implanting biocompatible light sources that emit lights with specific wavelength have been used to overcome the limited tissue penetration of excitation lights for optogenetics163. A portable acoustic system for neuromodulation has been developed to potentially broaden the use of ultrasound systems164. Most of these studies are still in their very early stage. However, with advances in smaller and portable energy sources, we will be one step closer to broader laboratory use and clinical translation of nanotransducer-enabled neuromodulation techniques.
A promising direction for neuromodulation is genetically engineered functional materials. Currently, electrical modulation with electrodes or nanotransducers do not show cell type specificity. Recently Liu et al. genetically modified neurons to express peroxidase enzyme to synthesize electrically functional polymers that are conductive or insulating on the cell membrane, upon local infusion of precursor reagents165. The in situ synthesized conductive polymers changes neuron membrane electrical properties and allows cell type-specific neuron and behavioral modulation in living animals. This work may inspire the creation of diverse and complex functional materials to seamlessly interface with the nervous system and allow next generation neuromodulation techniques.
Another area of significant interest is to integrate sensing capabilities for bidirectional closed-loop neuromodulation and allow bidirectional communication with the local brain microenvironment. Nanotransducers with sensing ability will enable neuronal activity-guided neuromodulation, increasing the temporal precision and avoiding unwanted side effects due to overstimulation. Furthermore, distributed transducer systems that can communicate individually or with each other provide powerful tools to sense and modulate more brain activity. This could be useful, especially for mapping complex neuronal signaling in different brain regions. Advances in genetics, materials science and engineering will provide us next generation nanotransducers with advanced functions. Ultimately, the emerging applications of new nanomaterials at the neural interfaces will lead towards promising nanotransducers to modulate neural activity without physical wiring and transgenes. The capability to precisely modulate neural activity in deep brain regions with high spatiotemporal control enabled by those tools will strongly enhance the power to understand, modify and control the human CNS.
In addition to enhancing understanding the signal transmission and processing in the CNS, nanotransducer-enabled wireless neuromodulation has shown the therapeutic potential for CNS disorders. Nanotransducer-enabled neural inhibition can restore normal firing of a hyperactive neuronal network and providing therapeutic effects for some brain disorders associated with neuronal hyperactivity, such as epilepsy and Parkinson’s disease63. Ye et al. showed that gold nanorods mediated neural inhibition of the left stellate ganglion can alleviate myocardial ischemia-induced ventricular arrhythmias44. Stimulation of nanotransducers has the potential to restore the ion channel equilibrium for diseases that exhibit off-balance ratio of excitatory to inhibitory ion channels/receptors, such as fragile X syndrome (FXS)14. Another emerging application for neuromodulation is the vision repair. Opto-stimulation of retinal neurons mediated by semiconducting polymer nanoparticles can rescue vision, suggesting its potential for retinitis pigmentosa treatment11. With more clinically translatable techniques being developed, nanotransducer-enabled neuromodulation will pave the way for therapies for many brain disorders.
Progress and Potential.
In the last 3~5 years, there have been significant interest and advances in the transformative potential of nanotransducers for neuromodulation. Many nanotransducer-based neuromodulation techniques have been developed recently, including sono-optogenetics enabled by mechanoluminescent nanoparticles and semiconducting polymer nanoparticles-mediated photoelectrical neuromodulation. Nanotransducers have demonstrated their clinical potentials, such as gold nanorods for restoring light sensitivity and alleviating ventricular arrhythmias. This review provides the current state-of-the-art for nanotransducer-enabled neuromodulation and discusses the latest major advances and debates in using nanotransducers to modulate and interface with the nervous system. Future directions include a better understanding of nanomaterials-brain interface and development of the next generation of nanotransducers with sensing ability to bidirectionally communicate with local environment.
Highlights.
Nanotransducers allow wireless neuromodulation by converting external stimuli into local signals.
Nanotransducers modulate brain activity through optogenetic, mechanical, thermal, electrical and chemical modalities.
Further translation of nanotransducer-based neuromodulation requires a better understanding of the nano-brain interface.
Future development includes nanotransducers with sensing ability to bidirectionally communicate with local brain environment.
Acknowledgement
This work was partially supported by National Science Foundation (1631910), and National Institute of Neurological Disorders and Stroke of the National Institutes of Health (RF1NS110499). Schematics were created with BioRender.com
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
The authors declare no competing interests.
References
- 1.Hong G and Lieber CM (2019). Novel electrode technologies for neural recordings. Nat. Rev. Neurosci 20, 330–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woods GA, Rommelfanger NJ and Hong G (2020). Bioinspired materials for in vivo bioelectronic neural interfaces. Matter. 3, 1087–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shemesh OA, Tanese D, Zampini V, Linghu C, Piatkevich K, Ronzitti E, Papagiakoumou E, Boyden ES and Emiliani V (2017). Temporally precise single-cell-resolution optogenetics. Nat. Neurosci 20, 1796–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang JB, Aryal M, Zhong Q, Vyas DB and Airan RD (2018). Noninvasive ultrasonic drug uncaging maps whole-brain functional networks. Neuron. 100, 728–738 e727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salatino JW, Ludwig KA, Kozai TDY and Purcell EK (2017). Glial responses to implanted electrodes in the brain. Nat. Biomed. Eng 1, 862–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Duan H, and Pu K (2019). Nanotransducers for near-Infrared photoregulation in biomedicine. Adv. Mater 31, e1901607. [DOI] [PubMed] [Google Scholar]
- 7.Chen R, Romero G, Christiansen MG, Mohr A and Anikeeva P (2015). Wireless magnetothermal deep brain stimulation. Science. 347, 1477–1480. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Che Z, Mazhar K, Price TJ and Qin Z (2017). Ultrafast near-Infrared light-triggered intracellular uncaging to probe cell signaling. Adv. Funct. Mater 27, 1605778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakatsuji H, Numata T, Morone N, Kaneko S, Mori Y, Imahori H, and Murakami T (2015). Thermosensitive ion channel activation in single neuronal cells by using surface-engineered plasmonic nanoparticles. Angew. Chem. Int. Ed 54, 11725–11729. [DOI] [PubMed] [Google Scholar]
- 10.DiFrancesco ML, Lodola F, Colombo E, Maragliano L, Bramini M, Paterno GM, Baldelli P, Serra MD, Lunelli L, Marchioretto M, Grasselli G, Cimo S, Colella L, Fazzi D, Ortica F, Vurro V, Eleftheriou CG, Shmal D, Maya-Vetencourt JF, Bertarelli C, Lanzani G and Benfenati F (2020). Neuronal firing modulation by a membrane-targeted photoswitch. Nat.Nanotechnol 15, 296–306. [DOI] [PubMed] [Google Scholar]
- 11.Maya-Vetencourt JF, Manfredi G, Mete M, Colombo E, Bramini M, Di Marco S, Shmal D, Mantero G, Dipalo M, Rocchi A, DiFrancesco ML, Papaleo ED, Russo A, Barsotti J, Eleftheriou C, Di Maria F, Cossu V, Piazza F, Emionite L, Ticconi F, Marini C, Sambuceti G, Pertile G, Lanzani G, and Benfenati F (2020). Subretinally injected semiconducting polymer nanoparticles rescue vision in a rat model of retinal dystrophy. Nat. Nanotechnol 15, 698–708. [DOI] [PubMed] [Google Scholar]
- 12.Guduru R, Liang P, Hong J, Rodzinski A, Hadjikhani A, Horstmyer J, Levister E and Khizroev S (2015). Magnetoelectric ‘spin’ on stimulating the brain. Nanomedicine (Lond). 10, 2051–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munshi R, Qadri SM, Zhang Q, Castellanos Rubio I, Del Pino P and Pralle A (2017). Magnetothermal genetic deep brain stimulation of motor behaviors in awake, freely moving mice. Elife. 6, e27069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tay A and Di Carlo D (2017). Magnetic nanoparticle-based mechanical stimulation for restoration of mechano-sensitive ion channel equilibrium in neural networks. Nano Lett. 17, 886–892. [DOI] [PubMed] [Google Scholar]
- 15.Engels J and Schlaeger EJ (1972). Synthesis, structure, and reactivity of adenosine cyclic 3’,5’-phosphate benzyl triesters. J. Med. Chem 20, 907–911. [DOI] [PubMed] [Google Scholar]
- 16.Bechtereva NP, Bondartchuk AN and Smirnov VM (1972). Therapeutic electrostimulations of the deep brain strucutres. Vopr. Neirokhir 36, 7–12. [PubMed] [Google Scholar]
- 17.Barker AT, Jalinous R and Freeston IL (1985). Non-invasive magnetic stimulation of human motor cortex. Lancet. 1, 1106–1107. [DOI] [PubMed] [Google Scholar]
- 18.Boyden ES, Zhang F, Bamberg E, Nagel G and Deisseroth K (2005). Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci 8, 1263–1268. [DOI] [PubMed] [Google Scholar]
- 19.Nagel G, Ollig D, Fuhrmann M, Kateriya S, Musti AM, Bamberg E and Hegemann P (2002) Channelrhodopsin-1: a light-gated proton channel in green algae. Science. 296, 2395–2398. [DOI] [PubMed] [Google Scholar]
- 20.Wells J, Kao C, Jansen ED, Konrad P and Mahadevan-Jansen A (2005). Application of infrared light for in vivo neural stimulation. J. Biomed. Opt 10, 064003. [DOI] [PubMed] [Google Scholar]
- 21.Lugo K, Miao X, Rieke F and Lin LY (2012). Remote switching of cellular activity and cell signaling using light in conjunction with quantum dots. J. Biomed. Opt 3, 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang H, Delikanli S, Zeng H, Ferkey DM and Pralle A (2010). Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nat. Nanotechnol 5, 602–606. [DOI] [PubMed] [Google Scholar]
- 23.Tufail Y, Matyushov A, Baldwin N, Tauchmann ML, Georges J, Yoshihiro A, Tillery SI and Tyler WJ (2010). Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron. 66, 681–694. [DOI] [PubMed] [Google Scholar]
- 24.Deisseroth KA, P.A. Upconversion of light for use in optogenetic methods. United States Patent 2011, PCT/US2011/ 059287.
- 25.Ghezzi D, Antognazza MR, Dal Maschio M, Lanzarini E, Benfenati F and Lanzani G (2011). A hybrid bioorganic interface for neuronal photoactivation. Nat. Commun 2, 166. [DOI] [PubMed] [Google Scholar]
- 26.Yong J, Needham K, Brown WG, Nayagam BA, McArthur SL, Yu A and Stoddart PR (2014). Gold-nanorod-assisted near-infrared stimulation of primary auditory neurons. Adv. Healthc. Mater 3, 1862–1868. [DOI] [PubMed] [Google Scholar]
- 27.Tay A, Sohrabi A, Poole K, Seidlits S and Di Carlo D (2018). A 3D Magnetic hyaluronic acid hydrogel for magnetomechanical neuromodulation of primary dorsal root ganglion neurons. Adv. Mater e1800927. [DOI] [PubMed] [Google Scholar]
- 28.Ibsen S, Tong A, Schutt C, Esener S and Chalasani SH (2015). Sonogenetics is a non-invasive approach to activating neurons in Caenorhabditis elegans. Nat. Commun 6, 8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marino A, Arai S, Hou Y, Sinibaldi E, Pellegrino M, Chang YT, Mazzolai B, Mattoli V, Suzuki M and Ciofani G (2015). Piezoelectric nanoparticle-assisted wireless neuronal stimulation. ACS. Nano 9, 7678–7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wheeler MA, Smith CJ, Ottolini M, Barker BS, Purohit AM, Grippo RM, Gaykema RP, Spano AJ, Beenhakker MP, Kucenas S, Patel MK, Deppmann CD and Guler AD (2016). Genetically targeted magnetic control of the nervous system. Nat. Neurosci 19, 756–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanley SA, Kelly L, Latcha KN, Schmidt SF, Yu X, Nectow AR, Sauer J, Dyke JP, Dordick JS and Friedman JM (2016). Bidirectional electromagnetic control of the hypothalamus regulates feeding and metabolism. Nature. 531, 647–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang HQ, Zhou XQ, Cui D, Liu RX, Tan RX, Wang X, Liu ZP and Yin T (2019). Comparative Study of Transcranial Magneto-Acoustic Stimulation and transcranial ultrasound stimulation of motor cortex. Front. Behav. Neurosci 13, 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romero G, Christiansen MG, Barbosa LS, Garcia F and Anikeeva P (2016). Localized excitation of neural activity via rapid magnetothermal drug Release. Adv. Funct. Mater 26, 6471–6478. [Google Scholar]
- 34.Airan RD, Meyer RA, Ellens NP, Rhodes KR, Farahani K, Pomper MG, Kadam SD, and Green JJ (2017). Noninvasive targeted transcranial neuromodulation via focused ultrasound gated drug release from nanoemulsions. Nano Lett. 17, 652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szablowski JO, Lee-Gosselin A, Lue B, Malounda D and Shapiro MG (2018). Acoustically targeted chemogenetics for the non-invasive control of neural circuits. Nat. Biomed. Eng 2, 475–484. [DOI] [PubMed] [Google Scholar]
- 36.Wu X, Zhu X, Chong P, Liu J, Andre LN, Ong KS, Brinson K Jr., Mahdi AI, Li J, Fenno LE, Wang H and Hong G (2019). Sono-optogenetics facilitated by a circulation-delivered rechargeable light source for minimally invasive optogenetics. Proc. Natl. Acad. Sci. U S A 116, 26332–26342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin JY, Knutsen PM, Muller A, Kleinfeld D and Tsien RY (2013). ReaChR, a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat. Neurosci 16, 1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berry MH, Holt A, Salari A, Veit J, Visel M, Levitz J, Aghi K, Gaub BM, Sivyer B, Flannery JG and Isacoff EY (2019). Restoration of high-sensitivity and adapting vision with a cone opsin. Nat. Commun 10, 1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin JY (2011). A user’s guide to channelrhodopsin variants: features, limitations and future developments. Exp. Physiol 96, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen S, Weitemier AZ, Zeng X, He L, Wang X, Tao Y, Huang AJY, Hashimotodani Y, Kano M, Iwasaki H, Parajuli LK, Okabe S, Teh DBL, All AH, Tsutsui-Kimura I, Tanaka KF, Liu X and McHugh TJ (2018). Near-infrared deep brain stimulation via upconversion nanoparticle-mediated optogenetics. Science. 359, 679–684. [DOI] [PubMed] [Google Scholar]
- 41.Wen S, Zhou J, Schuck PJ, Suh YD, Schmidt TW, and Jin D (2019). Future and challenges for hybrid upconversion nanosystems. Nat. Photonics 13, 828–838. [Google Scholar]
- 42.Owen SF, Liu MH and Kreitzer AC (2019). Thermal constraints on in vivo optogenetic manipulations. Nat. Neurosci 22, 1061–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ao Y, Zeng K, Yu B, Miao Y, Hung W, Yu Z, Xue Y, Tan TTY, Xu T, Zhen M, Yang X, Zhang Y and Gao S (2019). An upconversion nanoparticle enables near infrared-optogenetic manipulation of the caenorhabditis elegans motor circuit. ACS Nano. 13, 3373–3386. [DOI] [PubMed] [Google Scholar]
- 44.Ye TY, Lai YQ, Wang ZY, Zhang XG, Meng GN, Zhou LP, Zhang YF, Zhou Z, Deng JL, Wang M, Wang YH, Zhang QQ, Zhou XY, Yu LL, Jiang H and Xiao XH (2019). Precise modulation of gold nanorods for protecting against malignant ventricular arrhythmias via near-Infrared neuromodulation. Adv. Funct. Mater 29, 1902128. [Google Scholar]
- 45.Rastogi SK, Garg R, Scopelliti MG, Pinto BI, Hartung JE, Kim S, Murphey CGE, Johnson N, San Roman D, Bezanilla F, Cahoon JF, Gold MS, Chamanzar M and Cohen-Karni T (2020). Remote nongenetic optical modulation of neuronal activity using fuzzy graphene. Proc. Natl. Acad. Sci. U S A 117, 13339–13349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lyu Y, Xie C, Chechetka SA, Miyako E and Pu K (2016). Semiconducting polymer nanobioconjugates for targeted photothermal activation of neurons. J. Am. Chem. Soc 138, 9049–9052. [DOI] [PubMed] [Google Scholar]
- 47.Nelidova D, Morikawa RK, Cowan CS, Raics Z, Goldblum D, Scholl HPN, Szikra T, Szabo A, Hillier D, and Roska B (2020). Restoring light sensitivity using tunable near-infrared sensors. Science. 368, 1108–1113. [DOI] [PubMed] [Google Scholar]
- 48.Kang PY, Li XQ, Liu YN, Shiers SI, Xiong HJ, Giannotta M, Dejana E, Price TJ, Randrianalisoa J, Nielsen SO, and Qin ZP (2019). Transient photoinactivation of cell membrane protein activity without genetic modification by molecular hyperthermia. ACS Nano. 13, 12487–12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee JW, Jung H, Cho HH, Lee JH and Nam Y (2018). Gold nanostar-mediated neural activity control using plasmonic photothermal effects. Biomaterials. 153, 59–69. [DOI] [PubMed] [Google Scholar]
- 50.Duret G, Polali S, Anderson ED, Bell AM, Tzouanas CN, Avants BW and Robinson JT (2019). Magnetic entropy as a proposed gating mechanism for magnetogenetic ion channels. Biophys J. 116, 454–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Munshi R, Qadri SM and Pralle A (2018). Transient magnetothermal neuronal silencing using the chloride channel anoctamin 1 (TMEM16A). Front. Neurosci 12, 560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang Y, Jiang Y, Luo X, Wu J, Zong H, Shi L, heng R, Jiang S, Jia X, Mei J, Man H, Cheng J and Yang C. (2021). Neural stimulation in vitro and in vivo by photoacoustic nanotransducers. Matter. 4, 675–687. [Google Scholar]
- 53.Jiang Y, Lee HJ, Lan L, Tseng HA, Yang C, Man HY, Han X, and Cheng JX (2020). Optoacoustic brain stimulation at submillimeter spatial precision. Nat. Commun 11, 881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gregurec D, Senko AW, Chuvilin A, Reddy PD, Sankararaman A, Rosenfeld D, Chiang PH, Garcia F, Tafel I, Varnavides G, Ciocan E and Anikeeva P (2020). Magnetic vortex nanodiscs enable remote magnetomechanical neural stimulation. ACS Nano. 14, 8036–8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang G, Zhang P, Mendu SK, Wang Y, Zhang Y, Kang X, Desai BN, and Zhu JJ (2019). Revaluation of magnetic properties of Magneto. Nat. Neurosci 23, 1047–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wheeler MA, Deppmann CD, Patel MK and Guler AD (2019). Reply to, Magneto is ineffective in controlling electrical properties of cerebellar Purkinje cells, Assessing the utility of Magneto to control neuronal excitability in the somatosensory cortex and revaluation of magnetic properties of Magneto. Nat. Neurosci 23, 1051–1054. [DOI] [PubMed] [Google Scholar]
- 57.Kole K, Zhang Y, Jansen EJR, Brouns T, Bijlsma A, Calcini N, Yan X, Lantyer ADS and Celikel T(2019). Assessing the utility of Magneto to control neuronal excitability in the somatosensory cortex. Nat. Neurosci 23, 1044–1046. [DOI] [PubMed] [Google Scholar]
- 58.Jalali HB, Aria MM, Dikbas UM, Sadeghi S, Kumar BG, Sahin M, Kavakli IH, Ow-Yang CW and Nizamoglu S (2018). Effective neural photostimulation using indium-based type-II quantum dots. ACS Nano. 12, 8104–8114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rand D, Jakesova M, Lubin G, Vebraite I, David-Pur M, Derek V, Cramer T, Sariciftci NS, Hanein Y and Glowacki ED (2018). Direct electrical neurostimulation with organic pigment photocapacitors. Adv. Mater 30, e1707292. [DOI] [PubMed] [Google Scholar]
- 60.Tang J, Qin N, Chong Y, Diao Y, Yiliguma, Wang Z, Xue T, Jiang M, Zhang J and Zheng G (2018). Nanowire arrays restore vision in blind mice. Nat. Commun 9, 786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao X, Jiang Y, Lin Y, Kim KH, Fang Y, Yi J, Meng L, Lee HC, Lu Z, Leddy O, Zhang R, Tu Q, Feng W, Nair V, Griffin PJ, Shi F, Shekhawat GS, Dinner AR, Park HG and Tian B (2020). Structured silicon for revealing transient and integrated signal transductions in microbial systems. Sci. Adv 6, eaay2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang Y, Li X, Liu B, Yi J, Fang Y, Shi F, Gao X, Sudzilovsky E, Parameswaran R, Koehler K, Nair V, Yue J, Guo K, Fang Y, Tsai HM, Freyermuth G, Wong RCS, Kao CM, Chen CT, Nicholls AW, Wu X, Shepherd GMG and Tian B (2018). Rational design of silicon structures for optically controlled multiscale biointerfaces. Nat. Biomed. Eng 2, 508–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yue K, Guduru R, Hong J, Liang P, Nair M and Khizroev S (2012). Magneto-electric nano-particles for non-invasive brain stimulation. PloS One. 7, e44040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rojas C, Tedesco M, Massobrio P, Marino A, Ciofani G, Martinoia S, and Raiteri R (2018). Acoustic stimulation can induce a selective neural network response mediated by piezoelectric nanoparticles. J. Neural. Eng 15, 036016. [DOI] [PubMed] [Google Scholar]
- 65.Cabre G, Garrido-Charles A, Moreno M, Bosch M, Porta-de-la-Riva M, Krieg M, Gascon-Moya M, Camarero N, Gelabert R, Lluch JM, Busque F, Hernando J, Gorostiza P, and Alibes R (2019). Rationally designed azobenzene photoswitches for efficient two-photon neuronal excitation. Nat. Commun 10, 907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xiong H, Li X, Kang P, Perish J, Neuhaus F, Ploski JE, Kroener S, Ogunyankin MO, Shin JE, Zasadzinski JA, Wang H, Slesinger PA, Zumbuehl A and Qin Z (2020). Near-infrared light triggered-release in deep brain regions using ultra-photosensitive nanovesicles. Angew. Chem. Int 59, 8608–8615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kondaveeti S, Semeano ATS, Cornejo DR, Ulrich H and Petri DFS (2018). Magnetic hydrogels for levodopa release and cell stimulation triggered by external magnetic field. Colloids. Surf. B. Biointerfaces 167, 415–424. [DOI] [PubMed] [Google Scholar]
- 68.Rao S, Chen R, LaRocca AA, Christiansen MG, Senko AW, Shi CH, Chiang PH, Varnavides G, Xue J, Zhou Y, Park S, Ding R, Moon J, Feng G and Anikeeva P (2019). Remotely controlled chemomagnetic modulation of targeted neural circuits. Nat. Nanotechnol 14, 967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park J, Tabet A, Moon J, Chiang PH, Koehler F, Sahasrabudhe A and Anikeeva P (2020). Remotely controlled proton generation for neuromodulation. Nano Lett. 20, 6535–6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schreiber R, Ousingsawat J, Wanitchakool P, Sirianant L, Benedetto R, Reiss K and Kunzelmann K (2018). Regulation of TMEM16A/ANO1 and TMEM16F/ANO6 ion currents and phospholipid scrambling by Ca(2+) and plasma membrane lipid. J. Physiol 596, 217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yao J, Liu B, and Qin F (2010). Kinetic and energetic analysis of thermally activated TRPV1 channels. Biophys. J 99, 1743–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang YS, Fan CH, Hsu N, Chiu NH, Wu CY, Chang CY, Wu BH, Hong SR, Chang YC, Yan-Tang Wu A, Guo V, Chiang YC, Hsu WC, Chen L, Pin-Kuang Lai C, Yeh CK and Lin YC (2020). Sonogenetic modulation of cellular activities using an engineered auditory-sensing protein. Nano Lett. 20, 1089–1100. [DOI] [PubMed] [Google Scholar]
- 73.Ye J, Tang SY, Meng L, Li X, Wen XX, Chen SH, Niu LL, Li XY, Qiu WB, Hu HL, Jiang MZ, Shang SQ, Shu Q, Zheng HR, Duan SM and Li YZ (2018). Ultrasonic control of neural activity through activation of the mechanosensitive channel MscL. Nano Lett. 18, 4148–4155. [DOI] [PubMed] [Google Scholar]
- 74.Wu CY, Fan CH, Chiu NH, Ho YJ, Lin YC, and Yeh CK (2020). Targeted delivery of engineered auditory sensing protein for ultrasound neuromodulation in the brain. Theranostics. 10, 3546–3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lea-Banks H, O’Reilly MA, Hamani C, and Hynynen K (2020). Localized anesthesia of a specific brain region using ultrasound-responsive barbiturate nanodroplets. Theranostics. 10, 2849–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoo S, Hong S, Choi Y, Park JH, and Nam Y (2014). Photothermal inhibition of neural activity with near-infrared-sensitive nanotransducers. ACS Nano. 8, 8040–8049. [DOI] [PubMed] [Google Scholar]
- 77.Wang YX, Feng LH, and Wang S (2019). Conjugated polymer nanoparticles for imaging, cell activity regulation, and therapy. Adv. Funct. Mater 29, 1806818. [Google Scholar]
- 78.Roet M, Hescham SA, Jahanshahi A, Rutten BPF, Anikeeva PO, and Temel Y (2019). Progress in neuromodulation of the brain: A role for magnetic nanoparticles? Prog. Neurobiol 177, 1–14. [DOI] [PubMed] [Google Scholar]
- 79.Huang H, Delikanli S, Zeng H, Ferkey DM and Pralle A (2010). Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nat. Nanotechnol 5, 602–606. [DOI] [PubMed] [Google Scholar]
- 80.Thorne RG and Nicholson C (2006). In vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proc. Natl. Acad. Sci. U S A 103, 5567–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Householder KT, Dharmaraj S, Sandberg DI, Wechsler-Reya RJ and Sirianni RW (2019). Fate of nanoparticles in the central nervous system after intrathecal injection in healthy mice. Sci. Rep 9, 12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Svaasand LO, and Ellingsen R (1983). Optical properties of human brain. Photochem. Photobiol 38, 293–299. [DOI] [PubMed] [Google Scholar]
- 83.Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, Garlow SJ, Barrocas A, Wint D, Craighead MC, Kozarsky J, Chismar R, Moreines JL, Mewes K, Posse PR, Gutman DA, and Mayberg HS (2012). Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch. Gen. Psychiatry 69, 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mace E, Montaldo G, Cohen I, Baulac M, Fink M, and Tanter M (2011). Functional ultrasound imaging of the brain. Nat. Methods 8, 662–664. [DOI] [PubMed] [Google Scholar]
- 85.Ron M and Rob P (2015). Cell Biology by the Numbers 1st Edition. Garland Science [Google Scholar]
- 86.Ahmari SE and Smith SJ (2002). Knowing a nascent synapse when you see it. Neuron. 34, 333–336. [DOI] [PubMed] [Google Scholar]
- 87.Wright BL, Lai JT and Sinclair AJ (2012). Cerebrospinal fluid and lumbar puncture: a practical review. J. Neurol 259, 1530–1545. [DOI] [PubMed] [Google Scholar]
- 88.Pardridge WM (2016). CSF, blood-brain barrier, and brain drug delivery. Expert Opin. Drug. Deliv 13, 963–75. [DOI] [PubMed] [Google Scholar]
- 89.Tonnesen J, Inavalli V, and Nagerl UV (2018). Super-resolution imaging of the extracellular space in living brain tissue. Cell. 172, 1108–1121. e15. [DOI] [PubMed] [Google Scholar]
- 90.Kitagawa T (2012). Newborn screening for inborn errors of metabolism in Japan. A history of the development of newborn screening. Pediatr. Endocrinol. Rev 10 Suppl 1, 8–25. [PubMed] [Google Scholar]
- 91.Ferenczi EA, Tan X and Huang CL (2019). Principles of optogenetic methods and their application to cardiac experimental systems. Front. Physiol 10, 1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mackenzie LE, Goode JA, Vakurov A, Nampi PP, Saha S, Jose G and Millner PA (2018). The theoretical molecular weight of NaYF4 :RE upconversion nanoparticles. Sci. Rep 8, 1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cruz-Rangel S, De Jesus-Perez JJ, Contreras-Vite JA, Perez-Cornejo P, Hartzell HC and Arreola J (2015). Gating modes of calcium-activated chloride channels TMEM16A and TMEM16B. J. Physiol 593, 5283–5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shin KC, Park HJ, Kim JG, Lee IH, Cho H, Park C, Sung TS, Koh SD, Park SW and Bae YM (2019). The Piezo2 ion channel is mechanically activated by low-threshold positive pressure. Sci. Rep 9, 6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang L, Zhou H, Zhang M, Liu W, Deng T, Zhao Q, Li Y, Lei J, Li X and Xiao B (2019). Structure and mechanogating of the mammalian tactile channel PIEZO2. Nature. 573, 225–229. [DOI] [PubMed] [Google Scholar]
- 96.Jakesova M, Ejneby MS, Derek V, Schmidt T, Gryszel M, Brask J, Schindl R, Simon DT, Berggren M, Elinder F and Glowacki ED (2019). Optoelectronic control of single cells using organic photocapacitors. Sci. Adv 5, eaav5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miao Q, Xie C, Zhen X, Lyu Y, Duan H, Liu X, Jokerst JV, and Pu K (2017). Molecular afterglow imaging with bright, biodegradable polymer nanoparticles. Nat. Biotechnol 35,1102–1110. [DOI] [PubMed] [Google Scholar]
- 98.Li J and Pu K (2020). Semiconducting polymer nanomaterials as near-Infrared photoactivatable protherapeutics for cancer. Acc. Chem. Res 53, 752–762. [DOI] [PubMed] [Google Scholar]
- 99.Wu XYJ, Rommelfanger NJ, Yin R, Liu J, Cai S, Ren W, Shin A, Ong KS, Pu K, Hong G (2020). Through-scalp deep-brain stimulation in tether-free, naturally-behaving mice with widefield NIR-II illumination. BioRxiv, 10.1101/2020.05.29.123075. [DOI] [Google Scholar]
- 100.Honore E (2007). The neuronal background K2P channels: focus on TREK1. Nat. Rev. Neurosci 8, 251–261. [DOI] [PubMed] [Google Scholar]
- 101.Shapiro MG, Homma K, Villarreal S, Richter CP, and Bezanilla F (2012). Infrared light excites cells by changing their electrical capacitance. Nat. Commun 3, 736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tan X, Jahan I, Xu Y, Stock S, Kwan CC, Soriano C, Xiao X, Garcia-Anoveros J, Fritzsch B, and Richter CP (2018). Auditory neural activity in congenitally deaf mice induced by infrared neural stimulation. Sci. Rep 8, 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Feng HJ, Kao C, Gallagher MJ, Jansen ED, Mahadevan-Jansen A, Konrad PE and Macdonald RL (2010). Alteration of GABAergic neurotransmission by pulsed infrared laser stimulation. J. Neurosci. Methods 192, 110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cayce JM, Friedman RM, Chen G, Jansen ED, Mahadevan-Jansen A and Roe AW (2014) Infrared neural stimulation of primary visual cortex in non-human primates. NeuroImage. 84, 181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Izzo AD, Walsh JT Jr., Ralph H, Webb J, Bendett M, Wells J and Richter CP (2008). Laser stimulation of auditory neurons: effect of shorter pulse duration and penetration depth. Biophys. J 94, 3159–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Davis HC, Kang S, Lee JH, Shin TH, Putterman H, Cheon J and Shapiro MG (2020) Nanoscale Heat Transfer from Magnetic Nanoparticles and Ferritin in an Alternating Magnetic Field. Biophys. J 118, 1502–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wu J, Goyal R and Grandl J (2016). Localized force application reveals mechanically sensitive domains of Piezo1. Nat. Commun 7, 12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barbic M (2019). Possible magneto-mechanical and magneto-thermal mechanisms of ion channel activation in magnetogenetics. Elife. 8, e45807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hernandez-Morales M, Shang T, Chen J, Han V, and Liu C (2020). Lipid oxidation induced by RF waves and mediated by ferritin iron causes activation of ferritin-tagged ion channels. Cell. Rep 30, 3250–3260. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brier MI, Mundell JW, Yu X, Su L, Holmann A, Squeri J, Zhang B, Stanley SA, Friedman JM and Dordick JS (2020). Uncovering a possible role of reactive oxygen species in magnetogenetics. Sci. Rep 10, 13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mosabbir AA and Truong K (2018). Genetically encoded circuit for remote regulation of cell migration by magnetic fields. ACS Synth. Biol 7, 718–726. [DOI] [PubMed] [Google Scholar]
- 112.Hutson MR, Keyte AL, Hernandez-Morales M, Gibbs E, Kupchinsky ZA, Argyridis I, Erwin KN, Pegram K, Kneifel M, Rosenberg PB, Matak P, Xie L, Grandl J, Davis EE, Katsanis N, Liu C and Benner EJ (2017). Temperature-activated ion channels in neural crest cells confer maternal fever-associated birth defects. Sci. Signal 10, eaal4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xu FX, Zhou L, Wang XT, Jia F, Ma KY, Wang N, Lin L, Xu FQ and Shen Y (2019). Magneto is ineffective in controlling electrical properties of cerebellar Purkinje cells. Nat. Neurosci 23, 1041–1043. [DOI] [PubMed] [Google Scholar]
- 114.Meister M (2016). Physical limits to magnetogenetics. Elife. 5, e17210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Legon W, Ai L, Bansal P and Mueller JK (2018). Neuromodulation with single-element transcranial focused ultrasound in human thalamus. Hum. Brain Mapp 39, 1995–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Qiu Z, Guo J, Kala S, Zhu J, Xian Q, Qiu W, Li G, Zhu T, Meng L, Zhang R, Chan HC, Zheng H and Sun L (2019). The mechanosensitive ion channel Piezo1 significantly mediates in vitro ultrasonic stimulation of neurons. iScience. 21, 448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Oh SJ, Lee JM, Kim HB, Lee J, Han S, Bae JY, Hong GS, Koh W, Kwon J, Hwang ES, Woo DH, Youn I, Cho IJ, Bae YC, Lee S, Shim JW, Park JH and Lee CJ (2020). Ultrasonic neuromodulation via astrocytic TRPA1. Curr. Biol 30, 948. [DOI] [PubMed] [Google Scholar]
- 118.Qiu Z, Kala S, Guo J, Xian Q, Zhu J, Zhu T, Hou X, Wong KF, Yang M, Wang H and Sun L (2020). Targeted neurostimulation in mouse brains with non-invasive ultrasound. Cell. Rep 32, 108033. [DOI] [PubMed] [Google Scholar]
- 119.Towne CL and Andersen DE (2019). Minimally-invasive, high resolution neuromodulation of deep brain and cortical structures using circuit-specific promoters and focused ultrasound array (US20190321473A1, United States).
- 120.Sato T, Shapiro MG, and Tsao DY (2018). Ultrasonic neuromodulation causes widespread cortical activation via an indirect auditory mechanism. Neuron. 98, 1031–1041. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guo H, Hamilton M 2nd, Offutt SJ, Gloeckner CD, Li T, Kim, Legon W, Alford JK, and Lim HH (2018). Ultrasound produces extensive brain activation via a cochlear pathway. Neuron. 98, 1020–1030. e4. [DOI] [PubMed] [Google Scholar]
- 122.Parameswaran R, Carvalho-de-Souza JL, Jiang Y, Burke MJ, Zimmerman JF, Koehler K, Phillips AW, Yi J, Adams EJ, Bezanilla F, and Tian B (2018). Photoelectrochemical modulation of neuronal activity with free-standing coaxial silicon nanowires. Nat. Nanotechnol 13, 260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Patriarchi T, Cho JR, Merten K, Howe MW, Marley A, Xiong WH, Folk RW, Broussard GJ, Liang R, Jang MJ, Zhong H, Dombeck D, von Zastrow M, Nimmerjahn A, Gradinaru V, Williams JT, and Tian L (2018). Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science. 360, eaat4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sun F, Zeng J, Jing M, Zhou J, Feng J, Owen SF, Luo Y, Li F, Wang H, Yamaguchi T, Yong Z, Gao Y, Peng W, Wang L, Zhang S, Du J, Lin D, Xu M, Kreitzer AC, Cui G and Li Y (2018). A genetically encoded fluorescent sensor enables rapid and specific detection of dopamine in flies, fish, and mice. Cell. 174, 481–496 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sun F, Zhou J, Dai B, Qian T, Zeng J, Li X, Zhuo Y, Zhang Y, Wang Y, Qian C, Tan K, Feng J, Dong H, Lin D, Cui G, and Li Y (2020). Next-generation GRAB sensors for monitoring dopaminergic activity in vivo. Nat. Methods 17, 1156–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Patriarchi T, Mohebi A, Sun J, Marley A, Liang R, Dong C, Puhger K, Mizuno GO, Davis CM, Wiltgen B, von Zastrow M, Berke JD, and Tian L (2020). An expanded palette of dopamine sensors for multiplex imaging in vivo. Nat. Methods 17, 1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jing M, Li Y, Zeng J, Huang P, Skirzewski M, Kljakic O, Peng W, Qian T, Tan K, Zou J, Trinh S, Wu R, Zhang S, Pan S, Hires SA, Xu M, Li H, Saksida LM, Prado VF, Bussey TJ, Prado MAM, Chen L, Cheng H, and Li Y (2020). An optimized acetylcholine sensor for monitoring in vivo cholinergic activity. Nat. Methods 17, 1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wan J, Peng W, Li X, Qian T, Song K, Zeng J, Deng F, Hao S, Feng. J, Zhang P, Zhang Y, Zou J, Pan S, J. hu J, Jing M, Xu M and Yulong Li. (2020). A genetically encoded GRAB sensor for measuring serotonin dynamics in vivo. BioRxiv, 10.1101/2020.02.24.962282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Feng J, Zhang C, Lischinsky JE, Jing M, Zhou J, Wang H, Zhang Y, Dong A, Wu Z, Wu H, Chen W, Zhang P, Zou J, Hires SA, Zhu JJ, Cui G, Lin D, Du J and Li Y (2019). A genetically encoded fluorescent sensor for rapid and specific in vivo detection of norepinephrine. Neuron. 102, 745–761 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dong A, He K, Dudok B, Farrell JS, Guan W, Liput DJ, Puhl HL, Cai R, Duan J, Albarran E, Ding J, Lovinger DM, Li B, Soltesz I, and Li Y (2020). A fluorescent sensor for spatiotemporally resolved endocannabinoid dynamics. BioRxiv, doi: 10.1101/2020.10.08.329169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mignocchi N, Krussel. S, Jung K, Lee D and Kwon H. (2020). Development of a genetically-encoded oxytocin sensor. BioRxiv, 10.1101/2020.07.14.202598. [DOI] [Google Scholar]
- 132.Ding K, Han Y, Seid TW, Buser C, Karigo T, Zhang S, Dickman DK and Anderson DJ (2019). Imaging neuropeptide release at synapses with a genetically engineered reporter. Elife. 8, e46421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Beyene AG, Alizadehmojarad AA, Dorlhiac G, Goh N, Streets AM, Kral P, Vukovic L and Landry MP (2018). Ultralarge modulation of fluorescence by neuromodulators in carbon nanotubes functionalized with self-assembled oligonucleotide rings. Nano Lett. 18, 6995–7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Beyene AG, Delevich K, Del Bonis-O’Donnell JT, Piekarski DJ, Lin WC, Thomas AW, Yang SJ, Kosillo P, Yang D, Prounis GS, Wilbrecht L and Landry MP (2019). Imaging striatal dopamine release using a nongenetically encoded near infrared fluorescent catecholamine nanosensor. Sci. Adv 5, eaaw3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jeong S, Yang D, Beyene AG, Del Bonis-O’Donnell JT, Gest AMM, Navarro N, Sun X and Landry MP (2019). High-throughput evolution of near-infrared serotonin nanosensors. Sci. Adv 5, eaay3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bonis-O’Donnell JTD, Page RH, Beyene AG, Tindall EG, McFarlane IR and Landry MP, (2017). Dual near-infrared two-photon microscopy for deep-tissue dopamine nanosensor imaging. Adv. Funct. Mater 27, 1702112. [Google Scholar]
- 137.Yang W and Yuste R (2017). In vivo imaging of neural activity. Nat. Methods 14, 349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Allegra Mascaro AL, Silvestri L, Sacconi L and Pavone FS (2015). Towards a comprehensive understanding of brain machinery by correlative microscopy. J. Biomed. Opt 20, 61105. [DOI] [PubMed] [Google Scholar]
- 139.Vite CH, McGowan JC, Niogi SN, Passini MA, Drobatz KJ, Haskins ME and Wolfe JH (2005). Effective gene therapy for an inherited CNS disease in a large animal model. Ann. Neurol 57, 355–364. [DOI] [PubMed] [Google Scholar]
- 140.Nance E, Zhang F, Mishra MK, Zhang Z, Kambhampati SP, Kannan RM and Kannan S (2016). Nanoscale effects in dendrimer-mediated targeting of neuroinflammation. Biomaterials. 101, 96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schoch KM and Miller TM (2017). Antisense oligonucleotides: translation from mouse models to human neurodegenerative diseases. Neuron. 94, 1056–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hinderer C, Bell P, Katz N, Vite CH, Louboutin JP, Bote E, Yu H, Zhu Y, Casal ML, Bagel J, O’Donnell P, Wang P, Haskins ME, Goode T and Wilson JM (2018). Evaluation of intrathecal routes of administration for adeno-associated viral vectors in large animals. Hum. Gene. Ther 29, 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Blasberg RG, Patlak C and Fenstermacher JD (1975). Intrathecal chemotherapy: brain tissue profiles after ventriculocisternal perfusion. J. Pharmacol. Exp. Ther 195, 73–83. [PubMed] [Google Scholar]
- 144.Landis MS, Boyden T and Pegg S (2012). Nasal-to-CNS drug delivery: where are we now and where are we heading? An industrial perspective. Ther. Deliv 3, 195–208. [DOI] [PubMed] [Google Scholar]
- 145.Dal Magro R, Ornaghi F, Cambianica I, Beretta S, Re F, Musicanti C, Rigolio R, Donzelli E, Canta A, Ballarini E, Cavaletti G, Gasco P and Sancini G (2017). ApoE-modified solid lipid nanoparticles: A feasible strategy to cross the blood-brain barrier. J. Control. Release 249, 103–110. [DOI] [PubMed] [Google Scholar]
- 146.Jenkins SI, Weinberg D, Al-Shakli AF, Fernandes AR, Yiu HHP, Telling ND, Roach P and Chari DM (2016). ‘Stealth’ nanoparticles evade neural immune cells but also evade major brain cell populations: Implications for PEG-based neurotherapeutics. J. Control. Release 224, 136–145. [DOI] [PubMed] [Google Scholar]
- 147.Song E, Gaudin A, King AR, Seo YE, Suh HW, Deng Y, Cui J, Tietjen GT, Huttner A, and Saltzman WM (2017). Surface chemistry governs cellular tropism of nanoparticles in the brain. Nat. Commun 8, 15322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dante S, Petrelli A, Petrini EM, Marotta R, Maccione A, Alabastri A, Quarta A, De Donato F, Ravasenga T, Sathya A, Cingolani R, Proietti Zaccaria R, Berdondini L, Barberis A, and Pellegrino T (2017). Selective targeting of neurons with inorganic nanoparticles: revealing the crucial role of nanoparticle surface charge. ACS Nano. 11, 6630–6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Godin AG, Varela JA, Gao Z, Danne N, Dupuis JP, Lounis B, Groc L, and Cognet L (2017). Single-nanotube tracking reveals the nanoscale organization of the extracellular space in the live brain. Nat. Nanotechnol 12, 238–243. [DOI] [PubMed] [Google Scholar]
- 150.Soria FN, Paviolo C, Doudnikoff E, Arotcarena ML, Lee A, Danne N, Mandal AK, Gosset P, Dehay B, Groc L, Cognet L, and Bezard E (2020). Synucleinopathy alters nanoscale organization and diffusion in the brain extracellular space through hyaluronan remodeling. Nat. Commun 11, 3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Godin AG, Setaro A, Gandil M, Haag R, Adeli M, Reich S, and Cognet L (2019). Photoswitchable single-walled carbon nanotubes for super-resolution microscopy in the near-infrared. Sci. Adv 5, eaax1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lesniak A, Kilinc D, Blasiak A, Galea G, Simpson JC and Lee GU (2019). Rapid growth cone uptake and dynein-mediated axonal retrograde transport of negatively charged nanoparticles in neurons Is dependent on size and cell type. Small. 15, e1803758. [DOI] [PubMed] [Google Scholar]
- 153.Satpute-Krishnan P, DeGiorgis JA and Bearer EL (2003). Fast anterograde transport of herpes simplex virus: role for the amyloid precursor protein of alzheimer’s disease. Aging. Cell 2, 305–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kramer T, Greco TM, Taylor MP, Ambrosini AE, Cristea IM and Enquist LW (2012). Kinesin-3 mediates axonal sorting and directional transport of alphaherpesvirus particles in neurons. Cell. Host. Microbe 2012, 12, 806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hopkins LE, Patchin ES, Chiu PL, Brandenberger C, Smiley-Jewell S, and Pinkerton KE (2014). Nose-to-brain transport of aerosolised quantum dots following acute exposure. Nanotoxicology. 8, 885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Filler AG, Whiteside GT, Bacon M, Frederickson M, Howe FA, Rabinowitz MD, Sokoloff AJ, Deacon TW, Abell C, Munglani R, Griffiths JR, Bell BA and Lever AM (2010). Tri-partite complex for axonal transport drug delivery achieves pharmacological effect. BMC Neurosci. 11, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.He K, Luo W, Zhang Y, Liu F, Liu D, Xu L, Qin L, Xiong C, Lu Z, Fang X, and Zhang Y (2010). Intercellular transportation of quantum dots mediated by membrane nanotubes. ACS Nano. 4, 3015–3022. [DOI] [PubMed] [Google Scholar]
- 158.Tosi G, Vilella A, Chhabra R, Schmeisser MJ, Boeckers TM, Ruozi B, Vandelli MA, Forni F, Zoli M, and Grabrucker AM (2014). Insight on the fate of CNS-targeted nanoparticles. Part II: Intercellular neuronal cell-to-cell transport. J. Control. Release 177, 96–107. [DOI] [PubMed] [Google Scholar]
- 159.Zhou T, Hong G, Fu TM, Yang X, Schuhmann TG, Viveros RD, and Lieber CM (2017). Syringe-injectable mesh electronics integrate seamlessly with minimal chronic immune response in the brain. Proc. Natl. Acad. Sci. U S A 114, 5894–5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.McKenzie JL, Waid MC, Shi R and Webster TJ (2004). Decreased functions of astrocytes on carbon nanofiber materials. Biomaterials. 25, 1309–1317. [DOI] [PubMed] [Google Scholar]
- 161.Yang D, Yang SJ, Del Bonis-O’Donnell JT, Pinals RL, and Landry MP (2020). Mitigation of carbon nanotube neurosensor induced transcriptomic and morphological changes in mouse microglia with surface passivation. ACS Nano. 14, 13794–13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gu X, Song Q, Zhang Q, Huang M, Zheng M, Chen J, Wei D, Chen J, Wei X, Chen H, Zheng G, and Gao X (2020) Clearance of two organic nanoparticles from the brain via the paravascular pathway. J. Control. Release 322, 31–41. [DOI] [PubMed] [Google Scholar]
- 163.Matarese BFE, Feyen PLC, de Mello JC and Benfenati F (2019). Sub-millisecond control of neuronal firing by organic light-emitting diodes. Front. Bioeng. Biotechnol 7, 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Qiu W, Zhou J, Chen Y, Su M, Li G, Zhao H, Gu X, Meng, Wang C, Xiao Y, Lam KH, Dai J and Zheng H (2017). A portable ultrasound system for non-invasive ultrasonic neuro-stimulation. IEEE Trans. Neural. Syst. Rehabil. Eng 25, 2509–2515. [DOI] [PubMed] [Google Scholar]
- 165.Liu J, Kim YS, Richardson CE, Tom A, Ramakrishnan C, Birey F, Katsumata T, Chen S, Wang C, Wang X, Joubert LM, Jiang Y, Wang H, Fenno LE, Tok JB, Pasca SP, Shen K, Bao Z and Deisseroth K (2020). Genetically targeted chemical assembly of functional materials in living cells, tissues, and animals. Science. 367, 1372–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]


